Effects of Sugar Impregnation Methods on Physicochemical Properties and Flavor Profiles of Prune Preserves Using GC-IMS and Electronic Tongue
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Solute Uptake Determination
2.4. Determination of TA
2.5. Spectrophotometric Analysis
2.5.1. Determination of BI and Color Measurement
2.5.2. Determination of TPC and TFC
2.6. Measurement of Texture
2.7. GC-IMS Analysis of Volatile Compounds
2.8. Determination of E-Tongue
2.9. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Solute Uptake
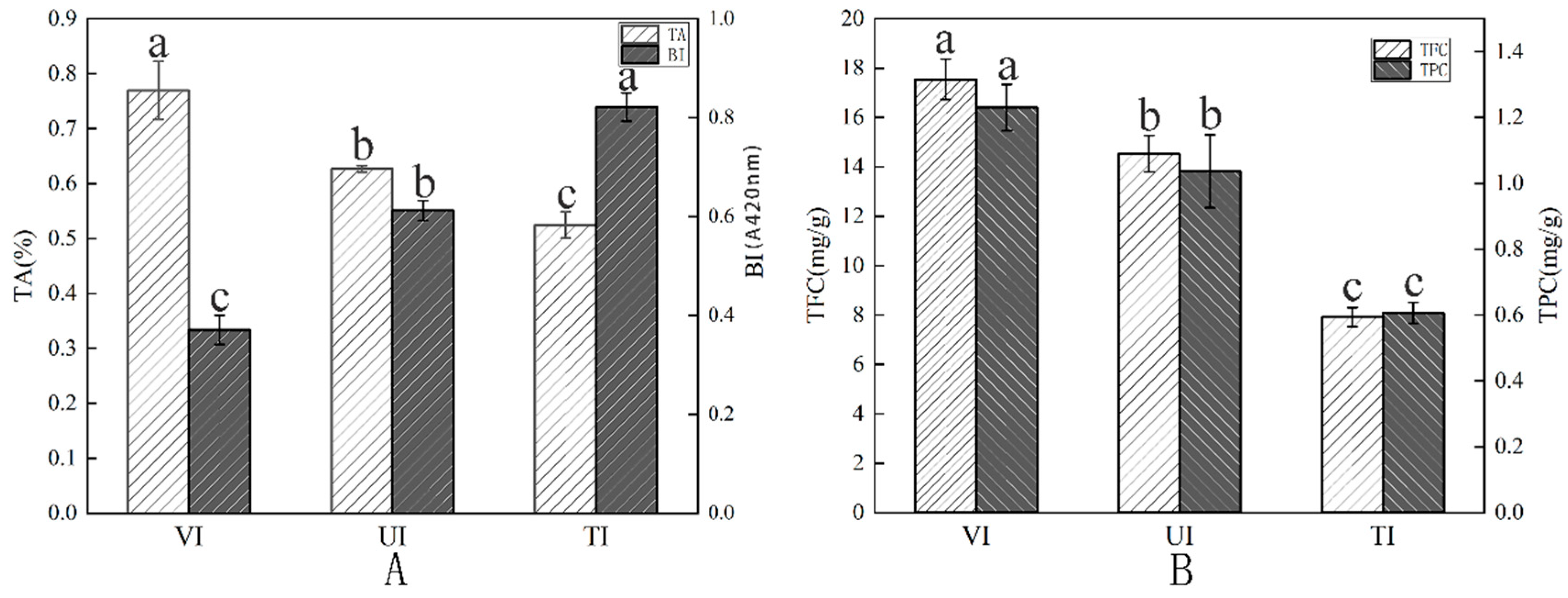
3.2. TA Analysis
3.3. Spectrophotometric Analysis
3.3.1. BI and Color Measurement Analysis
3.3.2. TPC and TFC Analysis
3.4. Texture Analysis
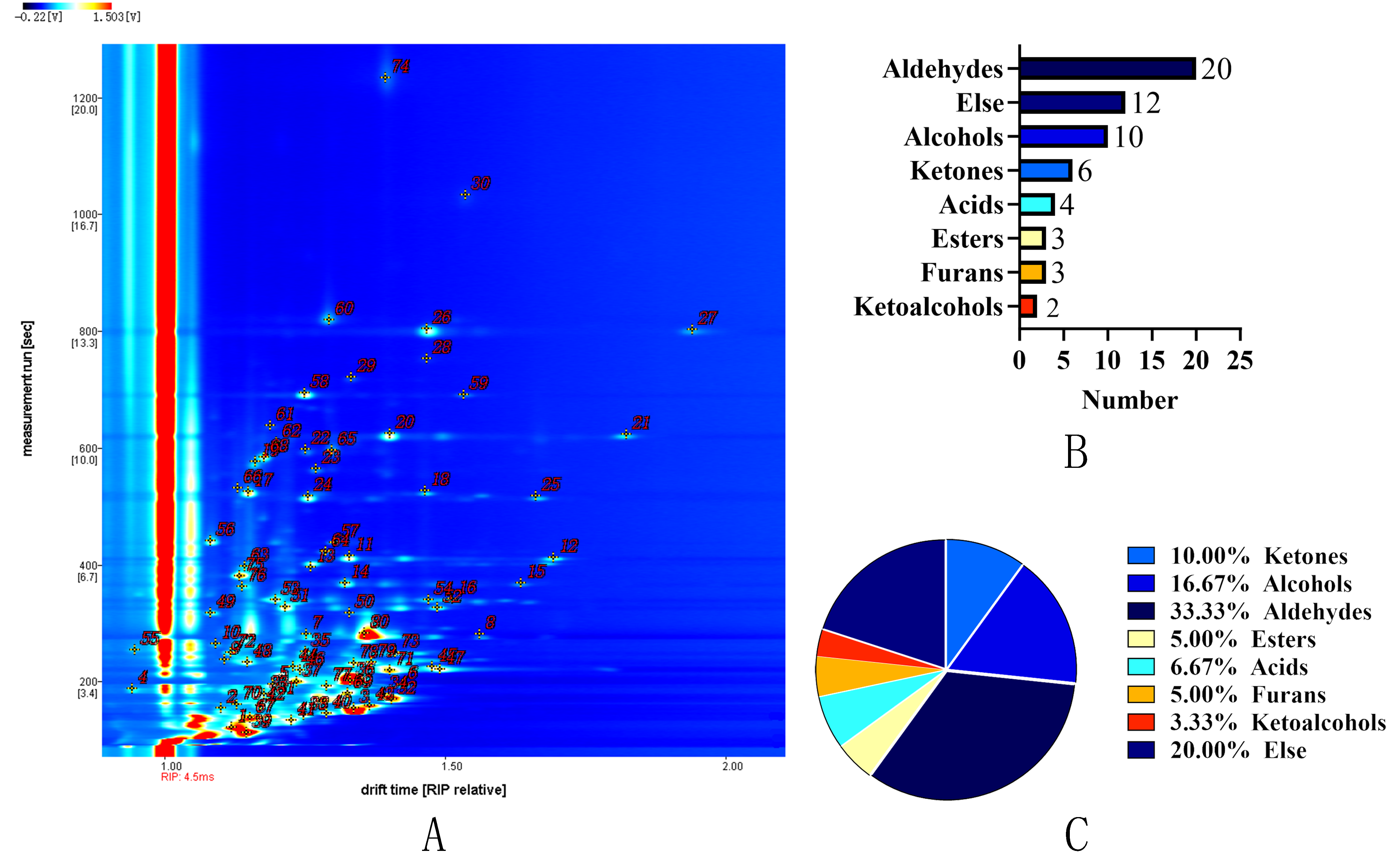
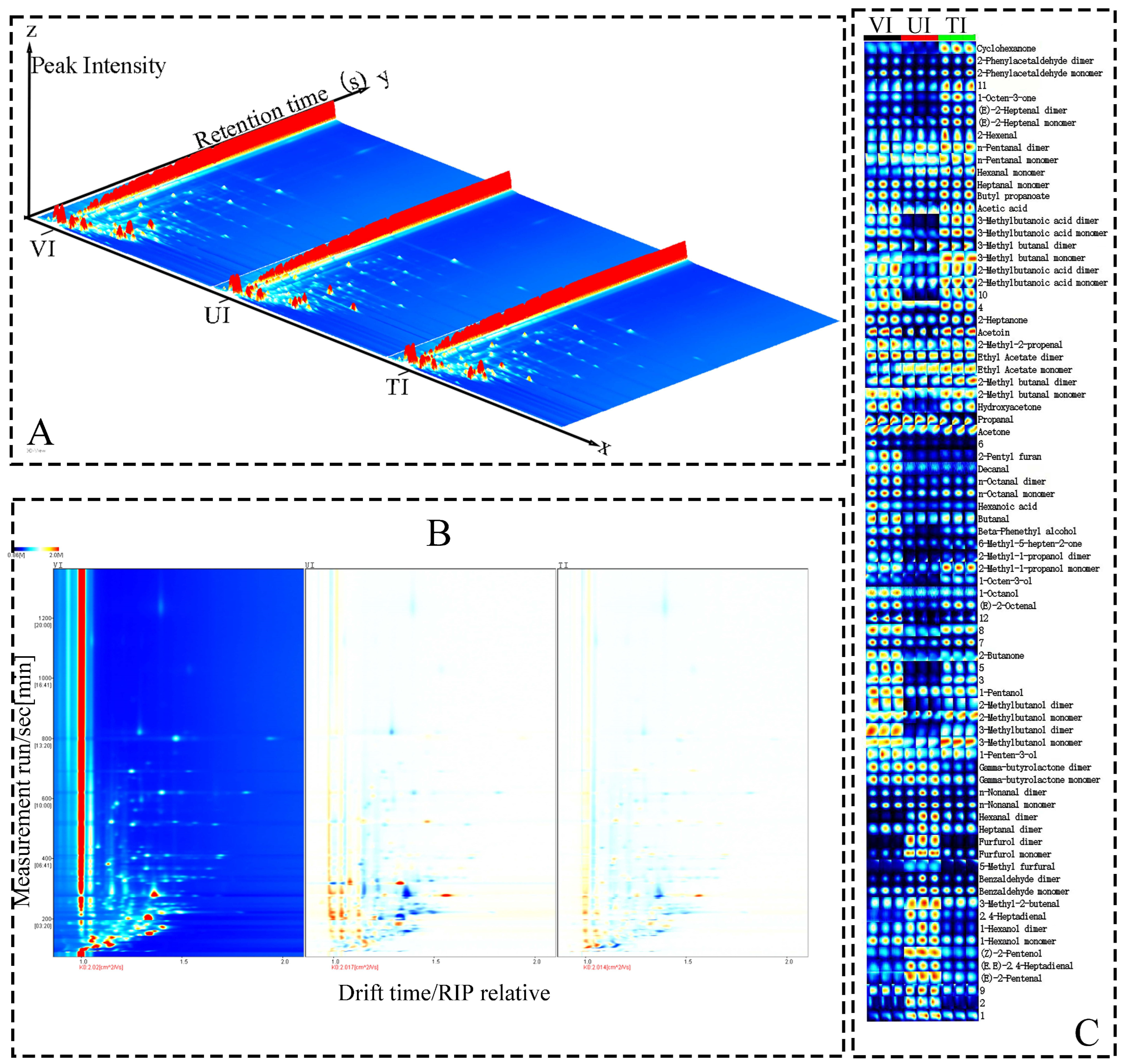
3.5. GC-IMS Analysis
3.5.1. GC-IMS Profiling and Key VOCs Identification
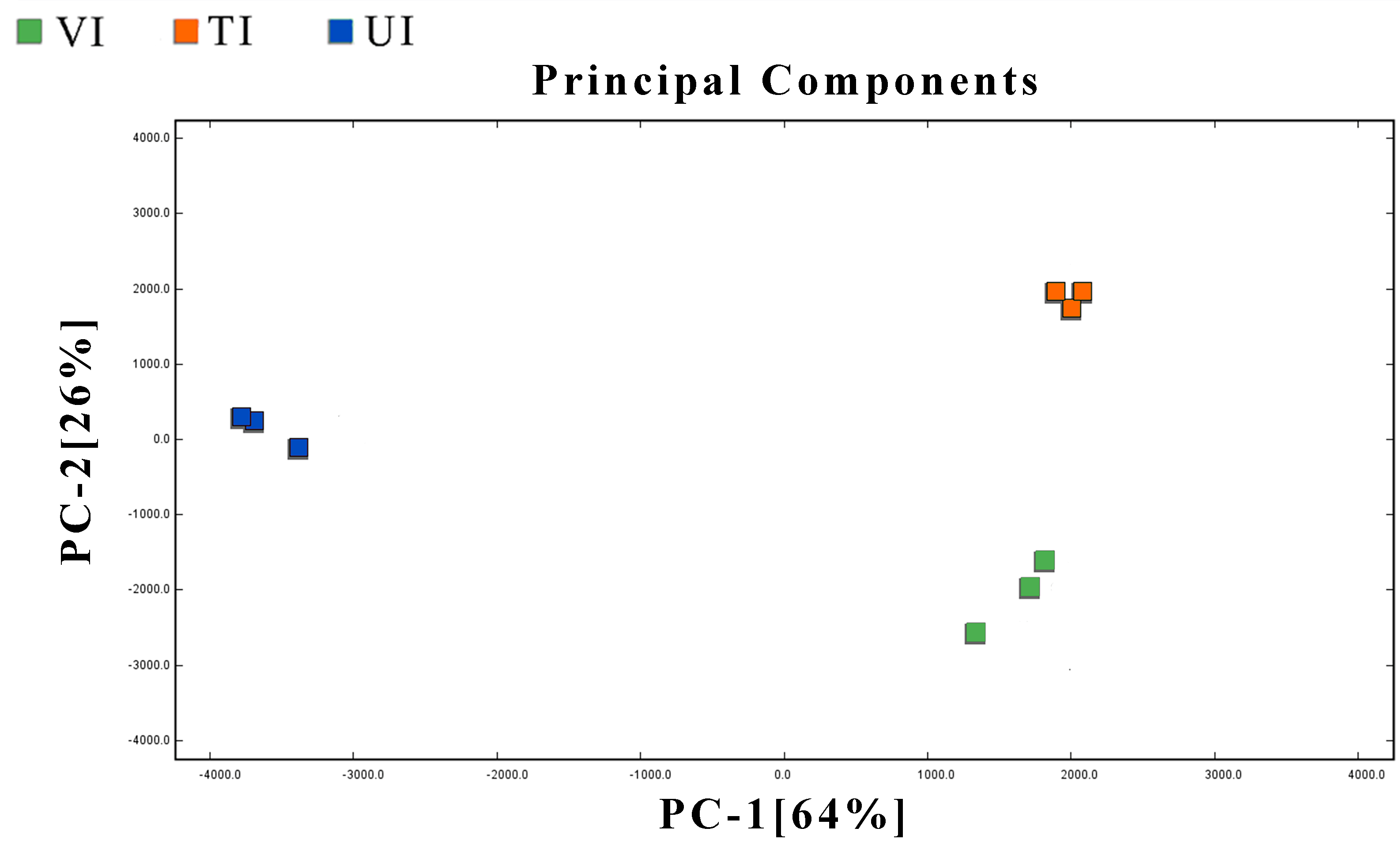
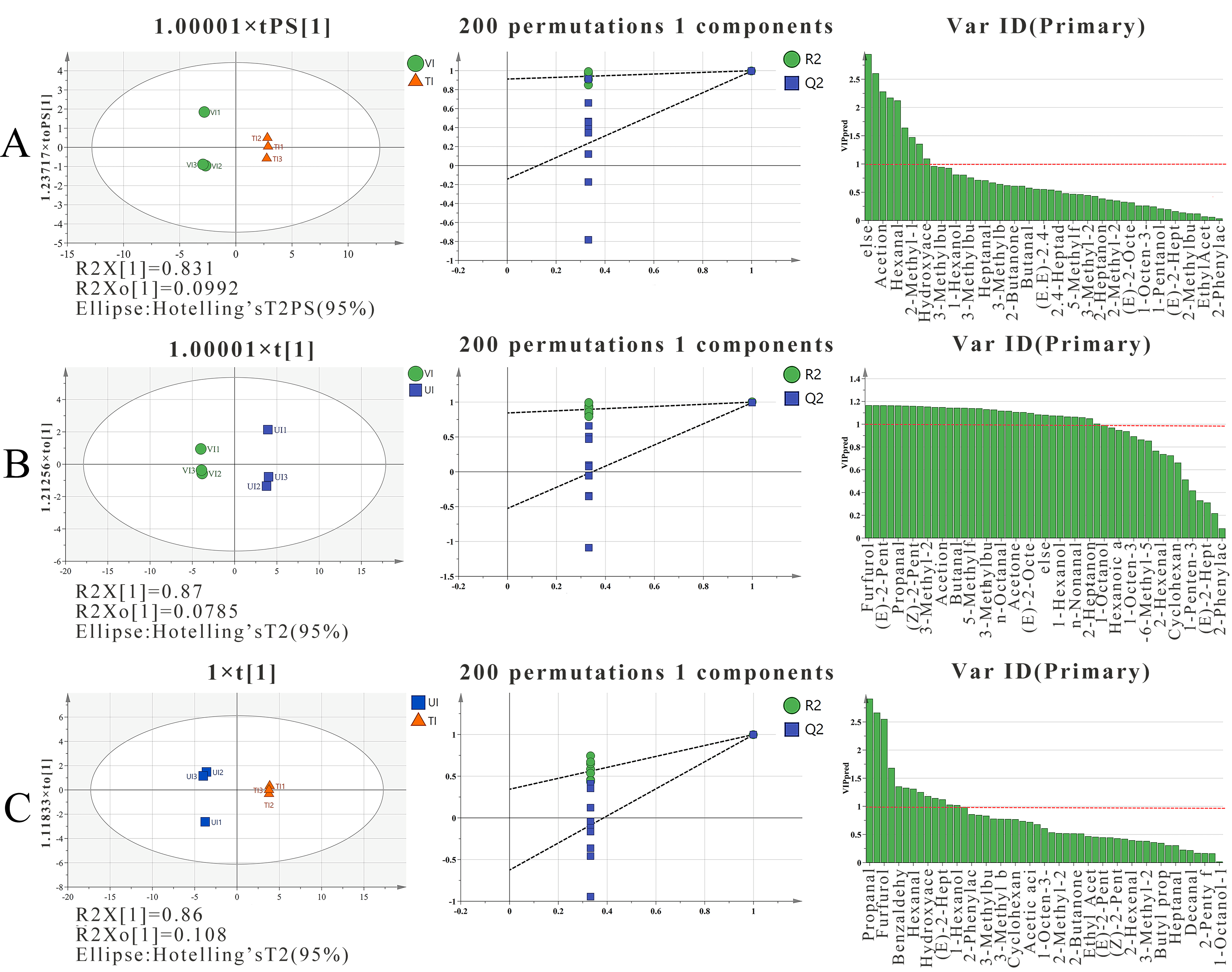
3.5.2. Multivariate Analysis of VOCs
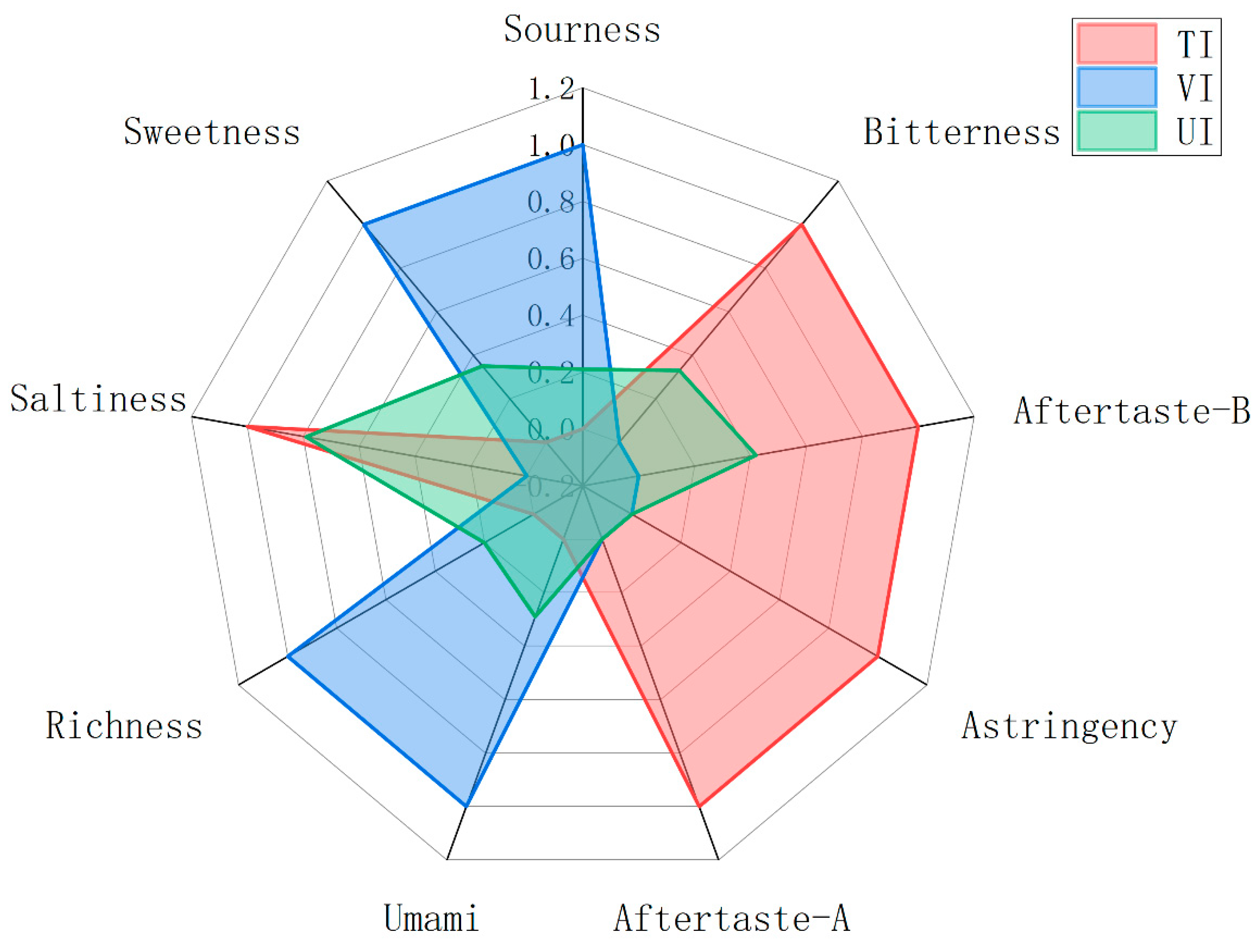
3.6. E-Tongue Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Savic, I.M.; Savic-Gajic, I.M. Optimization study on extraction of antioxidantsfrom plum seeds (Prunus domestica L.). Optim. Eng. 2020, 22, 141–158. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Zhang, W.D.; Cheng, S.B.; Liu, Y.X.; Yang, W.T.; Wang, Y.; Guo, M.R.; Chen, G.G. Postharvest storage at near-freezing temperature maintained the quality and antioxidant properties of Prunus domestica L. cv. Ximei fruit. Sci. Hortic. 2022, 293, 110720. [Google Scholar] [CrossRef]
- Li, L.; Yu, Y.Y.; Xu, Y.J.; Wu, J.J.; Yu, W.X.; Peng, J.; An, K.J.; Zou, B.; Yang, W.Y. Effect of ultrasound-assisted osmotic dehydration pretreatment on the drying characteristics and quality properties of Sanhua plum (Prunus salicina L.). LWT 2021, 138, 110653. [Google Scholar] [CrossRef]
- Almeida, A.R.; Mussi, L.P.; Oliveira, D.B.; Pereira, N.R. Effect of Temperature and Sucrose Concentration on the Retention of Polyphenol Compounds and Antioxidant Activity of Osmotically Dehydrated Bananas. J. Food Process. Preserv. 2014, 39, 1061–1069. [Google Scholar] [CrossRef]
- Rahaman, A.; Zeng, X.A.; Kumari, A.; Rafiq, M.; Siddeeg, A.; Manzoor, M.F.; Baloch, Z.; Ahmed, Z. Influence of ultrasound-assisted osmotic dehydration on texture, bioactive compounds and metabolites analysis of plum. Ultrason. Sonochem. 2019, 58, 104643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W. Simple Technology and Low Threshold: The Preserved Fruit Industry Needs to Develop into “Healthy Food”. China Food 2016, 20, 90–93. [Google Scholar]
- Zhu, M.; Liu, D.B.; Yang, X.Y.; Jing, W.N. Sugar Infiltration Methods on the Quality Characteristics of Preserved Passion Fruit. J. Shandong Agric. Univ. (Nat. Sci. Ed.) 2025, 56, 517–524. [Google Scholar]
- Cui, S.C.; Xiang, F.X.; Lu, H.X. The Influence of Sugar Preservation Technology on the Sugar Crystallization Phenomenon of Ganyingzi and Its Optimization. Zhejiang Agric. Sci. 2018, 07, 1267–1268. [Google Scholar]
- Neri, L.; Di-Biase, L.; Sacchetti, G.; Mattia, C.D.; Santarelli, V.; Mastrocola, D.; Pittia, P. Use of vacuum impregnation for the production of high quality fresh-like apple products. J. Food Eng. 2016, 179, 98–108. [Google Scholar] [CrossRef]
- Ji, X.L.; Guo, J.H.; Tian, J.Y.; Ma, K.; Liu, Y.Q. Research progress on degradation methods and product properties of plant polysaccharides. J. Light Ind. 2023, 38, 55–62. [Google Scholar]
