Poultry Food Assess Risk Model for Salmonella and Chicken Eggs in Riyadh, Saudi Arabia
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection Methods
2.2. Modeling Methods
3. Results and Discussion
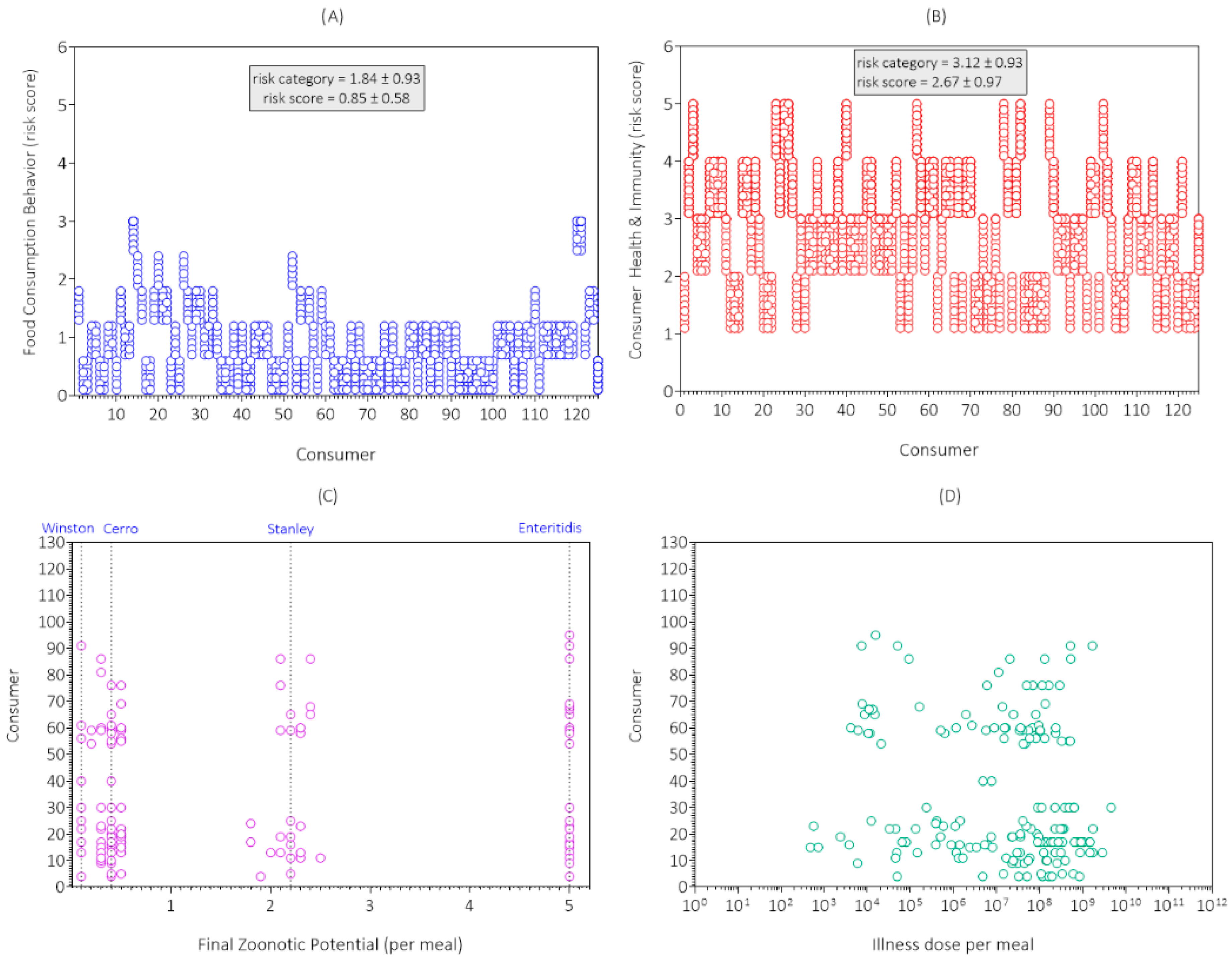
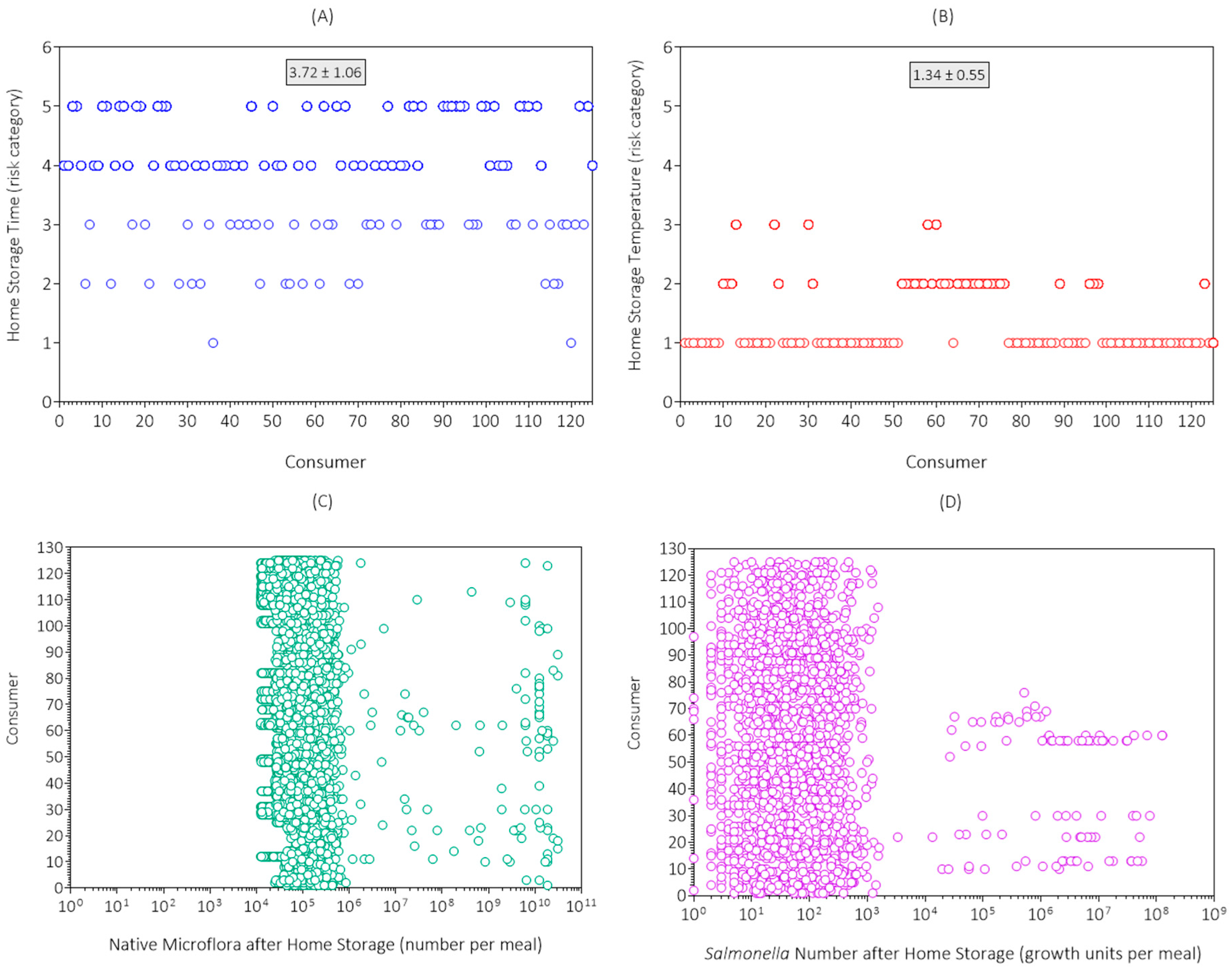
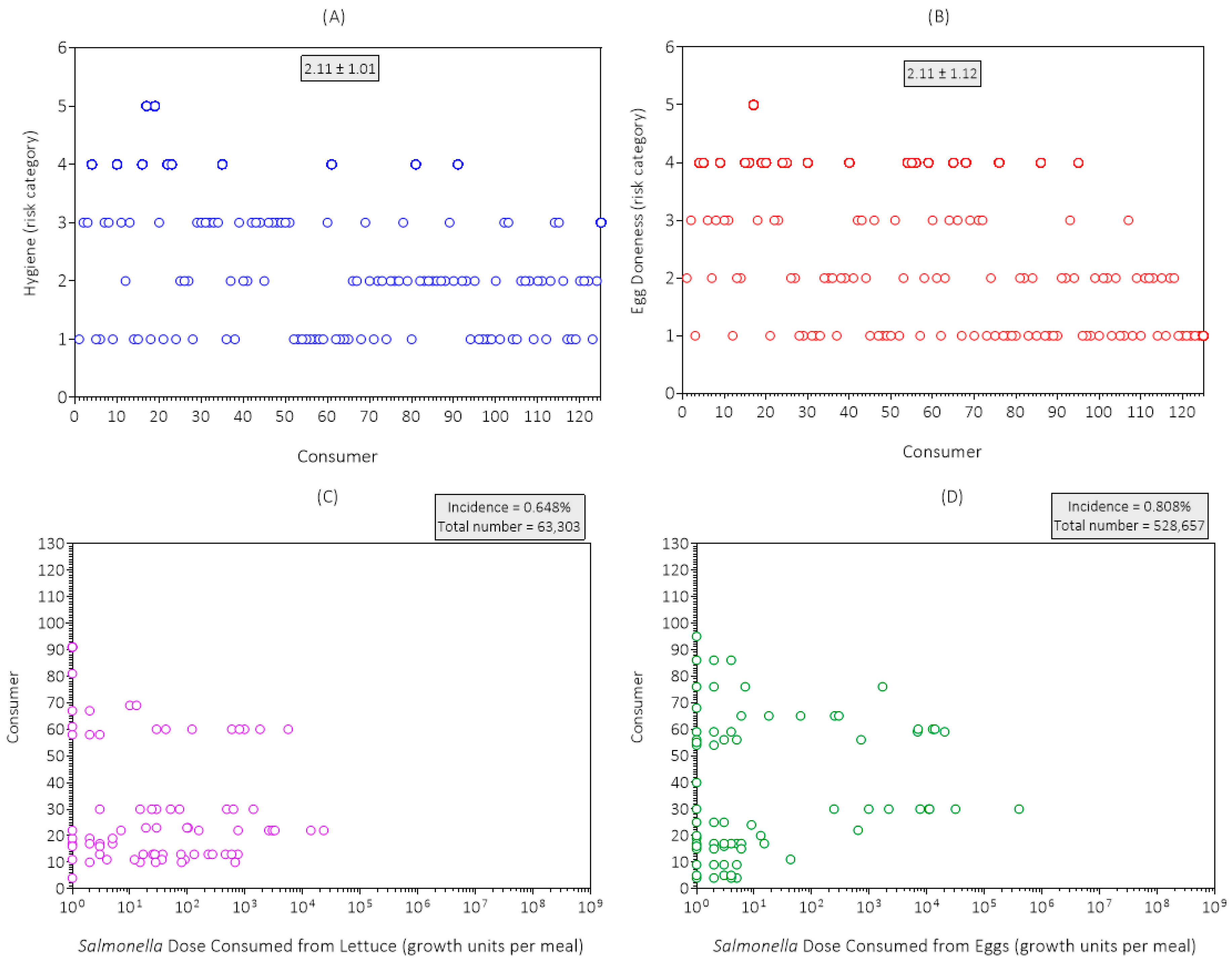
3.1. Data Collection Results and Discussion
3.2. Simulation Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardoso, M.J.; Nicolau, A.I.; Borda, D.; Nielsen, L.; Maia, R.L.; Møretrø, T.; Ferreira, V.; Knøchel, S.; Langsrud, S.; Teixeira, P. Salmonella in Eggs: From Shopping to Consumption—A Review Providing an Evidence-Based Analysis of Risk Factors. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2716–2741. [Google Scholar] [CrossRef]
- Popa, G.L.; Papa, M.I. Salmonella spp. Infection—A Continuous Threat Worldwide. Germs 2021, 11, 88–96. [Google Scholar] [CrossRef]
- Mohamed, M.-Y.I.; Habib, I.; Khalifa, H.O. Salmonella in the Food Chain within the Gulf Cooperation Council Countries. AIMS Microbiol. 2024, 10, 468–488. [Google Scholar] [CrossRef] [PubMed]
- Saudi Arabia: Poultry and Products Annual|USDA Foreign Agricultural Service. Available online: https://www.fas.usda.gov/data/saudi-arabia-poultry-and-products-annual-5 (accessed on 10 September 2025).
