Applicability of Controllable Normal Force Platform for Study of Bacteria Removal During Dry Cleaning in Dry Food Manufacturing Environments
Abstract
1. Introduction
2. Materials and Methods
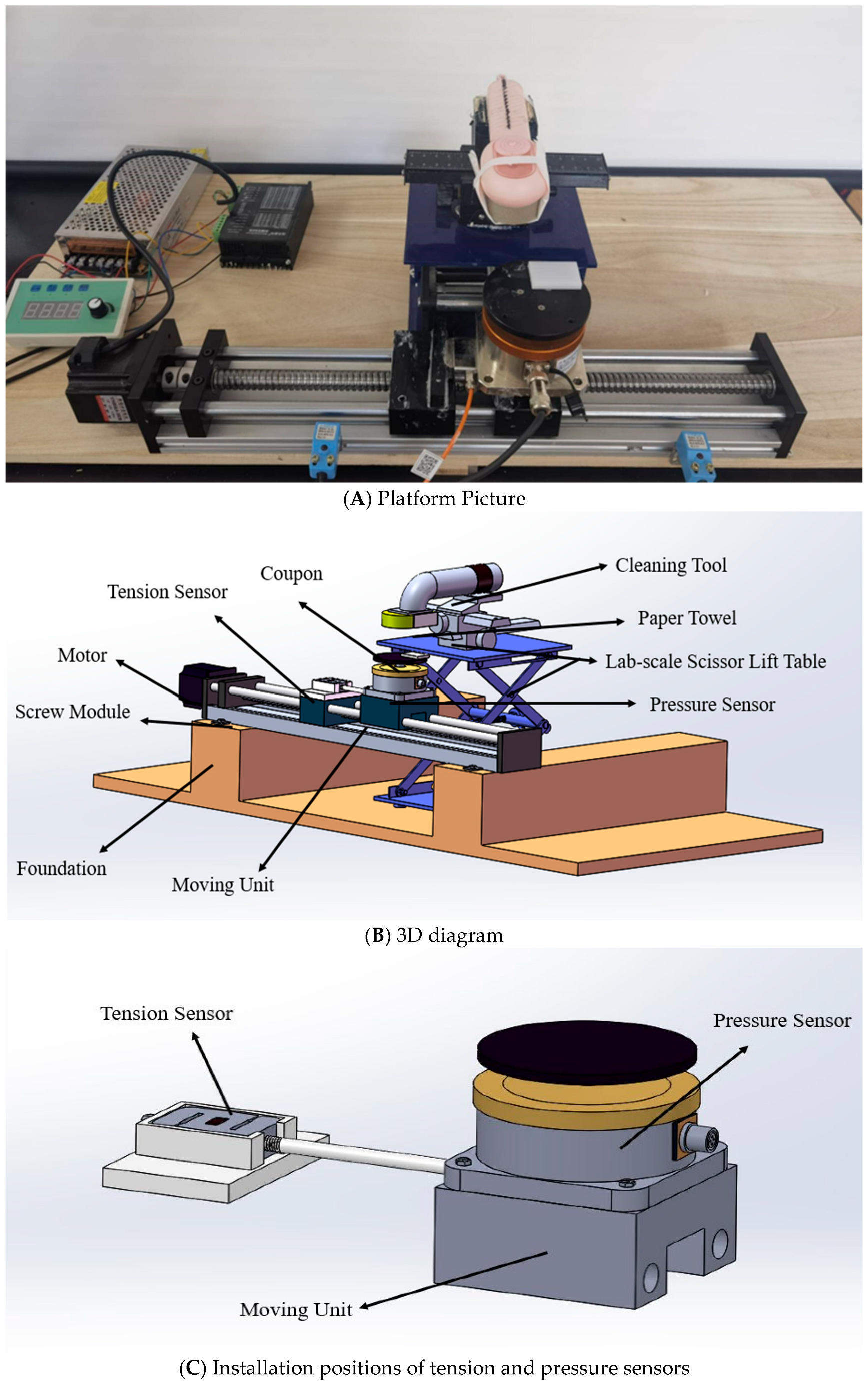
2.1. Fabrication of a Normal Force Controllable Dry-Cleaning Platform
2.1.1. Platform Development
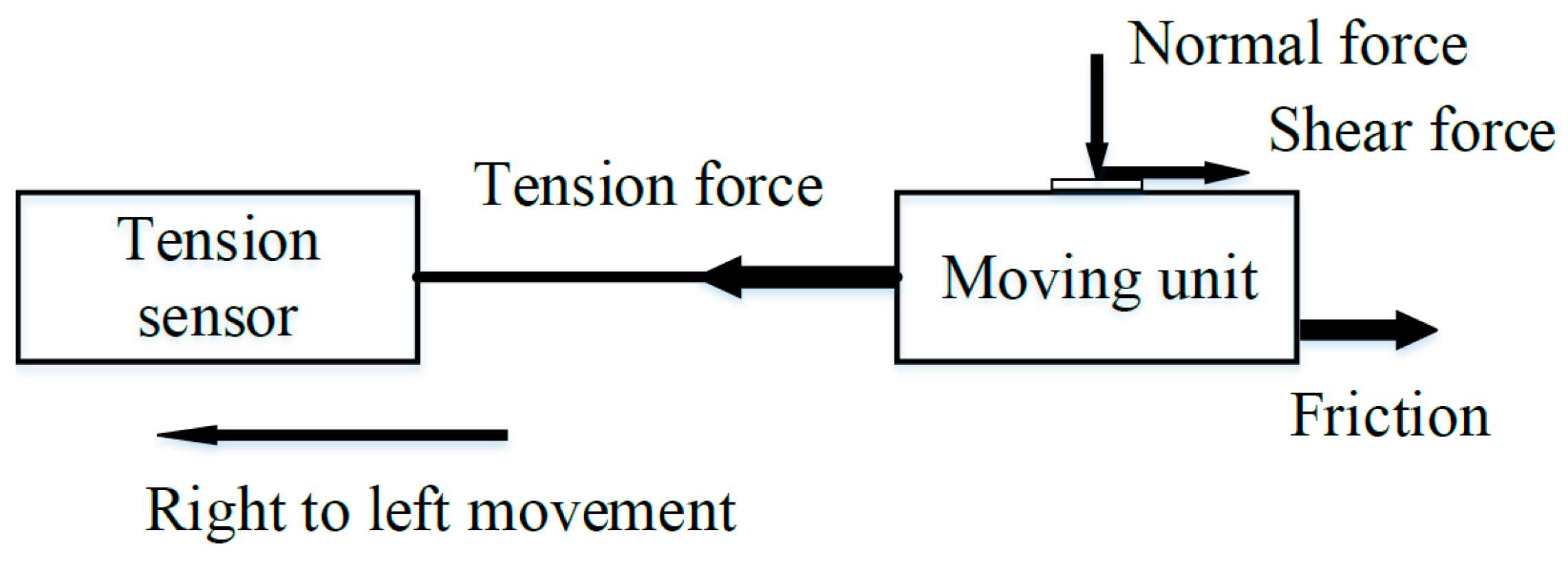
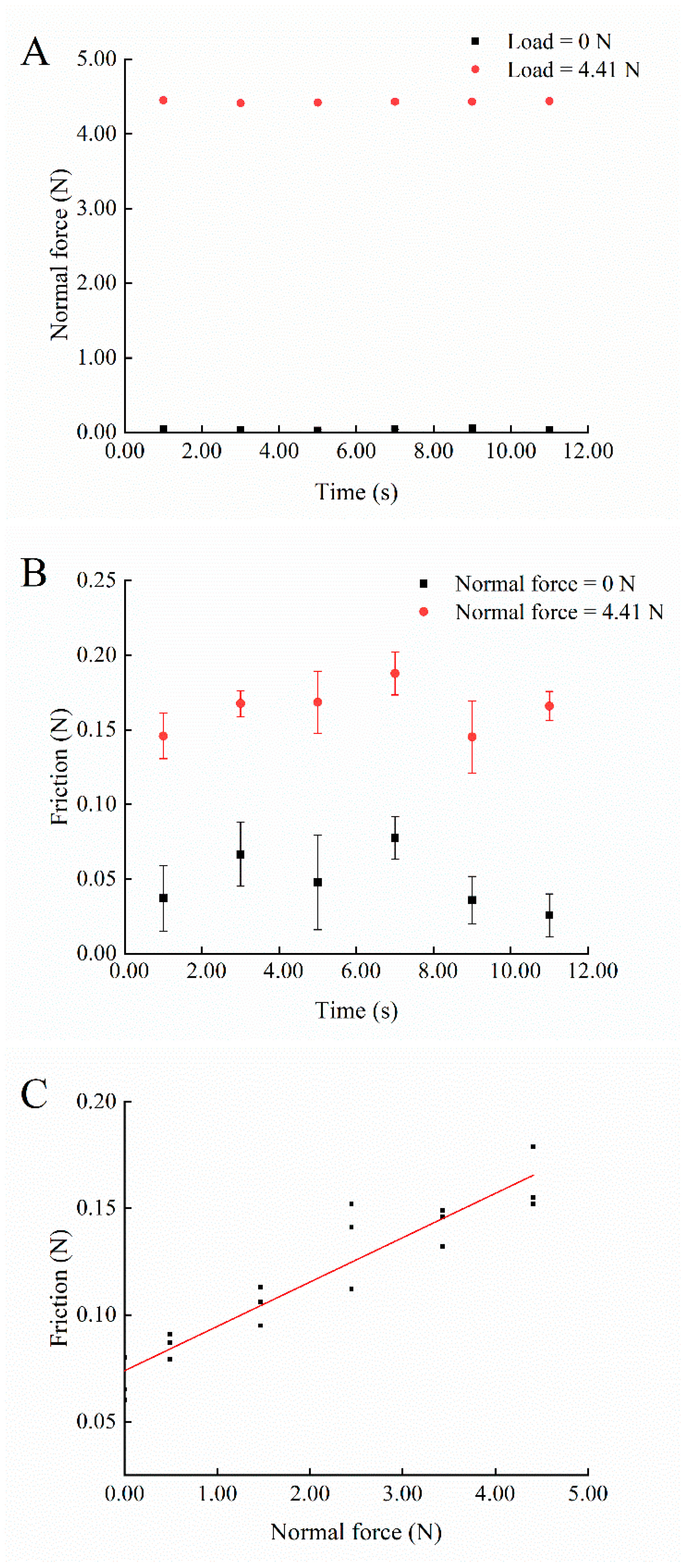
2.1.2. Platform Performance Evaluation
2.2. Surface Inoculation and Physical Bacterial Removal
2.2.1. Surface Materials
2.2.2. Bacterial Strains
2.2.3. Preparation of Inoculum
2.2.4. Inoculation of Surfaces
2.2.5. Bacteria Removal Experiments
2.3. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Performance of the Developed Dry-Cleaning Platform
3.2. Bacterial Removal on Three Surface Materials
| Microorganism | Surface Material | Normal Force (N) | Count Before Cleaning (log (CFU/Coupon)) | Count After Cleaning (log (CFU/Coupon)) | Log Reduction (log (CFU/Coupon)) | Shear Force (N) |
|---|---|---|---|---|---|---|
| S. PT 30 | SS | 1.10 ± 0.05 | 9.63 ± 0.09 | 9.56 ± 0.10 | 0.06 ± 0.04 | 0.30 ± 0.02 |
| 2.58 ± 0.03 | 9.53 ± 0.14 | 0.09 ± 0.06 | 0.61 ± 0.01 | |||
| 4.41 ± 0.10 | 9.55 ± 0.08 | 0.07 ± 0.06 | 0.93 ± 0.01 | |||
| HDPE | 1.10 ± 0.05 | 9.58 ± 0.19 | 9.52 ± 0.27 | 0.09 ± 0.07 | 0.13 ± 0.02 | |
| 2.58 ± 0.03 | 9.52 ± 0.25 | 0.04 ± 0.04 | 0.27 ± 0.01 | |||
| 4.41 ± 0.10 | 9.50 ± 0.22 | 0.08 ± 0.05 | 0.43 ± 0.01 | |||
| Rubber | 1.10 ± 0.05 | 9.51 ± 0.22 | 9.50 ± 0.24 | 0.02 ± 0.05 | 0.41 ± 0.03 | |
| 2.58 ± 0.03 | 9.53 ± 0.26 | 0.09 ± 0.05 | 1.02 ± 0.05 | |||
| 4.41 ± 0.10 | 9.50 ± 0.21 | 0.08 ± 0.05 | 1.60 ± 0.08 | |||
| E. faecium | SS | 1.10 ± 0.05 | 9.06 ± 0.09 | 8.97 ± 0.15 | 0.09 ± 0.07 | 0.29 ± 0.02 |
| 2.58 ± 0.03 | 8.92 ± 0.24 | 0.18 ± 0.09 | 0.61 ± 0.01 | |||
| 4.41 ± 0.10 | 8.88 ± 0.29 | 0.12 ± 0.13 | 0.92 ± 0.02 | |||
| HDPE | 1.10 ± 0.05 | 9.06 ± 0.11 | 9.02 ± 0.13 | 0.03 ± 0.02 | 0.15 ± 0.01 | |
| 2.58 ± 0.03 | 8.99 ± 0.10 | 0.09 ± 0.04 | 0.26 ± 0.02 | |||
| 4.41 ± 0.10 | 9.03 ± 0.08 | 0.07 ± 0.03 | 0.43 ± 0.03 | |||
| Rubber | 1.10 ± 0.05 | 9.05 ± 0.12 | 9.03 ± 0.03 | 0.04 ± 0.02 | 0.41 ± 0.07 | |
| 2.58 ± 0.03 | 8.98 ± 0.03 | 0.12 ± 0.02 | 1.04 ± 0.06 | |||
| 4.41 ± 0.10 | 9.00 ± 0.05 | 0.06 ± 0.03 | 1.56 ± 0.07 |
| Factor | n | Log Reduction Mean (log (CFU/Coupon)) | Shear Force Mean (N) |
|---|---|---|---|
| Microorganism Effect | |||
| S. PT 30 | 9 | 0.08 | 0.63 |
| E. faecium | 9 | 0.09 | 0.64 |
| Surface Material Effect | |||
| SS | 6 | 0.11 | 0.61 [0.50, 0.73] b |
| HDPE | 6 | 0.07 | 0.28 [0.23, 0.33] c |
| Rubber | 6 | 0.07 | 1.01 [0.79, 1.23] a |
| Normal Force Effect | |||
| Low FN | 6 | 0.07 | 0.29 [0.24, 0.34] c |
| Medium FN | 6 | 0.09 | 0.64 [0.49, 0.78] b |
