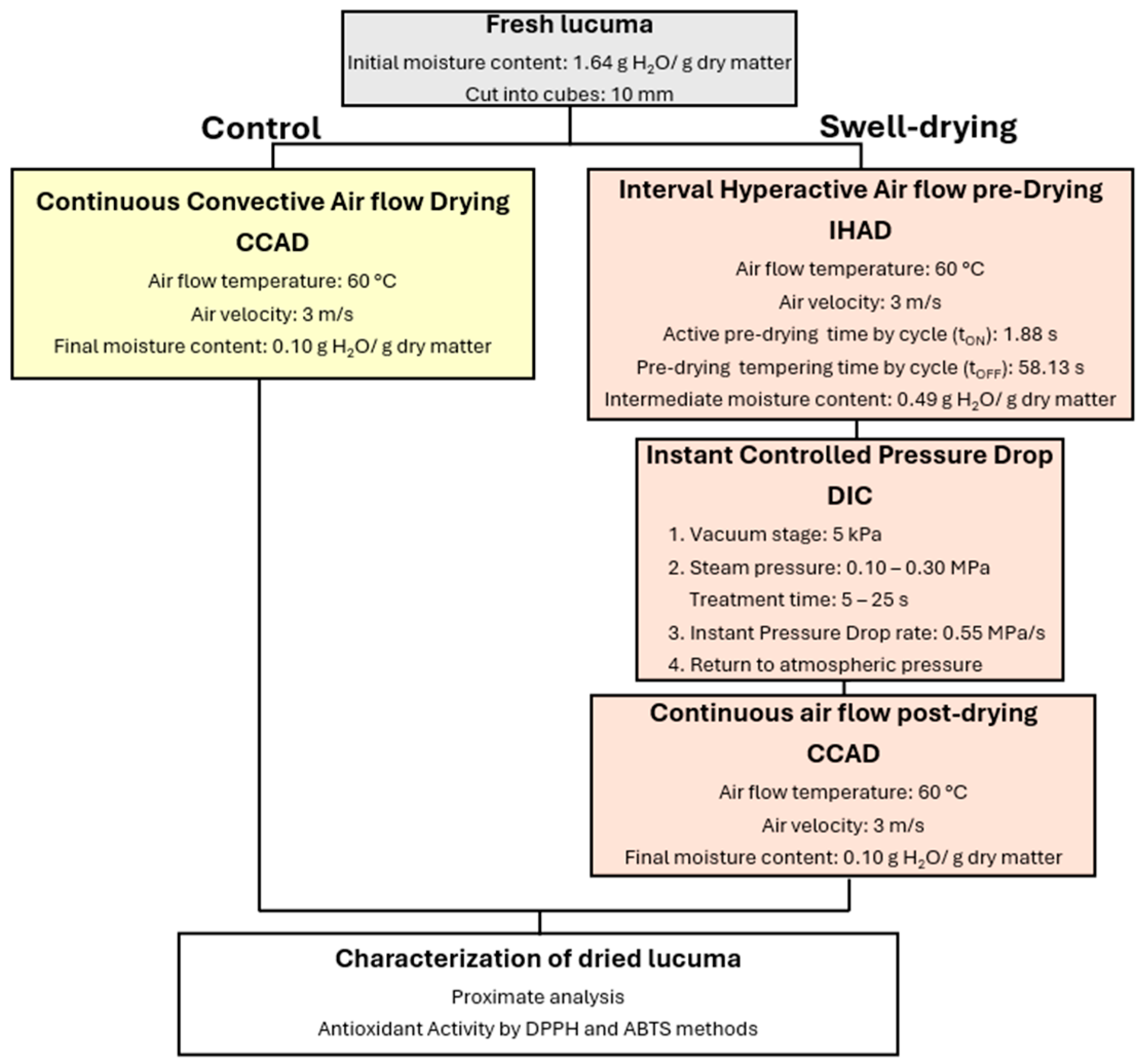
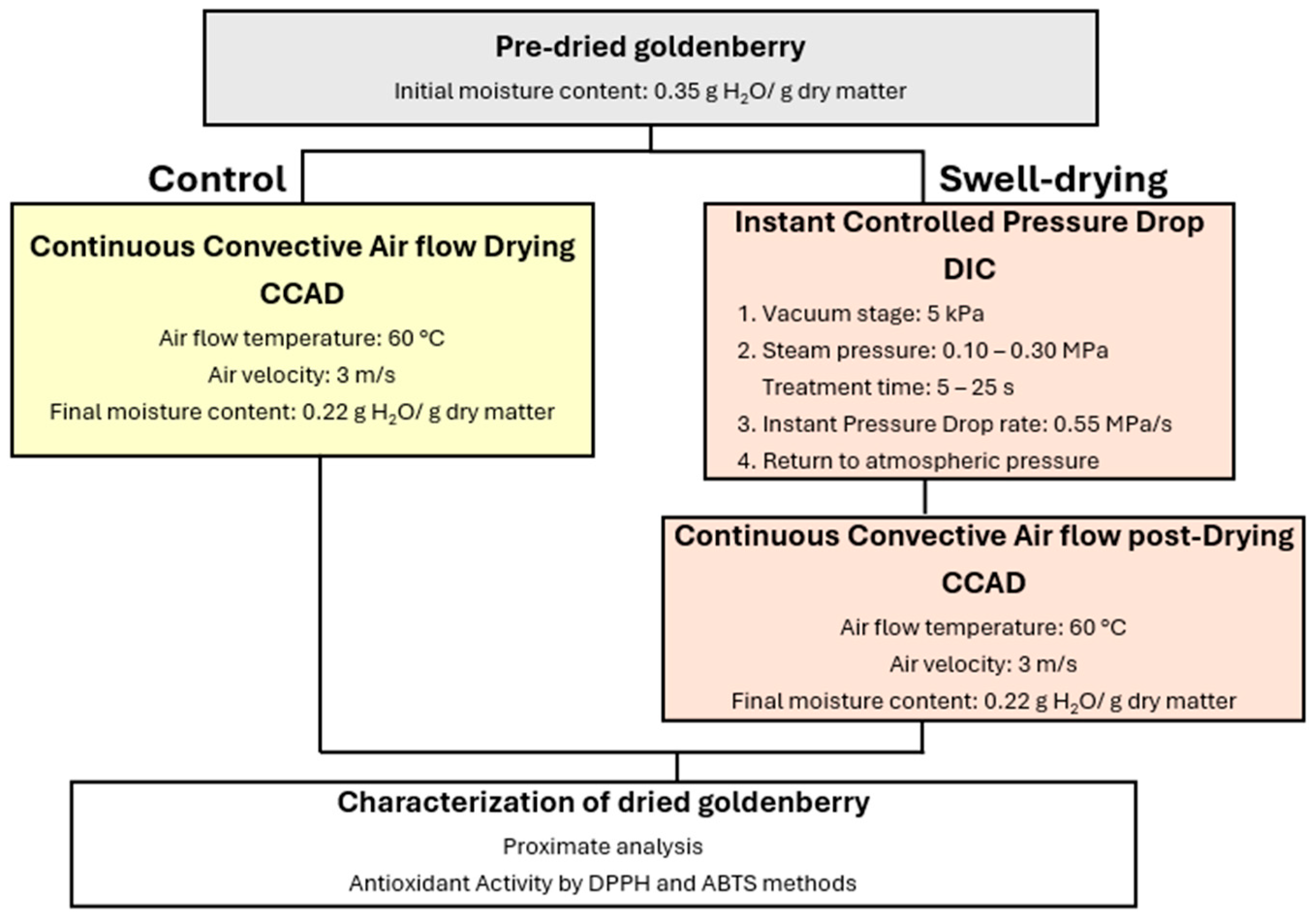
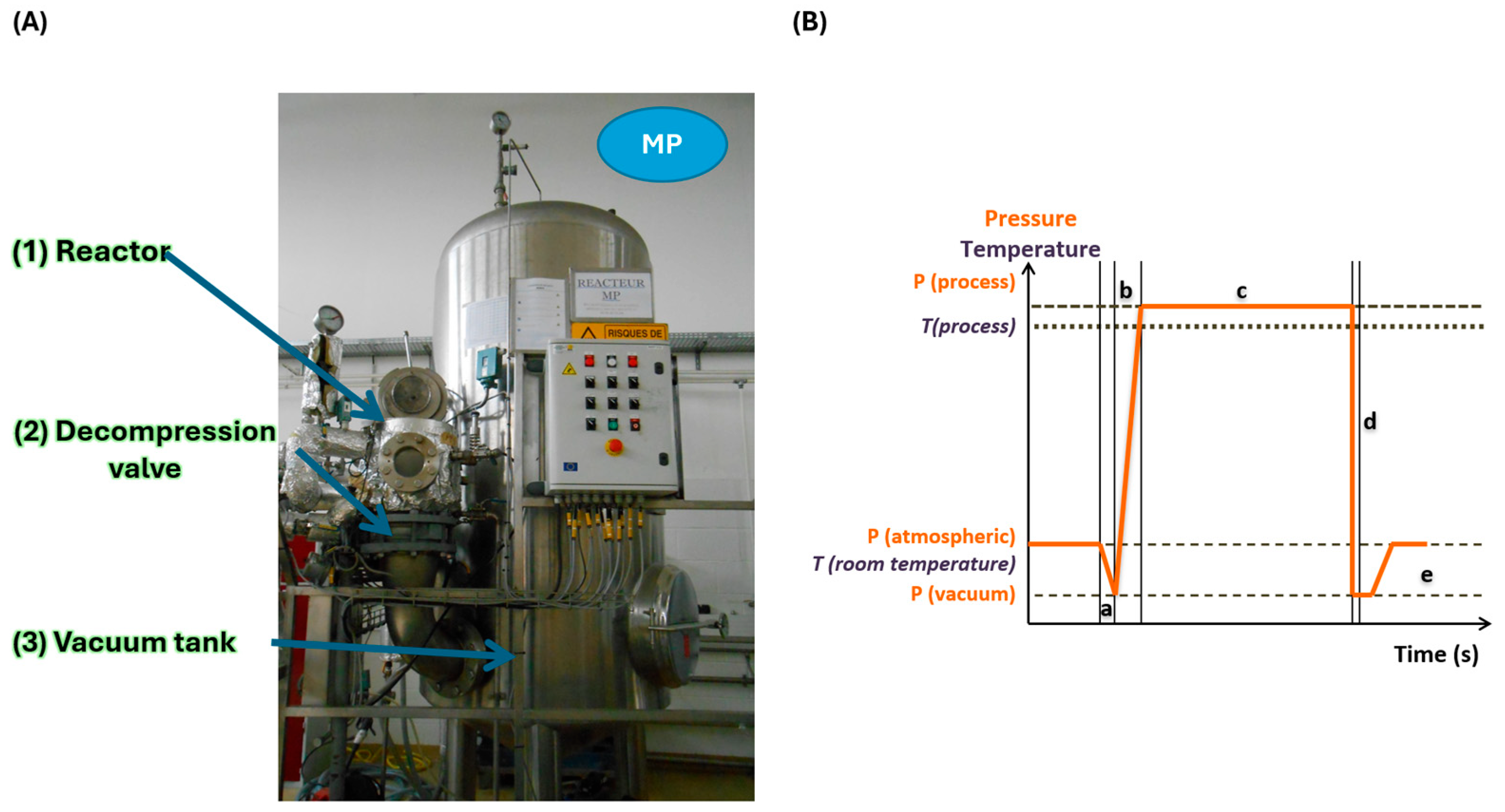
3.2. Bromatological Analysis Results
Table 3 displays the bromatological analysis of dried lucuma. As previously noted, the initial moisture content of fresh lucuma pulp was 62.12% w.b., and after drying, the final moisture content of all dried lucuma samples was 9.7% w.b. In fact, the final moisture content ranged from 7.98% to 11.8%. According to the study by Erazo, Escobar [
4], the moisture content of fresh lucuma (six varieties) ranged from 56.03% to 63.16% w.b. Similar results were reported by Maza-De la Quintana and Paucar-Menacho [
10], who observed moisture content in fresh lucuma between 62% and 72.3%. Regarding the final moisture content of dried lucuma, the study by Barrena Gurbillón and Quintana [
43] indicated that the equilibrium moisture content was 3.85% w.b. or 0.04 g H
2O/g dry matter. In this study, the goal was not to reach equilibrium moisture content but to achieve a safe storage moisture level. Typically, moisture levels in commercial products like lucuma flour or powder are kept below 10% for stability [
44]. Furthermore, the water activity of the control samples was 0.374 ± 0.011, while the a
w of SD samples treated under DIC central points was 0.294 ± 0.018. These results provide supplementary information on the stability of SD products. Previous studies have shown that the new expanded structure obtained by the DIC process increases the adsorption capacities of dried products, which directly impacts the water activity reduction [
35,
45,
46].
