Dynamics of Bioactive Compounds and Their Relationship with Antioxidant and Antimicrobial Activity in the Pulp, Peel, and Seeds of ‘Salak’ During Ripening
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Physicochemical Analyses
2.2.1. Mineral Determination
2.2.2. Phytochemical Screening
2.3. Analysis of Bioactive Compounds
2.3.1. Vitamin C
2.3.2. Organic Acid Profile
2.3.3. Carotenoid Profile
2.3.4. Chlorophylls and Their Derivatives
2.3.5. Phenol Profile
2.4. Antioxidant Activity Analyses
2.5. Antimicrobial Activity Analyses
2.6. Statistical Analysis
3. Results
3.1. Physico-Chemical Characteristics
| Pulp | Peel | Seed | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M1 | M2 | M3 | M1 | M2 | M3 | AM1 | AM2 | AM3 | |
| pH | 3.3 ± 0.1 b | 3.3 ± 0.1 b | 3.5 ± 0.0 a | 5.3 ± 0.0 a | 5.4 ± 0.1 a | 5.2 ± 0.0 a | 6.4 ± 0.1 a | 6.4 ± 0.1 a | 6.3 ± 0.0 a | *** | *** | *** |
| SS (°Brix) | 15.3 ± 2.1 b | 19.0 ± 1.0 ab | 20.3 ± 1.5 a | 1.0 ± 0.0 b | 1.3 ± 0.6 b | 2.7 ± 0.6 a | 2.0 ± 0.0 a | 2.7 ± 0.6 a | 2.3 ± 0.6 a | *** | *** | *** |
| TA (%) | 0.7 ± 0.1 ab | 0.9 ± 0.1 a | 0.7 ± 0.0 b | 0.2 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.2 ± 0.0 a | 0.2 ± 0.1 a | 0.2 ± 0.0 a | *** | *** | *** |
| Humidity (%) | 80.9 ± 0.8 a | 79.5 ± 0.3 b | 80.0 ± 0.4 ab | 69.5 ± 4.9 a | 73.2 ± 2.5 a | 56.7 ± 4.0 b | 64.9 ± 0.2 a | 57.3 ± 0.1 b | 59.2 ± 1.7 b | *** | *** | *** |
| Ash (%) | 0.4 ± 0.0 b | 0.4 ± 0.1 b | 0.6 ± 0.1 a | 1.9 ± 0.1 b | 1.3 ± 0.2 c | 3.1 ± 0.2 a | 0.9 ± 0.0 a | 0.9 ± 0.3 a | 1.2 ± 0.0 a | *** | * | *** |
| Mineral profile (mg/100 g DW) | ||||||||||||
| Ca | 124.8 ± 11.7 a | 92.2 ± 12.2 a | 111.5 ± 29.5 a | 1199.7 ± 154.9 a | 1105.5 ± 82.3 a | 1121.3 ± 59.9 a | 204.9 ± 8.8 a | 111.3 ± 1.7 b | 132.9 ± 21.3 b | ** | *** | *** |
| Fe | nd | nd | 12.4 ± 0.4 a | 6.3 ± 0.1 b | 6.4 ± 0.6 b | 10.2 ± 0.1 a | 5.6 ± 0.8 a | 6.2 ± 0.4 a | 6.3 ± 0.7 a | *** | *** | ** |
| K | 1197.2 ± 56.3 a | 988.0 ± 5.7 b | 1125.4 ± 34.0 ab | 1930.5 ± 263.8 a | 1462.9 ± 91.8 a | 1859.5 ± 120.2 a | 1073.9 ± 14.6 a | 1004.1 ± 62.4 a | 1066.5 ± 53.3 a | * | ** | ** |
| Mg | 606.5 ± 36.8 a | 497.1 ± 38.5 a | 499.2 ± 21.5 a | 782.4 ± 21.7 a | 900.1 ± 122.7 a | 864.0 ± 152.0 a | 1002.6 ± 34.8 a | 870.1 ± 75.1 a | 910.2 ± 148.6 a | ** | * | * |
| Na | 9172.4 ± 69.8 a | 7009.9 ± 36.2 b | 6193.6 ± 45.2 b | 16,064.9 ± 194.3 a | 12,362.9 ± 97.9 a | 16,384.5 ± 6.3 a | 4164.1 ± 923.7 a | 2698.4 ± 167.8 a | 2106.3 ± 513.2 a | ** | ** | *** |
3.2. Analysis of Bioactive Compounds
3.3. Antimicrobial Activity Analyses
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| QUPS | Universidad Politécnica Salesiana Sede Quito |
References
- Cosme, F.; Pinto, T.; Aires, A.; Morais, M.; Bacelar, E.; Anjos, R.; Ferreira, J.; Oliveira, I.; Vilela, A.; Gonçalves, B. Red Fruits Composition and Their Health Benefits—A Review. Foods 2022, 11, 644. [Google Scholar] [CrossRef] [PubMed]
- Dhalaria, R.; Verma, R.; Kumar, D.; Puri, S.; Tapwal, A.; Kumar, V.; Nepovimova, E.; Kuca, K. Bioactive Compounds of Edible Fruits with Their Anti-Aging Properties: A Comprehensive Review to Prolong Human Life. Antioxidants 2020, 9, 1123. [Google Scholar] [CrossRef]
- Vasconcelos, O.; Carvalhoo, R.; Teixeira-Costa, B. Sources of Carotenoids in Amazonian Fruits. Molecules 2024, 29, 2190. [Google Scholar] [CrossRef]
- Santos, A.; Catuzo, G.; Silva, C.; Rahal, I.; Sena, S.; Camargo, R.; Dias, A. Plantas Alimentícias Não Convencionais: Revisão. Arq. Ciências Saúde UNIPAR 2022, 26, 1068–1090. Available online: https://revistas.unipar.br/index.php/saude/article/view/8995 (accessed on 11 September 2025).
