Physicochemical Characterization of Camellia oleifera Husks from Different Regions and Microwave-Assisted RSM Optimization of Tea Saponin Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Comprehensive Physicochemical and Structural Characterization of C. oleifera Husks
2.2.1. Composition Analysis of C. oleifera Husks
2.2.2. Elemental Analysis of C. oleifera Husks
2.2.3. BET Analysis of C. oleifera Husks
2.2.4. SEM Analysis of C. oleifera Husks
2.2.5. FTIR Spectroscopy Analysis of C. oleifera Husks
2.3. Extraction and Determination of Tea Saponins, Tannins, and Flavonoids
2.3.1. Extraction and Determination of Tea Saponins
2.3.2. Extraction and Determination of Tannins
2.3.3. Extraction and Determination of Flavonoids
2.4. Single-Factor Experiment
2.5. Response Surface Experiment
2.6. Statistical Analysis
3. Results and Discussion
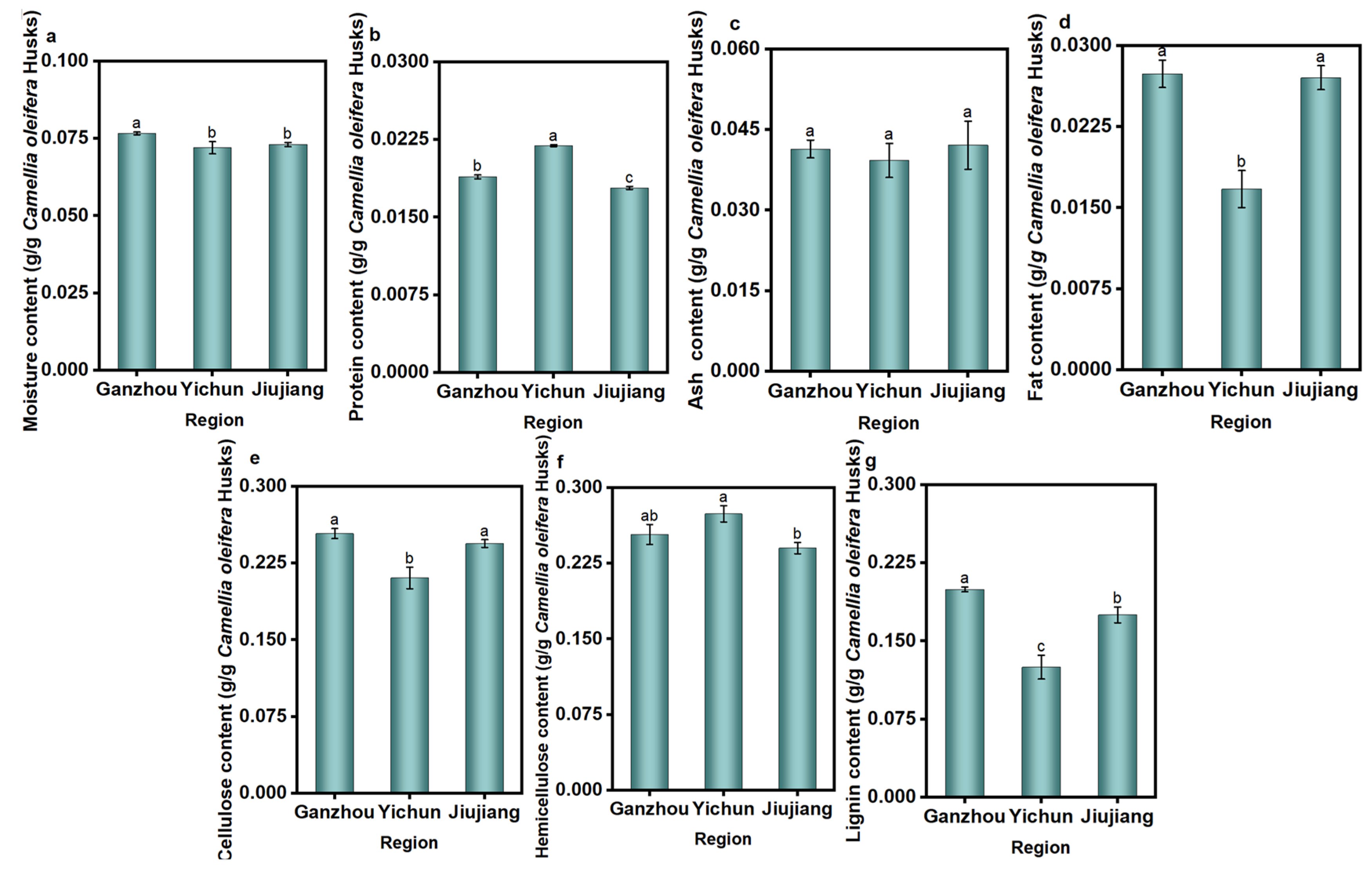
3.1. Physical and Chemical Properties of C. oleifera Husks in Three Regions
3.2. Structural Characterization of C. oleifera Husks in Three Regions
3.2.1. BET Analysis of C. oleifera Husks
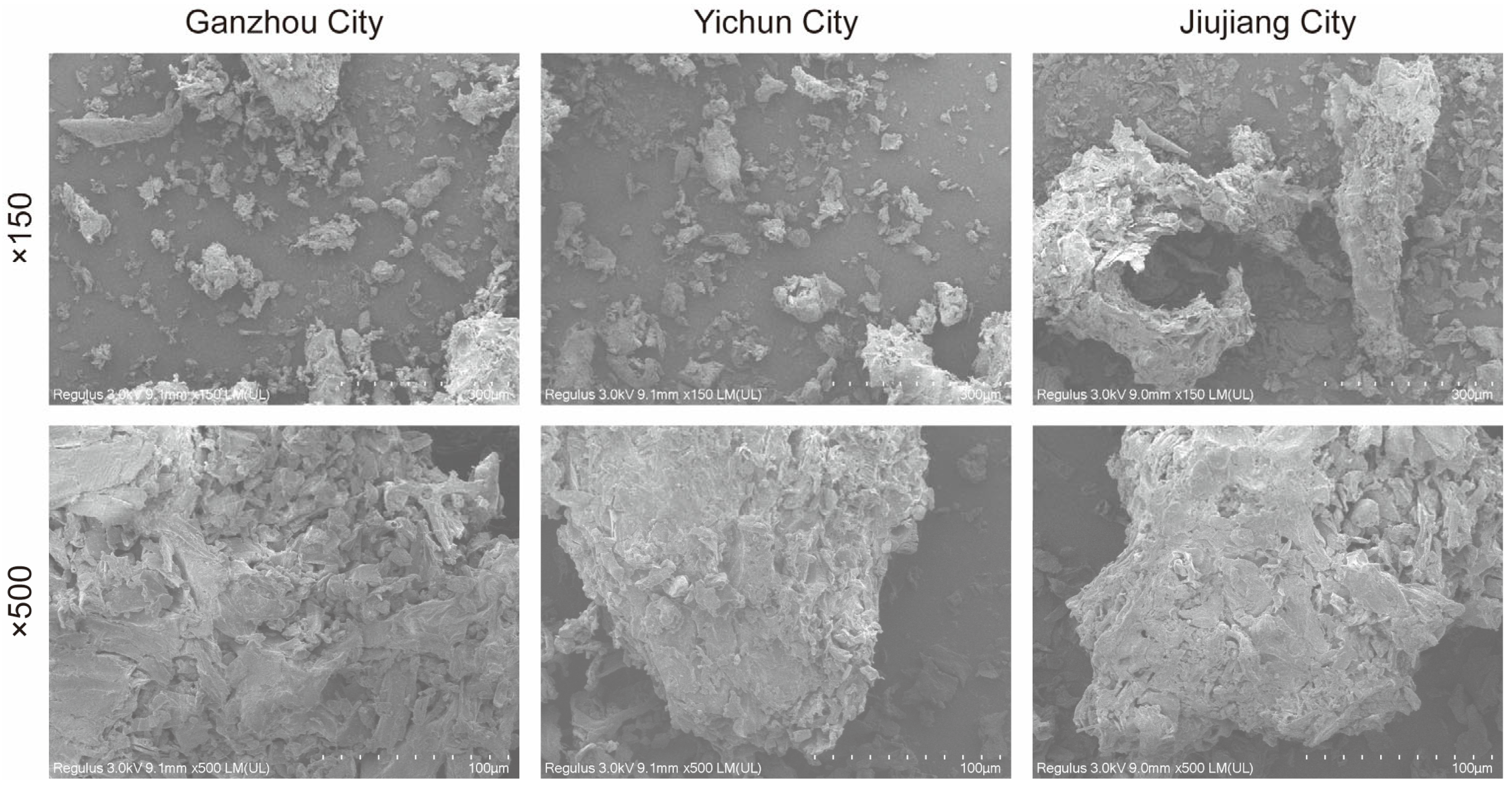
3.2.2. SEM Analysis of C. oleifera Husks
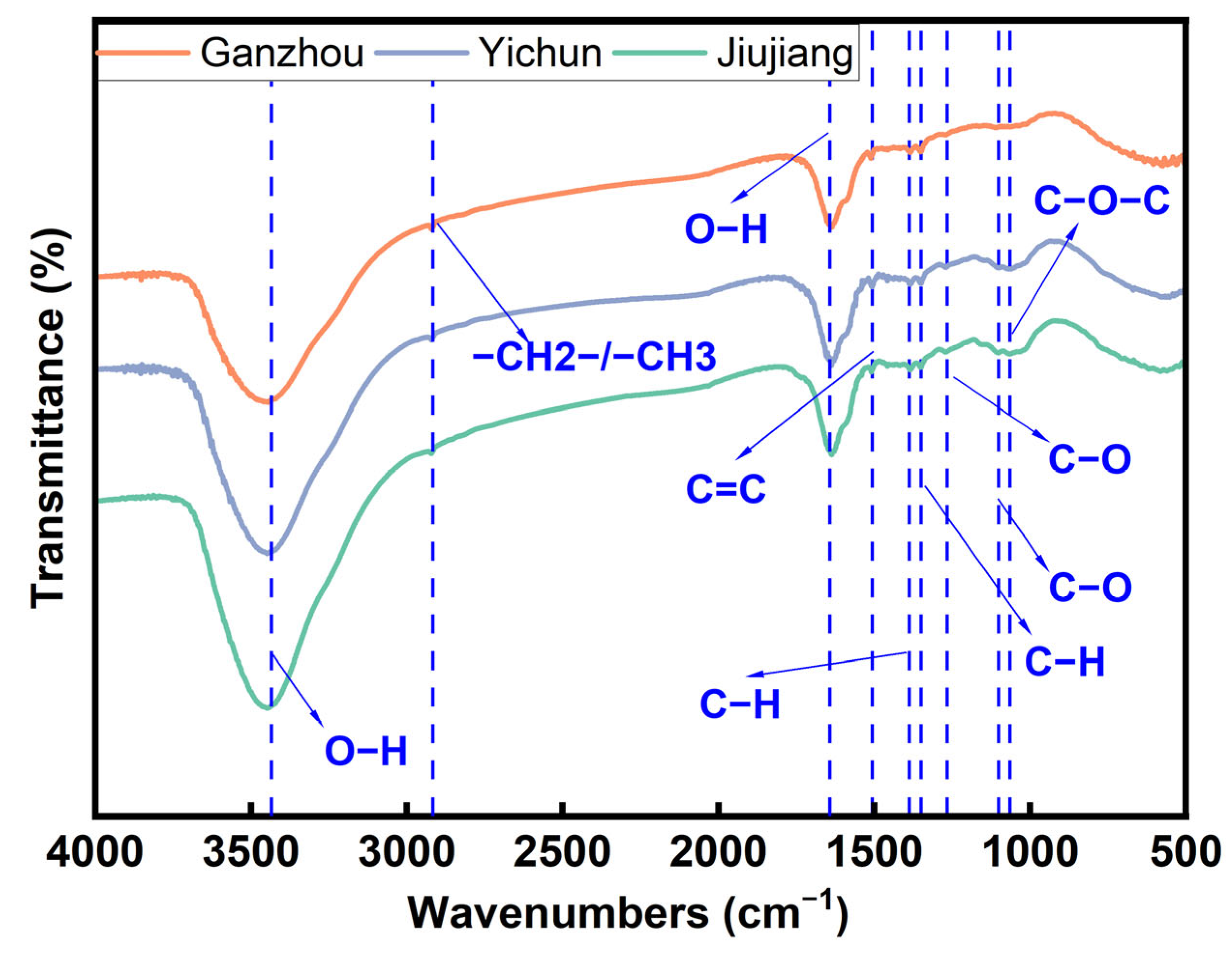
3.2.3. FTIR Spectrum Analysis of C. oleifera Husks
3.3. Effect of Extraction Parameters on the Yield of Tea Saponins
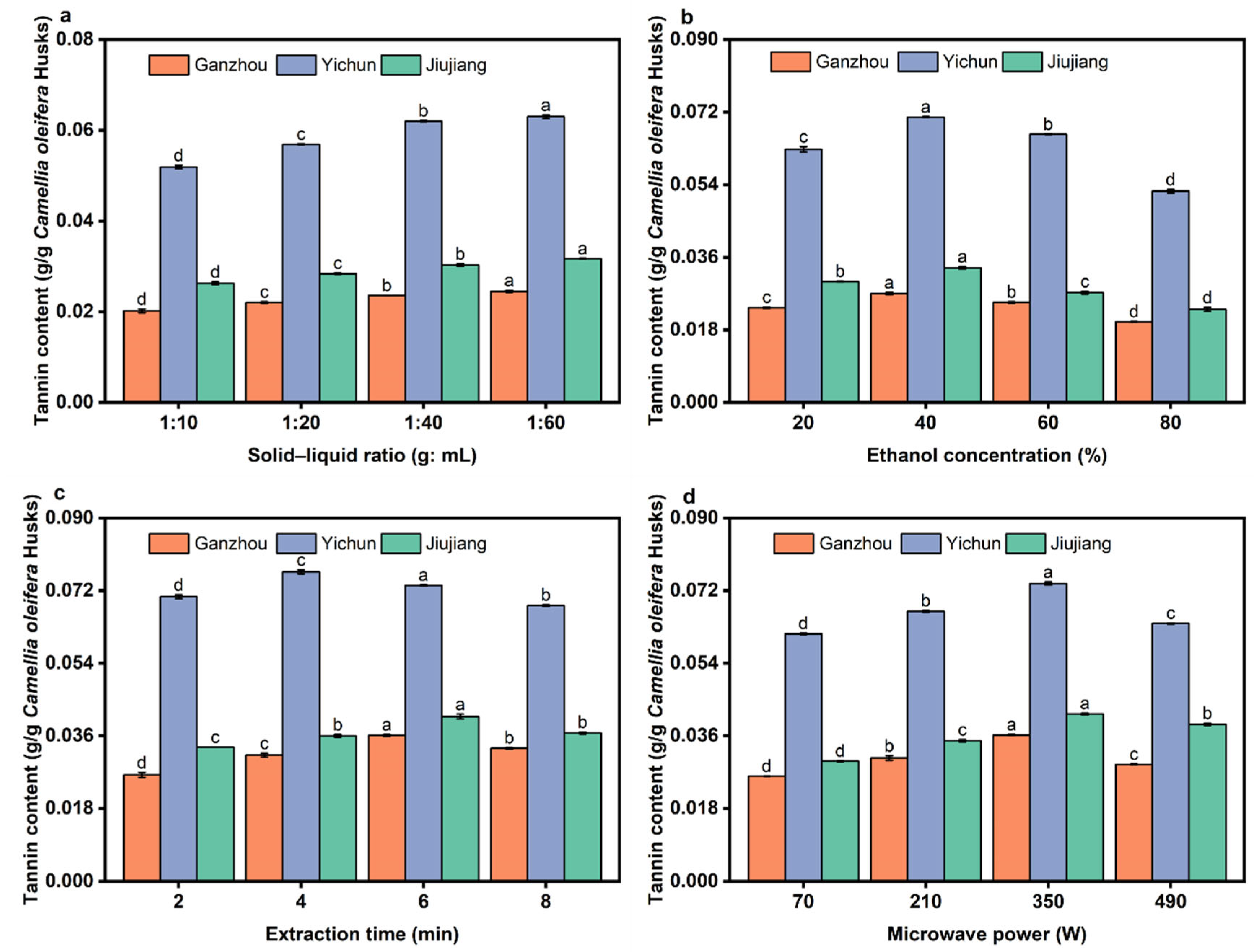
3.4. Effect of Extraction Parameters on the Yield of Tannins
3.5. Effect of Extraction Parameters on the Yield of Flavonoids
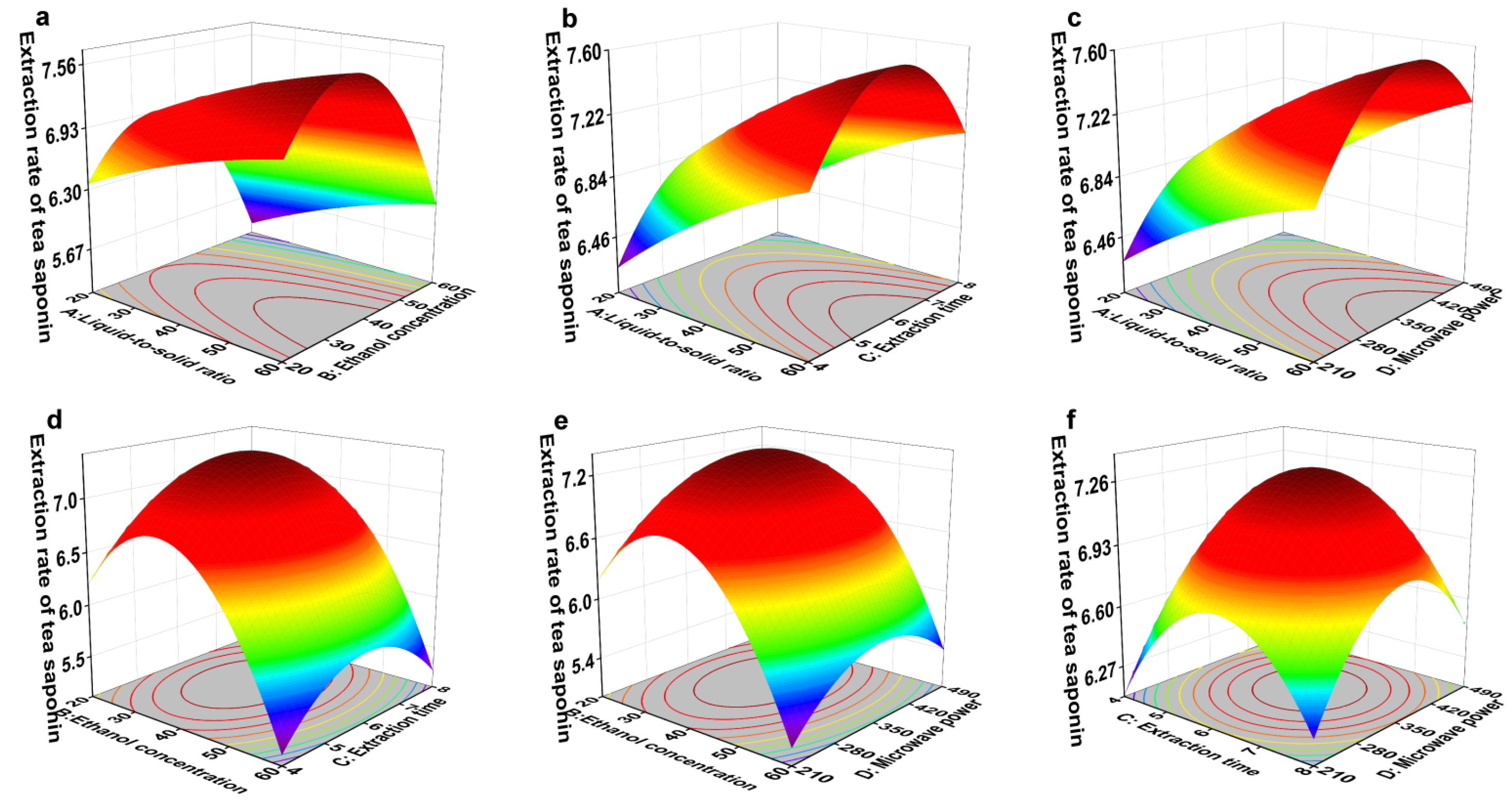
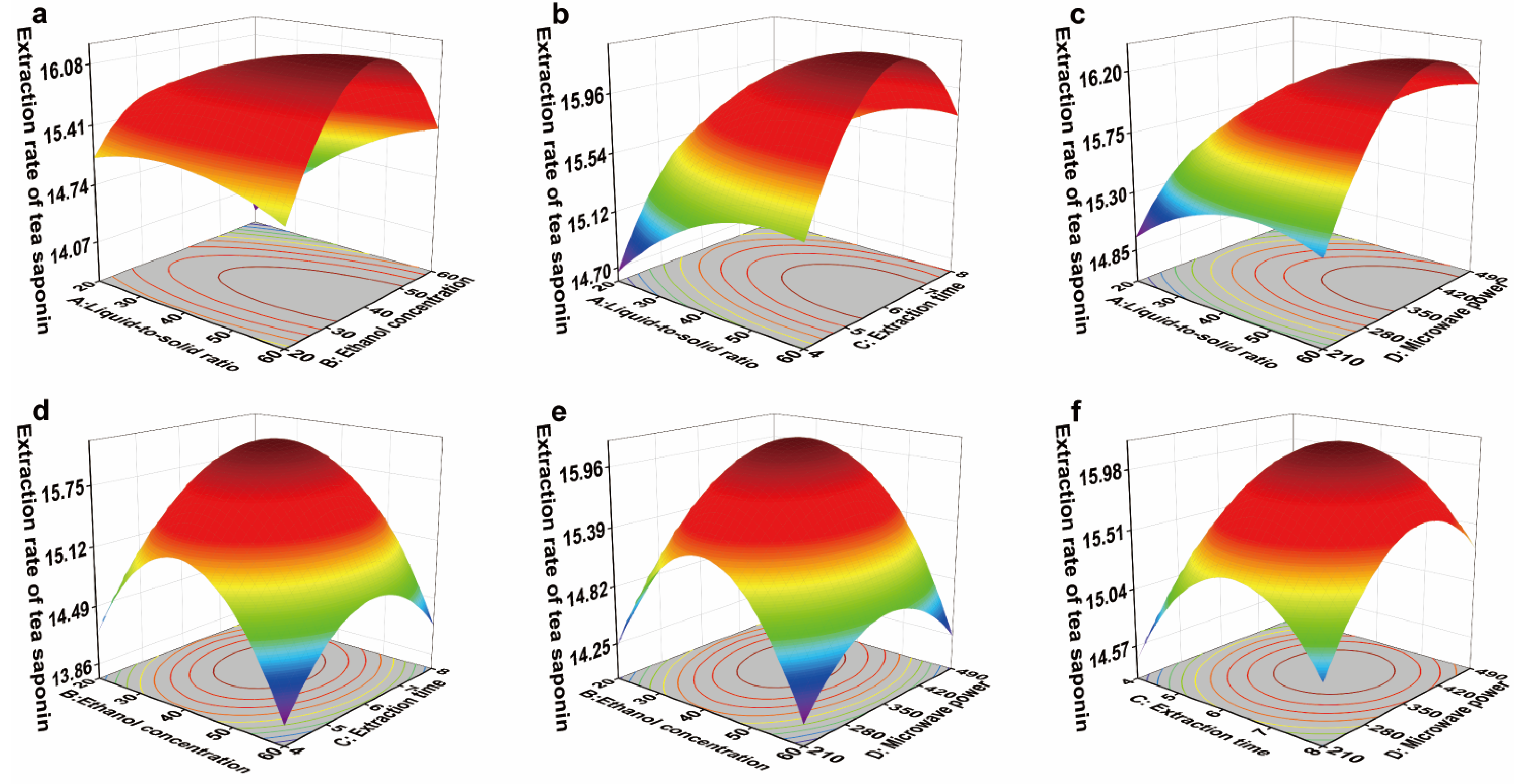
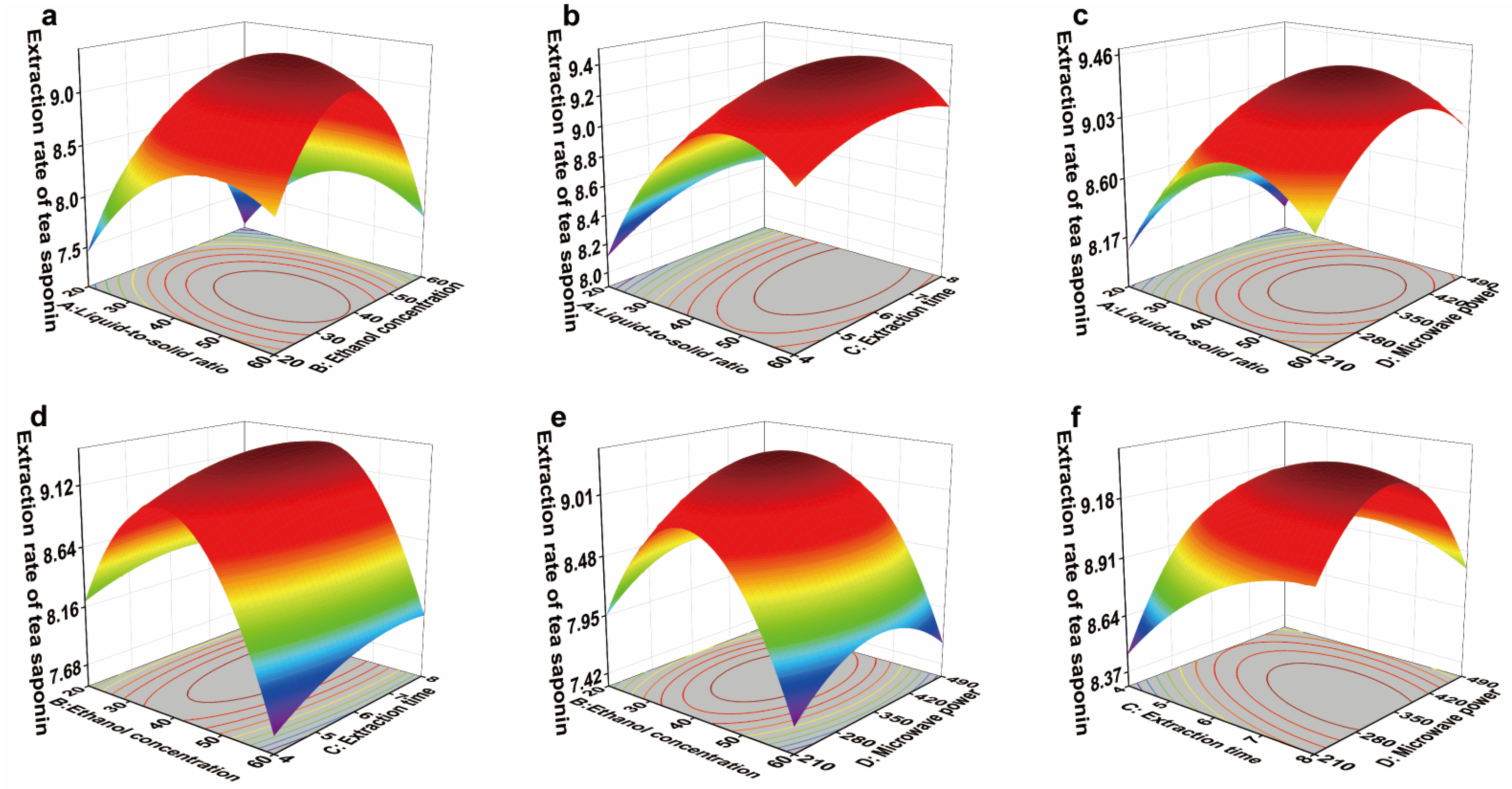
3.6. Analysis of Response Surface Optimization Experimental Results
3.6.1. Response Surface Optimization of Tea Saponin Extraction in GZ Region
+7.30 + 0.36 × A − 0.51 × B + 0.045 × C + 0.12 × D + 0.015 × AB − 0.03 × AC + 0.037 × AD − 0.012 × BC − 5 × 10−3 × BD − 0.018 × CD − 0.075 × A2 − 1.03 × B2 − 0.53 × C2 − 0.48 × D2
3.6.2. Response Surface Optimization of Tea Saponin Extraction in YC Region
3.6.3. Response Surface Optimization of Tea Saponin Extraction in the JJ Region
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luan, F.; Zeng, J.; Yang, Y.; He, X.; Wang, B.; Gao, Y.; Zeng, N. Recent Advances in Camellia oleifera Abel: A Review of Nutritional Constituents, Biofunctional Properties, and Potential Industrial Applications. J. Funct. Foods 2020, 75, 104242. [Google Scholar] [CrossRef]
- Yang, S.; Li, M.; Wang, Y.; Liu, X.; Qing, Y.; Li, X.; Wu, Y.; Liu, M.; Zhang, X. Industrial-Scale Manufacturing of Particleboards Using Agricultural Waste Camellia oleifera Shells. Constr. Build. Mater. 2024, 424, 135922. [Google Scholar] [CrossRef]
- Que Anh, H.N.; Tan Quoc, L.P.; Ngoc My, T.; Quynh Chi, L.N.; Phuong Khanh, P.T. A Review on Camellia oleifera Abel.: A Valuable Material in Food and Medicine. Food Sci. Preserv. 2024, 31, 333–345. [Google Scholar] [CrossRef]
- Wang, L.; Ok, Y.S.; Tsang, D.C.W.; Alessi, D.S.; Rinklebe, J.; Mašek, O.; Bolan, N.S.; Hou, D. Biochar Composites: Emerging Trends, Field Successes and Sustainability Implications. Soil Use Manag. 2022, 38, 14–38. [Google Scholar] [CrossRef]
- Wu, Y.; Xie, M.; Liu, X.; Qiu, S.; Zeng, W.; Jiang, Z.; Liu, R.; Xiao, Z.; Li, C.; Zhang, Y. Structural Characterization of Lignin Fractionated by Acidic Deep Eutectic Solvents and Fabrication of Lignin Nanoparticles from Camellia oleifera Shell. Ind. Crops Prod. 2024, 210, 118018. [Google Scholar] [CrossRef]
