Prevalence, Characterization, and Proteomic Relatedness Among β-Lactam-Resistant Bacteria Throughout the Poultry Production Chain in Greece
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Microbiological Examination, Identification, and Proteomic Relationship of the Isolated Strains
2.3. Antimicrobial Susceptibility Testing of the Isolated Strains
2.4. Confirmation of β-Lactamase Production
2.5. Molecular Screening of β-Lactamase Genes
2.6. Phylogenetic Analysis of E. coli Isolates
2.7. Statistical Analysis
3. Results
3.1. Prevalence, Seasonal, and Regional Variations in Isolates
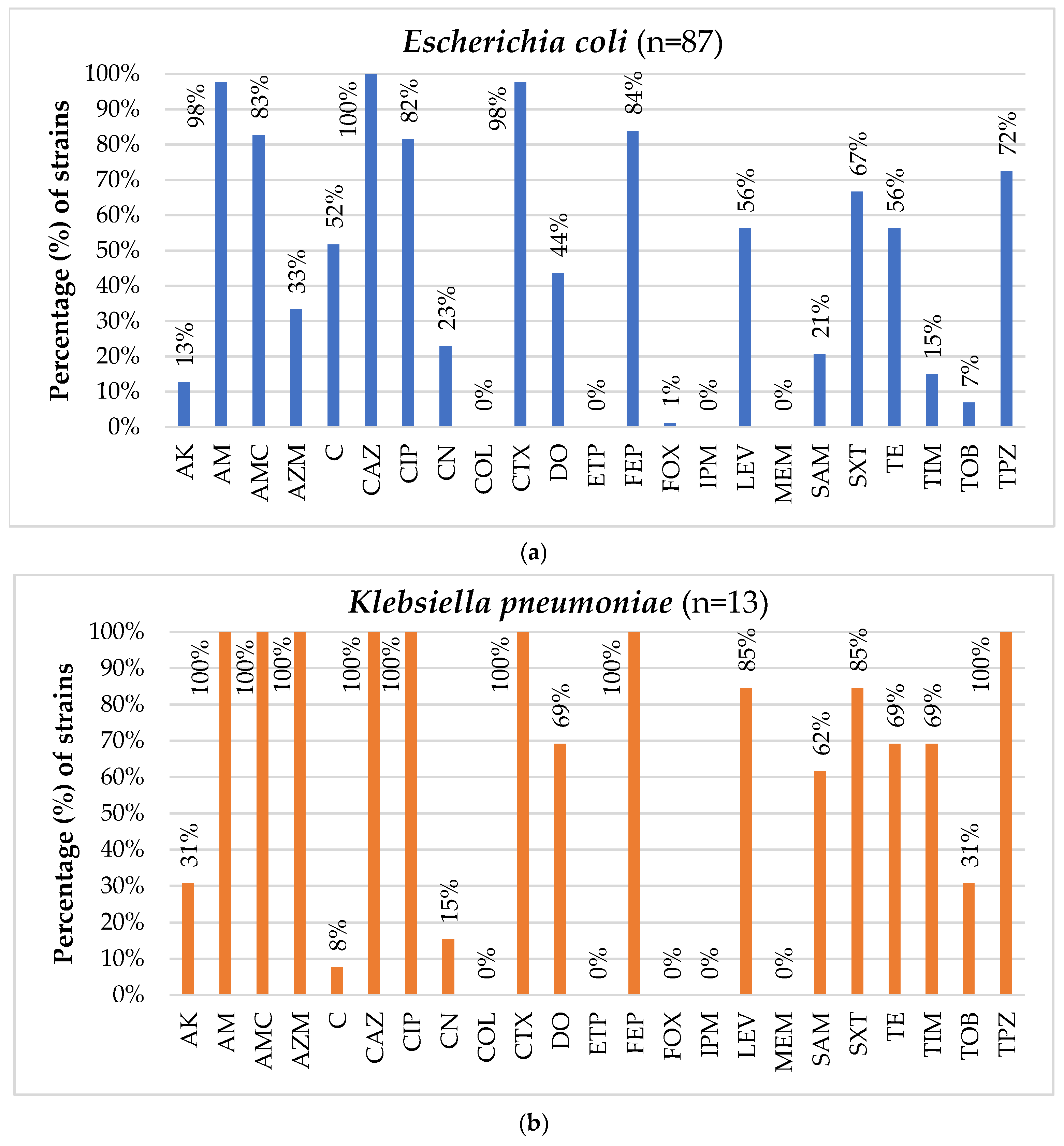
3.2. Antimicrobial Susceptibility Testing of the Isolated Strains
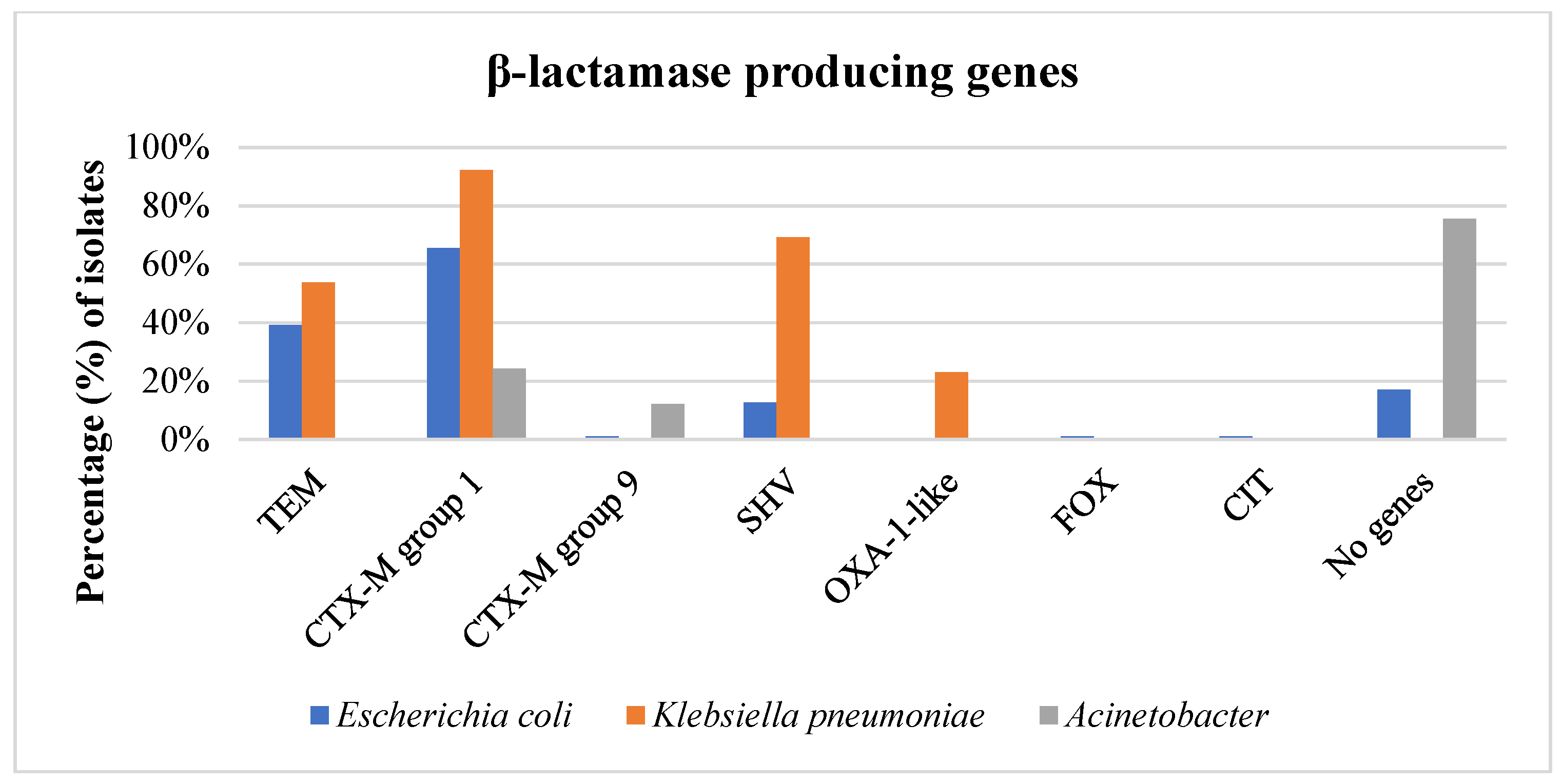
3.3. Phenotype of β-Lactam Resistance and Molecular Screening of β-Lactamase Genes
3.4. Proteomic Relationship of Isolates
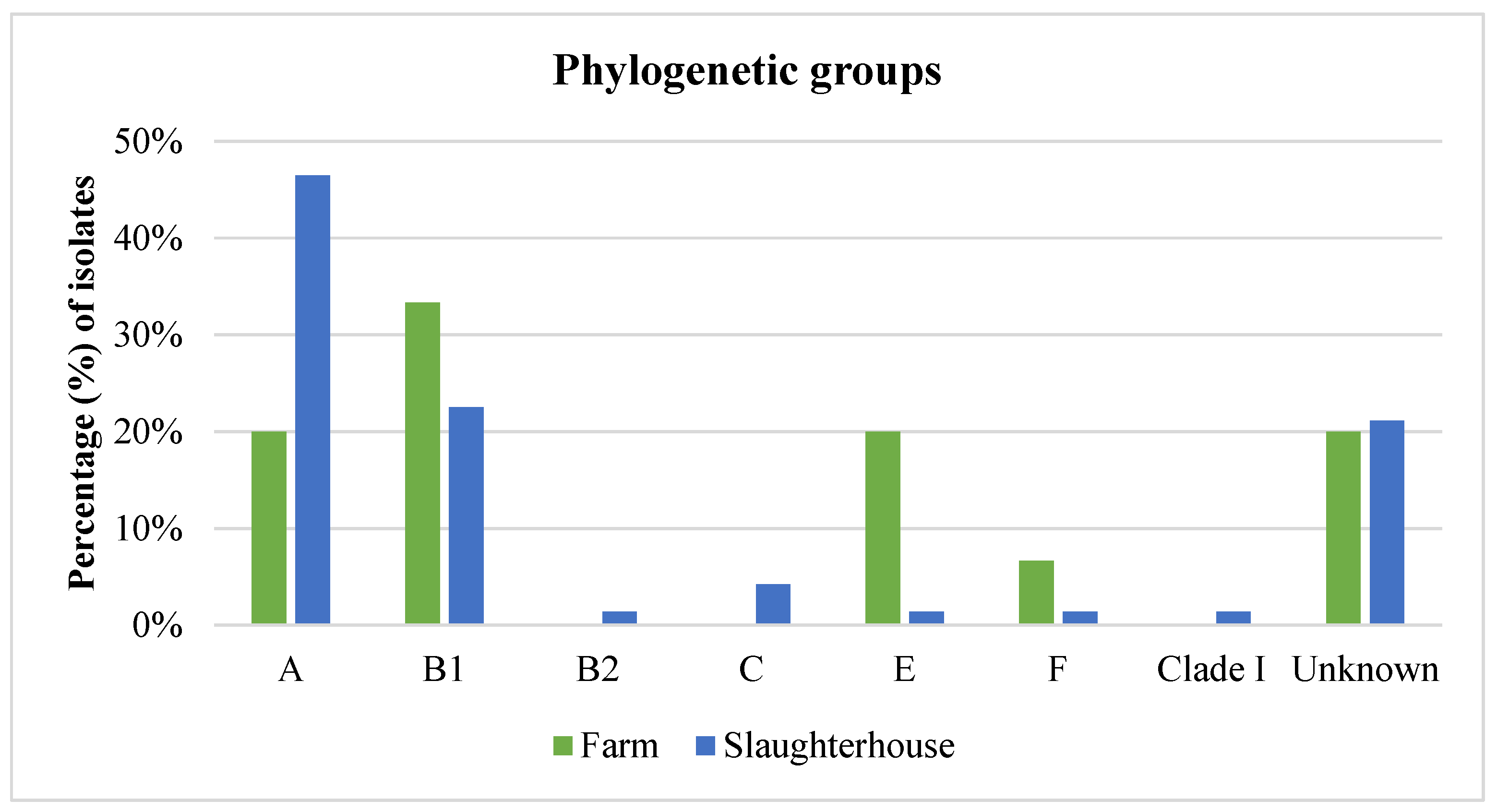
3.5. Phylogenetic Analysis of E. coli Isolates
3.6. Medical and Veterinary History Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Economou, V.; Gousia, P. Agriculture and Food Animals as a Source of Antimicrobial-Resistant Bacteria. Infect. Drug Resist. 2015, 8, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Argudín, M.; Deplano, A.; Meghraoui, A.; Dodémont, M.; Heinrichs, A.; Denis, O.; Nonhoff, C.; Roisin, S. Bacteria from Animals as a Pool of Antimicrobial Resistance Genes. Antibiotics 2017, 6, 12. [Google Scholar] [CrossRef]
- Worthington, R.J.; Melander, C. Overcoming Resistance to β-Lactam Antibiotics. J. Org. Chem. 2013, 78, 4207–4213. [Google Scholar] [CrossRef]
- Hussain, H.I.; Aqib, A.I.; Seleem, M.N.; Shabbir, M.A.; Hao, H.; Iqbal, Z.; Kulyar, M.F.-A.; Zaheer, T.; Li, K. Genetic Basis of Molecular Mechanisms in β-Lactam Resistant Gram-Negative Bacteria. Microb. Pathog. 2021, 158, 105040. [Google Scholar] [CrossRef]