- Zhang, Y.W.; Abatzoglou, N. Review: Fundamentals, applications and potentials of ultrasound-assisted drying. Chem. Eng. Res. Des. 2020, 154, 21–46. [Google Scholar] [CrossRef]
- Ji, X.L.; Hou, C.Y.; Yan, Y.Z.; Shi, M.M.; Liu, Y.Q. Comparison of structural characterization and antioxidant activity of polysaccharides from jujube (Ziziphus jujuba Mill.) fruit. Int. J. Biol. Macromol. 2020, 149, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.K.; Dong, R.; Yang, J.P.; Wang, G.Q. Objective Quantification Technique and Widely Targeted Metabolomics-Based Analysis of the Effects of Different Saccharidation Processes on Preserved French Plums. Molecules 2024, 29, 2011. [Google Scholar] [CrossRef] [PubMed]
- Fabela-Morón, M.F. Bioactive compounds, sensory attributes, and flavor perceptions involved in taste-active molecules in fruits and vegetables. Front. Nutr. 2024, 11, 1427857. [Google Scholar] [CrossRef]
- Yuan, J.J.; Li, H.B.; Cao, S.Q.; Liu, Z.B.; Li, N.; Xu, D.; Mo, H.Z.; Hu, L.B. Monitoring of Volatile Compounds of Ready-to-Eat Kiwifruit Using GC-IMS. Foods 2023, 12, 4394. [Google Scholar] [CrossRef]
- Xuan, X.T.; Sun, R.Y.; Zhang, X.Y.; Cui, Y.; Lin, X.D.; Sun, Y.; Deng, W.; Liao, X.J.; Ling, J.J. Novel application of HS-GC-IMS with PCA for characteristic fingerprints and flavor compound variations in NFC Chinese bayberry (Myrica rubra) juice during storage. LWT 2022, 167, 113882. [Google Scholar] [CrossRef]
- Gu, S.; Zhang, J.; Wang, J.; Wang, X.Y.; Du, D.D. Recent development of HS-GC-IMS technology in rapid and non-destructive detection of quality and contamination in agri-food products. Trends Anal. Chem. 2021, 144, 116435. [Google Scholar] [CrossRef]
- Aouadi, B.; Zaukuu, J.L.Z.; Vitális, F.; Bodor, Z.; Fehér, O.; Gillay, Z.; Bazar, G.; Kovacs, Z. Historical evolution and food control achievements of near infrared spectroscopy, electronic nose, and electronic tongue—Critical overview. Sensors 2020, 20, 5479. [Google Scholar] [CrossRef]
- De-Roos, K.B. Effect of texture and microstructure on flavour retention and release. Int. Dairy J. 2003, 13, 593–605. [Google Scholar] [CrossRef]
- Yu, Z.T.; Deng, J.; Ma, N.; Sun, Y.; Wang, J.; Liu, J.M.; Zhang, Y.; Lu, Y.S.; Wang, S. Comparative analysis of quality, structural, and flavor alterations in chestnuts (Castanea mollissima Blume) subjected to different thermal processing techniques. Food Chem. 2025, 474, 143149. [Google Scholar] [CrossRef]
- Gautam, S.; Kathuria, D.; Hamid; Dobhal, A.; Singh, N. Vacuum impregnation: Effect on food quality, application and use of novel techniques for improving its efficiency. Food Chem. 2024, 460, 140729. [Google Scholar] [CrossRef]
- Zhou, X.; Li, R.; Lyng, J.G.; Wang, S.J. Dielectric Properties of Kiwifruit Associated with a Combined Radio Frequency Vacuum and Osmotic Drying. J. Food Eng. 2018, 239, 72–82. [Google Scholar] [CrossRef]
- Pantelidou, D.; Gerogiannis, K.; Goula, A.; Gonas, C. Ultrasound-Assisted Osmotic Dehydration as a Method for Supplementing Potato with Unused Chokeberries Phenolics. Food Bioprocess Technol. 2021, 43, 133–141. [Google Scholar] [CrossRef]
- Kaur, D.; Singh, M.; Zalpouri, R.; Singh, I. Osmotic dehydration of fruits using unconventional natural sweeteners and non-thermal-assisted technologies: A review. J. Food Process. Preserv. 2022, 46, 16890. [Google Scholar] [CrossRef]
- Yu, Z.T.; Lu, Y.S.; Wei, F.; Zhang, Y.; Wang, S.; Dong, L. The impact of natural spices additions on hazards development and quality control in roast beef patties. Food Chem. 2024, 435, 137644. [Google Scholar] [CrossRef]
