Studies on the Ethyl Carbamate Content of Fermented Beverages and Foods: A Review
Abstract
1. Introduction
2. Identification in Fermented Beverages and Foods
| No.crt. | Food Products | Value of Ethyl Carbamate µg/kg | Country |
|---|---|---|---|
| 1 | Bread | 1.4–4.8 | Canada |
| ND–12 | Hong Kong, USA [24] | ||
| 2 | Soy sauce | ND–130 | China, Japan, USA, South |
| 8–108 | Korea [25,26] | ||
| 3 | Vinegar | ND–51 | China, South Korea, the USA, and Eu members |
| 4 | Sufu | 12–124 | China |
| 5 | Red sufu | 87–344 | China |
3. Alcoholic Beverages and Ethyl Carbamate
4. Wines Content of Ethyl Carbamate
5. Ethyl Carbamate Formation Mechanism
6. Formation of Ethyl Carbamate in Wines
7. Discussion
7.1. Bibliometric Analysis
7.2. Minimizing EC in Fermented Products
7.3. Detection Methods for EC in Fermented Foods and Beverages
7.4. EC in Distilled and Fermented Beverages
7.5. Health Implications and Carcinogenic Potential
7.6. Quantitative Detection Methods
7.7. Regional Exposure to EC
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.; Du, G.; Zou, H.; Fu, J.; Zhou, J.; Chen, J. Progress in preventing the accumulation of ethyl carbamate in alcoholic beverages. Trends Food Sci. Technol. 2013, 32, 97–107. [Google Scholar] [CrossRef]
- Battaglia, R.; Conacher, H.B.S.; Page, B.D. Ethyl carbamate (urethane) in alcoholic beverages and foods—A review. Food Addit. Contam. 1990, 7, 477–496. [Google Scholar] [CrossRef]
- Cairns, T.; Siegmund, E.G.; Luke, M.A.; Doose, G.M. Residue levels of ethyl carbamate in wines and spirits by gas chromatography and mass spectrometry/mass spectrometry. Anal. Chem. 1987, 59, 2055–2059. [Google Scholar] [CrossRef]
- Difford’s Guide. Available online: https://www.diffordsguide.com/encyclopedia/215/bws/umeshu (accessed on 14 June 2025).
- Zimmerli, B.; Schlatter, J. Ethyl carbamate: Analytical methodology, occurrence, formation, biological activity and risk assessment. Mutat. Res. 1991, 259, 325–350. [Google Scholar] [CrossRef]
- Schlatter, J.; Lutz, W.K. The carcinogenic potential of ethyl carbamate (urethane): Risk assessment at human dietary exposure levels. Food Chem. Toxicol. 1990, 28, 205–211. [Google Scholar] [CrossRef]
- Gowd, V.; Su, H.; Karlovsky, P.; Chen, W. Ethyl carbamate: An emerging food and environmental toxicant. Food Chem. 2018, 248, 312–321. [Google Scholar] [CrossRef]
- Gary, A.; Dahl, G.; Miller, E.; Miller, J. Comparative Carcinogenicities and Mutagenicities of Vinyl Carbamate, Ethyl Carbamate, and Ethyl N-Hydroxycarbamate. Cancer Res. 1980, 40, 1194–1203. [Google Scholar] [CrossRef]
- Sakano, K.; Oikawa, S.; Hiraku, Y.; Kawanishi, S. Metabolism of carcinogenic urethane to nitric oxide is involved in oxidative DNA damage. Free Radic. Biol. Med. 2002, 33, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Hofman-Bang, J. Nitrogen catabolite repression in Saccharomyces cerevisiae. Mol. Biotechnol. 1999, 12, 35–73. [Google Scholar] [CrossRef] [PubMed]
- Baffa Júnior, J.C.; Mendonça, R.C.S.; Kluge, J.M.A.T.; Pereira, J.A.M.; Soares, N.F.F. Ethyl-carbamate determination by gas chromatography–mass spectrometry at different stages of production of a traditional Brazilian spirit. Food Chem. 2011, 129, 1383–1387. [Google Scholar] [CrossRef]
- Cadranel, J.F.M.D.; Legendre, C.M.D.; Desaint, B.M.D.; Delamarre, N.M.D.; Florent, C.M.D.; Levy, V.G.M.D. Liver disease from surreptitious administration of urethane. J. Clin. Gastro. 1993, 17, 52–56. [Google Scholar] [CrossRef]
- Tannenbaum, A.; Silverstone, H. Urethan (Ethyl Carbamate) as a multipotential carcinogen. Cancer Res. 1958, 18, 1225–1231. [Google Scholar] [CrossRef]
- Weber, J.V.; Sharypov, V.I. Ethyl carbamate in foods and beverages: A review. Environ. Chem. Lett. 2009, 7, 233–247. [Google Scholar] [CrossRef]
- Benson, R.W.; Beland, F.A. Modulation of urethane (ethyl carbamate) carcinogenicity by ethyl alcohol: A review. Int. J. Toxicol. 1997, 16, 521–544. [Google Scholar] [CrossRef]
- World Health Organization. Safety Evaluation of Certain Contaminants in Food. WHO Food Additives, Series 55. In Proceedings of the Sixty-Fourth Meeting of the Joint FAO/WHO Expert Committee on Food Additives, Rome, Italy, 8–17 February 2006; Available online: https://www.who.int/publications/i/item/9241660554 (accessed on 14 July 2025).
- Ryu, D.; Choi, B.; Kim, E.; Park, S.; Paeng, H.; Kim, C.; Lee, J.; Yoon, H.J.; Koh, E. Determination of ethyl carbamate in alcoholic beverages and fermented foods sold in Korea. Toxicol. Res. 2015, 31, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Food Standards Australia, New Zeeland. Ethyl Carbamate in Australian Foods (2007), Survey Sampling and Analysis Conducted; FSANZ: Wellington, New Zealand, 2013; pp. 7–25. Available online: https://www.foodstandards.gov.au/science-data/monitoring-safety/ethylcarbamateinaustralia (accessed on 15 July 2025).
- Abt, E.; Incorvati, V.; Robin, L.P.; Redan, B.W. Occurrence of ethyl carbamate in foods and beverages: Review of the formation mechanisms. Advances in analytical methods, and mitigation strategies. J. Food Prot. 2021, 84, 2195–2212. [Google Scholar] [CrossRef]
- European Food Safety Authority Panel members. Ethyl carbamate and hydrocyanic acid in food and beverages, Scientific Opinion of the Panel on Contaminants. EFSA J. Eur. Food Saf. Auth. 2007, 551, 1–44. [Google Scholar] [CrossRef]
- Leça, J.M.; Pereira, V.; Pereira, A.; Marques, J.C. Rapid and sensitive methodology for determination of ethyl carbamate in fortified wines using microextraction by packed sorbent and gas chromatography with mass spectrometric detection. Anal. Chim. Acta 2014, 811, 29–35. [Google Scholar] [CrossRef]
- Andreiana, V.; Georgiana, S.D.; Constantinescu, A.; Bondac, G.T. Behavior of Romanian bread consumers. Ekon. Poljopr. 2023, 70, 611–626. [Google Scholar] [CrossRef]
- REGULAMENTUL (UE) NR. 1129/2011 AL COMISIEI din 11 Noiembrie 2011. Jurnalul Oficial al Uniunii Europene. L 295/2. Available online: https://old.ms.ro/documente/REGULAMENTUL UE NR. 1129 - 2011 AL COMISIEI_2934_7616.pdf (accessed on 15 September 2025).
