Valorization of Tomato Leaves: Optimization of Eco-Friendly Phenolic Extraction and Assessment of Biological Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Chemicals and Reagents
2.3. Dry Matter Content
2.4. Preparation of the Tomato Leaves Extract
2.5. Experimental Design for Optimization of Tomato Leaves Extraction
2.6. Determination of Total Phenolic Content
2.7. Antioxidant Activity Determination
2.8. Freeze-Drying
2.9. Antimicrobial Testing
2.9.1. Preparation of the Inoculum
2.9.2. Antimicrobial Susceptibility Testing
2.10. Evaluation of the Anticancer Activity of TLE
2.11. Evaluation of the Anti-Inflammatory Activity of TLE
2.12. Analysis of TLE Constituents by Liquid Chromatography-Mass Spectroscopy
2.13. Statistical Analysis
3. Results and Discussion
3.1. Determination of Solid-to-Liquid Extraction Ratio
3.2. Effect of Time and Temperature on TPC Yield and DPPH
3.3. Antimicrobial Activity of TLE
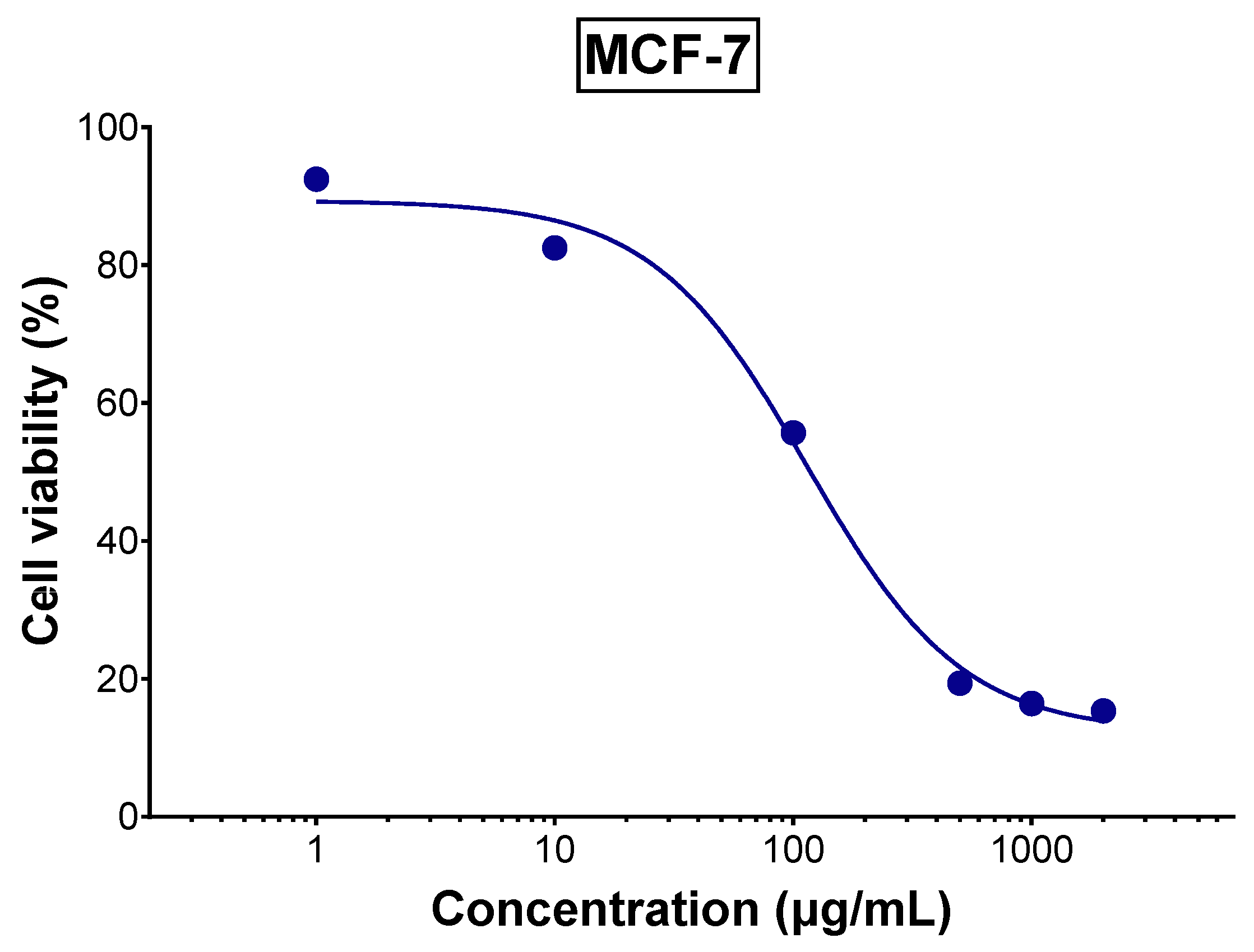
3.4. Anticancer Activity of TLE Against MCF-7 Cells
