Volatile Fingerprinting and Regional Differentiation of Safflower (Carthamus tinctorius L.) Using GC–IMS Combined with OPLS-DA
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Information
2.2. Instruments and Reagents
2.2.1. Reagents
2.2.2. Instruments
2.3. Analysis of Volatile Organic Compounds by GC–IMS
2.3.1. Sample Preparation and Injection Conditions
2.3.2. GC Conditions
2.3.3. IMS Conditions
2.4. Calculation of Relative Odor Activity Values (ROAVs)
2.5. Data Processing and Statistical Analysis
3. Results and Discussion
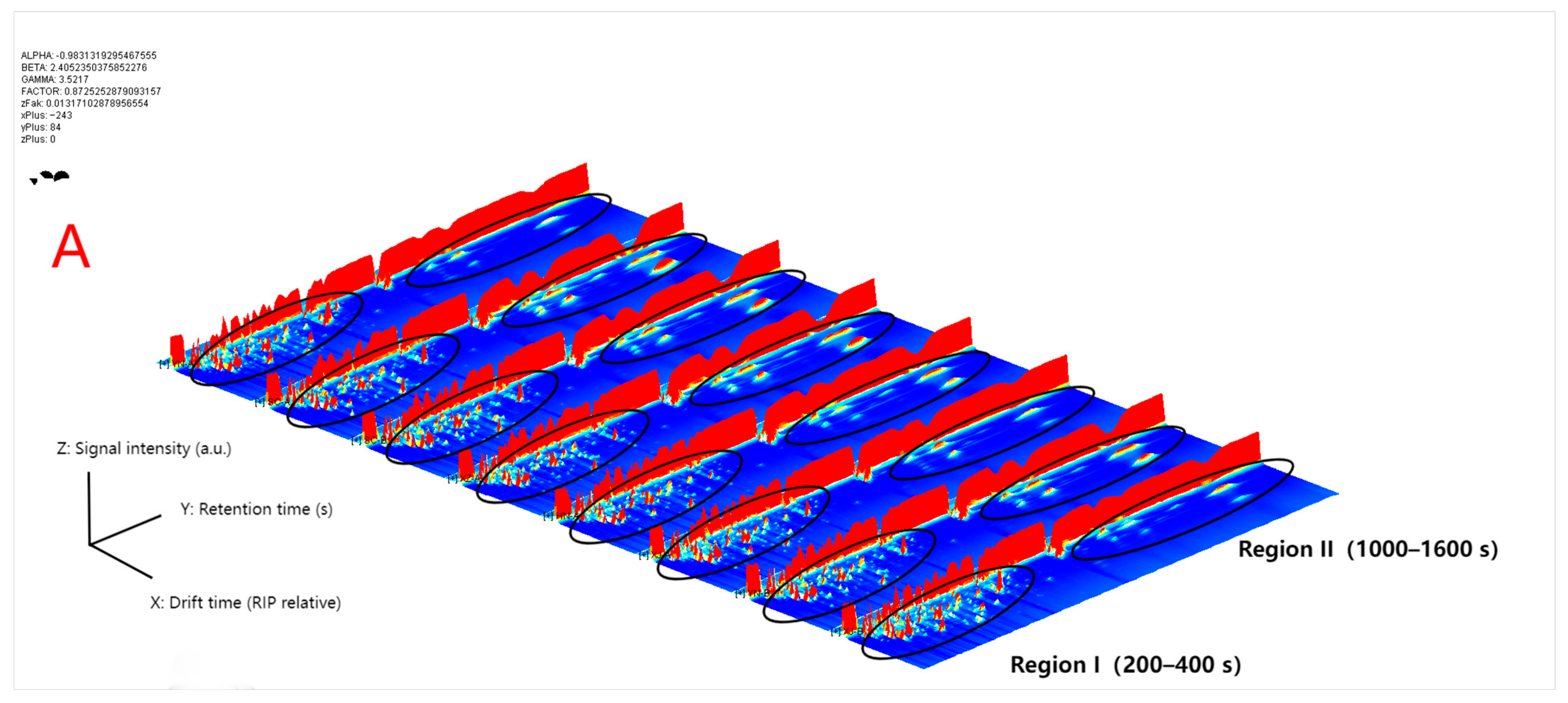
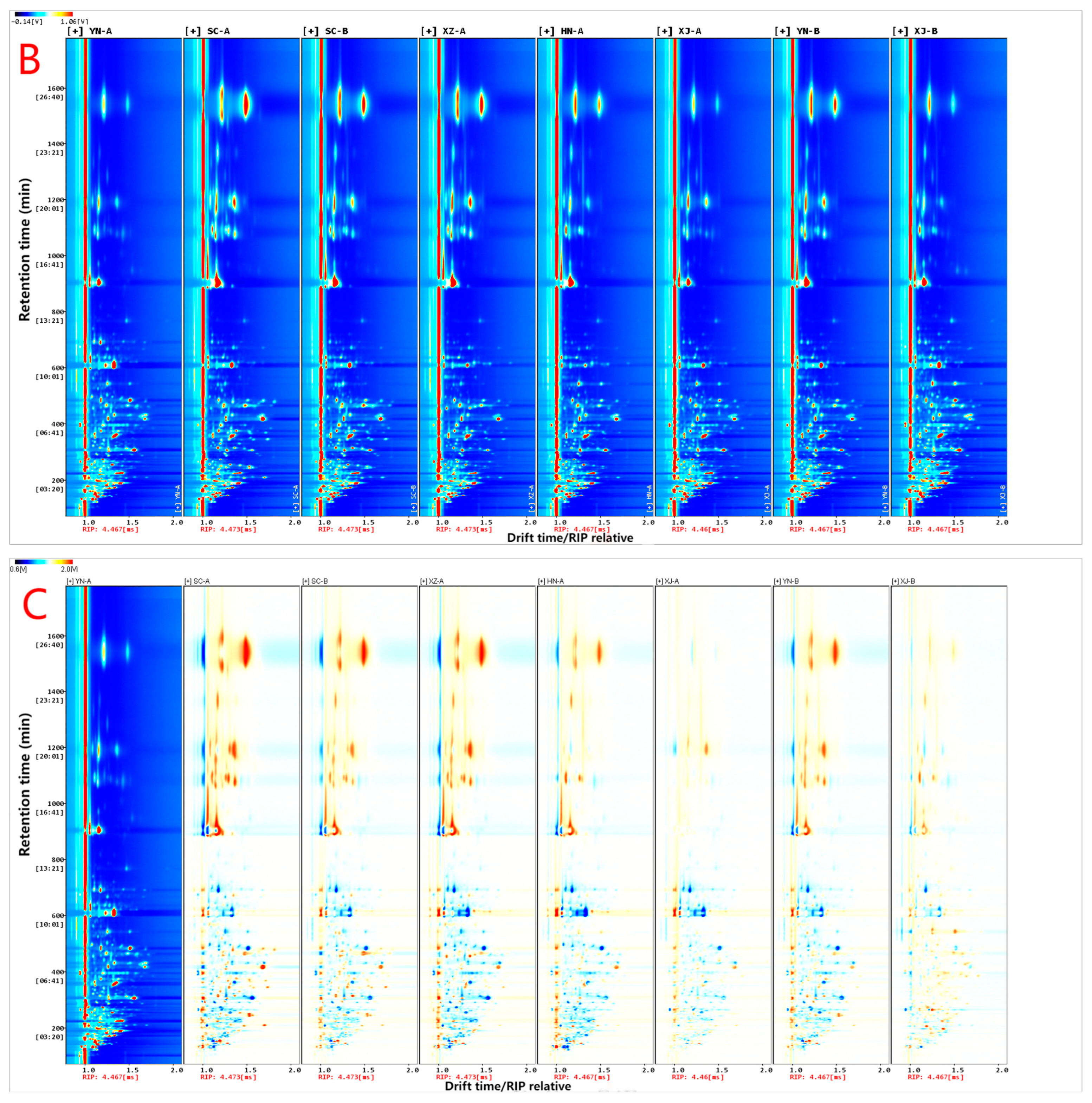
3.1. Overall GC–IMS Spectra and Preliminary Differences
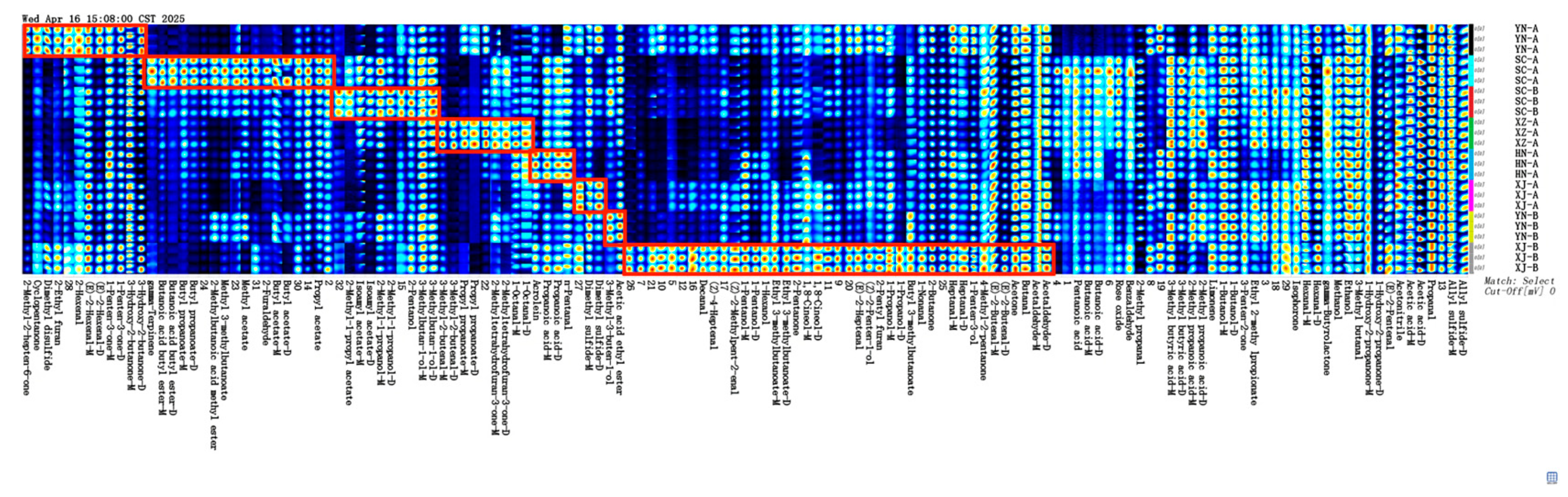
3.2. Fingerprint Profiles and Similarity Quantification
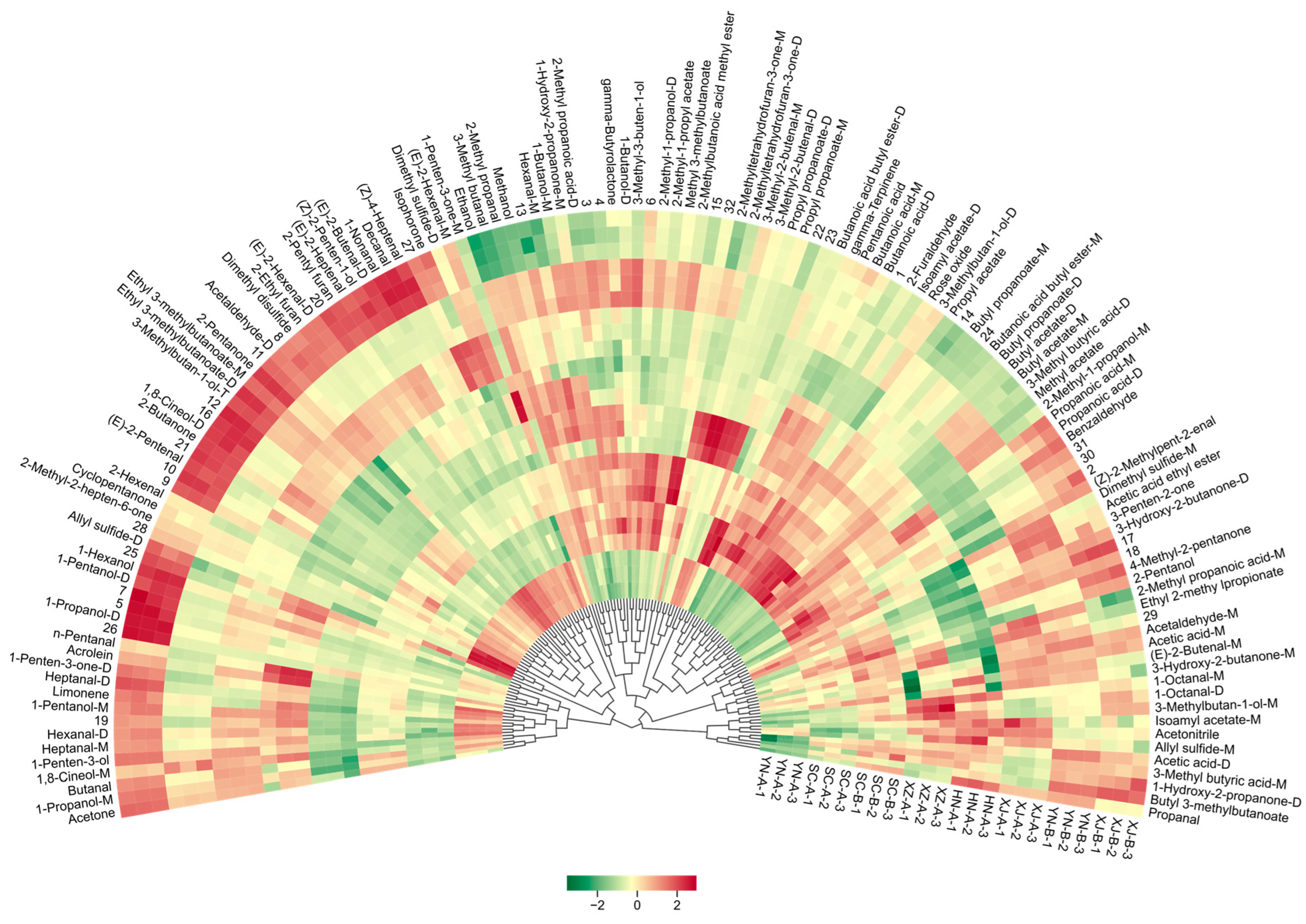
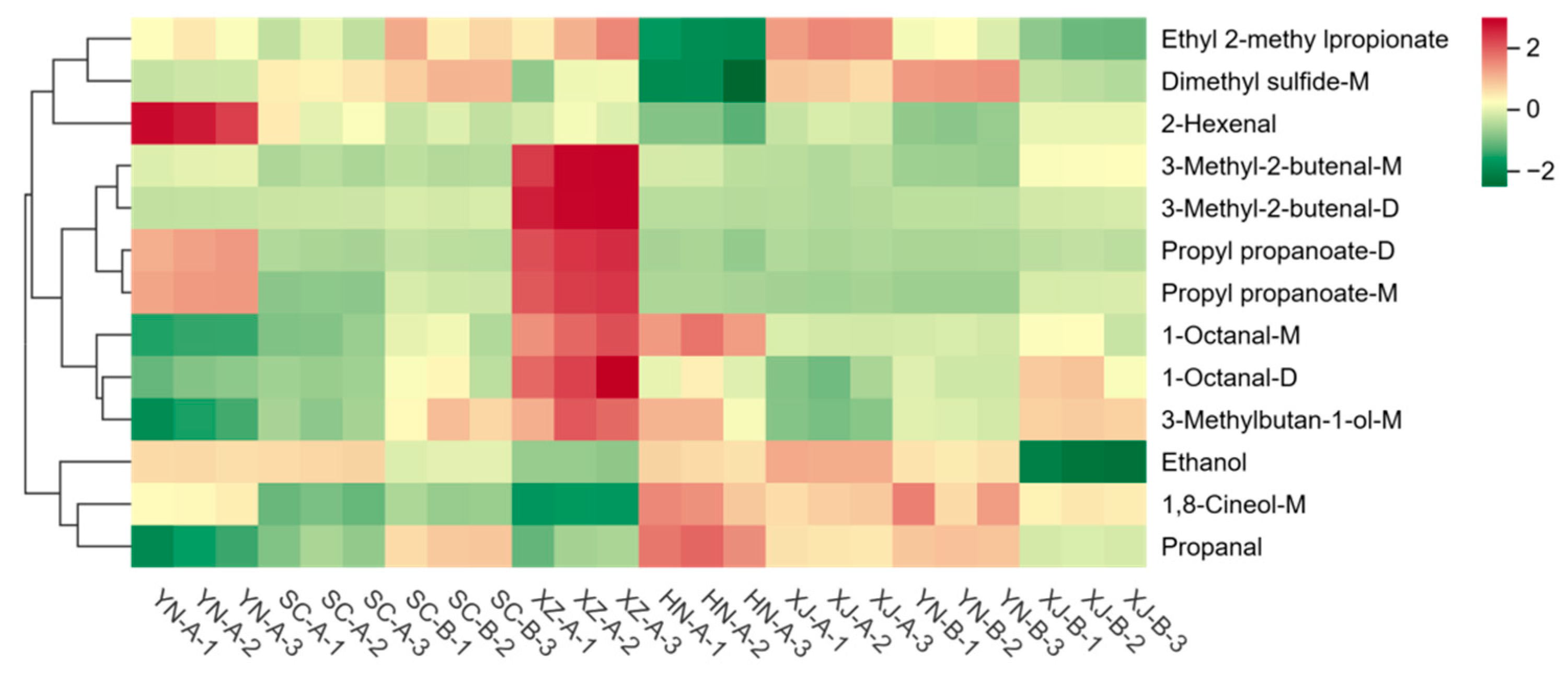
3.3. Identification and Classification of Volatile Compounds
3.4. Multivariate Statistical Separation and Model Validation
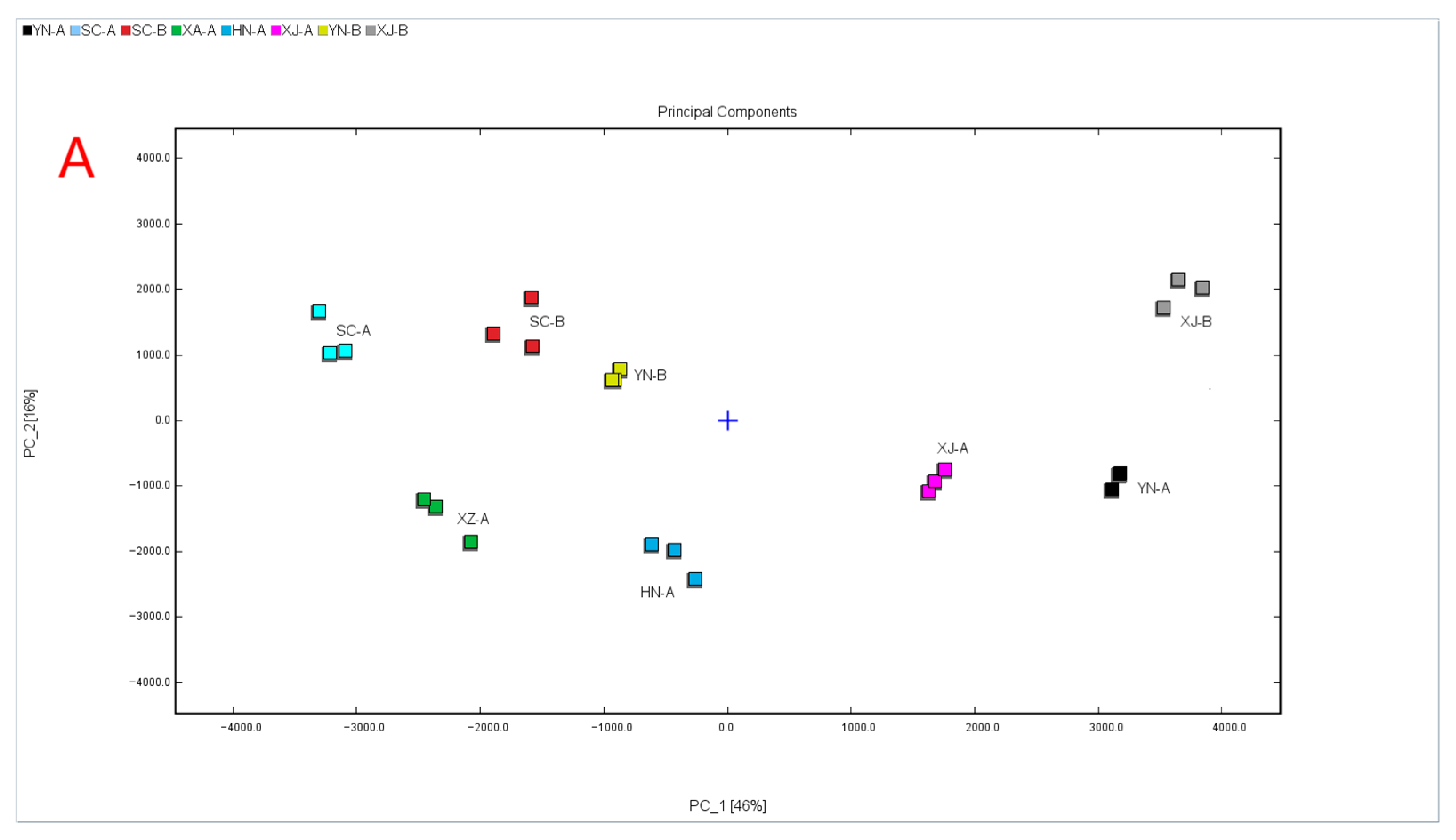
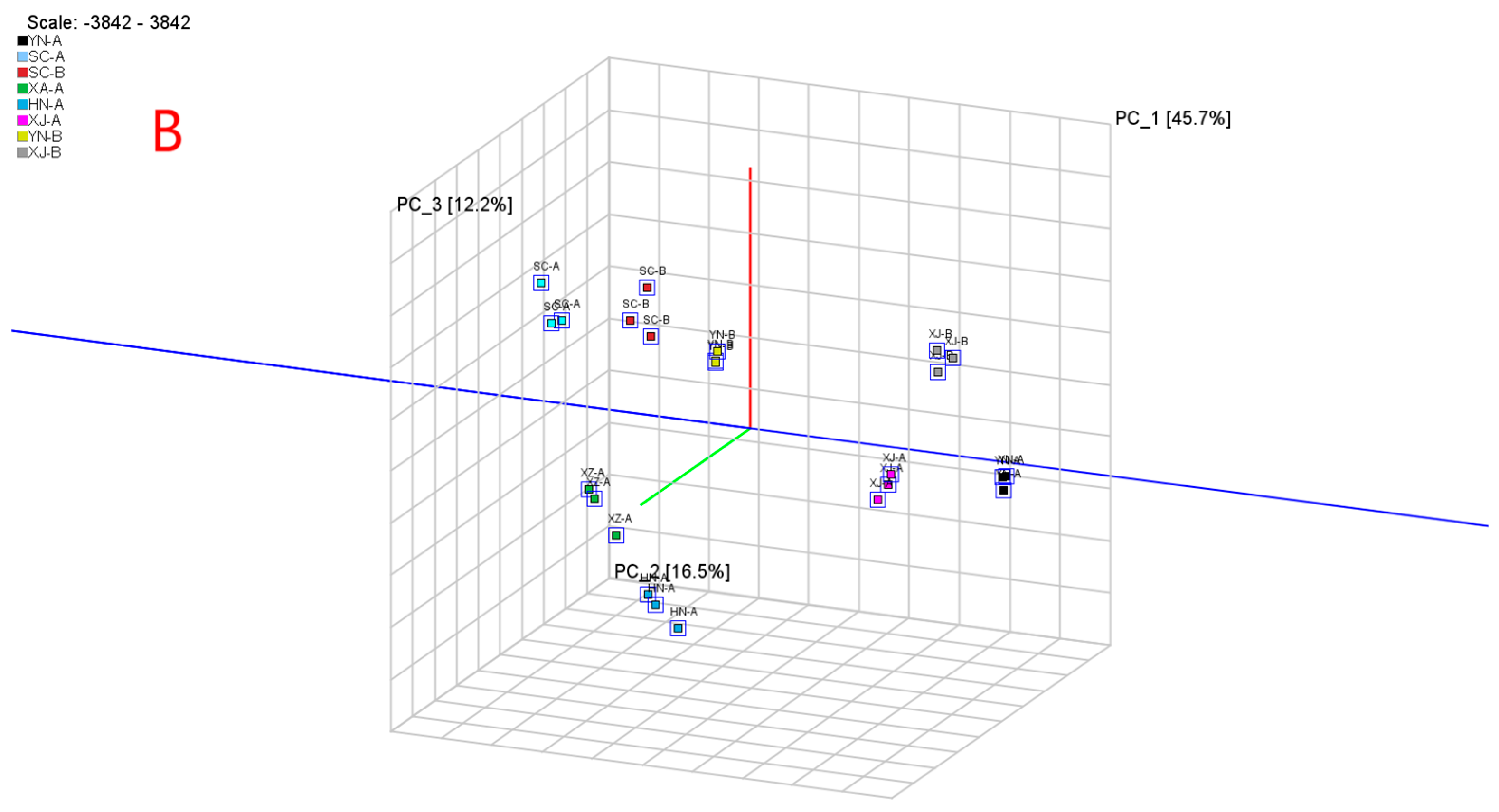
3.4.1. PCA
3.4.2. OPLS-DA and Permutation Test
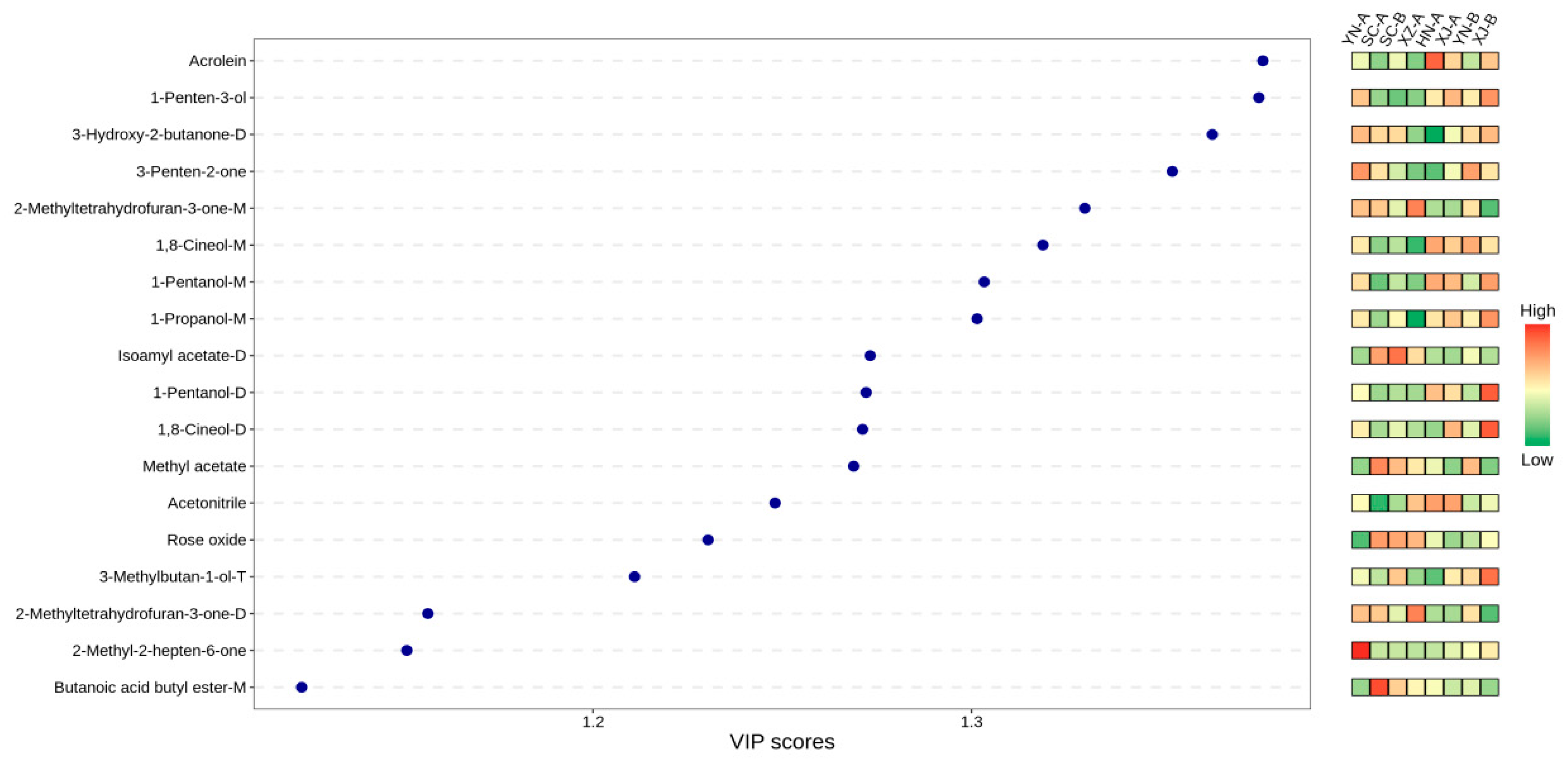
3.5. Characteristic Volatile Compounds (VIP) and Sensory Attribution
3.6. ROAV Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ye, G.; Guan, L.; Zhang, M. Research progress on processing and nutritional properties of fermented cereals. J. Food Sci. Technol. 2025, 62, 197–212. [Google Scholar] [CrossRef]
- Rahnama, A.; Salehi, F.; Meskarbashee, M.; Mehdi Khanlou, K.; Ghorbanpour, M.; Tom Harrison, M. High temperature perturbs physicochemical parameters and fatty acids composition of safflower (Carthamus tinctorius L.). BMC Plant Biol. 2024, 24, 1080. [Google Scholar] [CrossRef]
- Li, W.; Kim, E.-G.; Lee, D.; Choi, Y.; Lee, J.; Lee, S.; Lee, G.; Yoo, E. Flower Color and Seed Coat Color as a Phenotypic Marker: Correlations with Fatty Acid Composition, Antioxidant Properties, and Metabolite Profiles in Safflower (Carthamus tinctorius L.). Int. J. Mol. Sci. 2025, 26, 3105. [Google Scholar] [CrossRef]
- Hamid, M.T.; Hussein, N.N.; Sulaiman, G.M.; Moh Hamdoon, A. Green Synthesis of Silver Nanoparticles Using Carthamus tinctorius L. Extract and Assessment of Its Antioxidant, Anticancer, and Anti-Inflammatory Activities. Appl. Organomet. Chem. 2025, 39, e70278. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, L. Carthamus tinctorius L.: A natural neuroprotective source for anti-Alzheimer’s disease drugs. J. Ethnopharmacol. 2022, 298, 115656. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-C.; Li, M.-M.; Wu, Q.; Xu, W.-F.; Lin, S.; Chen, Z.-W.; Liu, L.; Shi, L.; Sheng, Q.; Li, T.-T.; et al. Hydroxysafflor Yellow A sensitizes ovarian cancer cells to chemotherapeutic agent by decreasing WSB1 expression. Eur. J. Integr. Med. 2019, 30, 100945. [Google Scholar] [CrossRef]
