Evaluating the Influence of Two Different Red Wines on the Physicochemical Properties, Volatile Compound Profiles, and Sensory Attributes of Wine-Soaked Pressed Cheeses
Abstract
1. Introduction
2. Materials and Methods
2.1. Cheesemaking
2.2. Cheese Sample Preparation for Analyses
2.3. Microbiological Analysis
2.4. Physical–Chemical Analysis
2.5. Solid Phase Microextraction Gas Chromatography–Mass Spectrometry Analysis
2.6. Sensory Evaluation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics
3.2. Microbiology
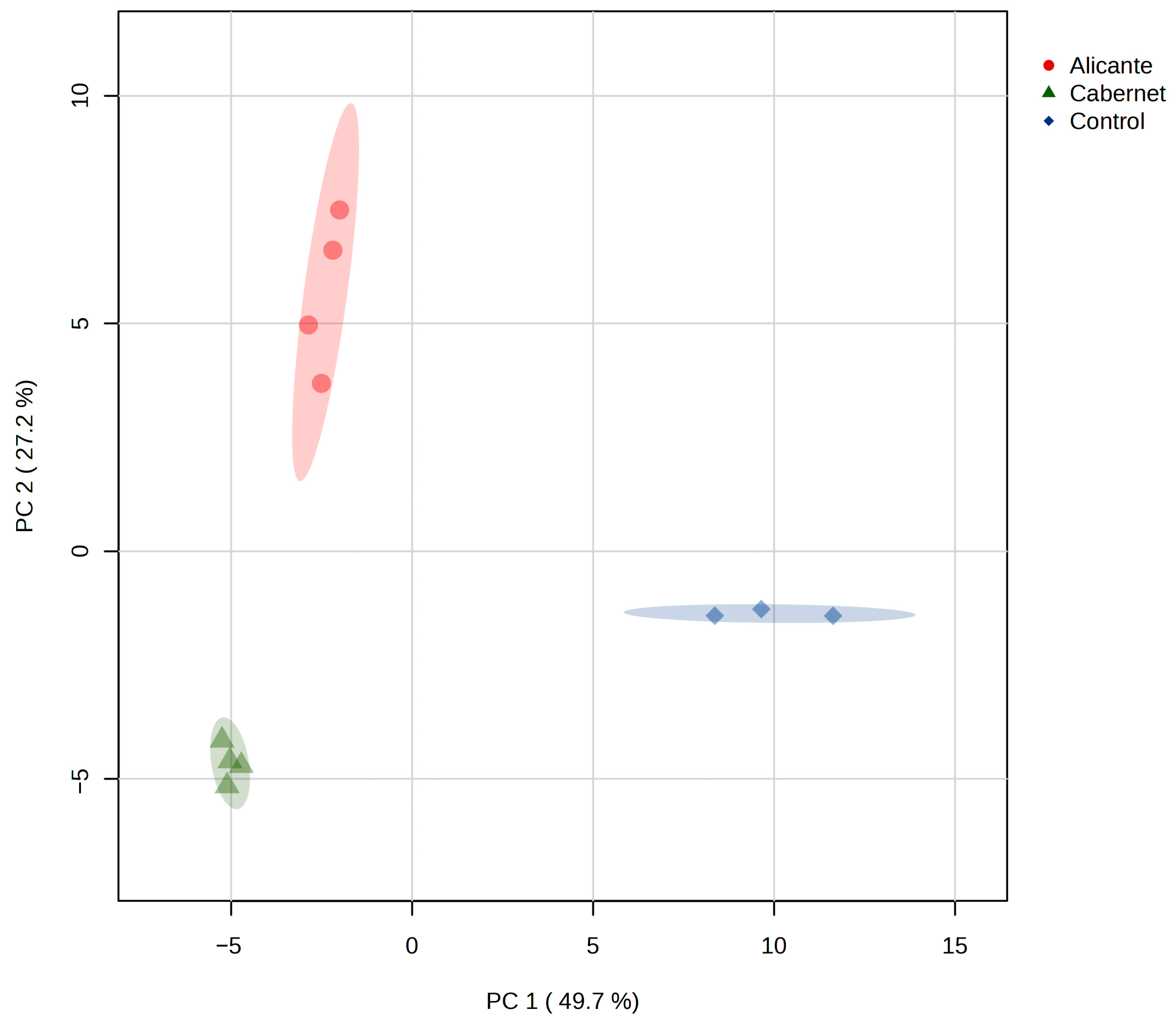
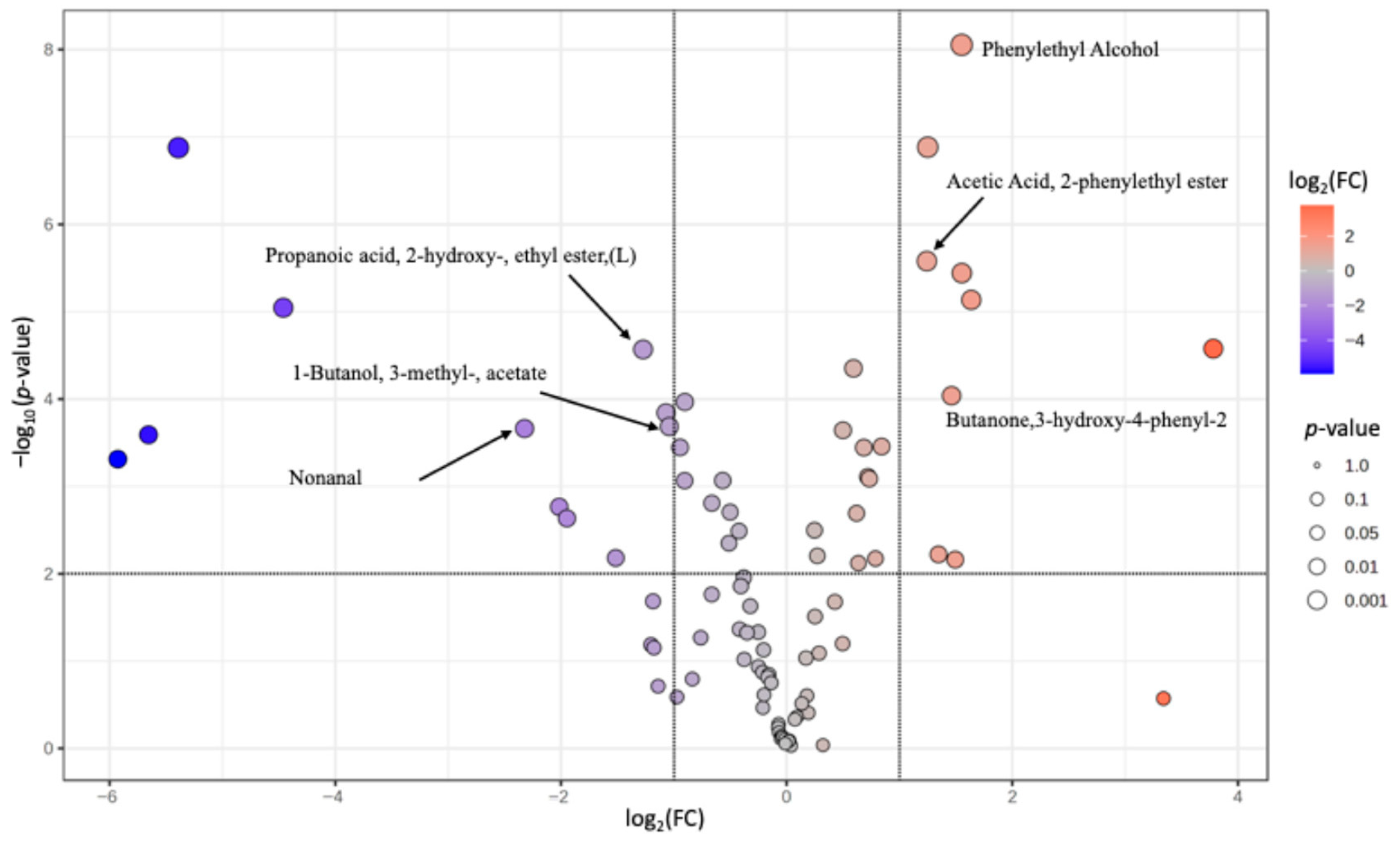
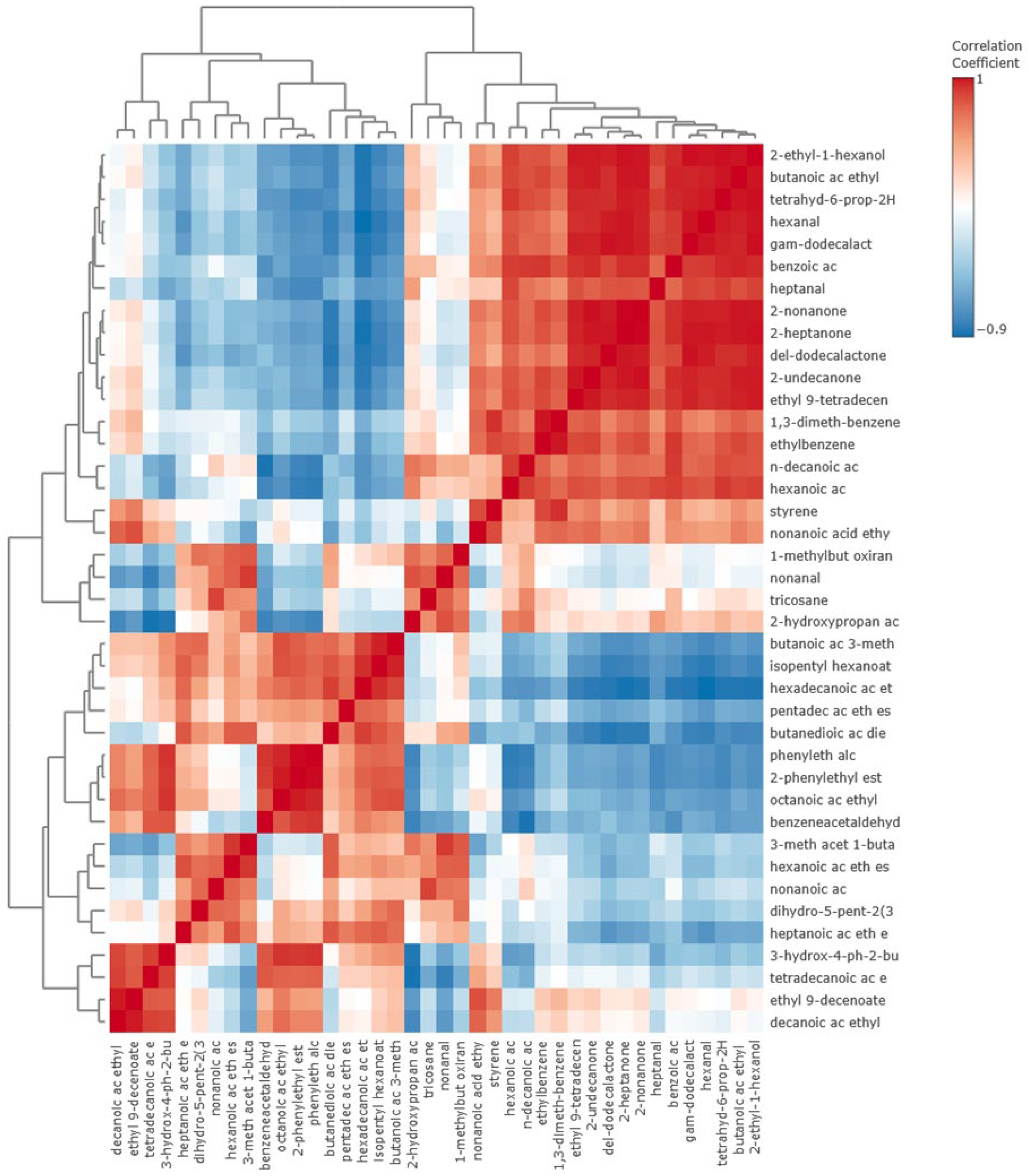
3.3. Volatile Compounds
3.4. Sensory Evaluation Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biango-daniels, M.N. American Artisan Cheese Quality and Spoilage: A Survey of Cheesemakers’ Concerns and Needs. J. Dairy Sci. 2021, 104, 6283–6294. [Google Scholar] [CrossRef]
- Becker, K.M.; Parsons, R.L.; Kolodinsky, J.; Matiru, G.N. A Cost and Returns Evaluation of Alternative Dairy Products to Determine Capital Investment and Operational Feasibility of a Small-Scale Dairy Processing Facility. J. Dairy Sci. 2007, 90, 2506–2516. [Google Scholar] [CrossRef]
- Dudić, B.; Mittelman, A.; Gubíniová, K.; Pajtinková Bartáková, G.; Kader, S.; Zejak, D.; Spalević, V. Wine Industry and Wine Markets: Dynamics, Challenges and Implications of Globalization. AGROFOR Int. J. 2024, 9, 27–39. [Google Scholar]
- Galmarini, M.V.; Dufau, L.; Loiseau, A.L.; Visalli, M.; Schlich, P. Wine and Cheese: Two Products or One Association? A New Method for Assessing Wine-Cheese Pairing. Beverages 2018, 4, 13. [Google Scholar] [CrossRef]
- “Guffanti Formaggi” Formaggio Ubriacato. Available online: https://guffantiformaggi.com/en/cheese/formaggio-ubriacato/ (accessed on 28 September 2025).