- Vahl, M. A Survey of ethyl carbamate in beverages, bread and acidified milks sold in Denmark. Food Addit. Contam. 1993, 10, 585–592. [Google Scholar] [CrossRef]
- Choi, B.; Jang, Y.; Koh, E. Determination of ethyl carbamate in soy sauce from Korean market. Food Control 2018, 93, 56–60. [Google Scholar] [CrossRef]
- Kim, Y.K.L.; Koh, E.; Chung, H.J.; Kwon, H. Determination of ethyl carbamate in some fermented Korean foods and beverages. Food Addit. Contam. 2000, 17, 469–475. [Google Scholar] [CrossRef]
- Sen, N.P.; Seaman, S.W.; Boyle, M.; Weber, D. Methyl carbamate and ethyl carbamate in alcoholic beveragesand other fermented foods. Food Chem. 1993, 4, 359–366. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, Y.; Cheng, Y.; Liu, Y.; Yadav, M.; Yin, L. Reduction of biogenic amines in sufu by ethanol addition during ripening stage. Food Chem. 2018, 239, 1244–1252. [Google Scholar] [CrossRef]
- Canas, B.J.; Joe, F.L., Jr.; Diachenko, G.W.; Burns, G. Determination of ethyl carbamate in alcoholic beverages and soy sauce by gas chromatography with mass selective detection: Collaborative study. J. AOAC Int. 1994, 77, 1530–1536. [Google Scholar] [CrossRef]
- Dennis, M.J.; Howarth, N.; Key, P.E.; Pointer, M.; Massey, R.C. Investigation of ethyl carbamate levels in some fermented foods and alcoholic beverages. Food Addit. Contam. 1989, 6, 383–389. [Google Scholar] [CrossRef]
- Jagerdeo, E.; Dugar, S.; Foster, G.D.; Schenck, H. Analysis of ethyl carbamate in wines using solid-phaseextraction and multidimensional gas chromatography/massspectrometry. J. Agric. Food Chem. 2002, 50, 5797–5802. [Google Scholar] [CrossRef]
- Lim, H.S.; Lee, K.G. Development and validation of analytical methods for ethyl carbamate in various fermented foods. Food Chem. 2011, 126, 1373–1379. [Google Scholar] [CrossRef]
- Tu, Q.; Qi, W.; Zhao, J.; Zhang, L.; Guo, Y. Quantification ethyl carbamate in wines using reaction-assisted-extraction with 9-xanthydrol and detection by heart-cutting multidimensional gas chromatography-mass spectrometry. Anal. Chim. Acta 2018, 1001, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Bărbulescu, I.-D.; Teodorescu, R.-I.; Dumitrache, C.; Begea, M.; Diguță, C.F.; Frîncu, M.; Baniță, D.C.; Mărculescu, S.-I.; Cîrîc, A.I.; Tudor, V.; et al. Obtaining active dry yeasts biomass for the production of Pietroasa wines. AgroLife Sci. J. 2023, 12, 18–24. [Google Scholar] [CrossRef]
- Šantrůčková, V.; Fischer, J.; Klikarová, J. A rapid and improved method for the determination of ethyl carbamate in foodstuffs of different matrices. Anal. Methods 2024, 16, 4733–4742. [Google Scholar] [CrossRef]
- Wu, H.; Chen, L.; Pan, G.; Tu, C.; Zhou, X.; Mo, L. Study on the changing concentration of ethyl carbamate in yellow rice wine during production and storage by gas chro-matography/mass spectrometry. Eur. Food Res. Technol. 2012, 235, 779–782. [Google Scholar] [CrossRef]
- Tang, A.S.; Chung, S.W.; Kwong, K.; Xiao, Y.; Chen, M.Y.; Ho, Y.Y.; Ma, S.W. Ethyl carbamate in fermented foods and beverages: Dietary exposure of the Hong Kong population in 2007–2008. Food Addit. Contam. Part B 2011, 4, 195–204. [Google Scholar] [CrossRef]
- Kim, D.H.; Jang, H.S.; Choi, G.I.; Kim, H.J.; Kim, H.J.; Kim, H.L.; Kim, K.S. Determination of residue levels of ethyl carbamate in alcoholic beverages by gas chromatography/tandem mass spectrometry (GC/MS/MS). J. Food Hyg. Saf. 2013, 1, 63–68. [Google Scholar] [CrossRef]
- Park, S.K.; Kim, C.T.; Lee, J.W.; Jhee, O.H.; Om, A.S.; Kang, J.S.; Moon, T.W. Analysis of ethyl carbamate in Korean soy sauce using high-performance liquid chromatography with fluorescence detection or tandem mass spectrometry and gas chromatography with mass spectrometry. Food Control 2007, 18, 975–982. [Google Scholar] [CrossRef]
- Diachenko, G.W.; Canas, B.J.; Joe, F.L.; DiNovi, M. Ethyl Carbamate in Alcoholic Beverages and Fermented Foods. In Food Safety Assessment; ACS Symposium Series; Finley, J.W., Robinson, S.F., Armstrong, D.J., Eds.; American Chemical Society: Washington, DC, USA, 1992; Volume 484, pp. 419–428. [Google Scholar] [CrossRef]
- Wang, Z.; Qu, P.; Zhao, Y.; Wu, Y.; Lyu, B.; Miao, H. A Nationwide Survey and Risk Assessment of Ethyl Carbamate Exposure Due to Daily Intake of Alcoholic Beverages in the Chinese General Population. Foods 2023, 12, 3129. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.S.; Hu, S.J.; Park, H.R.; Lee, H.M.; Kwon, K.S.; Han, E.M.; Kim, K.M.; Ko, E.J.; Ha, S.D.; Bae, D.H. Estimation of Korean adult’s daily intake of ethyl carbamate through Korean commercial alcoholic beverages based on the monitoring. Food Sci. Biotechnol. 2006, 15, 112–116. Available online: https://www.koreascience.kr/article/JAKO200609905800979.pdf (accessed on 25 July 2025).
- Alberts, P.; Stander, M.; De Villiers, A. Development of a novel solid-phase extraction, LC-MS/MS method for the analysis of ethyl carbamate in alcoholic beverages: Application to South African wine and spirits. Food Addit. Contam. Part A 2011, 28, 826–839. [Google Scholar] [CrossRef]
- Hwang, L.H.; Kim, A.K.; Park, K.A.; Kim, J.Y.; Hwang, I.S.; Chae, Y.Z. The effect of raw material, alcohol content, and trans-resveratrol on the formation of ethyl carbamate in plum wine. J. Food Saf. Hyg. 2009, 24, 194–199. Available online: https://koreascience.kr/article/JAKO200934939514576.pdf (accessed on 25 July 2025).