| High FN | 6 | 0.10 | 0.98 [0.76, 1.20] a |
| Microorganism–Surface Material Interaction Effect | |||
| S. PT 30 × SS | 3 | 0.08 [0.04, 0.11] ab | 0.62 |
| E. faecium × SS | 3 | 0.15 [0.08, 0.22] a | 0.61 |
| S. PT 30 × HDPE | 3 | 0.09 [0.05, 0.12] ab | 0.28 |
| E. faecium × HDPE | 3 | 0.05 [0.02, 0.08] ab | 0.29 |
| S. PT 30 × Rubber | 3 | 0.11 [0.08, 0.13] ab | 1.01 |
| E. faecium × Rubber | 3 | 0.04 [0.01, 0.08] b | 1.00 |
| Surface Material–Normal Force Interaction Effect | |||
| SS × Low FN | 2 | 0.08 | 0.31 [0.29, 0.32] f |
| SS × Medium FN | 2 | 0.14 | 0.61 [0.60, 0.62] d |
| SS × High FN | 2 | 0.13 | 0.93 [0.93, 0.93] c |
| HDPE × Low FN | 2 | 0.06 | 0.15 [0.14, 0.16] h |
| HDPE × Medium FN | 2 | 0.07 | 0.27 [0.26, 0.27] g |
| HDPE × High FN | 2 | 0.08 | 0.43 [0.42, 0.44] e |
| Rubber × Low FN | 2 | 0.04 | 0.42 [0.37, 0.47] e |
| Rubber × Medium FN | 2 | 0.05 | 1.03 [0.99, 1.07] b |
| Rubber × High FN | 2 | 0.09 | 1.58 [1.53, 1.63] a |
| Microorganism–Normal Force Interaction Effect | |||
| S. PT 30 × Low FN | 3 | 0.09 | 0.29 |
| E. faecium × Low FN | 3 | 0.05 | 0.29 |
| S. PT 30 × Medium FN | 3 | 0.09 | 0.63 |
| E. faecium × Medium FN | 3 | 0.08 | 0.64 |
| S. PT 30 × High FN | 3 | 0.09 | 0.99 |
| E. faecium × High FN | 3 | 0.11 | 0.97 |
| Microorganism–Surface Material–Normal Force Interaction Effect | |||
| S. PT 30 × SS × Low FN | 1 | / | / |
| S. PT 30 × SS × Medium FN | 1 | ||
| S. PT 30 × SS × High FN | 1 | ||
| S. PT 30 × HDPE × Low FN | 1 | ||
| S. PT 30 × HDPE × Medium FN | 1 | ||
| S. PT 30 × HDPE × High FN | 1 | ||
| S. PT 30 × Rubber × Low FN | 1 | ||
| S. PT 30 × Rubber × Medium FN | 1 | ||
| S. PT 30 × Rubber × High FN | 1 | ||
| E. faecium × SS × Low FN | 1 | ||
| E. faecium × SS × Medium FN | 1 | ||
| E. faecium × SS × High FN | 1 | ||
| E. faecium × HDPE × Low FN | 1 | ||
| E. faecium × HDPE × Medium FN | 1 | ||
| E. faecium × HDPE × High FN | 1 | ||
| E. faecium × Rubber × Low FN | 1 | ||
| E. faecium × Rubber × Medium FN | 1 | ||
| E. faecium × Rubber × High FN | 1 | ||
3.3. Bacterial Removal on SS with Three Surface Roughnesses
| Microorganism | Surface Roughness (Ra, μm) | Normal Force (N) | Count Before Cleaning (log (CFU/Coupon)) | Count After Cleaning (log (CFU/Coupon)) | Log Reduction (log (CFU/Coupon)) | Shear Force (N) |
|---|---|---|---|---|---|---|
| S. PT 30 | 0.6 ± 0.1 | 1.10 ± 0.05 | 9.63 ± 0.09 | 9.56 ± 0.10 | 0.06 ± 0.04 | 0.30 ± 0.02 |
| 2.58 ± 0.03 | 9.53 ± 0.14 | 0.09 ± 0.06 | 0.61 ± 0.01 | |||
| 4.41 ± 0.10 | 9.55 ± 0.08 | 0.07 ± 0.06 | 0.93 ± 0.01 | |||
| 1.1 ± 0.1 | 1.10 ± 0.05 | 9.48 ± 0.03 | 9.45 ± 0.09 | 0.07 ± 0.06 | 0.33 ± 0.01 | |
| 2.58 ± 0.03 | 9.34 ± 0.11 | 0.10 ± 0.04 | 0.64 ± 0.02 | |||
| 4.41 ± 0.10 | 9.45 ± 0.12 | 0.07 ± 0.06 | 0.95 ± 0.02 | |||