- Liu, D.Y.; Guo, W.C. Nondestructive determination of soluble solids content of persimmons by using dielectric spectroscopy. Int. J. Food Prop. 2017, 20, 1532–2386. [Google Scholar] [CrossRef]
- Xie, J.W.; Ni, Z.P.; Guo, M.Y.; Song, X.L. Sterilization Effect and Quality Change Analysis of Preserved Fruits Treated by Microwave and Ozone. Mod. Food Sci. Technol. 2023, 49, 7. [Google Scholar]
- Paul, V.; Singh, A.; Pandey, R. Determination of titrable acidity (TA). Postharvest Biol. Technol. 2010, 44, 17753. [Google Scholar]
- Lee, B.; Seo, J.D.; Rhee, J.K.; Kim, C.Y. Heated Apple Juice Supplemented with Onion Has Greatly Improved Nutritional Quality and Browning Index. Food Chem. 2016, 201, 315–319. [Google Scholar] [CrossRef]
- López, A.; Pittori, A.; Di-Sarli, A.Y. Architectural self-compacting concretes and their color stability for 10 years. Constr. Build. Mater. 2024, 451, 138650. [Google Scholar] [CrossRef]
- Dróżdż, P.; Šėžienė, V.; Wójcik, J.; Pyrzyńska, K.; Pyrzynska, K. Evaluation of Bioactive Compounds, Minerals and Antioxidant Activity of Lingonberry (Vaccinium vitis-idaea L.) Fruits. Molecules 2018, 23, 53. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A. Variation of the Phytochemical Constituents and Antioxidant Activities of Zingiber officinale var. rubrum Theilade Associated with Different Drying Methods and Polyphenol Oxidase Activity. Molecules 2016, 21, 780. [Google Scholar] [CrossRef]
- Zahari, I.; Ferawati, F.; Helstad, A.; Ahlstrm, C.; Östbring, K.; Rayner, M.; Purhagen, J.K. Development of High-Moisture Meat Analogues with Hemp and Soy Protein Using Extrusion Cooking. Foods 2020, 9, 772. [Google Scholar] [CrossRef]
- Zhang, D.; Ji, H.W.; Liu, S.C.; Gao, J. Similarity of aroma attributes in hot-air-dried shrimp (Penaeus vannamei) and its different parts using sensory analysis and GC-MS. Food Res. Int. 2020, 137, 109517. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Fan, Y.; Zhu, L.; Wang, Y.; Hou, H. Characteristic Flavor of Antarctic Krill (Euphausia Superba) and White Shrimp (Penaeus Vannamei) Induced by Thermal Treatment. Food Chem. 2022, 378, 132074. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Xie, J. Practical applications of vacuum impregnation in fruit and vegetable processing. Trends Food Sci. Tech. 2004, 15, 434–451. [Google Scholar] [CrossRef]
- Bich, L.; Pradeu, T.; Moreau, J.F. Understanding Multicellularity: The Functional Organization of the Intercellular Space. Front. Physiol. 2019, 10, 1170. [Google Scholar] [CrossRef]
- Betoret, E.; Betoret, N.; Rocculi, P.; Dalla Rosa, M. Strategies to improve food functionality: Structure-property relationships on high pressures homogenization, vacuum impregnation and drying technologies. Trends Food Sci. Technol. 2015, 46, 1–12. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Sadeghi, R. Soluting-in and soluting-out of water-soluble polymers inaqueous carbohydrate solutions studied by vaporpressure osmometry. J. Mol. Liq. 2017, 229, 405–416. [Google Scholar] [CrossRef]
- Barać, G.; Mastilović, J.; Kevrešan, Ž.; Milić, B.; Kovač, R.; Milović, M.; Keserović, Z. Effects of Plant Growth Regulators on Plum (Prunus Domestica L.) Grown on Two Rootstocks at Harvest and at the Postharvest Period. Horticulturae 2022, 8, 621. [Google Scholar] [CrossRef]
- Wolf, J.; Göttingerová, M.; Kaplan, J.; Kiss, T.; Venuta, R.; Nečas, T. Determination of the pomological and nutritional properties of selected plum cultivars and minor fruit species. Hort. Sci. 2020, 47, 181–193. [Google Scholar] [CrossRef]
