Novel Insights into Torrefacto and Natural Coffee Silverskin: Composition, Bioactivity, Safety, and Environmental Impact for Sustainable Food Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Collection and Pretreatment
2.2. Proximate Composition of Silverskin
2.3. Silverskin Characterization and Properties
2.3.1. Color Measurement
2.3.2. Total Phenolic Content Determination
2.3.3. Antioxidant Capacity (ABTS)
2.3.4. Determination and Characterization of Melanoidins
Extraction and Yield Evaluation of the Extract
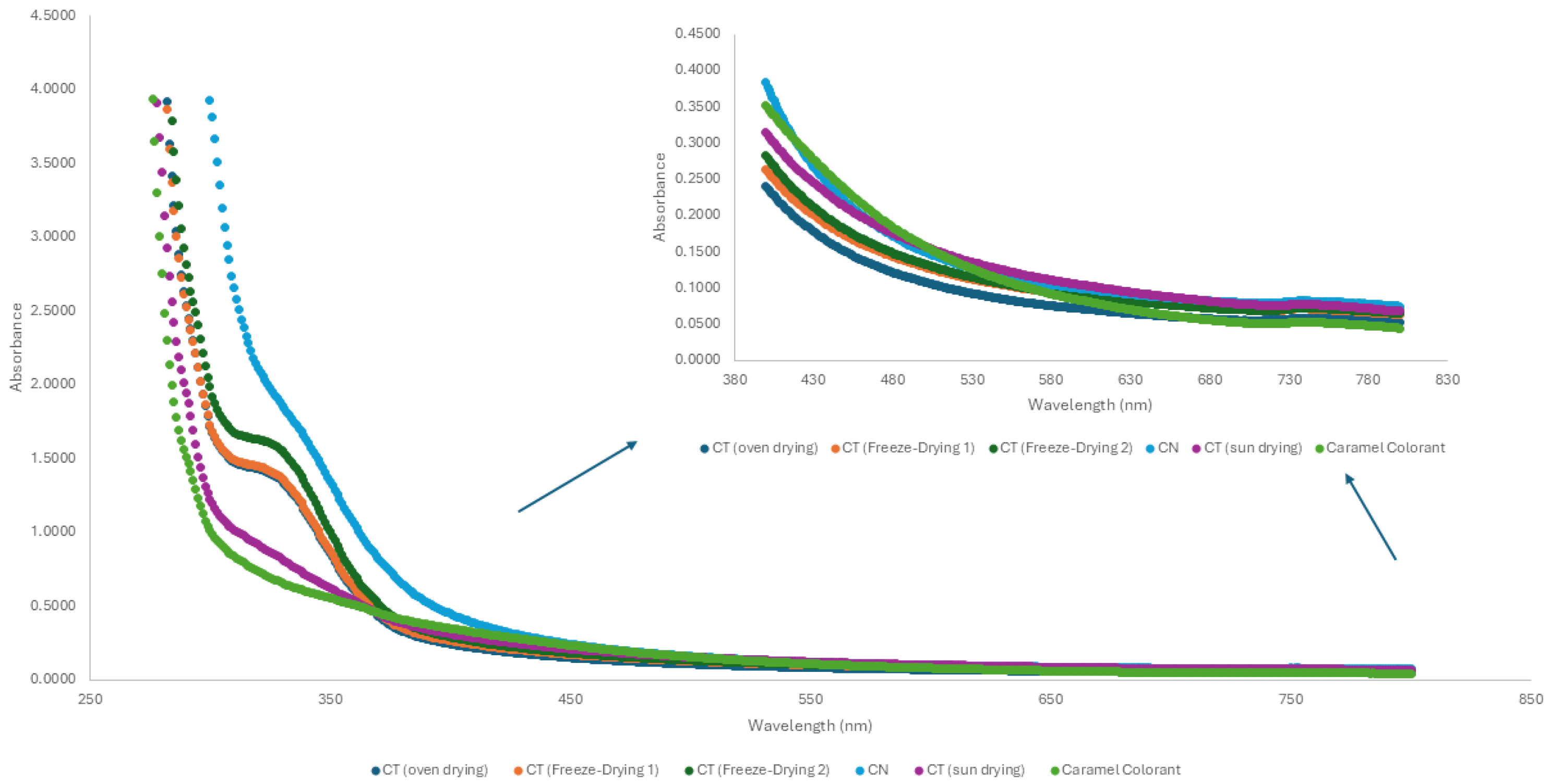
Color Evaluation by Reflectance Spectrophotometry
Color Evaluation by Absorbance Spectrophotometry
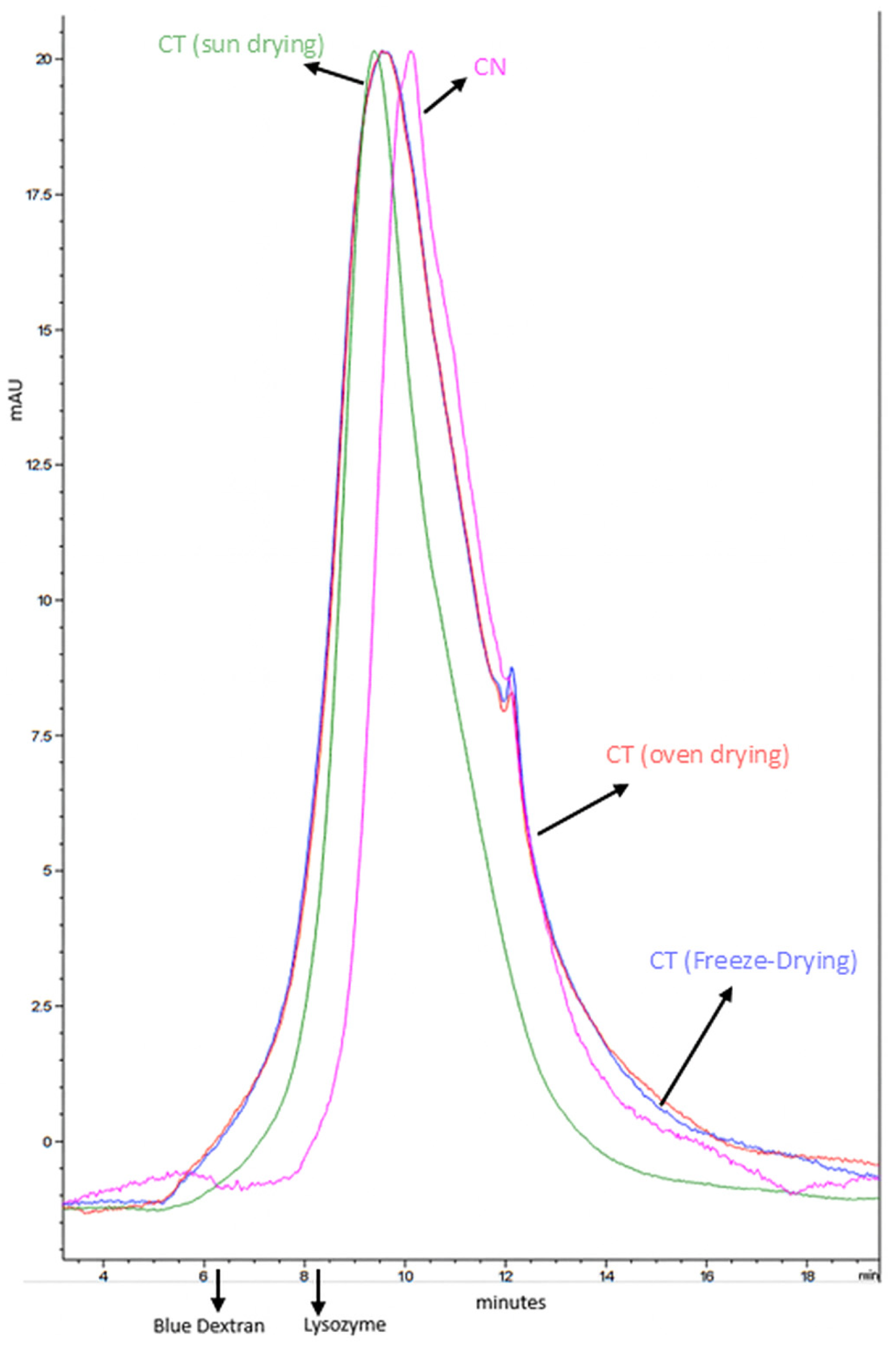
Molecular Weight Distribution of Melanoidins
Quantification of Melanoidins by Molecular Weight
Antioxidant Capacity of Melanoidin Fractions
2.3.5. Determination of Caffeine and Chlorogenic Acid Content
2.3.6. Determination of Hydroxymethylfurfural (HMF) Content
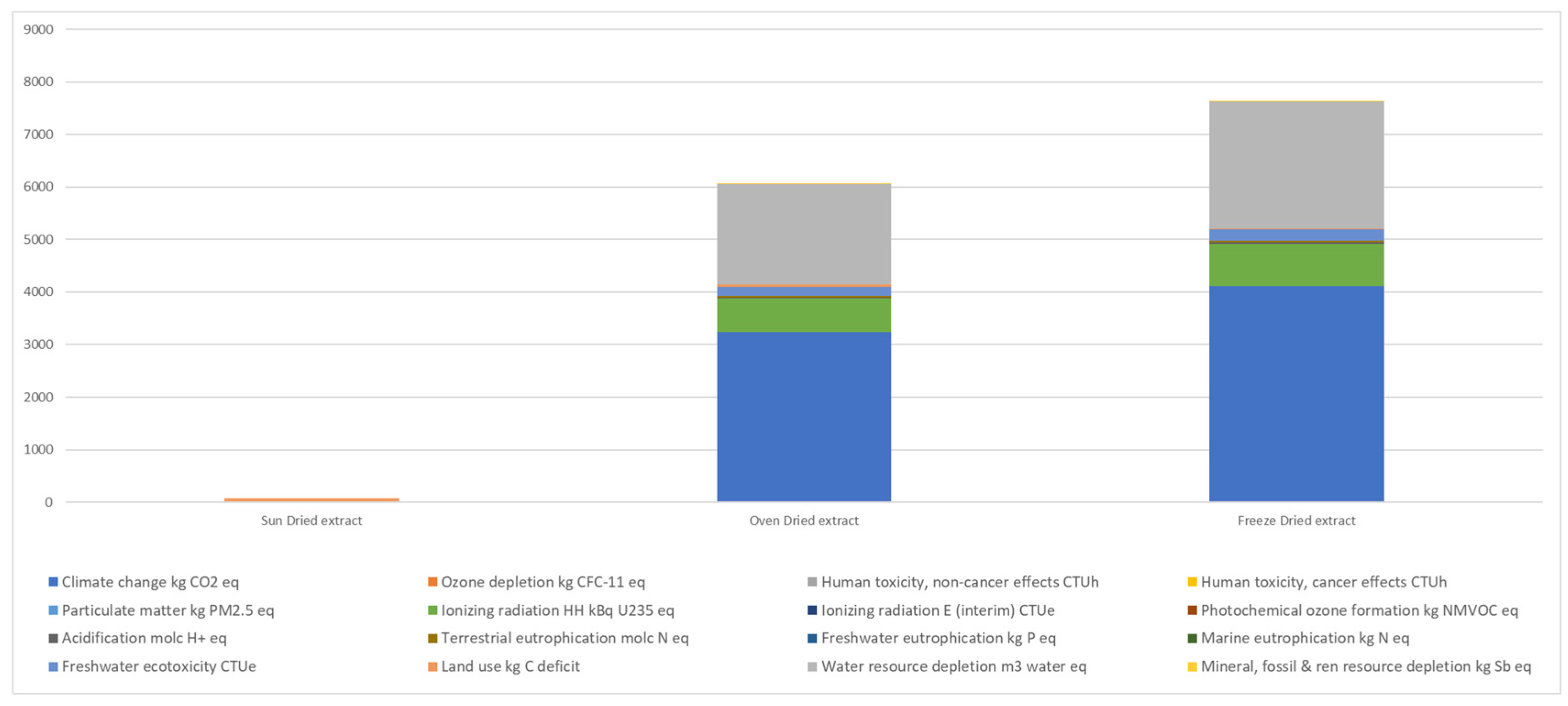
2.3.7. Life Cycle Assessment
2.3.8. Microbiological and Toxicological Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Analysis
3.2. Evaluation of the Extracts for Use as Colorants
3.3. Microbiological and Toxicological Analysis
3.4. Environmental Impacts of Drying Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CS | Coffee silverskin |
| CT | Torrefacto |
| CN | Natural |
| HMF | 5-hydroxymethylfurfural |
| SDG | Sustainable Development Goals |
| LCA | Life Cycle Assessment |
References
- Iriondo-DeHond, A.; Elizondo, A.S.; Iriondo-DeHond, M.; Ríos, M.B.; Mufari, R.; Mendiola, J.A.; Ibañez, E.; del Castillo, M.D. Assessment of Healthy and Harmful Maillard Reaction Products in a Novel Coffee Cascara Beverage: Melanoidins and Acrylamide. Foods 2020, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Papamatthaiakis, N.; Barbero-López, A.; Eronen, E.; Jänis, J.; Mola-Yudego, B.; Haapala, A. Hydrothermal Liquefaction of Coffee Silverskin and Spent Coffee Grounds: Bioenergy and Biochemical Potential. Bioenergy Res. 2025, 18, 65. [Google Scholar] [CrossRef]
- Hino, A.; Adachi, H.; Enomoto, M.; Furuki, K.; Shigetoh, Y.; Ohtsuka, M.; Kumagae, S.I.; Hirai, Y.; Jalaldin, A.; Satoh, A.; et al. Habitual Coffee but Not Green Tea Consumption Is Inversely Associated with Metabolic Syndrome. An Epidemiological Study in a General Japanese Population. Diabetes Res. Clin. Pract. 2007, 76, 383–389. [Google Scholar] [CrossRef]
- Carman, A.J.; Dacks, P.A.; Lane, R.F.; Shineman, D.W.; Fillit, H.M. Current Evidence for the Use of Coffee and Caffeine to Prevent Age-Related Cognitive Decline and Alzheimer’s Disease. J. Nutr. Health Aging 2014, 18, 383–392. [Google Scholar] [CrossRef]