- Munekata, P.; Pateiro, M.; Domínguez, R.; Nieto, G.; Kumar, M.; Dhama, K.; Lorenzo, J. Bioactive Compounds from Fruits as Preservatives. Foods 2023, 12, 343. [Google Scholar] [CrossRef]
- Kumoro, A.; Alhanif, M.; Wardhani, D. A Critical Review on Tropical Fruits Seeds as Prospective Sources of Nutritional and Bioactive Compounds for Functional Foods Development: A Case of Indonesian Exotic Fruits. Int. J. Food Sci. 2020, 2020, 1–15. [Google Scholar] [CrossRef]
- Hussain, H.; Mamadalieva, N.; Hussain, A.; Hassan, U.; Rabnawaz, A.; Ahmed, I.; Green, I. Fruit Peels: Food Waste as a Valuable Source of Bioactive Natural Products for Drug Discovery. Curr. Issues Mol. Biol. 2022, 44, 1960–1994. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Tumpa, A.; Zehravi, M.; Sarker, T.; Yamin; Islam, R.; Harun-Or-Rashid; Ahmed, M.; Ramproshad, S.; Mondal, B.; et al. An Overview of Antimicrobial Stewardship Optimization: The Use of Antibiotics in Humans and Animals to Prevent Resistance. Antibiotics 2022, 11, 667. [Google Scholar] [CrossRef]
- Salam, A.; Al-amin, Y.; Salam, M.; Pawar, J.; Akhter, N.; Rabaan, A.; Alqumber, M. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Sato, V.; Chewchinda, S.; Goli, A.; Sato, H.; Nontakham, J.; Vongsak, B. Oral Glucose Tolerance Test (OGTT) Evidence for the Postprandial Anti-Hyperglycemic Property of Salacca zalacca (Gaertn.) Voss Seed Extract. Molecules 2023, 28, 6775. [Google Scholar] [CrossRef] [PubMed]
- Coyago-Cruz, E.; Guachamin, A.; Villacís, M.; Rivera, J.; Neto, M.; Méndez, G.; Heredia-Moya, J.; Vera, E. Evaluation of Bioactive Compounds and Antioxidant Activity in 51 Minor Tropical Fruits of Ecuador. Foods 2023, 12, 4439. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Siddiqui, M.; Mediani, A.; Ismail, N.; Ahmed, Q.; Zation, S.; Saidi-Besbes, S. Salacca Zalacca: A Short Review of the Palm Botany, Pharmacological Uses and Phytochemistry. Asian Pac. J. Trop. Med. 2018, 11, 645. [Google Scholar] [CrossRef]
- Yahia, E. Salak (Salacca zalacca (Gaertner) Voss). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Woodhead Publishing, Limited: Cambridge, UK, 2011; Volume 4, pp. 334–348. ISBN 978-0-85709-090-4. [Google Scholar]
- Cepková, P.; Jágr, M.; Janovská, D.; Dvoracek, V.; Kozak, K.; Viehmannová, I. Comprehensive Mass Spectrometric Analysis of Snake Fruit: Salak (Salacca zalacca). J. Food Quali 2021, 6621811, 1–12. [Google Scholar]
- Wulansari, N.; Padmiswari, A.; Manik, I. The Effectiveness Probiotic Drink of Salak Bali (Salacca zalacca) in Inhibiting Growth of Escherichia Coli. J. Biol. Trop. 2022, 22, 934–939. [Google Scholar] [CrossRef]
- Djaafar, T.; Marwati, T.; Budiyanti, T.; Riska; Hadiati, S. Chemical and Sensory Evaluation on Several Varieties of Salak (Salacca zalacca) Fruit from Indonesia. Int. J. Adv. Sci. Eng. Inf. Technol. 2024, 14, 723–729. [Google Scholar] [CrossRef]
- Widowati, W.; Vera, D.; Yuninda, V. Antioxidant and Antiaging Potential of Salak Fruit Extract (Salacca zalacca (Gaert.) Voss)). Tradit. Med. J. 2023, 28, 230–236. [Google Scholar] [CrossRef]
- ISO 18421991; ISO Fruits, Vegetables and Derived Products. Determination of PH. ISO: Geneva, Switzerland, 2000.