- Mauludin, R.; Padjar, I.N.; Kurniati, N.F.; Utami, R.A. Enhanced immunomodulator activity of Carthamus tinctorius (L.) extracts, a traditional medicine using nanostructured lipid carrier approach. OpenNano 2025, 23, 100244. [Google Scholar] [CrossRef]
- Chen, J.; Wu, L.; Xie, X.; Peng, C. Carthamus tinctorius L. protects cerebral ischemia/reperfusion injury via arachidonic acid/p53-mediated apoptosis axis. Front. Pharmacol. 2024, 15, 1446631. [Google Scholar] [CrossRef]
- Bacchetti, T.; Morresi, C.; Bellachioma, L.; Ferretti, G. Antioxidant and Pro-Oxidant Properties of Carthamus tinctorius, Hydroxy Safflor Yellow A, and Safflor Yellow, A. Antioxidants 2020, 9, 119. [Google Scholar] [CrossRef]
- Alshareef, N.S.; AlSedairy, S.A.; Al-Harbi, L.N.; Alshammari, G.M.; Yahya, M.A. Carthamus tinctorius L. (Safflower) Flower Extract Attenuates Hepatic Injury and Steatosis in a Rat Model of Type 2 Diabetes Mellitus via Nrf2-Dependent Hypoglycemic, Antioxidant, and Hypolipidemic Effects. Antioxidants 2024, 13, 1098. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Cao, Y.; Zeng, M.; Zhang, Q.; Ren, Y.; Chen, X.; He, C.; Fan, X.; Zheng, X.; et al. Chemical Constituents from the Flowers of Carthamus tinctorius L. and Their Lung Protective Activity. Molecules 2022, 27, 3573. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, C.; Feng, K.; Su, J.; Luan, J.; Cao, Y.; Rahman, K. Characterization of Saffron from Different Origins by HS-GC-IMS and Authenticity Identification Combined with Deep Learning. Food Chem. X 2024, 24, 101981. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Qiao, L.; Liu, S.; He, Y.; Huang, D.; Wu, W.; Liu, Y.; Chen, L.; Huang, D. Explorative Study on Volatile Organic Compounds of Cinnamon Based on GC-IMS. Metabolites 2024, 14, 274. [Google Scholar] [CrossRef] [PubMed]
- Xian, B.; Wang, R.; Jiang, H.; Zhou, Y.; Yan, J.; Huang, X.; Chen, J.; Wu, Q.; Chen, C.; Xi, Z.; et al. Comprehensive Review of Two Groups of Flavonoids in Carthamus tinctorius L. Biomed. Pharmacother. 2022, 153, 113462. [Google Scholar] [CrossRef]
- Parastar, H.; Yazdanpanah, H.; Weller, P. Non-targeted Volatilomics for the Authentication of Saffron by Gas Chromatography–Ion Mobility Spectrometry and Multivariate Curve Resolution. Food Chem. 2024, 420, 136802. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Zhou, S.; Guo, J.; Yan, W. HS-GC-IMS Analysis of Volatile Organic Compounds in Different Varieties and Harvesting Times of Gastrodia elata in Yunnan Province. Molecules 2023, 28, 6705. [Google Scholar] [CrossRef]
