Anti-Protozoal Activity of Hops Essential Oil and Myrcene Against Cryptosporidium Parvum in Cell Culture
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Cytotoxicity Evaluation
2.3. C. parvum Preparation
2.4. In Vitro Anti-Cryptosporidium Activity
2.5. Immunofluorescence Determination of C. parvum Growth
2.6. Statistical Analyses
3. Results
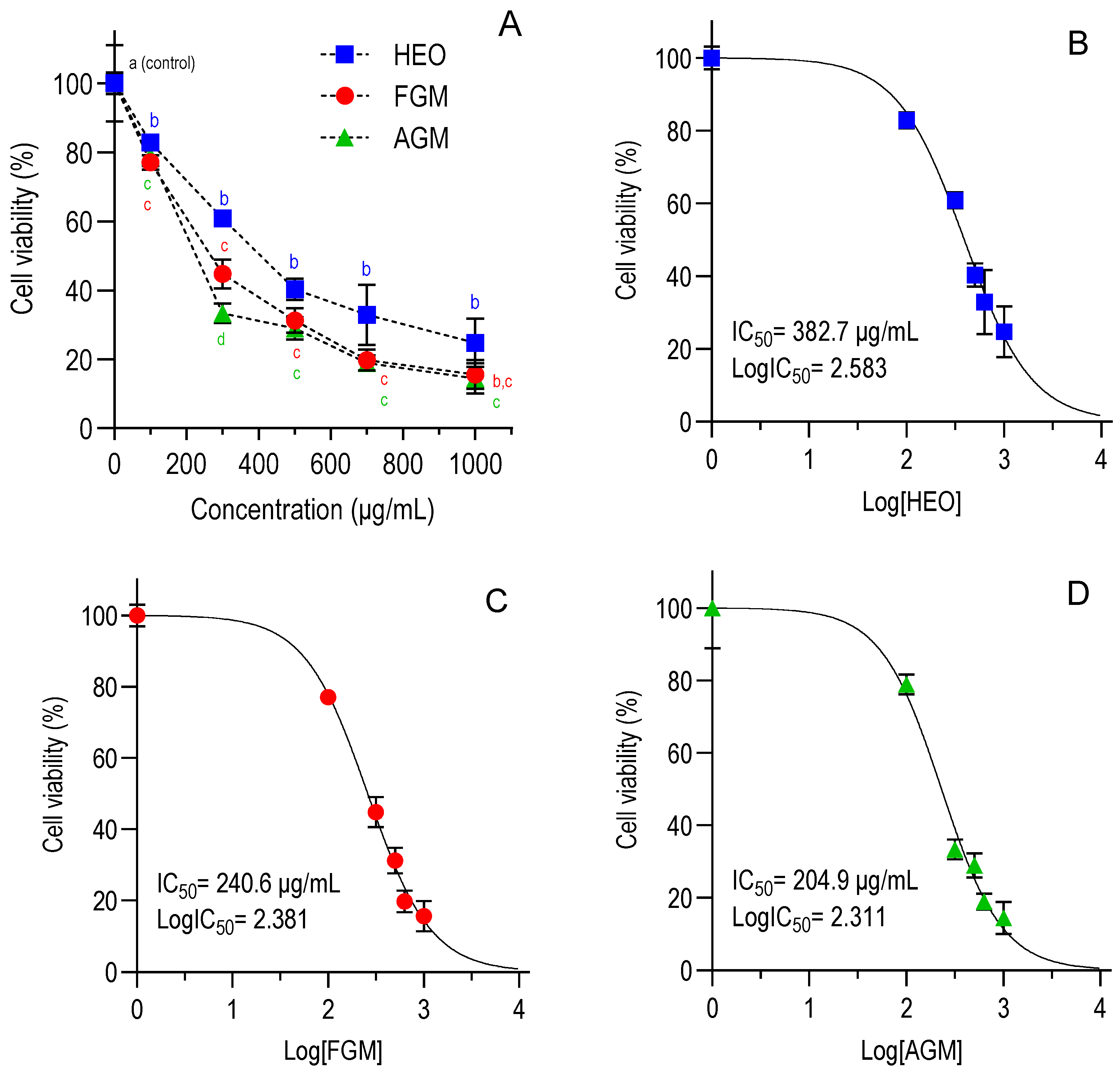
3.1. Evaluation of HEO and Myrcene Cytotoxicity
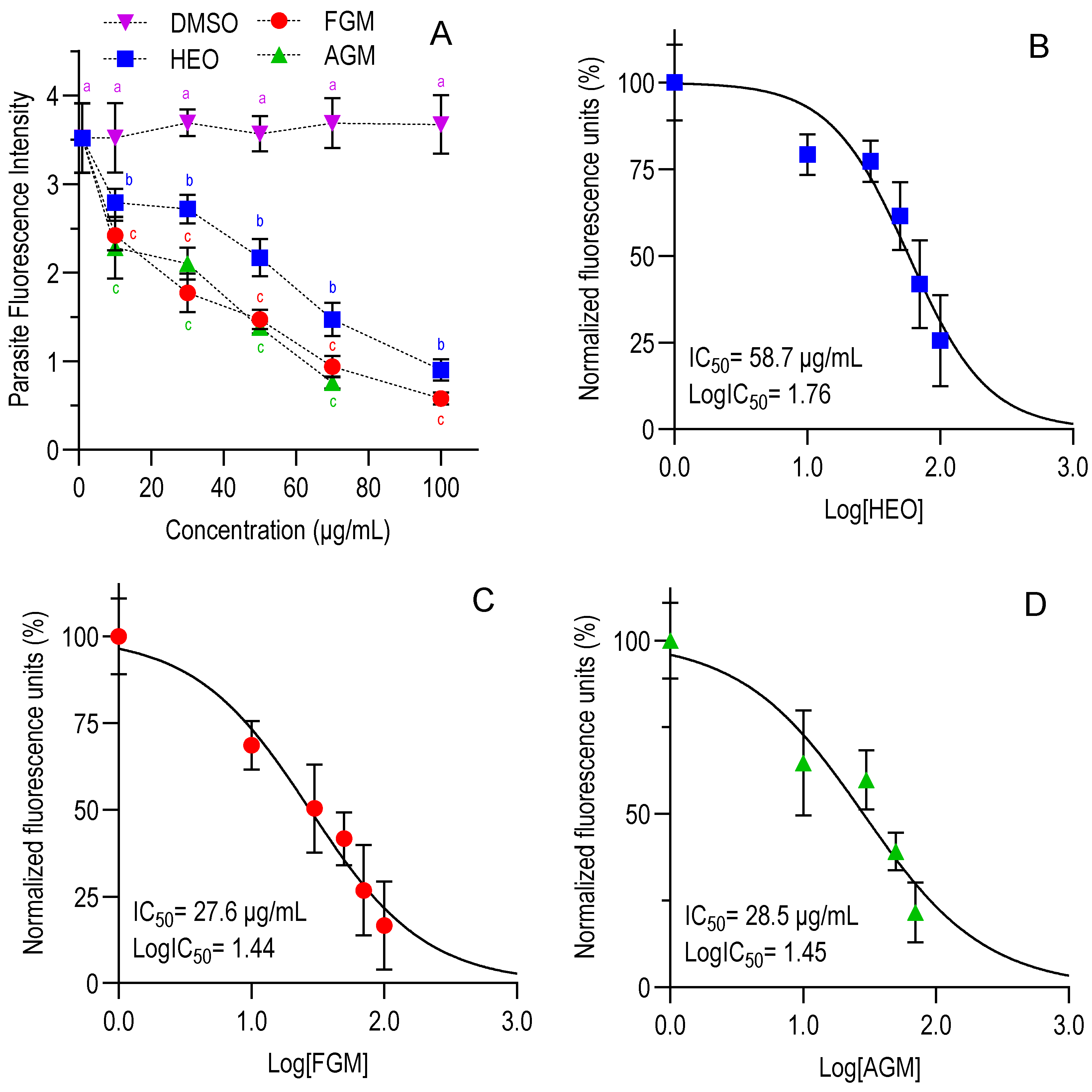
3.2. In Vitro Anti-Cryptosporidium Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UNICEF; World Health Organization; World Bank. Levels and Trends in Child Mortality; UN Inter-agency Group for Child Mortality Estimation: Washington, DC, USA, 2023; p. 96. [Google Scholar]
- Levine, M.M.; Nasrin, D.; Acácio, S.; Bassat, Q.; Powell, H.; Tennant, S.M.; Sow, S.O.; Sur, D.; Zaidi, A.K.M.; Faruque, A.S.G.; et al. Diarrhoeal disease and subsequent risk of death in infants and children residing in low-income and middle-income countries: Analysis of the gems case-control study and 12-month gems-1a follow-on study. Lancet Glob. Health 2020, 8, e204–e214. [Google Scholar] [CrossRef]
- Majorin, F.; Torondel, B.; Ka Seen Chan, G.; Clasen, T. Interventions to improve disposal of child faeces for preventing diarrhoea and soil-transmitted helminth infection. Cochrane Database Syst. Rev. 2019, 9, CD011055. [Google Scholar] [CrossRef] [PubMed]
- Ryan, U.; Zahedi, A.; Paparini, A. Cryptosporidium in humans and animals-a one health approach to prophylaxis. Parasite Immunol. 2016, 38, 535–547. [Google Scholar] [CrossRef]
- Ryan, U.M.; Feng, Y.; Fayer, R.; Xiao, L. Taxonomy and molecular epidemiology of Cryptosporidium and Giardia—A 50 year perspective (1971–2021). Int. J. Parasitol. 2021, 51, 1099–1119. [Google Scholar] [CrossRef] [PubMed]
- Sparks, H.; Nair, G.; Castellanos-Gonzalez, A.; White, A.C. Treatment of Cryptosporidium: What we know, gaps, and the way forward. Curr. Trop. Med. Rep. 2015, 2, 181–187. [Google Scholar] [CrossRef]
- Khan, S.M.; Witola, W.H. Past, current, and potential treatments for cryptosporidiosis in humans and farm animals: A comprehensive review. Front. Cell. Infect. Microbiol. 2023, 13, 1115522. [Google Scholar] [CrossRef]
- Khalil, I.A.; Troeger, C.; Rao, P.C.; Blacker, B.F.; Brown, A.; Brewer, T.G.; Colombara, D.V.; De Hostos, E.L.; Engmann, C.; Guerrant, R.L.; et al. Morbidity, mortality, and long-term consequences associated with diarrhoea from Cryptosporidium infection in children younger than 5 years: A meta-analyses study. Lancet Glob. Health 2018, 6, e758–e768. [Google Scholar] [CrossRef]