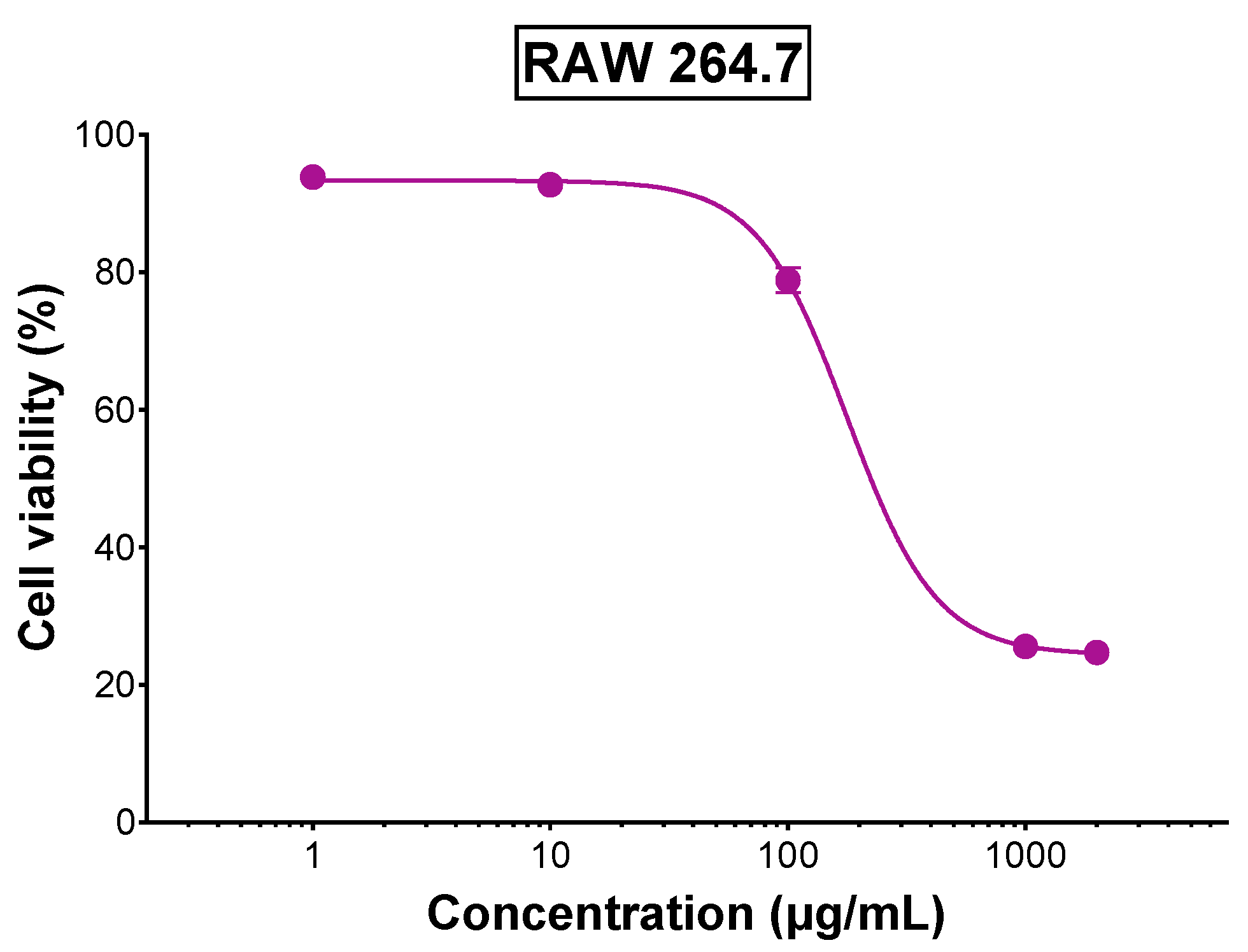
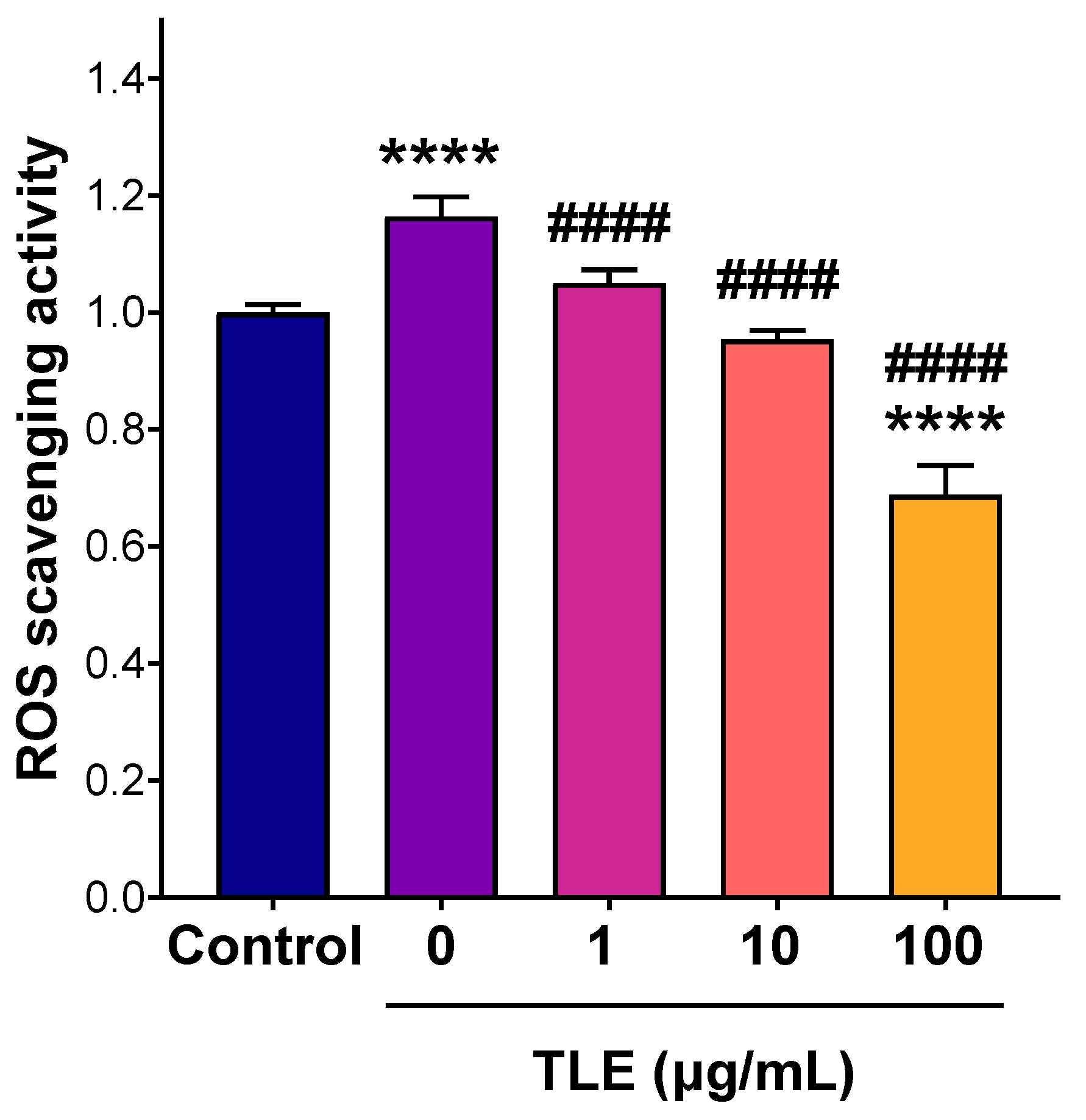
3.5. Anti-Inflammatory Activity of TLE in RAW 264.7 Macrophages
3.6. LC-MS Analysis of TLE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tiwari, J.K.; Behera, T.K.; Rai, N.; Yerasu, S.R.; Singh, M.K.; Singh, P.M. Tomato Breeding for Processing in India: Current Status and Prospects. Veg. Sci. 2022, 49, 123–132. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 7 October 2024).