- Yan, J.; Wang, H.; Yang, B.; Zhang, W.; Cao, Z.; Zhao, P.; Dong, Z.; Ren, F.; Chen, L. Characterization of the Flavor Profile of Hulatang Using GC-IMS Coupled with Sensory Analysis. Front. Nutr. 2024, 11, 1234. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, W.; Wang, H.; Wang, S.; Yan, J.; Dong, Z.; Zhao, P.; Ren, F.; Chen, L. Comparative Analysis of Texture Characteristics, Sensory Properties, and Volatile Components in Four Types of Marinated Tofu. Foods 2024, 13, 2068. [Google Scholar] [CrossRef]
- Shi, X.; Li, Y.; Huang, D.; Chen, S.; Zhu, S. Characterization and Discrimination of Volatile Compounds in Roasted Arabica Coffee Beans from Different Origins by Combining GC-TOFMS, GC-IMS, and GC-E-Nose. Food Chem. 2025, 481, 144079. [Google Scholar] [CrossRef]
- Yuan, J.; Li, H.; Cao, S.; Liu, Z.; Li, N.; Xu, D.; Mo, H.; Hu, L. Monitoring of Volatile Compounds of Ready-to-Eat Kiwifruit Using GC-IMS. Foods 2023, 12, 4394. [Google Scholar] [CrossRef]
- Liu, G.; Duan, H.; Zheng, Y.; Guo, J.; Wang, D.; Yan, W. Differences in the Determination of Volatile Organic Compounds Between Chrysanthemum morifolium Ramat. and Chrysanthemum indicum L. by HS-GC-IMS. Molecules 2024, 29, 4609. [Google Scholar] [CrossRef]
- Meng, L.; Nie, Y.; Zhou, Q. Effect of Hot-Air Drying Processing on the Volatile Organic Compounds and Maillard Precursors of Dictyophora rubrovalvata Based on GC-IMS, HPLC and LC-MS. Food Chem. 2024, 420, 136789. [Google Scholar] [CrossRef]
- Xu, Y.; Li, R.; Zhang, L.; Bai, X.; Zhang, W. Advancements in Gas Chromatography-Ion Mobility Spectrometry for Analysing Natural Medicines. Anal. Methods 2025, 17, 5621–5635. [Google Scholar] [CrossRef] [PubMed]
- Mena-García, A.; Herrero-Gutiérrez, D.; Sanz, M.L.; Diez-Municio, M.; Ruiz-Matute, A.I. Fingerprint of Characteristic Saffron Compounds as Novel Standardization of Commercial Crocus sativus Extracts. Foods 2023, 12, 1634. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, J.; Qin, Z.; Wang, Y.; Zhao, P.; Gao, H. Insights into the Composition and Antibacterial Activity of Amomum tsao-ko Essential Oils from Different Regions Based on GC-MS and GC-IMS. Foods 2022, 11, 1402. [Google Scholar] [CrossRef]
- Zheng, A.-R.; Wei, C.-K.; Wang, M.-S.; Ju, N.; Fan, M. Characterization of the Key Flavor Compounds in Cream Cheese by GC-MS, GC-IMS, Sensory Analysis and Multivariable Statistics. Curr. Res. Food Sci. 2024, 7, 100226. [Google Scholar] [CrossRef]
