A Review of Nutrition, Bioactivities, and Health Benefits of Custard Apple (Annona squamosa): From Phytochemicals to Potential Application
Abstract
1. Introduction
2. Chemical Composition and Nutritional Profiles
2.1. Fatty Acids Profile
2.2. Mineral Elements
2.3. Essential Oils
2.4. Vitamins
2.5. Carbohydrates
2.6. Volatile Profiles
3. Phytochemical Compounds
3.1. Phenolic Compounds
3.2. Terpenoids
3.3. Annonaceous Acetogenins (ACGs)
3.4. Other Bioactive Compounds
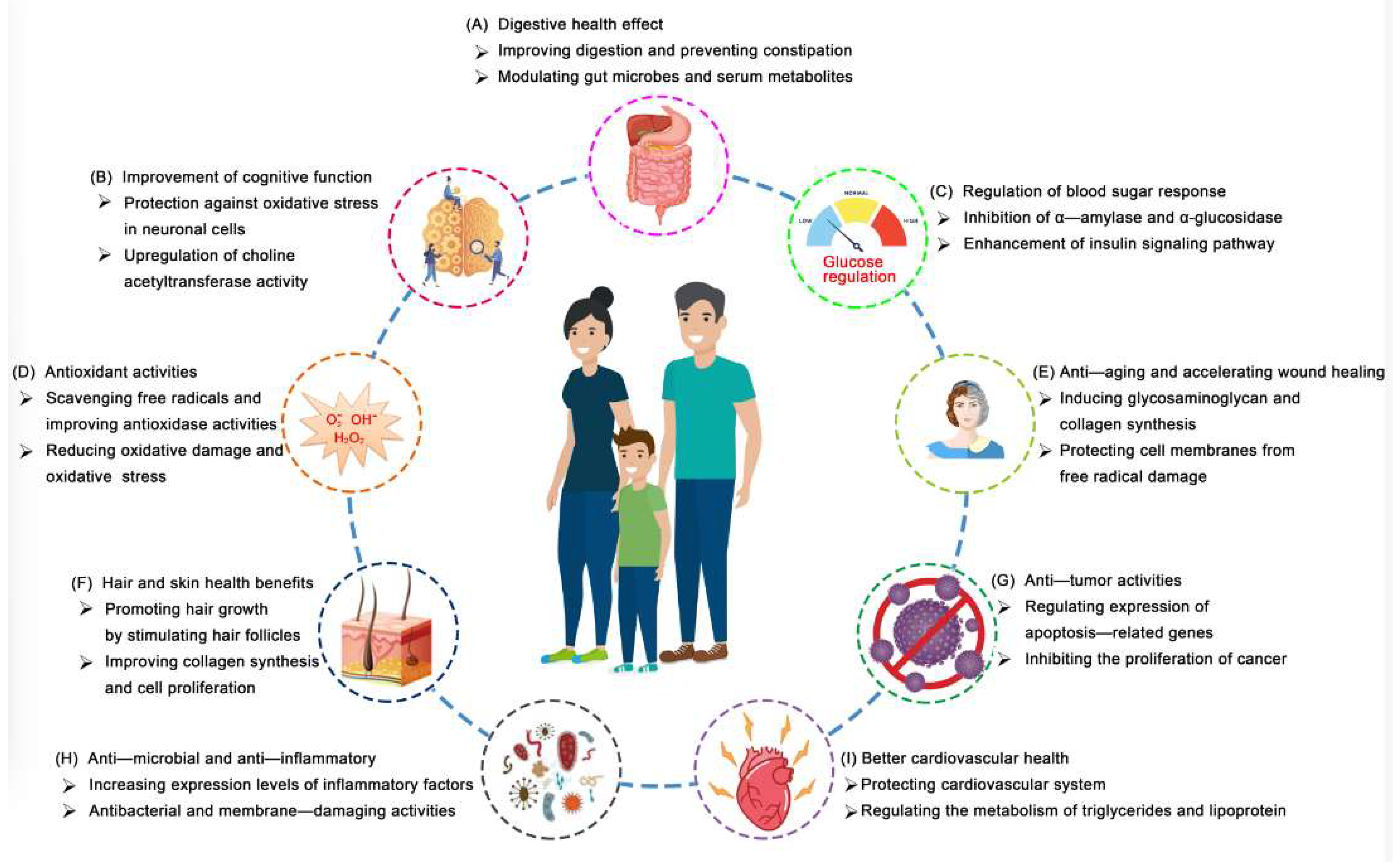
4. Health Benefits
4.1. Antioxidant, Anti-Inflammatory, and Wound-Healing Activities
4.2. Anti-Tumor Activities
4.3. Regulation of Blood Sugar Response
4.4. Improvement of Cognitive Function
4.5. Prevention of Cardiovascular and Cerebrovascula Diseases
4.6. Hair Conditioning
5. Industrial Applications
5.1. Nutritional Incorporation
5.1.1. Puree, Jam, and Nectar
5.1.2. Milkshake
5.1.3. Fruit Powder
5.1.4. Ready to Serve (RTS) Beverages
5.2. Fermented Product Applications
5.2.1. LAB Fermentation
5.2.2. Alcoholic Fermentation
5.2.3. Acetic Fermentation
5.2.4. Meat Preservation
5.3. Production of High Added-Value Products
5.3.1. Polysaccharide and Pectin
5.3.2. Bio-Based Bioenergy
5.3.3. Bio-Based Materials
5.3.4. Biological Regulator
6. Potential for Toxicity
7. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leal, F.; Paull, R.E. The genus Annona: Botanical characteristics, horticultural requirements and uses. Crop Sci. 2023, 63, 1030–1049. [Google Scholar] [CrossRef]
- Padmanabhan, P.; Paliyath, G. Annonaceous Fruits. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 169–173. [Google Scholar]
- Al-ghazzawi, A.M. Anti-cancer activity of new benzylisoquinoline alkaloid from Saudi plant Annona squamosa. BMC Chem. 2019, 13, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Changan, S.; Tomar, M.; Prajapati, U.; Saurabh, V.; Hasan, M.; Sasi, M.; Maheshwari, C.; Singh, S.; Dhumal, S.; et al. Custard apple (Annona squamosa L.) leaves: Nutritional composition, phytochemical profile, and health-promoting biological activities. Biomolecules 2021, 11, 614. [Google Scholar] [CrossRef] [PubMed]
- Trindade, M.L.M.D.; Radünz, M.; Ramos, A.H.; Silveira, C.S.; Gandra, E.A.; Helbig, E. Chemical characterization, antimicrobial and antioxidant activity of sugar-apple (Annona squamosa L.) pulp extract. Rev. Chil. Nutr. 2020, 47, 281–285. [Google Scholar] [CrossRef]
- Prasad, S.K.; Devananda, D. Evaluating the anticancer potentials of methanol extracted Annona muricata fruit pulp and seed(s) phytochemicals. Clin. Oncol. Res. 2020, 3, 1–8. [Google Scholar] [CrossRef]
- Ebenyi, N.L.; Chigozie, U.V.; Destiny, D.; Anyanwu, C.B. Antioxidative, anti-androgenic, and inhibitory activities of ethanolic extract of Annona muricata leaf on sex hormones-induced benign prostate hyperplasia through in vivo and in silico studies. Nat. Prod. Res. 2024, 1–8. [Google Scholar] [CrossRef]
- Hiwale, S. Custard Apple (Annona squamosa L.). In Sustainable Horticulture in Semiarid Dry Lands; Springer: New Delhi, India, 2015; pp. 135–152. [Google Scholar]
- Pareek, S.; Yahia, E.M.; Pareek Kaushik, R.A. Postharvest physiology and technology of Annona fruits. Food Res. Int. 2011, 44, 1141–1151. [Google Scholar] [CrossRef]
- Santos, W.N.L.D.; Sauthier, M.C.S.; Cavalcante, D.D.; Benevides, C.M.J.; Dias, F.S.; Santos, D.C.M.B. Mineral composition, nutritional properties, total phenolics and flavonoids compounds of the atemoya fruit (Annona squamosa L. × Annona cherimola Mill.) and evaluation using multivariate analysis techniques. An. Da Acad. Bras. De. Cie. Ciei 2016, 88, 1–10. [Google Scholar] [CrossRef]
