Co-Formulation of Edamame-Based Beverage with Coconut Derivatives Enhances Nutritional Quality, Antioxidant Capacity, Flavor Profile, and Physical Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Edamame-Based Beverage
2.2. Proximate Composition
2.3. Mineral Composition
2.4. Amino Acid Profile
2.5. Preparation of Hydroalcoholic Extracts
2.6. Total Phenolic Content
2.7. Total Flavonoid Content
2.8. Antioxidant Activity
2.9. Particle Size and Distribution
2.10. Rheological Behavior
2.11. Separation Index
2.12. Phenolic Compounds
2.13. Volatile Compound
2.14. Statistical Analysis
3. Results and Discussion
3.1. Nutritional Composition
3.2. Mineral Composition
3.3. Amino Acid Profile and Concentrations
3.4. Total Phenolics, Total Flavonoids, and Antioxidant Activity
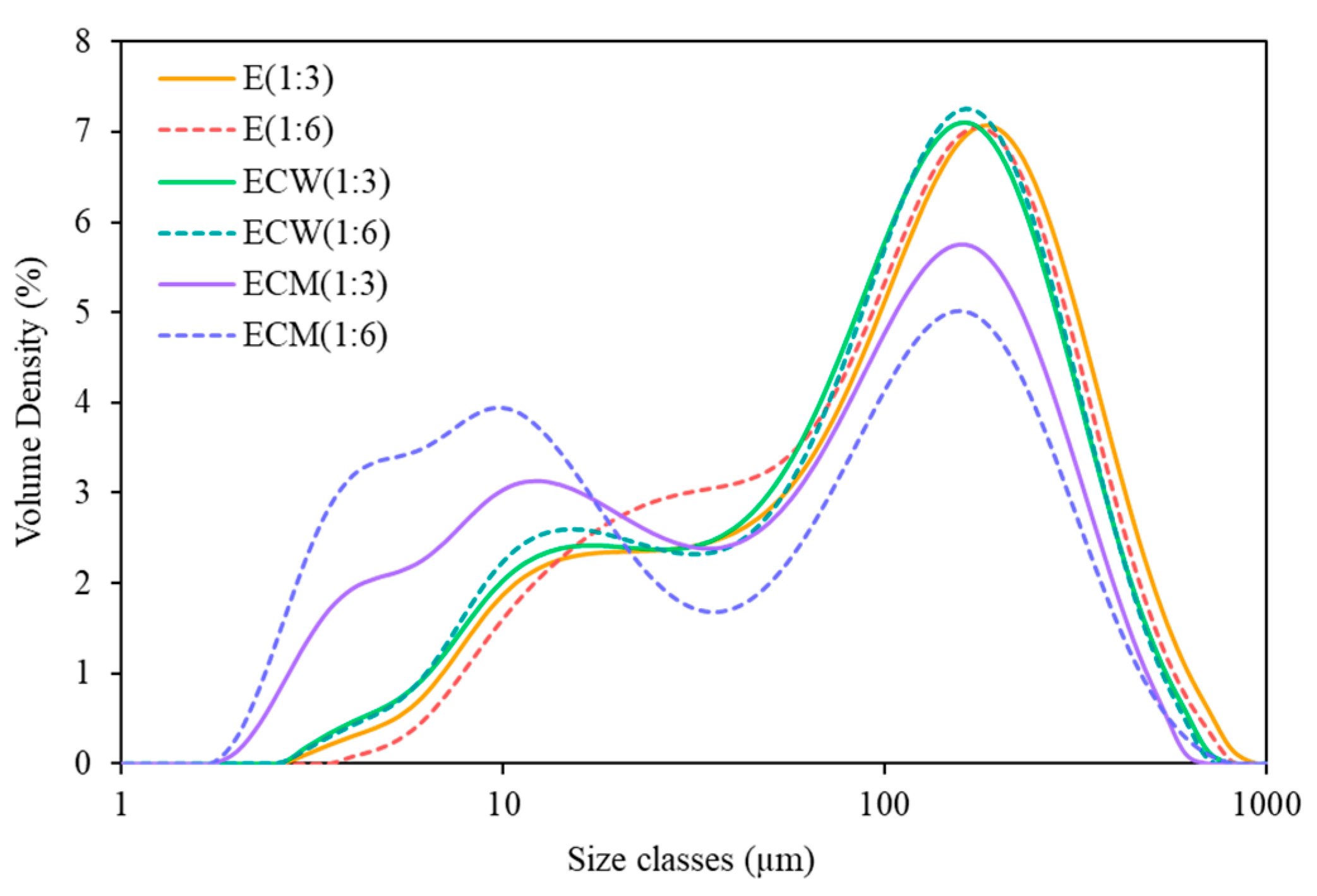
3.5. Particle Size Distribution
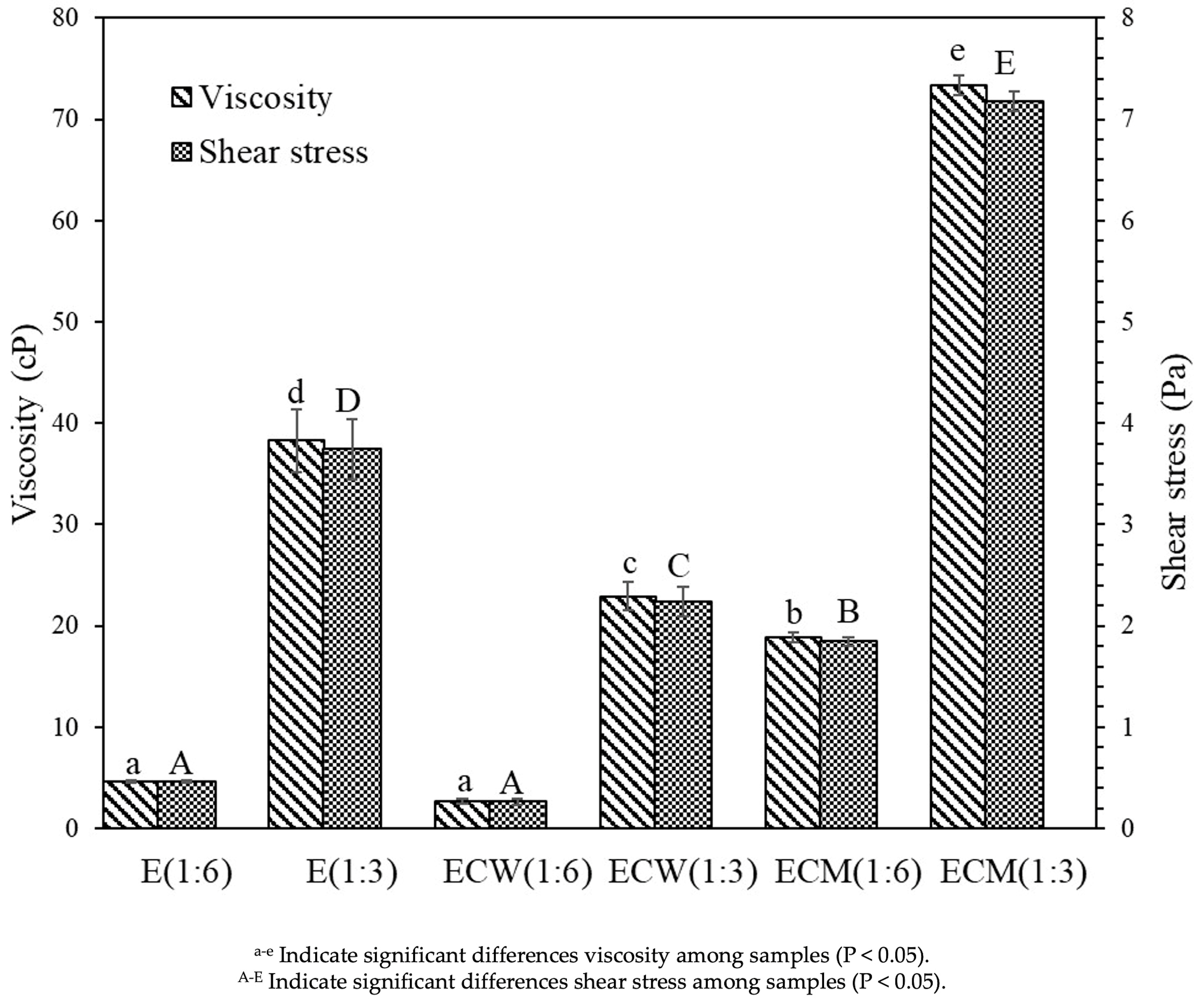
3.6. Rheological Behavior
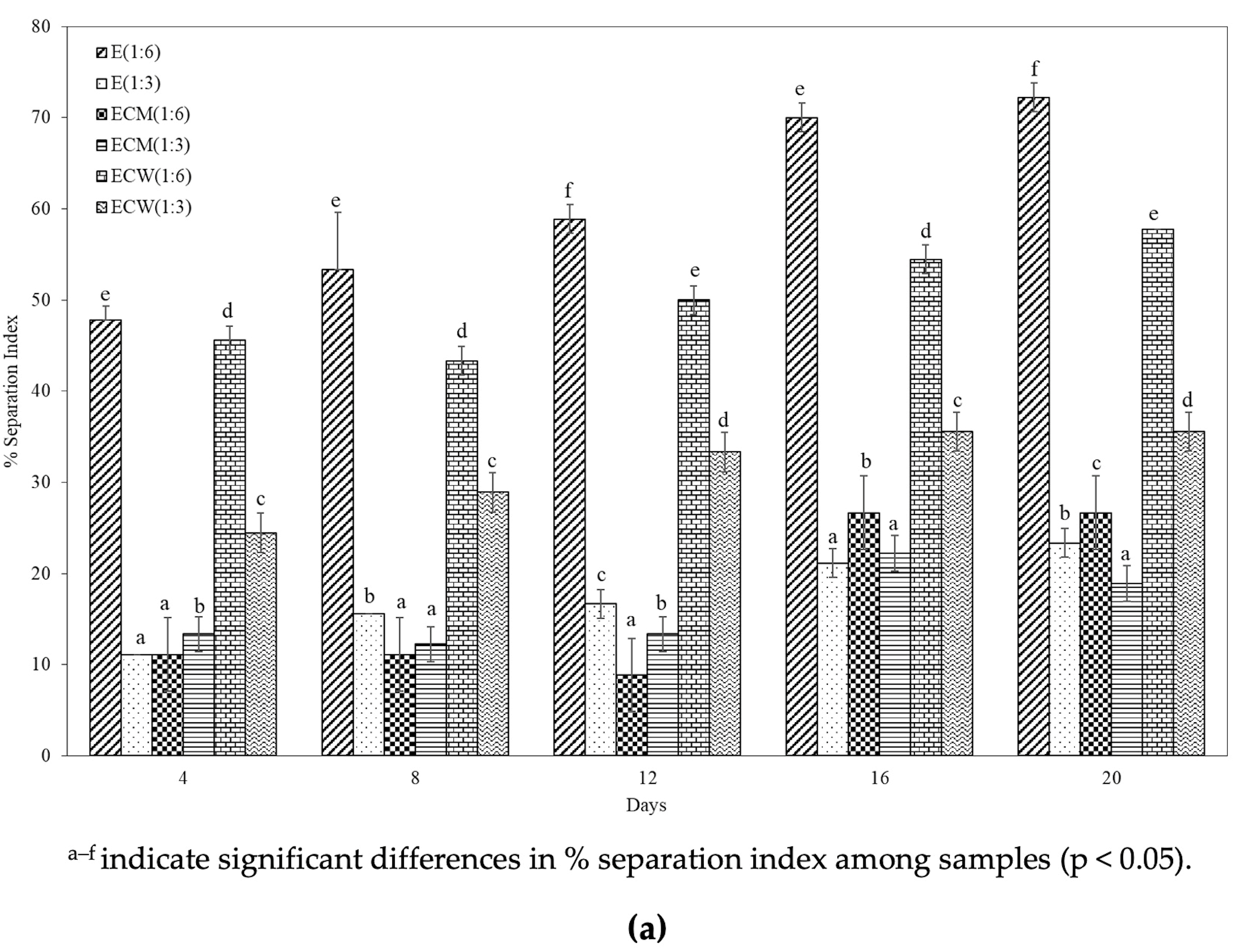
3.7. Separation Index
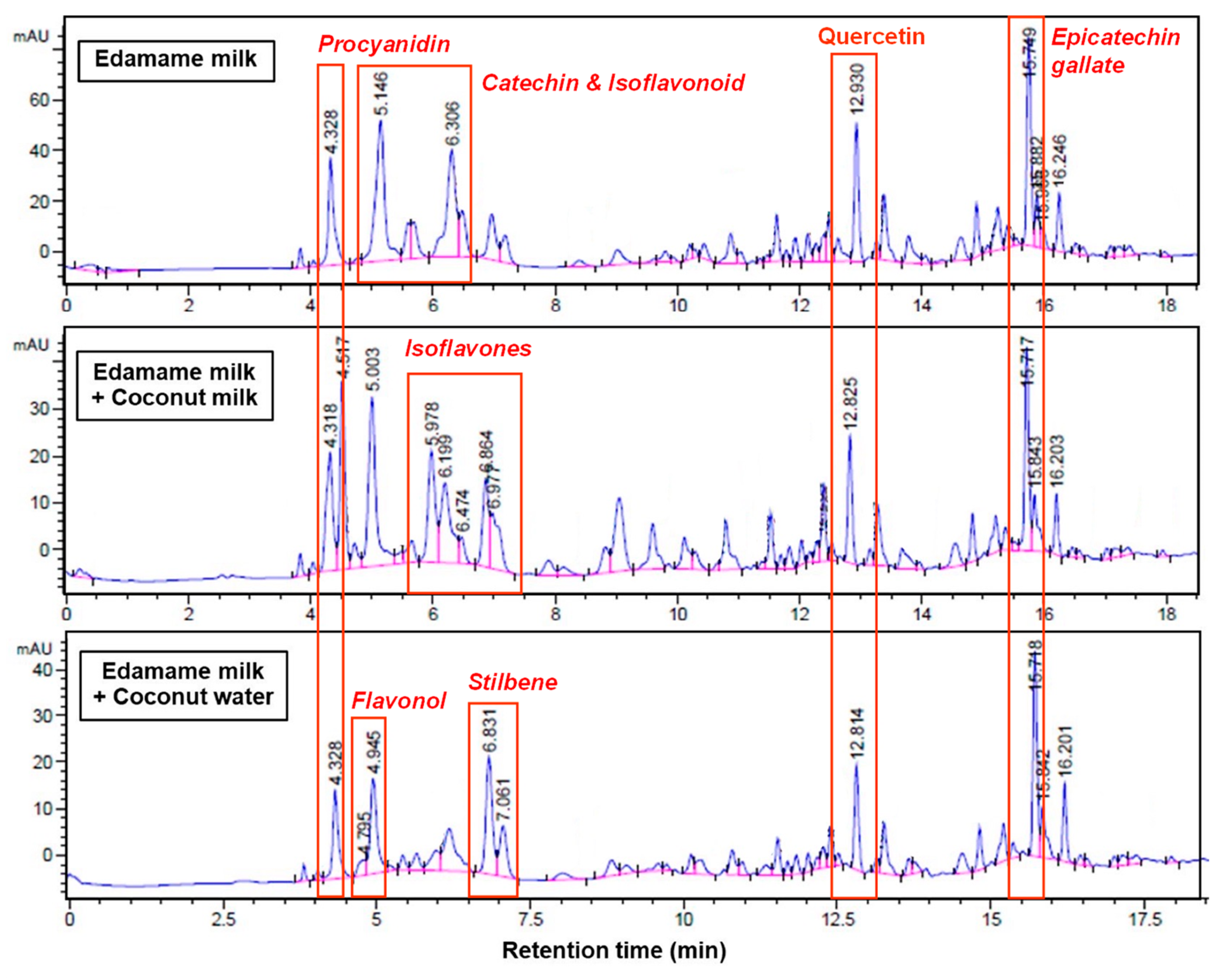
3.8. Phenolic Compounds Profile
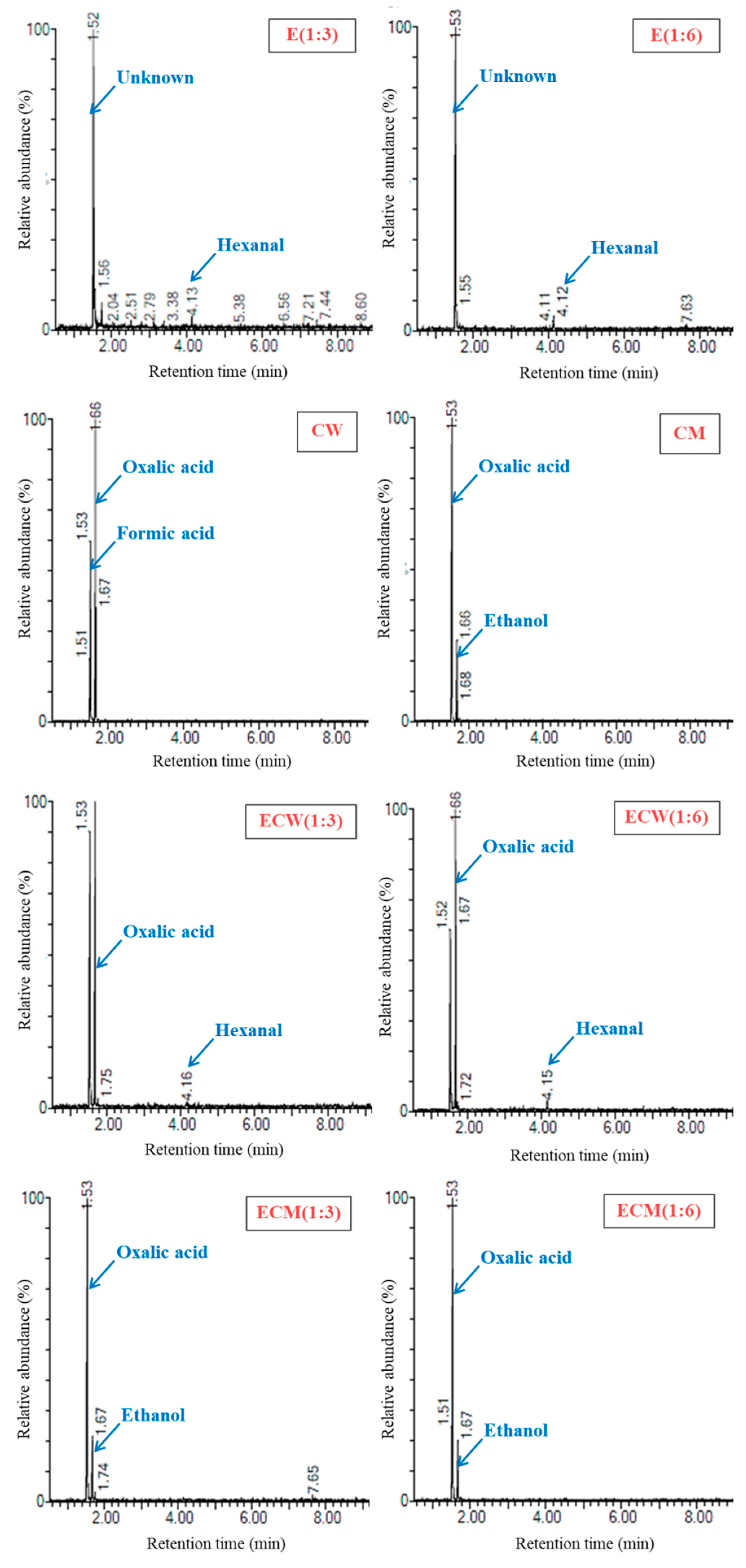
3.9. Volatile Compound Profile
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McClements, D.J. Development of next-generation nutritionally fortified plant-based milk substitutes: Structural design principles. Foods 2020, 9, 421. [Google Scholar] [CrossRef]
- Aydar, E.F.; Tutuncu, S.; Ozcelik, B. Plant-based milk substitutes: Bioactive compounds, conventional and novel processes, bioavailability studies, and health effects. J. Funct. Foods 2020, 70, 103975. [Google Scholar] [CrossRef]
- Yao, Y.; He, W.; Cai, X.; Bekhit, A.E.D.A.; Xu, B. Sensory, physicochemical and rheological properties of plant-based milk alternatives made from soybean, peanut, adlay, adzuki bean, oat and buckwheat. Int. J. Food Sci. Technol. 2022, 57, 4868–4878. [Google Scholar] [CrossRef]