- Avula, B.; Katragunta, K.; Wang, Y.-H.; Upton, R.; Khan, I. Analysis of Crocetins and Safranal Variations in Saffron (Crocus sativus) Stigma Samples and Dietary Supplements Using HPLC/UHPLC-PDA-MS: Chemical Profiling and Chemometric Analysis Using LC-QToF. Food Anal. Methods 2022, 15, 1234–1247. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Z.; Song, Z.; Wang, F.; Lin, L.; Tao, N. Effect of Short-Term Depuration on the Flavor of Crucian Carp (Carassius auratus) and Exploration of Prediction Model for Fishy Odor. J. Food Compos. Anal. 2025, 123, 105678. [Google Scholar] [CrossRef]
- Che, X.; Cao, F.; Yan, T.; Liu, X.; Cai, Q.; Liu, S.; Ma, Y.; Ren, D.; Zhou, H.; Wang, Q. Analysis of Flavor Differences Between Undaria pinnatifida Produced Using Different Processing Methods and from Different Origins Based on GC-IMS. Foods 2025, 14, 2107. [Google Scholar] [CrossRef]
- Li, Z.; Wang, T.; Jiang, H.; Wang, W.; Lan, T.; Xu, L.; Yun, Y.; Zhang, W. Comparative Key Aroma Compounds and Sensory Correlations of Aromatic Coconut Water Varieties: Insights from GC×GC-O-TOF-MS, E-Nose, and Sensory Analysis. Food Chem. X 2024, 23, 101987. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zou, Y.; Liao, G.; Zheng, Z.; Chen, G.; Zhong, Y.; Wang, G. Identification of Characteristic Flavor Compounds and Small Molecule Metabolites during the Ripening Process of Nuodeng Ham by GC-IMS, GC-MS Combined with Metabolomics. Food Chem. 2023, 410, 135498. [Google Scholar] [CrossRef]
- Zou, M.; Yu, X.; Liu, Y.; Zhu, L.; Ou, F.; Lei, C. Comparative Analysis of Volatile Organic Compounds in Different Parts of Ginseng Powder Using Gas Chromatography-Ion Mobility Spectrometry. Molecules 2025, 30, 1965. [Google Scholar] [CrossRef]
- Yang, B.; Wang, W.; Zhang, J.; Gao, W.; Fan, L.; Chitrakar, B.; Sang, Y. Comparative Study on Organoleptic Properties and Volatile Organic Compounds in Turmeric, Turmeric Essential Oil, and By-Products Using E-Nose, HS-GC-IMS, and HS-GC-MS. Food Chem. X 2024, 22, 101952. [Google Scholar] [CrossRef]
- Zhang, T.; Liao, Z.; Li, Z.; Liu, Y.; Song, Y.; Qin, Y. Revealing the Flavor Differences of Sauvignon Blanc Wines Fermented in Different Oak Barrels and Stainless-Steel Tanks through GC-MS, GC-IMS, Electronic, and Artificial Sensory Analyses. Food Chem. X 2025, 25, 102045. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Zhang, Z.; Chen, J.; Wu, H.; Jiang, H.; Teng, C.; Dai, Z. Enhanced Understanding of Dark Tea Quality through Integrated GC-IMS and E-Nose Analysis. LWT-Food Sci. Technol. 2025, 182, 115678. [Google Scholar] [CrossRef]