- Mariod, A.A.; Elkheir, S.; Ahmed, Y.M.; Matthäus, B. Annona squamosa and Catunaregam nilotica seeds, the effect of the extraction method on the oil composition. J. Am. Oil Chem. Soc. 2010, 87, 763–769. [Google Scholar] [CrossRef]
- Yathish, K.V.; Omkaresh, B.R.; Suresh, R. Biodiesel production from custard apple seed (Annona squamosa) oil and its characteristics study. Int. J. Eng. 2013, 2, 31–36. [Google Scholar]
- Rana, V.S. Fatty oil and fatty acid composition of Annona squamosa Linn. seed kernels. Int. J. Fruit. Sci. 2015, 15, 79–84. [Google Scholar] [CrossRef]
- Bala, S.; Nigam, V.K.; Singh, S.S.; Kumar, A.; Kumar, S. Evaluation of Nutraceutical Applications of Annona squamosa L. based Food Products. J. Pharmacogn. Phytochem. 2018, SP1, 827–831. [Google Scholar]
- Kachhadiya, S.; Jethva, K.R. Physico-chemical properties of custard apple. Int. J. Biochem. Res. Rev. 2017, 20, 1–13. [Google Scholar] [CrossRef]
- Zahid, M.; Mujahid, M.; Singh, P.; Farooqui, S.; Singh, K.; Shahla Arif, M. Annona squamosa Linn. (custard apple): An aromatic medicinal plant fruit with immense nutraceutical and therapeutic potentials. Int. J. Pharm. Sci. Res. 2018, 9, 1745–1759. [Google Scholar]
- Noman, S.; Kabeel, A.E.; Manokar, A.M. Experimental analysis of tubular solar still with custard apple seeds a bio-waste material: An energy, exergy, economic, environmental and sustainability analysis. J. Energy Storage 2024, 100, 113531. [Google Scholar] [CrossRef]
- Awada, N.; Ayoub, A.; Jaber, A.; Ibrahim, F.; El Ghotmi, N.; Cheble, E. Evaluation of the anticancer, anti-inflammatory, and antioxidant properties of various extracts of Annona squamosa L. Pharm. Sci. 2023, 29, 384–394. [Google Scholar]
- Gutierrez, M.; Sola, M.M.; Vargas, A.M. Fatty acid composition of phospholipids in mesocarp of cherimoya fruit during ripening. Food Chem. 2005, 90, 341–346. [Google Scholar] [CrossRef]
- Rabelo, S.V.; Quintans, J.D.S.S.; Costa, E.V.; da Silva Almeida, J.R.G.; Quintans-Junior, L.J. Chapter 24-Annona Species (Annonaceae) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, R.V., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 221–229. [Google Scholar]
- Neha, T.; Amit, K.D. Beneficial aspects of custard apple (Annona squamosa): A perspective review. Int. J. Res. Appl. Sci. Eng. Technol. 2022, 10, 1108–1115. [Google Scholar] [CrossRef]
- Wang, L.Y.; Liu, C.Y.; Geng, X.Q.; Jiang, W.; Bao, K.S.; Zhu, Z.Y. Cell wall polysaccharides from Annona squamosa: Chemical and functional characterization. Process Biochem. 2024, 136, 136–146. [Google Scholar] [CrossRef]
- Gong, X.; Wu, X.S.; Qi, N.L.; Li, J.H.; Zhang, H.; Huo, Y.J. Changes in the biochemical characteristics and volatile fingerprints of atemoya during postharvest ripening at room temperature. Qual. Assur. Saf. Crop. 2020, 12, 26–35. [Google Scholar] [CrossRef]
- Madhumitha, G.; Rajakumar, G.; Roopan, S.M.; Rahuman, A.A.; Priya, M.K.; Saral, A.M.; Khan, F.R.N.; Khanna, V.G.; Velayutham, K.; Jayaseelan, C.; et al. Acaricidal, insecticidal, and larvicidal efficacy of fruit peel aqueous extract of Annona squamosa and its compounds against blood-feeding parasites. Parasitol. Res. 2012, 111, 2189–2199. [Google Scholar] [CrossRef]
- Yang, Y.L.; Chang, F.R.; Wu, C.C.; Wang, W.Y.; Wu, Y.C. New ent-kaurane diterpenoids with anti-platelet aggregation activity from Annona squamosa. J. Nat. Prod. 2002, 65, 1462–1467. [Google Scholar] [CrossRef]
- Baskaran, R.; Pullencheri, D.; Somasundaram, R. Characterization of free, esterified and bound phenolics in custard apple (Annona squamosa L.) fruit pulp by UPLC-ESI-MS/MS. Food Res. Int. 2016, 82, 121–127. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Li, F.Q.; Zhu, X.L.; Chen, J.W.; Li, X. Chemical composition and anti-hepatoma effect of Annona squamosa L. pericarp oil. Nat. Prod. Res. 2021, 36, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.X.; Sun, L.R.; Feng, F.; Mo, J.X.; Zhu, H.; Yang, B.; He, Q.J.; Gan, L.S. Cytotoxic diterpenoids from the stem bark of Annona squamosa L. Helv. Chim. Acta 2013, 96, 656–662. [Google Scholar] [CrossRef]