- Lachenmeier, D.W.; Kanteres, F.; Kuballa, T.; López, M.G.; Rehm, J. Ethyl carbamate in alcoholic beverages from Mexico (tequila, mezcal, bacanora, sotol) and Guatemala (cuxa): Market survey and risk assessment. Int. J. Environ. Res. Public Health 2009, 6, 349–360. [Google Scholar] [CrossRef]
- Bortoletto, A.M.; Alcarde, A.R. Assessment of chemical quality of Brazilian sugar cane spirits and cachaças. Food Control 2015, 54, 1–6. [Google Scholar] [CrossRef]
- D’Avila, G.B.; Cardoso, M.D.G.; Santiago, W.D.; Rodrigues, L.M.A.; Da Silva, B.L.; Cardoso, R.R.; Caetano, A.R.S.; Ribeiro, C.D.F.S.; Nelson, D.L. Quantification of ethyl carbamate in cachaça produced in different agro-industrial production systems. J. Inst. Brew. 2016, 122, 299–303. [Google Scholar] [CrossRef]
- Nóbrega, I.C.; Pereira, J.A.; Paiva, J.E.; Lachenmeier, D.W. Ethyl carbamate in cachaça (Brazilian sugarcane spirit): Extended survey confirms simple mitigation approaches in pot still distillation. Food Chem. 2011, 127, 1243–1247. [Google Scholar] [CrossRef]
- Lachenmeier, D.W. Rapid screening for ethyl carbamate in stone-fruit spirits using FTIR spectroscopy and chemometrics. Anal. Bioanal. Chem. 2005, 382, 1407–1412. [Google Scholar] [CrossRef]
- Pomohaci, N.; Cioltean, I.; Popa, A.; Modoran, D.; Vişan, L.; Chioran, I. Ţara palincii, Ed. Ceres. 2008, p. 408. Available online: https://www.librariadelfin.ro/carte/producerea-bauturilor-alcoolice-traditionale-in-tara-palincii-n-pomohaci (accessed on 25 July 2025).
- Lachenmeier, D.W.; Sarsh, B.; Rehm, J. The composition of alcohol products from markets in Lithuania and Hungary, and potential health consequences: A pilot study. Alcohol Alcohol. 2009, 44, 93–102. [Google Scholar] [CrossRef]
- Institutul Naţional De Statistică (INS). Consumul de Băuturi în Anul. 2022. Available online: https://insse.ro/cms/sites/default/files/field/publicatii/consumul_de_bauturi_in_anul_2022.pdf (accessed on 25 July 2025).
- Choi, B.; Koh, E. Changes of ethyl carbamate and its precursors in maesil (Prunus mume) extract during one-year fermentation. Food Chem. 2016, 209, 318–322. [Google Scholar] [CrossRef]
- Nan-Young, K.; Mi-Na, E.; Young-Sook, D. Determination of ethyl carbamate in maesil wine by alcohol content and ratio of maesil (Prunus mume) during ripening period. Kor. J. Food Pres. 2013, 20, 429–434. [Google Scholar] [CrossRef][Green Version]
- Chen, D.; Ren, Y.; Zhong, Q.; Shao, Y.; Zhao, Y.; Wu, Y. Ethyl carbamate in alcoholic beverages from China: Levels, dietary intake, and risk assessment. Food Control 2017, 72, 283–288. [Google Scholar] [CrossRef]
- Choi, B.; Ryu, D.; Kim, C.I.; Lee, J.Y.; Choi, A.; Koh, E. Probabilistic dietary exposure to ethyl carbamate from fermented foods and alcoholic beverages in the Korean population. Food Addit. Contam. Part A 2017, 34, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Kamimura, H.; Ibe, A.; Tabata, S.; Yasuda, K.; Nishijima, M. Formation of ethyl carbamate in umeshu (plum liqueur). Shokuhin Eiseigaku Zasshi J. Food Hyg. Soc. Jpn. 2001, 42, 354–358. [Google Scholar] [CrossRef]
- Wu, P.; Pan, X.; Wang, L.; Shen, X.; Yang, D. A survey of ethyl carbamate in fermented foods and beverages from Zhejiang, China. Food Control 2012, 23, 286–288. [Google Scholar] [CrossRef]
- Liu, Y.P.; Dong, B.; Qin, Z.S.; Yang, N.J.; Lu, Y.; Yang, L.X.; Chang, F.Q.; Wu, Y.N. Ethyl carbamate levels in wine and spirits from markets in Hebei Province, China. Food Addit. Contam. 2011, 4, 1–5. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, W.M.; Clyne, A.H.; MacDonald, L.S. Ethyl carbamate formation in grain based spirits. II. The identification and determination of cyanide related species involved in ethyl carbamate formation in Scotch grain whisky. J. Inst. Brew. 1990, 96, 223–232. [Google Scholar] [CrossRef]
- Perreira, V.S.; Medeiros, M.M.; Telles, D.; Albuquerque, E., Jr.; Oliveira, J.; Lachenmeier, D. Improved sample preparation for GC–MS–SIM analysis of ethyl carbamate in wine. Food Chem. 2015, 177, 23–28. [Google Scholar] [CrossRef]
- Wogan, G.N.; Hecht, S.S.; Felton, J.S.; Conney, A.H.; Loeb, L.A. Environmental and chemical carcinogenesis. Semin. Cancer Biol. 2004, 14, 473–486. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Lima, M.; Nóbrega, I.; Pereira, J.; Kerr-Corrêa, F.; Kanteres, F.; Rehm, J. Cancer risk assessment of ethyl carbamate in alcoholic beverages from Brazil with special consideration to the spirits cachaça and tiquira. BMC Cancer 2010, 10, 266. [Google Scholar] [CrossRef]
- Uthurry, C.A.; Varela, F.; Colomo, B.; Suárez Lepe, J.A.; Lombardero, J.; García del Hierro, J.R. Ethyl carbamate concentrations of typical Spanish red wines. Food Chem. 2004, 88, 329–336. [Google Scholar] [CrossRef]
- Hasnip, S.; Crews, C.; Potter, N.; Christy, J.; Chan, D.; Bondu, T.; Matthews, W.; Walters, B.; Patel, K. Survy of ethyl carbamate in fermented foods sold in the United Kingdom in 2004. J. Agric. Food Chem. 2007, 55, 2755–2759. [Google Scholar] [CrossRef]
- Fu, Z.; Yang, L.; Ma, L.; Liu, X.; Li, J. Occurrence of ethyl carbamate in three types of Chinese wines and its possible reasons. Food Sci. Biotechnol. 2016, 25, 949–953. [Google Scholar] [CrossRef]
- Perestrelo, R.; Petronilho, S.; Câmara, J.S.; Rocha, S.M. Comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry combined with solid phase microextraction as a powerful tool for quantification of ethyl carbamate in fortified wines. The case study of Madeira wine. J. Chromatogr. A 2010, 1217, 3441–3445. [Google Scholar] [CrossRef]
- Fu, M.L.; Liu, J.; Chen, Q.H.; Liu, X.J.; He, G.Q.; Chen, J.C. Determination of ethyl carbamate in Chinese yellow rice wine using high-performance liquid chromatography with fluorescence detection. Int. J. Food Sci. Technol. 2010, 45, 1297–1302. [Google Scholar] [CrossRef]
- Tredoux, G.J.; Ferreira, A.C.S. Fortified Wines: Styles, Production and Flavour Chemistry. In Alcoholic Beverages: Sensory Evaluation and Consumer; Piggott, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 159–179. [Google Scholar] [CrossRef]
- Antoce, A.O.; Stockley, C. An overview of the implications of wine on human health, with special consideration of the wine-derived phenolic compounds. AgroLife Sci. J. 2019, 8, 21–34. Available online: https://agrolifejournal.usamv.ro/index.php/agrolife/article/view/412/408 (accessed on 25 July 2025).