- Chen, P.; Hu, C.; Gu, J.; Lin, X.; Yang, C.; Leu, S.-Y.; Guan, L. Pyrolysis Characteristics of Tea Oil Camellia (Camellia oleifera Abel.) Shells and Their Chemically Pre-Treated Residues: Kinetics, Mechanisms, Product Evaluation and Joint Optimization. J. Anal. Appl. Pyrolysis 2022, 164, 105526. [Google Scholar] [CrossRef]
- Negm, N.A.; Betiha, M.H.A.; El-Wakeel, N.M.H.; Mohamed, E.A. An Insight into Recent Developments in Sustainable Biofuel Production Using Activated Carbon Catalyst Produced via Valorization of Agricultural Biomass: Challenges, and Environmental Prospective. Ind. Crops Prod. 2024, 209, 117991. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, W.; Lu, N.; Yu, J.; Chen, S.; Liang, Z. The Role of Camellia Shell Substrates in Modulating the Nutritional Characteristics of Pleurotus pulmonarius. Foods 2024, 13, 2946. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, T.; Hu, Y.; Shu, Q.; Xu, W.; Fan, C.; Zhou, G. Two New Aryltetralin-Type Lignans from Camellia oleifera Husk. Nat. Prod. Res. 2024, 38, 2264–2271. [Google Scholar] [CrossRef]
- Jiang, L.; Wu, J.; Liu, S.; Wu, W.; Liao, L. Effect of Alkaline Microcrystalline Cellulose Deacidification on Chemical Composition, Antioxidant Activity and Volatile Compounds of Camellia Oil. LWT 2023, 186, 115214. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Y.; Huang, S.; Luo, J.; Sun, M.; Chen, Y.; Jiang, S.; Guo, G.; Chang, Y. Comparison of Fatty Acid Composition in Seed Kernels of Tea Plant (Camellia sinensis), between Conventional Cultivars and Albino Cultivars. Cogent Food Agric. 2023, 9, 2272464. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, N.; Zhao, Y.; Jin, H.; Sun, G.; Yu, J.; Zhang, H.; Shao, J.; Yu, M.; Yang, D.; et al. The Extraction Using Deep Eutectic Solvents and Evaluation of Tea Saponin. Biology 2024, 13, 438. [Google Scholar] [CrossRef] [PubMed]
- Niu, A.; Wang, C.; Liu, F.; Ling, G.; Wang, Y.; Liu, S.; Hu, X. Mechanism and Regulation of Tea Saponin Extraction from C. oleifera Seed Meal in Subcritical Water. Foods 2025, 14, 1849. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; He, Y. Optimization of Tea-leaf Saponins Water Extraction and Relationships between Their Contents and Tea (Camellia sinensis) Tree Varieties. Food Sci. Nutr. 2018, 6, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, S.; Wan, F.; Zhou, Y.; Wang, Z.; Fan, G.; Wang, P.; Luo, H.; Liao, S.; He, L.; et al. Quantitative Analysis of Camellia oleifera Seed Saponins and Aqueous Two-Phase Extraction and Separation. Molecules 2023, 28, 2132. [Google Scholar] [CrossRef]
- Yu, X.-L.; He, Y. Development of a Rapid and Simple Method for Preparing Tea-Leaf Saponins and Investigation on Their Surface Tension Differences Compared with Tea-Seed Saponins. Molecules 2018, 23, 1796. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.; Chi, X.; Chen, S. Green and Efficient Extraction of Podophyllotoxin from Sinopodophyllum hexandrum by Optimized Subcritical Water Extraction Combined with Macroporous Resin Enrichment. Ind. Crops Prod. 2018, 121, 267–276. [Google Scholar] [CrossRef]