- Ma, D.; Li, Y.; Zhang, J.; Wang, C.; Qin, H.; Ding, H.; Xie, Y.; Guo, T. Accumulation of Phenolic Compounds and Expression Profiles of Phenolic Acid Biosynthesis-Related Genes in Developing Grains of White, Purple, and Red Wheat. Front. Plant Sci. 2016, 7, 528. [Google Scholar] [CrossRef]
- Abu Khalaf, R.; Alhusban, A.A.; Al-Shalabi, E.; Al-Sheikh, I.; Sabbah, D.A. Isolation and Structure Elucidation of Bioactive Polyphenols. Stud. Nat. Prod. Chem. 2019, 63, 267–337. [Google Scholar] [CrossRef]
- Stiller, A.; Garrison, K.; Gurdyumov, K.; Kenner, J.; Yasmin, F.; Yates, P.; Song, B.H. From Fighting Critters to Saving Lives: Polyphenols in Plant Defense and Human Health. Int. J. Mol. Sci. 2021, 22, 8995. [Google Scholar] [CrossRef]
- Zhao, Q.; Dong, M.; Li, M.; Jin, L.; Paré, P.W. Light-Induced Flavonoid Biosynthesis in Sinopodophyllum hexandrum with High-Altitude Adaptation. Plants 2023, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- Arab, M.; Bahramian, B.; Schindeler, A.; Valtchev, P.; Dehghani, F.; McConchie, R. Extraction of Phytochemicals from Tomato Leaf Waste Using Subcritical Carbon Dioxide. Innov. Food Sci. Emerg. Technol. 2019, 57, 102204. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Ornelas-Paz, J.D.J.; López-Mata, M.A.; Del-Toro-Sánchez, C.L.; Ayala-Zavala, J.F.; Márquez-Ríos, E. Total Phenolic, Flavonoid, Tomatine, and Tomatidine Contents and Antioxidant and Antimicrobial Activities of Extracts of Tomato Plant. Int. J. Anal. Chem. 2015, 2015, 284071. [Google Scholar] [CrossRef]
- Kim, D.S.; Kwack, Y.; Lee, J.H.; Chun, C. Antimicrobial Activity of Various Parts of Tomato Plants Varied with Different Solvent Extracts. Plant Pathol. J. 2019, 35, 149. [Google Scholar] [CrossRef]
- Fujimaki, J.; Sayama, N.; Shiotani, S.; Suzuki, T.; Nonaka, M.; Uezono, Y.; Oyabu, M.; Kamei, Y.; Nukaya, H.; Wakabayashi, K.; et al. The Steroidal Alkaloid Tomatidine and Tomatidine-Rich Tomato Leaf Extract Suppress the Human Gastric Cancer-Derived 85As2 Cells In Vitro and In Vivo via Modulation of Interferon-Stimulated Genes. Nutrients 2022, 14, 1023. [Google Scholar] [CrossRef]
- Figueiredo-González, M.; Valentão, P.; Pereira, D.M.; Andrade, P.B. Further Insights on Tomato Plant: Cytotoxic and Antioxidant Activity of Leaf Extracts in Human Gastric Cells. Food Chem. Toxicol. 2017, 109, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Rajha, H.N.; Khabbaz, S.; Rached, R.A.; Debs, E.; Maroun, R.G.; Louka, N. Optimization of Polyphenols Extraction from Purple Com Cobs Using SS-Cyclodextrin as a Green Solvent. In Proceedings of the 2020 5th International Conference on Renewable Energies for Developing Countries, REDEC 2020, Marrakech, Morocco, 29–30 June 2020. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Rajha, H.N.; Abi-Khattar, A.M.; El Kantar, S.; Boussetta, N.; Lebovka, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Comparison of Aqueous Extraction Efficiency and Biological Activities of Polyphenols from Pomegranate Peels Assisted by Infrared, Ultrasound, Pulsed Electric Fields and High-Voltage Electrical Discharges. Innov. Food Sci. Emerg. Technol. 2019, 58, 102212. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts against Food Pathogens and Spoilage Microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef] [PubMed]