- Heeger, A.; Kosińska-Cagnazzo, A.; Cantergiani, E.; Andlauer, W. Bioactives of Coffee Cherry Pulp and Its Utilisation for Production of Cascara Beverage. Food Chem. 2017, 221, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-deHond, A.; Iriondo-deHond, M.; del Castillo, M.D. Applications of Compounds from Coffee Processing By-Products. Biomolecules 2020, 10, 1219. [Google Scholar] [CrossRef]
- Marzorati, S.; Jiménez-Quero, A.; Massironi, A.; Nasti, R.; Verotta, L. Circular Valorization of Coffee Silverskin through Supercritical CO2 for the Production of Functional Extracts. RSC Sustain. 2023, 1, 563–573. [Google Scholar] [CrossRef]
- Folmer, B. The Craft and Science of Coffee, 1st ed.; Folmer, B., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-0-12-803520-7. [Google Scholar]
- Gottstein, V.; Bernhardt, M.; Dilger, E.; Keller, J.; Breitling-Utzmann, C.M.; Schwarz, S.; Kuballa, T.; Lachenmeier, D.W.; Bunzel, M. Coffee Silver Skin: Chemical Characterization with Special Consideration of Dietary Fiber and Heat-Induced Contaminants. Foods 2021, 10, 1705. [Google Scholar] [CrossRef]
- Illy, A.; Viano, R. Espresso Coffee: The Science of Quality, 2nd ed.; Illy, A., Rinantonio, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; ISBN 0123703719. [Google Scholar]
- Arya, S.S.; Venkatram, R.; More, P.R.; Vijayan, P. The Wastes of Coffee Bean Processing for Utilization in Food: A Review. J. Food Sci. Technol. 2022, 59, 429–444. [Google Scholar] [CrossRef]
- Nzekoue, F.; Borsetta, G.; Navarini, L.; Abouelenein, D.; Xiao, J.; Sagratini, G.; Vittori, S.; Caprioli, G.; Angeloni, S. Coffee Silverskin: Characterization of B-Vitamins, Macronutrients, Minerals and Phytosterols. Food Chem. 2022, 372, 131188. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gomez, B.; Lezama, A.; Amigo-Benavent, M.; Ullate, M.; Herrero, M.; Martín, M.Á.; Mesa, M.D.; del Castillo, M.D. Insights on the Health Benefits of the Bioactive Compounds of Coffee Silverskin Extract. J. Funct. Foods 2016, 25, 197–207. [Google Scholar] [CrossRef]
- Nolasco, A.; Squillante, J.; Velotto, S.; D’Auria, G.; Ferranti, P.; Mamone, G.; Errico, M.E.; Avolio, R.; Castaldo, R.; Cirillo, T.; et al. Valorization of Coffee Industry Wastes: Comprehensive Physicochemical Characterization of Coffee Silverskin and Multipurpose Recycling Applications. J. Clean. Prod. 2022, 370, 133520. [Google Scholar] [CrossRef]
- Nunes, F.M.; Coimbra, M.A. Melanoidins from Coffee Infusions. Fractionation, Chemical Characterization, and Effect of the Degree of Roast. J. Agric. Food Chem. 2007, 55, 3967–3977. [Google Scholar] [CrossRef]
- Andriot, I.; Le Quéré, J.L.; Guichard, E. Interactions between Coffee Melanoidins and Flavour Compounds: Impact of Freeze-Drying (Method and Time) and Roasting Degree of Coffee on Melanoidins Retention Capacity. Food Chem. 2004, 85, 289–294. [Google Scholar] [CrossRef]