- López, M.B.; Ferrandini, E.; Rodriguez, M.; Roca, J.D.; Haba, E.; Luna, A.; Rovira, S. Physicochemical Study of Murcia al Vino Cheese. Small Rumin. Res. 2012, 106, 154–159. [Google Scholar] [CrossRef]
- Di Cagno, R.; Buchin, S.; De Candia, S.; De Angelis, M.; Fox, P.F.; Gobbetti, M. Characterization of Italian Cheeses Ripened under Nonconventional Conditions. J. Dairy Sci. 2007, 90, 2689–2704. [Google Scholar] [CrossRef] [PubMed]
- Gyenge, L.; Erdő, K.; Albert, C.; Laslo, É.; Salamon, R.V. The Effects of Soaking in Salted Blackcurrant Wine on the Properties of Cheese. Heliyon 2024, 10, e34060. [Google Scholar] [CrossRef] [PubMed]
- Innocente, N.; Biasutti, M.; Comuzzo, P. Characterization of a Traditional Semi-Hard Italian Cheese Produced by Soaking in Wine. Food Chem. 2007, 105, 1452–1456. [Google Scholar] [CrossRef]
- Ferrandini, E.; Castillo, M.; de Renobales, M.; Virto, M.D.; Garrido, M.D.; Rovira, S.; López, M.B. Influence of Lamb Rennet Paste on the Lipolytic and Sensory Profile of Murcia al Vino Cheese. J. Dairy Sci. 2012, 95, 2788–2796. [Google Scholar] [CrossRef]
- Bertuzzi, A.S.; McSweeney, P.L.H.; Rea, M.C.; Kilcawley, K.N. Detection of Volatile Compounds of Cheese and Their Contribution to the Flavor Profile of Surface-ripened Cheese. Compr. Rev. Food Sci. Food Saf. 2018, 17, 371–390. [Google Scholar] [CrossRef]
- Andiç, S.; Tunçtürk, Y.; Boran, G. Chapter 28. Changes in Volatile Compounds of Cheese. In Processing and Impact on Active Components in Food; Preedy, V., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 231–239. [Google Scholar]
- Rizzo, P.V.; Del Toro-Gipson, R.S.; Cadwallader, D.C.; Drake, M.A. Identification of Aroma-Active Compounds in Cheddar Cheese Imparted by Wood Smoke. J. Dairy Sci. 2022, 105, 5622–5640. [Google Scholar]
- Faulkner, H.; O’Callaghan, T.F.; McAuliffe, S.; Hennessy, D.; Stanton, C.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Effect of Different Forage Types on the Volatile and Sensory Properties of Bovine Milk. J. Dairy Sci. 2018, 101, 1034–1047. [Google Scholar] [PubMed]
- Garofalo, G.; Busetta, G.; Alfonzo, A.; Francesca, N.; Moschetti, G.; Settanni, L.; Gaglio, R. Effetto Dell’immersione in Vino Rosso Sul Profilo Microbiologico, Contenuto Totale in Composti Fenolici e Aspetti Sensoriali Di Un Formaggio Ovino a Pasta Pressata. Sci. E Tec. Latt. Casearia 2022, 72, 56. [Google Scholar] [CrossRef]
- Tejada, L.; Abellán, A.; Cayuela, J.M.; Martínez-Cacha, A. Sensorial Characteristics during Ripening of the Murcia al Vino Goat’s Milk Cheese: The Effect of the Type of Coagulant Used and the Size of the Cheese. J. Sens. Stud. 2006, 21, 333–347. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Method 989.10. Coliform and Escherichia Count in Food. Dry Rehydratable Film Method (PetrifilmTM Method). In Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Gaithersburg MD, USA, 2000. [Google Scholar]
- AOAC. AOAC Official Method 996.02. Coliform Count in Dairy Products. In Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Washington, DC, USA, 2002. [Google Scholar]
- Licón, C.C.; Carmona, M.; Molina, A.; Berruga, M.I. Chemical, Microbiological, Textural, Color, and Sensory Characteristics of Pressed Ewe Milk Cheeses with Saffron (Crocus sativus L.) during Ripening. J. Dairy Sci. 2012, 95, 4263–4274. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Magariño, S.; González-Sanjosé, M.L. Application of Absorbance Values Used in Wineries for Estimating CIELAB Parameters in Red Wines. Food Chem. 2003, 81, 301–306. [Google Scholar] [CrossRef]
- ColorDesigner LAB to RGB Color Converter. Available online: https://colordesigner.io/convert/labtorgb (accessed on 7 July 2025).
- Osunbade, O.A.; Ajiboye, T.S.; Adisa, O.A.; Olatunji, O.; Oyewo, I. Evaluation of Consumers Acceptability of Cassava Cooked Paste (EBA) Using 9-Points Hedonic, Jar, and Cata Methods. Int. J. Multidiscip. Res. Publ. (IJMRAP) 2021, 4, 48–54. [Google Scholar]
- Rahayu, N.I.; Muktiarni, M.; Hidayat, Y. An Application of Statistical Testing: A Guide to Basic Parametric Statistics in Educational Research Using SPSS. ASEAN J. Sci. Eng. 2024, 4, 569–582. [Google Scholar] [CrossRef]
- Guler, Z.; Park, Y.; Sekerli, Y. Evaluation of Volatile Compounds of Red and White Wine Treated Dil Cheese during Refrigerated Storage. Front. Food Sci. Technol. 2014, 1, 30–37. [Google Scholar]
- Gaglio, R.; Barbaccia, P.; Barbera, M.; Restivo, I.; Attanzio, A.; Maniaci, G.; Di Grigoli, A.; Francesca, N.; Tesoriere, L.; Bonanno, A.; et al. The Use of Winery By-Products to Enhance the Functional Aspects of the Fresh Ovine “Primosale” Cheese. Foods 2021, 10, 461. [Google Scholar] [CrossRef]
- Tunick, M.H. Aging of Hispanic Cheese. ACS Symp. Ser. 2022, 1406, 1–10. [Google Scholar] [CrossRef]
- Giroux, H.J.; Lemaire, N.; Britten, M. Effect of Cheese Composition and Cheese-Making Conditions on Salt and Moisture Transfer during Brining. Int. Dairy J. 2022, 129, 105325. [Google Scholar] [CrossRef]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.H. Fundamentals of Cheese Science; Springer: Berlin/Heidelberg, Germany, 2017; Volume 1. [Google Scholar]
- Guinee, T.P.; Fox, P.F. Salt in Cheese: Physical, Chemical and Biological Aspects. In Cheese: Chemistry, Physics and Microbiology; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 217–375. [Google Scholar]
- Revilla, E.; Losada, M.M.; Gutiérrez, E. Phenolic Composition and Color of Single Cultivar Young Red Wines Made with Mencia and Alicante-Bouschet Grapes in AOC Valdeorras (Galicia, NW Spain). Beverages 2016, 2, 18. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, Y.; Luo, J.; Wang, P.; Liu, B.; Du, Y.; Jiao, Y.; Hu, Y.; Chen, C.; Ren, F. Effect of Anthocyanin-Absorbed Whey Protein Microgels on Physicochemical and Textural Properties of Reduced-Fat Cheddar Cheese. J. Dairy Sci. 2021, 104, 228–242. [Google Scholar]
- Vaquero, M.J.R.; Alberto, M.R.; de Nadra, M.C.M. Antibacterial Effect of Phenolic Compounds from Different Wines. Food Control 2007, 18, 93–101. [Google Scholar] [CrossRef]
- Cabezas, L.; Sanchez, I.; Poveda, J.M.; Seseña, S.; Palop, M. Comparison of Microflora, Chemical and Sensory Characteristics of Artisanal Manchego Cheeses from Two Dairies. Food Control 2005, 18, 11–17. [Google Scholar] [CrossRef]
- Poveda, J.M.; Chicón, R.; Cabezas, L. Biogenic Amine Content and Proteolysis in Manchego Cheese Manufactured with Lactobacillus Paracasei Subsp. Paracasei as Adjunct and Other Autochthonous Strains as Starters. Int. Dairy J. 2015, 47, 94–101. [Google Scholar] [CrossRef]
- USDA Food Safety and Inspection Service Microbiological Testing by Industry of Ready-to-Eat Foods Under FDA’s Jurisdiction for Pathogens (or Appropriate Indicator Organisms): Verification of Preventive Controls. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/2022-03/NACMCF_2018-2020_RTE_Testing.pdf (accessed on 16 June 2025).