- Alharbi, N.A.; Alsaeed, T.S.; Aljohany, A.S.; Alwehaibi, K.K.; Almasaad, M.A.; Alotaibi, R.M.; Alotaibi, B.J.; Alamoudi, E.A. Extra-Intestinal Salmonellosis in a Tertiary Care Center in Saudi Arabia. Sudan. J. Paediatr. 2021, 21, 152–161. [Google Scholar] [CrossRef] [PubMed]
- WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance. Available online: https://www.who.int/publications/i/item/9789240093461 (accessed on 10 September 2025).
- Qureshi, S.; Naveed, A.B.; Yousafzai, M.T.; Ahmad, K.; Ansari, S.; Lohana, H.; Mukhtar, A.; Qamar, F.N. Response of Extensively Drug Resistant Salmonella Typhi to Treatment with Meropenem and Azithromycin, in Pakistan. PLoS Negl. Trop. Dis. 2020, 14, e0008682. [Google Scholar] [CrossRef] [PubMed]
- Latimer, H.K.; Marks, H.M.; Coleman, M.E.; Schlosser, W.D.; Golden, N.J.; Ebel, E.D.; Kause, J.; Schroeder, C.M. Evaluating the Effectiveness of Pasteurization for Reducing Human Illnesses from Salmonella spp. in Egg Products: Results of a Quantitative Risk Assessment. Foodborne Pathog. Dis. 2008, 5, 59–68. [Google Scholar] [CrossRef]
- Pouillot, R.; Schlosser, W.; Van Doren, J.M.; Dennis, S.B.; Kause, J.R. Assessment of the Risk of Salmonellosis Linked to the Consumption of Liquid Egg Products Made from Internally Contaminated Shell Eggs Initially Stored at 65 °F (18 °C) Compared with Eggs Stored at 45 °F (7 °C). J. Food Prot. 2020, 83, 767–778. [Google Scholar] [CrossRef]
- Schroeder, C.M.; Latimer, H.K.; Schlosser, W.D.; Golden, N.J.; Marks, H.M.; Coleman, M.E.; Hogue, A.T.; Ebel, E.D.; Quiring, N.M.; Kadry, A.-R.M.; et al. Overview and Summary of the Food Safety and Inspection Service Risk Assessment for Salmonella Enteritidis in Shell Eggs, October 2005. Foodborne Pathog. Dis. 2006, 3, 403–412. [Google Scholar] [CrossRef]
- Oscar, T.P. Course Corrections Are Needed to Better Address the Risk of Salmonellosis from Poultry Food. In Salmonella-Molecular Biology, Pathogenesis, and Public Health Impact; IntechOpen: London, UK, 2025; ISBN 978-1-83635-628-8. [Google Scholar]
- Oscar, T.P. Poultry Food Assess Risk Model for Salmonella and Chicken Gizzards: III. Dose Consumed Step. J. Food Prot. 2024, 87, 100242. [Google Scholar] [CrossRef]
- Kim, M.; Barnett-Neefs, C.; Chavez, R.A.; Kealey, E.; Wiedmann, M.; Stasiewicz, M.J. Risk Assessment Predicts Most of the Salmonellosis Risk in Raw Chicken Parts Is Concentrated in Those Few Products with High Levels of High-Virulence Serotypes of Salmonella. J. Food Prot. 2024, 87, 100304. [Google Scholar] [CrossRef]
- Oscar, T.P. Poultry Food Assess Risk Model for Salmonella and Chicken Gizzards: I. Initial Contamination. J. Food Prot. 2023, 86, 100036. [Google Scholar] [CrossRef]
- Oscar, T.P. Poultry Food Assess Risk Model for Salmonella and Chicken Gizzards: II. Illness Dose Step. J. Food Prot. 2023, 86, 100091. [Google Scholar] [CrossRef] [PubMed]
- Oscar, T.P. Poultry Food Assess Risk Model for Salmonella and Chicken Gizzards: IV. Consumer Response Step. J. Food Saf. 2025, 45, e70026. [Google Scholar] [CrossRef]
- Oscar, T.P. Risk of Salmonellosis from Chicken Parts Prepared from Whole Chickens Sold in Flow Pack Wrappers and Subjected to Temperature Abuse. J. Food Prot. 2017, 80, 1496–1505. [Google Scholar] [CrossRef]