- Huang, X.; Wang, H.K.; Luo, W.J.; Xue, S.; Hayat, F.; Gao, Z.H. Prediction of loquat soluble solids and titratable acid content using fruit mineral elements by artificial neural network and multiple linear regression. Sci. Hortic. 2021, 278, 109873. [Google Scholar] [CrossRef]
- Panayampadan, A.S.; Alam, M.S.; Aslam, R.; Kaur, J. Vacuum impregnation process and its potential in modifying sensory, physicochemical and nutritive characteristics of food products. Food Eng. Rev. 2022, 14, 229–256. [Google Scholar] [CrossRef]
- Sadras, V.O.; Petrie, P.R.; Moran, M.A. Effects of elevated temperature in grapevine. II juice pH, titratable acidity and wine sensory attributes. Aust. J. Grape Wine Res. 2012, 19, 107–115. [Google Scholar] [CrossRef]
- Obenland, D.; Collin, S.; Mackey, B.; Sievert, J.; Arpaia, M.L. Storage temperature and time influences sensory quality of mandarins by altering soluble solids, acidity and aroma volatile composition. Postharvest Biol. Technol. 2011, 59, 187–193. [Google Scholar] [CrossRef]
- Dias, L.G.; Sequeira, C.; Veloso, A.C.; Sousa, M.E.; Peres, A.M. Evaluation of healthy and sensory indexes of sweetened beverages using an electronic tongue. Anal. Chim. Acta. 2014, 848, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Tsikrika, K.; Tzima, K.; Rai, D.K. Recent advances in anti-browning methods in minimallyprocessed potatoes—A review. J. Food Process. Preserv. 2022, 46, 16298. [Google Scholar] [CrossRef]
- Nath, P.; Pandey, N.; Samota, M.; Sharma, K.; Kale, S.; Kannaujia, P.; Chauhan, O.P. Saxena, D.C., Ed.; Browning Reactions in Foods. In Advances in Food Chemistry: Food Components, Processing and Preservation; Springer: Singapore, 2022; pp. 117–159. [Google Scholar]
- Qu, W.J.; Ruan, W.Y.; Feng, Y.H.; Liu, Y.; Tuly, J.A.; Zhou, C.S. Assessing the dry inactivation mechanism of polyphenol oxidase (PPO) and peroxidase (POD) employing catalytic infrared treatment based on experiments and molecular simulations. Innov. Food Sci. Emerg. Technol. 2025, 100, 103943. [Google Scholar] [CrossRef]
- Min, T.; Xie, J.; Zheng, M.L.; Yi, Y.; Hou, W.F.; Wang, L.M.; Ai, Y.W.; Wang, H.X. The effect of different temperatures on browning incidence and phenol compound metabolism in fresh-cut lotus (Nelumbo nucifera G.) root. Postharvest Biol. Technol. 2017, 123, 69–76. [Google Scholar] [CrossRef]
- Gómez-Martínez, H.; Bermejo, A.; Zuriaga, E.; Badenes, M.L. Polyphenol content in apricot fruits. Sci. Hortic. 2021, 277, 109828. [Google Scholar] [CrossRef]
- Réblová, Z. Effect of temperature on the antioxidant activity of phenolic acids. Czech J. Food Sci. 2012, 30, 171–175. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Mazzara, E.; Abouelenein, D.; Angeloni, S.; Nunez, S.; Sagratini, G.; Maggi, F. Optimization of solvent-free microwave-assisted hydrodiffusion and gravity extraction of Morus nigra L. fruits maximizing polyphenols, sugar content, and biological activities using central composite design. Pharmaceuticals 2022, 15, 99. [Google Scholar] [CrossRef]
- Aaby, K.; Amundsen, M.R. The stability of phenolic compounds and the colour of lingonberry juice with the addition of different sweeteners during thermal treatment and storage. Heliyon 2023, 05, 15959. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Deng, G.; Zhang, Y.C. Multiple free radical scavenging reactions of flavonoids. Dyes Pigm. 2022, 198, 109877. [Google Scholar] [CrossRef]