- Blaak, H.; van Hoek, A.H.A.M.; Hamidjaja, R.A.; van der Plaats, R.Q.J.; Kerkhof-de Heer, L.; de Roda Husman, A.M.; Schets, F.M. Distribution, Numbers, and Diversity of ESBL-Producing E. coli in the Poultry Farm Environment. PLoS ONE 2015, 10, e0135402. [Google Scholar] [CrossRef]
- Cantón, R.; Akóva, M.; Carmeli, Y.; Giske, C.G.; Glupczynski, Y.; Gniadkowski, M.; Livermore, D.M.; Miriagou, V.; Naas, T.; Rossolini, G.M.; et al. Rapid Evolution and Spread of Carbapenemases among Enterobacteriaceae in Europe. Clin. Microbiol. Infect. 2012, 18, 413–431. [Google Scholar] [CrossRef]
- Ramatla, T.; Mafokwane, T.; Lekota, K.; Monyama, M.; Khasapane, G.; Serage, N.; Nkhebenyane, J.; Bezuidenhout, C.; Thekisoe, O. “One Health” Perspective on Prevalence of Co-Existing Extended-Spectrum β-Lactamase (ESBL)-Producing Escherichia coli and Klebsiella pneumoniae: A Comprehensive Systematic Review and Meta-Analysis. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 88. [Google Scholar] [CrossRef]
- Day, M.J.; Hopkins, K.L.; Wareham, D.W.; Toleman, M.A.; Elviss, N.; Randall, L.; Teale, C.; Cleary, P.; Wiuff, C.; Doumith, M.; et al. Extended-Spectrum β-Lactamase-Producing Escherichia coli in Human-Derived and Foodchain-Derived Samples from England, Wales, and Scotland: An Epidemiological Surveillance and Typing Study. Lancet Infect. Dis. 2019, 19, 1325–1335. [Google Scholar] [CrossRef]
- Montelin, H.; Camporeale, A.; Hallgren, A.; Angelin, M.; Hogvall, J.; Östholm Balkhed, Å.; Vading, M.; Giske, C.G.; Tängdén, T.; Angelin, M.; et al. Treatment, Outcomes and Characterization of Pathogens in Urinary Tract Infections Caused by ESBL-Producing Enterobacterales: A Prospective Multicentre Study. J. Antimicrob. Chemother. 2024, 79, 531–538. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America 2022 Guidance on the Treatment of Extended-Spectrum β-Lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin. Infect. Dis. 2022, 75, 187–212. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, N.; Lübbert, C.; Kaiser, T.; Kaisers, U.X.; Rodloff, A.C. Clinical Epidemiology of Klebsiella pneumoniae Carbapenemases. Lancet Infect. Dis. 2014, 14, 271–272. [Google Scholar] [CrossRef] [PubMed]
- Vrancianu, C.O.; Gheorghe, I.; Czobor, I.B.; Chifiriuc, M.C. Antibiotic Resistance Profiles, Molecular Mechanisms and Innovative Treatment Strategies of Acinetobacter baumannii. Microorganisms 2020, 8, 935. [Google Scholar] [CrossRef] [PubMed]
- Ayoub Moubareck, C.; Hammoudi Halat, D. Insights into Acinetobacter baumannii: A Review of Microbiological, Virulence, and Resistance Traits in a Threatening Nosocomial Pathogen. Antibiotics 2020, 9, 119. [Google Scholar] [CrossRef]
- Harding, C.M.; Hennon, S.W.; Feldman, M.F. Uncovering the Mechanisms of Acinetobacter baumannii Virulence. Nat. Rev. Microbiol. 2018, 16, 91–102. [Google Scholar] [CrossRef]
- Economou, V.; Gousia, P.; Sakkas, H.; Papadopoulou, C. Environmental Reservoirs of Antimicrobial Resistance of Foodborne Pathogens. In Encyclopedia of Environmental Health; Elsevier: Amsterdam, The Netherlands, 2019; pp. 615–626. [Google Scholar]
- Larsson, D.G.J.; Flach, C.-F. Antibiotic Resistance in the Environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- Schulz, F.; Eloe-Fadrosh, E.A.; Bowers, R.M.; Jarett, J.; Nielsen, T.; Ivanova, N.N.; Kyrpides, N.C.; Woyke, T. Towards a Balanced View of the Bacterial Tree of Life. Microbiome 2017, 5, 140. [Google Scholar] [CrossRef]
- Forsberg, K.J.; Reyes, A.; Wang, B.; Selleck, E.M.; Sommer, M.O.A.; Dantas, G. The Shared Antibiotic Resistome of Soil Bacteria and Human Pathogens. Science 2012, 337, 1107–1111. [Google Scholar] [CrossRef]