- Liechti, C.; Mack, G.; Walther, B.; Ammann, J. Transforming plant-based milk alternatives for better health. LWT 2025, 223, 117787. [Google Scholar] [CrossRef]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef]
- Tangyu, M.; Muller, J.; Bolten, C.J.; Wittmann, C. Fermentation of plant-based milk alternatives for improved flavour and nutritional value. Appl. Microbiol. Biotechnol. 2019, 103, 9263–9275. [Google Scholar] [CrossRef] [PubMed]
- Taesuk, N.; Wang, A.; Srikaew, M.; Chumroenphat, T.; Barile, D.; Siriamornpun, S.; Bunyatratchata, A. Phytochemical profiling of Thai plant-based milk alternatives: Insights into bioactive compounds, antioxidant activities, prebiotics, and amino acid abundance. Food Chem. X 2025, 27, 102402. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, R.C.V.; Yin, Y.; Duncan, S.E.; O’Keefe, S.F. Edamame Flavor Characteristics Driving Consumer Acceptability in the United States: A Review. ACS Food Sci. Technol. 2021, 1, 1748–1756. [Google Scholar] [CrossRef]
- Olakanmi, S.; Barbhuiya, R.I.; Wroblewski, C.; Ramalingam, S.; Wang, J.; Nair, G.R.; Singh, A. Effect of microwave and conventional heat treatment on trypsin inhibitor activity and in vitro digestibility of edamame-based beverage protein. Food Bioeng. 2023, 2, 114–126. [Google Scholar] [CrossRef]
- Yang, J.; Chi, Y.; Burkhardt, B.R.; Guan, Y.; Wolf, B.A. Leucine metabolism in regulation of insulin secretion from pancreatic beta cells. Nutr. Rev. 2010, 68, 270–279. [Google Scholar] [CrossRef]
- Forzano, I.; Avvisato, R.; Varzideh, F.; Jankauskas, S.S.; Cioppa, A.; Mone, P.; Salemme, L.; Kansakar, U.; Tesorio, T.; Trimarco, V.; et al. L-Arginine in diabetes: Clinical and preclinical evidence. Cardiovasc. Diabetol. 2023, 22, 89. [Google Scholar] [CrossRef] [PubMed]