- Coyago-Cruz, E.; Méndez, G.; Escobar-Quiñonez, R.; Cerna, M.; Heredia-Moya, J. Lacmellea Oblongata and Other Undervalued Amazonian Fruits as Functional, Antioxidant, and Antimicrobial Matrices. Antioxidants 2025, 14, 23. [Google Scholar] [CrossRef]
- US ISO 2173; ISO Fruit and Vegetable Products. Determination of Soluble Solids. Refractometric Method. ISO: Geneva, Switzerland, 2009.
- ISO-5520; Fruits, Vegetables and Derived Products—Determination of Alkalinity of Total Ash and of Water-Soluble Ash. International Standard ISO: Geneva, Switzerland, 1981.
- León-Fernández, A.; Balois Morales, R.; Bautista-Rosales, P.; Palomino-Hermosillo, Y.; Bello-Lara, J.; López-Rivas, C. Extracción de Compuestos Fitoquímicos de Inflorescencia y Frutos de Guanábana (Annona muricata L.). Acta Agrícola Pecu. 2021, 7, 1–12. [Google Scholar] [CrossRef]
- Coyago-Cruz, E.; Guachamin, A.; Méndez, G.; Moya, M. Marti Functional and Antioxidant Evaluation of Two Ecotypes of Control and Grafted Tree Tomato (Solanum betaceum) at Different Altitudes. Foods 2023, 12, 3494. [Google Scholar] [CrossRef] [PubMed]
- Macrae, R.; Schweigert, B. HPLC in Food Analysis (Food Science and Technology), 2nd ed.; Academic Press: Cambridge, MA, USA, 1988; Volume 1. [Google Scholar]
- Rivero-Pérez, M.D.; Muñiz, P.; González-Sanjosé, M.L. Antioxidant Profile of Red Wines Evaluated by Total Antioxidant Capacity, Scavenger Activity, and Biomarkers of Oxidative Stress Methodologies. J. Agric. Food Chem. 2007, 55, 5476–5483. [Google Scholar] [CrossRef] [PubMed]
- Vinha, A.; Alves, R.; Barreira, S.; Castro, A.; Costa, A.; Oliveira, M. Effect of Peel and Seed Removal on the Nutritional Value and Antioxidant Activity of Tomato (Lycopersicon esculentum L.) Fruits. LWT-Food Sci. Technol. 2014, 55, 197–202. [Google Scholar] [CrossRef]
- CLSI M02; Performance Standards for Antimicrobial Disk Suspectibility Tests, Approved Standard-Eleventh Edition. Clinical and Laboratory Standards Institue: Malvern, PA, USA, 2018.