- Yang, P.; Zhou, M.; Zhou, C.; Wang, Q.; Zhang, F.; Chen, J. Separation and Purification of Both Tea Seed Polysaccharide and Saponin from Camellia Cake Extract Using Macroporous Resin. J. Sep. Sci. 2015, 38, 656–662. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Y.; Liu, Y.; Kong, M.; Ma, K.; Wang, J.; Wang, H.; Zhao, Y. Ultrasonic Pretreatment Combined with Microwave-Assisted Hydrodistillation for the Situ Extraction of Essential Oil from Pinus koraiensis Seed Scales Induced by Tea Saponin: Functional Activity, Composition, Thermal Stability and Material Characterization. Ind. Crops Prod. 2024, 210, 118191. [Google Scholar] [CrossRef]
- Zhang, S.; Niu, L.; Si, X.; Li, L.; Sheng, Z. Microwave-Assisted Extraction of Luteolin from Peanut Shells Using Natural Deep Eutectic Solvents and Its Molecular Mechanism. Ind. Crops Prod. 2025, 225, 120578. [Google Scholar] [CrossRef]
- Jurić, M.; Golub, N.; Galić, E.; Radić, K.; Maslov Bandić, L.; Vitali Čepo, D. Microwave-Assisted Extraction of Bioactive Compounds from Mandarin Peel: A Comprehensive Biorefinery Strategy. Antioxidants 2025, 14, 722. [Google Scholar] [CrossRef]
- Dhotre, I. A Comprehensive Review on Progression and Innovations in Microwave Assisted Extraction Technology for Essential Oils. J. Chem. Technol. Biotechnol. 2025, 100, 894–907. [Google Scholar] [CrossRef]
- Mobasheri, F.; Khajeh, M.; Ghaffari-Moghaddam, M.; Piri, J.; Bohlooli, M. Machine Learning Optimization of Microwave-Assisted Extraction of Phenolics and Tannins from Pomegranate Peel. Sci. Rep. 2025, 15, 19439. [Google Scholar] [CrossRef]
- Soest, P.J.V. Use of Detergents in the Analysis of Fibrous Feeds. II. A Rapid Method for the Determination of Fiber and Lignin. J. Assoc. Off. Agric. Chem. 1963, 46, 829–835. [Google Scholar] [CrossRef]
- Yan, H.; Lu, R.; Liu, Y.; Cui, X.; Wang, Y.; Yu, Z.; Ruan, R.; Zhang, Q. Development of Microalgae-Bacteria Symbiosis System for Enhanced Treatment of Biogas Slurry. Bioresour. Technol. 2022, 354, 127187. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Lu, R.; Hong, B.; Wang, Y.; Cui, X.; Liu, C.; Liu, Y.; Wu, X.; Ruan, R.; Zhang, Q. Microalgal Biofilm Cultivation on Lignocellulosic Based Bio-Carriers: Effects of Material Physical Characteristics on Microalgal Biomass Production and Composition. Chem. Eng. J. 2025, 510, 161656. [Google Scholar] [CrossRef]
- Gu, Z.; Liu, Y.; Zhu, L.; Fan, B.; Li, Y.; Liu, C.; Wang, Y.; Cui, X.; Yu, Z.; Ruan, R.; et al. Hormetic Effect of Dissolved Organic Matter from Pig Manure Anaerobic Digestion Effluents on Chlorella sp.: Physiological and Transcriptomic Responses. Water Res. 2025, 283, 123877. [Google Scholar] [CrossRef]
- Tomlinson, G.H.; Hibbert, H. Studies on Lignin and Related Compounds. XXIV. The Formation of Vanillin from Waste Sulfite Liquor1. J. Am. Chem. Soc. 1936, 58, 345–348. [Google Scholar] [CrossRef]
- Musci, M.; Yao, S. Optimization and Validation of Folin–Ciocalteu Method for the Determination of Total Polyphenol Content of Pu-Erh Tea. Int. J. Food Sci. Nutr. 2017, 68, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Gudej, J.; Tomczyk, M. Determination of Flavonoids, Tannins and Ellagic Acid in Leaves from Rubus L. Species. Arch. Pharm. Res. 2004, 27, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhao, H.; Zhang, X.; Zhang, W.; Liu, X.; Gao, S. Comparison of Different Extraction Techniques and Optimization of the Microwave-Assisted Extraction of Saponins from Aralia elata (Miq.) Seem Fruits and Rachises. Chem. Pap. 2020, 74, 3077–3087. [Google Scholar] [CrossRef]