- Christmann, J.; Rohn, S.; Weller, P. gc-ims-tools—A New Python Package for Chemometric Analysis of GC-IMS Data. Food Chem. 2022, 373, 131558. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, D.; Duan, H.; Zhou, S.; Guo, J.; Yan, W. Detection and Analysis of Volatile Flavor Compounds in Different Varieties and Origins of Goji Berries Using HS-GC-IMS. LWT-Food Sci. Technol. 2023, 175, 114550. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Liu, Y. Geographical Origins and Varieties Identification of Hops (Humulus lupulus L.) by Multi-Metal Elements Fingerprinting and the Relationships with Functional Ingredients. Food Chem. 2019, 276, 353–361. [Google Scholar] [CrossRef]
- Song, J.; Shao, Y.; Yan, Y.; Li, X.; Peng, J.; Guo, L. Characterization of Volatile Profiles of Three Colored Quinoas Based on GC-IMS and PCA. LWT-Food Sci. Technol. 2021, 146, 111411. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, J.; Wu, B.; Sun, J.; Dai, C. Discrimination of Tea Varieties Using FTIR Spectroscopy and Allied Gustafson-Kessel Clustering. Comput. Electron. Agric. 2018, 155, 385–392. [Google Scholar] [CrossRef]
- Li, M.; Yang, R.; Zhang, H.; Wang, S.; Chen, D.; Lin, S. Development of a flavor fingerprint by HS-GC-IMS with PCA for volatile compounds of Tricholoma matsutake Singer. Food Chem. 2019, 290, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Wang, J.; He, Y.; Ji, X.-J.; Tang, H.; Dong, Y.-M.; Yan, W.-J. HS-GC-IMS detection of volatile organic compounds in Acacia honey powders under vacuum belt drying at different temperatures. Food Sci. Nutr. 2021, 9, 4085–4093. [Google Scholar] [CrossRef]
- Song, J.; Chen, Q.; Bi, J.; Meng, X.; Wu, X.; Qiao, Y.; Lyu, Y. GC/MS coupled with MOS e-nose and flash GC e-nose for volatile characterization of Chinese jujubes as affected by different drying methods. Food Chem. 2020, 331, 127201. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Pérez, A.; Romero-González, R.; Garrido Frenich, A. Application of an innovative metabolomics approach to discriminate geographical origin and processing of black pepper by untargeted UHPLC-Q-Orbitrap-HRMS analysis and mid-level data fusion. Food Res. Int. 2021, 150, 110722. [Google Scholar] [CrossRef]