- Becker, E.; Projahn, M.; Burow, E.; Käsbohrer, A. Are There Effective Intervention Measures in Broiler Production against the ESBL/AmpC Producer Escherichia coli? Pathogens 2021, 10, 608. [Google Scholar] [CrossRef]
- Castanheira, M.; Simner, P.J.; Bradford, P.A. Extended-Spectrum β-Lactamases: An Update on Their Characteristics, Epidemiology and Detection. JAC Antimicrob. Resist. 2021, 3, dlab092. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on the Public Health Risks of Bacterial Strains Producing Extended-Spectrum β-Lactamases and/or AmpC β-Lactamases in Food and Food-Producing Animals. EFSA J. 2011, 9, 2322. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on Carbapenem Resistance in Food Animal Ecosystems. EFSA J. 2013, 11, 3501. [Google Scholar] [CrossRef]
- Carvalheira, A.; Ferreira, V.; Silva, J.; Teixeira, P. Enrichment of Acinetobacter Spp. from Food Samples. Food Microbiol. 2016, 55, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Tsitsos, A.; Damianos, A.; Boutel, M.; Gousia, P.; Soultos, N.; Papa, A.; Tirodimos, I.; Economou, V. Prevalence, Characterization, and Epidemiological Relationships between ESBL and Carbapenemase-Producing Escherichia coli, Klebsiella pneumoniae, and Acinetobacter spp. Isolated from Humans and the Kitchen Environment of Two Greek Hospitals. Antibiotics 2024, 13, 934. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing, 34th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024. [Google Scholar]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. EUCAST Guidelines for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance; European Committee on Antimicrobial Susceptibility Testing: Växjö, Sweden, 2017; Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_170711.pdf (accessed on 10 May 2024).