- Oscar, T. Salmonella Prevalence Alone Is Not a Good Indicator of Poultry Food Safety. Risk Anal. 2021, 41, 110–130. [Google Scholar] [CrossRef]
- Bonardi, S.; Morganti, M.; Pupillo, G.; Brindani, F. Salmonella Brandenburg in the Pork Chain in Italy: Genetic Comparison with the Human Isolates. Ital. J. Food Saf. 2018, 7, 21–23. [Google Scholar] [CrossRef]
- Tsuchida, S.; Umemura, H.; Nakayama, T. Current Status of Matrix-Assisted Laser Desorption/Ionization–Time-of-Flight Mass Spectrometry (MALDI-TOF MS) in Clinical Diagnostic Microbiology. Molecules 2020, 25, 4775. [Google Scholar] [CrossRef] [PubMed]
- Guibourdenche, M.; Roggentin, P.; Mikoleit, M.; Fields, P.I.; Bockemühl, J.; Grimont, P.A.D.; Weill, F.-X. Supplement 2003–2007 (No. 47) to the White-Kauffmann-Le Minor Scheme. Res. Microbiol. 2010, 161, 26–29. [Google Scholar] [CrossRef]
- Martínez-Chávez, L.; Cabrera-Diaz, E.; Pérez-Montaño, J.A.; Garay-Martínez, L.E.; Varela-Hernández, J.J.; Castillo, A.; Lucia, L.; Ávila-Novoa, M.G.; Cardona-López, M.A.; Gutiérrez-González, P.; et al. Quantitative Distribution of Salmonella spp. and Escherichia coli on Beef Carcasses and Raw Beef at Retail Establishments. Int. J. Food Microbiol. 2015, 210, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Bai, Y.; Wang, Y.; Song, X.; Cui, S.; Xu, H.; Jiao, X.; Li, F. A Risk Assessment of Salmonellosis Linked to Chicken Meals Prepared in Households of China. Food Control 2017, 79, 279–287. [Google Scholar] [CrossRef]
- Latimer, H.K.; Jaykus, L.-A.; Morales, R.A.; Cowen, P.; Crawford-Brown, D. Sensitivity Analysis of Salmonella Enteritidis Levels in Contaminated Shell Eggs Using a Biphasic Growth Model. Int. J. Food Microbiol. 2002, 75, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.P. Spoilage of Shell Eggs by Pseudomonads. Appl. Microbiol. 1954, 2, 158–164. [Google Scholar] [CrossRef]
- Jameson, J.E. A Discussion of the Dynamics of Salmonella Enrichment. J. Hyg. 1962, 60, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Ohkochi, M.; Koseki, S.; Kunou, M.; Sugiura, K.; Tsubone, H. Growth Modeling of Listeria monocytogenes in Pasteurized Liquid Egg. J. Food Prot. 2013, 76, 1549–1556. [Google Scholar] [CrossRef]
- Singh, A.; Korasapati, N.R.; Juneja, V.K.; Subbiah, J.; Froning, G.; Thippareddi, H. Dynamic Predictive Model for the Growth of Salmonella spp. in Liquid Whole Egg. J. Food Sci. 2011, 76, M225–M232. [Google Scholar] [CrossRef]
- Carson, M.O.; Lillard, H.S.; Hamdy, M.K. Transfer of Firmly Attached 32P-Salmonella Typhimurium from Raw Poultry Skin to Other Surfaces. J. Food Prot. 1987, 50, 327–329. [Google Scholar] [CrossRef]
- Chen, Y.; Jackson, K.M.; Chea, F.P.; Schaffner, D.W. Quantification and Variability Analysis of Bacterial Cross-Contamination Rates in Common Food Service Tasks. J. Food Prot. 2001, 64, 72–80. [Google Scholar] [CrossRef]
- Montville, R.; Chen, Y.; Schaffner, D.W. Glove Barriers to Bacterial Cross-Contamination between Hands to Food. J. Food Prot. 2001, 64, 845–849. [Google Scholar] [CrossRef]