| 1.6 ± 0.1 | 1.10 ± 0.05 | 9.58 ± 0.12 | 9.58 ± 0.07 | 0.07 ± 0.03 | 0.38 ± 0.02 | |
| 2.58 ± 0.03 | 9.55 ± 0.13 | 0.05 ± 0.03 | 0.70 ± 0.02 | |||
| 4.41 ± 0.10 | 9.50 ± 0.17 | 0.08 ± 0.05 | 0.99 ± 0.04 | |||
| E. faecium | 0.6 ± 0.1 | 1.10 ± 0.05 | 9.06 ± 0.09 | 8.97 ± 0.15 | 0.09 ± 0.07 | 0.29 ± 0.02 |
| 2.58 ± 0.03 | 8.92 ± 0.24 | 0.18 ± 0.09 | 0.61 ± 0.01 | |||
| 4.41 ± 0.10 | 8.88 ± 0.29 | 0.12 ± 0.13 | 0.92 ± 0.02 | |||
| 1.1 ± 0.1 | 1.10 ± 0.05 | 9.14 ± 0.11 | 9.08 ± 0.17 | 0.08 ± 0.05 | 0.33 ± 0.01 | |
| 2.58 ± 0.03 | 9.10 ± 0.13 | 0.07 ± 0.04 | 0.66 ± 0.01 | |||
| 4.41 ± 0.10 | 9.11 ± 0.18 | 0.05 ± 0.05 | 0.96 ± 0.01 | |||
| 1.6 ± 0.1 | 1.10 ± 0.05 | 8.93 ± 0.19 | 8.98 ± 0.09 | 0.12 ± 0.04 | 0.40 ± 0.01 | |
| 2.58 ± 0.03 | 9.01 ± 0.18 | 0.06 ± 0.02 | 0.71 ± 0.01 | |||
| 4.41 ± 0.10 | 9.01 ± 0.12 | 0.06 ± 0.03 | 1.03 ± 0.05 |
| Factor | n | Log Reduction Mean (log (CFU/Coupon)) | Shear Force Mean (N) |
|---|---|---|---|
| Microorganism Effect | |||
| S. PT 30 | 9 | 0.07 | 0.65 [0.57, 0.76] b |
| E. faecium | 9 | 0.09 | 0.66 [0.55, 0.74] a |
| Surface Roughness Effect | |||
| Low roughness | 6 | 0.11 | 0.61 [0.50, 0.73] c |
| Medium roughness | 6 | 0.08 | 0.65 [0.53, 0.77] b |
| High roughness | 6 | 0.06 | 0.71 [0.59, 0.83] a |
| Normal Force Effect | |||
| Low FN | 6 | 0.07 | 0.35 [0.33, 0.37] c |
| Medium FN | 6 | 0.09 | 0.66 [0.64, 0.68] b |
| High FN | 6 | 0.08 | 0.97 [0.95, 0.99] a |
| Microorganism–Surface Roughness Interaction Effect | |||
| S. PT 30 × Low roughness | 3 | 0.08 | 0.62 [0.45, 0.78] d |
| E. faecium × Low roughness | 3 | 0.15 | 0.61 [0.45, 0.78] d |
| S. PT 30 × Medium roughness | 3 | 0.10 | 0.65 [0.48, 0.80] c |
| E. faecium × Medium roughness | 3 | 0.06 | 0.66 [0.49, 0.82] c |
| S. PT 30 × High roughness | 3 | 0.06 | 0.69 [0.53, 0.85] b |
| E. faecium × High roughness | 3 | 0.05 | 0.73 [0.56, 0.90] a |
| Surface Roughness–Normal Force Interaction Effect | |||
| Low roughness × Low FN | 2 | 0.08 | 0.31 [0.29, 0.32] h |
| Low roughness × Medium FN | 2 | 0.14 | 0.61 [0.60, 0.62] e |
| Low roughness × High FN | 2 | 0.13 | 0.93 [0.92, 0.94] b |
| Medium roughness × Low FN | 2 | 0.07 | 0.34 [0.34, 0.35] g |
| Medium roughness × Medium FN | 2 | 0.10 | 0.65 [0.64, 0.66] d |
| Medium roughness × High FN | 2 | 0.06 | 0.96 [0.94, 0.97] b |
| High roughness × Low FN | 2 | 0.06 | 0.40 [0.39, 0.41] f |
| High roughness × Medium FN | 2 | 0.04 | 0.71 [0.70, 0.72] c |
| High roughness × High FN | 2 | 0.06 | 1.02 [1.00, 1.06] a |
| Microorganism–Normal Force Interaction Effect | |||
| S. PT 30 × Low FN | 3 | 0.07 | 0.35 |
| E. faecium × Low FN | 3 | 0.07 | 0.35 |
| S. PT 30 × Medium FN | 3 | 0.09 | 0.65 |
| E. faecium × Medium FN | 3 | 0.10 | 0.66 |
| S. PT 30 × High FN | 3 | 0.07 | 0.96 |