- Aresta, M.; Boscolo, M.; Franco, D.W. Copper (II) catalysis in cyanate conversion into ethyl carbamate in spirits, and relevant reactions. J. Agric. Food Chem. 2001, 49, 2819–2824. [Google Scholar] [CrossRef]
- Mildau, G.; Preuss, A.; Frank, W.; Heering, W. Ethyl carbamate (urethane) in alcoholic beverages: Improved analysis and light-dependent formation. Dtsch. Lebensm.-Rundsch. 1987, 83, 69–74. [Google Scholar]
- Lofroth, G.; Gejvall, T. Diethyl pyrocarbonate: Formation of urethan in treated beverages. Science 1971, 174, 1248–1250. [Google Scholar] [CrossRef] [PubMed]
- Ough, C.S.; Crowell, E.A.; Gutlove, B.R. Carbamyl compound reactions with ethanol. Am. J. Enol. Vitic. 1988, 39, 239–242. [Google Scholar] [CrossRef]
- Kliew, W.M.; Cook, J.A. Arginine and total free amino acids as indicators of the nitrogen status of grapevines. J. Am. Soc. Hortic. Sci. 1971, 96, 581–587. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Garde-Cerdán, T.; Gonzalo-Diago, A.; Moreno-Simunovic, Y.; Martínez-Gil, A. Effect of different foliar nitrogen applications on the must amino acids and glutathione composition in Cabernet Sauvignon vineyard. J. Food Sci. Technol. 2017, 75, 147–154. [Google Scholar] [CrossRef]
- Stines, A.P.; Grubb, J.; GockowiaK, H.; Henschke, P.A.; Hoj, P.B.; Van Heeswijck, R. Proline and arginine accumulation in developing berries of Vitis vinifera L. in Australian vineyards: Influence of vine cultivar, berry maturity and tissue type. Aust. J. Grape Wine Res. 2000, 6, 150–158. [Google Scholar] [CrossRef]
- Ancín-Azpilicueta, C.; Nieto-Rojo, R.; Gómez-Cordón, J. Influence of fertilisation with foliar urea on the content of amines in wine. Food Addit. Contam. Part A 2011, 28, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Ancín-Azpilicueta, C.; Nieto-Rojo, R.; Gómez-Cordón, J. Effect of foliar urea fertilisation on volatile compounds in Tempranillo wine. J. Sci. Food Agric. 2013, 93, 1481–1485. [Google Scholar] [CrossRef]