- Moretto, L.; Tonolo, F.; Folda, A.; Scalcon, V.; Bindoli, A.; Bellamio, M.; Feller, E.; Rigobello, M.P. Comparative analysis of the antioxidant capacity and lipid and protein oxidation of soy and oats beverages. Food Prod. Process. Nutr. 2021, 3, 1. [Google Scholar] [CrossRef]
- da Silveira Maia, I.C.; Macedo, E.B.C.; dos Santos, L.P.; de Oliveira Bordin, E.; de Oliveira Lima, L.; Feihrmann, A.C.; Marcolino, V.A.; Barão, C.E.; Pimentel, T.C. Ultrasound as an alternative for pasteurization of cashew nut milks: Improvements in the rheological and technological properties, fatty acid profile and acceptance. Food Biosci. 2025, 63, 105649. [Google Scholar] [CrossRef]
- Dai, T.; Shuai, X.; Chen, J.; Li, C.; Wang, J.; Liu, W.; Liu, C.; Wang, R. Whole peanut milk prepared by an industry-scale microfluidization system: Physical stability, microstructure, and flavor properties. LWT 2022, 171, 114140. [Google Scholar] [CrossRef]
- Deng, B.; Li, Y.; Yang, Y.; Xie, W. Advantages of UHT in retaining coconut milk aroma and insights into thermal changes of aroma compounds. Food Res. Int. 2024, 194, 114937. [Google Scholar] [CrossRef]
- Miller, R.J.; Duncan, S.E.; Carneiro, R.; Lahne, J.; Kuhar, T.; Zhang, B.; Yin, Y. Determining Aroma Compounds and Their Relation to Consumer Acceptability in United States Edamame. ACS Food Sci. Technol. 2025, 5, 2469–2479. [Google Scholar] [CrossRef]
- Yong, J.W.; Ge, L.; Ng, Y.F.; Tan, S.N. The chemical composition and biological properties of coconut (Cocos nucifera L.) water. Molecules 2009, 14, 5144–5164. [Google Scholar] [CrossRef]
- Alchoubassi, G.; Kińska, K.; Bierla, K.; Lobinski, R.; Szpunar, J. Speciation of essential nutrient trace elements in coconut water. Food Chem. 2021, 339, 127680. [Google Scholar] [CrossRef]