- Wan, X.; Sun, D.; Nie, Y.; Wang, Q.; Zhang, T.; Wang, R.; Li, F.; Zhao, X.; Gao, C. Analysis and Evaluation of Camellia oleifera Abel. Germplasm Fruit Traits from the High-Altitude Areas of East Guizhou Province, China. Sci. Rep. 2024, 14, 18440. [Google Scholar] [CrossRef]
- Wardana, M.S.; Jufri, M.; Mun’Im, A. Physicochemical Properties and Nutrition of Moringa oleifera Lam. Leaf Extract: A Preliminary Study on Preparation Phytosomes as Herbal Supplement for Children. Int. J. App Pharm. 2022, 14, 281–287. [Google Scholar] [CrossRef]
- Mao, Y.; Robinson, J.; Binner, E. Understanding Heat and Mass Transfer Processes during Microwave-Assisted and Conventional Solvent Extraction. Chem. Eng. Sci. 2021, 233, 116418. [Google Scholar] [CrossRef]
- Huang, J.; Liao, J.; Li, X.; Zhao, H.; Li, H.; Kuang, J.; Li, J.; Guo, J.; Huang, T.; Li, J. Tea Saponin-Zein Binary Complex as a Quercetin Delivery Vehicle: Preparation, Characterization, and Functional Evaluation. Int. J. Biol. Macromol. 2024, 279, 135485. [Google Scholar] [CrossRef]
- Rath, S.; Pradhan, D.; Du, H.; Mohapatra, S.; Thatoi, H. Transforming Lignin into Value-Added Products: Perspectives on Lignin Chemistry, Lignin-Based Biocomposites, and Pathways for Augmenting Ligninolytic Enzyme Production. Adv. Compos. Hybrid. Mater. 2024, 7, 27. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Du, M.; Yao, X.; Hu, L. Physicochemical Properties of Camellia Nut Shell and Its Thermal Degradation Characteristics. BioResources 2014, 10, 647–659. [Google Scholar] [CrossRef]
- Zhang, L.; He, Y.; Zhu, Y.; Liu, Y.; Wang, X. Camellia oleifera Shell as an Alternative Feedstock for Furfural Production Using a High Surface Acidity Solid Acid Catalyst. Bioresour. Technol. 2018, 249, 536–541. [Google Scholar] [CrossRef]
- Dahmoune, B.; Houma-Bachari, F.; Chibane, M.; Akrour-Aissou, C.; Guégan, J.-P.; Vives, T.; Jéhan, P.; Dahmoune, F.; Mouni, L.; Ferrières, V.; et al. Microwave Assisted Extraction of Bioactive Saponins from the Starfish Echinaster sepositus: Optimization by Response Surface Methodology and Comparison with Ultrasound and Conventional Solvent Extraction. Chem. Eng. Process. —Process Intensif. 2021, 163, 108359. [Google Scholar] [CrossRef]
- Feng, K.; Liu, G.; Zhang, Z.; Liu, H.; Lv, R.; Wang, X.; Chang, P.; Lin, J.; Barakos, G. Fractal Strategy for Improving Characterization of N2 Adsorption–Desorption in Mesopores. Fractal Fract. 2024, 8, 617. [Google Scholar] [CrossRef]
- Kocer, S.; Utku Copur, O.; Ece Tamer, C.; Suna, S.; Kayahan, S.; Uysal, E.; Cavus, S.; Akman, O. Optimization and Characterization of Chestnut Shell Pigment Extract Obtained Microwave Assisted Extraction by Response Surface Methodology. Food Chem. 2024, 443, 138424. [Google Scholar] [CrossRef] [PubMed]
- Raiolo, A.; Stockinger, C.; Tuttlies, U.; Ivleva, N.P.; Shadloo, M.S.; Nieken, U. Effect of Nanostructure and BET Surface Area on the Oxygen Reactivity of Soot Filter Cakes. Carbon 2024, 227, 119251. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Du, X.; Fu, B.; Jiang, P.; Qi, L.; Shang, S. Characterization of Bamboo Shoots Dietary Fiber Modified by Ball Milling and Its Role in Altering the Physicochemical Properties of Shrimp Surimi. Int. J. Biol. Macromol. 2024, 271, 131979. [Google Scholar] [CrossRef]