- Regmi, P.; Jones, D.R.; Gast, R.K.; Guard, J.Y.; Karcher, D.M. Egg Carton and Eggshell: Is There a Possibility of Salmonella Cross-Contamination? J. Appl. Poult. Res. 2021, 30, 100185. [Google Scholar] [CrossRef]
- Oscar, T.P. Predictive Model for Growth of Salmonella Newport on Romaine Lettuce. J. Food Saf. 2020, 40, e12786. [Google Scholar] [CrossRef]
- Bemrah, N.; Bergis, H.; Colmin, C.; Beaufort, A.; Millemann, Y.; Dufour, B.; Benet, J.J.; Cerf, O.; Sanaa, M. Quantitative Risk Assessment of Human Salmonellosis from the Consumption of a Turkey Product in Collective Catering Establishments. Int. J. Food Microbiol. 2003, 80, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Porto-Fett, A.C.S.; Shoyer, B.A.; Henry, E.; Shane, L.E.; Osoria, M.; Luchansky, J.B. Prevalence, Levels, and Viability of Salmonella in and on Raw Chicken Livers. J. Food Prot. 2019, 82, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Jakočiūnė, D.; Pasquali, F.; da Silva, C.S.; Löfström, C.; Hoorfar, J.; Klein, G.; Manfreda, G.; Olsen, J.E. Enumeration of Salmonellae in Table Eggs, Pasteurized Egg Products, and Egg-Containing Dishes by Using Quantitative Real-Time PCR. Appl. Environ. Microbiol. 2014, 80, 1616–1622. [Google Scholar] [CrossRef]
- Malorny, B.; Löfström, C.; Wagner, M.; Krämer, N.; Hoorfar, J. Enumeration of Salmonella Bacteria in Food and Feed Samples by Real-Time PCR for Quantitative Microbial Risk Assessment. Appl. Environ. Microbiol. 2008, 74, 1299–1304. [Google Scholar] [CrossRef]
- Krämer, N.; Löfström, C.; Vigre, H.; Hoorfar, J.; Bunge, C.; Malorny, B. A Novel Strategy to Obtain Quantitative Data for Modelling: Combined Enrichment and Real-Time PCR for Enumeration of Salmonellae from Pig Carcasses. Int. J. Food Microbiol. 2011, 145, S86–S95. [Google Scholar] [CrossRef]
- Oscar, T.P. Monte Carlo Simulation Model for Predicting Salmonella Contamination of Chicken Liver as a Function of Serving Size for Use in Quantitative Microbial Risk Assessment. J. Food Prot. 2021, 84, 1824–1835. [Google Scholar] [CrossRef]
- Archer, E.W.; Chisnall, T.; Tano-Debrah, K.; Card, R.M.; Duodu, S.; Kunadu, A.P.-H. Prevalence and Genomic Characterization of Salmonella Isolates from Commercial Chicken Eggs Retailed in Traditional Markets in Ghana. Front. Microbiol. 2023, 14, 1283835. [Google Scholar] [CrossRef]
- Tessema, K.; Bedu, H.; Ejo, M.; Hiko, A. Prevalence and Antibiotic Resistance of Salmonella Species Isolated from Chicken Eggs by Standard Bacteriological Method. J. Vet. Sci. Technol. 2017, 8, 2. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Zhang, H.; Jia, H.; Liu, X.; Yu, B.; Zeng, Y.; Zhang, Y.; Pei, X.; Yang, D. Prevalence and Antimicrobial Susceptibility of Salmonella in the Commercial Eggs in China. Int. J. Food Microbiol. 2020, 325, 108623. [Google Scholar] [CrossRef]