- Joy, B.; Remani, P. Antitumor constituents from Annona squamosa fruit pericarp. Med. Chem. Res. 2008, 17, 345–355. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, L.; Yu, J.Q.; Cui, L.; Ali, I.; Song, X.Y.; Park, J.H.; Wang, D.J.; Wang, X. Flavonoid epimers from custard apple leaves, a rapid screening and separation by HSCCC and their antioxidant and hypoglycaemic activities evaluation. Sci. Rep. 2020, 10, 8819. [Google Scholar] [CrossRef]
- Panda, S.; Kar, A. Antidiabetic and antioxidative effects of Annona squamosa leaves are possibly mediated through quercetin-3-O-glucoside. BioFactors 2008, 31, 201–210. [Google Scholar] [CrossRef]
- Pandey, N.; Barve, D. Phytochemical and pharmacological review on Annona squamosa Linn. Int. J. Res. Pharm. Biomed. Sci. 2011, 2, 1404–1412. [Google Scholar]
- Panda, S.; Kar, A. Protective effects of 5,7,4′-trihydroxy-6,3′dimethoxy-flavone 5-O-α-l-rhamnopyranoside, isolated from Annona squamosa leaves in thyrotoxicosis and in hepatic lipid peroxidation in rats. Bioorg. Med. Chem. Lett. 2015, 25, 5726–5728. [Google Scholar] [CrossRef]
- Neethu, S.K.; Santhoshkumar, R.; Kumar, N.S. Phytochemical analysis and antimicrobial activities of Annona squamosa (L.) leaf extracts. J. Pharmacog. Phytochem. 2016, 5, 128–131. [Google Scholar]
- Shiekh, K.A.; Olatunde, O.O.; Zhang, B.; Huda, N.; Benjakul, S. Pulsed electric field assisted process for extraction of bioactive compounds from custard apple (Annona squamosa) leaves. Food Chem. 2021, 359, 129976. [Google Scholar] [CrossRef]
- Christophe, W.; Au, T.S.; Yusof, M.; Hassan, H.; Mazdida, S. 16 α Hydroxy-(-)-kauran-19-oic Acid: An antibacterial diterpene from sweet apple (Annona squamosa L. Annonaceae). Int. J. Pharmacol. 2005, 1, 296–298. [Google Scholar]
- Yang, Y.L.; Hua, K.F.; Chuang, P.H.; Wu, S.H.; Wu, K.Y.; Chang, F.R.; Wu, Y.C. New cyclic peptides from the seeds of Annona squamosa L. and their anti-inflammatory activities. J. Agric. Food Chem. 2008, 56, 386–392. [Google Scholar] [CrossRef]
- Ma, C.Y.; Wang, Q.W.; Shi, Y.Y.; Li, Y.; Wang, X.N.; Li, X.; Chen, Y.; Chen, J.W. Three new antitumor Annonaceous acetogenins from the seeds of Annona squamosa. Nat. Prod. Res. 2017, 31, 2085–2090. [Google Scholar] [CrossRef]
- Li, R.S.; Li, L.Y.; Zhu, X.F.; Li, X.; Qiu, S.J.; Zhou, J.; Fan, J.; Hu, B.; Mu, Q. Annonaceous acetogenins synergistically inhibit hepatocellular carcinoma with sorafenib. J. Nat. Prod. 2024, 87, 14–27. [Google Scholar] [CrossRef]
- Manisha Sharma, M.C. In-vitro evaluation of antioxidant activity of isolated phytochemicals from methanolic seed extract of Annona squamosa. Int. J. Bio. Pharm. Alli. Sci. 2023, 12, 5756–5766. [Google Scholar]
- Fu, L.; Xu, B.T.; Xu, X.R.; Gan, R.Y.; Zhang, Y.; Xia, E.Q.; Li, H.B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Ma, C.Y.; Li, Y.; Lu, J.H.; Wang, M.L.; Li, X.; Chen, J.W.; Chen, Y.; Lu, W.Z. Three new cytotoxic Annonaceous acetogenins from the seeds of Annona squamosa. Nat. Prod. Res. 2022, 38, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Ranganatha, K.S.; Venugopal, A.; Chinthapalli, D.K.; Subramanyam, R.; Nadimpalli, S.K. Purification, biochemical and biophysical characterization of an acidic alpha-galactosidase from the seeds of Annona squamosa (custard apple). Int. J. Biol. Macromol. 2021, 175, 558–571. [Google Scholar] [CrossRef] [PubMed]