- Knoll, M.A.; Samardzic, E.; Posch, W.; Lass-Flörl, C. Evaluation of Inoculum Preparation for Etest and EUCAST Broth Dilution to Detect Anidulafungin Polyresistance in Candida Glabrata. Antimicrob. Agents Chemother. 2022, 66, e00168-22. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, S.J.; Microbiology, A.S. Manual of Antimicrobial Susceptibility Testing; American Society for Microbiology: Washington, DC, USA, 2005; ISBN 9781555813499. [Google Scholar]
- Asmerom, D.; Kalay, T.H.; Tafere, G.G. Antibacterial and Antifungal Activities of the Leaf Exudate of Aloe megalacantha Baker. Int. J. Microbiol. 2020, 2020, 8840857. [Google Scholar] [CrossRef]
- Muhamad, P.; Panrit, L.; Chaijaroenkul, W.; Na-Bangchang, K. Cytotoxicity, Cell Cycle Arrest, and Apoptosis Induction Activity of Ethyl-p-Methoxycinnamate in Cholangiocarcinoma Cell. Asian Pac. J. Cancer Prev. 2020, 21, 927. [Google Scholar] [CrossRef]
- Petiti, J.; Revel, L.; Divieto, C. Standard Operating Procedure to Optimize Resazurin-Based Viability Assays. Biosensors 2024, 14, 156. [Google Scholar] [CrossRef]
- Souza, G.R.; Molina, J.R.; Raphael, R.M.; Ozawa, M.G.; Stark, D.J.; Levin, C.S.; Bronk, L.F.; Ananta, J.S.; Mandelin, J.; Georgescu, M.M.; et al. Three-Dimensional Tissue Culture Based on Magnetic Cell Levitation. Nat. Nanotechnol. 2010, 5, 291–296. [Google Scholar] [CrossRef]
- Kim, M.; An, J.; Shin, S.A.; Moon, S.Y.; Kim, M.; Choi, S.; Kim, H.; Phi, K.H.; Lee, J.H.; Youn, U.J.; et al. Anti-Inflammatory Effects of TP1 in LPS-Induced Raw264.7 Macrophages. Appl. Biol. Chem. 2024, 67, 16. [Google Scholar] [CrossRef]
- Taciak, B.; Białasek, M.; Braniewska, A.; Sas, Z.; Sawicka, P.; Kiraga, Ł.; Rygiel, T.; Król, M. Evaluation of Phenotypic and Functional Stability of RAW 264.7 Cell Line through Serial Passages. PLoS ONE 2018, 13, e0198943. [Google Scholar] [CrossRef]
- Yang, R.; Sun, S.; Guo, Y.; Meng, Y.; Liu, H.; Shi, M.; Guan, S.; Xu, J. Anti-Inflammatory Effect of Dimethyl Fumarate Associates with the Inhibition of Thioredoxin Reductase 1 in RAW 264.7 Cells. Molecules 2022, 28, 107. [Google Scholar] [CrossRef]
- More, G.K.; Makola, R.T. In-Vitro Analysis of Free Radical Scavenging Activities and Suppression of LPS-Induced ROS Production in Macrophage Cells by Solanum Sisymbriifolium Extracts. Sci. Rep. 2020, 10, 6493. [Google Scholar] [CrossRef] [PubMed]
- Dunkhunthod, B.; Talabnin, C.; Murphy, M.; Thumanu, K.; Sittisart, P.; Hengpratom, T.; Eumkeb, G. Intracellular ROS Scavenging and Anti-Inflammatory Activities of Oroxylum indicum Kurz (L.) Extract in LPS plus IFN-γ-Activated RAW264.7 Macrophages. Evid.-Based Complement. Altern. Med. 2020, 2020, 7436920. [Google Scholar] [CrossRef]