| E. faecium × High FN | 3 | 0.10 | 0.98 |
| Microorganism–Surface Roughness–Normal Force Interaction Effect | |||
| S. PT 30 × Low roughness × Low FN | 1 | / | / |
| S. PT 30 × Low roughness × Medium FN | 1 | ||
| S. PT 30 × Low roughness × High FN | 1 | ||
| S. PT 30 × Medium roughness × Low FN | 1 | ||
| S. PT 30 × Medium roughness × Medium FN | 1 | ||
| S. PT 30 × Medium roughness × High FN | 1 | ||
| S. PT 30 × High roughness × Low FN | 1 | ||
| S. PT 30 × High roughness × Medium FN | 1 | ||
| S. PT 30 × High roughness × High FN | 1 | ||
| E. faecium × Low roughness × Low FN | 1 | ||
| E. faecium × Low roughness × Medium FN | 1 | ||
| E. faecium × Low roughness × High FN | 1 | ||
| E. faecium × Medium roughness × Low FN | 1 | ||
| E. faecium × Medium roughness × Medium FN | 1 | ||
| E. faecium × Medium roughness × High FN | 1 | ||
| E. faecium × High roughness × Low FN | 1 | ||
| E. faecium × High roughness × Medium FN | 1 | ||
| E. faecium × High roughness × High FN | 1 | ||
4. Potential Applications of the Developed Dry Cleaning Platform
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Wei, X.; Irmak, S.; Chaves, B.D.; Subbiah, J. Inactivation of Salmonella Enterica and Enterococcus faecium NRRL B-2354 in cumin seeds by radiofrequency heating. Food Control 2019, 103, 59–69. [Google Scholar] [CrossRef]
- Anderson, N.M.; Benyathiar, P.; Mishra, D.K. Aseptic processing and packaging. In Food Safety Engineering; Demirci, A., Feng, H., Krishnamurthy, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 661–692. [Google Scholar]
- Flock, G.; Pacitto, D.; Cowell, C.; Marek, P.; Senecal, A. Investigating the effects of environmental stresses on Salmonella enterica serovar Tennessee survival in a low moisture food model. J. Food Process. Preserv. 2020, 44, e14906. [Google Scholar] [CrossRef]
- Acuff, J.C.; Dickson, J.S.; Farber, J.M.; Grasso-Kelley, E.M.; Hedberg, C.; Lee, A.; Zhu, M.J. Practice and progress: Updates on outbreaks, advances in research, and processing technologies for low-moisture food safety. Food Prot. Trends 2023, 86, 100018. [Google Scholar] [CrossRef]
- Alonso, V.P.P.; Goncalves, M.; de Brito, F.A.E.; Barboza, G.R.; Rocha, L.D.; Silva, N.C.C. Dry surface biofilms in the food processing industry: An overview on surface characteristics, adhesion and biofilm formation, detection of biofilms, and dry sanitization methods. Compr. Rev. Food Sci. Food Saf. 2023, 22, 688–713. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, J.; Liu, Z.; Wang, S.; Chen, L. An accurate approach to predict Salmonella Enteritidis PT 30 survival based on dynamic thermal resistance during hot air assisted radio frequency pasteurization of in-shell walnuts. Int. J. Food Microbiol. 2025, 437, 111216. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Tang, J.; Wang, S.J. A new strategy to improve heating uniformity of low moisture foods in radio frequency treatment for pathogen control. J. Food Eng. 2014, 141, 128–138. [Google Scholar] [CrossRef]