- Daskalaki, D.; Kefi, G.; Kotsiou, K.; Tasioula-Margari, M. Evaluation of phenolic compounds degradation in virgin olive oil during storage and heating. J. Food Nutr. Res. 2009, 48, 31–41. [Google Scholar]
- Schaefer, H.M. Why fruits go to the dark side. Acta Oecol. 2011, 37, 604–610. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Lin, Z.Q.; Chen, X.M.; Chen, J.W.; Wu, J.S.; Chen, H.G.; Zeng, X.F. Characterization of structures and gel properties of ultra-high-pressure treated-myofibrillar protein extracted from mud carp (Cirrhinus molitorella) and quality characteristics of heat-induced sausage products. LWT 2022, 165, 113691. [Google Scholar] [CrossRef]
- Li, C.S.; Cui, Q.Y.; Li, L.H.; Huang, H.; Chen, S.J.; Zhao, Y.Q.; Wang, Y. Formation and improvement mechanism of physical property and volatile flavor of fermented tilapia surimi by newly isolated lactic acid bacteria based on two dimensional correlation networks. Food Chem. 2024, 440, 138260. [Google Scholar] [CrossRef]
- Ruan, J.W.; Xue, G.; Liu, Y.; Ye, B.; Li, M.; Xu, Q. Optimization of the Vacuum Microwave Drying of Tilapia Fillets Using Response Surface Analysis. Foods. 2025, 14, 873. [Google Scholar] [CrossRef]
- Nath, K.G.; Pandiselvam, R.; Sunil, C.K. High-pressure processing: Effect on textural properties of food-A review. J. Food Eng. 2023, 351, 111521. [Google Scholar] [CrossRef]
- Li, M.Q.; Yang, R.W.; Zhang, H.; Wang, S.L.; Chen, D.; Lin, S.Y. Development of a flavor fingerprint by HS-GC-IMS with PCA for volatile compounds of Tricholoma matsutake Singer. Food Chem. 2019, 290, 32–39. [Google Scholar] [CrossRef]
- Abbas, F.; Zhou, Y.; O’Neill-Rothenberg, D.; Alam, I.; Ke, Y.; Wang, H.C. Aroma components in horticultural crops: Chemical diversity and usage of metabolic engineering for industrial applications. Plants 2023, 12, 1748. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Y.; Chen, X.C.; Li, Y.; Li, L. Effect of Drying Methods on Volatile Compounds of Citrus Reticulata Ponkan and Chachi Peels as Characterized by GC-MS and GC-IMS. Foods 2022, 11, 2662. [Google Scholar] [CrossRef]
- Johnson, D.R.; Decker, E.A. The role of oxygen in lipid oxidation reactions: A review. Annu. Rev. Food Sci. Technol. 2015, 06, 171–190. [Google Scholar] [CrossRef]
- Man, L.M.; Ren, W.; Sun, M.G.; Du, Y.R.; Chen, H.; Qin, H.X.; Chai, W.Q.; Zhu, M.X.; Liu, G.Q.; Wang, C.F.; et al. Characterization of donkey-meat flavor profiles by GC-IMS and multivariate analysis. Front. Nutr. 2023, 10, 1079799. [Google Scholar] [CrossRef]
- Song, X.; Capanoglu, E.; Simal-Gandara, J.; Chen, F.; Xiao, J. Simal-Gandara, J., Ed.; Different Food Processing Technologies: A General Background. In Retention of Bioactives in Food Processing; Springer: Cham, Switzerland, 2022; pp. 37–89. [Google Scholar]
- Oliveira, S.M.; Brandao, T.R.; Silva, C.L. Influence of drying processes and pretreatments on nutritional and bioactive characteristics of dried vegetables: A review. Food Eng. Rev. 2015, 8, 134–163. [Google Scholar] [CrossRef]
- Mahanta, B.P.; Bora, P.K.; Kemprai, P.; Borah, G.; Lal, M.; Haldar, S. Thermolabile Essential Oils, Aromas and Flavours: Degradation Pathways, Effect of Thermal Processing and Alteration of Sensory Quality. Food Res. Int. 2021, 145, 110404. [Google Scholar] [CrossRef]
- Saleena, P.; Jayashree, E.; Anees, K. A Comprehensive Review on Vacuum Impregnation: Mechanism, Applications and Prospects. Food Bioprocess Technol. 2023, 17, 1434–1447. [Google Scholar] [CrossRef]