- De La Cruz, S.T.; Iriondo-DeHond, A.; Herrera, T.; Lopez-Tofiño, Y.; Galvez-Robleño, C.; Prodanov, M.; Velazquez-Escobar, F.; Abalo, R.; Del Castillo, M.D. An Assessment of the Bioactivity of Coffee Silverskin Melanoidins. Foods 2019, 8, 68. [Google Scholar] [CrossRef]
- Cao, P.; Zhang, L.; Yang, Y.; Wang, X.-D.; Liu, Z.-P.; Li, J.-W.; Wang, L.-Y.; Chung, S.; Zhou, M.; Deng, K.; et al. Analysis of Furan and Its Major Furan Derivatives in Coffee Products on the Chinese Market Using HS-GC–MS and the Estimated Exposure of the Chinese Population. Food Chem. 2022, 387, 132823. [Google Scholar] [CrossRef] [PubMed]
- Strocchi, G.; Rubiolo, P.; Cordero, C.; Bicchi, C.; Liberto, E. Acrylamide in Coffee: What Is Known and What Still Needs to Be Explored. A Review. Food Chem. 2022, 393, 133406. [Google Scholar] [CrossRef] [PubMed]
- Li, Z. Extraction Technology and Influencing Factors of Cold Brew Coffee. In The International Conference on Modern Medicine and Global Health; EWA Publishing: Dublin, Ireland, 2023; Volume 6, pp. 459–466. [Google Scholar]
- García-Serna, E.; Martinez-Saez, N.; Mesias, M.; Morales, F.J.; Castillo, M.D. Use of Coffee Silverskin and Stevia to Improve the Formulation of Biscuits. Pol. J. Food Nutr. Sci. 2014, 64, 243–251. [Google Scholar] [CrossRef]
- Sazesh, B.; Goli, M. Quinoa as a Wheat Substitute to Improve the Textural Properties and Minimize the Carcinogenic Acrylamide Content of the Biscuit. J. Food Process. Preserv. 2020, 44, e14563. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Aparicio García, N.; Fernandez-Gomez, B.; Guisantes-Batan, E.; Velázquez Escobar, F.; Blanch, G.P.; San Andres, M.I.; Sanchez-Fortun, S.; del Castillo, M.D. Validation of Coffee By-Products as Novel Food Ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Haza, A.I.; Ávalos, A.; del Castillo, M.D.; Morales, P. Validation of Coffee Silverskin Extract as a Food Ingredient by the Analysis of Cytotoxicity and Genotoxicity. Food Res. Int. 2017, 100, 791–797. [Google Scholar] [CrossRef]
- Iriondo-Dehond, A.; Rios, M.B.; Herrera, T.; Rodriguez-Bertos, A.; Nuñez, F.; Andres, M.I.S.; Sanchez-Fortun, S.; del Castillo, M.D. Coffee Silverskin Extract: Nutritional Value, Safety and Effect on Key Biological Functions. Nutrients 2020, 11, 2693. [Google Scholar] [CrossRef]
- Papargyropoulou, E.; Lozano, R.; Steinberger, J.K.; Wright, N.; Ujang, Z. Bin The Food Waste Hierarchy as a Framework for the Management of Food Surplus and Food Waste. J. Clean. Prod. 2014, 76, 106–115. [Google Scholar] [CrossRef]
- EFSA. Guidance on the Preparation and Presentation of an Application for Authorisation of a Novel Food in the Context of Regulation (EU) 2015/2283. EFSA J. 2016, 14, 4594. [Google Scholar] [CrossRef]