- Gautam, D.K.; Vishvajeet, S.; Sachin, K.S.; Tiwari, P.; Prabakaran, S.; Verma, S.; Tripathy, S.; Luthra, S.; Chawla, R. Custard apple (Annona squamosa L.): Exploring its health benefits and medicinal properties. Eur. J. Nutr. Food Saf. 2024, 16, 129–140. [Google Scholar] [CrossRef]
- Dellai, A.; Maricic, I.; Kumar, V.; Arutyunyan, S.; Bouraoui, A.; Nefzi, A. Parallel synthesis and anti-inflammatory activity of cyclic peptides cyclosquamosin D and Met-cherimolacyclopeptide B and their analogs. Bioorg. Med. Chem. Lett. 2010, 20, 5653–5657. [Google Scholar] [CrossRef] [PubMed]
- Nhung, T.T.P.; Quoc, L.P.T. Exploring the analgesic, antipyretic, and anti-inflammatory properties of Annona squamosa Linnaeus fruit peel extract in a mouse model. Trop. J. Nat. Prod. Res. 2024, 8, 8537. [Google Scholar]
- Rihana, F.H.; Neelakanta, R.P. Effect of Asoka Bark (Saraca indica) and custard apple pulp (Annona squamosa) on wound healing in female albino rats. Res. J. Pharm. Technol. 2011, 4, 928–931. [Google Scholar]
- Ponrasu, T.; Suguna, L. Efficacy of Annona squamosa on wound healing in streptozotocin-induced diabetic rats. Int. Wound J. 2012, 9, 613–623. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Rouhollahi, E.; Hajrezaie, M.; Karimian, H.; Abdulla, M.A.; Kadir, H.A. Annona muricata leaves accelerate wound healing in rats via involvement of Hsp70 and antioxidant defence. Int. J. Surg. 2015, 18, 110–117. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Shehata, M.G.; Abu-Serie, M.M.; Abd El-Aziz, N.M.; El-Sohaimy, S.A. Nutritional, phytochemical, and in vitro anticancer potential of sugar apple (Annona squamosa) fruits. Sci. Rep. 2021, 11, 6224. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Heinrich, H. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J. Ethnopharmacol. 2005, 99, 325–348. [Google Scholar] [CrossRef]
- Agu, K.C.; Eluehike, N.; Ofeimun, R.O.; Abile, D.; Ideho, G.; Ogedengbe, M.O.; Onose, P.O.; Elekofehinti, O.O. Possible anti-diabetic potentials of Annona muricata (soursop): Inhibition of α-amylase and α-glucosidase activities. Clin. Phytosci. 2019, 5, 21. [Google Scholar] [CrossRef]
- Ponce-Sánchez, C.; Oidor-Chan, V.H.; Álvarez-Ramírez, E.L.; Gómez-Cansino, R.; Zarza-García, A.L.; Gómez-Olivares, J.L.; de León-Sánchez, F.D.; Mendoza-Espinoza, J.A. Chemical profile and study of the antidiabetic effect of Annona squamosa L. peel. Waste Biomass Valori. 2024, 15, 1053–1063. [Google Scholar] [CrossRef]
- Tomar, R.S.; Sisodia, S. Antidiabetic activity of Annona squamosa L. in experimental induced diabetic rats. Int. J. Pharm. Biol. Arch. 2012, 3, 1492–1495. [Google Scholar]
- Sohn, E.; Lim, H.-S.; Kim, Y.-J.; Kim, B.-Y.; Jeong, S.-J. Annona atemoya leaf extract improves scopolamine-induced memory impairment by preventing hippocampal cholinergic dysfunction and neuronal cell death. Int. J. Mol. Sci. 2019, 20, 3538. [Google Scholar] [CrossRef]
- Lim, H.S.; Kim, Y.J.; Sohn, E.; Yoon, J.; Kim, B.Y.; Jeong, S.J. Annona atemoya leaf extract ameliorates cognitive impairment in amyloid-beta injected Alzheimer’s disease-like mouse mode. Exp. Biol. Med. 2019, 244, 1665–1679. [Google Scholar] [CrossRef] [PubMed]
- Madhuri, Y.; Saloni, M.; Sandhyarani, S.; Sonal, S.; Jayshri, H. Formulation and evaluation of herbal hair conditioner from custard apple and curry leaves. Educ. Admin. Theory Pract. 2024, 30, 2516–2518. [Google Scholar]
- Souza, F.T.; Santos, E.R.; Silva, J.D.; Valentim, I.; Rabelo, T.C.; Andrade, N.D.; Silva, L.K. Production of nutritious flour from residue custard apple (Annona squamosa L.) for the development of new products. J. Food Quality 2018, 2018, 5281035. [Google Scholar] [CrossRef]