- Denagamage, T.; Jayarao, B.; Patterson, P.; Wallner-Pendleton, E.; Kariyawasam, S. Risk Factors Associated with Salmonella in Laying Hen Farms: Systematic Review of Observational Studies. Avian Dis. 2015, 59, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Birk, T.; Kristensen, K.; Harboe, A.; Hansen, T.B.; Ingmer, H.; De Jonge, R.; Takumi, K.; Aabo, S. Dietary Proteins Extend the Survival of Salmonella Dublin in a Gastric Acid Environment. J. Food Prot. 2012, 75, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Mennah-Govela, Y.A.; Bornhorst, G.M. Food Buffering Capacity: Quantification Methods and Its Importance in Digestion and Health. Food Funct. 2021, 12, 543–563. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L. The Role of Gastric Acid in Preventing Foodborne Disease and How Bacteria Overcome Acid Conditions. J. Food Prot. 2003, 66, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, C.A.; Martin, L.B.; Micoli, F. Vaccines against Invasive Salmonella Disease. Hum. Vaccines Immunother. 2014, 10, 1478–1493. [Google Scholar] [CrossRef]
- Demirtas, E.D.; Barnard, R.; Lee, J.; Jit, M. A Systematised Review and Evidence Synthesis on the Broader Societal Impact of Vaccines against Salmonella. NPJ Vaccines 2025, 10, 21. [Google Scholar] [CrossRef]
- McCullough, N.B.; Eisele, C.W. Experimental Human Salmonellosis. II. Immunity Studies Following Experimental Illness with Salmonella Meleagridis and Salmonella Anatum. J. Immunol. Baltim. Md 1951, 66, 595–608. [Google Scholar]
- Hoffmann, S.; Scallan Walter, E. Acute Complications and Sequelae from Foodborne Infections: Informing Priorities for Cost of Foodborne Illness Estimates. Foodborne Pathog. Dis. 2020, 17, 172–177. [Google Scholar] [CrossRef]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M. The Global Burden of Nontyphoidal Salmonella Gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef]





| Salmonella | |||||
|---|---|---|---|---|---|
| Date | log10 Growth Units/Egg | ||||
| (dd.mm.yyyy) | CT a | Lower | X | Upper | Serotype |
| 08.05.2023 | 25 | 2.009 | 2.299 | 2.571 | Cerro |
| 15.05.2023 | ND | 0 b | Stanley | ||
| 15.05.2023 | 35 | 0.000 | 0.061 | 0.369 | Cerro |
| 22.05.2023 | ND | 0 b | Cerro | ||
| 22.05.2023 | 33 | 0.000 | 0.431 | 0.723 | Cerro |
| 22.05.2023 | 26 | 1.753 | 2.047 | 2.325 | Enteritidis |
| 22.05.2023 | 22 | 2.808 | 3.077 | 3.338 | Winston |
| Consumer Survey | Risk Category | ||||||
|---|---|---|---|---|---|---|---|
| Code | Item | 1 | 2 | 3 | 4 | 5 | Mean |
| S1 | Home storage time a | 2 | 16 | 33 | 38 | 36 | 3.7 |
| S2 | Home storage temperature b | 88 | 32 | 5 | 0 | 0 | 1.3 |
| S3 | Hygiene | 42 | 40 | 32 | 9 | 2 | 2.1 |
| S4 | Meal preparation time c | 115 | 10 | 0 | 0 | 0 | 1.1 |
| S5 | Kitchen temperature d | 45 | 47 | 29 | 3 | 1 | 1.9 |
| S6 | Fried egg doneness e | 49 | 35 | 20 | 20 | 1 | 2.1 |
| S7 | Portion size f | 28 | 47 | 32 | 10 | 8 | 2.4 |
| S8 | Food consumption behavior | 53 | 49 | 16 | 4 | 3 | 1.8 |
| S9 | Consumer health and immunity | 0 | 38 | 44 | 33 | 10 | 3.1 |
| Seed | No Exposure | No Response | Infection | Illness | Hospital | Death |
|---|---|---|---|---|---|---|
| 1 | 12,337 | 147 | 15 | 1 | 0 | 0 |
| 8 | 12,358 | 136 | 6 | 0 | 0 | 0 |
| 11 | 12,355 | 136 | 9 | 0 | 0 | 0 |
| 28 | 12,327 | 158 | 14 | 1 | 0 | 0 |
| 32 | 12,354 | 133 | 13 | 0 | 0 | 0 |
| 46 | 12,320 | 163 | 17 | 0 | 0 | 0 |
| 52 | 12,334 | 159 | 7 | 0 | 0 | 0 |
| 69 | 12,345 | 148 | 7 | 0 | 0 | 0 |
| total | 98,730 | 1180 | 88 | 2 | 0 | 0 |
| Consumer 59 | Consumer 22 | |||
|---|---|---|---|---|
| Yolk Membrane Integrity Lost | Retail | fraction | 0.61 | 0.40 |