- Amadi, B.; Mwiya, M.; Musuku, J.; Watuka, A.; Sianongo, S.; Ayoub, A.; Kelly, P. Effect of nitazoxanide on morbidity and mortality in zambian children with cryptosporidiosis: A randomised controlled trial. Lancet 2002, 360, 1375–1380. [Google Scholar] [CrossRef]
- Checkley, W.; White, A.C.; Jaganath, D.; Arrowood, M.J.; Chalmers, R.M.; Chen, X.M.; Fayer, R.; Griffiths, J.K.; Guerrant, R.L.; Hedstrom, L.; et al. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for Cryptosporidium. Lancet Infect. Dis. 2015, 15, 85–94. [Google Scholar] [CrossRef]
- Bartelt, L.A.; Bolick, D.T.; Kolling, G.L.; Stebbins, E.; Huston, C.D.; Guerrant, R.L.; Hoffman, P.S. Amixicile reduces severity of cryptosporidiosis but does not have in vitro activity against Cryptosporidium. Antimicrob. Agents Chemother. 2018, 62, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.P.; Fyfe, L.; Smith, H. Plant active components—A resource for antiparasitic agents? Trends Parasitol. 2005, 21, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Elbahaie, E.; El Gamal, R.; Fathy, G.; Al-Ghandour, A.; El-Akabawy, N.; Abd El Hameed, B.; Yahia, S. The controverted therapeutic efficacy of Allium sativum and Artemisia herba-alba extracts on Cryptosporidium-infected mice. J. Infect. Dev. Ctries. 2023, 17, 732–743. [Google Scholar] [CrossRef]
- Woolsey, I.; Valente, A.; Williams, A.; Thamsborg, S.; Simonsen, H.; Enemark, H. Anti-protozoal activity of extracts from chicory (Cichorium intybus) against Cryptosporidium parvum in cell culture. Sci. Rep. 2019, 9, 20414. [Google Scholar] [CrossRef]
- Asadpour, M.; Namazi, F.; Razavi, S.; Nazifi, S. Comparative efficacy of curcumin and paromomycin against Cryptosporidium parvum infection in a balb/c model. Vet. Parasitol. 2018, 250, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Salanta, L.; Farcas, A.; Borsa, A.; Pop, C. Current strategies for the management of valuable compounds from hops waste for a circular economy. Food Chem. X 2023, 19, 100876. [Google Scholar] [CrossRef]
- Duarte, P.; do Nascimento, L.; Bandiera, V.; Fischer, B.; Fernandes, I.; Paroul, N.; Junges, A. Exploring the versatility of hop essential oil (Humulus lupulus L.): Bridging brewing traditions with modern industry applications. Ind. Crops Prod. 2024, 218, 118974. [Google Scholar] [CrossRef]
- Cimini, A.; Moresi, M. Circular economy in the brewing chain. Ital. J. Food Sci. 2021, 33, 47–69. [Google Scholar] [CrossRef]
- Pereira, O.; Santos, G.; Sousa, M. Hop by-products: Pharmacological activities and potential application as cosmetics. Cosmetics 2022, 9, 139. [Google Scholar] [CrossRef]