- Frempong, T.F.; Boadi, N.O.; Badu, M. Optimization of Extraction Conditions for Polyphenols from the Stem Bark of Funtumia elastica (Funtum) Utilizing Response Surface Methodology. AAS Open Res. 2021, 4, 46. [Google Scholar] [CrossRef]
- Lima, R.C.; de Carvalho, A.P.A.; da Silva, B.D.; Torres Neto, L.; de Figueiredo, M.R.D.S.; Chaves, P.H.T.; de Almeida, A.E.C.C.; Conte-Junior, C.A. Green Ultrasound-Assisted Extraction of Bioactive Compounds of Babassu (Attalea speciosa) Mesocarp: Effects of Solid-Liquid Ratio Extraction, Antioxidant Capacity, and Antimicrobial Activity. Appl. Food Res. 2023, 3, 100331. [Google Scholar] [CrossRef]
- Shkeir, B.; El Darra, N.; Azakir, B.; Khazaal, S.; Sokhn, E.S.; Koubaa, M.; Maroun, R.G.; Louka, N.; Debs, E. Optimized Extraction of Polyphenols from Kiwifruit Peels and Their Biological Activities. BioTech 2024, 13, 54. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Amir Hamzah, N.; Morad, N.; Nordin, M.; Ilia Anisa, A.; Yusof, Y.M.; Azian Morad, N. Effect of Extraction Time and Temperature on the Extraction of Phenolic Compounds from Orthosiphon stamineus Leaves. Aust. J. Basic Appl. Sci. 2017, 11, 54100. [Google Scholar]
- Rajha, H.N.; Louka, N.; El Darra, N.; Hobaika, Z.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Centre d’Analyses et de Recherche; UR TVA; Faculte des Sciences; et al. Multiple Response Optimization of High Temperature, Low Time Aqueous Extraction Process of Phenolic Compounds from Grape Byproducts. Food Nutr. Sci. 2014, 5, 351–360. [Google Scholar] [CrossRef]
- Okur, I.; Namlı, S.; Oztop, M.H.; Alpas, H. High-Pressure-Assisted Extraction of Phenolic Compounds from Olive Leaves: Optimization and Comparison with Conventional Extraction. ACS Food Sci. Technol. 2023, 3, 161–169. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Hirun, S.; Roach, P.D.; Bowyer, M.C.; Phillips, P.A.; Scarlett, C.J. Effect of Extraction Conditions on Total Phenolic Compounds and Antioxidant Activities of Carica Papaya Leaf Aqueous Extracts. J. Herb. Med. 2013, 3, 104–111. [Google Scholar] [CrossRef]
- Mendoza, Y.; Arias-Londoño, M.; Sánchez-Garzón, J.; Rojas-Vahos, D.F.; Robledo-Sierra, J. Antioxidant and Inhibitory Capacity of Tomato Leaf Ethanolic Extract against Streptococcus mutans, Porphyromonas gingivalis, and Candida albicans. Vitae 2022, 29, 1–9. [Google Scholar] [CrossRef]
- Gutiérrez-Venegas, G.; Gómez-Mora, J.A.; Meraz-Rodríguez, M.A.; Flores-Sánchez, M.A.; Ortiz-Miranda, L.F. Effect of Flavonoids on Antimicrobial Activity of Microorganisms Present in Dental Plaque. Heliyon 2019, 5, e03013. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Khan, F.; Qari, H.A.; Oves, M. Rutin (Bioflavonoid) as Cell Signaling Pathway Modulator: Prospects in Treatment and Chemoprevention. Pharmaceuticals 2021, 14, 1069. [Google Scholar] [CrossRef] [PubMed]