- Sabel, A.; Bredefeld, S.; Schlander, M.; Claus, H. Wine Phenolic Compounds: Antimicrobial Properties against Yeasts, Lactic Acid and Acetic Acid Bacteria. Beverages 2017, 3, 29. [Google Scholar] [CrossRef]
- Torres-Guardado, R.; Esteve-Zarzoso, B.; Reguant, C.; Bordons, A. Microbial Interactions in Alcoholic Beverages. Int. Microbiol. 2022, 25, 1–15. [Google Scholar]
- Marín, P.; Palmero, D.; Jurado, M. Occurrence of Moulds Associated with Ovine Raw Milk and Cheeses of the Spanish Region of Castilla La Mancha. Int. J. Dairy Technol. 2015, 68, 565–572. [Google Scholar] [CrossRef]
- Jurado, M.; Vicente, C.J. Penicillium Commune Affects Textural Properties and Water Distribution of Hard and Extra-Hard Cheeses. J. Dairy Res. 2020, 87, 117–122. [Google Scholar]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Gürbüz, O.; Rouseff, J.M.; Rouseff, R.L. Comparison of Aroma Volatiles in Commercial Merlot and Cabernet Sauvignon Wines Using Gas Chromatography− Olfactometry and Gas Chromatography− Mass Spectrometry. J. Agric. Food Chem. 2006, 54, 3990–3996. [Google Scholar] [CrossRef] [PubMed]
- de Macêdo Morais, S.; de Sousa Galvão, M.; Olegario, L.S.; de Carvalho, L.M.; Pereira, G.E.; de Andrade Lima, L.L.; da Silva, F.L.H.; Madruga, M.S. Identification of Chemical Markers of Commercial Tropical Red Wine Candidates for the São Francisco Valley Geographical Indication. Food Anal. Methods 2022, 15, 1237–1255. [Google Scholar] [CrossRef]
- Le Quéré, J.-L.; Pierre, A.; Riaublanc, A.; Demaizières, D. Characterization of Aroma Compounds in the Volatile Fraction of Soft Goat Cheese during Ripening. Le Lait 1998, 78, 279–290. [Google Scholar] [CrossRef]
- Curioni, P.M.G.; Bosset, J.O. Key Odorants in Various Cheese Types as Determined by Gas Chromatography-Olfactometry. Int. Dairy J. 2002, 12, 959–984. [Google Scholar] [CrossRef]
- Carunchia Whetstine, M.E.; Cadwallader, K.R.; Drake, M. Characterization of Aroma Compounds Responsible for the Rosy/Floral Flavor in Cheddar Cheese. J. Agric. Food Chem. 2005, 53, 3126–3132. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2024; ISBN 1119894093. [Google Scholar]
- Roger, S.; Degas, C.; Gripon, J.C. Production of Phenyl Ethyl Alcohol and Its Esters during Ripening of Traditional Camembert. Food Chem. 1988, 28, 129–140. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhou, X.; Xiao, Z.; Niu, Y. Characterization of the Differences in the Aroma of Cherry Wines from Different Price Segments Using Gas Chromatography–Mass Spectrometry, Odor Activity Values, Sensory Analysis, and Aroma Reconstitution. Food Sci. Biotechnol. 2017, 26, 331–338. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Du, Z.; Li, B.; Liu, Y.; Gao, Y.; Zhang, Y.; Zhang, K.; Wang, Q.; Lu, S. Impact of NSLAB on Kazakh Cheese Flavor. Food Res. Int. 2021, 144, 110315. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, W.; Qian, M.C. Characterization of Aroma Compounds in Apple Cider Using Solvent-Assisted Flavor Evaporation and Headspace Solid-Phase Microextraction. J. Agric. Food Chem. 2007, 55, 3051–3057. [Google Scholar]
- Sgarbi, E.; Lazzi, C.; Tabanelli, G.; Gatti, M.; Neviani, E.; Gardini, F. Nonstarter Lactic Acid Bacteria Volatilomes Produced Using Cheese Components. J. Dairy Sci. 2013, 96, 4223–4234. [Google Scholar] [PubMed]