- Shalamitskiy, M.Y.; Tanashchuk, T.N.; Cherviak, S.N.; Vasyagin, E.A.; Ravin, N.V.; Mardanov, A.V. Ethyl carbamate in fermented food products: Sources of appearance, hazards and methods for reducing its content. Foods 2023, 12, 3816. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qian, M.; Dong, H.; Bai, W.; Zhao, W.; Li, X.; Liu, G. Effect of ageing process on carcinogen ethyl carbamate (EC), its main precursors and aroma compound variation in Hakka Huangjiu produced in southern China. Int. J. Food Sci. Technol. 2020, 55, 1773–1780. [Google Scholar] [CrossRef]
- Broughton, J.D.; Blackburn, P.; Evans, R.J.; Hugenholtz, J. Applications of the bacteriosin, nisin. Anton. Leeuw. Int. J. Gen. 1996, 69, 193–202. [Google Scholar] [CrossRef]
- Mihăescu, C.; Neagu Frăsin, L.B. The antifungal effect of some natural and synthetic chemical compounds on the bitter rot apple. AgroLife Sci. J. 2020, 9, 179–184. Available online: https://agrolifejournal.usamv.ro/index.php/agrolife/article/view/270/269 (accessed on 28 July 2025).
- Jovanović-Cvetković, T.; Grbić, R.; Grobelnik Mlakar, S.; Bosančić, B.; Cvetković, M. Physicochemical evaluation of the grape and wine of the Blatina, Trnjak and Vranac in different vintages. AgroLife Sci. J. 2023, 12, 105–115. [Google Scholar] [CrossRef]
- Meng, C.; Liu, H.; Sun, B. Molecularly Imprinted Polymers: A New Solution for Controlling Ethyl Carbamate in Fermented Alcoholic Beverages. Sep. Purif. Rev. 2025, 54, 17–36. [Google Scholar] [CrossRef]
- Perestrelo, R. Nanostrategy for selective ethyl carbamate removal from fermented alcoholic beverages via molecular imprinting technology. Beverages 2025, 11, 30. [Google Scholar] [CrossRef]
- Deng, H.; Ji, L.; Han, X.; Wu, T.; Han, B.; Li, C.; Zhan, J.; Huang, W.; You, Y. Research progress on the application of different controlling strategies to minimizing ethyl carbamate in grape wine. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1495–1516. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, W.; Fang, F.; Yu, S.; Zhou, J. Elimination of ethyl carbamate in fermented foods. Food Biosci. 2022, 47, 101725. [Google Scholar] [CrossRef]
- Han, L.; Zhu, P.; Liu, H.; Sun, B. Molecularly imprinted bulk and solgel optosensing based on biomass carbon dots derived from watermelon peel for detection of ethyl carbamate in alcoholic beverages. Microchim. Acta 2022, 189, 286. [Google Scholar] [CrossRef]