- Kang, C.; Zhang, Y.; Zhang, M.; Qi, J.; Zhao, W.; Gu, J.; Guo, W.; Li, Y. Screening of specific quantitative peptides of beef by LC–MS/MS coupled with OPLS-DA. Food Chem. 2022, 387, 132932. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, K.; Guo, H.; Geng, Y.; Lv, W.; Wang, S.; Guo, Y.; Qin, P.; Ren, G. Characterization of volatile compounds in differently coloured Chenopodium quinoa seeds before and after cooking by headspace-gas chromatography-ion mobility spectrometry. Food Chem. 2021, 344, 128586. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Chen, J.; Li, X.; Qi, Y.; Jiang, R. Flavor components detection and discrimination of isomers in Huaguo tea using headspace-gas chromatography-ion mobility spectrometry and multivariate statistical analysis. Anal. Chim. Acta 2023, 1243, 340842. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, R.; Chen, Y.; Ho, C.T.; Hou, A.; Zhang, X.; Zhu, M.; Zhang, C.; Wang, Y.; Liu, Z.; et al. Dynamics changes in volatile profile, non-volatile metabolites and antioxidant activities of dark tea infusion during submerged fermentation with Eurotium cristatum. Food Biosci. 2023, 55, 102966. [Google Scholar] [CrossRef]
- Wang, F.; Chen, M.; Luo, R.; Huang, G.; Wu, X.; Zheng, N.; Zhang, Y.; Wang, J. Fatty acid profiles of milk from Holstein cows, Jersey cows, buffalos, yaks, humans, goats, camels, and donkeys based on gas chromatography–mass spectrometry. J. Dairy Sci. 2021, 104, 1234–1245. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, Y.; Huang, Y.; Song, H.; Yang, P. Aroma identification and classification in 18 kinds of teas (Camellia sinensis) by sensory evaluation, HS-SPME-GC-IMS/GC×GC-MS, and chemometrics. Foods 2023, 12, 2433. [Google Scholar] [CrossRef]
- Tian, H.; Xiong, J.; Yu, H.; Chen, C.; Lou, X. Flavor optimization in dairy fermentation: From strain screening and metabolic diversity to aroma regulation. Trends Food Sci. Technol. 2023, 135, 53–65. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, H.D.; Jang, Y.J. Delivery systems designed to enhance stability and suitability of lipophilic bioactive compounds in food processing: A review. Food Chem. 2023, 395, 133581. [Google Scholar] [CrossRef]
- Xu, Y.; Bi, S.; Niu, X.; Chen, Y.; Liu, Y.; Zhou, Q. Comparison of aroma active compounds in cold- and hot-pressed walnut oil by comprehensive two-dimensional gas chromatography-olfactory-mass spectrometry and headspace-gas chromatography-ion mobility spectrometry. Food Res. Int. 2022, 155, 111054. [Google Scholar] [CrossRef] [PubMed]
- Urango, A.C.M.; Meireles, M.A.A.; Silva, E.K. Maillard conjugates produced from proteins and prebiotic dietary fibers: Technological properties, health benefits and challenges. Trends Food Sci. Technol. 2024, 146, 252–266. [Google Scholar] [CrossRef]