- Zakidou, P.; Varka, E.M.; Paraskevopoulou, A. Foaming properties and sensory acceptance of plant-based beverages as alternatives in the preparation of cappuccino style beverages. Int. J. Gastron. Food Sci. 2022, 30, 100623. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Cohen, S.A.; Michaud, D.P. Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal. Biochem. 1993, 211, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Yang, Y.; Liu, Q.; Wang, Y.; Jin, Z.; Jiao, A. Effects of enzymatic extrusion on the structure and physicochemical properties of oat flour and its application in oat milk production. Int. J. Food Sci. Technol. 2023, 58, 4638–4651. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Y.; Yun, Y.; Li, C.; Fang, Y.; Zhang, W. Discrimination and characterization of different coconut water (CW) by their phenolic composition and volatile organic compounds (VOCs) using LC-MS/MS, HS-SPME-GC-MS, and HS-GC-IMS. J. Food Sci. 2023, 88, 3758–3772. [Google Scholar] [CrossRef]
- Manousi, N.; Zachariadis, G.A. Determination of Volatile Compounds in Nut-Based Milk Alternative Beverages by HS-SPME Prior to GC-MS Analysis. Molecules 2019, 24, 3091. [Google Scholar] [CrossRef]
- Daryani, D.; Pegua, K.; Aryaa, S.S. Review of plant-based milk analogue: Its preparation, nutritional, physicochemical, and organoleptic properties. Food Sci. Biotechnol. 2024, 33, 1059–1073. [Google Scholar] [CrossRef]
- Promhuad, K.; Prasopdee, T.; Smitthipong, W. Effect of natural extract from waste corn meal on stabilization of natural rubber. SPE Polymers. 2021, 2, 172–178. [Google Scholar] [CrossRef]
- Stöckl, M.; Pferdmenges, L.E.; Brühl, L.; Greiner, R.; Hüsken, A.; Krüger, R.; Langenkämper, G.; Lencioni, A.; Müller, A.; Schmidt, M.; et al. Characterization of the nutritional profile of three plant-based drinks. J. Food Compos. Anal. 2024, 135, 106553. [Google Scholar] [CrossRef]