- Culleré, L.; Ferreira, V.; Cacho, J. Analysis, Occurrence and Potential Sensory Significance of Aliphatic Aldehydes in White Wines. Food Chem. 2011, 127, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Martín, A.; Carrillo, F.; Trillo, L.M.; Roselló, A. A Quick Method for Obtaining Partition Factor of Congeners in Spirits. Eur. Food Res. Technol. 2009, 229, 697–703. [Google Scholar] [CrossRef]
- Yu, C.; Zhu, J.; Chen, J.; Yu, W.; Wang, C.; Gu, S.; Huang, D.; Pu, L.; Qiu, S.; Dai, Y. (R)-Ethyl Lactate Is an Important Contributor to Fruity Flavor of Baijiu as Revealed by Chiral GC–MS and Sensory Evaluation. Food Chem. X 2025, 31, 102525. [Google Scholar]
- Van Wyk, C.J.; Augustyn, O.P.H.; De Wet, P.; Joubert, W.A. Isoamyl Acetate—A Key Fermentation Volatile of Wines of Vitis Vinifera Cv Pinotage. Am. J. Enol. Vitic. 1979, 30, 167–173. [Google Scholar]
- Chira, K.; Jourdes, M.; Teissedre, P.-L. Cabernet Sauvignon Red Wine Astringency Quality Control by Tannin Characterization and Polymerization during Storage. Eur. Food Res. Technol. 2012, 234, 253–261. [Google Scholar] [CrossRef]
- OIV. Distribution of the World’s Grapevine Varieties; OIV: Dijon, France, 2017; ISBN 9791091799898. [Google Scholar]
- Fox, P.F.; McSweeney, P.L.H. Cheese: An Overview. In Cheese: Chemistry, Physics and Microbiology, 4th ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 1, pp. 5–21. ISBN 9780122636530. [Google Scholar]

| Day | Protein (%) | Fat (%) | Salt (%) | Moisture (%) |
|---|---|---|---|---|
| Start | ||||
| CCS | 15.2 ± 0.8 a | 20.0 ± 0.5 a | 4.4 ± 0.1 a | 51.8 ± 0.2 a |
| CAB | 17.2 ± 0.8 b | 22.5 ± 0.0 b | 2.9 ± 0.2 b | 50.5 ± 0.4 a |
| Day 30 | ||||
| CCS | 17.8 ± 0.0 a | 23.2 ± 0.2 a | 5.7 ± 0.0 b | 44.3 ± 0.4 b |
| CAB | 19.4 ± 0.1 b | 25.4 ± 0.1 b | 5.8 ± 0.0 b | 40.7 ± 0.0 a |
| CS | 20.4 ± 0.1 c | 23.1 ± 0.2 a | 2.3 ± 0.0 a | 46.2 ± 0.0 c |
| AB | 22.1 ± 0.2 d | 25.4 ± 0.9 b | 2.2 ± 0.0 a | 43.1 ± 0.8 b |
| Day 60 | ||||
| CCS | 20.6 ± 0.0 b | 27.0 ± 0.1 c | 5.5 ± 0.1 b | 38.7 ± 0.1 a |
| CAB | 18.1 ± 0.2 a | 22.9 ± 0.2 a | 5.4 ± 0.2 b | 45.1 ± 0.5 c |
| CS | 21.3 ± 0.1 c | 24.8 ± 0.1 b | 2.2 ± 0.0 a | 44.4 ± 0.2 b,c |
| AB | 21.3 ± 0.1 c | 25.0 ± 0.1 b | 2.2 ± 0.0 a | 43.8 ± 0.2 b |
| L* | a* | b* | Red | Green | Blue | |
|---|---|---|---|---|---|---|
| Cabernet Sauvignon | 36.13 ± 3.13 a | 12.95 ± 1.06 a | 1.95 ± 1.04 a | 106 ± 10 a | 77 ± 6.5 a | 82 ± 5.5 a |
| Alicante Bouchet | 30.91 ± 7.88 a | 12.87 ± 3.50 a | 3.53 ± 1.44 b | 95 ± 25 a | 65 ± 15.5 a | 66 ± 16 a |
| Day | TPC | Coliforms | Yeast and Mold | Listeria |
|---|---|---|---|---|
| Start | ||||
| C | 5.2 × 108 | 6.5 × 103 | 1.9 × 104 | (-) |
| Day 30 | ||||
| C | 9.3 × 106 | 5.0 × 102 | 1.4 × 104 | (-) |
| CS | 2.6 × 104 | 3.2 × 101 | 7.6 × 104 | (-) |
| AB | 5.3 × 106 | 1.3 × 102 | 7.6 × 103 | (-) |
| Day 60 | ||||
| C | 3.3 × 104 | < | 2.8 × 104 | (-) |
| CS | 2.0 × 104 | < | 5.0 × 103 | (-) |
| AB | 2.0 × 106 | < | 4.8 × 103 | (-) |
| Compound | CAS | m/z | Experimental Mean RI | NIST Median RI | Control Mean | Alicante Mean | Cabernet Mean | p-Value |
|---|---|---|---|---|---|---|---|---|
| Mean SD = 0.46 | Mean SD = 3.79 | 95% CI of %RSD: 17 ± 5 | 95% CI of %RSD: 14 ± 5 | 95% CI of %RSD: 16 ± 4 | ||||
| Esters (n = 16) | ||||||||
| Acetic acid, 2-phenylethyl ester@ | 103-45-7 | 104 | 1262 | 1258 | 1.75 × 10−4 a | 5.69 × 10−4 b | 1.35 × 10−3 c | 1.90 × 10−8 |
| Butanedioic acid, diethyl ester@ | 123-25-1 | 101 | 1188 | 1181 | 4.43 × 10−4 a | 3.29 × 10−3 b | 2.31 × 10−3 c | 9.23 × 10−8 |