- Mao, C.; Chen, Y.R.; Ye, P.F.; Chang, Z.; Sun, S.J.; Liu, R.; Wang, Y.Q.; Chen, X.W.; Fu, H.F.; Wang, Y.Y.; et al. Sugar boiling pre-treatment improves radio frequency explosion puffing quality on modifying the physicochemical and functional properties of purple sweet potato flour. Int. J. Biol. Macromol. 2025, 294, 139543. [Google Scholar] [CrossRef] [PubMed]
- Stacewicz-Sapuntzakis, M.; Bowen, P.E.; Hussain, E.A.; Damayanti-Wood, B.I.; Farnsworth, N.R. Chemical composition and potential health effects of prunes: A functional food? Crit. Rev. Food Sci. Nutr. 2001, 41, 251–286. [Google Scholar] [CrossRef]
- Xia, R.J.; Qiao, Y.T.; Xu, H.; Hou, H.S.; Qian, G.L.; Wang, Y.F.; Li, Y.T.; Yan, M.; Pan, S.; Xin, G. Unlocking the Potential of the Umami Taste-Presenting Compounds: A Review of the Health Benefits, Metabolic Mechanisms and Intelligent Detection Strategies. Food Rev. Int. 2024, 41, 323–343. [Google Scholar] [CrossRef]
- Pagliarini, E.; Proserpio, C.; Spinelli, S.; Lavelli, V.; Laureati, M.; Arena, E.; Dinnella, C. The role of sour and bitter perception in liking, familiarity and choice for phenol-rich plant-based foods. Food Qual. Prefer. 2021, 93, 104250. [Google Scholar] [CrossRef]
- Huang, R.; Xu, C. An overview of the perception and mitigation of astringency associated with phenolic compounds. Compr. Rev. Food Sci. Food Saf. 2022, 20, 1036–1074. [Google Scholar] [CrossRef] [PubMed]
- ElGamal, R.; Song, C.; Rayan, A.M.; Liu, C.; Al-Rejaie, S.; ElMasry, G. Thermal degradation of bioactive compounds during drying process of horticultural and agronomic products: A comprehensive overview. Agronomy 2023, 13, 1580. [Google Scholar] [CrossRef]
- Buckow, R.; Weiss, U.; Knorr, D. Inactivation kinetics of apple polyphenol oxidase in different pressure–temperature domains. Innov. Food Sci. Emerg. Technol. 2009, 10, 441–448. [Google Scholar] [CrossRef]
- Mu, Y.W.; Zhang, B.H.; Zeng, C.Z.; Zhu, T.D.; Hu, S.H. Mechanistic and Multi-Parametric Insights into Preserving Nutritional, Bioactive, and Flavor Attributes of Daylily (Hemerocallis citrina): A Comparative Evaluation of Freeze-Drying, Hot-Air Drying, and Sun Drying. LWT 2025, 218, 117510. [Google Scholar] [CrossRef]
| Indicators | OD Techniques | ||
|---|---|---|---|
| VI | UI | TI | |
| TSS (°Brix) | 30.10 ± 0.26 a | 27.47 ± 0.60 b | 23.47 ± 0.71 c |
| aw | 0.86 ± 0.01 c | 0.91 ± 0.01 b | 0.93 ± 0.01 a |
| L* | 31.85 ± 1.56 a | 28.05 ± 0.83 b | 25.17 ± 0.85 c |
| a* | 17.03 ± 0.58 a | 12.88 ± 0.72 b | 11.13 ± 0.95 c |
| b* | 16.88 ± 0.16 b | 17.68 ± 0.63 b | 18.79 ± 0.36 a |
| Hardness (g) | 187.63 ± 4.04 b | 176.53 ± 5.81 b | 156.25 ± 4.55 a |
| Springiness (mm) | 0.82 ± 0.03 b | 0.73 ± 0.02 ab | 0.62 ± 0.06 a |
| Chewiness (mJ) | 113.99 ± 6.61 c | 93.52 ± 5.70 b | 69.56 ± 4.65 a |
| Cohesiveness | 0.80 ± 0.04 b | 0.72 ± 0.02 ab | 0.67 ± 0.03 a |
| Gumminess | 129.38 ± 6.42 a | 106.53 ± 4.27 ab | 84.05 ± 6.25 a |
| No. | Compound | CAS# | Formula | MW | (RI) | Rt [s] | Dt [a.u.] |
|---|---|---|---|---|---|---|---|
| 1 | Acetone | C67641 | C3H6O | 58.1 | 529.6 | 122.764 | 1.12109 |
| 2 | Ethyl Acetate monomer | C141786 | C4H8O2 | 88.1 | 613.2 | 154.71 | 1.10222 |
| 3 | Ethyl Acetate dimer | C141786 | C4H8O2 | 88.1 | 613.2 | 154.71 | 1.33808 |
| 4 | 1-Penten-3-ol | C616251 | C5H10O | 86.1 | 683.5 | 187.733 | 0.94289 |
| 5 | n-Pentanal monomer | C110623 | C5H10O | 86.1 | 695.6 | 195.271 | 1.19342 |
| 6 | n-Pentanal dimer | C110623 | C5H10O | 86.1 | 696.1 | 195.63 | 1.42404 |
| 7 | Hexanal monomer | C66251 | C6H12O | 100.2 | 797.2 | 282.135 | 1.25317 |