- Ariyaprakai, S.; Limpachoti, T.; Pradipasena, P. Interfacial and emulsifying properties of sucrose ester in coconut milk emulsions in comparison with Tween. Food Hydrocoll. 2013, 30, 358–367. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Jiang, L.; Huang, Z.; Zhang, W.; Yun, Y. Improvement of emulsifying stability of coconut globulin by noncovalent interactions with coffee polyphenols. Food Chem. X 2023, 20, 100954. [Google Scholar] [CrossRef] [PubMed]
- Bano, S.; Sommano, S.R.; Leksawasdi, N.; Taesuwan, S.; Rachtanapun, P.; Techapun, C.; Sumonsiri, N.; Khemacheewakul, J. Innovative Cold Plasma Pretreatment and Enzyme-Assisted Extraction of Genistein from Edamame and Storage Stability of Dried Extract Powder. Foods 2025, 14, 2118. [Google Scholar] [CrossRef] [PubMed]
- Pabich, M.; Marciniak, B.; Kontek, R. Phenolic Compound Composition and Biological Activities of Fractionated Soybean Pod Extract. Appl. Sci. 2021, 11, 10233. [Google Scholar] [CrossRef]
- Kazi, M.; Alhajri, A.; Alshehri, S.M.; Elzayat, E.M.; Al Meanazel, O.T.; Shakeel, F.; Noman, O.; Altamimi, M.A.; Alanazi, F.K. Enhancing Oral Bioavailability of Apigenin Using a Bioactive Self-Nanoemulsifying Drug Delivery System (Bio-SNEDDS): In Vitro, In Vivo and Stability Evaluations. Pharmaceutics 2020, 12, 749. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhao, M.; Meng, K.; Xia, G.; Pan, Y.; Li, C.; Zhang, W. The Diuretic Effects of Coconut Water by Suppressing Aquaporin and Renin-Angiotensin-Aldosterone System in Saline-Loaded Rats. Front. Nutr. 2022, 9, 930506. [Google Scholar] [CrossRef] [PubMed]
- Imtiyaz, K.; Shafi, M.; Fakhri, K.U.; Uroog, L.; Zeya, B.; Anwer, S.T.; Rizvi, M.M.A. Polydatin: A natural compound with multifaceted anticancer properties. J. Tradit. Complement. Med. 2024, 15, 447–466. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, H.; Chen, W.; Zhong, Q.; Zhang, M.; Chen, W. Effect of ultrasonic treatment on the activity of sugar metabolism relative enzymes and quality of coconut water. Ultrason. Sonochem. 2021, 79, 105780. [Google Scholar] [CrossRef] [PubMed]