| Hexadecanoic acid, ethyl ester@ | 628-97-7 | 88 | 1994 | 1993 | 5.85 × 10−4 a | 1.33 × 10−3 a,b | 1.40 × 10−3 b | 2.15 × 10−6 |
| Propanoic acid, 2-hydroxy-,ethyl ester, (L)@ | 687-47-8 | 45 | 813 | 848 * | 5.26 × 10−3 a | 6.31 × 10−3 a | 2.61 × 10−3 b | 3.56 × 10−6 |
| Octanoic acid, ethyl ester@ | 106-32-1 | 88 | 1198 | 1196 | 7.91 × 10−3 a | 1.16 × 10−2 b | 2.08 × 10−2 c | 4.80 × 10−5 |
| Butanoic acid, ethyl ester@ | 105-54-4 | 71 | 803 | 802 | 3.90 × 10−3 a | 1.65 × 10−3 a,b | 1.44 × 10−3 b | 6.16 × 10−5 |
| Tetradecanoic acid, ethyl ester | 124-06-1 | 88 | 1794 | 1793 | 2.96 × 10−3 a | 2.57 × 10−3 b | 3.64 × 10−3 c | 0.00013 |
| Ethyl 9-tetradecenoate | 24880-50-0 | 55 | 1783 | 1787 * | 1.59 × 10−4 a | 1.06 × 10−4 a,b | 1.05 × 10−4 b | 0.00027 |
| Isopentyl hexanoate@ | 2198-61-0 | 70 | 1255 | 1250 | 9.20 × 10−5 a | 1.62 × 10−4 a,b | 1.77 × 10−4 b | 0.00053 |
| Decanoic acid, ethyl ester | 110-38-3 | 88 | 1395 | 1396 | 1.63 × 10−2 a | 1.23 × 10−2 b | 1.98 × 10−2 a | 0.00062 |
| Butanoic acid, 3-methylbutyl ester@ | 106-27-4 | 70 | 1059 | 1056 | 1.26 × 10−4 a | 2.26 × 10−4 a,b | 2.58 × 10−4 b | 0.00133 |
| Nonanoic acid, ethyl ester | 123-29-5 | 88 | 1296 | 1295 | 1.12 × 10−3 a | 6.05 × 10−4 b | 9.40 × 10−4 a | 0.00294 |
| Ethyl 9-decenoate | 67233-91-4 | 55 | 1388 | 1388 | 3.13 × 10−4 a | 2.34 × 10−4 b | 3.59 × 10−4 a | 0.00465 |
| Hexanoic acid, ethyl ester | 123-66-0 | 88 | 1001 | 999 | 4.77 × 10−3 a | 7.17 × 10−3 a | 5.51 × 10−3 a | 0.01245 |
| Heptanoic acid, ethyl ester@ | 106-30-9 | 88 | 1101 | 1098 | 7.72 × 10−5 a | 1.68 × 10−4 a | 1.46 × 10−4 a | |
| Pentadecanoic acid, ethyl ester | 41114-00-5 | 88 | 1894 | 1894 | 6.49 × 10−5 a | 7.77 × 10−5 a | 7.86 × 10−5 a | |
| Acids (n = 4) | ||||||||
| Hexanoic acid | 142-62-1 | 60 | 988 | 990 | 2.65 × 10−3 a | 1.98 × 10−3 b | 1.34 × 10−3 c | 0.00011 |
| Benzoic acid | 65-85-0 | 105 | 1171 | 1177 | 1.91 × 10−3 a | 7.40 × 10−4 a,b | 4.36 × 10−4 b | 0.00045 |
| n-Decanoic acid | 334-48-5 | 60 | 1365 | 1372 | 2.19 × 10−3 a | 1.67 × 10−3 a | 1.06 × 10−3 b | 0.00203 |
| Nonanoic acid@ | 112-05-0 | 60 | 1273 | 1273 | 1.84 × 10−3 a | 6.48 × 10−3 a | 3.30 × 10−3 a | |
| Alcohols (n = 2) | ||||||||
| Phenylethyl Alcohol@ | 60-12-8 | 91 | 1115 | 1116 | 1.69 × 10−2 a | 9.10 × 10−2 b | 2.67 × 10−1 c | 7.67 × 10−1 |
| 1-Hexanol, 2-ethyl- | 104-76-7 | 57 | 1031 | 1030 | 4.37 × 10−4 a | 1.88 × 10−4 b | 1.50 × 10−4 c | 1.59 × 10−6 |
| Ketones (n = 9) | ||||||||
| 2-Heptanone | 110-43-0 | 43 | 892 | 891 | 3.45 × 10−3 a | 1.79 × 10−4 b | 1.35 × 10−4 c | 6.06 × 10−1 |
| 2-Nonanone | 821-55-6 | 58 | 1093 | 1092 | 1.32 × 10−3 a | 2.81 × 10−4 a,b | 3.17 × 10−4 b | 7.24 × 10−8 |
| 2H-Pyran-2-one, tetrahydro-6-propyl- | 698-76-0 | 99 | 1287 | 1288 | 1.64 × 10−4 a | 8.33 × 10−5 b | 6.21 × 10−5 c | 1.24 × 10−6 |
| .delta.-Dodecalactone | 713-95-1 | 99 | 1721 | 1720 | 4.64 × 10−4 a | 1.53 × 10−4 a,b | 1.73 × 10−4 b | 9.51 × 10−6 |
| 2-Undecanone | 112-12-9 | 58 | 1295 | 1294 | 5.63 × 10−4 a | 2.54 × 10−4 a,b | 2.71 × 10−4 b | 1.88 × 10−5 |
| Butanone,3-hydroxy-4-phenyl-2- | 5355-63-5 | 91 | 1353 | 1361 ** | 5.30 × 10−5 a | 4.62 × 10−5 a | 1.27 × 10−4 b | 2.55 × 10−5 |
| 1-Butanol, 3-methyl-, acetate@ | 123-92-2 | 43 | 880 | 876 | 5.26 × 10−4 a | 1.23 × 10−3 b | 5.96 × 10−4 a | 4.41 × 10−5 |
| .gamma.-Dodecalactone | 2305-05-7 | 85 | 1690 | 1681 | 2.92 × 10−4 a | 1.42 × 10−4 a | 1.29 × 10−4 a | |