Furthermore,
Table 3 shows the bromatological analysis of dried lucuma. In fact, the average macronutrient contents for control and swell-dried samples for carbohydrates, proteins, lipids, fiber, and ash of lucuma were 88.73%, 7.28%, 1.18%, 1.88%, and 0.92%, respectively. The lipid content in dried lucuma ranged from 1.16 to 1.46% of dry matter. These results align with those from studies by Maza-De la Quintana and Paucar-Menacho [
10], and Erazo and Escobar [
4], which reported lipid contents between 0.52 and 2.17% of dry matter. Regarding the protein content in dried lucuma from this study, it ranged from 7.42 to 9.01%. In contrast, the studies by Maza-De la Quintana and Paucar-Menacho [
10], as well as Erazo and Escobar [
4], showed lower protein levels, between 4.81 and 6.05% of dry matter. Additionally, fiber content ranged from 0.11 to 1.85%. Erazo and Escobar [
4] reported a fiber range between 1.97 and 2.80%, whereas Maza-De la Quintana and Paucar-Menacho [
10] found values from 2.89 to 26.63% of dry matter.
Likewise, the ash content of dried lucuma samples ranged from 1.29% to 2.42% of dry matter; in this regard, Erazo and Escobar [
4] reported an ash range between 1.6% and 2.79% of dry matter. Finally, examining the carbohydrate content of lucuma samples, they varied between 86.72% and 91%. Maza-De la Quintana and Paucar-Menacho [
10], as well as Erazo and Escobar [
4], indicated similar results with a carbohydrate content between 87.29% and 91.12%.
Additionally,
Table 4 with the analysis of variance for bromatological analysis of SD lucuma and
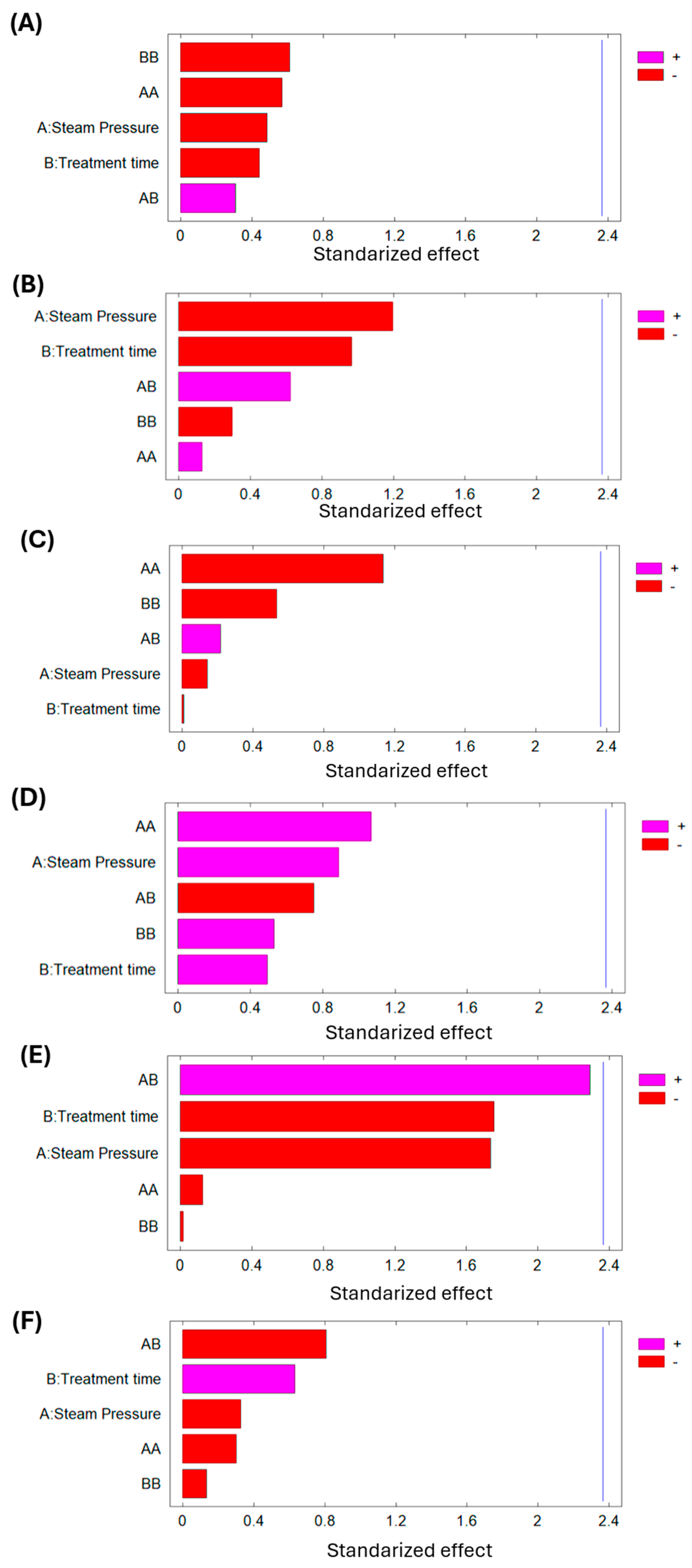
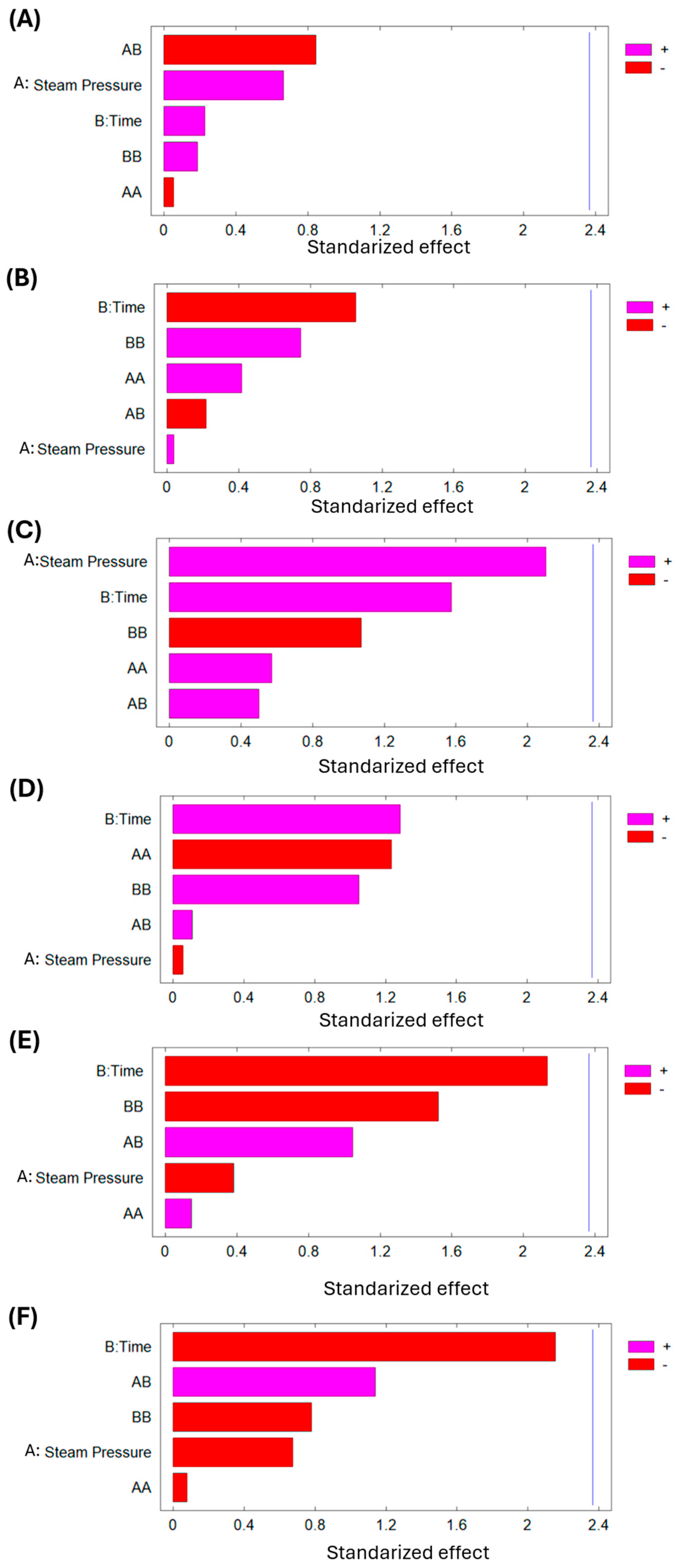
Figure 7 (Pareto chart) demonstrate that any of the studied DIC variables (P and t) significantly affect the final moisture content, lipid content, protein content, carbohydrate content, fiber content, and ash content of dried lucuma material.