- Shi, B.; Guo, X.; Liu, H.; Jiang, K.; Liu, L.; Yan, N.; Farag, M.; Liu, L. Dissecting Maillard reaction production in fried foods: Formation mechanisms, sensory characteristic attribution, control strategy, and gut homeostasis regulation. Food Chem. 2023, 407, 134846. [Google Scholar] [CrossRef] [PubMed]



| Code | Location | Latitude | Longitude | Elevation | Climate Type | Mean Annual Precip. |
|---|---|---|---|---|---|---|
| HN-A | Henan (Xinxiang), China | 35.3086° N | 114.0511° E | 73 m | Warm-temperate monsoon climate | Annual precipitation ranges from approximately 600 to 700 mm, with the majority occurring during the summer months. |
| SC-A | Sichuan (Jianyang), China | 31.7774° N | 104.7421° E | 400–580 m | Humid subtropical climate | Approximately 900–1100 mm. |
| SC-B | Sichuan (Jiangyou), China | 30.3931° N | 104.5532° E | 800–1500 m | Humid subtropical climate | Approximately 1000–1200 mm. |
| XZ-A | Rikaze (Tibet Autonomous Region), China | 28.3683° N | 87.7634° E | 3836 m | Alpine plateau climate | Approximately 400 mm, characterized by arid and low-rainfall conditions. |
| XJ-A | Xinjiang (Yili), China | 43.9052° N | 81.2747° E | 1600–1900 m | Temperate continental climate with oasis irrigation | Approximately 400–500 mm, representing a relatively humid area within Xinjiang. |
| XJ-B | Xinjiang (Tacheng), China | 46.7510° N | 82.9838° E | 1100–5000 m | Arid continental climate | Approximately 200–250 mm, extremely arid. |
| YN-A | Yunnan (Tengchong), China | 25.017° N | 98.483° E | 1640 m | Mild subtropical highland climate | Approximately 1500 mm, with a long rainy season. |
| YN-B | Yunnan (Dali), China | 25.6065° N | 100.2676° E | 1970 m | Subtropical plateau monsoon climate | Approximately 1050 mm. |
| Full Distance | HN-A | SC-A | SC-B | XJ-A | XJ-B | XZ-A | YN-A | YN-B |
|---|---|---|---|---|---|---|---|---|
| HN-A | 0 | 17.71778 | 15.83031 | 15.32197 | 20.15048 | 15.10411 | 18.67716 | 15.35231 |
| SC-A | 17.71778 | 0 | 11.14107 | 19.78153 | 23.81304 | 14.76061 | 22.18853 | 12.94494 |
| SC-B | 15.83031 | 11.14107 | 0 | 15.0435 | 18.69994 | 13.83421 | 19.0351 | 8.945528 |
| XJ-A | 15.32197 | 19.78153 | 15.0435 | 0 | 15.79017 | 17.19138 | 12.04877 | 12.04061 |
| XJ-B | 20.15048 | 23.81304 | 18.69994 | 15.79017 | 0 | 23.18586 | 15.69517 | 18.25957 |
| XZ-A | 15.10411 | 14.76061 | 13.83421 | 17.19138 | 23.18586 | 0 | 20.74933 | 14.09947 |
| YN-A | 18.67716 | 22.18853 | 19.0351 | 12.04877 | 15.69517 | 20.74933 | 0 | 16.9434 |
| YN-B | 15.35231 | 12.94494 | 8.945528 | 12.04061 | 18.25957 | 14.09947 | 16.9434 | 0 |
| No. | Compound | CAS | RI | Odor Description | Odor Threshold (mg/kg) | ROAV Range (Across Samples) | Key Sample Enrichment |