- de Medeiros, F.G.M.; Pereira, G.B.C.; da Silva Pedrini, M.R.; Hoskin, R.T.; Nunes, A.O. Evaluation of the Environmental Performance of the Production of Polyphenol-Rich Fruit Powders: A Case Study on Acerola. J. Food Eng. 2024, 372, 112010. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Bravo, J.; De Peña, M.P.; Cid, C. Effect of Sugar Addition (Torrefacto) during Roasting Process on Antioxidant Capacity and Phenolics of Coffee. LWT—Food Sci. Technol. 2013, 51, 553–559. [Google Scholar] [CrossRef]
- Freitas, V.V.; Rodrigues Borges, L.L.; Dias Castro, G.A.; Henrique dos Santos, M.; Teixeira Ribeiro Vidigal, M.C.; Fernandes, S.A.; Stringheta, P.C. Impact of Different Roasting Conditions on the Chemical Composition, Antioxidant Activities, and Color of Coffea Canephora and Coffea arabica L. Samples. Heliyon 2023, 9, e19580. [Google Scholar] [CrossRef]
- Dauber, C.; Romero, M.; Chaparro, C.; Ureta, C.; Ferrari, C.; Lans, R.; Frugoni, L.; Echeverry, M.V.; Calvo, B.S.; Trostchansky, A.; et al. Cookies Enriched with Coffee Silverskin Powder and Coffee Silverskin Ultrasound Extract to Enhance Fiber Content and Antioxidant Properties. Appl. Food Res. 2024, 4, 100373. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Fernández-Fernández, A.M.; Iriondo-DeHond, A.; Dellacassa, E.; Medrano-Fernandez, A.; del Castillo, M.D. Assessment of Antioxidant, Antidiabetic, Antiobesity, and Anti-Inflammatory Properties of a Tannat Winemaking by-Product. Eur. Food Res. Technol. 2019, 245, 1539–1551. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Rodríguez, A.; Lema, P.; Bessio, M.I.; Moyna, G.; Panizzolo, L.A.; Ferreira, F. Isolation and Characterization of Melanoidins from Dulce de Leche, a Confectionary Dairy Product. Molecules 2019, 24, 4163. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Calani, L.; Dall’Asta, M.; Brighenti, F. Polyphenolic Composition of Hazelnut Skin. J. Agric. Food Chem. 2011, 59, 9935–9941. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Alves, R.C.; Costa, A.S.G.; Nunes, M.A.; Oliveira, M.B.P.P. Coffea Canephora Silverskin from Different Geographical Origins: A Comparative Study. Sci. Total Environ. 2018, 645, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Martuscelli, M.; Esposito, L.; Di Mattia, C.D.; Ricci, A.; Mastrocola, D. Characterization of Coffee Silver Skin as Potential Food-Safe Ingredient. Foods 2021, 10, 1367. [Google Scholar] [CrossRef]
- Mirón-Mérida, V.A.; Yáñez-Fernández, J.; Montañez-Barragán, B.; Barragán Huerta, B.E. Valorization of Coffee Parchment Waste (Coffea arabica) as a Source of Caffeine and Phenolic Compounds in Antifungal Gellan Gum Films. LWT 2019, 101, 167–174. [Google Scholar] [CrossRef]
