Physicochemical Properties and Sensory Evaluation of Low-Sugar Collagen Jelly Using Fruit and Vegetable Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Jelly Preparation
2.3. Proximate Composition and Sugar Content Analysis
2.4. Chromaticity Determination
2.5. Texture Profile Analysis (TPA)
2.6. Antioxidant Capacity Analysis
2.7. Total Flavonoid Content Analysis
2.8. Total Phenolic Content Analysis
2.9. DPPH (2,2-diphenyl-1-picrylhydrazyl) Radical-Scavenging Activity
2.10. ABTS (2,2ʹ-Azino-bis[3-ethylbenzothiazoline-6-sulfonic acid]) Radical-Scavenging Assay
2.11. Sensory Evaluation
2.12. Statistical Analysis
3. Results and Discussion
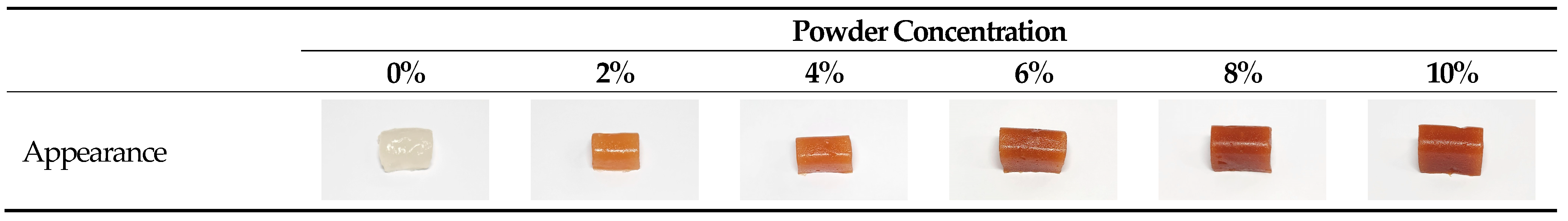
3.1. Appearance and Color Analysis
3.2. Proximate Composition and Sugar Content Analysis
3.3. Texture Profile Analysis
3.4. Total Flavonoid and Polyphenol Content
3.5. DPPH and ABTS Radical-Scavenging Activity
3.6. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blinnikova, O.; Babushkin, V.; Akindinov, V.; Perfilova, O.; Novikova, I. Production technology and mathematical method for modeling the formulation of fruit and jelly candies enriched with collagen. IOP Conf. Ser. Mater. Sci. Eng. 2020, 919, 052036. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Borumand, M.; Sibilla, S. Effects of a nutritional supplement containing collagen peptides on skin elasticity, hydration and wrinkles. J. Med. Nutr. Nutraceuticals 2015, 4, 47. [Google Scholar]
- Proksch, E.; Segger, D.; Degwert, J.; Schunck, M.; Zague, V.; Oesser, S. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: A double-blind, placebo-controlled study. Skin Pharmacol. Physiol. 2013, 27, 47–55. [Google Scholar] [CrossRef]
- Genovese, L.; Corbo, A.; Sibilla, S. An insight into the changes in skin texture and properties following dietary intervention with a nutricosmeceutical containing a blend of collagen bioactive peptides and antioxidants. Skin Pharmacol. Physiol. 2017, 30, 146–158. [Google Scholar] [CrossRef]
- Cai, L.; Feng, J.; Regenstein, J.; Lv, Y.; Li, J. Confectionery gels: Effects of low calorie sweeteners on the rheological properties and microstructure of fish gelatin. Food Hydrocoll. 2017, 67, 157–165. [Google Scholar] [CrossRef]
- Gurung, P.; Zubair, M.; Jialal, I. Plasma Glucose; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- De Cock, P. Erythritol functional roles in oral-systemic health. Adv. Dent. Res. 2018, 29, 104–109. [Google Scholar] [CrossRef]
- Ruan, Y. The advantages of erythritol compared with the common natural sweeteners in the market and current uses in the food and food industries. In Proceedings of the Second International Conference on Biological Engineering and Medical Science (ICBioMed 2022), Oxford, UK, 7–13 November 2022; pp. 441–453. [Google Scholar]
- den Hartog, G.J.; Boots, A.W.; Adam-Perrot, A.; Brouns, F.; Verkooijen, I.W.; Weseler, A.R.; Haenen, G.R.; Bast, A. Erythritol is a sweet antioxidant. Nutrition 2010, 26, 449–458. [Google Scholar] [CrossRef]
- Rubio-Arraez, S.; Capella, J.V.; Castelló, M.L.; Ortolá, M.D. Physicochemical characteristics of citrus jelly with non cariogenic and functional sweeteners. J. Food Sci. Technol. 2016, 53, 3642–3650. [Google Scholar] [CrossRef]
