Antioxidant Activity of Pomegranate Husk Ellagitannins in Enhancing Oxidative Stability of Canola Oil During Frying
Abstract
1. Introduction
2. Materials and Methods
2.1. Drying of Pomegranate Husk
2.2. Extraction of Polyphenols from Pomegranate Husk
2.3. Purification of Polyphenols
2.4. HPLC-ESI-MS/MS Characterization
2.5. Evaluation of the Antioxidant Effect of Polyphenols in Canola Vegetable Oil
2.6. Application of Polyphenols to Vegetable Oil for Frying Potatoes
2.7. Evaluation of Primary Lipid Oxidation
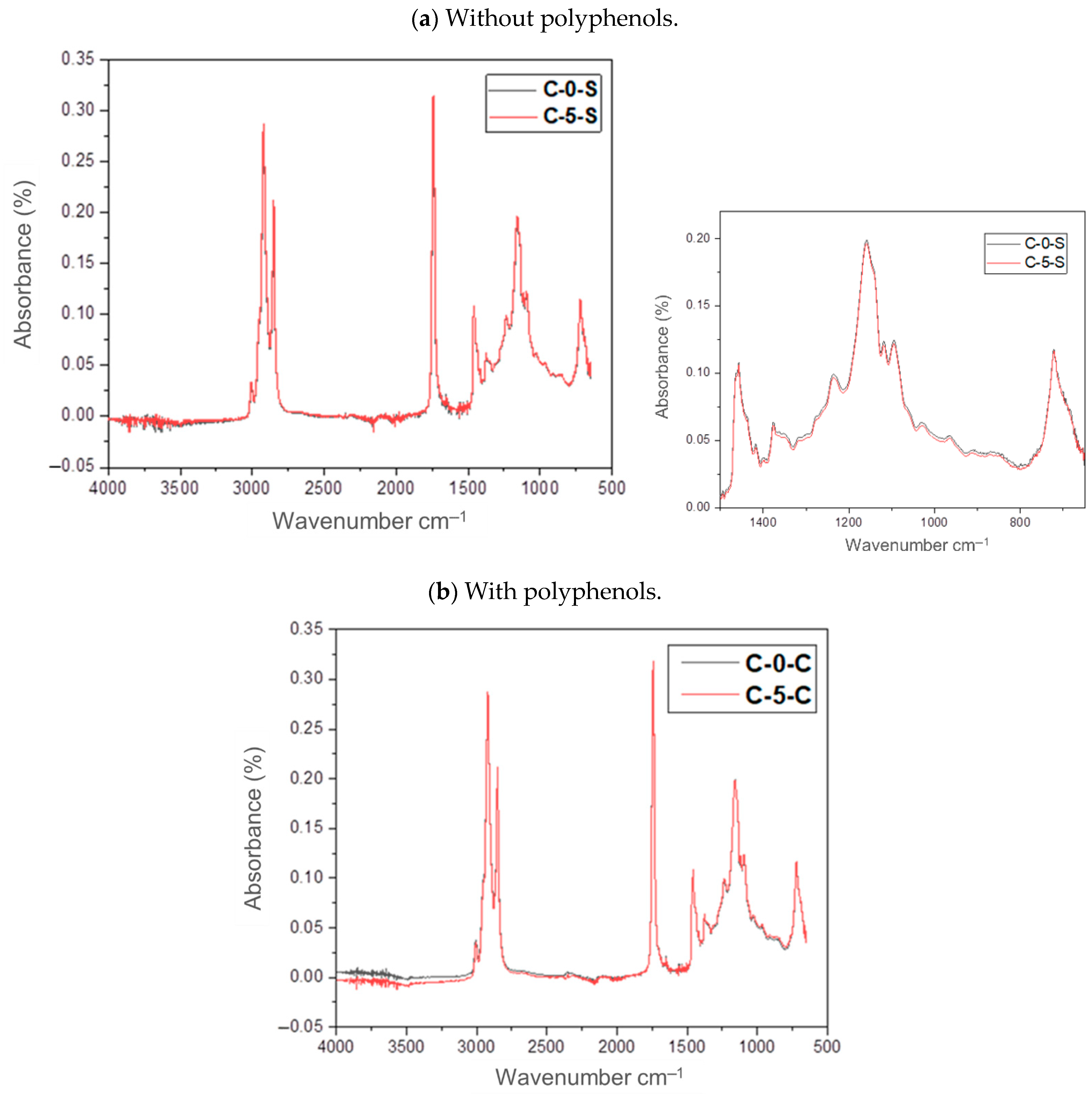
2.8. Identification of Peroxide Formation by Infrared Spectroscopy
2.9. Statistical Analysis
3. Results and Discussion
3.1. Polyphenols from Pomegranate Husk
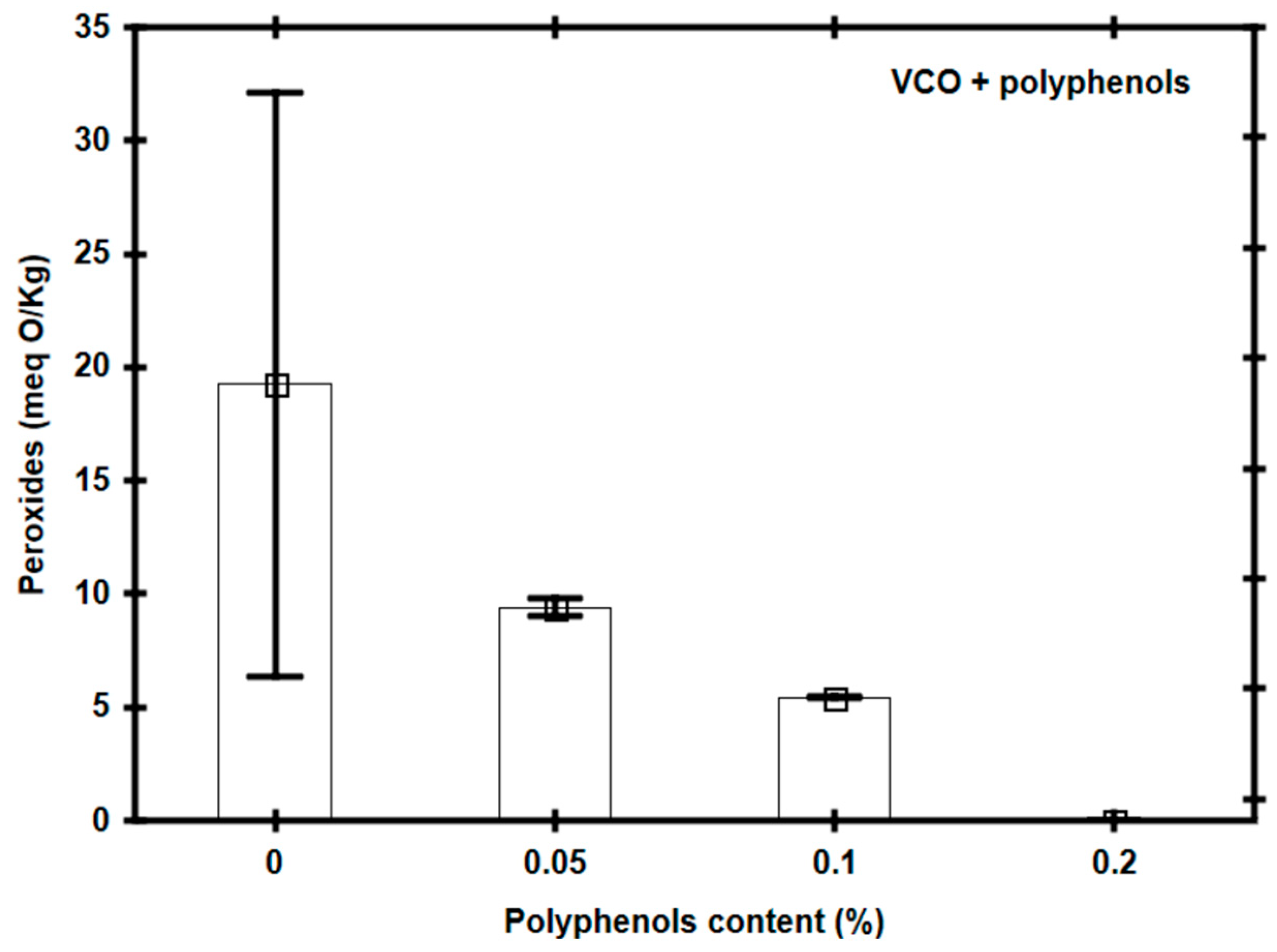
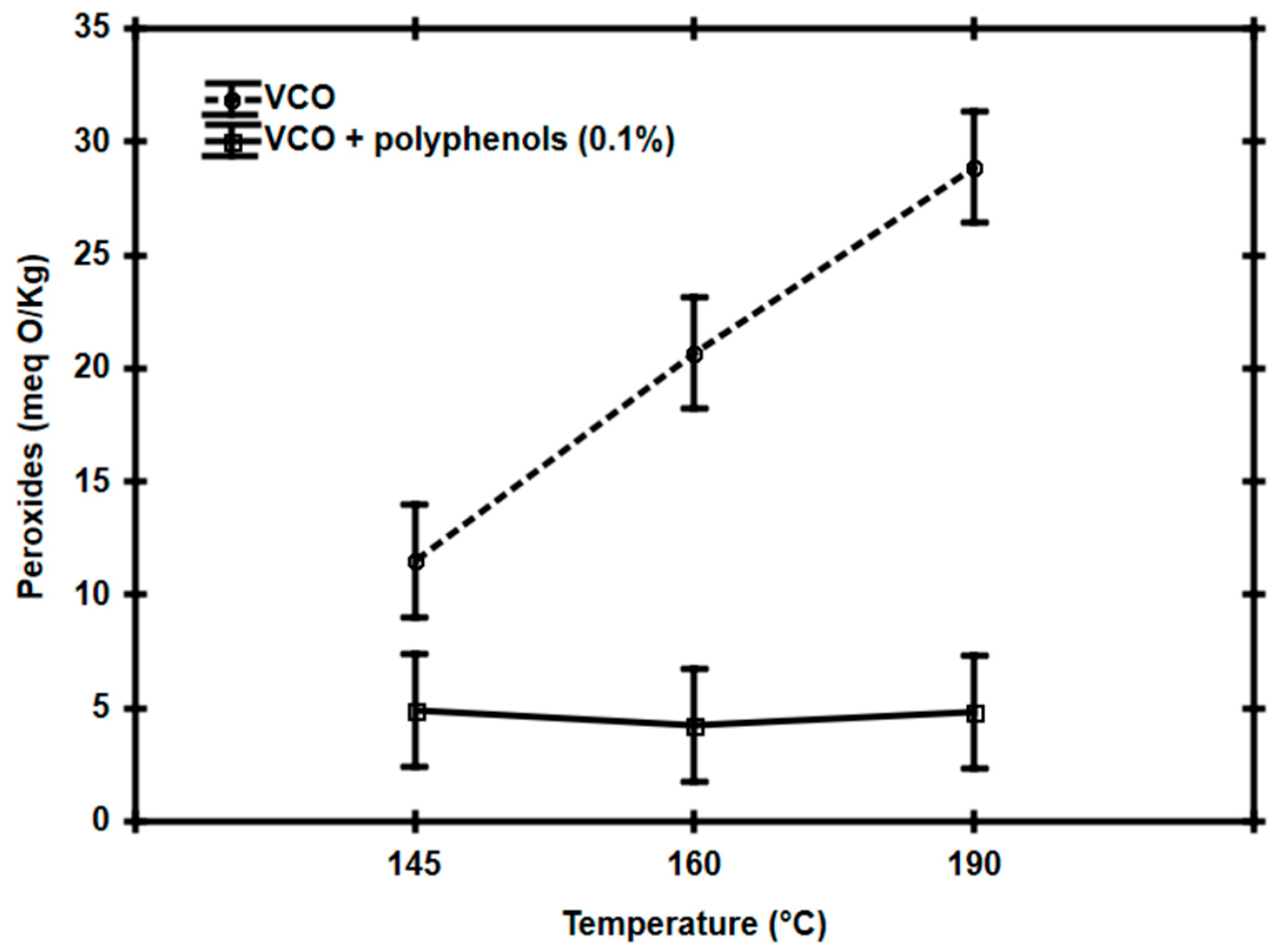
3.2. Effect of Polyphenol Addition in Vegetable Canola Oil for Frying
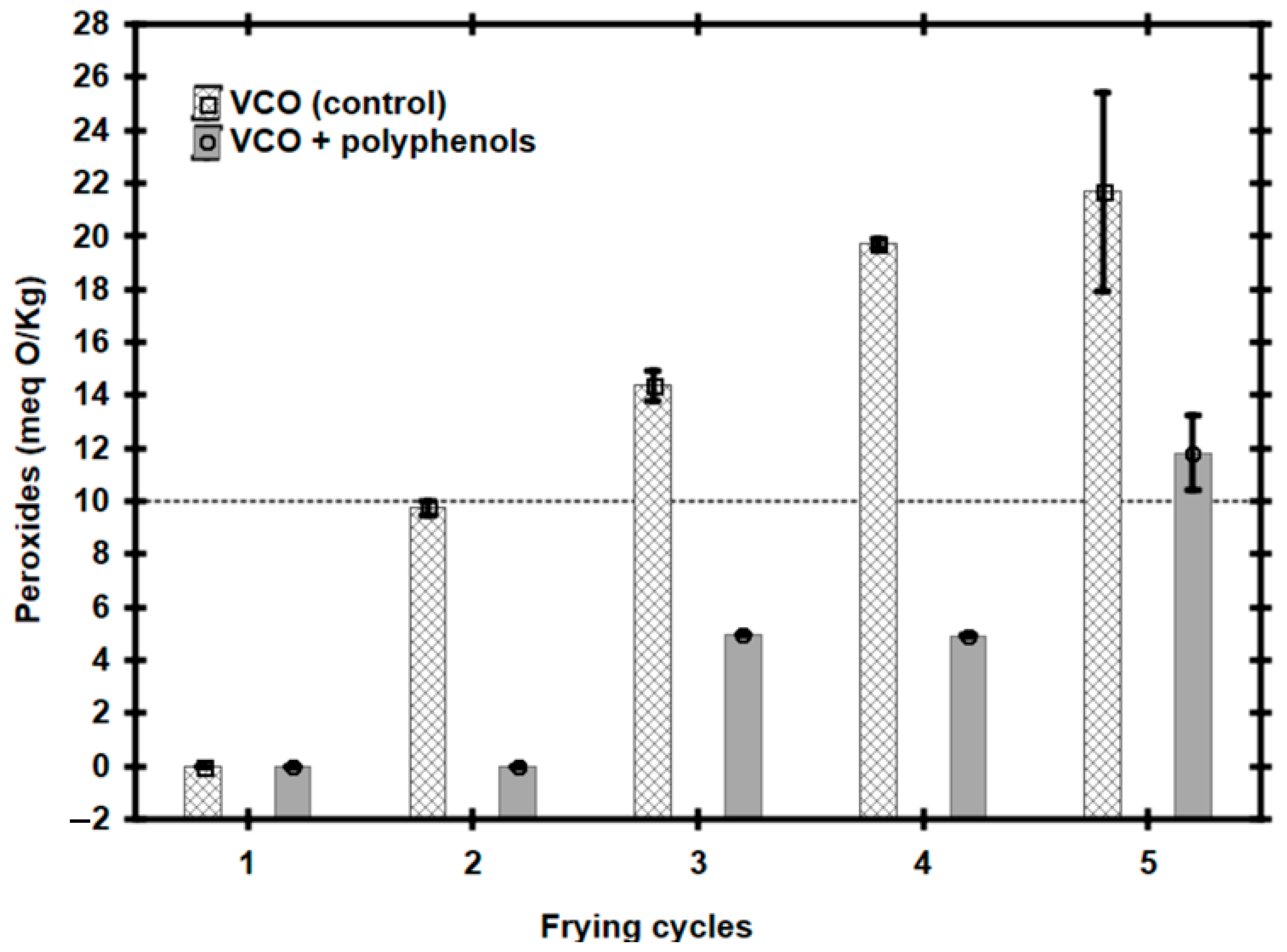