- Chou, C.-H.; Wang, C.-Y.; Shyu, Y.-T.; Wu, S.-J. The effect of high-pressure processing on reducing the glycaemic index of atemoya puree. J. Sci. Food Agric. 2021, 101, 1546–1553. [Google Scholar] [CrossRef]
- Saket, R.K.; Bisen, B.P.; Pandey, S.K.; Pandey, S.; Sharma, H.L. Effect of various recipes on organoleptic evaluation of custard apple jam. J. Pharm. Phytochem. 2018, SP2, 300–304. [Google Scholar]
- Gautam, D.; Jain, S.K.; Bhatnagar, P.; Meena, N.K.; Chhipa, H. Utilization of custard apple pulp for preparation of blended nectar. Indian J. Hortic. 2021, 78, 229–235. [Google Scholar] [CrossRef]
- Meshram, S.K.; Nage, S.P.; Chavan, S.D.; KAHATE, P.A.; Raut, U.A. Preparation of value added cow milk lassi blended with custard apple (Annona squamosa L.) pulp. J. Adv. Food Sci. Technol. 2024, 11, 47–52. [Google Scholar] [CrossRef]
- Soni, N.; Mudgal, V.D.; Kumar, A. Influence of drying temperature on drying characteristics and moisture diffusivity of custard apple pulp. Environ. Ecol. 2023, 41, 1394–1399. [Google Scholar] [CrossRef]
- Khodifad, B.C.; Kumar, N. Foaming properties of custard apple pulp and mathematical modelling of foam mat drying. J. Food Sci. Tech. 2020, 57, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Tripathi, A.D.; Paul, V.; Rai, D.C. Optimization of spray drying parameters for custard apple (Annona squamosa L.) pulp powder development using response surface methodology (RSM) with improved physicochemical attributes and phytonutrients. LWT-Food Sci. Technol. 2021, 151, 112091. [Google Scholar] [CrossRef]
- Soni, N.; Sharma, M.; Mudgal, V.D.; Champawat, P.S.; Kohli, D. Physical characteristics and flow behavior of custard apple powder. Octa J. Biosci. 2021, 9, 119–122. [Google Scholar]
- Thube, R.; Purohit, S.; Gothoskar, A. Study of effect of custard apple pulp powder as an excipient on the properties of acetaminophen tablet. World Appl. Sci. J. 2011, 12, 364–371. [Google Scholar]
- Shifa, S.K.K.M.; Sujatha, G.; Narayanan, R. Effect of cold plasma on the quality parameters of custard apple juice milk beverage. Indian J. Dairy Sci. 2023, 76, 356–363. [Google Scholar]
- Tien, Y.Y.; Ng, C.C.; Chang, C.C.; Tseng, W.S.; Kotwal, S.; Shyu, Y.T. Studies on the Lactic-fermentation of sugar apple (Annona squamosa L.) puree. J. Food Drug Anal. 2005, 13, 377–381. [Google Scholar] [CrossRef]
- Arunkumar, M.; Divya, S.K.; Mahesh, N. Development of improved strategies for the survival of Lactobacillus Plantarum MTCC 1407 in probioticated custard apple juice. Proc. Natl. Acad. Sci. India B 2021, 91, 217–226. [Google Scholar] [CrossRef]
- Kumar, V.; Goud, P.V.; Babu, J.D.; Reddy, R.S. Preparation and evaluation of custard apple wine: Effect of dilution of pulp on physico-chemical and sensory quality characteristics. Int. J. Food Ferm. Technol. 2011, 1, 247–253. [Google Scholar]
- Jagtap, U.B.; Bapat, V.A. Phenolic composition and antioxidant capacity of wine prepared from custard apple (Annona squamosal L.) fruits. J. Food Process. Pres. 2015, 39, 175–182. [Google Scholar] [CrossRef]
- Phan, H.T.; Nguyen, H.M. Optimization of alcoholic fermentation of custard apple juice by Saccharomyces cerevisiae using response surface methodology. J. Agric. Dev. 2019, 18, 70–78. [Google Scholar] [CrossRef]
- Raichurkar, S.J.; Dadagkhair, R.A. Studies on preparation of custard apple vinegar. Int. J. Adv. Eng. Res. Sci. 2017, 4, 101–106. [Google Scholar] [CrossRef]
- Kadam, B.R.; Ambadkar, R.K.; Rathod, K.S. Effect of custard apple (Annona squamosa) peel extract on shelf life of chicken breast fillets. J. Environ. Biol. Sci. 2018, 32, 9–14. [Google Scholar]