The composition of lucuma varies according to genetic, environmental, and postharvest factors, which could explain the slight differences in lucuma’s bromatological composition between this study and the referenced literature. Lucuma varieties may differ in seed, skin, and pulp ratios. Furthermore, the ripening stage directly affects macronutrient levels, and postharvest handling can lead to enzymatic degradation of nutrients [
1,
47]. Therefore, selecting the optimal ripeness stage before drying is very important.
Table 5 presents the bromatological analysis of dried goldenberries and shows that, starting with an initial moisture content of 25.93% w.b. in pre-dried goldenberries, the DIC treatment and drying resulted in a final moisture content ranging from 9.61% to 22.95%. Nawirska-Olszańska and Stępień [
7] noted that the thick, stiff, and waxy skin of goldenberry makes dehydration difficult because it acts as a barrier that limits water loss. To improve mass transfer through the skin, several methods have been studied, including physical pretreatments such as puncturing the peel with a penetrator [
7], blanching at 96 °C for 25 min [
48], and microwaving [
7], as well as chemical pretreatments like applying 9.48% olive oil combined with 4.74% K
2CO
3 at 28 °C for 60 min [
48]. In this study, the lowest moisture content (9.61%) of goldenberry was observed after a DIC treatment at 0.30 MPa for 30 s, suggesting that these conditions help promote moisture evaporation from inside the fruit. In addition, the water activity of control samples was 0.421 ± 0.013, while the a
w of SD samples treated under DIC central points was 0.373 ± 0.006. Dried fruits and powders often have final a
w values between 0.20 and 0.40, which ensures long shelf life if packaged to avoid moisture uptake and under temperatures around 20 °C [
49,
50].