- Han, L.; Meng, C.; Zhang, D.; Liu, H.; Sun, B. Fabrication of a fluorescence probe via molecularly imprinted polymers on carbazole-based covalent organic frameworks for optosensing of ethyl carbamate in fermented alcoholic beverages. Anal. Chim. Acta 2022, 1192, 339381. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Chen, H.; Liu, R.; Wang, S.; Liu, T.; Hu, Z.; Lan, W.; Yu, Y.; She, Y.; Fu, H. Fluorescent sensor based on quantum dots and nano-porphyrin for highly sensitive and specific determination of ethyl carbamate in fermented food. J. Sci. Food Agric. 2021, 101, 6193–6201. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Lin, X.; Lei, Y.; Zhu, J.; Sa, R.; Chen, Y. Contactless photoelectrochemical biosensors based on hierarchical MXene/Bi2S3 nanosheets with the branched hybridization chain reaction. Biosens. Bioelectron. 2023, 243, 115764. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Di, C.; Zhang, R.; Shi, L. Ethyl carbamate regulate esters degradation by activating hydrolysis during Baijiu ripening. Food Res. Int. 2022, 156, 111157. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Zhang, M. Ethyl carbamate in Chinese liquor (Baijiu): Presence, analysis, formation, and control. Appl. Microbiol. Biotechnol. 2021, 105, 4383–4395. [Google Scholar] [CrossRef]
- Wang, W.; Han, Z.; Guo, D.; Xiang, Y. Renal transcriptomics reveals the carcinogenic mechanism of ethyl carbamate in Musalais. OncoTargets Ther. 2021, 14, 1401–1416. [Google Scholar] [CrossRef]
- Crain, S.L.; Coffey, R.T. “Occurrence of Ethyl Carbamate in Foods and Beverages: Review of the Formation Mechanisms, Advances in Analytical Methods, and Mitigation Strategies.” A Comment on: J Food Prot. 84(12):2195–2212 (2021). https://doi.org/10.4315/JFP-21-219. PMID: 34347857. J. Food Prot. 2022, 85, 1104–1106. [Google Scholar] [CrossRef]
- Xia, Q.; Yang, C.; Wu, C.; Zhou, R.; Li, Y. Quantitative strategies for detecting different levels of ethyl carbamate (EC) in various fermented food matrices: An overview. Food Control 2018, 84, 499–512. [Google Scholar] [CrossRef]