- Rufián-Henares, J.A.; Delgado-Andrade, C.; Morales, F.J. Analysis of Heat-Damage Indices in Breakfast Cereals: Influence of Composition. J. Cereal Sci. 2006, 43, 63–69. [Google Scholar] [CrossRef]
- AOAC. AOAC 991.45 Total Aflatoxin Levels in Peanut Butter. In Official Methods of Analysis; AOAC, International, Ed.; AOAC: Rockville, MD, USA, 2016. [Google Scholar]
- ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. ISO: Geneva, Switzerland, 2013. Available online: https://www.iso.org/standard/53728.html (accessed on 11 June 2025).
- APHA (American Public Health Association). Compendium of Methods for the Microbiological Examination of Foods, 4th ed.; Pouch Downes, F., Ito, K., Eds.; APHA: Washington, DC, USA, 2001; ISBN 0-87553-175-X. [Google Scholar]
- Ballesteros, L.F.; Texeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Li, Y.O.; Komarek, A.R. Dietary Fibre Basics: Health, Nutrition, Analysis, and Applications. Food Qual. Saf. 2017, 1, 47–59. [Google Scholar] [CrossRef]
- Veronese, N.; Solmi, M.; Caruso, M.G.; Giannelli, G.; Osella, A.R.; Evangelou, E.; Maggi, S.; Fontana, L.; Stubbs, B.; Tzoulaki, I. Dietary Fiber and Health Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Am. J. Clin. Nutr. 2018, 107, 436–444. [Google Scholar] [CrossRef]
- WHO. Carbohydrate Intake for Adults and Children; WHO Guideline: Geneva, Switzerland, 2023. [Google Scholar]
- Borrelli, R.C.; Esposito, F.; Napolitano, A.; Ritieni, A.; Fogliano, V. Characterization of a New Potential Functional Ingredient: Coffee Silverskin. J. Agric. Food Chem. 2004, 52, 1338–1343. [Google Scholar] [CrossRef]
- Napolitano, A.; Fogliano, V.; Tafuri, A.; Ritieni, A. Natural Occurrence of Ochratoxin A and Antioxidant Activities of Green and Roasted Coffees and Corresponding Byproducts. J. Agric. Food Chem. 2007, 55, 10499–10504. [Google Scholar] [CrossRef]
- Beltrán-Medina, E.A.; Guatemala-Morales, G.M.; Padilla-Camberos, E.; Corona-González, R.I.; Mondragón-Cortez, P.M.; Arriola-Guevara, E. Evaluation of the Use of a Coffiee Industry By-Product in a Cereal-Based Extruded Food Product. Foods 2020, 9, 1008. [Google Scholar] [CrossRef]
- Aroufai, İ.; Sabuncu, M.; Dülger Altıner, D.; Sahan, Y. Antioxidant Properties and Bioaccesibility of Coffee Beans and Their Coffee Silverskin Grown in Different Countries. J. Food Meas. Charact. 2022, 16, 1873–1888. [Google Scholar] [CrossRef]
- Zainuri; Paramartha, D. N.A.; Fatinah, A.; Nofrida, R.; Rahayu, N.; Anggraini, I.M.D.; Utama, Q.D. The Chemical Characteristics of Arabica and Robusta Green Coffee Beans from Geopark Rinjani, Indonesia. Biotropia 2023, 30, 318–328. [Google Scholar] [CrossRef]
- CXS 12-1981; FAO/WHO Codex Alimentarius Commission Codex Alimentarius Standard for Honey. International Food Standards: Rome, Italy, 2022.