- Peratikos, P.; Tsitsos, A.; Damianos, A.; Kyritsi, M.A.; Hadjichristodoulou, C.; Soultos, N.; Economou, V. Listeria Monocytogenes from Marine Fish and the Seafood Market Environment in Northern Greece: Prevalence, Molecular Characterization, and Antibiotic Resistance. Appl. Sci. 2024, 14, 2725. [Google Scholar] [CrossRef]
- Dallenne, C.; Da Costa, A.; Decré, D.; Favier, C.; Arlet, G. Development of a Set of Multiplex PCR Assays for the Detection of Genes Encoding Important β-Lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 2010, 65, 490–495. [Google Scholar] [CrossRef]
- Clermont, O.; Christenson, J.K.; Denamur, E.; Gordon, D.M. The Clermont Escherichia coli Phylo-typing Method Revisited: Improvement of Specificity and Detection of New Phylo-groups. Environ. Microbiol. Rep. 2013, 5, 58–65. [Google Scholar] [CrossRef]
- Tansawai, U.; Walsh, T.R.; Niumsup, P.R. Extended Spectrum SS-Lactamase-Producing Escherichia coli among Backyard Poultry Farms, Farmers, and Environments in Thailand. Poult. Sci. 2019, 98, 2622–2631. [Google Scholar] [CrossRef]
- Dierikx, C.; van der Goot, J.; Fabri, T.; van Essen-Zandbergen, A.; Smith, H.; Mevius, D. Extended-Spectrum-β-Lactamase- and AmpC-β-Lactamase-Producing Escherichia coli in Dutch Broilers and Broiler Farmers. J. Antimicrob. Chemother. 2013, 68, 60–67. [Google Scholar] [CrossRef]
- Huijbers, P.M.C.; Graat, E.A.M.; Haenen, A.P.J.; van Santen, M.G.; van Essen-Zandbergen, A.; Mevius, D.J.; van Duijkeren, E.; van Hoek, A.H.A.M. Extended-Spectrum and AmpC β-Lactamase-Producing Escherichia coli in Broilers and People Living and/or Working on Broiler Farms: Prevalence, Risk Factors and Molecular Characteristics. J. Antimicrob. Chemother. 2014, 69, 2669–2675. [Google Scholar] [CrossRef] [PubMed]
- Laube, H.; Friese, A.; von Salviati, C.; Guerra, B.; Rösler, U. Transmission of ESBL/AmpC-Producing Escherichia coli from Broiler Chicken Farms to Surrounding Areas. Vet. Microbiol. 2014, 172, 519–527. [Google Scholar] [CrossRef] [PubMed]
- von Tippelskirch, P.; Gölz, G.; Projahn, M.; Daehre, K.; Friese, A.; Roesler, U.; Alter, T.; Orquera, S. Prevalence and Quantitative Analysis of ESBL and AmpC Beta-Lactamase Producing Enterobacteriaceae in Broiler Chicken during Slaughter in Germany. Int. J. Food Microbiol. 2018, 281, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Savin, M.; Bierbaum, G.; Hammerl, J.A.; Heinemann, C.; Parcina, M.; Sib, E.; Voigt, A.; Kreyenschmidt, J. ESKAPE Bacteria and Extended-Spectrum-β-Lactamase-Producing Escherichia coli Isolated from Wastewater and Process Water from German Poultry Slaughterhouses. Appl. Environ. Microbiol. 2020, 86, e02748-19. [Google Scholar] [CrossRef]
- Veloo, Y.; Thahir, S.S.A.; Rajendiran, S.; Hock, L.K.; Ahmad, N.; Muthu, V.; Shaharudin, R. Multidrug-Resistant Gram-Negative Bacteria and Extended-Spectrum β-Lactamase-Producing Klebsiella pneumoniae from the Poultry Farm Environment. Microbiol. Spectr. 2022, 10, e0269421. [Google Scholar] [CrossRef]