On the other hand,
Table 5 shows the bromatological analysis of dried goldenberry. The average carbohydrates, proteins, lipids, fiber, and ash of control and swell-dried goldenberry were 71.87%, 7.18% 7.01%, 6.60%, and 6.77%, respectively. The lipid content of dried goldenberry ranged from 5.25 to 8.84% of dry matter. These results are higher than those reported by Sierra and Escobar [
51] and Campos and Chirinos [
52], who noted a lipid content between 1 and 3% of dry matter. Regarding the protein content of dried goldenberry, it varied from 5.15 to 8.61% of dry matter. In this context, Sierra and Escobar [
51] found a wide range of proteins, between 0.15 and 8% of dry matter. Additionally, for fiber, the values ranged from 2.48 to 8.25% of dry matter. Campos and Chirinos [
52] reported a fiber content of 3.97% of dry matter. It should be noted that Siddiqui and Ucak [
53] reported that higher drying temperatures can lead to nutritional losses of macronutrients. However, to the best of our knowledge, no studies have established how goldenberry fiber is affected when subjected to saturated steam pressure. Referring to
Table 5, the fiber reduction observed in the DIC 3 goldenberry sample may be associated with the longest thermal treatment time applied (55 s), which could have triggered hydrolysis and structural fiber damage. Future studies should include additional trials to better clarify the impact of extended treatment times on goldenberry nutrients.
Furthermore, the ash content of goldenberry samples ranged from 5.97% to 7.34% of dry matter. In this regard, Campos and Chirinos [
52] reported an ash content of 3.95% of dry matter. Lastly, concerning the carbohydrate content in goldenberry samples, it varied between 69.4% and 77.25% of dry matter. Sierra and Escobar [
51] and Campos and Chirinos [
52] indicated a carbohydrate content in goldenberry between 81% and 98% of dry matter.
In addition,
Table 6 with the analysis of variance for bromatological analysis of SD goldenberry and the Pareto chart of
Figure 8 demonstrate that any of the studied DIC variables (P and t) significantly affect the final moisture content, lipid content, protein content, carbohydrate content, fiber content, and ash content of dried goldenberry material. Numerical differences in macronutrient composition are negligible between controls and treated samples, which means that swell-drying does not affect the nutritional composition of goldenberry.
3.3. Antioxidant Activity
The antioxidant activity of dried lucuma, measured by the percentage of ABTS inhibition and DPPH inhibition, is shown in
Table 7.
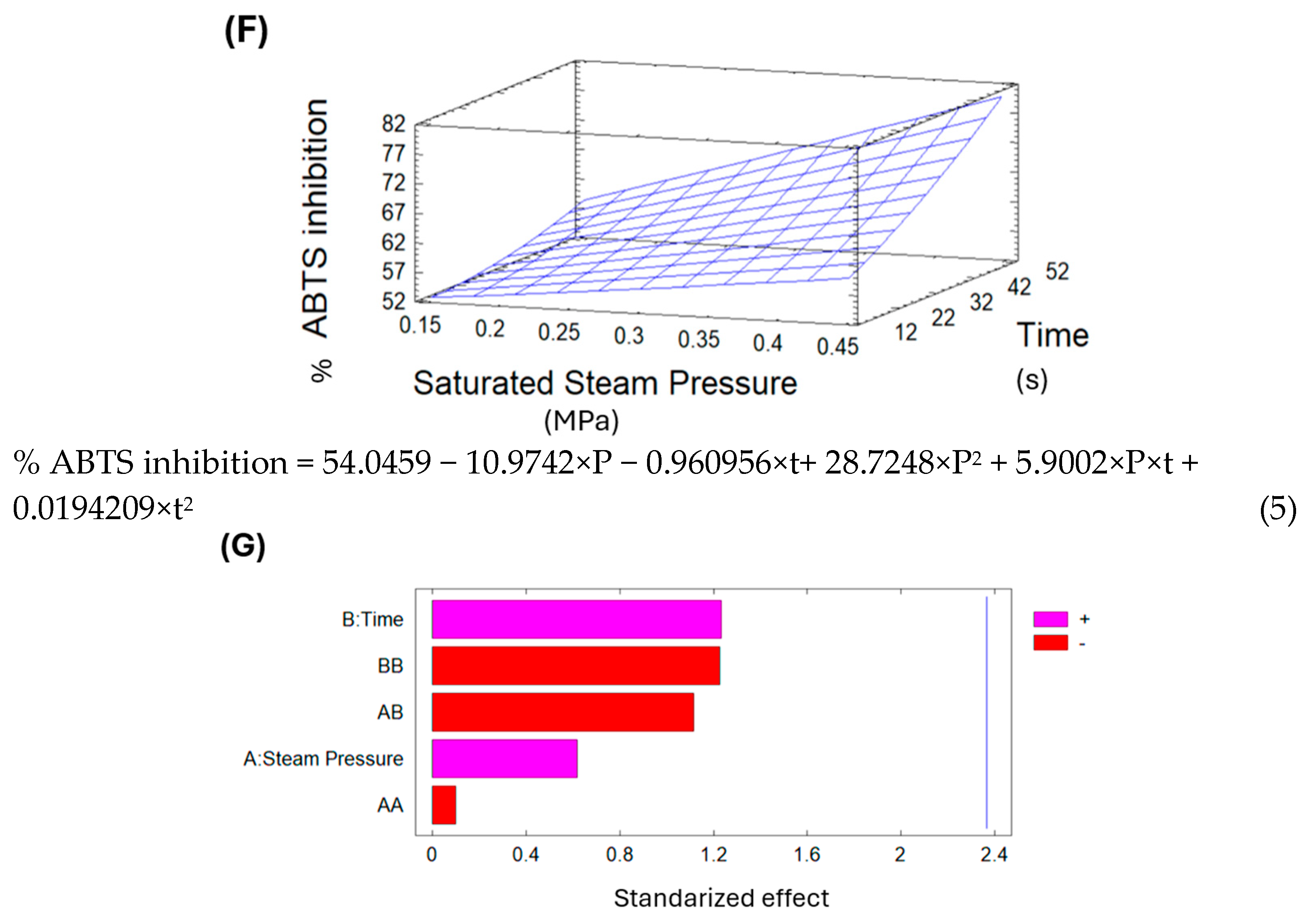
The percentage of ABTS inhibition in the lucuma control was 39.62%, while for lucuma DIC-treated samples, it ranged from 35.73% to 47.96%. Comparing the control to DIC 5 (0.27 MPa and 22 s), this treatment increased the ABTS inhibition by 21%. However, DIC 8 (0.13 MPa and 8 s) reduced the ABTS inhibition by 10% relative to the control.