- Park, J.J.; Olawuyi, I.F.; Park, G.D.; Lee, W.Y. Effects of gelling agents and sugar substitutes on the quality characteristics of carrot jelly. Food Sci. Preserv. 2021, 28, 469–479. [Google Scholar] [CrossRef]
- Gómez-Gaete, C.; Avendaño-Godoy, J.; Escobar-Avello, D.; Campos-Requena, V.H.; Rogel-Castillo, C.; Estevinho, L.M.; Martorell, M.; Sharifi-Rad, J.; Calina, D. Revolutionizing fruit juice: Exploring encapsulation techniques for bioactive compounds and their impact on nutrition, flavour and shelf life. Food Prod. Process. Nutr. 2024, 6, 8. [Google Scholar] [CrossRef]
- Brown, H.; Williams, J. Packaged product quality and shelf life. In Food Packaging Technology; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 65–94. [Google Scholar]
- Kschonsek, J.; Wolfram, T.; Stöckl, A.; Böhm, V. Polyphenolic compounds analysis of old and new apple cultivars and contribution of polyphenolic profile to the in vitro antioxidant capacity. Antioxidants 2018, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Luo, M.; Wei, S. The bioprotective effects of polyphenols on metabolic syndrome against oxidative stress: Evidences and perspectives. Oxidative Med. Cell. Longev. 2019, 2019, 6713194. [Google Scholar] [CrossRef] [PubMed]
- Moßhammer, M.R.; Stintzing, F.C.; Carle, R. Evaluation of different methods for the production of juice concentrates and fruit powders from cactus pear. Innov. Food Sci. Emerg. Technol. 2006, 7, 275–287. [Google Scholar] [CrossRef]
- Kiefer, I.; Prock, P.; Lawrence, C.; Wise, J.; Bieger, W.; Bayer, P.; Rathmanner, T.; Kunze, M.; Rieder, A. Supplementation with mixed fruit and vegetable juice concentrates increased serum antioxidants and folate in healthy adults. J. Am. Coll. Nutr. 2004, 23, 205–211. [Google Scholar] [CrossRef]
- Chavan, G.; Kad, V.; Kamble, K.; Salve, V.; Shelke, G.; Lad, P. Physico-chemical, reconstitution, flow and functional properties of mix fruit juice powder. Int. J. Adv. Biochem. Res. 2025, 9, 630–638. [Google Scholar] [CrossRef]
- Utomo, B.S.B.; Darmawan, M.; Hakim, A.R.; Ardi, D.T. Physicochemical properties and sensory evaluation of jelly candy made from different ratio of k-carrageenan and konjac. Squalen Bull. Mar. Fish. Postharvest Biotechnol. 2014, 9, 25–34. [Google Scholar] [CrossRef][Green Version]
- Brenner, T.; Wang, Z.; Achayuthakan, P.; Nakajima, T.; Nishinari, K. Rheology and synergy of κ-carrageenan/locust bean gum/konjac glucomannan gels. Carbohydr. Polym. 2013, 98, 754–760. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Arlington, VA, USA, 2012; Volume 19. [Google Scholar]
- Lekjing, S.; Venkatachalam, K. Influences of storage time and temperature on sensory and measured quality of green gram savory crackers. LWT 2019, 113, 108310. [Google Scholar] [CrossRef]
- Kanzler, S.; Manschein, M.; Lammer, G.; Wagner, K.-H. The nutrient composition of European ready meals: Protein, fat, total carbohydrates and energy. Food Chem. 2015, 172, 190–196. [Google Scholar] [CrossRef]
- Borilova, G.; Hulankova, R.; Svobodova, I.; Jezek, F.; Hutarova, Z.; Vecerek, V.; Steinhauserova, I. The effect of storage conditions on the hygiene and sensory status of wild boar meat. Meat Sci. 2016, 118, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Joung, M.; Shin, Y. Physicochemical quality, antioxidant compounds, and activity of’Beta Tiny’and’TY Nonari’cherry tomatoes during storage. Korean J. Food Sci. Technol. 2021, 53, 63–71. [Google Scholar]