- Ryu, D.; Choi, B.; Kim, N.; Koh, E. Validation of analytical methods for ethyl carbamate in nine food matrices. Food Chem. 2016, 211, 770–775. [Google Scholar] [CrossRef]
- Huang, Z.; Pan, X.-D.; Wu, P.-G.; Chen, Q.; Han, J.-L.; Shen, X.-H. Validation (in-house and collaboratory) of the quantification method for ethyl carbamate in alcoholic beverages and soy sauce by GC–MS. Food Chem. 2013, 141, 4161–4165. [Google Scholar] [CrossRef] [PubMed]
- de Resende Machado, A.M.; Cardoso, M.; Saczk, A.A.; dos Anjos, J.P.; Zacaroni, L.M.; Dórea, H.S.; Nelson, D.L. Determination of ethyl carbamate in cachaça produced from copper stills by HPLC. Food Chem. 2013, 138, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Park, S.K.; Yoon, H.J.; Kang, D.H.; Kim, M. Exposure assessment and risk characterisation of ethyl carbamate from Korean traditional fermented rice wine, Takju and Yakju. Food Addit. Contam. Part A 2016, 33, 207–214. [Google Scholar] [CrossRef]
- Constantin, E.-A.; Constantinescu-Aruxandei, D.; Matei, F.; Shaposhnikov, S.; Oancea, F. Biochemical and microbiological characterization of traditional Romanian fermented drinks—Socata and Borș—A Review. AgroLife Sci. J. 2023, 12, 53–61. [Google Scholar] [CrossRef]
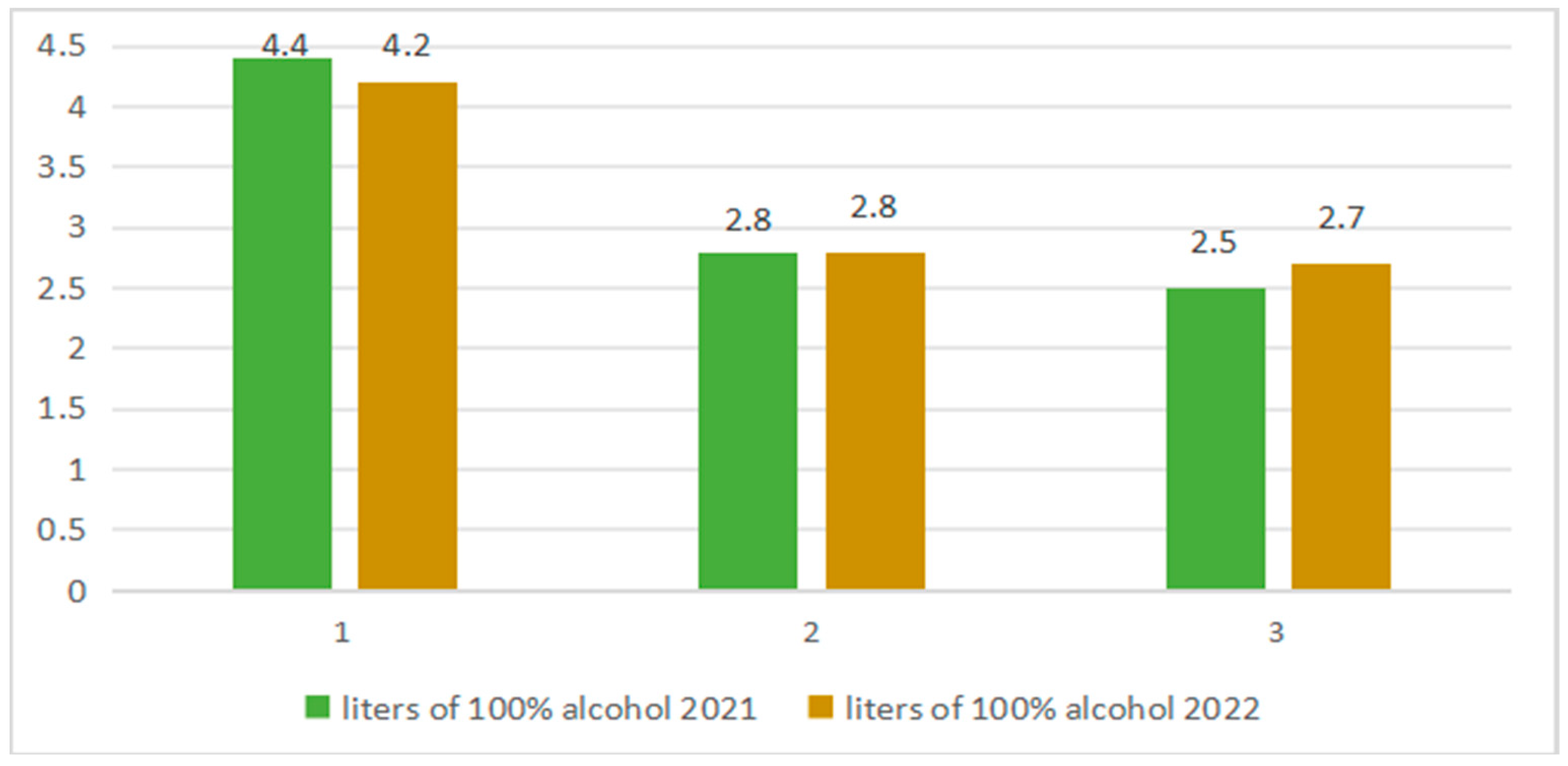
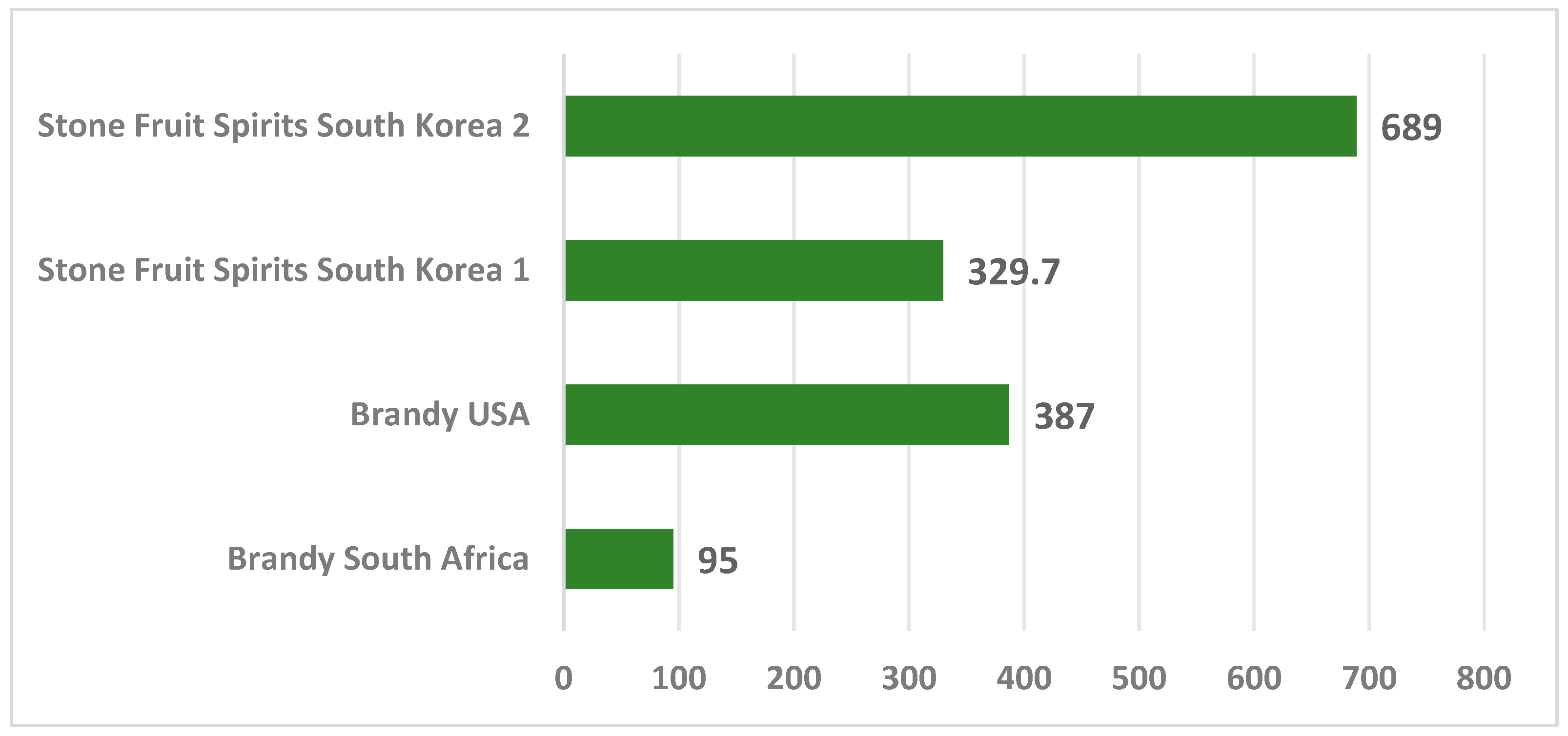
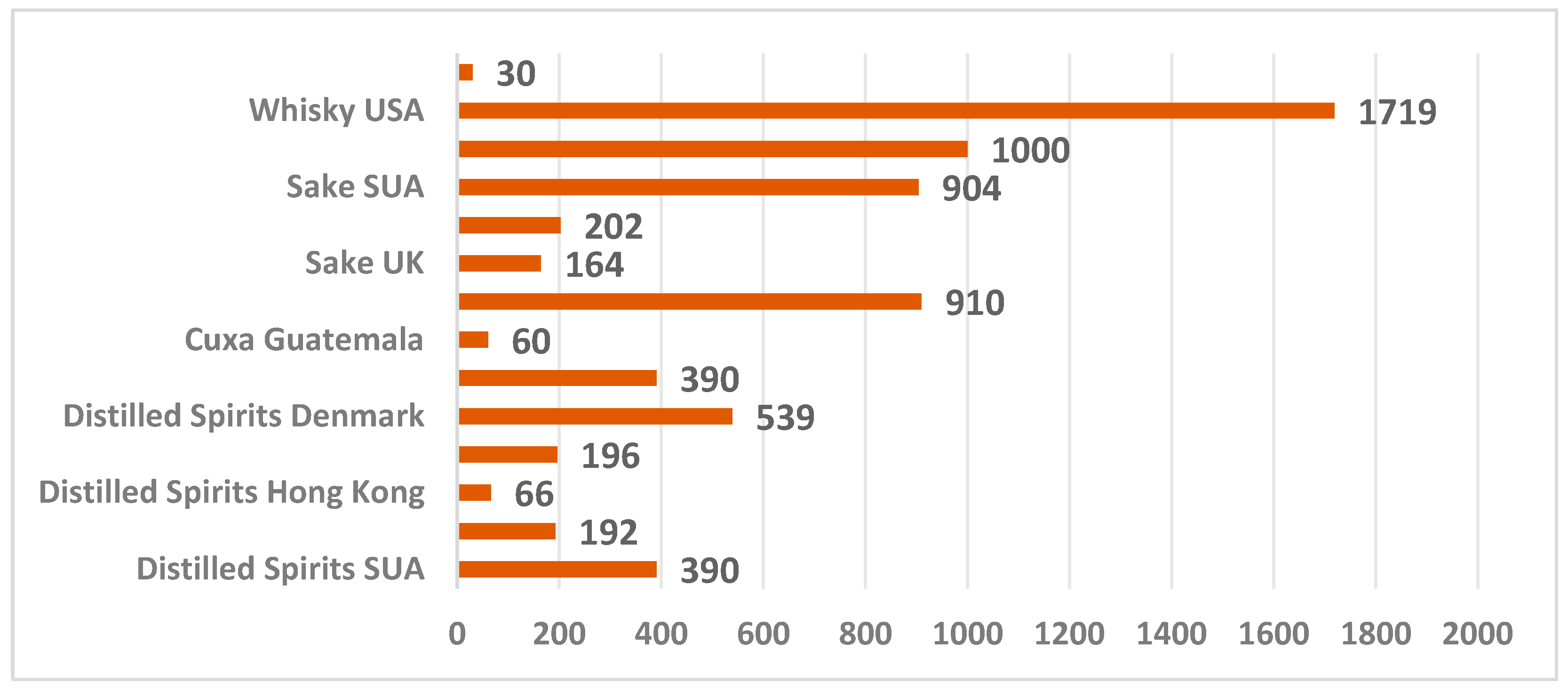
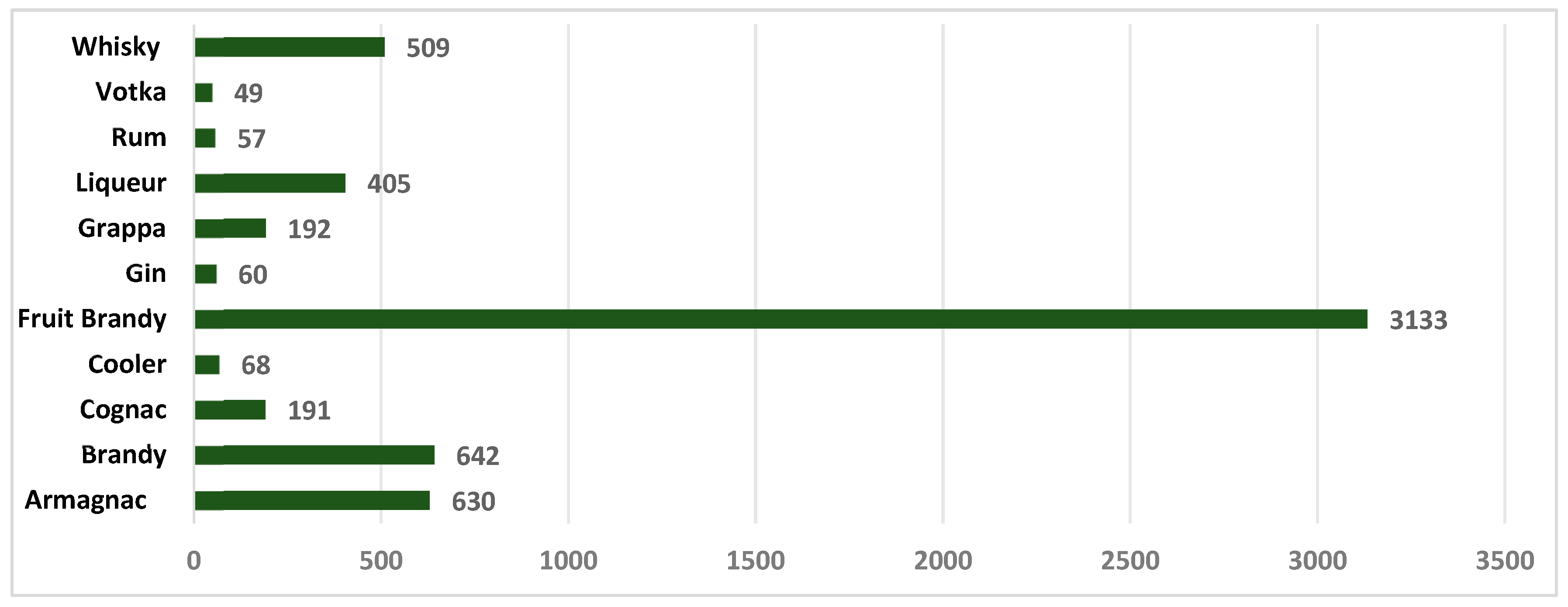
| Alcoholic Beverages | Value of Ethyl Carbamate µg/ L | Country | References |
|---|---|---|---|
| Distilled spirits | 5–5000 | Denmark | [35] |
| 192 | China | [36] | |
| 20–66 | Hong Kong | [37] | |
| 196 | South Korea | [17,38,39] | |
| 390 | USA | [19,40] | |
| Stone fruit spirits | 22,000 | UE | [16,17] |
| 18,000 | Germany | [28] | |
| Whisky | ND-1000 | UE | [17,41] |
| 1719 | USA | [17] | |
| 30 | Korea | [42] | |
| Maesil | 67.9 | Korea | [38] |
| 151.06 | Korea | [17] | |
| Brandy | 4.4–95 | South Africa | [43] |
| 387 | USA | [17,44] | |
| Fruit brandy | 5100 | USA | [19,40] |
| 329.7 | Korea | [19,42] | |
| 689 | Korea | [42] | |
| Agave spirits (tequila) | 390 | Mexico | [19,45] |