- Jiang, H.; Hu, Y.; Jiang, X.; Zhou, H. Maturity Stage Discrimination of Camellia oleifera Fruit Using Visible and Near-Infrared Hyperspectral Imaging. Molecules 2022, 27, 6318. [Google Scholar] [CrossRef]
- Giang, N.T.N.; Khai, T.V. Effects of Temperature and Duration on Extraction Process Utilising Aqueous Solvent towards Quality of Tea Derived from Dried Roots of Asparagus officinalis L. Mal. J. Nutr. 2024, 30, 417–428. [Google Scholar] [CrossRef]
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M.; Alsaggaf, A.H.A.; Ahmed, N. LC-QTOF-MS Analysis of Phenolics and Saponins Extracted from Aloe vera Leaves via Microwave Technology in Optimal Condition. S. Afr. J. Bot. 2021, 139, 362–373. [Google Scholar] [CrossRef]
- Cao, B.; Hu, D.; Wang, Z.; Nawaz, N.; Chen, H.; Huang, J.; Chen, F.; Lei, X.; Liu, Y. Asymmetric Responses of Soil Multifunctionality to Black Ground Fabric Mulching of Different Camellia oleifera Abel. Varieties. Ind. Crops Prod. 2025, 229, 120894. [Google Scholar] [CrossRef]
- Waziiroh, E.; Kamilia, K. Microwave-Assisted Extraction (MAE) of Bioactive Saponin from Mahogany Seed (Swietenia mahogany Jacq). IOP Conf. Ser. Earth Environ. Sci. 2018, 131, 012006. [Google Scholar] [CrossRef]
- Bin Mokaizh, A.A.; Hamid Nour, A.; Ali, G.A.M.; Ishmael Ukaegbu, C.; Faraj Hawege, E. Eco-Friendly and Efficient Extraction of Phenolic Compounds from Commiphora gileadensis Bark Using Microwave-Assisted Extraction. J. Ind. Eng. Chem. 2025, 142, 321–328. [Google Scholar] [CrossRef]
- Ciuperca (Apreutesei), O.T.; Ionescu, E.; Secula, M.S.; Volf, I. Microwave-Assisted Extraction of Condensed Tannins from Branches of Prunus spinosa L.: Response Surface Modeling and Optimization. Processes 2023, 11, 2024. [Google Scholar] [CrossRef]
- Tambun, R.; Husna, R.; Fitri, M.D.; Ginting, Y.; Alexander, V. The Use of Soxhletation Method and Microwave-Assisted Extraction in Extracting Tannin from Jengkol Peel (Pithecellobium jiringa). IOP Conf. Ser.: Mater. Sci. Eng. 2021, 1122, 012092. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, H.; Wang, M.; Zhao, S.; Sun, G.; Li, Z. Recent Advancements in Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioprocess. Technol. 2025, 18, 2083–2100. [Google Scholar] [CrossRef]
- Ramírez-Brewer, D.; Quintana, S.E.; García-Zapateiro, L.A. Modeling and Optimization of Microwave-Assisted Extraction of Total Phenolics Content from Mango (Mangifera indica) Peel Using Response Surface Methodology (RSM) and Artificial Neural Networks (ANN). Food Chem. X 2024, 22, 101420. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Peng, M.-J.; Wang, Z.-H.; Yang, Q.-L.; Peng, S. Ultrasound-Microwave Assisted Extraction of Flavonoid Compounds from Eucommia ulmoides Leaves and an Evaluation of Their Antioxidant and Antibacterial Activities. Arch Biol Sci. 2020, 72, 211–221. [Google Scholar] [CrossRef]
- Cervantes-Güicho, V.D.J.; Ríos-González, L.J.; Reyes-Alvarado, A.G.; Morales-Martínez, T.K. Microwave-Assisted Extraction of Flavonoids from Lechuguilla guishe. Ing. Agrícola Biosist. 2024, 16, 39–53. [Google Scholar] [CrossRef]
- He, L.; Ku, C.; Guo, H.; Peng, Y.; Chen, Y.; Yan, L. Microwave-Assisted Extraction of Camellia oleifera Shell Lignin via Dual-Acidic Deep Eutectic Solvent and Its Mechanism. Int. J. Biol. Macromol. 2025, 323, 147077. [Google Scholar] [CrossRef]