- López-Galilea, I.; Andueza, S.; Di Leonardo, I.; De Peña, M.P.; Cid, C. Influence of Torrefacto Roast on Antioxidant and Pro-Oxidant Activity of Coffee. Food Chem. 2006, 94, 75–80. [Google Scholar] [CrossRef]
- Bagdonaite, K.; Derler, K.; Murkovic, M. Determination of Acrylamide during Roasting of Coffee. J. Agric. Food Chem. 2008, 56, 6081–6086. [Google Scholar] [CrossRef]
- Chen, H.; Gu, Z. Effect of Ascorbic Acid on the Properties of Ammonia Caramel Colorant Additives and Acrylamide Formation. J. Food Sci. 2014, 79, 1678–1682. [Google Scholar] [CrossRef]
- Sung, W.C.; Chi, M.H.; Chiou, T.Y.; Lin, S.H.; Lee, W.J. Influence of Caramel and Molasses Addition on Acrylamide and 5-Hydroxylmethylfurfural Formation and Sensory Characteristics of Non-Centrifugal Cane Sugar during Manufacturing. J. Sci. Food Agric. 2020, 100, 4512–4520. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Rodríguez Casas, A.; del Castillo, M.D. Interest of Coffee Melanoidins as Sustainable Healthier Food Ingredients. Front. Nutr. 2021, 8, 730343. [Google Scholar] [CrossRef]
- Ministerio de Salud Pública. Reglamento Bromatológico Nacional; IMPO: Montevideo, Uruguay, 2012. [Google Scholar]
- EC Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Off. J. Eur. Union 2005, L338, 1–26.
- ISO 14040; Environmental Management—Life Cycle Assessment—Principles and Framework. International Organization for Standardization (ISO): Geneva, Switzerland, 2020.
- ISO 14044; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. International Organization for Standardization (ISO): Geneva, Switzerland, 2020.
- Prosapio, V.; Norton, I.; De Marco, I. Optimization of Freeze-Drying Using a Life Cycle Assessment Approach: Strawberries’ Case Study. J. Clean. Prod. 2017, 168, 1171–1179. [Google Scholar] [CrossRef]
- Borzova, M.; Lenigk, V.; Gauvin, F.; Schollbach, K. Life Cycle Assessment of Silica Aerogel Produced from Waste Glass via Ambient Pressure Drying Method. J. Clean. Prod. 2024, 477, 143839. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Tian, W.; Li, Y.; Song, Y.; Zhang, P. Multi-Objective Optimization of Milk Powder Spray Drying System Considering Environmental Impact, Economy and Product Quality. J. Clean. Prod. 2022, 369, 133353. [Google Scholar] [CrossRef]
- Kumar, S.; Jadhav, S.V.; Thorat, B.N. Life Cycle Assessment of Tomato Drying in Heat Pump and Microwave Vacuum Dryers. Mater. Today Proc. 2022, 57, 1700–1705. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Ramírez, B.; Escobar, F.V.; del Castillo, M.D. Antioxidant Properties of High Molecular Weight Compounds from Coffee Roasting and Brewing Byproducts. Bioact. Compd. Health 2019, 2, 48–63. [Google Scholar] [CrossRef]