| Salmonella | Retail | per egg meal | 132 | 257 |
| Salmonella | Home Storage | per egg meal | 1,301,715 | 179,073,773 |
| Salmonella | Kitchen Fomites | per egg meal | 650 | 931,183 |
| Salmonella | Lettuce | per egg meal | 0 | 100,940 |
| Salmonella | Abused Lettuce | per egg meal | 0 | 100,940 |
| Salmonella | Egg(s) | per egg meal | 6896 | 0 |
| Native Microflora | Retail | per egg meal | 77,253 | 234,699 |
| Native Microflora | After Home Storage | per egg meal | 18,600,000,000 | 18,600,000,000 |
| Native Microflora | Kitchen Fomites | per egg meal | 2,388,527 | 96,226,770 |
| Native Microflora | Lettuce | per egg meal | 1738 | 9,605,058 |
| Native Microflora | Consumption | per egg meal | 89,055,082 | 279,895 |
| Consumer Survey | Home Storage Time | Risk Category | 4 | 4 |
| Consumer Survey | Home Storage Temperature | Risk Category | 2 | 3 |
| Consumer Survey | Hygiene | Risk Category | 1 | 4 |
| Consumer Survey | Meal Preparation Time | Risk Category | 2 | 1 |
| Consumer Survey | Kitchen Temperature | Risk Category | 1 | 3 |
| Consumer Survey | Egg Doneness | Risk Category | 4 | 3 |
| Consumer Survey | Portion Size | Risk Category | 3 | 3 |
| Consumer Survey | Food Consumption Behavior | Risk Category | 3 | 3 |
| Consumer Survey | Consumer Health and Immunity | Risk Category | 3 | 2 |
| Consumption | Food Consumption Behavior | Score | 1.8 | 1.8 |
| Consumption | Consumer Health and Immunity | Score | 2.6 | 1.2 |
| Consumption | Zoonotic Potential | Score | 5.0 | 5.0 |
| Consumption | Disease Triangle Score | Score | 9.4 | 7.9 |
| Consumption | Dose Consumed | per egg meal | 6896 | 100,940 |
| Consumption | Illness Dose | per egg meal | 6109 | 84,883 |
| Consumption | Consumer response | illness | 1.13 | 1.19 |
| Consumption | Consumer Response | per egg meal | 1.13 | 1.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsufyani, A.T.; Alotaibi, N.M.; Alreshoodi, F.M.; Mukhtar, L.E.; Althubaiti, A.; Almusa, M.; Althubyani, M.; Bin Jaddua, R.; Alsulaiman, B.; Alsaleh, S.; et al. Poultry Food Assess Risk Model for Salmonella and Chicken Eggs in Riyadh, Saudi Arabia. Foods 2025, 14, 3382. https://doi.org/10.3390/foods14193382
Alsufyani AT, Alotaibi NM, Alreshoodi FM, Mukhtar LE, Althubaiti A, Almusa M, Althubyani M, Bin Jaddua R, Alsulaiman B, Alsaleh S, et al. Poultry Food Assess Risk Model for Salmonella and Chicken Eggs in Riyadh, Saudi Arabia. Foods. 2025; 14(19):3382. https://doi.org/10.3390/foods14193382
Chicago/Turabian StyleAlsufyani, Amani T., Norah M. Alotaibi, Fahad M. Alreshoodi, Lenah E. Mukhtar, Afnan Althubaiti, Manal Almusa, Maha Althubyani, Rashed Bin Jaddua, Bassam Alsulaiman, Sarah Alsaleh, and et al. 2025. "Poultry Food Assess Risk Model for Salmonella and Chicken Eggs in Riyadh, Saudi Arabia" Foods 14, no. 19: 3382. https://doi.org/10.3390/foods14193382
APA StyleAlsufyani, A. T., Alotaibi, N. M., Alreshoodi, F. M., Mukhtar, L. E., Althubaiti, A., Almusa, M., Althubyani, M., Bin Jaddua, R., Alsulaiman, B., Alsaleh, S., Alakeel, S. I., Oscar, T. P., & Alajel, S. M. (2025). Poultry Food Assess Risk Model for Salmonella and Chicken Eggs in Riyadh, Saudi Arabia. Foods, 14(19), 3382. https://doi.org/10.3390/foods14193382