- Ban, C.; Lee, D.H.; Jo, Y.; Bae, H.; Seong, H.; Kim, S.O.; Lim, S.; Choi, Y.J. Use of superheated steam to inactivate Salmonella enterica serovars Typhimurium and Enteritidis contamination on black peppercorns, pecans, and almonds. J. Food Eng. 2018, 222, 284–291. [Google Scholar] [CrossRef]
- Subedi, S.; Roopesh, M.S. Simultaneous drying of pet food pellets and Salmonella inactivation by 395 nm light pulses in an LED reactor. J. Food Eng. 2020, 286, 110110. [Google Scholar] [CrossRef]
- Illera, A.E.; Souza, V.R.; Nikmaram, N.; Tang, L.Y.; Keener, K.M. High voltage atmospheric cold plasma decontamination of Salmonella Enteritidis on chicken eggs. Innov. Food Sci. Emerg. Technol. 2022, 82, 103210. [Google Scholar] [CrossRef]
- Salazar, J.K.; Tesfaldet, B.; Zamperlini, M.; Streufert, R.; Fay, M.; Keller, S.E. Desiccation survival of Salmonella enterica, Escherichia coli, and Enterococcus faecium related to initial cell level and cellular components. J. Food Prot. 2022, 85, 398–405. [Google Scholar] [CrossRef]
- Tonti, M.; Verheyen, D.; Kozak, D.; Coombes, C.; Hossain, M.A.; Skara, T.; Van Impe, J.F.M. Inactivation of Salmonella Typhimurium and Listeria monocytogenes in dairy systems: Effect of fat and food matrix structure under radio frequency heating. Innov. Food Sci. Emerg. Technol. 2024, 94, 103684. [Google Scholar] [CrossRef]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef]
- Finn, S.; Condell, O.; McClure, P.; Amézquita, A.; Fanning, S. Mechanisms of survival, responses, and sources of Salmonella in low-moisture environments. Front. Microbiol. 2013, 4, 331. [Google Scholar] [CrossRef]
- Gomba, A.; Chidamba, L.; Korsten, L. Prevalence and serovar diversity of Salmonella spp. in primary horticultural fruit production environments. Food Control 2016, 69, 13–19. [Google Scholar] [CrossRef]
- Miao, S.; Liu, L.; Fu, Z. Prevalence of Salmonella in Chinese food commodities: A meta-analysis. J. Food Prot. 2022, 85, 859–870. [Google Scholar] [CrossRef]
- Prestes, F.S.; Yotsuyanagi, S.E.; Alonso, V.P.P.; Nascimento, M.S. Dry sanitization in the food industry: A review. Curr. Opin. Food Sci. 2024, 57, 101166. [Google Scholar] [CrossRef]
- Kim, W.J.; Karuppuchamy, V.; Heldman, D.R. Evaluation of maximum wall shear stress from air impingement to remove food deposits from stainless steel surfaces. J. Food Eng. 2022, 316, 110825. [Google Scholar] [CrossRef]