- Sucha, L.; Hroch, M.; Rezacova, M.; Rudolf, E.; Havelek, R.; Sispera, L.; Cmielova, J.; Kohlerova, R.; Bezrouk, A.; Tomsik, P. The Cytotoxic Effect of α-Tomatine in MCF-7 Human Adenocarcinoma Breast Cancer Cells Depends on Its Interaction with Cholesterol in Incubation Media and Does Not Involve Apoptosis Induction. Oncol. Rep. 2013, 30, 2593. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.Q.C.; Binh, T.D.; Pham, T.L.A.; Nguyen, Y.D.H.; Trang, D.T.X.; Nguyen, T.T.; Kanaori, K.; Kamei, K. Anti-Inflammatory Effects of Lasia spinosa Leaf Extract in Lipopolysaccharide-Induced RAW 264.7 Macrophages. Int. J. Mol. Sci. 2020, 21, 3439. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhou, B.; Bao, L.; Yang, Y.; Guo, K. Alpha-Tomatine Exhibits Anti-Inflammatory Activity in Lipopolysaccharide-Activated Macrophages. Inflammation 2015, 38, 1769–1776. [Google Scholar] [CrossRef]
- Semwal, R.; Joshi, S.K.; Semwal, R.B.; Semwal, D.K. Health Benefits and Limitations of Rutin—A Natural Flavonoid with High Nutraceutical Value. Phytochem. Lett. 2021, 46, 119–128. [Google Scholar] [CrossRef]
- Gȩgotek, A.; Rybałtowska-Kawałko, P.; Skrzydlewska, E. Rutin as a Mediator of Lipid Metabolism and Cellular Signaling Pathways Interactions in Fibroblasts Altered by UVA and UVB Radiation. Oxid. Med. Cell. Longev. 2017, 2017, 4721352. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, X.; Yue, C. Rutin Ameliorates Inflammation and Oxidative Stress in Ulcerative Colitis by Inhibiting NLRP3 Inflammasome Signaling Pathway. Cell Biochem. Biophys. 2024, 82, 3715–3726. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Li, Y.; Zhao, C.; Yang, Y.; Xiong, C.; Zhang, D.; Feng, S.; Wu, J.; Wang, X. Rutin Supplementation Reduces Oxidative Stress, Inflammation and Apoptosis of Mammary Gland in Sheep During the Transition Period. Front. Vet. Sci. 2022, 9, 907299. [Google Scholar] [CrossRef] [PubMed]


| Run | Temperature (T) °C | Time (t) Min | TPC (mg GAE/g DM) | DPPH (mg TE/g DM) | DPPH AA (mg AAE/g DM) | |
|---|---|---|---|---|---|---|
| Factorial Points | 1 2 3 4 | 40 (−1) 80 (+1) 40 (−1) 80 (+1) | 15 (−1) 15 (−1) 45 (+1) 45 (+1) | 11.03 ± 0.15 23.58 ± 0.39 12.77 ± 0.18 21.39 ± 0.77 | 7.74 ± 0.05 11.00 ± 0.09 7.96 ± 0.16 10.81 ± 0.10 | 6.92 ± 0.04 10.09 ± 0.08 7.14 ± 0.15 9.88 ± 0.09 |
| Star Points | 5 6 7 8 | 32 (–α) 88 (+α) 60 (0) 60 (0) | 30 (0) 30 (0) 8.8 (–α) 51.2 (+α) | 13.57 ± 0.41 30.16 ± 0.39 15.04 ± 0.15 15.40 ± 0.29 | 8.57 ± 0.07 10.98 ± 0.10 10.61 ± 0.02 10.10 ± 0.11 | 7.72 ± 0.07 10.04 ± 0.10 9.68 ± 0.02 9.19 ± 0.11 |
| Center Points | 9 10 11 12 | 60 (0) 60 (0) 60 (0) 60 (0) | 30 (0) 30 (0) 30 (0) 30 (0) | 27.50 ± 0.35 26.62 ± 0.27 28.68 ± 0.79 26.40 ± 0.38 | 10.76 ± 0.04 10.30 ± 0.06 10.65 ± 0.11 10.86 ± 0.04 | 9.82 ± 0.03 9.39 ± 0.6 9.72 ± 0.11 9.92 ± 0.04 |
| (1) TPC = −48.4 + 1.29 × T + 1.9 × t − 0.007 × T2 − 0.003 × T × t − 0.028 × t2 (2) TPC = −42.45 + 1.19 × T + 1.7 × t − 0.007 × T2 − 0.028 × t2 |
| (3) DPPH = −0.082 + 0.25× T + 0.098 × t − 0.0015 × T2 − 0.0003 × T × t − 0.0014× t2 (4) DPPH = 2.11 + 0.22 × T − 0.0057 × t − 0.0013 × T2 |