- Olatunde, O.O.; Shiekh, K.A.; Ma, L.K.; Ying, X.G.; Zhang, B.; Benjakul, S. Effect of the extract from custard apple (Annona squamosa) leaves prepared with pulsed electric field-assisted process on the diversity of microorganisms and shelf-life of refrigerated squid rings. Int. J. Food Sci. Technol. 2021, 56, 6527–6538. [Google Scholar] [CrossRef]
- Huang, C.H.; Tu, W.S.; Zhang, M.; Peng, D.; Guo, Z.; Huang, W.; Zhu, J.; Yu, R.; Song, L.; Wang, Y. A novel heteropolysaccharide isolated from custard apple pulp and its immunomodulatory activity in mouse macrophages and dendritic cells. Heliyon 2023, 9, e18521. [Google Scholar] [CrossRef]
- Shivamathi, C.S.; Moorthy, I.G.; Kumar, R.V.; Soosai, M.R.; Maran, J.P.; Kumar, R.S.; Varalakshmi, P. Optimization of ultrasound assisted extraction of pectin from custard apple peel: Potential and new source. Carbohyd. Polym. 2019, 225, 115240. [Google Scholar] [CrossRef]
- Hotti, S.R.; Hebbal, O.D. Biodiesel production process optimization from sugar apple seed oil (Annona squamosa) and its characterization. J. Renew. Ener. 2015, 2015, 148587. [Google Scholar] [CrossRef]
- Sivanesh, S.; Aswin, K.N.; Antony, A.; Varma, M.S.; Kamalesh, K.; Naageshwaran, M.; Soundarya, S.; Subramanian, S. Biodiesel production from custard apple seeds and Euglena sanguinea using CaO nano-catalyst. Bioresour. Technol. 2022, 344, 126418. [Google Scholar] [CrossRef]
- Shiney Judith, M.; Shiney Judith, M.P.; Muthusamy, P. The production of bioethanol from custard apple peels (Annona squamosa) using Saccharomyces cerevisiae. Int. J. Agric. Sci. Res. 2019, 9, 43–50. [Google Scholar]
- Dey, S.; Veerendra, G.T.N.; Manoj Phani, A.V.; Padavala, S.S.A.B.; Swaroop, A.H.L. Synthesize and applications of green custard apples leaves biosorbents for adsorptions of sulphates and fluorides from water. Environ. Sur. Interf. 2025, 3, 1–23. [Google Scholar] [CrossRef]
- Prashant, M.; Dipika, J.I.; Arti, M. Adsorption of dyes using custard apple and wood apple waste: A review. J. Indian Chem. Soc. 2023, 100, 100948. [Google Scholar]
- Saravanan, K.; Subramaniam, Y.; Kathirvel, P. Valorization of custard apple (Annona squamosa) waste through polyhydroxybutyrate (PHB) production by Bacillus megaterium MAPCS4: Optimization, characterization, and biodegradation studies. Biomass Convers. Bior. 2024, 14, 26121–26137. [Google Scholar] [CrossRef]
- Kharat, B.M.; More, A.P. Synthesis of bio-based plasticizer from custard apple seed oil for PVC application. Vietnam J. Chem. 2024, 62, 337–345. [Google Scholar] [CrossRef]
- Mukke, A.P.; More, A.P. Custard apple seed oil-based polyesteramide polyol-a precursor for layered double hydroxide (LDH)-modified polyurethane coatings. Ind. Crops Prod. 2024, 214, 11860. [Google Scholar] [CrossRef]
- Sarathamani, T.; Kalaiselvi, V.; Blessymol, B.; Gopi, S.; Khuwaja, G.; Palanismy, S.; Ismail, K.S.; Ali, S.K.; Sillanpaa, M.; Ayrilmis, N. Annona squamosa seeds capped calcium oxide nano particles-anti-microbial, antioxidant, anti-ulcer analysis. RSC Adv. 2025, 15, 4904–4914. [Google Scholar]
- Scheunemann, T.; Krüger, A.P.; Piovesan, B.; Vieira, J.G.A.; Do Prado Ribeiro, L.; Schiedeck, G.; Bernardi, D.; Nava, D.E. Potential use of Annona (Annonaceae) by-product to Palpita forficifera management: Lethal and sublethal toxicities and residual effect in olive plants. Crop Prot. 2022, 160, 106035. [Google Scholar] [CrossRef]
- Ilakkia, M.; Premalatha, K.; Vellaikumar, S.; Shanmugam, P.S.; Harish, S.; Jayarajan Nelson, S. Laboratory studies on chromatographic profile, toxicity and repellent activity of custard apple (Annona squamosa L.) seed extract. Int. J. Environ. Clim. Change 2023, 13, 2949–2955. [Google Scholar]