- Karuppuchamy, V.; Heldman, D.R.; Snyder, A.B. A review of food safety in low-moisture foods with current and potential dry-cleaning methods. J. Food Sci. 2024, 89, 793–810. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Y.; Ma, J.; Kim, W.-J.; Chen, L. Developing an air impingement dry cleaning device and investigating the factors affecting nonfat dry milk residue removal from stainless steel surfaces. J. Dairy Sci. 2025, 108, 9345–9358. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Zhao, S.Y. Application of pulsating vacuum cleaning technology to medical devices cleaning. J. Biomater. Tissue Eng. 2022, 12, 984–988. [Google Scholar] [CrossRef]
- He, Q.R.; Chen, L.; Snyder, A.B. The physicochemical properties of fruit powders and their residence time on stainless steel surfaces are associated with their ease of removal by brushing. Food Res. Int. 2022, 158, 111569. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Rana, Y.S.; Heldman, D.R.; Snyder, A.B. Environment, food residue, and dry cleaning tool all influence the removal of food powders and allergenic residues from stainless steel surfaces. Innov. Food Sci. Emerg. Technol. 2022, 75, 102877. [Google Scholar] [CrossRef]
- Daeschel, D.; Chen, L.; Zoellner, C.; Snyder, A.B. A simulation model to quantify the efficacy of dry cleaning interventions on a contaminated milk powder line. Appl. Environ. Microbiol. 2025, 91, e02086-24. [Google Scholar] [CrossRef] [PubMed]
- Parvin, F.; Hu, H.; Whiteley, G.S.; Glasbey, T.; Vickery, K. Difficulty in removing biofilm from dry surfaces. J. Hosp. Infect. 2019, 103, 465–467. [Google Scholar] [CrossRef]
- Chen, L.; Snyder, A.B. Surface inoculation method impacts microbial reduction and transfer of Salmonella Enteritidis PT 30 and potential surrogates during dry sanitation. Int. J. Food Microbiol. 2023, 406, 110405. [Google Scholar] [CrossRef]
- Magens, O.M.; Liu, Y.; Hofmans, J.F.A.; Nelissen, J.A.; Ian Wilson, D. Adhesion and cleaning of foods with complex structure: Effect of oil content and fluoropolymer coating characteristics on the detachment of cake from baking surfaces. J. Food Eng. 2017, 197, 48–59. [Google Scholar] [CrossRef]
- Koo, O.K.; Martin, E.M.; Story, R.; Lindsay, D.; Ricke, S.C.; Crandall, P.G. Comparison of cleaning fabrics for bacterial removal from food-contact surfaces. Food Control 2013, 30, 292–297. [Google Scholar] [CrossRef]
- Mizuno, M.; Matsuda, J.; Watanabe, K.; Shimizu, N.; Sekiya, I. Effect of disinfectants and manual wiping for processing the cell product changeover in a biosafety cabinet. Regen. Ther. 2023, 22, 169–175. [Google Scholar] [CrossRef]
- Perez-Rodríguez, F.; Valero, A.; Carrasco, E.; García, R.M.; Zurera, G. Understanding and modelling bacterial transfer to foods: A review. Trends Food Sci. Technol. 2008, 19, 131–144. [Google Scholar] [CrossRef]