- Meyers, K.J.; Watkins, C.B.; Pritts, M.P.; Liu, R.H. Antioxidant and antiproliferative activities of strawberries. J. Agric. Food Chem. 2003, 51, 6887–6892. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Kapoor, H.C. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int. J. Food Sci. Technol. 2002, 37, 153–161. [Google Scholar] [CrossRef]
- Yang, H.; Kim, Y.-J.; Shin, Y. Influence of ripening stage and cultivar on physicochemical properties and antioxidant compositions of aronia grown in South Korea. Foods 2019, 8, 598. [Google Scholar] [CrossRef]
- Gaber, N.B.; El-Dahy, S.I.; Shalaby, E.A. Comparison of ABTS, DPPH, permanganate, and methylene blue assays for determining antioxidant potential of successive extracts from pomegranate and guava residues. Biomass Convers. Biorefinery 2021, 13, 4011–4020. [Google Scholar] [CrossRef]
- Granato, D.; Masson, M.L.; Ribeiro, J.C.B. Sensory acceptability and physical stability evaluation of a prebiotic soy-based dessert developed with passion fruit juice. Food Sci. Technol. 2012, 32, 119–126. [Google Scholar] [CrossRef]
- Rosa, A.; Masala, C. Labeled Hedonic Scale for the Evaluation of Sensory Perception and Acceptance of an Aromatic Myrtle Bitter Liqueur in Consumers with Chemosensory Deficits. Appl. Sci. 2023, 13, 13083. [Google Scholar] [CrossRef]
- Brandt, S.; Pék, Z.; Barna, É.; Lugasi, A.; Helyes, L. Lycopene content and colour of ripening tomatoes as affected by environmental conditions. J. Sci. Food Agric. 2006, 86, 568–572. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. Characterization of băbească neagră grape pomace and incorporation into jelly candy: Evaluation of phytochemical, sensory, and textural properties. Foods 2023, 13, 98. [Google Scholar] [CrossRef]
- Zambrano, M.V.; Dutta, B.; Mercer, D.G.; MacLean, H.L.; Touchie, M.F. Assessment of moisture content measurement methods of dried food products in small-scale operations in developing countries: A review. Trends Food Sci. Technol. 2019, 88, 484–496. [Google Scholar] [CrossRef]
- Delgado, P.; Bañón, S. Determining the minimum drying time of gummy confections based on their mechanical properties. CyTA-J. Food 2015, 13, 329–335. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y.; Rosenthal, A. Human oral processing and texture profile analysis parameters: Bridging the gap between the sensory evaluation and the instrumental measurements. J. Texture Stud. 2019, 50, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Culetu, A.; Duta, D.E.; Papageorgiou, M.; Varzakas, T. The role of hydrocolloids in gluten-free bread and pasta; rheology, characteristics, staling and glycemic index. Foods 2021, 10, 3121. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Gorjanović, S.; Zlatanović, S.; Laličić-Petronijević, J.; Dodevska, M.; Micić, D.; Stevanović, M.; Pastor, F. Enhancing composition and functionality of jelly candies through apple and beetroot pomace flour addition. npj Sci. Food 2024, 8, 85. [Google Scholar] [CrossRef]
- Biedrzycka, E.; Amarowicz, R. Diet and health: Apple polyphenols as antioxidants. Food Rev. Int. 2008, 24, 235–251. [Google Scholar] [CrossRef]