| Parameters | TPC | DPPH | |
|---|---|---|---|
| Optimized operating conditions | Time (min) | 29 | 29 |
| Temperature (°C) | 78.5 | 78.5 | |
| Model-predicted optimal TPC and DPPH under optimized parameters | TPC optimal value (mg GAE/g DM) | 30 | - |
| DPPH optimal value (mg TE/g DM) | - | 11 | |
| Validation of model optimum under optimized conditions | TPC observed value (mg GAE/g DM) | 27.7 | - |
| DPPH observed value (mg TE/mL) | 10.22 | ||
| Model’s R-squared (%) | 96 | 87 |
| Microbial Strain | TLE Concentration (mg/mL) | Zone of Inhibition (mm ± SD) |
|---|---|---|
| E. coli | 40 | 17.6 ± 1.5 |
| 20 | 15.2 ± 0.6 | |
| 10 | 14.3 ± 0.3 | |
| 5 | 10.7 ± 0.3 | |
| 2.5 | 8.6 ± 0.5 | |
| 1.25 | 0 | |
| Gentamicin (10 µg) | 22.4 ± 0.4 | |
| C. albicans | 40 | 21.4 ± 0.4 |
| 20 | 17.7 ± 1.7 | |
| 10 | 12.0 ± 2.0 | |
| 5 | 9.6 ± 0.1 | |
| 2.5 | 0 | |
| 1.25 | 0 | |
| Fluconazole (25 µg) | 30.4 ± 0.3 |
| Compound | Retention Time (min) | m/z | Measured Molar Mass | Percentage (%) a |
|---|---|---|---|---|
| Rutin | 12.8 | 611.16 | 610.15 | 45.28 |
| 4-Hydroxycoumarin | 4.47 | 163.039 | 162.03 | 13.62 |
| α-tomatine | 19.47 | 1034.54 | 1033.54 | 12.40 |
| Dehydrotomatine | 18.88 | 1032.52 | 1031.53 | 9 |
| Quercetin | 12.8 | 303.05 | 302.04 | 6.74 |
| Chlorogenic acid | 4.48 | 355.10 | 354.09 | 5.66 |
| 4-Methylumbelliferone | 6.08 | 177.0 | 176.04 | 4.78 |
| Aesculetin | 6.36 | 179.03 | 178.02 | 1.90 |
| Capsaicin | 12.2 | 306.20 | 305.19 | 0.20 |
| Pinolenic acid | 27.9 | 281.24 | 280.23 | 0.15 |
| (Z)-3-Hydroxyoctadec-7-enoic acid | 26.85 | 299.25 | 298.24 | 0.14 |
| Artocaprin | 26.06 | 437.19 | 436.18 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helmi, L.; Sunoqrot, S.; Abusulieh, S.; Huwaitat, R.; Debs, E.; Khazaal, S.; El-Dakdouki, M.H.; Louka, N.; El Darra, N. Valorization of Tomato Leaves: Optimization of Eco-Friendly Phenolic Extraction and Assessment of Biological Activities. Foods 2025, 14, 3383. https://doi.org/10.3390/foods14193383
Helmi L, Sunoqrot S, Abusulieh S, Huwaitat R, Debs E, Khazaal S, El-Dakdouki MH, Louka N, El Darra N. Valorization of Tomato Leaves: Optimization of Eco-Friendly Phenolic Extraction and Assessment of Biological Activities. Foods. 2025; 14(19):3383. https://doi.org/10.3390/foods14193383
Chicago/Turabian StyleHelmi, Layan, Suhair Sunoqrot, Samah Abusulieh, Rawan Huwaitat, Espérance Debs, Salma Khazaal, Mohammad H. El-Dakdouki, Nicolas Louka, and Nada El Darra. 2025. "Valorization of Tomato Leaves: Optimization of Eco-Friendly Phenolic Extraction and Assessment of Biological Activities" Foods 14, no. 19: 3383. https://doi.org/10.3390/foods14193383
APA StyleHelmi, L., Sunoqrot, S., Abusulieh, S., Huwaitat, R., Debs, E., Khazaal, S., El-Dakdouki, M. H., Louka, N., & El Darra, N. (2025). Valorization of Tomato Leaves: Optimization of Eco-Friendly Phenolic Extraction and Assessment of Biological Activities. Foods, 14(19), 3383. https://doi.org/10.3390/foods14193383