- Li, Z.; Xin, L.; Peng, C.; Liu, C.; Wang, P.; Yu, L.; Liu, M.; Wang, F. Prevalence and Antimicrobial Susceptibility Profiles of ESBL-Producing Klebsiella pneumoniae from Broiler Chicken Farms in Shandong Province, China. Poult. Sci. 2022, 101, 102002. [Google Scholar] [CrossRef]
- Barnes, N.M.; Wu, H. Mechanisms Regulating the Airborne Survival of Klebsiella pneumoniae under Different Relative Humidity and Temperature Levels. Indoor Air 2022, 32, e12991. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhou, Y.; Chang, Y.; Liang, X.; Zhang, H.; Lin, X.; Qing, K.; Zhou, X.; Luo, Z. The Effects of Ventilation, Humidity, and Temperature on Bacterial Growth and Bacterial Genera Distribution. Int. J. Environ. Res. Public Health 2022, 19, 15345. [Google Scholar] [CrossRef]
- Projahn, M.; von Tippelskirch, P.; Semmler, T.; Guenther, S.; Alter, T.; Roesler, U. Contamination of Chicken Meat with Extended-Spectrum Beta-Lactamase Producing-Klebsiella pneumoniae and Escherichia coli during Scalding and Defeathering of Broiler Carcasses. Food Microbiol. 2019, 77, 185–191. [Google Scholar] [CrossRef]
- Schmitz, A.; Hanke, D.; Lüschow, D.; Schwarz, S.; Higgins, P.G.; Feßler, A.T. Acinetobacter baumannii from Samples of Commercially Reared Turkeys: Genomic Relationships, Antimicrobial and Biocide Susceptibility. Microorganisms 2023, 11, 759. [Google Scholar] [CrossRef]
- Wadepohl, K.; Müller, A.; Seinige, D.; Rohn, K.; Blaha, T.; Meemken, D.; Kehrenberg, C. Association of Intestinal Colonization of ESBL-Producing Enterobacteriaceae in Poultry Slaughterhouse Workers with Occupational Exposure—A German Pilot Study. PLoS ONE 2020, 15, e0232326. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, D.M.; O’Doherty, Á.; Burgess, C.M.; Howe, N.; McMahon, F.; Murphy, D.; Leonard, F.; Morris, D.; Harrington, C.; Carty, A.; et al. Critically Important Antimicrobial Resistant Enterobacteriaceae in Irish Farm Effluent and Their Removal in Integrated Constructed Wetlands. Sci. Total Environ. 2022, 806, 151269. [Google Scholar] [CrossRef] [PubMed]
- Fares, A. Factors Influencing the Seasonal Patterns of Infectious Diseases. Int. J. Prev. Med. 2013, 4, 128–132. [Google Scholar] [PubMed]
- Deeny, S.R.; van Kleef, E.; Bou-Antoun, S.; Hope, R.J.; Robotham, J.V. Seasonal Changes in the Incidence of Escherichia coli Bloodstream Infection: Variation with Region and Place of Onset. Clin. Microbiol. Infect. 2015, 21, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Wielders, C.C.H.; Van Duijkeren, E.; Van Den Bunt, G.; Meijs, A.P.; Dierikx, C.M.; Bonten, M.J.M.; Van Pelt, W.; Franz, E.; De Greeff, S.C. Seasonality in Carriage of Extended-Spectrum β-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae in the General Population: A Pooled Analysis of Nationwide Cross-Sectional Studies. Epidemiol. Infect. 2020, 148, e68. [Google Scholar] [CrossRef]