- Kifle, F.; Girma, M.; Gebresilassie, A.; Woldehawariat, Y.; Ele, E.; Tomanovic, Z. Evaluation of crude oil extracts from plants for controlling maize infestations caused by the Weevil Sitophilus zeamais (Motschulsky) (Coleoptera: Curculionidae). Psyche-J. Entomol. 2024, 1, 5993907. [Google Scholar] [CrossRef]
- Nuriyasa, I.M.; Dewi, G.A.M.K.; Dewi, N.M.A.K. Effects of sugar apple (Annona squamosa L.) extract on cockerel’s growth performance, carcass, digestive tract microbial count, and meat cholesterol. Indian J. Anim. Sci. 2023, 93, 469–474. [Google Scholar] [CrossRef]
- Sohn, E.; Kim, B.-Y.; Kim, Y.J.; Jeong, S.-J. Non-clinical safety assessment of Annona atemoya leaf extract: Evaluation of genotoxicity. Toxicol. Res. 2024, 40, 473–485. [Google Scholar] [CrossRef]
- Sohn, E.; Kim, Y.-J.; Lim, H.-S.; Jeong, S.-J. Single acute and repeated subacute toxicity evaluations of Annona atemoya leaf extract with in vitro anti-inflammatory potential. Drug Chem. Toxicol. 2024, 48, 864–875. [Google Scholar] [CrossRef]
- Sharma, P.N.; Shrestha, P.D.D.; Chauhan, S.R.; Sharma, V. Effects of neem (Azadirachta indic) and custard apple (Annona reticulata) diets on sterility of house rat (rattus rattus). J. Nepal Agric. Res. Counc. 2016, 1, 37–40. [Google Scholar] [CrossRef][Green Version]
- Nagaraja, H.; Kugar, T.; Shivanna, Y.; Agrawal, A.; Shetty, R. Ocular toxicity by seeds of Annona squamosa (custard apple). Indian J. Ophthalmol. 2016, 64, 611–613. [Google Scholar] [CrossRef]
- Prasad, N.; Singh, P.; Pillay, G. Custard apple seeds (Annona squamosa)-induced bilateral toxic keratopathy. Indian J. Ophthalmol. Case Rep. 2024, 4, 838–839. [Google Scholar] [CrossRef]
- Thite, S.; Patil, K.; Jadhav, R.; Suryawanshi, Y.; Chumchu, P. Empowering agricultural research: A comprehensive custard apple (Annona squamosa) disease dataset for precise detection. Data Brief 2024, 53, 110078. [Google Scholar] [CrossRef]

| Classifications | Nutrition Compositions | Methods of Detection | Pulp | Seed | Leaf | References |
|---|---|---|---|---|---|---|
| Contents (Dried Weight) | ||||||
| Carbohydrate | Total carbohydrate | AOCS official method | 18.2–59.0 g/100 g | [8,9,10] | ||
| Crude fiber | 2.55–11.0 g/100 g | 16.80 g/100 g | ||||
| Amino acids | Threonine | Amino acid analyzer | 5.67 g/100 g | [11] | ||
| Valine | 11.24 g/100 g | |||||
| Isoleucine | 8.12 g/100 g | |||||
| Leucine | 14.79 g/100 g | |||||
| Histidine | 2.43 g/100 g | |||||
| Lysine | 7.12 g/100 g | |||||
| Arginine | 12.32 g/100 g | |||||
| Glutamic acid | 17.41 g/100 g | |||||
| Serine | 5.23 g/100 g | |||||
| Glycine | 6.86 g/100 g | |||||
| Aspartic acid | 11.97 g/100 g | |||||
| Alanine | 6.86 g/100 g | |||||
| Fatty acids | Palmitic acid (C16:0) | GC-FID, GC-MS | 1.82–4.65 g/100 g | [11,12,13] | ||
| Stearic acid (C18:0) | 1.12–2.76 g/100 g | |||||
| Oleic acid (C18:1) | 7.12–13.13 g/100 g | |||||
| Linoleic acid (C18:2) | 3.45–7.57 g/100 g | |||||
| Vitamins | Ascorbic acid | HPLC-MS/MS | 9.22–300 mg/100 g | 0.01–0.02 mg/g FW | [4,8,9,10,11,14,15,16,17,18] | |
| Thiamine | 0.05–0.28 mg/100 g | |||||
| Riboflavin | 0.07–0.28 mg/100 g | |||||
| Niacin | 0.80–2.2 mg/100 g | |||||
| Panthothenic acid | 0.20–1.28 mg/100 g | |||||
| Pyridoxine | 0–0.5 mg/100 g | |||||
| Folic acid | 35.00 µg/100 g | 8.12–11.98 g/g FW | ||||
| Vitamin B12 | 0.057–0.167 mg/100 g | |||||
| Vitamin A | 1.80–7.00 µg/100 g | |||||
| Vitamin E | 0.60 mg/100 g | 15.50–16.60 mg/100 g | ||||
| Minerals | Ca | FAES, ICP-MS/MS | 4.92–75.47 mg/100 g | 3.41–68.79 mg/100 g | 0.28 g/100 g | |
| P | 20.00–235.61 mg/100 g | 328 mg/100 g | ||||
| Fe | 0.30–105.00 mg/100 g | 1.09 mg/100 g | 37.23–49.55 µmol/g | |||
| K | 250–1362.25 mg/100 g | 252.47–386.98 µmol/g | 363 mg/100 g | |||
| Na | 4.50–62.75 mg/100 g | 61 mg/100 g | 61.2–95.18 µmol/g | |||