3.3. Using Vegetable Canola Oil with Polyphenols for Frying Potatoes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Metin, S.; Hartel, R.W. Edible Oil and Fat Products: Chemistry, Properties, and Health Effects. Crystallization of Fats and Oils. In Bailey’s Industrial Oil and Fat Products; Wiley: Hoboken. NY, USA, 2005; pp. 45–76. ISBN 0-471-38552-2. [Google Scholar]
- Awogbemi, O.; Onuh, E.I.; Inambao, F.L. Comparative Study of Properties and Fatty Acid Composition of Some Neat Vegetable Oils and Waste Cooking Oils. Int. J. Low-Carbon Technol. 2019, 14, 417–425. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms of Antioxidants in the Oxidation of Foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Oh, S.; Lee, E.; Choe, E. Light Effects on Lipid Oxidation, Antioxidants, and Pigments in Dried Laver (Porphyra) during Storage. Food Sci. Biotechnol. 2014, 23, 701–709. [Google Scholar] [CrossRef]
- Sun, M.; Wang, J.; Dong, J.; Lu, Y.; Zhang, Y.; Dong, L.; Wang, S. Effects of Different Frying Oils Composed of Various Fatty Acids on the Formation of Multiple Hazards in Fried Pork Balls. Foods 2023, 12, 4182. [Google Scholar] [CrossRef]