- Mugwagwa, L.R.; Chimphango, A.F.A. Box-Behnken Design Based Multi-Objective Optimisation of Sequential Extraction of Pectin and Anthocyanins from Mango Peels. Carbohydr. Polym. 2019, 219, 29–38. [Google Scholar] [CrossRef]
- Khan, A.A.; Zaidi, S.; Qureshi, F.; Yusuf, M.; Al-Kahtani, A.A.; Kamyab, H.; Gupta, M.; Pandit, B.; Gill, H.S.; Ibrahim, H. Response Surface Optimization and Support Vector Regression Modeling of Microwave-Assisted Essential Oil Extraction from Cumin Seeds. Ind. Crops Prod. 2024, 208, 117895. [Google Scholar] [CrossRef]
- Du, G.; Liu, Y.; Zhang, J.; Fang, S.; Wang, C. Microwave-Assisted Extraction of Dandelion Root Polysaccharides: Extraction Process Optimization, Purification, Structural Characterization, and Analysis of Antioxidant Activity. Int. J. Biol. Macromol. 2025, 299, 139732. [Google Scholar] [CrossRef]
- Chang, C.-W.; Yen, C.-C.; Wu, M.-T.; Hsu, M.-C.; Wu, Y.-T. Microwave-Assisted Extraction of Cannabinoids in Hemp Nut Using Response Surface Methodology: Optimization and Comparative Study. Molecules 2017, 22, 1894. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Wu, D.; Xu, M.; Chen, J. Microwave-Assisted Extraction of Polyphenols from Camellia oleifera Fruit Hull. Molecules 2011, 16, 4428–4437. [Google Scholar] [CrossRef]
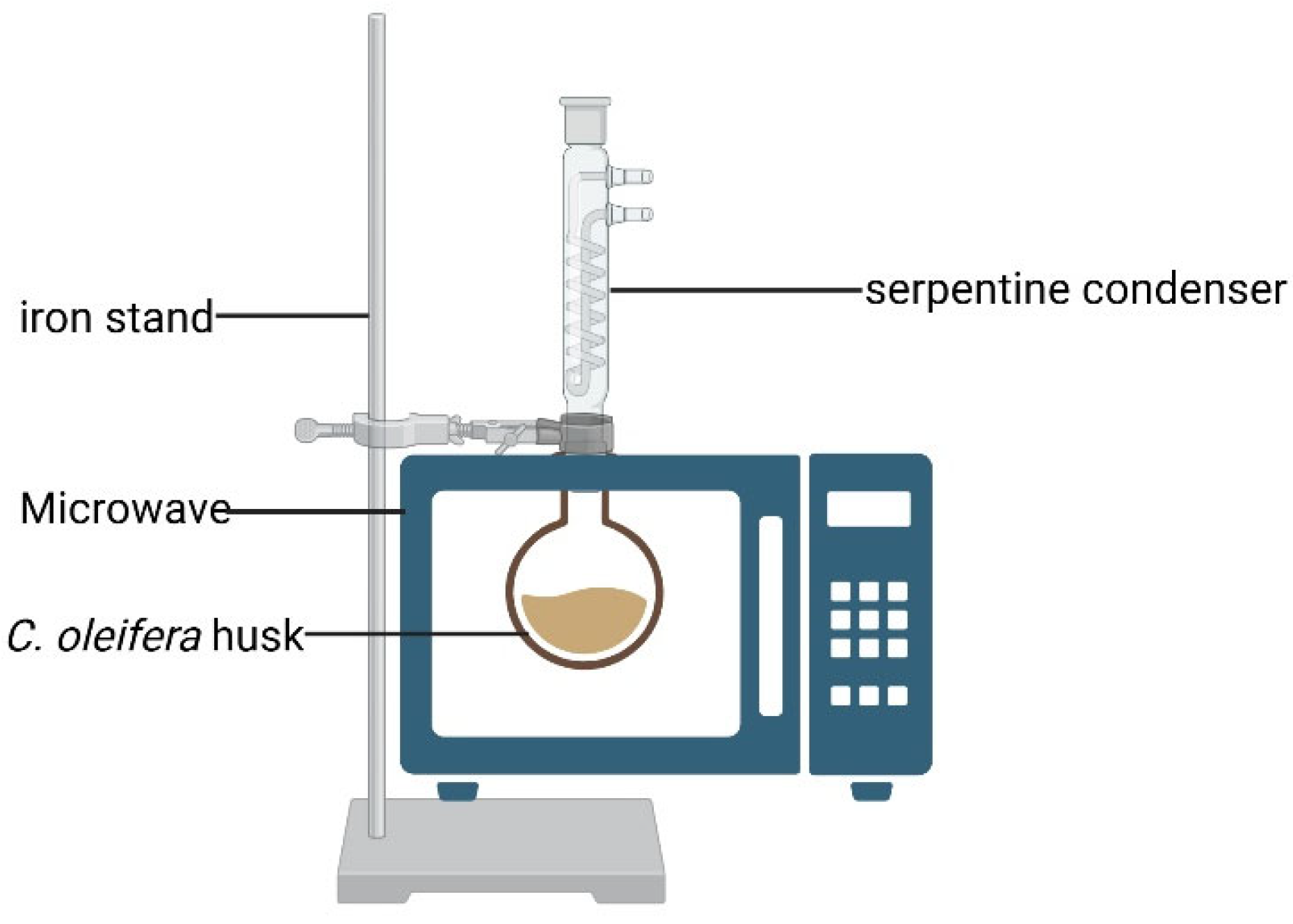



| Experimental Factors | Label | Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Liquid-to-solid ratio (mL/g) | A | 20 | 40 | 60 |
| Ethanol concentration (%) | B | 20 | 40 | 60 |
| Extraction time (min) | C | 4 | 6 | 8 |
| Microwave power (W) | D | 210 | 350 | 490 |
| The origin of C. oleifera husks | Elemental Analysis (%) | ||||
|---|---|---|---|---|---|
| C | H | O | N | S | |
| GZ | 46.24 ± 0.07 b | 5.72 ± 0.03 a | 47.61 ± 0.11 b | 0.42 ± 0.02 a | 0.02 ± 0.00 b |
| YC | 45.76 ± 0.03 c | 5.68 ± 0.01 b | 48.14 ± 0.03 a | 0.41 ± 0.01 ab | 0.02 ± 0.00 b |
| JJ | 46.97 ± 0.06 a | 5.71 ± 0.01 a | 46.88 ± 0.08 c | 0.39 ± 0.01 b | 0.06 ± 0.01 a |
| Sample (Mesh Size) | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) | Most Frequent Pore Diameter (nm) |
|---|---|---|---|---|
| GZ40~60 | 1.57 ± 0.02 c | 0.004 ± 0.00 | 9.66 ± 0.04 f | 1.88 ± 0.01 a |
| GZ60~80 | 1.28 ± 0.01 e | 0.003 ± 0.00 | 10.22 ± 0.05 e | 1.83 ± 0.01 b |
| GZ80~100 | 1.23 ± 0.01 f | 0.004 ± 0.00 | 13.46 ± 0.07 a | 1.58 ± 0.01 c |
| YC40~60 | 1.53 ± 0.02 d | 0.004 ± 0.00 | 10.75 ± 0.06 d | 1.86 ± 0.01 ab |
| YC60~80 | 1.48 ± 0.01 d | 0.005 ± 0.00 | 12.63 ± 0.06 c | 1.85 ± 0.01 ab |
| YC80~100 | 1.50 ± 0.01 d | 0.005 ± 0.00 | 12.67 ± 0.06 c | 1.57 ± 0.01 c |
| JJ40~60 | 4.15 ± 0.04 a | 0.007 ± 0.00 | 6.77 ± 0.04 h | 1.86 ± 0.01 ab |
| JJ60~80 | 3.41 ± 0.03 b | 0.006 ± 0.00 | 7.31 ± 0.05 g | 1.86 ± 0.01 ab |
| JJ80~100 | 0.89 ± 0.01 g | 0.003 ± 0.00 | 12.95 ± 0.07 b | 1.59 ± 0.01 c |
| Test Number | A | B | C | D | Extraction Yield of Tea Saponins (%) |
|---|---|---|---|---|---|
| 1 | 20 | 20 | 6 | 350 | 6.36 |
| 2 | 40 | 40 | 4 | 210 | 7.10 |
| 3 | 60 | 40 | 6 | 210 | 5.31 |
| 4 | 40 | 60 | 6 | 210 | 6.11 |
| 5 | 40 | 40 | 6 | 350 | 6.13 |
| 6 | 40 | 20 | 8 | 350 | 6.25 |
| 7 | 20 | 40 | 4 | 350 | 6.42 |
| 8 | 40 | 40 | 6 | 350 | 6.47 |
| 9 | 60 | 40 | 8 | 350 | 6.30 |
| 10 | 60 | 60 | 6 | 350 | 6.95 |
| 11 | 20 | 40 | 8 | 350 | 6.48 |
| 12 | 40 | 40 | 6 | 350 | 7.28 |
| 13 | 60 | 40 | 4 | 350 | 6.21 |
| 14 | 40 | 20 | 6 | 210 | 5.22 |
| 15 | 20 | 40 | 6 | 210 | 6.31 |
| 16 | 40 | 40 | 8 | 490 | 5.27 |