- Sun, T.; Simon, P.W.; Tanumihardjo, S.A. Antioxidant phytochemicals and antioxidant capacity of biofortified carrots (Daucus carota L.) of various colors. J. Agric. Food Chem. 2009, 57, 4142–4147. [Google Scholar] [CrossRef]
- Borguini, R.G.; Ferraz da Silva Torres, E.A. Tomatoes and tomato products as dietary sources of antioxidants. Food Rev. Int. 2009, 25, 313–325. [Google Scholar] [CrossRef]
- Hwang, E.-S.; Moon, S.J. Quality Characteristics and Antioxidant Activity of Stick Jelly Made with Different Amount of Tomato Juice. J. Korean Soc. Food Sci. Nutr. 2021, 50, 476–482. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, P.; Li, S.; Shah, N.P. Antioxidant, antibacterial, and antiproliferative activities of free and bound phenolics from peel and flesh of fuji apple. J. Food Sci. 2016, 81, M1735–M1742. [Google Scholar] [CrossRef]
- Carvalho, V.S.; Damiani, C.; Asquieri, E.R.; Orsi, D.C.; Nishi, A.C.F. Development and antioxidant capacity of sapota pulp jelly (Quararibea cordata Vischer). Ciênc. Agrotecnologia 2012, 36, 341–347. [Google Scholar] [CrossRef]
- Kafache, D.; Deli, M.; Galani, B.R.T.; Agume, A.N.; Bouba, A.A.; Njintang, N.Y. Physicochemical and in Vitro Antioxidant Properties of Juice and Cake Filters from Carissa edulis Vahl Fruits. J. Explor. Res. Pharmacol. 2022, 7, 134–145. [Google Scholar] [CrossRef]
- Muñoz-Fariña, O.; López-Casanova, V.; García-Figueroa, O.; Roman-Benn, A.; Ah-Hen, K.; Bastias-Montes, J.M.; Quevedo-León, R.; Ravanal-Espinosa, M.C. Bioaccessibility of phenolic compounds in fresh and dehydrated blueberries (Vaccinium corymbosum L.). Food Chem. Adv. 2023, 2, 100171. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Chen, Q.; Guan, W. Effects of different drying methods on the antioxidant profiles and volatile organic compounds of blueberry pomace. Food Chem. X 2025, 27, 102505. [Google Scholar] [CrossRef]
| Ingredients | Powder Concentration | |||||
|---|---|---|---|---|---|---|
| 0% | 2% | 4% | 6% | 8% | 10% | |
| Fruit and vegetable powder (g) * | 0 | 2 | 4 | 6 | 8 | 10 |
| Erythritol (g) | 8.5 | 8.5 | 8.5 | 8.5 | 8.5 | 8.5 |
| Sugar (g) | 8.5 | 8.5 | 8.5 | 8.5 | 8.5 | 8.5 |
| Citric acid (g) | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 |
| Salt (g) | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Mixed gellant (g) ** | 2 | 2 | 2 | 2 | 2 | 2 |
| Collagen (g) | 5 | 5 | 5 | 5 | 5 | 5 |
| Water (g) | 75.1 | 73.1 | 71.1 | 69.1 | 67.1 | 65.2 |
| Powder Concentration | ||||||
|---|---|---|---|---|---|---|
| 0% | 2% | 4% | 6% | 8% | 10% | |
| Lightness (L) | 34.83 ± 0.04 a | 31.17 ± 0.07 b | 28.33 ± 0.05 c | 27.39 ± 0.18 d | 25.27 ± 0.12 e | 23.60 ± 0.19 f |
| Redness (a) | −1.45 ± 0.01 e | 10.82 ± 0.04 d | 14.90 ± 0.07 c | 17.28 ± 0.17 b | 17.49 ± 0.11 b | 18.93 ± 0.23 a |
| Yellowness (b) | 2.48 ± 0.02 e | 15.07 ± 0.03 e | 20.84 ± 0.02 d | 23.08 ± 0.20 c | 24.29 ± 0.07 b | 25.59 ± 0.10 a |
| Powder Concentration | ||||||
|---|---|---|---|---|---|---|
| 0% | 2% | 4% | 6% | 8% | 10% | |
| Moisture (%) | 50.89 ± 0.50 a | 47.80 ± 3.72 ab | 45.80 ± 2.78 bc | 43.81 ± 1.05 cd | 41.52 ± 0.97 de | 39.70 ± 1.20 e |
| Crude protein (%) | 6.28 ± 0.20 e | 6.63 ± 0.20 de | 6.86 ± 0.34 cd | 7.08 ± 0.20 bc | 7.43 ± 0.20 ab | 7.66 ± 0.20 a |
| Crude fat (%) | 0.31 ± 0.09 d | 0.43 ± 0.03 de | 0.57 ± 0.06 cd | 0.70 ± 0.12 bc | 0.90 ± 0.13 ab | 1.09 ± 0.21 a |