- Goswami, G.; Bora, R.; Rathore, M.S. Oxidation Of Cooking Oils Due To Repeated Frying And Human Health. Int. J. Sci. Technol. Manag. 2015, 4, 495–501. [Google Scholar]
- Park, J.M.; Koh, J.H.; Kim, J.M. Determining the Reuse of Frying Oil for Fried Sweet and Sour Pork According to Type of Oil and Frying Time. Food Sci. Anim. Resour. 2020, 40, 785–794. [Google Scholar] [CrossRef]
- Fan, L.; Eskin, N.A.M. The Use of Antioxidants in the Preservation of Edible Oils; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; ISBN 9781782420972. [Google Scholar]
- Cutimbo, M.C.; Aro, J.M.A.; Vivanco, Z.L.T. Estabilidad Oxidativa Del Aceite de Soya Con Adición de Antioxidante De. Rev. Investig. Altoandinas J. High Andean Res. 2016, 18, 395–402. [Google Scholar] [CrossRef]
- Taghvaei, M.; Jafari, S.M. Application and Stability of Natural Antioxidants in Edible Oils in Order to Substitute Synthetic Additives. J. Food Sci. Technol. 2015, 52, 1272–1282. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 14–16. [Google Scholar] [CrossRef]
- Rashid, R.; Masoodi, F.A.; Wani, S.M.; Manzoor, S.; Gull, A. Ultrasound Assisted Extraction of Bioactive Compounds from Pomegranate Peel, Their Nanoencapsulation and Application for Improvement in Shelf Life Extension of Edible Oils. Food Chem. 2022, 385, 132608. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Zárate, P.; Wong-Paz, J.; Buenrostro-Figueroa, J.; Ascacio, J.; Contreras-Esquivel, J.; Aguilar, C. Ellagitannins: Bioavailability, Purification and Biotechnological Degradation. Mini Rev. Med. Chem. 2017, 18, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Haleem, S.; Akhtar, M.; Zia-ul-Haq, M.; Akbar, J. Efficiency of Pomegranate Peel Extracts in Stabilization of Sunflower Oil under Accelerated Conditions. Food Res. Int. 2008, 41, 194–200. [Google Scholar] [CrossRef]
- El-Hadary, A.E.; Taha, M. Pomegranate Peel Methanolic-Extract Improves the Shelf-Life of Edible-Oils under Accelerated Oxidation Conditions. Food Sci. Nutr. 2020, 8, 1798–1811. [Google Scholar] [CrossRef]