| E (1:3) | ECM (1:3) | ECW (1:3) | E (1:6) | ECM (1:6) | ECW (1:6) | |
|---|---|---|---|---|---|---|
| Ash (g/100 g) | 0.28 ± 0.00 b | 0.48 ± 0.01 e | 0.51 ± 0.01 f | 0.17 ± 0.00 a | 0.36 ± 0.01 c | 0.39 ± 0.01 d |
| Calories (Kcal/100 g) | 36.09 ± 0.04 c | 114.90 ± 0.15 f | 42.46 ± 0.40 d | 18.89 ± 0.03 a | 114.06 ± 0.49 e | 29.50 ± 0.23 b |
| Calories from fat (Kcal/100 g) | 15.39 ± 0.18 c | 86.72 ± 0.41 e | 19.92 ± 0.18 d | 7.42 ± 0.05 a | 94.82 ± 0.77 f | 9.86 ± 0.14 b |
| Carbohydrates (g/100 g) | 2.23 ± 0.03 c | 3.37 ± 0.05 d | 3.54 ± 0.19 d | 1.39 ± 0.02 a | 1.97 ± 0.05 b | 3.44 ± 0.11 d |
| Fat (g/100 g) | 1.71 ± 0.02 c | 9.64 ± 0.05 e | 1.88 ± 0.02 d | 0.83 ± 0.01 a | 10.54 ± 0.09 f | 1.10 ± 0.02 b |
| Moisture (g/100 g) | 92.84 ± 0.02 d | 82.85 ± 0.02 a | 91.23 ± 0.12 c | 96.14 ± 0.00 f | 84.30 ± 0.02 b | 93.61 ± 0.07 e |
| Protein (g/100 g) | 2.95 ± 0.01 c | 3.68 ± 0.01 d | 2.85 ± 0.03 b | 1.48 ± 0.01 a | 2.84 ± 0.02 b | 1.48 ± 0.01 a |
| Total dietary fiber (g/100 g) | 1.86 ± 0.00 c | 2.43 ± 0.01 e | 2.57 ± 0.01 f | 0.62 ± 0.00 a | 1.89 ± 0.01 d | 1.09 ± 0.00 b |
| Element | Mass Concentration (Mass%) | |||||
|---|---|---|---|---|---|---|
| E (1:3) | ECM (1:3) | ECW (1:3) | E (1:6) | ECM (1:6) | ECW (1:6) | |
| Mg | 0.10 bc | 0.23 b | 0.23 a | 0.40 cd | 0.12 d | 0.26 a |
| P | 0.11 c | 0.60 b | 0.15 a | 0.86 d | 0.18 b | 0.15 a |
| S | 0.42 c | 2.41 b | 0.70 a | 2.40 d | 0.81 c | 0.71 a |
| Cl | 0.40 a | 0.60 b | 0.31 d | 0.21 a | 1.47 c | 0.48 e |
| K | 0.50 b | 1.84 c | 0.94 c | 2.52 a | 0.19 b | 0.61 c |
| Ca | 0.28 d | 2.18 c | 0.86 b | 0.35 b | 0.87 b | 0.56 a |
| Zn | 0.04 b | 0.02 c | 0.04 a | 0.02 a | 0.08 bc | 0.02 a |
| Type | Amino Acid (mg/mL) | |||||