- van Elsas, J.D.; Semenov, A.V.; Costa, R.; Trevors, J.T. Survival of Escherichia coli in the Environment: Fundamental and Public Health Aspects. ISME J. 2011, 5, 173–183. [Google Scholar] [CrossRef]
- Friese, A.; Schulz, J.; Laube, H.; Von Salviati, C.; Hartung, J.; Roesler, U. Faecal Occurrence and Emissions of Live-Stock-Associated Methicillin-Resistant Staphylococcus Aureus (LaMRSA) and ESBL/AmpC-Producing E. coli from Animal Farms in Germany. Berl. Münch. Tierärztl. Wochenschr. 2013, 126, 117–180. [Google Scholar]
- Dahms, C.; Hübner, N.-O.; Kossow, A.; Mellmann, A.; Dittmann, K.; Kramer, A. Occurrence of ESBL-Producing Escherichia coli in Livestock and Farm Workers in Mecklenburg-Western Pomerania, Germany. PLoS ONE 2015, 10, e0143326. [Google Scholar] [CrossRef]
- Xexaki, A.; Papadopoulos, D.K.; Alvanou, M.V.; Giantsis, I.A.; Papageorgiou, K.V.; Delis, G.A.; Economou, V.; Kritas, S.K.; Sossidou, E.N.; Petridou, E. Prevalence of Antibiotic Resistant E. coli Strains Isolated from Farmed Broilers and Hens in Greece, Based on Phenotypic and Molecular Analyses. Sustainability 2023, 15, 9421. [Google Scholar] [CrossRef]
- King, D.T.; Sobhanifar, S.; Strynadka, N.C.J. The Mechanisms of Resistance to β-Lactam Antibiotics. In Handbook of Antimicrobial Resistance; Springer: New York, NY, USA, 2014; pp. 1–22. [Google Scholar]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a Successful Pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef]
- Savin, M.; Parcina, M.; Schmoger, S.; Kreyenschmidt, J.; Käsbohrer, A.; Hammerl, J.A. Draft Genome Sequences of Acinetobacter baumannii Isolates Recovered from Sewage Water from a Poultry Slaughterhouse in Germany. Microbiol. Resour. Announc. 2019, 8, e00553-19. [Google Scholar] [CrossRef] [PubMed]
- Savin, M.; Sib, E.; Heinemann, C.; Eichel, V.M.; Nurjadi, D.; Klose, M.; Andre Hammerl, J.; Binsker, U.; Mutters, N.T. Tracing Clinically-Relevant Antimicrobial Resistances in Acinetobacter baumannii-Calcoaceticus Complex across Diverse Environments: A Study Spanning Clinical, Livestock, and Wastewater Treatment Settings. Environ. Int. 2024, 186, 108603. [Google Scholar] [CrossRef] [PubMed]
- Egli, A.; Tschudin-Sutter, S.; Oberle, M.; Goldenberger, D.; Frei, R.; Widmer, A.F. Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass-Spectrometry (MALDI-TOF MS) Based Typing of Extended-Spectrum β-Lactamase Producing E. coli—A Novel Tool for Real-Time Outbreak Investigation. PLoS ONE 2015, 10, e0120624. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Xu, H.; Ren, X.; Li, X.; Ma, X.; Zhou, H.; Hong, G.; Liang, X. Epidemiology and Risk Factors for Carbapenem-Resistant Klebsiella pneumoniae and Subsequent MALDI-TOF MS as a Tool to Cluster KPC-2-Producing Klebsiella pneumoniae, a Retrospective Study. Front. Cell. Infect. Microbiol. 2020, 10, 462. [Google Scholar] [CrossRef]