- Alsufiani, H.; Mansouri, R.; Almalki, A.; Alsolami, A.; Kuddah, L.; Yamani, R.; Alghashmari, N.; Omar, U. Effect of Punicalagin as Natural Antioxidant on the Oxidative Stability of Canola Oil during Storage. Egypt. J. Chem. 2020, 63, 2369–2378. [Google Scholar] [CrossRef]
- Ascacio-Valdés, J.; Burboa, E.; Aguilera-Carbo, A.F.; Aparicio, M.; Pérez-Schmidt, R.; Rodríguez, R.; Aguilar, C.N. Antifungal Ellagitannin Isolated from Euphorbia antisyphilitica Zucc. Asian Pac. J. Trop. Biomed. 2013, 3, 41–46. [Google Scholar] [CrossRef]
- Aguilar-Zárate, P.; Wong-Paz, J.E.; Michel, M.; Buenrostro-Figueroa, J.; Díaz, H.R.; Ascacio, J.A.; Contreras-Esquivel, J.C.; Gutiérrez-Sánchez, G.; Aguilar, C.N. Characterisation of Pomegranate-Husk Polyphenols and Semi-Preparative Fractionation of Punicalagin. Phytochem. Anal. 2017, 28, 433–438. [Google Scholar] [CrossRef]
- NMX-F-808-SCFI-2018; Secretaría de Economía. Norma of Mexicana. Secretaria de Gobernación: Mexico City, Mexico, 2018.