|---|---|---|---|---|---|---|
| E (1:3) | ECM (1:3) | ECW (1:3) | E (1:6) | ECM (1:6) | ECW (1:6) | |
| Hydrophilic | 11.68 | 11.97 | 10.10 | 6.90 | 10.97 | 6.65 |
| Aspartic | 3.06 | 2.83 | 2.65 | 1.81 | 2.49 | 1.75 |
| Glutamic | 5.08 | 5.25 | 4.42 | 2.96 | 4.78 | 2.88 |
| Lysine * | 1.80 | 1.68 | 1.51 | 1.06 | 1.40 | 1.05 |
| Arginine | 1.74 | 2.21 | 1.52 | 1.07 | 2.30 | 0.97 |
| Hydrophobic | 6.98 | 6.66 | 6.08 | 4.05 | 6.32 | 3.84 |
| Alanine | 1.12 | 1.17 | 0.98 | 0.65 | 1.07 | 0.62 |
| Valine * | 1.03 | 1.03 | 0.84 | 0.56 | 1.00 | 0.50 |
| Cystine | 0.07 | 0.05 | 0.05 | 0.03 | 0.07 | 0.01 |
| Methionine * | 0.27 | 0.29 | 0.28 | 0.18 | 0.29 | 0.19 |
| Phenylalanine * | 1.36 | 1.27 | 1.23 | 0.83 | 1.22 | 0.80 |
| Isoleucine * | 1.15 | 1.03 | 0.99 | 0.67 | 0.96 | 0.63 |
| Leucine * | 1.98 | 1.82 | 1.71 | 1.13 | 1.71 | 1.09 |
| Neutral | 6.19 | 5.82 | 5.31 | 3.49 | 5.28 | 3.22 |
| Serine | 1.59 | 1.54 | 1.43 | 0.93 | 1.41 | 0.88 |
| Histidine * | 0.67 | 0.63 | 0.60 | 0.40 | 0.55 | 0.38 |
| Glycine | 1.06 | 1.10 | 0.95 | 0.63 | 1.03 | 0.60 |
| Threonine * | 0.89 | 0.83 | 0.78 | 0.50 | 0.76 | 0.46 |
| Tyrosine | 0.76 | 0.60 | 0.51 | 0.36 | 0.54 | 0.22 |
| Proline | 1.22 | 1.12 | 1.04 | 0.67 | 0.99 | 0.68 |
| Total | 24.85 | 24.45 | 21.49 | 14.44 | 22.57 | 13.71 |
| Sample | Total Phenolics (mg GAE/100 mL) | Total Flavonoids (mg QE/100 mL) | DPPH Scavenging (%) |
|---|---|---|---|
| E (1:3) | 16.25 ± 0.39 c | 6.42 ± 0.53 c | 14.85 ± 0.72 d |
| E (1:6) | 9.45 ± 0.38 a | 5.10 ± 0.57 b | 7.12 ± 0.65 a |
| ECW (1:3) | 10.42 ± 0.43 b | 3.48 ± 0.66 a | 11.08 ± 2.20 bc |
| ECW (1:6) | 8.94 ± 0.22 a | 3.15 ± 0.27 a | 10.32 ± 0.62 b |
| ECM (1:3) | 10.50 ± 0.91 b | 6.10 ± 0.40 c | 12.33 ± 0.47 c |
| ECM (1:6) | 9.20 ± 0.82 a | 5.18 ± 0.40 b | 9.95 ± 0.67 b |
| Samples | Particle Size (μm) | |||
|---|---|---|---|---|