| 8 | Hexanal dimer | C66251 | C6H12O | 100.2 | 796.1 | 281.059 | 1.56346 |
| 9 | (E)-2-Pentenal | C1576870 | C5H8O | 84.1 | 750.4 | 238.344 | 1.10746 |
| 10 | 3-Methyl-2-butenal | C107868 | C5H8O | 84.1 | 780.6 | 265.983 | 1.09174 |
| 11 | Heptanal monomer | C111717 | C7H14O | 114.2 | 904.8 | 415.906 | 1.33096 |
| 12 | Heptanal dimer | C111717 | C7H14O | 114.2 | 903.2 | 413.173 | 1.69531 |
| 13 | 2-Heptanone | C110430 | C7H14O | 114.2 | 893.1 | 396.777 | 1.26165 |
| 14 | 1-Hexanol monomer | C111273 | C6H14O | 102.2 | 872.6 | 368.767 | 1.32208 |
| 15 | 1-Hexanol dimer | C111273 | C6H14O | 102.2 | 872.6 | 368.767 | 1.63666 |
| 16 | 2-Hexenal | C505577 | C6H10O | 98.1 | 852 | 342.806 | 1.51403 |
| 17 | Benzaldehyde monomer | C100527 | C7H6O | 106.1 | 963.9 | 526.58 | 1.14967 |
| 18 | Benzaldehyde dimer | C100527 | C7H6O | 106.1 | 964.2 | 527.263 | 1.46604 |
| 19 | 1-Octen-3-ol | C3391864 | C8H16O | 128.2 | 986.5 | 576.452 | 1.16212 |
| 20 | n-Octanal monomer | C124130 | C8H16O | 128.2 | 1011.9 | 624.957 | 1.40205 |
| 21 | n-Octanal dimer | C124130 | C8H16O | 128.2 | 1011.1 | 623.591 | 1.82506 |
| 22 | 2-Pentyl furan | C3777693 | C9H14O | 138.2 | 995.8 | 598.314 | 1.25276 |
| 23 | 1-Octen-3-one | C4312996 | C8H14O | 126.2 | 981.7 | 565.521 | 1.27053 |
| 24 | (E)-2-Heptenal monomer | C18829555 | C7H12O | 112.2 | 960 | 518.382 | 1.25631 |
| 25 | (E)-2-Heptenal dimer | C18829555 | C7H12O | 112.2 | 960 | 518.382 | 1.66332 |
| 26 | n-Nonanal monomer | C124196 | C9H18O | 142.2 | 1106.9 | 804.46 | 1.46895 |
| 27 | n-Nonanal dimer | C124196 | C9H18O | 142.2 | 1106.5 | 803.747 | 1.9419 |
| 28 | 1-Octanol | C111875 | C8H18O | 130.2 | 1082 | 753.088 | 1.46895 |
| 29 | (E)-2-Octenal | C2548870 | C8H14O | 126.2 | 1065.7 | 720.98 | 1.3341 |
| 30 | Decanal | C112312 | C10H20O | 156.3 | 1201 | 1033.035 | 1.53767 |
| 31 | 3-Methyl butanal monomer | C590863 | C5H10O | 86.1 | 649.5 | 170.974 | 1.19066 |
| 32 | 3-Methyl butanal dimer | C590863 | C5H10O | 86.1 | 649.5 | 170.974 | 1.40516 |
| 33 | 2-Methyl butanal monomer | C96173 | C5H10O | 86.1 | 667.8 | 179.786 | 1.17906 |
| 34 | 2-Methyl butanal dimer | C96173 | C5H10O | 86.1 | 666.9 | 179.366 | 1.39212 |
| 35 | 1-Pentanol | C71410 | C5H12O | 88.1 | 764.7 | 251.007 | 1.25226 |
| 36 | Acetoin | C513860 | C4H8O2 | 88.1 | 704.3 | 201.526 | 1.33233 |
| 37 | Hydroxyacetone | C116096 | C3H6O2 | 74.1 | 702.6 | 200.302 | 1.23621 |
| 38 | 2-Butanone | C78933 | C4H8O | 72.1 | 583.7 | 142.622 | 1.24876 |
| 39 | Propanal | C123386 | C3H6O | 58.1 | 499.9 | 113.056 | 1.14671 |
| 40 | Butanal | C123728 | C4H8O | 72.1 | 593.5 | 146.532 | 1.28983 |
| 41 | 2-Methyl-2-propenal | C78853 | C4H6O | 70.1 | 563.4 | 134.803 | 1.22636 |
| 42 | 2-Methyl-1-propanol monomer | C78831 | C4H10O | 74.1 | 625.4 | 159.971 | 1.17285 |
| 43 | 2-Methyl-1-propanol dimer | C78831 | C4H10O | 74.1 | 625.4 | 159.971 | 1.36699 |
| 44 | 2-Methylbutanol monomer | C137326 | C5H12O | 88.1 | 736.4 | 226.467 | 1.23023 |
| 45 | 2-Methylbutanol dimer | C137326 | C5H12O | 88.1 | 736.4 | 226.467 | 1.47787 |
| 46 | 3-Methylbutanol monomer | C123513 | C5H12O | 88.1 | 729.3 | 220.739 | 1.24255 |
| 47 | 3-Methylbutanol dimer | C123513 | C5H12O | 88.1 | 730.9 | 221.967 | 1.49155 |
| 48 | Furfurol monomer | C98011 | C5H4O2 | 96.1 | 831.3 | 318.529 | 1.08247 |
| 49 | Furfurol dimer | C98011 | C5H4O2 | 96.1 | 830.6 | 317.711 | 1.33011 |
| 50 | 3-Methylbutanoic acid monomer | C503742 | C5H10O2 | 102.1 | 839.9 | 328.349 | 1.21655 |