- Zhao, P.C.; Chan, P.O.; Gao, Y.S.; Lai, O.W.; Zhang, T.; Li, Y.G. Physical factors that affect microbial transfer during surface touch. Build. Environ. 2019, 158, 28–38. [Google Scholar] [CrossRef]
- European Hygienic Engineering and Design Group (EHEDG). Hygienic Design Principles, 3rd ed.; European Hygienic Engineering and Design Group: Amsterdam, The Netherlands, 2018; p. 10. [Google Scholar]
- Lv, C.Y.; Zhang, J.R.; Li, F.; Shi, H.; Kong, F.B.; Jiao, Y. Effect of environmental conditions on the survival and transfer of Enterococcus faecium between wheat flour and selected contact surfaces. Food Microbiol. 2025, 129, 104761. [Google Scholar] [CrossRef]
- Tadapaneni, R.K.; Xu, J.; Yang, R.; Tang, J.M. Improving design of thermal water activity cell to study thermal resistance of Salmonella in low-moisture foods. LWT-Food Sci. Technol. 2018, 92, 371–379. [Google Scholar] [CrossRef]
- Liu, S.X.; Ozturk, S.; Xu, J.; Kong, F.B.; Gray, P.; Zhu, M.J.; Sablani, S.S.; Tang, J.M. Microbial validation of radio frequency pasteurization of wheat flour by inoculated pack studies. J. Food Eng. 2018, 217, 68–74. [Google Scholar] [CrossRef]
- Wei, X.Y.; Vasquez, S.; Thippareddi, H.; Subbiah, J. Evaluation of Enterococcus faecium NRRL B-2354 as a surrogate for Salmonella in ground black pepper at different water activities. Int. J. Food Microbiol. 2021, 344, 109114. [Google Scholar] [CrossRef] [PubMed]
- Casado, E.G.; Yao, Y.J.; Zaffora, B.; Battaggia, D.; Schnabel, U.; Zubera, S.; den Besten, H.M.W. Inactivation of Salmonella, Enterococcus faecium and natural microbiota on dry food matrices with microwave-driven plasma-processed air. Innov. Food Sci. Emerg. Technol. 2024, 97, 103822. [Google Scholar] [CrossRef]
- Xie, Y.C.; Long, X.N.; Kim, Y.; Harris, L.J.; Nitin, N. Survival of Salmonella enterica and Enterococcus faecium on abiotic surfaces during storage at low relative humidity. J. Food Prot. 2024, 87, 100292. [Google Scholar] [CrossRef]
- Weyrich, A.; Salvi, D. Ultraviolet-C light (254 nm) treatment using a batch-style powder redistribution system for the inactivation of Salmonella surrogate, Enterococcus faecium, in wheat flour. J. Food Eng. 2025, 401, 112657. [Google Scholar] [CrossRef]
- Xie, Y.C.; Nitin, N.; Harris, L.J. Transfer of Enterococcus faecium and Salmonella enterica during simulated postharvest handling of yellow onions (Allium cepa). Food Microbiol. 2023, 115, 104340. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Z.; Liu, Y.; Wang, S.; Weller, C.L.; Chen, L. Factors affecting microbial transfer of Salmonella enterica Enteritidis PT 30 and potential surrogate Enterococcus faecium NRRL B2354 during dry cleaning. LWT-Food Sci. Technol. 2025, 233, 118544. [Google Scholar] [CrossRef]