- U. S. Food and Drug Administration. CFR-Code of Federal Regulations Title 21; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2024. [Google Scholar]
- AOCS Cd 8-53 SURPLUS. Peroxide Value—Acetic Acid-Chloroform Method. In Official Methods and Recommended Practices of the AOCS; The American Oil Chemists’ Society: Champaign, IL USA, 2024; p. 53. [Google Scholar]
- Michel-Michel, M.R.; Martínez-Torres, P.F.; Ávila-Hernández, J.G.; Rojas, R.; Martínez-Ávila, G.C.G.; Ascacio-Valdés, J.A.; Aguilar-Zárate, M.; Aguilar-Zárate, P. Effect of Spray-Dried Pomegranate Peel Polyphenols on the Inhibition of Lipid Oxidation. Int. J. Food Sci. Technol. 2023, 58, 6744–6751. [Google Scholar] [CrossRef]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and Quantification of Phenolic Compounds from Pomegranate (Punica granatum L.) Peel, Mesocarp, Aril and Differently Produced Juices by HPLC-DAD–ESI/MSn. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef]
- Mena, P.; Calani, L.; Dall’Asta, C.; Galaverna, G.; García-Viguera, C.; Bruni, R.; Crozier, A.; Del Rio, D. Rapid and Comprehensive Evaluation of (Poly) Phenolic Compounds in Pomegranate (Punica granatum L.) Juice by UHPLC-MSn. Molecules 2012, 17, 14821–14840. [Google Scholar] [CrossRef]
- Álvarez-Díaz, N.G.; Michel-Michel, M.R.; Aguilar-Zárate, P.; Ascacio-Valdés, J.A.; Wong-Paz, J.E.; Muñiz-Márquez, D.B.; Aguilar-Zárate, M. Antioxidant Effect of Polyphenols Extracted from Pomegranate Peel on Vegetable Oil/Ethylcellulose-Based Oleogels. Rev. Int. Investig. E Innov. Tecnol. 2023, 10, 31–48. [Google Scholar]
- Hager, T.J.; Howard, L.R.; Liyanage, R.; Lay, J.O.; Prior, R.L. Ellagitannin Composition of Blackberry as Determined by HPLC-ESI-MS and MALDI-TOF-MS. J. Agric. Food Chem. 2008, 56, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Gasperotti, M.; Masuero, D.; Vrhovsek, U.; Guella, G.; Mattivi, F. Profiling and Accurate Quantification of Rubus Ellagitannins and Ellagic Acid Conjugates Using Direct UPLC-Q-TOF HDMS and HPLC-DAD Analysis. J. Agric. Food Chem. 2010, 58, 4602–4616. [Google Scholar] [CrossRef] [PubMed]