- Al Sattar, A.; Chisty, N.N.; Irin, N.; Uddin, M.H.; Hasib, F.M.Y.; Hoque, M.A. Knowledge and Practice of Antimicrobial Usage and Resistance among Poultry Farmers: A Systematic Review, Meta-Analysis, and Meta-Regression. Vet. Res. Commun. 2023, 47, 1047–1066. [Google Scholar] [CrossRef]
- Habiba, U.E.; Khan, A.; Mmbaga, E.J.; Green, I.R.; Asaduzzaman, M. Use of Antibiotics in Poultry and Poultry Farmers- a Cross-Sectional Survey in Pakistan. Front. Public Health 2023, 11, 1154668. [Google Scholar] [CrossRef]
- Kariuki, J.W.; Jacobs, J.; Ngogang, M.P.; Howland, O. Antibiotic Use by Poultry Farmers in Kiambu County, Kenya: Exploring Practices and Drivers of Potential Overuse. Antimicrob. Resist. Infect. Control 2023, 12, 3. [Google Scholar] [CrossRef]
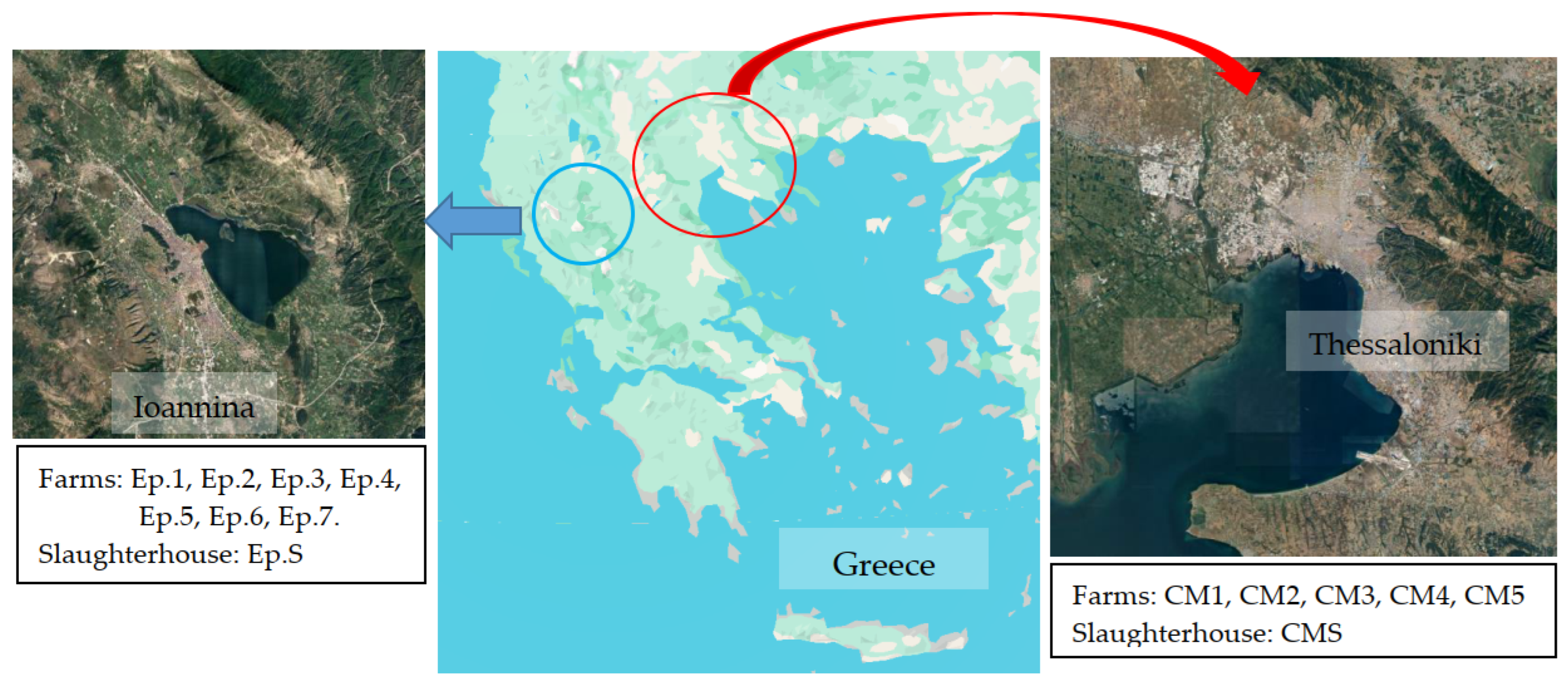






| Sample | Samples per Unit per Season | Total Number of Samples | |
|---|---|---|---|
| Farm environment | Watering troughs | 2 | 40 |
| Cooling pads | 1 | 20 | |
| Ventilation fans | 1 | 20 | |
| Poultry litter | 1 | 20 | |
| Total | 5 | 100 | |
| Slaughterhouse environment | Chicken transport boxes | 5 * | 20 |
| Chicken pluckers | 5 * | 20 | |
| Evisceration machines | 5 * | 20 | |
| Carcass cutting equipment | 5 * | 20 | |
| Cutting boards | 5 * | 20 | |
| Drains of dirty area | 2 | 8 | |
| Drains of clean area | 2 | 8 | |
| Sewage (influent) | 1 | 4 | |
| Sewage (effluent) | 1 | 4 | |
| Total | 31 | 124 | |
| Human samples | Skin | 1 | 40 |
| Oropharynx | 1 | 40 | |
| Total | 2 | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsitsos, A.; Damianos, A.; Kiskinis, K.; Tsiouris, V.; Tirodimos, I.; Soultos, N.; Papa, A.; Economou, V. Prevalence, Characterization, and Proteomic Relatedness Among β-Lactam-Resistant Bacteria Throughout the Poultry Production Chain in Greece. Foods 2025, 14, 224. https://doi.org/10.3390/foods14020224
Tsitsos A, Damianos A, Kiskinis K, Tsiouris V, Tirodimos I, Soultos N, Papa A, Economou V. Prevalence, Characterization, and Proteomic Relatedness Among β-Lactam-Resistant Bacteria Throughout the Poultry Production Chain in Greece. Foods. 2025; 14(2):224. https://doi.org/10.3390/foods14020224
Chicago/Turabian StyleTsitsos, Anestis, Alexandros Damianos, Konstantinos Kiskinis, Vasilios Tsiouris, Ilias Tirodimos, Nikolaos Soultos, Anna Papa, and Vangelis Economou. 2025. "Prevalence, Characterization, and Proteomic Relatedness Among β-Lactam-Resistant Bacteria Throughout the Poultry Production Chain in Greece" Foods 14, no. 2: 224. https://doi.org/10.3390/foods14020224
APA StyleTsitsos, A., Damianos, A., Kiskinis, K., Tsiouris, V., Tirodimos, I., Soultos, N., Papa, A., & Economou, V. (2025). Prevalence, Characterization, and Proteomic Relatedness Among β-Lactam-Resistant Bacteria Throughout the Poultry Production Chain in Greece. Foods, 14(2), 224. https://doi.org/10.3390/foods14020224