- Cai, L.; Wu, D.; Xia, J.H.; Shi, H.H.; Kim, H. Influence of physicochemical surface properties on the adhesion of bacteria onto four types of plastics. Sci. Total Environ. 2019, 671, 1101–1107. [Google Scholar] [CrossRef]
- Velkavrh, I.; Lungevics, J.; Jansons, E.; Klien, S.; Voyer, J.; Ausserer, F. The influence of isotropic surface roughness of steel sliders on ice friction under different testing conditions. Lubricants 2019, 7, 106. [Google Scholar] [CrossRef]
- Waldhans, C.; Hebel, M.; Herbert, U.; Spoelstra, P.; Barbut, S.; Kreyenschmidt, J. Microbial investigation of cleanability of different plastic and metal surfaces used by the food industry. J. Food Sci. Technol. 2023, 60, 2581–2590. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.F.; Chmielewski, R. Influence of surface finish on the cleanability of stainless steel. J. Food Prot. 2001, 64, 1178–1182. [Google Scholar] [CrossRef] [PubMed]
- Tannera, M.; Singhb, R.; Svellentic, L.; Hamzad, B.; Attine, T.; Wegehauptf, F.J. Effect of toothbrush bristle stiffness and brushing force on cleaning efficacy. Oral Health Prev. Dent. 2023, 21, 153–162. [Google Scholar]
- Middleton, K.E.; Holah, J.T.; Timperley, A.W. Guidelines for the Hygienic Design, Selection and Use of Dry Cleaning Equipment; Guideline No. 40; Campden & Chorleywood Food Research Association: Chipping Campden, UK, 2003. [Google Scholar]
- Lasagabaster, A.; Arboleya, J.C.; de Marañón, I.M. Pulsed light technology for surface decontamination of eggs: Impact on Salmonella inactivation and egg quality. Innov. Food Sci. Emerg. Technol. 2011, 12, 124–128. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Weller, C.L.; Wang, S.; Liu, Y.; Liu, Z.; Chen, L. Applicability of Controllable Normal Force Platform for Study of Bacteria Removal During Dry Cleaning in Dry Food Manufacturing Environments. Foods 2025, 14, 3459. https://doi.org/10.3390/foods14203459
Ma J, Weller CL, Wang S, Liu Y, Liu Z, Chen L. Applicability of Controllable Normal Force Platform for Study of Bacteria Removal During Dry Cleaning in Dry Food Manufacturing Environments. Foods. 2025; 14(20):3459. https://doi.org/10.3390/foods14203459
Chicago/Turabian StyleMa, Jincheng, Curtis L. Weller, Shaojin Wang, Yu Liu, Zhipeng Liu, and Long Chen. 2025. "Applicability of Controllable Normal Force Platform for Study of Bacteria Removal During Dry Cleaning in Dry Food Manufacturing Environments" Foods 14, no. 20: 3459. https://doi.org/10.3390/foods14203459
APA StyleMa, J., Weller, C. L., Wang, S., Liu, Y., Liu, Z., & Chen, L. (2025). Applicability of Controllable Normal Force Platform for Study of Bacteria Removal During Dry Cleaning in Dry Food Manufacturing Environments. Foods, 14(20), 3459. https://doi.org/10.3390/foods14203459