- Balouiri, M.; Sadiki, M.; Ibnsouda, S. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- CLSI M44-A2; Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts. Approved Guideline—Second Edition. Clinical and Laboratory Standards Institue: Malvern, PA, USA, 2009.
- Coyago-Cruz, E.; Corell, M.; Moriana, A.; Hernanz, D.; Benítez-González, A.M.; Stinco, C.M.; Meléndez-Martínez, A.J. Antioxidants (Carotenoids and Phenolics) Profile of Cherry Tomatoes as Influenced by Deficit Irrigation, Ripening and Cluster. Food Chem. 2018, 240, 870–884. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, G.; Ishibashi, Y.; Iwaya-Inoue, M. Ontogenetic Changes of the Water Status and Accumulated Soluble Compounds in Developing and Ripening Mume (Prunus mume) Fruit Measured by 1H-NMR Analysis. Adv. Hortic. Sci. 2015, 29, 3–12. [Google Scholar]
- Harhar, H.; Gharby, S.; Idrissi, Y.; Pioch, D.; Charrouf, Z.; Tabyaoui, M. Effect of Maturity Stage on the Chemical Composition of Argan Fruit Pulp. OCL J. 2019, 26, 15. [Google Scholar] [CrossRef]
- Coyago-Cruz, E.; Corell, M.; Moriana, A.; Mapelli-Brahm, P.; Hernanz, D.; Stinco, C.M.; Beltrán-Sinchiguano, E.; Meléndez-Martínez, A. Study of Commercial Quality Parameters, Sugars, Phenolics, Carotenoids and Plastids in Different Tomato Varieties. Food Chem. 2019, 277, 480–489. [Google Scholar] [CrossRef]
- Ma, W.; Li, Y.; Nai, G.; Liang, G.; Ma, Z.; Chen, B.; Mao, J. Changes and Response Mechanism of Sugar and Organic Acids in Fruits under Water Deficit Stress. PeerJ 2022, 10, e13691. [Google Scholar] [CrossRef]
- González, J.; Hinojosa, L.; Mercado, M.; Fernández-Turiel, J.; Bazile, D.; Ponessa, G.; Eisa, S.; González, D.; Rejas, M.; Hussin, S.; et al. A Long Journey of Cica-17 Quinoa Variety to Salinity Conditions in Egypt: Mineral Concentration in the Seeds. Plants 2021, 10, 407. [Google Scholar] [CrossRef]
- Popova, V.; Petkova, Z.; Mazova, N.; Ivanova, T.; Petkova, N.; Stoyanova, M.; Stoyanova, A.; Ercisli, S.; Okcu, Z.; Skrovankova, S.; et al. Chemical Composition Assessment of Structural Parts (Seeds, Peel, Pulp) of Physalis alkekengi L. Fruits. Molecules 2022, 27, 5787. [Google Scholar] [CrossRef]