| Ash (%) | 0.40 ± 0.14 f | 0.72 ± 0.03 e | 0.93 ± 0.07 d | 1.21 ± 0.08 c | 1.47 ± 0.10 b | 1.73 ± 0.18 a |
| Total carbohydrates (%) | 42.11 ± 10.46 d | 44.42 ± 3.90 cd | 45.84 ± 2.61 bcd | 47.19 ± 1.03 abc | 48.68 ± 0.77 ab | 49.83 ± 1.10 a |
| Sugar (°Brix) | 2.43 ± 0.06 f | 2.67 ± 0.06 e | 2.93 ± 0.06 d | 3.17 ± 0.06c | 3.43 ± 0.06b | 3.63 ± 0.06 a |
| Powder Concentration | ||||||
|---|---|---|---|---|---|---|
| 0% | 2% | 4% | 6% | 8% | 10% | |
| Hardness (N) | 1.32 ± 0.15 d | 1.50 ± 0.07 cd | 1.61 ± 0.01 cd | 1.77 ± 0.11 c | 2.51 ± 0.36 b | 3.39 ± 0.27 a |
| Adhesiveness (N·s) | 0.04 ± 0.03 c | 0.06 ± 0.04 c | 0.06 ± 0.00 c | 0.07 ± 0.01 c | 0.19 ± 0.07 b | 0.26 ± 0.03 a |
| Cohesiveness (-) | 0.65 ± 0.03 a | 0.66 ± 0.01 a | 0.68 ± 0.03 a | 0.64 ± 0.02 a | 0.53 ± 0.04 b | 0.34 ± 0.03 c |
| Springiness (-) | 0.52 ± 0.08 b | 0.59 ± 0.06 ab | 0.68 ± 0.05 a | 0.69 ± 0.05 a | 0.72 ± 0.05 a | 0.60 ± 0.09 ab |
| Gumminess (N) | 0.86 ± 0.13 c | 1.00 ± 0.04 bc | 1.09 ± 0.04 ab | 1.13 ± 0.09 ab | 1.33 ± 0.22 a | 1.17 ± 0.17 ab |
| Chewiness (N) | 0.45 ± 0.08 d | 0.59 ± 0.07 cd | 0.74 ± 0.08 bc | 0.79 ± 0.11 b | 0.95 ± 0.10 a | 0.70 ± 0.07 bc |
| Powder Concentration | ||||||
|---|---|---|---|---|---|---|
| 0% | 2% | 4% | 6% | 8% | 10% | |
| Total flavonoid contents (mg QE/g) | 1.58 ± 0.07 f | 5.13 ± 0.29 e | 8.57 ± 0.34 d | 10.74 ± 0.28 c | 12.80 ± 0.22 b | 14.99 ± 0.07 a |
| Total polyphenol contents (mg GAE/g) | 12.72 ± 0.12 f | 16.91 ± 0.12 e | 22.48 ± 0.10 d | 28.32 ± 0.05 c | 34.31 ± 0.10 b | 39.99 ± 0.20 a |
| Powder Concentration | ||||||
|---|---|---|---|---|---|---|
| 0% | 2% | 4% | 6% | 8% | 10% | |
| DPPH (mg VCE/100g) | 25.68 ± 0.71 f | 36.48 ± 0.44 e | 50.39 ± 0.66 d | 63.40 ± 0.48 c | 78.57 ± 0.28 b | 90.67 ± 0.24 a |
| ABTS (mg VCE/100g) | 12.68 ± 0.34 f | 19.37 ± 0.22 e | 25.81 ± 0.30 d | 34.10 ± 0.05 c | 42.26 ± 0.25 b | 51.72 ± 0.25 a |
| Powder Concentration | |||||
|---|---|---|---|---|---|
| 2% | 4% | 6% | 8% | 10% | |
| Color | 3.47 ± 1.14 c | 4.50 ± 1.31 ab | 4.87 ± 0.97 a | 4.67 ± 1.03 ab | 4.13 ± 1.20 b |
| Odor | 3.17 ± 1.18 c | 3.40 ± 1.10 bc | 3.93 ± 1.05 ab | 4.30 ± 1.39 a | 3.80 ± 1.21 abc |
| Taste | 3.67 ± 1.30 b | 4.13 ± 1.11 ab | 4.13 ± 1.33 ab | 4.63 ± 1.10 a | 4.03 ± 1.47 ab |
| Texture | 3.40 ± 1.28 ns | 3.23 ± 1.41 | 3.93 ± 1.17 | 3.87 ± 1.14 | 3.40 ± 1.45 |
| Overall preference | 3.17 ± 1.21 b | 3.77 ± 1.30 ab | 4.13 ± 1.14 a | 4.30 ± 1.09 a | 3.70 ± 1.42 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Park, S.-J.; Lee, H.-J. Physicochemical Properties and Sensory Evaluation of Low-Sugar Collagen Jelly Using Fruit and Vegetable Powder. Foods 2025, 14, 3407. https://doi.org/10.3390/foods14193407
Yu J, Park S-J, Lee H-J. Physicochemical Properties and Sensory Evaluation of Low-Sugar Collagen Jelly Using Fruit and Vegetable Powder. Foods. 2025; 14(19):3407. https://doi.org/10.3390/foods14193407
Chicago/Turabian StyleYu, Junho, Seon-Joo Park, and Hae-Jeung Lee. 2025. "Physicochemical Properties and Sensory Evaluation of Low-Sugar Collagen Jelly Using Fruit and Vegetable Powder" Foods 14, no. 19: 3407. https://doi.org/10.3390/foods14193407
APA StyleYu, J., Park, S.-J., & Lee, H.-J. (2025). Physicochemical Properties and Sensory Evaluation of Low-Sugar Collagen Jelly Using Fruit and Vegetable Powder. Foods, 14(19), 3407. https://doi.org/10.3390/foods14193407