| Mg | 21.0–64.45 mg/100 g | 98 mg/100 g | ||||
| Cu | 0.11–2.75 mg/100 g | 1.09 mg/100 g | ||||
| Mn | 0.21–1.75 mg/100 g | 2.93 mg/100 g | ||||
| Zn | 0.57–1.21 mg/100 g | 2.84 mg/100 g | ||||
| Ba | 0.028–0.23 mg/100 g | |||||
| Se | 1.50 µg/100 g | |||||
| Parts | Compounds | Biological Activities Identified | Type of Study | References |
|---|---|---|---|---|
| Pulp | Ent-kaurane diterpenes (e.g., 16β,17-dihydroxy-ent-kauran-19-oic acid, 17-hydroxy-16β-ent-kauran-19-al) | Anti-HIV, anti-tumor | In vivo against 95-D lung and ovarian A2780 cancer cells | [24,25] |
| Phenolics and derivatives (e.g., gallic acid, ferulic acid, protocatechuic acid, caffeic acid, p-coumaric acid, sinapic acid, quinic acid, decycloxybenzoic acid, procyanidin B2, procyanidin trimer, catechin, epicatechin, epigallocatechin gallate, 4-(β-D-glucopyranosyloxy) benzoic acid, epigallocatechin, 7-hydroxycoumarin 7-glucoside, dihydroquercetin, xanthotaxol acetate, caffeoyl hexoside) | Antioxidant | In vitro, rats and mice | [26] | |
| Peel | Fatty acids and diterpenes (e.g., (9Z)-9-octadecenoic acid, ent-kaur-16-en-19-oic acid, (-)-ent-kaur-16-en-19-oic acid, 16α,17-dihydroxy-ent-kauran-19-oic acid, 4β,17-dihydroxy-16α-acetoxy-18-nor-ent-kaurane) | Anti-tumor | In vivo against SMMC-7721 and HepG2 cell line | [27,28,29] |
| Azulene derivative (e.g., 1H-cycloprop[e]azulen-7-ol decahydro-1,1,7-trimethyl-4-methylene-[1ar-(1aα, 4aα, 7β, 7a, β, 7bα)]) | Anti-parasitic activity | In vitro | [24] | |
| Leaf | Flavonoids and derivatives (e.g., rutin kaempferol-3-O-rutinoside, quercetin-3-O-robinobioside, quercetin-3-O-β-D-glucoside, quercetin-3-O-glucoside) | Antioxidant and hypoglycemic activity | In vitro, diabetic rats | [30,31] |
| Acetogenins (e.g., annoreticuin, isoannoreticuin) | Anti-tumor | In vivo against AD-5 tumor | [32] | |
| Flavonoid derivatives (e.g., 5,7,4′-trihydroxy-6,3′dimethoxy-flavone 5-O-α-L-rhamnopyranoside (THDMF-Rha) | Hepatoprotective | Cellular levels, rats | [33] | |
| Acetogenins (e.g., annotemoyin, purpureacin 2 | Anti-microbes | In vivo against H22 liver cancer mice | [34,35] | |
| Seed | Diterpenes (e.g., 16-ahydroxy-(−)-kauran-19-oic) | Anti-microbes | In vitro | [36] |
| Cyclopeptides (e.g., cyclosquamosin D) | Anti-inflammatory | In vitro | [37] | |
| Acetogenins (e.g., dieporeticenin B, squamocin P, annosquatin III, annonacin) | Anti-tumor (cytotoxic) | In vitro cytotoxic activity, cellular levels | [38,39] | |
| Phenolic and Flavonoids (e.g., quercetin, ferulic acid, kaempferol, 6-methoxy isovitexin) | Antioxidant | In vitro | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, N.; Gong, X.; Luo, Y.; Zhang, C.; Chen, J.; Chen, T. A Review of Nutrition, Bioactivities, and Health Benefits of Custard Apple (Annona squamosa): From Phytochemicals to Potential Application. Foods 2025, 14, 3413. https://doi.org/10.3390/foods14193413
Qi N, Gong X, Luo Y, Zhang C, Chen J, Chen T. A Review of Nutrition, Bioactivities, and Health Benefits of Custard Apple (Annona squamosa): From Phytochemicals to Potential Application. Foods. 2025; 14(19):3413. https://doi.org/10.3390/foods14193413
Chicago/Turabian StyleQi, Ningli, Xiao Gong, Yang Luo, Chenghan Zhang, Jingjing Chen, and Tinghui Chen. 2025. "A Review of Nutrition, Bioactivities, and Health Benefits of Custard Apple (Annona squamosa): From Phytochemicals to Potential Application" Foods 14, no. 19: 3413. https://doi.org/10.3390/foods14193413
APA StyleQi, N., Gong, X., Luo, Y., Zhang, C., Chen, J., & Chen, T. (2025). A Review of Nutrition, Bioactivities, and Health Benefits of Custard Apple (Annona squamosa): From Phytochemicals to Potential Application. Foods, 14(19), 3413. https://doi.org/10.3390/foods14193413