- Zugravu, C.; Bohiltea, R.; Salmen, T.; Pogurschi, E.; Otelea, M. Antioxidants in hops: Bioavailability, health effects and perspectives for new products. Antioxidants 2022, 11, 241. [Google Scholar] [CrossRef]
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.; Becker, T. Humulus lupulus—A story that begs to be told. A review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
- Karabin, M.; Hudcova, T.; Jelinek, L.; Dostalek, P. Biotransformations and biological activities of hop flavonoids. Biotech. Adv. 2015, 33, 1063–1090. [Google Scholar] [CrossRef]
- Bocquet, L.; Rivière, C.; Dermont, C.; Samaillie, J.; Hilbert, J.; Halama, P.; Siah, A.; Sahpaz, S. Antifungal activity of hop extracts and compounds against the wheat pathogen Zymoseptoria tritici. Ind. Crops Prod. 2018, 122, 290–297. [Google Scholar] [CrossRef]
- Albayrak, G.; Yoruk, E.; Teker, T.; Sefer, O. Investigation of antifungal activities of myrcene on fusarium reference strains. Arch. Microbiolo. 2023, 205, 82. [Google Scholar] [CrossRef] [PubMed]
- Móricz, A.; Bartoszek, M.; Polak, J.; Marczewska, P.; Knas, M.; Böszörményi, A.; Fodor, J.; Kowalska, T.; Sajewicz, M. A comparison of quantitative composition and bioactivity of oils derived from seven north american varieties of hops (Humulus lupulus L.). Separations 2023, 10, 402. [Google Scholar] [CrossRef]
- Iglesias, A.; Mitton, G.; Szawarski, N.; Cooley, H.; Ramos, F.; Arcerito, F.; Brasesco, C.; Ramirez, C.; Gende, L.; Eguaras, M.; et al. Essential oils from Humulus lupulus as novel control agents against varroa destructor. Ind. Crops Prod. 2020, 158, 113043. [Google Scholar] [CrossRef]
- Chen, N.; Cai, Q.; Wang, S.; Song, Q.; Xie, Y.; Shi, H.; Li, H.; Zhao, X.; Zhao, N.; Zhang, X. Evaluation of the efficicacy of myrcene in the treatment of Eimeria tenella and Toxoplasma gondii infection. J. Vet. Med. Sci. 2025, 87, 32–42. [Google Scholar] [CrossRef]
- Jeliazkova, E.; Zheljazkov, V.; Kacániova, M.; Astatkie, T.; Tekwani, B. Sequential elution of essential oil constituents during steam distillation of hops (Humulus lupulus L.) and influence on oil yield and antimicrobial activity. J. Oleo Sci. 2018, 67, 871–883. [Google Scholar] [CrossRef]
- Polec, K.; Olechowska, K.; Klejdysz, A.; Dymek, M.; Rachwalik, R.; Sikora, E.; Hac-Wydro, K. The influence of ergosterol on the action of the hop oil and its major terpenes on model fungi membranes. Towards understanding the mechanism of action of phytocompounds for food and plant protection. Chem. Phy. Lipids 2021, 238, 105092. [Google Scholar] [CrossRef]