Table 8 shows the analysis of variance of the antioxidant activity of SD lucuma.
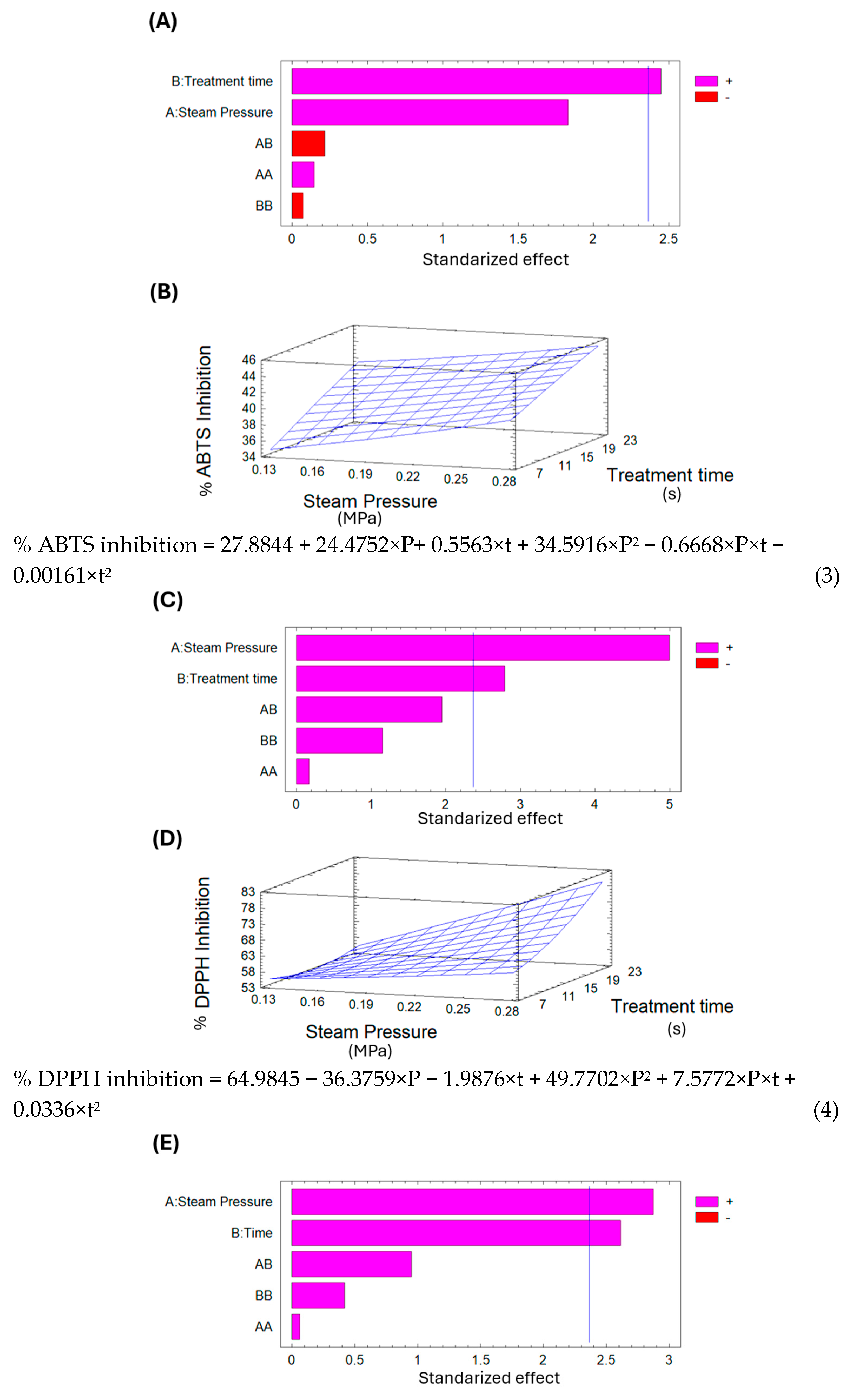
The Pareto chart in
Figure 9A shows that treatment time significantly affects the ABTS inhibition percentage. Additionally, the surface response in
Figure 9B indicates that, under the selected parameters for DIC treatment, longer treatment times and higher saturated steam pressures lead to increased ABTS inhibition of dried lucuma. Using the surface response equation, the predicted optimal DIC conditions to maximize ABTS inhibition of pre-dried lucuma were 0.29 MPa and 25 s.