| 2(3H)-Furanone, dihydro-5-pentyl- | 104-61-0 | 85 | 1368 | 1365 | 2.07 × 10−4 a | 2.45 × 10−4 a | 2.37 × 10−4 a | |
| Aldehydes (n = 4) | ||||||||
| Hexanal | 66-25-1 | 56 | 803 | 801 | 9.24 × 10−4 a | 1.72 × 10−4 b | 8.95 × 10−5 c | 1.35 × 10−8 |
| Nonanal * | 124-19-6 | 57 | 1108 | 1104 | 7.56 × 10−4 a | 1.78 × 10−3 b | 3.57 × 10−4 c | 8.62 × 10−5 |
| Benzeneacetaldehyde@ | 122-78-1 | 91 | 1047 | 1045 | 4.22 × 10−4 a | 5.12 × 10−4 a | 8.84 × 10−4 b | 0.00209 |
| Heptanal | 111-71-7 | 70 | 905 | 901 | 1.82 × 10−4 a | 9.35 × 10−5 a | 4.25 × 10−5 a | 0.01641 |
| Miscellaneous (n = 5) | ||||||||
| Ethylbenzene | 100-41-4 | 91 | 859 | 855 | 4.87 × 10−4 a | 3.11 × 10−4 a,b | 2.96 × 10−4 b | 0.01840 |
| Benzene, 1,3-dimethyl- | 108-38-3 | 91 | 868 | 866 | 4.13 × 10−4 a | 2.92 × 10−4 a | 2.97 × 10−4 a | |
| Oxirane, (1-methylbutyl)- | 53229-39-3 | 56 | 870 | 743 * | 1.01 × 10−3 a | 1.25 × 10−3 a | 9.32 × 10−4 a | |
| Styrene | 100-42-5 | 104 | 890 | 893 | 9.56 × 10−4 a | 6.61 × 10−4 a | 8.07 × 10−4 a | |
| Tricosane | 638-67-5 | 57 | 2300 | 2300 *** | 2.79 × 10−4 a | 3.71 × 10−4 a | 1.64 × 10−4 a |
| Unknown | m/z | Experimental Mean RI | Control Mean | Alicante Mean | Cabernet Mean | p-Value |
|---|---|---|---|---|---|---|
| 95% CI of %RSD: 21 ± 5 | 95% CI of %RSD: 17 ± 4 | 95% CI of %RSD: 29 ± 9 | ||||
| Unknown 41 | 105 | 677 | 4.70 × 10−4 | 4.43 × 10−4 | 3.83 × 10−4 | |
| Unknown 42 | 55 | 687 | 1.76 × 10−2 | 2.22 × 10−2 | 2.29 × 10−2 | |
| Unknown 44 | 57 | 692 | 4.14 × 10−3 | 5.69 × 10−3 | 5.50 × 10−3 | |
| Unknown 52 | 45 | 772 | 6.26 × 10−3 | 2.74 × 10−3 | 3.43 × 10−3 | |
| Unknown 60 | 55 | 804 | 3.90 × 10−4 | 1.52 × 10−4 | 1.28 × 10−4 | 3.13 × 10−6 |
| Unknown 67 | 60 | 840 | 1.91 × 10−4 | 1.50 × 10−4 | 1.30 × 10−4 | 0.005286 |
| Unknown 74 | 91 | 867 | 3.02 × 10−4 | 2.40 × 10−4 | 2.29 × 10−4 | |
| Unknown 78 | 87 | 880 | 6.57 × 10−5 | 1.54 × 10−4 | 7.32 × 10−5 | 1.32 × 10−5 |
| Unknown 79 | 43 | 883 | 1.54 × 10−4 | 2.49 × 10−4 | 1.10 × 10−4 | 0.021946 |
| Unknown 81 | 91 | 891 | 2.14 × 10−4 | 1.46 × 10−4 | 1.42 × 10−4 | |
| Unknown 83 | 85 | 895 | 5.87 × 10−5 | 1.59 × 10−5 | 3.94 × 10−6 | 0.000195 |
| Unknown 85 | 45 | 905 | 2.12 × 10−4 | 1.27 × 10−4 | 6.78 × 10−5 | 7.01 × 10−7 |
| Unknown 86 | 106 | 969 | 6.38 × 10−4 | 3.87 × 10−5 | 1.09 × 10−4 | 1.56 × 10−5 |
| Unknown 89 | 105 | 991 | 1.01 × 10−4 | 7.75 × 10−5 | 7.67 × 10−5 | |
| Unknown 90 | 81 | 991 | 1.12 × 10−4 | 8.46 × 10−5 | 4.52 × 10−5 | 0.000118 |
| Unknown 92 | 29 | 1002 | 1.72 × 10−3 | 2.57 × 10−3 | 2.16 × 10−3 | |
| Unknown 95 | 81 | 1025 | 4.55 × 10−4 | 1.39 × 10−4 | 1.09 × 10−4 | 0.000253 |
| Unknown 100 | 71 | 1060 | 1.39 × 10−4 | 2.11 × 10−4 | 1.88 × 10−4 | 0.018067 |
| Unknown 102 | 87 | 1061 | 9.85 × 10−6 | 3.01 × 10−4 | 7.66 × 10−4 | 7.03 × 10−8 |
| Unknown 103 | 105 | 1067 | 1.88 × 10−4 | 7.88 × 10−5 | 9.52 × 10−5 | 7.16 × 10−6 |
| Unknown 105 | 60 | 1077 | 1.42 × 10−4 | 1.29 × 10−4 | 7.21 × 10−5 | |
| Unknown 1117 | 267 | 1140 | 2.57 × 10−3 | 1.10 × 10−3 | 1.30 × 10−3 | 3.10 × 10−7 |
| Unknown 118 | 77 | 1171 | 1.45 × 10−3 | 5.58 × 10−4 | 2.42 × 10−4 | 0.004415 |
| Unknown 120 | 60 | 1180 | 2.75 × 10−3 | 1.73 × 10−3 | 1.34 × 10−3 | 0.002391 |
| Unknown 121 | 104 | 1183 | 2.35 × 10−5 | 3.81 × 10−5 | 9.05 × 10−5 | 1.38 × 10−6 |
| Unknown 125 | 85 | 1200 | 1.00 × 10−4 | 1.49 × 10−4 | 2.48 × 10−4 | 0.000259 |
| Unknown 1268 | 57 | 2479 | 1.00 × 10−5 | 4.20 × 10−4 | 1.00 × 10−5 | 1.22 × 10−9 |