|---|---|---|---|---|---|---|---|
| 1 | Hexanal | C66251 | 1090.3 | Green, grassy, fatty | 4.50 | 15–42 | SC-B, XJ-B |
| 2 | Nonanal | C124196 | 1399.1 | Waxy, citrus, oily | 1 | 12–35 | SC-B, XJ-B |
| 3 | 3-Methylbutanoic acid | C503742 | 1669.4 | Fruity, sweet | 30 | 18–40 | YN-B |
| 4 | Butanoic acid | C107926 | 1625.5 | Fruity, fermented | 42 | 4–10 | XJ-B |
| 5 | 2-Methyl-1-propyl acetate | C110190 | 1021.0 | Fruity, raw pear and raspberrie | 0.01 | 4.5–10 | SC-B |
| 6 | Isoamyl acetate | C123922 | 1122.5 | Fruity, banana, sweet | 17 | 7–20 | SC-A, SC-B |
| 7 | Butanoic acid butyl ester | C109217 | 1219.2 | Fruity, pineapple, sweet | 4.80 | 8–19 | SC-A |
| 8 | Rose oxide | C16409431 | 1341.9 | Floral, rose-like | 0.32 | 57–100 | YN-A, SC-B |
| 9 | 1,8-Cineole | C470826 | 1204.7 | Camphor, herbal, cooling | 1.20 | 17–100 | XJ-B |
| 10 | 3-Penten-2-one | C625332 | 1133.0 | Fruity, spicy | 0.94 | 16–87 | YN-A |
| 11 | Acetic acid | C64197 | 1464.3 | Sulfurous, onion-like | 0.05 | 5–20 | YN-A |
| 12 | 2-Furaldehyde | C98011 | 1458.4 | sweet, woody, almond, bready | 0.02 | 3–12 | XJ-A |
| 13 | Acrolein | C107028 | 870.3 | Pungent, acrid | 105 | 0.8–3.9 | HN-A |
| 14 | 2-Methyl-2-hepten-6-one | C110930 | 1355.9 | Citrus, fruity | 50 | 0.3–9.3 | YM-A |
| 15 | 2-Methyltetrahydrofuran-3-one | C3188009 | 1355.9 | Roasted, nutty, buttery | 2 | 7.4–29 | All samples |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Duan, H.; Wang, L.; Qin, R.; Liu, J.; Liu, H.; Bao, S.; Yan, W. Volatile Fingerprinting and Regional Differentiation of Safflower (Carthamus tinctorius L.) Using GC–IMS Combined with OPLS-DA. Foods 2025, 14, 3381. https://doi.org/10.3390/foods14193381
Liu J, Duan H, Wang L, Qin R, Liu J, Liu H, Bao S, Yan W. Volatile Fingerprinting and Regional Differentiation of Safflower (Carthamus tinctorius L.) Using GC–IMS Combined with OPLS-DA. Foods. 2025; 14(19):3381. https://doi.org/10.3390/foods14193381
Chicago/Turabian StyleLiu, Jiaqi, Hao Duan, Li Wang, Rui Qin, Jiao Liu, Hong Liu, Shuyuan Bao, and Wenjie Yan. 2025. "Volatile Fingerprinting and Regional Differentiation of Safflower (Carthamus tinctorius L.) Using GC–IMS Combined with OPLS-DA" Foods 14, no. 19: 3381. https://doi.org/10.3390/foods14193381
APA StyleLiu, J., Duan, H., Wang, L., Qin, R., Liu, J., Liu, H., Bao, S., & Yan, W. (2025). Volatile Fingerprinting and Regional Differentiation of Safflower (Carthamus tinctorius L.) Using GC–IMS Combined with OPLS-DA. Foods, 14(19), 3381. https://doi.org/10.3390/foods14193381