- Pouysegu, L.; Deffieux, D.; Malik, G.; Natangelo, A.; Quideau, S. Synthesis of Ellagitannin Natural Products. Nat. Prod. Rep. 2011, 28, 853–874. [Google Scholar] [CrossRef]
- Peyronel, F. Medium Chain Triacylglycerides; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, ISBN 9780128140451. [Google Scholar]
- Mekawi, E.; Fawzy, M.; Hassanien, R. Reduction of Acrylamide Formation in Potato Chips during Deep-Frying in Sunflower Oil Using Pomegranate Peel Nanoparticles Extract Reduction o Acrylamide Ormation in Potato Chips during Deep-Rying in Sunfower Oil Using Pomegranate Peel Nanoparticles Extract. J. Food Meas. Charact. 2019, 13, 3298–3306. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; McClements, D.J.; Meng, C.; Zhang, M.; Chen, H.; Deng, Q. Recent Advances in Understanding the Interfacial Activity of Antioxidants in Association Colloids in Bulk Oil. Adv. Colloid Interface Sci. 2024, 325, 103117. [Google Scholar] [CrossRef]
- Fukasawa, R.; Kanda, A.; Hara, S. Anti-Oxidative Effects of Rooibos Tea Extract on Autoxidation and Thermal Oxidation of Lipids. J. Oleo Sci. 2009, 283, 275–283. [Google Scholar] [CrossRef][Green Version]
- Aladedunye, F.; Przybylski, R.; Matthaus, B. Performance of Antioxidative Compounds under Frying Conditions: A Review. Crit. Rev. Food Sci. Nutr. 2017, 11, 1539–1561. [Google Scholar] [CrossRef]
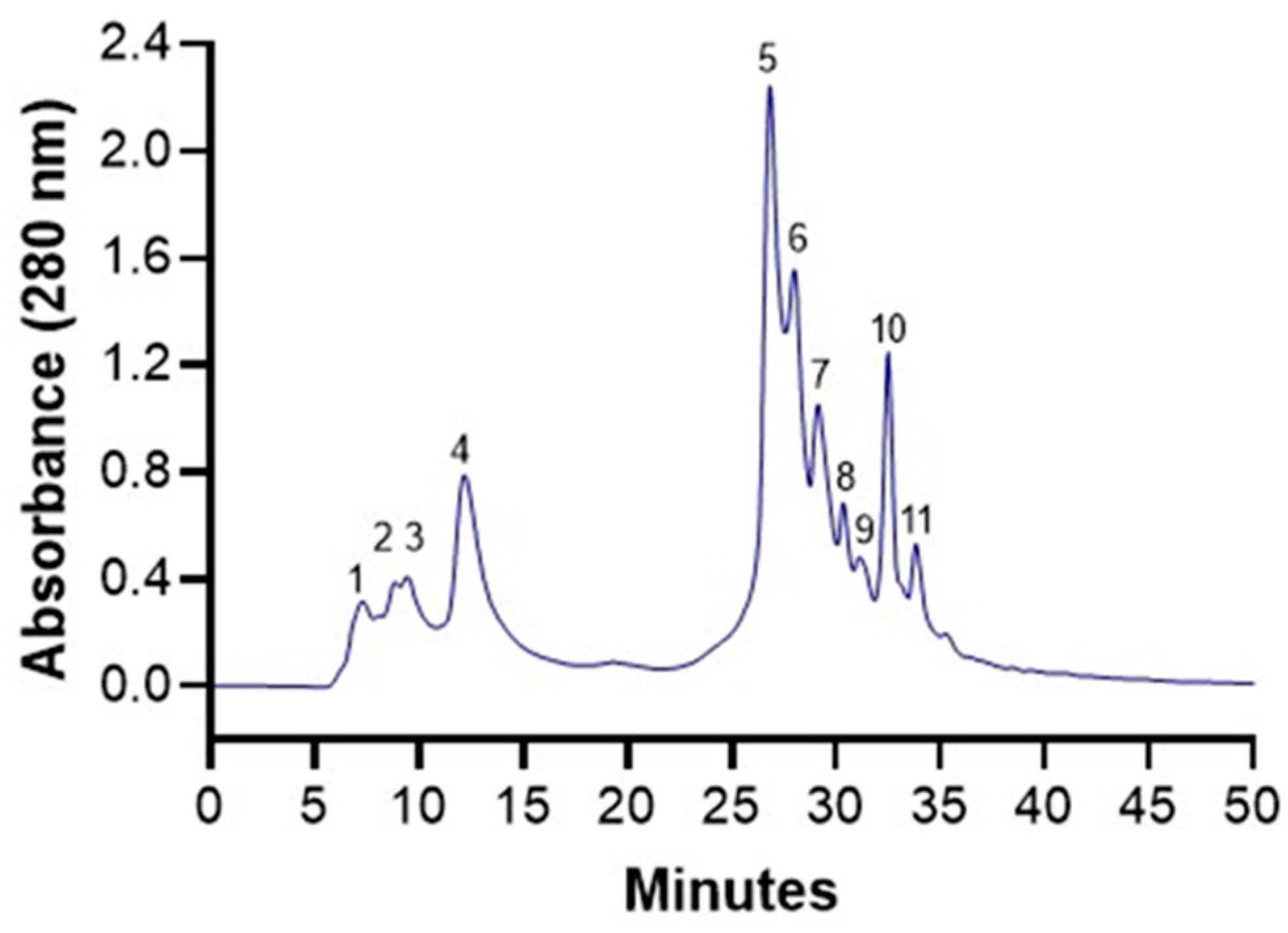
| Peak No. | Tentative Identification | RT (min) | Wavelength | [M−H]− m/z | MS2 Ion Fragment (m/z) |
|---|---|---|---|---|---|
| 1 | Galloyl-hex | 7.21 | 273, 380 | 331 | 169, 271 |
| 2 | Di(HHDP-galloylglucose)-pentose | 8.89 | 278, 380 | 707 * | 613, 633, 461, 635 |
| 3 | Pedunculagin I | 9.46 | 260, 388 | 783 | 481, 301 |