- Djordjevic, B. Phenolics Compounds in Fruits of Different Types of Berries and Their Beneficent for Human Health. Annals Univ. Craiova-Agric. Mont. Cadastre Ser. 2023, 53, 97–107. [Google Scholar] [CrossRef]
- Nhon, T.; Phan, T.; Lien, T.; Van, N.; Dao, T.; Anh, T. Phytochemical Screening, Extraction, and Determination of the Bioactivities of the Extract-Enriched Polyphenols and Saponins from Musa Balbisiana Fruit. J. Food Process. Preserv. 2023, 2023. [Google Scholar] [CrossRef]
- Santamaría, M.; Quintero, A.; Piloni, J.; López, C. Metabolitos Secundarios Con Efectos Tóxicos Presentes En La Semilla de Moringa (Moringa oleifera). Revisión. Cienc. Lat. Rev. Científica Multidiscip. 2023, 7, 9637–9646. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, G.; Ji, X.; Liu, J.; Zhao, T.; Gao, Y.; Gao, S.; Hao, Y.; Gao, Y.; Wang, L. Metabolome and Transcriptome Analysis Reveals Components Regulating Triterpenoid Saponin Biosynthesis of Soapberry. BioRxiv 2022, 2002–2022. [Google Scholar] [CrossRef]
- Gao, M.; Zhao, H.; Zheng, L.; Zhang, L.; Peng, Y.; Ma, W.; Tian, R.; Yuan, Y.; Ma, F.; Li, M.; et al. Overexpression of Apple Ma12, a Mitochondrial Pyrophosphatase Pump Gene, Leads to Malic Acid Accumulation and the Upregulation of Malate Dehydrogenase in Tomato and Apple Calli. Hortic. Res. 2022, 9, uhab053. [Google Scholar] [CrossRef] [PubMed]
- Brasil, I.; Siddiqui, M. Postharvest Quality of Fruits and Vegetables: An Overview. In Preharvest Modulation of Postharvest Fruit and Vegetable Quality; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–40. ISBN 9780128098080. [Google Scholar]
- Coyago-Cruz, E.; Moya, M.; Méndez, G.; Villacís, M.; Rojas-Silva, P.; Corell, M.; Mapelli-Brahm, P.; Vicario, I.; Meléndez-Martínez, A. Exploring Plants with Flowers: From Therapeutic Nutritional Benefits to Innovative Sustainable Uses. Foods 2023, 12, 67. [Google Scholar] [CrossRef]
- Liu, X.; Le, C.; Renard, C. Interactions between Cell Wall Polysaccharides and Polyphenols: Effect of Molecular Internal Structure. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3574–3617. [Google Scholar] [CrossRef] [PubMed]