| 17 | 40 | 20 | 4 | 350 | 6.27 |
| 18 | 20 | 60 | 6 | 350 | 6.98 |
| 19 | 60 | 20 | 6 | 350 | 6.44 |
| 20 | 60 | 40 | 6 | 490 | 7.03 |
| 21 | 40 | 40 | 4 | 490 | 6.17 |
| 22 | 40 | 40 | 8 | 210 | 5.18 |
| 23 | 40 | 60 | 4 | 350 | 6.38 |
| 24 | 40 | 60 | 8 | 350 | 5.37 |
| 25 | 40 | 20 | 6 | 490 | 7.44 |
| 26 | 20 | 40 | 6 | 490 | 7.21 |
| 27 | 40 | 60 | 6 | 490 | 7.26 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model | 11.43 | 14 | 0.82 | 211.04 | <0.0001 b |
| A—Liquid-to-solid ratio | 1.53 | 1 | 1.53 | 396.57 | <0.0001 b |
| B—Ethanol concentration | 3.07 | 1 | 3.07 | 793.93 | <0.0001 b |
| C—Extraction time | 0.024 | 1 | 0.024 | 6.28 | 0.0276 a |
| D—Microwave power | 0.17 | 1 | 0.17 | 43.45 | <0.0001 b |
| AB | 9.00 × 10−4 | 1 | 9.00 × 10−4 | 0.23 | 0.6382 |
| AC | 3.60 × 10−3 | 1 | 3.60 × 10−3 | 0.93 | 0.3537 |
| AD | 5.63 × 10−3 | 1 | 5.63 × 10−3 | 1.45 | 0.2511 |
| BC | 6.25 × 10−4 | 1 | 6.25 × 10−4 | 0.16 | 0.6947 |
| BD | 1.00 × 10−4 | 1 | 1.00 × 10−4 | 0.026 | 0.8749 |
| CD | 1.23 × 10−3 | 1 | 1.23 × 10−3 | 0.32 | 0.5839 |
| A2 | 0.03 | 1 | 0.03 | 7.76 | 0.0165 a |
| B2 | 5.63 | 1 | 5.63 | 1455.95 | <0.0001 b |
| C2 | 1.48 | 1 | 1.48 | 381.92 | <0.0001 b |
| D2 | 1.22 | 1 | 1.22 | 316.08 | <0.0001 b |
| Residual | 0.046 | 12 | 3.87 × 10−3 | ||
| Lack of fit | 0.017 | 10 | 1.71 × 10−3 | 0.12 | 0.9931 |
| Pure error | 0.029 | 2 | 0.015 | ||
| Cor total | 11.47 | 26 |
| Test Number | A | B | C | D | Extraction Yield of Tea Saponin (%) |
|---|---|---|---|---|---|
| 1 | 20 | 20 | 6 | 350 | 15.03 |
| 2 | 40 | 40 | 4 | 210 | 14.75 |
| 3 | 60 | 40 | 6 | 210 | 13.78 |
| 4 | 40 | 60 | 6 | 210 | 15.24 |
| 5 | 40 | 40 | 6 | 350 | 14.41 |
| 6 | 40 | 20 | 8 | 350 | 14.73 |
| 7 | 20 | 40 | 4 | 350 | 14.75 |
| 8 | 40 | 40 | 6 | 350 | 15.27 |
| 9 | 60 | 40 | 8 | 350 | 14.98 |
| 10 | 60 | 60 | 6 | 350 | 15.34 |
| 11 | 20 | 40 | 8 | 350 | 15.02 |
| 12 | 40 | 40 | 6 | 350 | 16.18 |
| 13 | 60 | 40 | 4 | 350 | 14.31 |
| 14 | 40 | 20 | 6 | 210 | 13.91 |
| 15 | 20 | 40 | 6 | 210 | 14.88 |
| 16 | 40 | 40 | 8 | 490 | 14.22 |
| 17 | 40 | 20 | 4 | 350 | 14.66 |
| 18 | 20 | 60 | 6 | 350 | 15.32 |
| 19 | 60 | 20 | 6 | 350 | 14.98 |
| 20 | 60 | 40 | 6 | 490 | 15.74 |
| 21 | 40 | 40 | 4 | 490 | 14.18 |
| 22 | 40 | 40 | 8 | 210 | 14.13 |
| 23 | 40 | 60 | 4 | 350 | 14.92 |
| 24 | 40 | 60 | 8 | 350 | 14.27 |
| 25 | 40 | 20 | 6 | 490 | 16.15 |
| 26 | 20 | 40 | 6 | 490 | 15.96 |
| 27 | 40 | 60 | 6 | 490 | 16.29 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model | 12.37 | 14 | 0.88 | 74.09 | <0.0001 c |
| A—Liquid-to-solid ratio | 1.41 | 1 | 1.41 | 118.65 | <0.0001 c |
| B—Ethanol concentration | 0.53 | 1 | 0.53 | 44.39 | <0.0001 c |
| C—Extraction time | 0.5 | 1 | 0.5 | 42.3 | <0.0001 c |
| D—Microwave power | 0.58 | 1 | 0.58 | 48.72 | <0.0001 c |
| AB | 0.76 | 1 | 0.76 | 63.49 | <0.0001 c |
| AC | 2.50 × 10−3 | 1 | 2.50 × 10−3 | 0.21 | 0.6552 |
| AD | 0.16 | 1 | 0.16 | 13.42 | 0.0032 b |
| BC | 0.017 | 1 | 0.017 | 1.42 | 0.2568 |
| BD | 0.09 | 1 | 0.09 | 7.55 | 0.0177 a |
| CD | 1.00 × 10−2 | 1 | 1.00 × 10−2 | 0.84 | 0.3778 |
| A2 | 0.28 | 1 | 0.28 | 23.84 | 0.0004 b |
| B2 | 7.13 | 1 | 7.13 | 597.63 | <0.0001 c |
| C2 | 2.69 | 1 | 2.69 | 226.04 | <0.0001 c |
| D2 | 1.83 | 1 | 1.83 | 153.53 | <0.0001 c |
| Residual | 0.14 | 12 | 0.012 | ||
| Lack of fit | 0.088 | 10 | 8.82 × 10−3 | 0.32 | 0.9109 |