- Paventi, G.; de Acutis, L.; De Cristofaro, A.; Pistillo, M.; Germinara, G.; Rotundo, G. Biological activity of Humulus lupulus L. essential oil and its main components against Sitophilus granarius L. Biomolecules 2020, 10, 1108. [Google Scholar]
- Kuhlenschmidt, T.; Rutaganira, F.; Long, S.; Tang, K.; Shokat, K.; Kuhlenschmidt, M.; Sibley, L. Inhibition of calcium-dependent protein kinase 1 (cdpk1) in vitro by pyrazolopyrimidine derivatives does not correlate with sensitivity of Cryptosporidium parvum growth in cell culture. Antimicrob. Agents Chemother. 2016, 60, 570–579. [Google Scholar] [CrossRef]
- Pincigher, L.; Valenti, F.; Bergamini, C.; Prata, C.; Fato, R.; Amorati, R.; Jin, Z.; Farruggia, G.; Fiorentini, D.; Calonghi, N.; et al. Myrcene: A natural compound showing anticancer activity in Hela cells. Molecules 2023, 28, 6728. [Google Scholar] [CrossRef]
- Chaouki, W.; Leger, D.; Liagre, B.; Beneytout, J.; Hmamouchi, M. Citral inhibits cell proliferation and induces apoptosis and cell cycle arrest in MCF-7 cells. Fundam. Clin. Pharmacol. 2009, 23, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Tang, J. Myrcene exhibits antitumor activity against lung cancer cells by inducing oxidative stress and apoptosis mechanisms. Nat. Prod. Commun. 2020, 15. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Z.; Liang, Y. Myrcene exerts anti-tumor effects on oral cancer cells in vitro via induction of apoptosis. Trop. J. Pharmaceut Res. 2022, 21, 933–938. [Google Scholar] [CrossRef]
- Surendran, S.; Qassadi, F.; Surendran, G.; Lilley, D.; Heinrich, M. Myrcene-what are the potential health benefits of this flavouring and aroma agent? Front. Nutr. 2021, 8, 699666. [Google Scholar] [CrossRef]
- Mendanha, S.; Alonso, A. Effects of terpenes on fluidity and lipid extraction in phospholipid membranes. Biophys. Chem. 2015, 198, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Usta, J.; Kreydiyyeh, S.; Knio, K.; Barnabe, P.; Bou-Moughlabay, Y.; Dagher, S. Linalool decreases hepg2 viability by inhibiting mitochondrial complexes i and ii, increasing reactive oxygen species and decreasing ATP and GSH levels. Chem.-Biol. Interact. 2009, 180, 39–46. [Google Scholar] [CrossRef] [PubMed]
- El Hachlafi, N.; Elbouzidi, A.; Batbat, A.; Taibi, M.; Jeddi, M.; Addi, M.; Mrabti, H.; Fikri-Benbrahim, K. Chemical composition and assessment of the anti-inflammatory, antioxidant, cytotoxic and skin enzyme inhibitory activities of Citrus sinensis L. osbeck essential oil and its major compound limonene. Pharmaceuticals 2024, 17, 1652. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Gaur, S.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Andrade, J.E. Effect of oregano essential oil and carvacrol on Cryptosporidium parvum infectivity in HCT-8 cells. Parasitol. Int. 2018, 67, 170–175. [Google Scholar] [CrossRef]