| 4 | Punicalin | 12.20 | 285, 388 | 781 | 301, 479, 601, 723 |
| 5 | Punicalagin | 26.85 | 280, 388 | 1083 | 781, 601, 575 |
| 6 | Ellagic acid der | 28.03 | 258, 380 | 799 | 301, 479 |
| 7 | Sanguiin-H6 | 29.07 | 234, 320 | 934 * | 801 |
| 8 | Pedunculagin II | 30.29 | 254, 355 | 785 | 633, 483, 301, 765 |
| 9 | Galloyl-HHDP-hex | 31.11 | 262, 373 | 633 | 301, 435, 463 |
| 10 | Granatin B | 32.45 | 266, 370 | 951 | 301, 587, 613, 896 |
| 11 | Ellagic acid | 33.79 | 254, 373 | 301 | 185, 229, 257, 272, 284, 301 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michel, M.R.; Pacheco-Lara, M.; Rojas, R.; Martínez-Ávila, G.C.G.; Ascacio-Valdés, J.A.; Aguilar-Zárate, M.; Aguilar-Zárate, P. Antioxidant Activity of Pomegranate Husk Ellagitannins in Enhancing Oxidative Stability of Canola Oil During Frying. Foods 2025, 14, 226. https://doi.org/10.3390/foods14020226
Michel MR, Pacheco-Lara M, Rojas R, Martínez-Ávila GCG, Ascacio-Valdés JA, Aguilar-Zárate M, Aguilar-Zárate P. Antioxidant Activity of Pomegranate Husk Ellagitannins in Enhancing Oxidative Stability of Canola Oil During Frying. Foods. 2025; 14(2):226. https://doi.org/10.3390/foods14020226
Chicago/Turabian StyleMichel, Mariela R., Maritza Pacheco-Lara, Romeo Rojas, Guillermo Cristian G. Martínez-Ávila, Juan Alberto Ascacio-Valdés, Mayra Aguilar-Zárate, and Pedro Aguilar-Zárate. 2025. "Antioxidant Activity of Pomegranate Husk Ellagitannins in Enhancing Oxidative Stability of Canola Oil During Frying" Foods 14, no. 2: 226. https://doi.org/10.3390/foods14020226
APA StyleMichel, M. R., Pacheco-Lara, M., Rojas, R., Martínez-Ávila, G. C. G., Ascacio-Valdés, J. A., Aguilar-Zárate, M., & Aguilar-Zárate, P. (2025). Antioxidant Activity of Pomegranate Husk Ellagitannins in Enhancing Oxidative Stability of Canola Oil During Frying. Foods, 14(2), 226. https://doi.org/10.3390/foods14020226