| Pure error | 0.055 | 2 | 0.027 | ||
| Cor total | 12.51 | 26 |
| Test Number | A | B | C | D | Extraction Yield of Tea Saponins (%) |
|---|---|---|---|---|---|
| 1 | 20 | 20 | 6 | 350 | 7.46 |
| 2 | 40 | 40 | 4 | 210 | 8.28 |
| 3 | 60 | 40 | 6 | 210 | 7.18 |
| 4 | 40 | 60 | 6 | 210 | 7.72 |
| 5 | 40 | 40 | 6 | 350 | 8.42 |
| 6 | 40 | 20 | 8 | 350 | 9.01 |
| 7 | 20 | 40 | 4 | 350 | 8.84 |
| 8 | 40 | 40 | 6 | 350 | 8.86 |
| 9 | 60 | 40 | 8 | 350 | 8.07 |
| 10 | 60 | 60 | 6 | 350 | 8.66 |
| 11 | 20 | 40 | 8 | 350 | 7.91 |
| 12 | 40 | 40 | 6 | 350 | 8.92 |
| 13 | 60 | 40 | 4 | 350 | 8.23 |
| 14 | 40 | 20 | 6 | 210 | 7.63 |
| 15 | 20 | 40 | 6 | 210 | 8.37 |
| 16 | 40 | 40 | 8 | 490 | 8.02 |
| 17 | 40 | 20 | 4 | 350 | 8.15 |
| 18 | 20 | 60 | 6 | 350 | 8.88 |
| 19 | 60 | 20 | 6 | 350 | 8.43 |
| 20 | 60 | 40 | 6 | 490 | 9.01 |
| 21 | 40 | 40 | 4 | 490 | 7.90 |
| 22 | 40 | 40 | 8 | 210 | 7.48 |
| 23 | 40 | 60 | 4 | 350 | 8.08 |
| 24 | 40 | 60 | 8 | 350 | 7.61 |
| 25 | 40 | 20 | 6 | 490 | 9.35 |
| 26 | 20 | 40 | 6 | 490 | 9.24 |
| 27 | 40 | 60 | 6 | 490 | 9.37 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model | 9.97 | 14 | 0.71 | 214.02 | <0.0001 c |
| A—Liquid-to-solid ratio | 1.52 | 1 | 1.52 | 456.68 | <0.0001 c |
| B—Ethanol concentration | 0.6 | 1 | 0.6 | 179.9 | <0.0001 c |
| C—Extraction time | 0.2 | 1 | 0.2 | 60.18 | <0.0001 c |
| D—Microwave power | 0.039 | 1 | 0.039 | 11.58 | 0.0052 b |
| AB | 0.02 | 1 | 0.02 | 5.89 | 0.0319 a |
| AC | 5.63 × 10−3 | 1 | 5.63 × 10−3 | 1.69 | 0.2179 |
| AD | 0.044 | 1 | 0.044 | 13.25 | 0.0034 b |
| BC | 0.016 | 1 | 0.016 | 4.70 | 0.0511 |
| BD | 6.25 × 10−4 | 1 | 6.25 × 10−4 | 0.19 | 0.6724 |
| CD | 0.08 | 1 | 0.08 | 24.41 | 0.0003 b |
| A2 | 1.55 | 1 | 1.55 | 466.72 | <0.0001 c |
| B2 | 6.79 | 1 | 6.79 | 2040.84 | <0.0001 c |
| C2 | 0.11 | 1 | 0.11 | 32.36 | 0.0001 c |
| D2 | 0.87 | 1 | 0.87 | 260.77 | <0.0001 c |
| Residual | 0.04 | 12 | 3.33 × 10−3 | ||
| Lack of fit | 0.03 | 10 | 3.01 × 10−3 | 0.61 | 0.7554 |
| Pure error | 9.80 × 10−3 | 2 | 4.90 × 10−3 | ||
| Cor total | 10.01 | 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Liu, Y.; Huang, J.; Liu, X.; Zhang, G.; Gu, Z.; Huang, S.; Wang, Y.; Zhang, Q. Physicochemical Characterization of Camellia oleifera Husks from Different Regions and Microwave-Assisted RSM Optimization of Tea Saponin Extraction. Foods 2025, 14, 3380. https://doi.org/10.3390/foods14193380
Wu W, Liu Y, Huang J, Liu X, Zhang G, Gu Z, Huang S, Wang Y, Zhang Q. Physicochemical Characterization of Camellia oleifera Husks from Different Regions and Microwave-Assisted RSM Optimization of Tea Saponin Extraction. Foods. 2025; 14(19):3380. https://doi.org/10.3390/foods14193380
Chicago/Turabian StyleWu, Weixian, Yuhuan Liu, Jian Huang, Xiaoyan Liu, Guangda Zhang, Zhiqiang Gu, Shuangquan Huang, Yunpu Wang, and Qi Zhang. 2025. "Physicochemical Characterization of Camellia oleifera Husks from Different Regions and Microwave-Assisted RSM Optimization of Tea Saponin Extraction" Foods 14, no. 19: 3380. https://doi.org/10.3390/foods14193380
APA StyleWu, W., Liu, Y., Huang, J., Liu, X., Zhang, G., Gu, Z., Huang, S., Wang, Y., & Zhang, Q. (2025). Physicochemical Characterization of Camellia oleifera Husks from Different Regions and Microwave-Assisted RSM Optimization of Tea Saponin Extraction. Foods, 14(19), 3380. https://doi.org/10.3390/foods14193380