- Dominguez-Uscanga, A.; Aycart, D.F.; Li, K.; Witola, W.H.; Andrade Laborde, J.E. Anti-protozoal activity of thymol and a thymol ester against Cryptosporidium parvum in cell culture. Int. J. Parasitol. Drugs Drug Resist. 2021, 15, 126–133. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.; dos Santos, K.; Mendes, T.; Garcia, V.; Oliveira, A.; Jeraldo, V.; Allegretti, S. Sesquiterpenes evaluation on Schistosoma mansoni: Survival, excretory system and membrane integrity. Biomed. Pharmacother. 2017, 90, 813–820. [Google Scholar] [CrossRef]
- Menezes, S.; Tasca, T. Essential oils and terpenic compounds as potential hits for drugs against amitochondriate protists. Trop. Med. Infect. Dis. 2023, 8, 37. [Google Scholar] [CrossRef]
- da Silva, B.; do Rosario, D.; Weitz, D.; Conte-Junior, C. Essential oil nanoemulsions: Properties, development, and application in meat and meat products. Trend Food Sci. Technol. 2022, 121, 1–13. [Google Scholar] [CrossRef]
- Papada, E.; Gioxari, A.; Amerikanou, C.; Galanis, N.; Kaliora, A. An absorption and plasma kinetics study of monoterpenes present in mastiha oil in humans. Foods 2020, 9, 1019. [Google Scholar] [CrossRef] [PubMed]
- Raj, G.; Wilson, D.; Jenitha; Kokelavani, N.; Srividhya, M.; Vijay, N.; Grace, V. Different in vivo administration routes of essential oil for various therapies: A review. Fitoterapia 2025, 184, 106577. [Google Scholar] [CrossRef] [PubMed]
- Gaur, S.; Lopez, E.C.; Ojha, A.; Andrade, J.E. Functionalization of lipid-based nutrient supplement with β-cyclodextrin inclusions of oregano essential oil. J. Food Sci. 2018, 83, 1748–1756. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.; Bergonzi, M.; Guccione, C.; Manconi, M.; Fadda, A.; Sinico, C. Vesicles and micelles: Two versatile vectors for the delivery of natural products. J. Drug Deliv. Sci. Technol. 2016, 32, 241–255. [Google Scholar] [CrossRef]
- Nor, M.; Kamal, N.; Khairuddean, M.; Chear, N.; Tong, W.; Leong, C.; Tan, W. Nanotechnology-based combination approach using essential oils and chemotherapeutic drugs for targeting cancer cells. Int. J. Polym. Mater. Polym. Biomater. 2025, 74, 1529–1545. [Google Scholar] [CrossRef]
- Tanghort, M.; Chefchaou, H.; Mzabi, A.; Moussa, H.; Chami, N.; Chami, F.; Remma, A. Oocysticidal effect of essential oils (eos) and their major components on Cryptosporidium baileyi and Cryptosporidium galli. Int. J. Poult. Sci. 2019, 18, 475–482. [Google Scholar] [CrossRef]