Conversely, analyzing the DPPH percentage inhibition of dried lucuma in
Table 7 shows that the control sample had 49.22%, while the DIC samples ranged from 47.36% to 72.26%. DIC 11 (0.1 MPa and 15 s) exhibited the lowest inhibition percentage, 4% less than the control; in contrast, DIC 5 (0.27 MPa and 22 s) increased the DPPH inhibition percentage by 1.5 times. Additionally, the Pareto chart in
Figure 9C indicates that both saturated steam pressure and thermal treatment time influence the DPPH inhibition percentage. Furthermore, the surface response graph in
Figure 9D shows that, under the chosen DIC treatment parameters, the higher the saturated steam pressure and the longer the treatment time, the higher the DPPH inhibition in dried lucuma. Using the surface response model equation, the predicted optimal DIC conditions to maximize the percentage of DPPH inhibition in pre-dried lucuma can be set at 0.29 MPa and 25 s.
Among the reported antioxidants of lucuma, Aguilar-Galvez and García-Ríos [
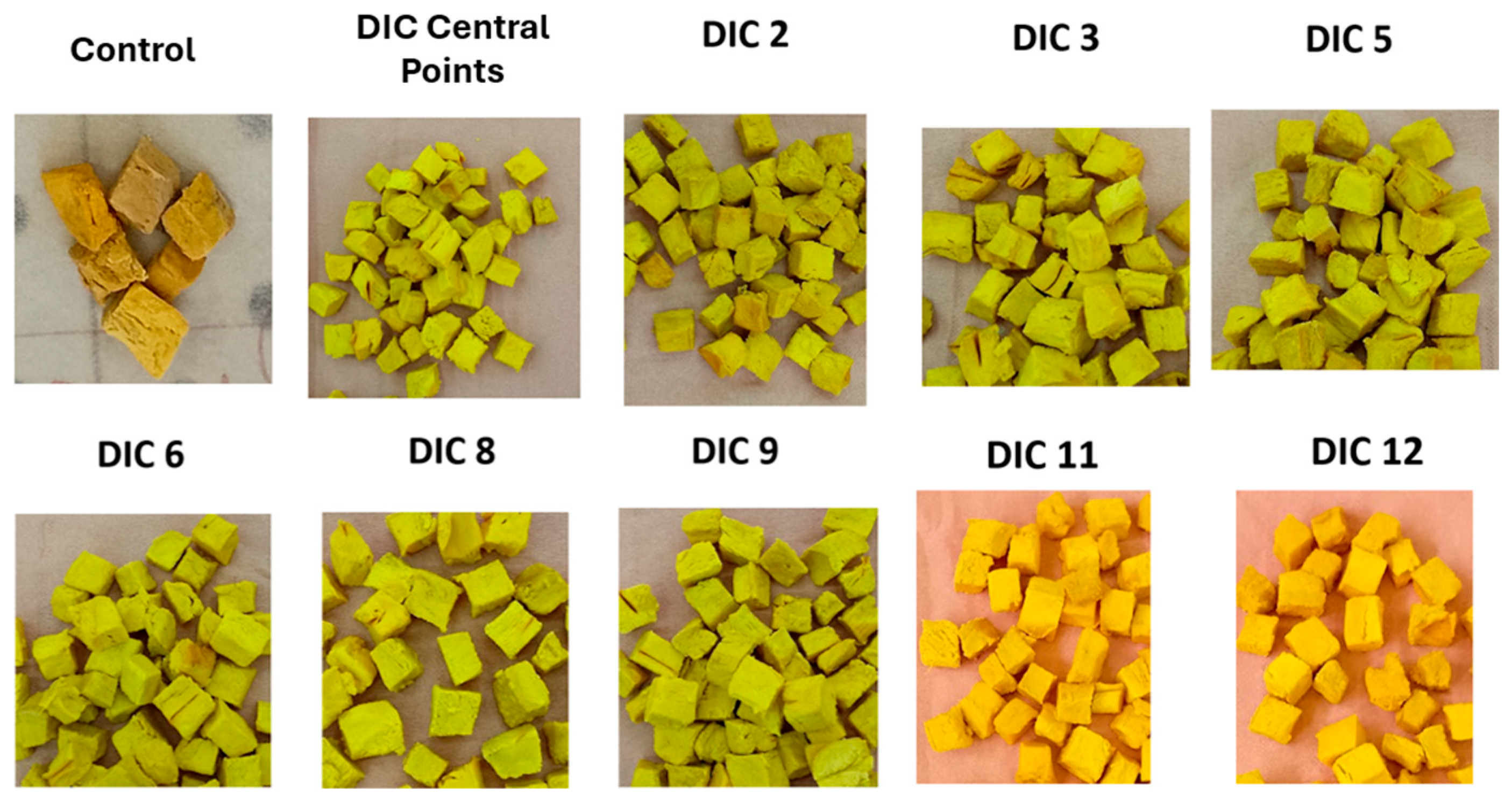
1] indicate that ripe lucuma is rich in carotenoids (0.15 mg β-carotene equivalent/g dw), phenols (69.3 mg gallic acid equivalent/g dw), sterols (6.5 μg β-sitosterol/g dw and 0.86 μg cycloartenol/g dw), α-amyrin (25.4 μg/g dw), myo-inositol (3.17 mg/g dw), and α-tocopherol (5.1 mg/100 g dw). In this regard, it can be noted that after swell-drying, the yellow-orange color of lucuma pulp was maintained, which may indicate good preservation of carotenoids. Therefore, future studies could analyze the impact of IHAD and DIC on each of these molecules and their relation to the antioxidant activity of dried lucuma.