| Cuxa | 60 | Guatemala | [19,45] |
| Sugar cane spirits (cachaҫa) | 12–910 | Brasil | [46,47,48] |
| Alcoholic Beverage | Values | Country | References |
|---|---|---|---|
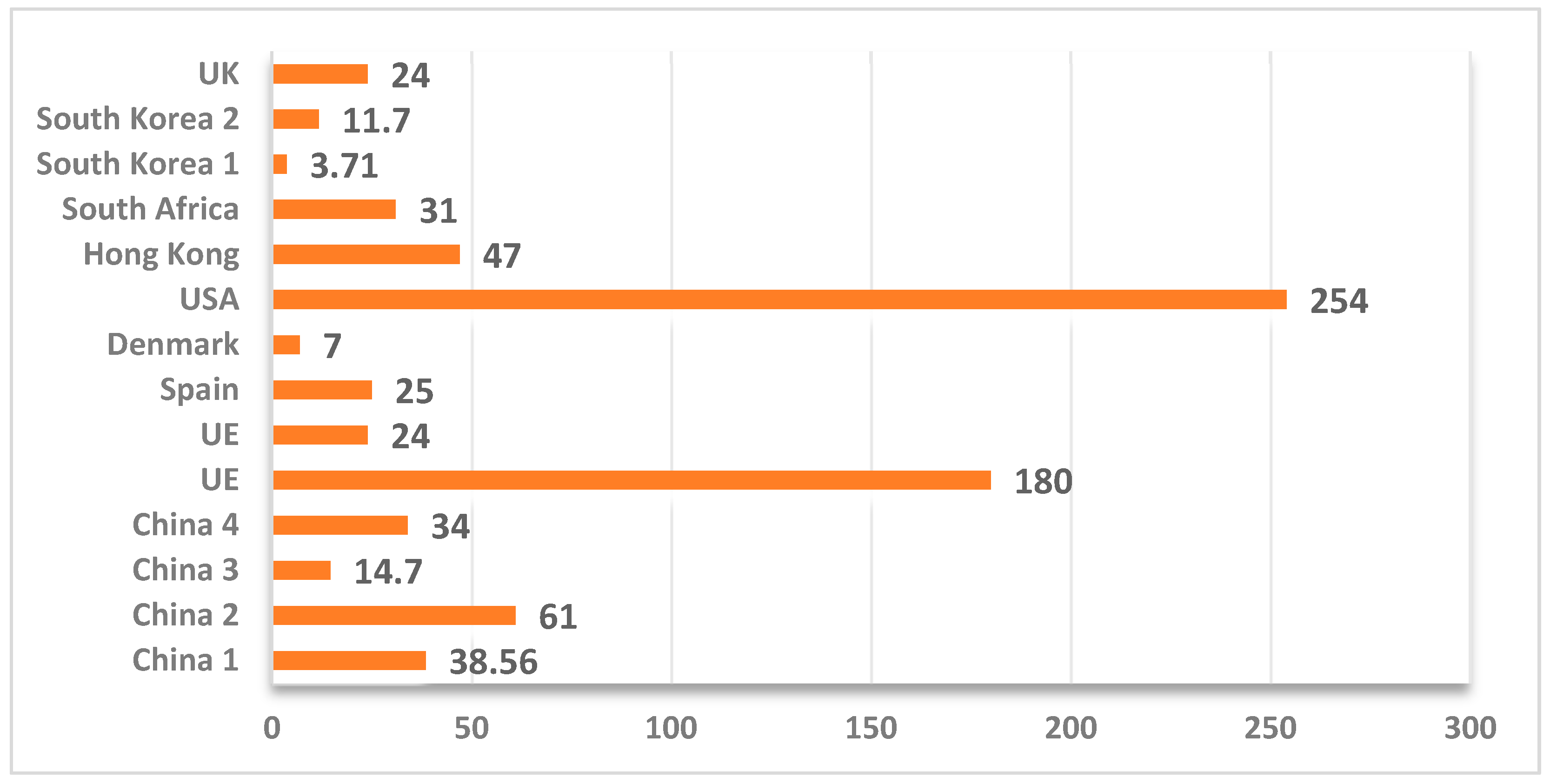
| Wines without additives | ND–25 | Spain | [64] |
| ND–24 | UE | [17,19] | |
| ND–180 | China | [41] | |
| 1.8–31 | South Africa | [43] | |
| 254 | USA | [17,44] | |
| 5–7 | Denmark | [24] | |
| 11–24 | UK | [65] | |
| 6–47 | Hong Kong | [17,37] | |
| 3.71–11.7 | South Korea | [17,42] | |
| 1.16–38.56 | China | [17,36,66] | |
| 61 | China | [19] | |
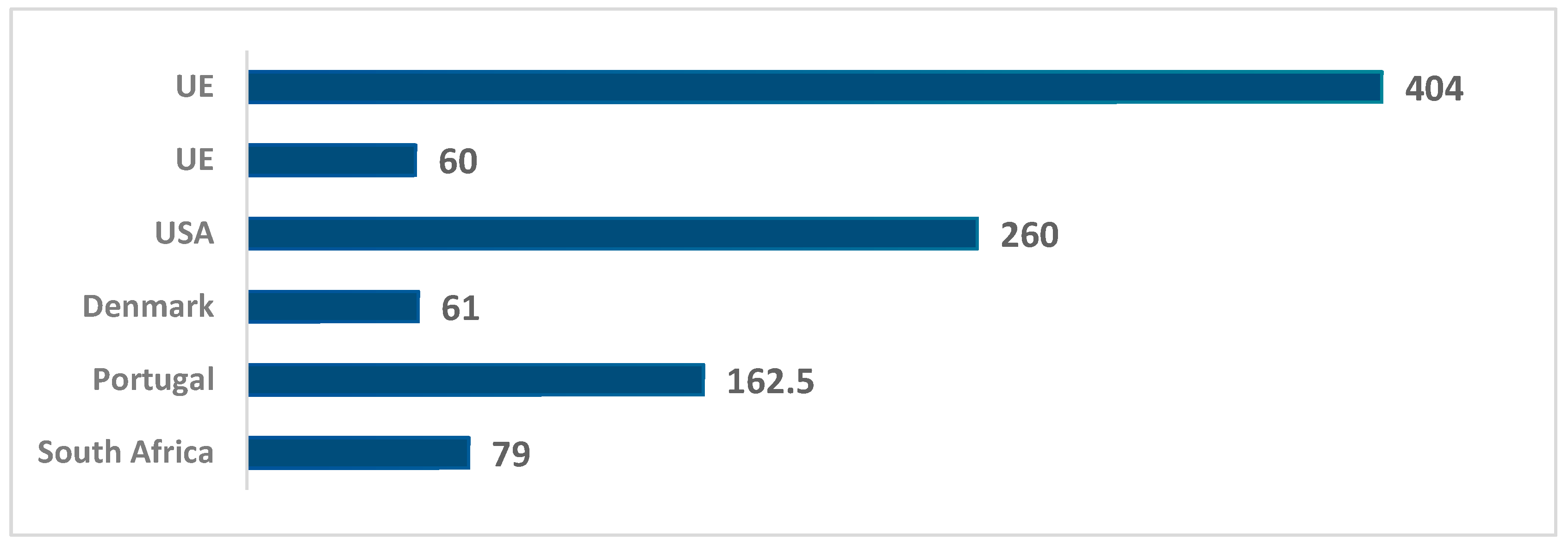
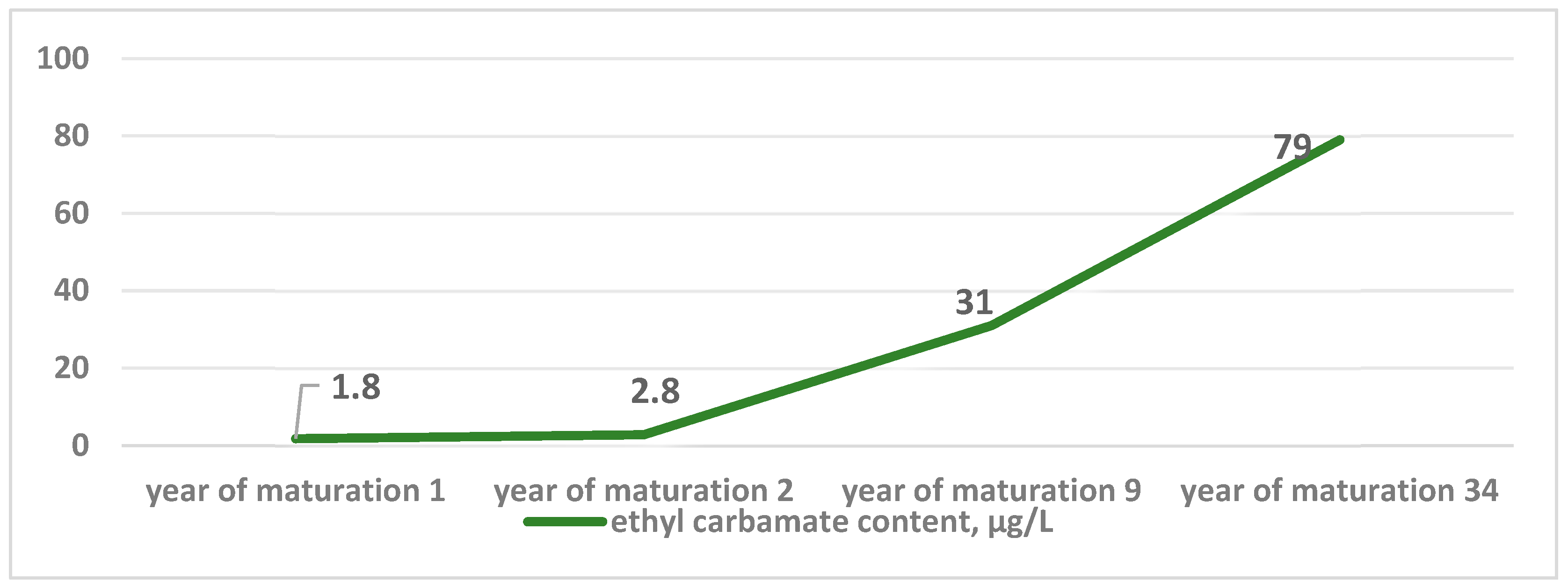
| Fortified wines | 1.8–31 | South Africa (age 1–9) | [43] |
| 2.8–79 | South Africa (age 2–34) | [43] | |
| 54.1–162.5 | Portugal (Madeira) | [67] | |
| 7–61 | Denmark | [35] | |
| 14–60 | UE | [17,65] | |
| ND–404 | UE | [20] | |
| ND–260 | USA | [19] | |
| Rice wine | 8–515 | China | [36] |
| ND–580 | China | [19] | |
| 242.2 | China (yellow rice wine) | [68] | |
| 14.11 | South Korea | [17,38,39] | |
| Sake | 81–164 | UE members | [17,20] |
| ND–202 | Japan | [20] | |
| 904 | USA | [17,44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simion, V.; Vişan, V.L.; Dobrinoiu, R.V.; Dănăilă-Guidea, S.M. Studies on the Ethyl Carbamate Content of Fermented Beverages and Foods: A Review. Foods 2025, 14, 3292. https://doi.org/10.3390/foods14193292
Simion V, Vişan VL, Dobrinoiu RV, Dănăilă-Guidea SM. Studies on the Ethyl Carbamate Content of Fermented Beverages and Foods: A Review. Foods. 2025; 14(19):3292. https://doi.org/10.3390/foods14193292
Chicago/Turabian StyleSimion, Valentina, Valerica Luminiţa Vişan, Ricuţa Vasilica Dobrinoiu, and Silvana Mihaela Dănăilă-Guidea. 2025. "Studies on the Ethyl Carbamate Content of Fermented Beverages and Foods: A Review" Foods 14, no. 19: 3292. https://doi.org/10.3390/foods14193292
APA StyleSimion, V., Vişan, V. L., Dobrinoiu, R. V., & Dănăilă-Guidea, S. M. (2025). Studies on the Ethyl Carbamate Content of Fermented Beverages and Foods: A Review. Foods, 14(19), 3292. https://doi.org/10.3390/foods14193292