- Girsang, E.; Ginting, C.; Lister, I.; Wijayanti, C.; Widowati, W.; Rizal, R. Antioxidant Properties of Salacca zalacca (Gaertn.) Voss Peel Ethanolic Extract Compared to Chlorogenic Acid. In Proceedings of the 1st International Conference on Emerging Issues in Technology, Engineering and Science, Online Virtual, 1–2 July 2021; SCITEPRESS—Science and Technology Publications: Setúbal, Portugal, 2021; pp. 87–94. [Google Scholar]
- Zouine, N.; Ghachtouli, N.; Abed, S.; Koraichi, S. A Comprehensive Review on Medicinal Plant Extracts as Antibacterial Agents: Factors, Mechanism Insights and Future Prospects. Sci. Afr. 2024, 26, 25. [Google Scholar] [CrossRef]
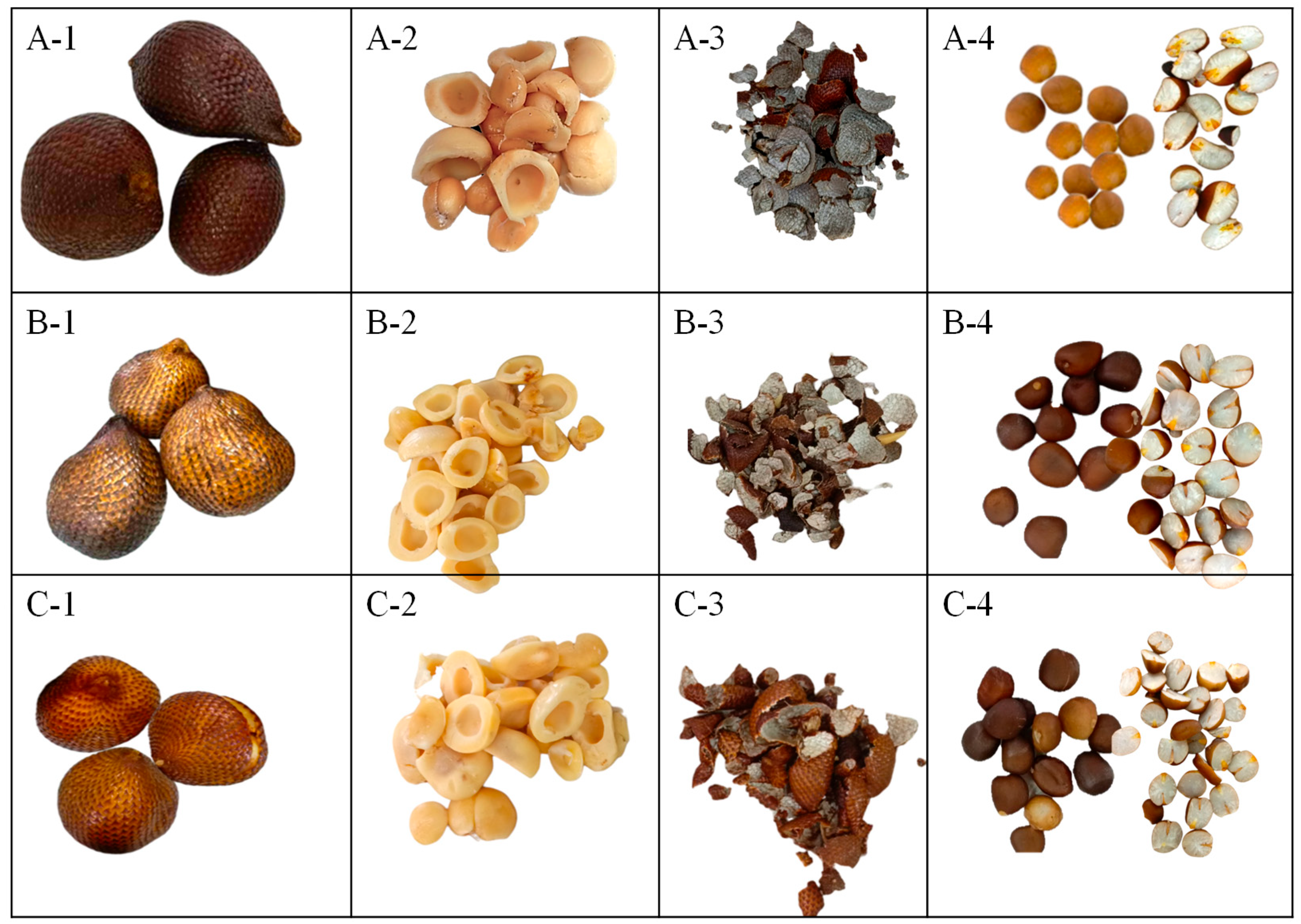
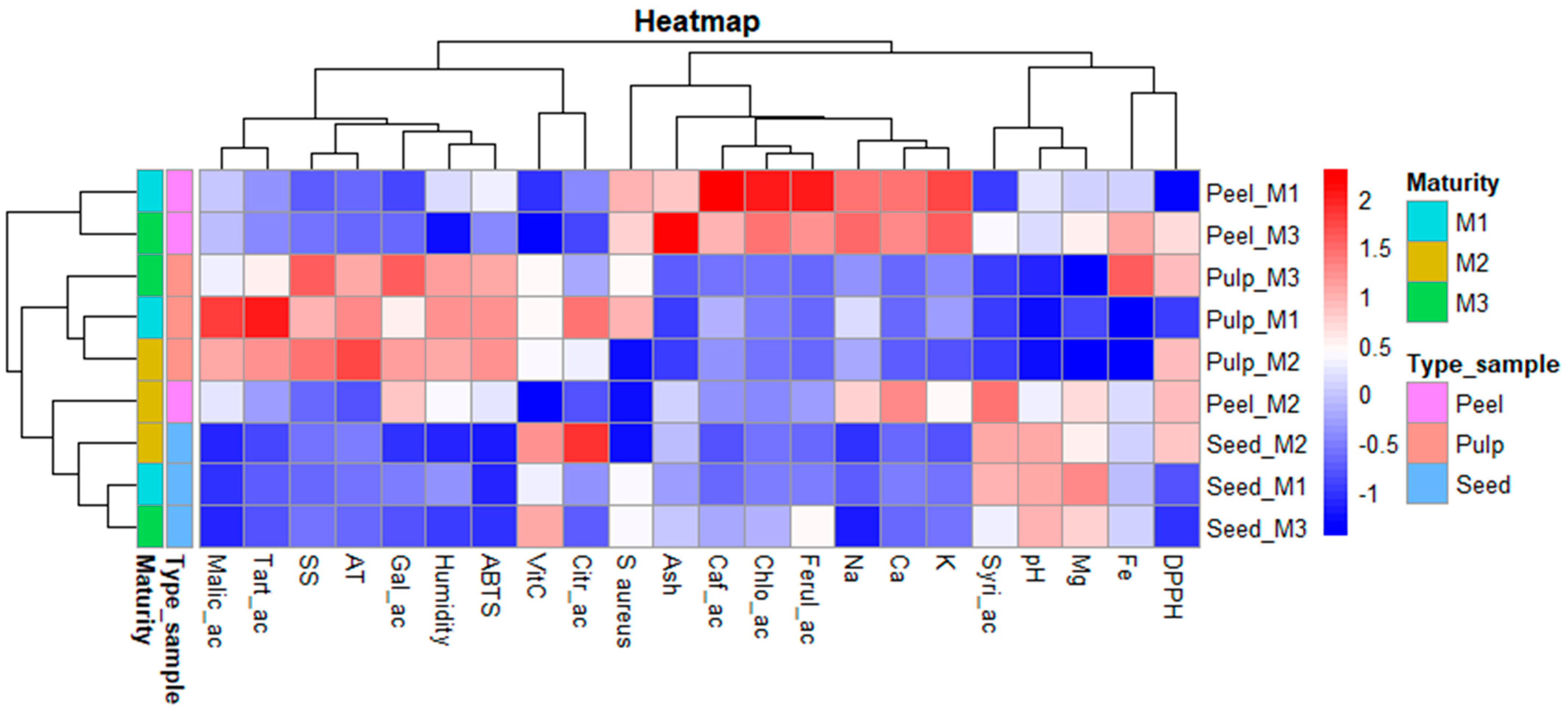
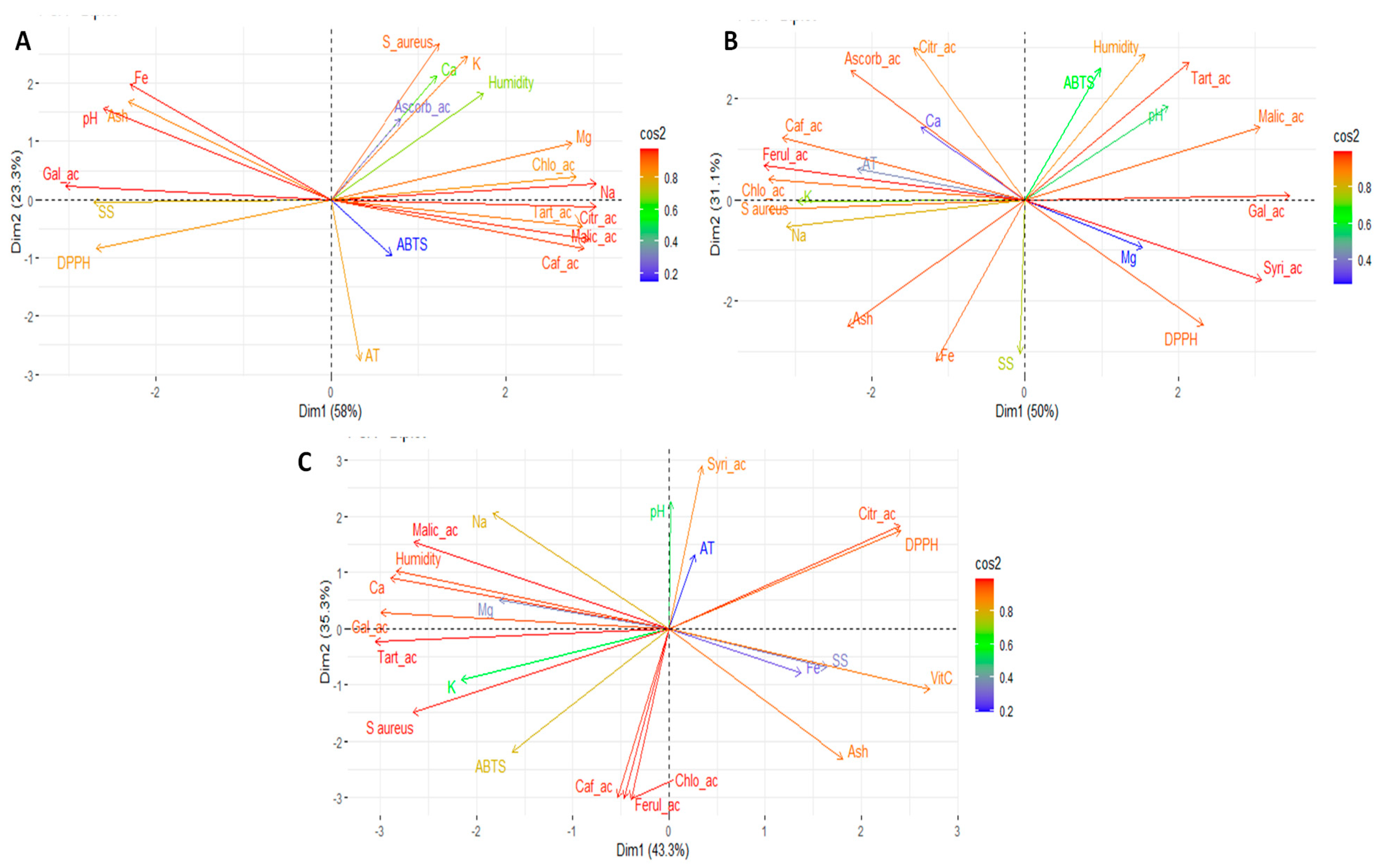
| Pulp | Seed | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M1 | M2 | M3 | AM1 | AM2 | AM3 | |
| Weight (g) | 28.7 ± 3.8 c | 46.2 ± 3.8 b | 67.1 ± 5.9 a | 4.1 ± 0.5 a | 4.3 ± 1.4 a | 4.9 ± 0.7 a | *** | *** | *** |
| LD (mm) | 44.1 ± 3.4 b | 51.3 ± 4.6 ab | 55.9 ± 7.4 a | 16.3 ± 1.8 b | 21.5 ± 0.3 a | 22.3 ± 1.7 a | *** | *** | *** |
| ED (mm) | 35.3 ± 8.9 b | 46.5 ± 1.4 a | 51.9 ± 2.7 a | 15.3 ± 1.7 b | 19.56 ± 0.3 a | 21.2 ± 2.7 a | *** | *** | *** |
| Pulp | Peel | Seed | |||||||
|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M1 | M2 | M3 | M1 | M2 | M3 | |
| Steroids | − | − | − | − | − | − | − | − | − |
| Terpenoids | − | − | − | − | − | − | − | − | − |
| Phenols | + | + | + | + | + | + | + | + | + |
| Tannins | + | + | + | + | + | + | + | + | + |
| Alkaloids | − | − | − | − | − | − | − | − | − |
| Flavonoids | − | − | − | − | − | − | − | − | − |
| Anthraquinones | − | − | − | − | − | − | − | − | − |
| Saponins | + | + | + | − | − | − | + | + | − |
| Acetogenins | − | − | − | − | − | − | − | − | − |
| Pulp | Peel | Seed | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M1 | M2 | M3 | M1 | M2 | M3 | AM1 | AM2 | AM3 | |
| Vitamin C (mg/100 g DW) | 12.2 ± 0.6 a | 11.7 ± 1.12 a | 12.1 ± 0.0 a | 3.5 ± 0.2 a | 1.5 ± 0.1 b | 1.6 ± 0.1 b | 11.4 ± 0.6 b | 16.8 ± 0.9 a | 15.9 ± 0.6 a | *** | *** | *** |
| Organic acid (mg/100 g DW) | ||||||||||||