- Ullah, F.; Hasrat, K.; Iqbal, S.; Kumar, S.; Mu, M.; Wang, S. Performance Evaluation of Improved Indirect Flat-Plate Solar Collector Drier with Integrated Phase Change Material for Drying Kiwifruit (Actinidia deliciosa A. Chev.). J. Stored Prod. Res. 2025, 111, 102551. [Google Scholar] [CrossRef]

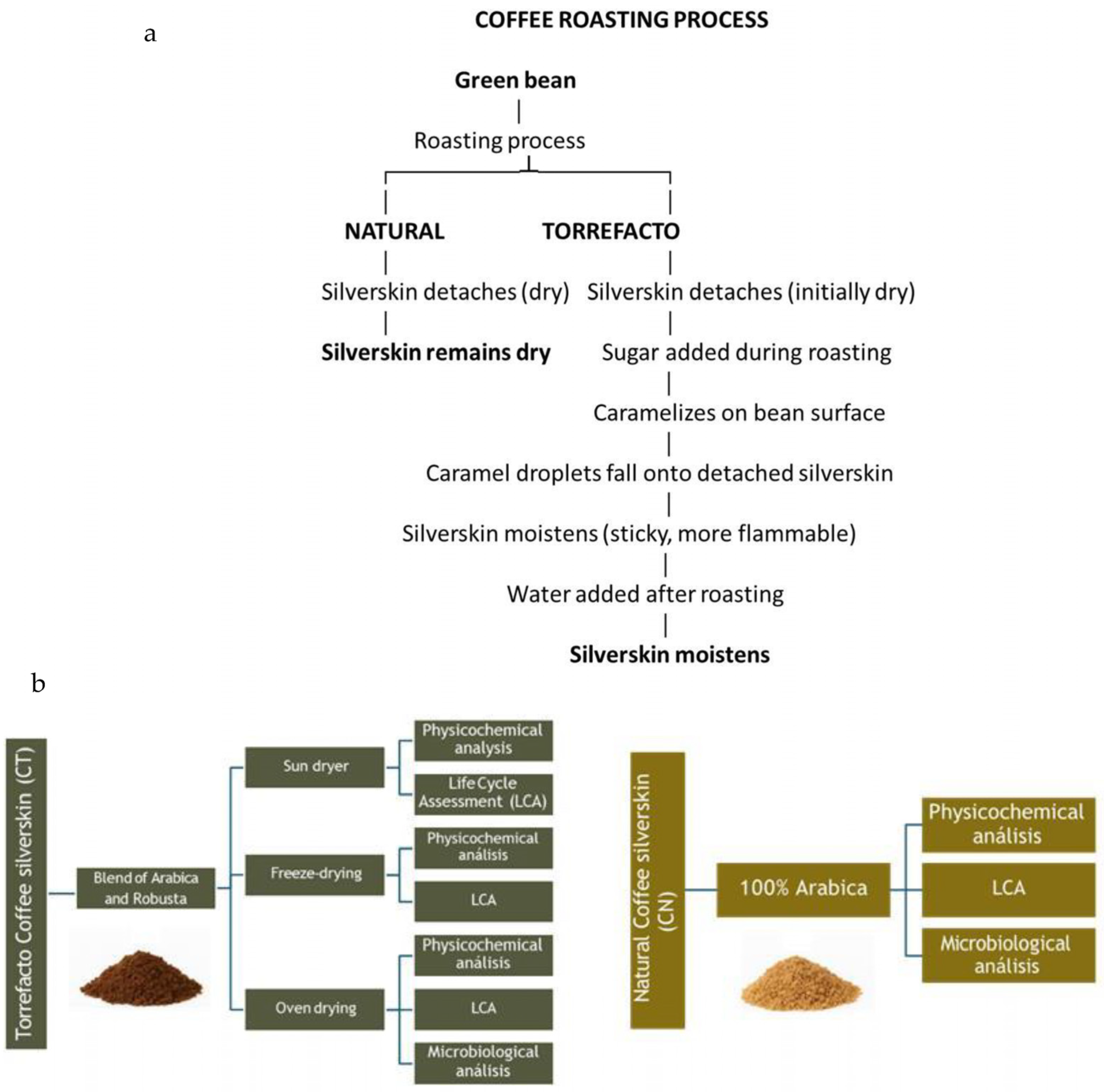

| Content (% Dry Basis) | ||
|---|---|---|
| Component | Arábica | Robusta |
| Soluble carbohydrates | 9–12.5 | 6–11.5 |
| Insoluble carbohydrates | 46–53 | 34–44 |
| Lipids | 15–18 | 12–18 |
| Protein | 8.5–12 | 8.5–12 |
| Chlorogenic acid | 6.7–9.2 | 7.1–12.1 |
| Caffeine | 0.8–1.4 | 1.7–4.0 |
| Extraction Method | Chlorogenic Acid (ppm) | Caffeine (ppm) |
|---|---|---|
| Water at 100 °C 10 min, (500 mg, 10 mL) | 2962 ± 324 b | 5877 ± 417 b |
| 1% Aqueous formic acid at 70 °C 1 h, (5 mL, 200 mg) | 1780 ± 27 c | 3895 ± 46 c |
| EtOH:Water 1:1 at 40 °C for 60 min (25 mL, 500 mg) | 3327 ± 183 a | 7198 ± 395 a |
| MeOH:Agua 70:30, (1 g, 10 mL) | 2381 ± 66 b | 5240 ± 77 b |
| CT (Freeze-Dried) | CT (Oven-Dried) | CT (Sun-Dried) | CN | |
|---|---|---|---|---|
| Fiber (%) | 66.22 ± 0.28 a.b | 67.25 ± 0.61 a | 63.79 ± 1.01 b | 66.50 ± 0.89 a.b |
| Protein (%) | 15.68 ± 0.68 a | 15.69 ± 0.22 a | 15.48 ± 0.42 a.b | 14.23 ± 0.28 b |
| Lipids (%) | 3.32 ± 0.29 a.b | 2.72 ± 0.14 b | 3.04 ± 0.11 a.b | 4.81 ± 0.51 a |
| Ashes (%) | 6.63 ± 0.56 a | 6.93 ± 0.46 a | 6.93 ± 0.68 a | 6.43 ± 0.61 a |
| Carbohydrates (by difference) (%) | 8.16 | 7.41 | 10.76 | 8.03 |
| Total Phenolics (mg GAE/g silverskin) | 25.14 ± 0.92 a | 27.02 ± 0.51 a | 26.63 ± 1.06 a | 26.63 ± 0.79 a |
| TEAC (µmol TE/g silverskin) | 105.98 ± 0.47 a | 95.53 ± 0.39 a | 16.72 ± 0.85 c | 52.10 ± 0.62 b |
| Caffeine (ppm) | 7198 ± 395 a | 8097 ± 293 a | 1419 ± 14 c | 5657 ± 234 b |
| Chlorogenic Acid (ppm) | 3327 ± 183 a | 3016 ± 85 a | 60 ± 4 c | 616 ± 95 b |
| Melanoidins (% PM > 10 kDa/total) | 33.80 ± 1.02 b | 30.49 ± 1.15 b | 38.53 ± 0.72 a | 19.66 ± 0.54 c |
| TEAC (µmol TE/g melanoidins PM > 10 kDa) | 592.71 ± 10.12 b | 627.76 ± 11.56 a | 445.09 ± 7.75 c | 178.87 ± 9.22 d |
| TEAC (µmol/g melanoidins PM < 10 kDa/total) | 770.59 ± 68.19 b | 1028.63 ± 51.11 a | 538.47 ± 32.19 c | 419.42 ± 27.81 d |
| HMF (ppm) | 14 ± 0.7 b | 4 ± 0.07 c | 1 ± 0.04 d | 35 ± 1 a |
| Sample | L* | a* | b* | dE*ab | Digital Color Image |
|---|---|---|---|---|---|
| CT (wet) | 38.30 ± 2.29 c | 1.20 ± 1.14 d | 2.08 ± 0.25 d | - | |
| CT (oven-dried) | 43.81 ± 1.91 b | 3.05 ± 0.22 c | 6.32 ± 0.59 c | 7.34 b | |
| CT (sun-dried) | 42.79 ± 0.26 b | 1.36 ± 0.11 d | 2.49 ± 0.23 d | 4.52 c | |