| 51 | 3-Methylbutanoic acid dimer | C503742 | C5H10O2 | 102.1 | 838.5 | 326.712 | 1.48745 |
| 52 | 2-Methylbutanoic acid monomer | C116530 | C5H10O2 | 102.1 | 849.9 | 340.215 | 1.19877 |
| 53 | 2-Methylbutanoic acid dimer | C116530 | C5H10O2 | 102.1 | 849.9 | 340.215 | 1.4724 |
| 54 | (Z)-2-Pentenol | C1576950 | C5H10O | 86.1 | 768.7 | 254.7 | 0.94702 |
| 55 | γ-butyrolactone monomer | C96480 | C4H6O2 | 86.1 | 919.5 | 440.908 | 1.08266 |
| 56 | γ-butyrolactone dimer | C96480 | C4H6O2 | 86.1 | 918.1 | 438.46 | 1.30323 |
| 57 | 2-Phenylacetaldehyde monomer | C122781 | C8H8O | 120.2 | 1051.3 | 693.99 | 1.25011 |
| 58 | 2-Phenylacetaldehyde dimer | C122781 | C8H8O | 120.2 | 1050.3 | 692.223 | 1.53526 |
| 59 | β-Phenethyl alcohol | C60128 | C8H10O | 122.2 | 1114.2 | 820.361 | 1.29427 |
| 60 | (E,E)-2,4-Heptadienal | C4313035 | C7H10O | 110.2 | 1019.8 | 638.316 | 1.18941 |
| 61 | 2,4-Heptadienal | C5910850 | C7H10O | 110.2 | 1002.8 | 610.038 | 1.20044 |
| 62 | Cyclohexanone | C108941 | C6H10O | 98.1 | 894.1 | 398.438 | 1.14433 |
| 63 | Butyl propanoate | C590012 | C7H14O2 | 130.2 | 909.3 | 423.463 | 1.28891 |
| 64 | Hexanoic acid | C142621 | C6H12O2 | 116.2 | 995.1 | 596.667 | 1.29888 |
| 65 | 5-Methyl furfural | C620020 | C6H6O2 | 110.1 | 966.2 | 531.469 | 1.13103 |
| 66 | Acetic acid | C64197 | C2H4O2 | 60.1 | 573.6 | 138.681 | 1.15396 |
| 67 | 6-Methyl-5-hepten-2-one | C110930 | C8H14O | 126.2 | 989.9 | 584.397 | 1.17773 |
| 68 | Unidentified compound 1 | * | * | * | 669.7 | 180.716 | 1.3274 |
| 69 | Unidentified compound 2 | * | * | * | 628 | 161.13 | 1.12899 |
| 70 | Unidentified compound 3 | * | * | * | 728.5 | 220.082 | 1.40308 |
| 71 | Unidentified compound 4 | * | * | * | 764.3 | 250.608 | 1.11941 |
| 72 | Unidentified compound 5 | * | * | * | 763.4 | 249.79 | 1.4122 |
| 73 | Unidentified compound 6 | * | * | * | 1268 | 1234.62 | 1.3949 |
| 74 | Unidentified compound 7 | * | * | * | 882 | 381.315 | 1.13435 |
| 75 | Unidentified compound 8 | * | * | * | 868.7 | 363.734 | 1.13896 |
| 76 | Unidentified compound 9 | * | * | * | 693 | 193.415 | 1.2893 |
| 77 | Unidentified compound 10 | * | * | * | 742.8 | 231.812 | 1.33913 |
| 78 | Unidentified compound 11 | * | * | * | 745.2 | 233.886 | 1.37029 |
| 79 | Unidentified compound 12 | * | * | * | 798 | 282.984 | 1.35759 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Q.; Yang, R.; Wang, W.; Li, W.; Sun, T.; Huang, S.; Han, X.; Ai, M. Effects of Sugar Impregnation Methods on Physicochemical Properties and Flavor Profiles of Prune Preserves Using GC-IMS and Electronic Tongue. Foods 2025, 14, 2852. https://doi.org/10.3390/foods14162852
Du Q, Yang R, Wang W, Li W, Sun T, Huang S, Han X, Ai M. Effects of Sugar Impregnation Methods on Physicochemical Properties and Flavor Profiles of Prune Preserves Using GC-IMS and Electronic Tongue. Foods. 2025; 14(16):2852. https://doi.org/10.3390/foods14162852
Chicago/Turabian StyleDu, Qingping, Rui Yang, Wei Wang, Wei Li, Tongle Sun, Shihao Huang, Xinyao Han, and Mingxun Ai. 2025. "Effects of Sugar Impregnation Methods on Physicochemical Properties and Flavor Profiles of Prune Preserves Using GC-IMS and Electronic Tongue" Foods 14, no. 16: 2852. https://doi.org/10.3390/foods14162852
APA StyleDu, Q., Yang, R., Wang, W., Li, W., Sun, T., Huang, S., Han, X., & Ai, M. (2025). Effects of Sugar Impregnation Methods on Physicochemical Properties and Flavor Profiles of Prune Preserves Using GC-IMS and Electronic Tongue. Foods, 14(16), 2852. https://doi.org/10.3390/foods14162852