| Unknown 147 | 73 | 1313 | 7.80 × 10−6 | 3.17 × 10−5 | 4.48 × 10−5 | 5.67 × 10−6 |
| Unknown 149 | 74 | 1326 | 8.87 × 10−5 | 6.77 × 10−5 | 6.43 × 10−5 | 0.033612 |
| Unknown 150 | 71 | 1334 | 4.18 × 10−5 | 5.75 × 10−5 | 5.16 × 10−5 | 0.010128 |
| Unknown 151 | 121 | 1353 | 1.50 × 10−5 | 1.43 × 10−5 | 4.46 × 10−5 | 3.26 × 10−5 |
| Unknown 166 | 70 | 1448 | 5.20 × 10−5 | 5.85 × 10−5 | 7.87 × 10−5 | 0.011226 |
| Unknown 169 | 85 | 1474 | 1.68 × 10−4 | 1.03 × 10−4 | 7.28 × 10−5 | 3.80 × 10−6 |
| Unknown 171 | 88 | 1493 | 3.58 × 10−4 | 2.37 × 10−4 | 2.83 × 10−4 | 0.001454 |
| Unknown 175 | 57 | 1506 | 1.05 × 10−4 | 7.77 × 10−5 | 7.86 × 10−4 | |
| Unknown 178 | 74 | 1523 | 1.56 × 10−4 | 1.28 × 10−4 | 8.06 × 10−5 | 0.000858 |
| Unknown 185 | 88 | 1593 | 1.10 × 10−2 | 6.67 × 10−3 | 1.01 × 10−2 | 1.21 × 10−5 |
| Unknown 257 | 57 | 2340 | 2.96 × 10−4 | 6.40 × 10−4 | 1.66 × 10−4 | 0.002612 |
| Unknown 259 | 57 | 2405 | 2.93 × 10−4 | 4.77 × 10−4 | 1.67 × 10−4 | 0.009889 |
| Unknown 260 | 145 | 2437 | 4.70 × 10−6 | 1.31 × 10−4 | 2.16 × 10−6 | 0.002230 |
| Unknown 268 | 57 | 2495 | 2.84 × 10−4 | 1.00 × 10−5 | 1.38 × 10−4 | 7.51 × 10−7 |
| Unknown 276 | 99 | 2530 | 4.09 × 10−5 | 3.64 × 10−4 | 1.66 × 10−5 | 7.87 × 10−7 |
| Unknown 285 | 127 | 1121 | 3.98 × 10−7 | 6.52 × 10−5 | 1.29 × 10−6 | 1.96 × 10−5 |
| Unknown 2001 | 71 | 1200 | 1.87 × 10−4 | 2.60 × 10−4 | 4.28 × 10−4 | 6.66 × 10−5 |
| Unknown 2002 | 79 | 1037 | 2.45 × 10−4 | 1.16 × 10−4 | 3.41 × 10−4 | 1.17 × 10−6 |
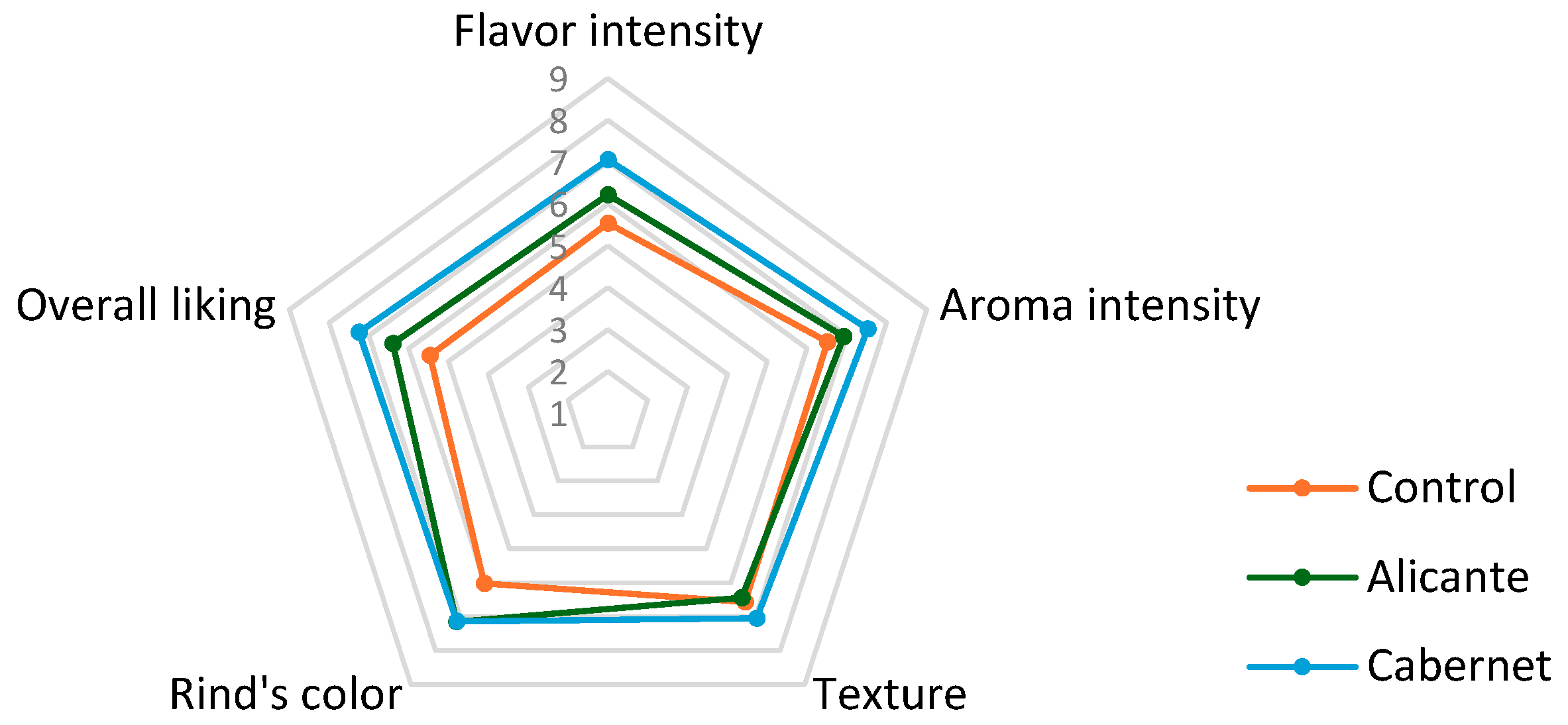
| Sensory Parameter | Control | Cabernet Sauvignon | Alicante Bouchet |
|---|---|---|---|
| Flavor intensity | 5.51 ± 1.96 a | 6.22 ± 1.89 b | 7.06 ± 1.49 b |
| Aroma intensity | 6.44 ± 1.48 a | 6.92 ± 1.87 b | 7.52 ± 1.30 b |
| Texture | 6.64 ± 1.65 a | 6.44 ± 1.79 a | 7.05 ± 1.49 a |
| Rind’s color | 6.23 ± 1.73 a | 7.15 ± 1.75 b | 7.14 ± 1.67 b |
| Overall liking | 5.43 ± 1.94 a | 6.40 ± 1.85 b | 7.25 ± 1.50 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freire, P.; Olmos, D.; Pedroza, M.A.; Adamson, J.; Elkhalil, R.; Atwood, M.; Miller-Schulze, J.P.; Licon, C.C. Evaluating the Influence of Two Different Red Wines on the Physicochemical Properties, Volatile Compound Profiles, and Sensory Attributes of Wine-Soaked Pressed Cheeses. Foods 2025, 14, 3475. https://doi.org/10.3390/foods14203475
Freire P, Olmos D, Pedroza MA, Adamson J, Elkhalil R, Atwood M, Miller-Schulze JP, Licon CC. Evaluating the Influence of Two Different Red Wines on the Physicochemical Properties, Volatile Compound Profiles, and Sensory Attributes of Wine-Soaked Pressed Cheeses. Foods. 2025; 14(20):3475. https://doi.org/10.3390/foods14203475
Chicago/Turabian StyleFreire, Paulina, Daniel Olmos, Miguel A. Pedroza, Jack Adamson, Reem Elkhalil, Madison Atwood, Justin P. Miller-Schulze, and Carmen C. Licon. 2025. "Evaluating the Influence of Two Different Red Wines on the Physicochemical Properties, Volatile Compound Profiles, and Sensory Attributes of Wine-Soaked Pressed Cheeses" Foods 14, no. 20: 3475. https://doi.org/10.3390/foods14203475
APA StyleFreire, P., Olmos, D., Pedroza, M. A., Adamson, J., Elkhalil, R., Atwood, M., Miller-Schulze, J. P., & Licon, C. C. (2025). Evaluating the Influence of Two Different Red Wines on the Physicochemical Properties, Volatile Compound Profiles, and Sensory Attributes of Wine-Soaked Pressed Cheeses. Foods, 14(20), 3475. https://doi.org/10.3390/foods14203475