- Machado, M.; Dinis, A.; Salgueiro, L.; Custódio, J.; Cavaleiro, C.; Sousa, M. Anti-giardia activity of Syzygium aromaticum essential oil and eugenol: Effects on growth, viability, adherence and ultrastructure. Exp. Parasitol. 2011, 127, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Calzada, F.; Yepez-Mulia, L.; Tapia-Contreras, A.; Bautista, E.; Maldonado, E.; Ortega, A. Evaluation of the antiprotozoal activity of neo-clerodane type diterpenes from Salvia polystachya against Entamoeba histolytica and Giardia lamblia. Phytother. Res. 2010, 24, 662–665. [Google Scholar] [CrossRef]
- Vieira, P.; Silva, N.; da Silva, G.; Silva, D.; Lopes, N.; Gnoatto, S.; da Silva, M.; Macedo, A.; Bastida, J.; Tasca, T. Caatinga plants: Natural and semi-synthetic compounds potentially active against Trichomonas vaginalis. Bioorg. Med. Chem. Lett. 2016, 26, 2229–2236. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Peng, C.; Peng, F.; Xie, C.; Wang, P.; Sun, F. Anti-trichomonas vaginalis properties of the oil of amomum tsao-ko and its major component, geraniol. Pharm. Biol. 2016, 54, 445–450. [Google Scholar] [CrossRef] [PubMed]

| Cell Viability | Anti-Cryptosporidium Activity | ||||
|---|---|---|---|---|---|
| IC50 24 h [CI 95%] | IC50 48 h [CI 95%] | IC50 Invasion [CI 95%] | IC50 Growth [CI 95%] | Selectivity Index | |
| HEO | 675.2 [551.7–829.6] | 382.7 [338.9–431.7] | 45.8 [35.6–58.8] | 58.7 [46.4–72.9] | 6.5–8.4 |
| FGM | 980.9 [823.0–1178] | 240.6 [210.1–274.7] | 16.4 [13.3–19.1] | 27.6 [19.9–36.3] | 8.7–14.7 |
| AGM | 506.6 [434.9–590.1] | 204.9 [166.7–249.7] | 19.0 [14.6–24.3] | 28.5 [19.0–40.6] | 7.2–10.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aycart, D.F.; Domínguez-Uscanga, A.; Witola, W.H.; Andrade Laborde, J.E. Anti-Protozoal Activity of Hops Essential Oil and Myrcene Against Cryptosporidium Parvum in Cell Culture. Foods 2025, 14, 3352. https://doi.org/10.3390/foods14193352
Aycart DF, Domínguez-Uscanga A, Witola WH, Andrade Laborde JE. Anti-Protozoal Activity of Hops Essential Oil and Myrcene Against Cryptosporidium Parvum in Cell Culture. Foods. 2025; 14(19):3352. https://doi.org/10.3390/foods14193352
Chicago/Turabian StyleAycart, Danielle F., Astrid Domínguez-Uscanga, William H. Witola, and Juan E. Andrade Laborde. 2025. "Anti-Protozoal Activity of Hops Essential Oil and Myrcene Against Cryptosporidium Parvum in Cell Culture" Foods 14, no. 19: 3352. https://doi.org/10.3390/foods14193352
APA StyleAycart, D. F., Domínguez-Uscanga, A., Witola, W. H., & Andrade Laborde, J. E. (2025). Anti-Protozoal Activity of Hops Essential Oil and Myrcene Against Cryptosporidium Parvum in Cell Culture. Foods, 14(19), 3352. https://doi.org/10.3390/foods14193352