| CT (freeze-dried) | 47.84 ± 1.58 a | 3.71 ± 0.39 b | 8.92 ± 0.57 b | 12.03 a | |
| CN | 46.82 ± 0.85 a | 7.52 ± 0.23 a | 23.13 ± 0.53 a | - |
| Extracts (7 mg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Extract | Yield (% mg Extract/mg Silverskin) | mg eq Colorant/g Silverskin | L* | a* | b* | h*ab | C*ab | Absorbance at 420 nm |
| CT (oven-dried) | 5.86 | 300.86 c | 12.51 | 16.88 | 15.76 | 43.03 | 23.09 | 1.2153 |
| CT (sun-dried) | 6.05 | 455.27 a | 6.79 | 6.35 | 4.13 | 33.04 | 7.57 | 1.6264 |
| CT (freeze-dried) | 6.18 | 374.33 b | 13.74 | 13.91 | 15.44 | 47.97 | 20.78 | 1.2646 |
| CN | 8.52 | 123.75 d | 47.39 | 7.24 | 42.66 | 80.37 | 43.27 | 1.7711 |
| Class IV Caramel Colorant (0.7 mg/mL) | 19.51 | 20.42 | 27.03 | 52.93 | 33.88 | 1.6951 | ||
| Test/Assay | CN | CT (Oven) |
|---|---|---|
| Ochratoxin A | 6.2 µg/kg | 3.9 µg/kg |
| Aerobic Mesophilic Count at 30 °C | 2.9 × 106 ufc/g | 8.3 × 103 ufc/g |
| Mold Count | <10 ufc/g | 7.7 × 102 ufc/g |
| Yeast Count | 10 ufc/g | 3.9 × 103 ufc/g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quagliata, E.; Gazzara, S.; Dauber, C.; Rodríguez, A.; Panizzolo, L.; Irigaray, B.; Gámbaro, A.; Mendiola, J.A.; Vieitez, I.; del Castillo, M.D. Novel Insights into Torrefacto and Natural Coffee Silverskin: Composition, Bioactivity, Safety, and Environmental Impact for Sustainable Food Applications. Foods 2025, 14, 3388. https://doi.org/10.3390/foods14193388
Quagliata E, Gazzara S, Dauber C, Rodríguez A, Panizzolo L, Irigaray B, Gámbaro A, Mendiola JA, Vieitez I, del Castillo MD. Novel Insights into Torrefacto and Natural Coffee Silverskin: Composition, Bioactivity, Safety, and Environmental Impact for Sustainable Food Applications. Foods. 2025; 14(19):3388. https://doi.org/10.3390/foods14193388
Chicago/Turabian StyleQuagliata, Ernesto, Silvina Gazzara, Cecilia Dauber, Analía Rodríguez, Luis Panizzolo, Bruno Irigaray, Adriana Gámbaro, José A. Mendiola, Ignacio Vieitez, and María Dolores del Castillo. 2025. "Novel Insights into Torrefacto and Natural Coffee Silverskin: Composition, Bioactivity, Safety, and Environmental Impact for Sustainable Food Applications" Foods 14, no. 19: 3388. https://doi.org/10.3390/foods14193388
APA StyleQuagliata, E., Gazzara, S., Dauber, C., Rodríguez, A., Panizzolo, L., Irigaray, B., Gámbaro, A., Mendiola, J. A., Vieitez, I., & del Castillo, M. D. (2025). Novel Insights into Torrefacto and Natural Coffee Silverskin: Composition, Bioactivity, Safety, and Environmental Impact for Sustainable Food Applications. Foods, 14(19), 3388. https://doi.org/10.3390/foods14193388