Regarding untreated and DIC-treated goldenberries,
Table 9 shows their antioxidant activity measured by the percentage of ABTS and DPPH inhibition.
The control goldenberry sample exhibited 56% ABTS inhibition, while DIC-treated samples ranged from 50.65% to 74.08%. The lowest ABTS inhibition among DIC samples was observed with DIC 11 (0.1 MPa for 30 s), whereas DIC 2 (0.5 MPa for 30 s) and DIC 3 (0.3 MPa for 55 s) increased the ABTS inhibition by 1.3 times.
Table 10 shows the analysis of variance of the antioxidant activity of SD goldenberry.
The Pareto chart in
Figure 9E indicates that both saturated steam pressure and treatment time influence the percentage of ABTS inhibition. Additionally, the surface response graph in
Figure 9F shows that within the selected variable range for DIC treatment, higher saturated steam pressure and longer treatment times result in greater ABTS inhibition. Using the surface response model equation, the predicted optimal conditions to maximize ABTS inhibition in goldenberry are 0.49 MPa and 55 s. Naranjo-Durán and Quintero-Quiroz [
54] state that goldenberry has a good capacity to trap free radicals. And Narváez-Cuenca and Mateus-Gómez [
55] also observed higher ABTS values in dried goldenberry fruits processed with an airflow at 60 °C compared to fresh fruits. This increase in ABTS values could be linked to a good preservation of bioactive compounds and/or to the generation of Maillard products with a good radical scavenger activity.
Finally, regarding the DPPH percentage of inhibition of goldenberry, the control showed 75.91%, while DIC samples ranged from 58.02% to 79.6%. DIC 8 (0.16 MPa and 12 s) exhibited the lowest DPPH percentage of inhibition, 24% less than the control. In the Pareto chart of
Figure 9G, none of the selected variables could explain the variations in DPPH percentage of inhibition. For this reason, it was not possible to present the surface response graph and the corresponding equation.
The study by İzli and Yıldız [
56] indicates that by comparing the DPPH antioxidant activity of fresh fruit vs. dried goldenberry (convective, microwave, and microwave + convective drying), fresh samples had significantly higher antioxidant capacity than the dried samples. Conversely, DIC 11 (0.10 MPa, 30 s) showed the highest DPPH percentage of inhibition, 4.9% higher than the control. This slight increase could be linked to a good preservation of bioactive compounds such as phenolics. Yıldız and İzli [
42] also studied the antioxidant capacity of fresh goldenberries, and they found a 57.67% inhibition of DPPH. On the other hand, Olivares-Tenorio and Verkerk [
57] studied the antioxidant activity of rehydrated freeze-dried goldenberries heated from 40 to 120 °C, and their results showed that after heating (100 °C during 120 min), antioxidant activity was reduced from 547.6 to 355 μg Trolox Equivalent 100 g
−1 FW. Moreover, among the several bioactive molecules responsible for the antioxidant activity of goldenberry, the study highlights ascorbic acid as the main molecule that contributes to DPPH inhibition.
Furthermore, the antioxidant capacity of goldenberry may be linked to the levels of phenols, flavonoids, and carotenoids. In fact, these compounds act as scavengers of free radicals produced during oxidation reactions [
58,
59]. Biasi and Huber [
60] identified 23 bioactive compounds in goldenberry flour, including benzoic acid, chlorogenic acid, 2,4-dihydroxybenzoic acid, 2,5-dihydroxybenzoic acid, 3,4-dihydroxybenzoic acid, synaptic acid, ferulic acid, p-coumaric acid, caffeic acid, salicylic acid, synapaldehyde, syringaldehyde, syringic acid, pinocembrin, galangin, apigenin, kaempferol, epicatechin, catechin, hesperidin, quercetin, naringenin, and naringin.
Olivares-Tenorio and Verkerk [
57] also identified the presence of catechin, epicatechin, rutin, quercetin dihydrate, myricetin, and kaempferol in goldenberries; however, after heat treatment, only catechin and epicatechin were measured. Nocetti and Núñez [
61] reported the presence of tocopherols and sterols in goldenberry, with β-tocopherol and campesterol being the most abundant. Furthermore, the study by Jéssica and Vega-Gálvez [
62] showed that air drying goldenberry at 50 °C resulted in a 28% loss of β-carotene compared to the control, and drying at 90 °C showed no significant difference from the control. The authors observed the same pattern with total phenol content (TPC), where higher air-drying temperatures reduced the initial TPC in goldenberry, except at 90 °C. TPC increased from 321.05 to 356.68 mg gallic acid per 100 g of dry matter. These findings demonstrate that the bioavailability of antioxidant compounds can be influenced by various factors, such as the binding of phenolic compounds within the food matrix, differences in cell wall structures, and the location of bioactive compounds within cells—all directly related to fruit drying conditions. Therefore, it is possible that, after DIC treatment, the extractability of certain bioactive compounds was improved due to the formation of new porous structures during the process of autovaporization.
In fact, various studies have shown that adequate DIC treatment can improve the antioxidant activity of food products, such as beetroots, amaranth, okra, pepper, lentils, and cardamom [
21,
22,
63,
64,
65,
66]. During the instant controlled pressure drop step, the system immediately undergoes autovaporization, causing quick expansion, which creates a porous structure and rapidly cools biological matrices. This cooling protects thermosensitive compounds, and the porous structure enhances the extraction of active molecules, both significantly increasing the nutritional value of dried foods. Moreover, IHAD can prevent the degradation of thermosensitive molecules by significantly reducing the exposure time of fruits to high temperatures. This study also showed that DIC treatment could boost the antioxidant activity of dried lucuma and goldenberry, with IHAD offering an innovative pre-drying process to enhance the nutritional quality of dried fruits.