| Citric acid | 283.9 ± 30.8 a | 187.0 ± 5.1 b | 137.8 ± 11.2 b | 118.7 ± 2.8 a | 83.7 ± 3.2 b | 73.7 ± 12.5 b | 121.7 ± 11.5 b | 322.8 ± 29.3 a | 87.0 ± 17.1 b | ** | ** | * |
| Malic acid | 7232.8 ± 840.6 a | 5459.0 ± 20.1 ab | 3650.3 ± 201.9 b | 2909.2 ± 21.9 b | 3387.3 ± 118.3 a | 2713.6 ± 132.3 b | 197.5 ± 8.6 a | 33.6 ± 1.1 b | 35.4 ± 2.6 b | ** | *** | *** |
| Tartaric acid | 501.9 ± 105.6 a | 364.5 ± 10.3 a | 251.7 ± 13.1 a | 106.0 ± 0.5 a | 112.0 ± 4.4 a | 89.9 ± 0.6 b | 45.5 ± 1.9 a | 16.6 ± 3.8 c | 33.3 ± 2.0 b | *** | *** | *** |
| Total organic acid | 8018.6 a ± 977.0 a | 6010.5 ± 216.7 ab | 4039.8 ± 226.3 b | 3133.9 ± 18.7 ab | 3583.9 ± 125.9 a | 2877.2 ± 145.4 b | 364.8 c ± 22.0 a | 373.1 ± 26.6 a | 155.7 ± 21.7 b | ** | *** | *** |
| Carotenoids (mg/100 g DW) | nd | nd | nd | nd | nd | nd | nd | nd | ||||
| Pheophytin b (mg/100 g DW) | 2.6 ± 0.0 a | 0.7 ± 0.0 b | 0.2 ± 0.0 c | |||||||||
| Phenolics profile (mg/100 g DW) | ||||||||||||
| Gallic acid | 7.5 ± 0.5 b | 9.9 ± 0.5 a | 11.6 ± 0.4 a | 1.8 ± 0.1 b | 8.7 ± 0.6 a | 2.8 ± 0.0 b | 3.5 ± 0.0 a | 1.4 ± 0.1 c | 2.3 ± 0.2 b | *** | *** | *** |
| Syringic acid | 8.4 ± 0.8 a | 4.8 ± 0.4 b | 6.8 ± 0.4 a | 7.1 ± 0.7 a | 4.5 ± 0.3 b | *** | ns | ns | ||||
| Chlorogenic acid | 12.2 ± 1.3 a | 9.0 ± 0.3 ab | 8.3 ± 0.4 b | 705.0 ± 18.9 a | 39.0 ± 3.5 b | 533.2 ± 39.4 a | 18.1 ± 0.3 b | 4.1 ± 0.1 b | 114.4 ± 12.2 a | * | ** | *** |
| Caffeic acid | 78.5 ± 4.9 a | 58.7 ± 1.5 b | 31.1 ± 2.3 c | 321.0 ± 69.8 a | 52.0 ± 3.3 b | 190.8 ± 8.7 ab | 23.8 ± 0.9 b | 14.1 ± 0.0 b | 71.2 ± 9.1 a | *** | *** | *** |
| Ferulic acid | 173.5 ± 0.3 a | 26.3 ± 1.1 c | 123.8 ± 3.1 b | 11.4 ± 0.4 b | nd | 74.2 ± 4.7 a | *** | ns | ** | |||
| Total phenolics | 98.3 ± 6.8 a | 77.7 ± 1.3 b | 51.1 ± 1.5 c | 1201.3 ± 25.8 a | 134.32 ± 0.9 b | 855.5 ± 51.8 a | 63.6 ± 1.2 b | 26.6 ± 0.7 b | 266.7 ± 26.0 a | ** | *** | *** |
| Antioxidant activity (mmol ET/100 g DW) | ||||||||||||
| ABTS | 4.0 ± 0.2 a | 4.1 ± 0.0 a | 3.8 ± 0.4 a | 2.7 ± 0.5 a | 2.7 ± 0.4 a | 1.7 ± 0.2 b | 0.8 ± 0.0 a | 0.6 ± 0.1 b | 0.9 ± 0.1 a | *** | *** | *** |
| DPPH | 2.3 ± 0.1 b | 5.5 ± 0.7 a | 5.5 ± 0.4 a | 1.8 ± 0.3 a | 5.5 ± 0.5 a | 5.1 ± 0.9 a | 2.6 ± 0.5 a | 5.4 ± 0.8 a | 2.3 ± 0.1 b | *** | ns | *** |