| Dav | D (10) | D (50) | D (90) | |
| E (1:3) | 70.27 ± 0.06 f | 8.31 ± 0.08 e | 57.90 ± 0.26 e | 151.00 ± 0.00 e |
| E (1:6) | 65.80 ± 0.17 e | 9.36 ± 0.06 f | 54.17 ± 0.23 d | 140.33 ± 0.58 d |
| ECW (1:3) | 62.83 ± 0.49 d | 7.43 ± 0.04 d | 52.00 ± 0.36 c | 135.00 ± 1.73 c |
| ECW (1:6) | 61.80 ± 0.30 c | 7.23 ± 0.05 c | 51.97 ± 0.31 c | 132.67 ± 0.58 c |
| ECM (1:3) | 50.07 ± 0.42 b | 3.85 ± 0.04 b | 35.63 ± 0.45 b | 121.00 ± 1.00 b |
| ECM (1:6) | 43.53 ± 1.20 a | 2.86 ± 0.04 a | 22.20 ± 0.46 a | 113.33 ± 2.52 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klinmalai, P.; Promhuad, K.; Srisa, A.; Sathawarintu, A.; Harnkarnsujarit, N. Co-Formulation of Edamame-Based Beverage with Coconut Derivatives Enhances Nutritional Quality, Antioxidant Capacity, Flavor Profile, and Physical Stability. Foods 2025, 14, 3321. https://doi.org/10.3390/foods14193321
Klinmalai P, Promhuad K, Srisa A, Sathawarintu A, Harnkarnsujarit N. Co-Formulation of Edamame-Based Beverage with Coconut Derivatives Enhances Nutritional Quality, Antioxidant Capacity, Flavor Profile, and Physical Stability. Foods. 2025; 14(19):3321. https://doi.org/10.3390/foods14193321
Chicago/Turabian StyleKlinmalai, Phatthranit, Khwanchat Promhuad, Atcharawan Srisa, Aiyaporn Sathawarintu, and Nathdanai Harnkarnsujarit. 2025. "Co-Formulation of Edamame-Based Beverage with Coconut Derivatives Enhances Nutritional Quality, Antioxidant Capacity, Flavor Profile, and Physical Stability" Foods 14, no. 19: 3321. https://doi.org/10.3390/foods14193321
APA StyleKlinmalai, P., Promhuad, K., Srisa, A., Sathawarintu, A., & Harnkarnsujarit, N. (2025). Co-Formulation of Edamame-Based Beverage with Coconut Derivatives Enhances Nutritional Quality, Antioxidant Capacity, Flavor Profile, and Physical Stability. Foods, 14(19), 3321. https://doi.org/10.3390/foods14193321