| Minimal Inhibitory Concentration (mg/mL) | |||||||
|---|---|---|---|---|---|---|---|
| Bacterial Strain | Fungal Strain | ||||||
| E. coli ATCC 8739 | S. aureus ATCC 6538P | P. aeruginosa ATCC 9027 | S. mutans ATCC 25175 | C. albicans ATCC 1031 | C. tropicalis ATCC 13803 | ||
| Pulp | M1 | 31.9 | 42.6 | 42.6 | 84.9 | na | na |
| M2 | 42.5 | 84.9 | 84.9 | na | na | na | |
| M3 | 41.8 | 31.4 | 83.7 | na | na | na | |
| Peel | M1 | 84.3 | 42.1 | na | na | na | na |
| M2 | 84.2 | 42.1 | na | na | na | na | |
| M3 | 83.3 | 83.3 | na | na | na | na | |
| Seed | M1 | na | na | na | na | na | na |
| M2 | na | na | na | na | na | na | |
| M3 | na | na | na | na | na | na | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coyago-Cruz, E.; Méndez, G.; Zúñiga-Miranda, J.; Jami, N.; Acurio-Vásconez, R.; Heredia-Moya, J. Dynamics of Bioactive Compounds and Their Relationship with Antioxidant and Antimicrobial Activity in the Pulp, Peel, and Seeds of ‘Salak’ During Ripening. Foods 2025, 14, 3476. https://doi.org/10.3390/foods14203476
Coyago-Cruz E, Méndez G, Zúñiga-Miranda J, Jami N, Acurio-Vásconez R, Heredia-Moya J. Dynamics of Bioactive Compounds and Their Relationship with Antioxidant and Antimicrobial Activity in the Pulp, Peel, and Seeds of ‘Salak’ During Ripening. Foods. 2025; 14(20):3476. https://doi.org/10.3390/foods14203476
Chicago/Turabian StyleCoyago-Cruz, Elena, Gabriela Méndez, Johana Zúñiga-Miranda, Nubia Jami, Ramiro Acurio-Vásconez, and Jorge Heredia-Moya. 2025. "Dynamics of Bioactive Compounds and Their Relationship with Antioxidant and Antimicrobial Activity in the Pulp, Peel, and Seeds of ‘Salak’ During Ripening" Foods 14, no. 20: 3476. https://doi.org/10.3390/foods14203476
APA StyleCoyago-Cruz, E., Méndez, G., Zúñiga-Miranda, J., Jami, N., Acurio-Vásconez, R., & Heredia-Moya, J. (2025). Dynamics of Bioactive Compounds and Their Relationship with Antioxidant and Antimicrobial Activity in the Pulp, Peel, and Seeds of ‘Salak’ During Ripening. Foods, 14(20), 3476. https://doi.org/10.3390/foods14203476











