1. Introduction
In 2024, the global production of pork reached 116.02 billion tons. China, the European Union and the United States accounted for 48.91%, 18.32% and 10.93% (United States Department of Agriculture, USDA). Pork quality directly influences consumer purchasing preferences and serves as a core indicator for evaluating the profitability of pig farming [
1]. Consumers increasingly demand diversity in pork flavor and a natural taste profile. However, the accumulation of flavor-related compounds in muscle, including essential amino acids and polyunsaturated fatty acids, is being neglected due to the excessive pursuit of growth rate and lean meat percentage. This leads to the homogenization of pork flavor. For instance, a reduction in lipid-soluble flavor compounds (such as aldehydes) weakens the fatty aroma and meatiness of pork [
2]. Therefore, it is of great importance to find effective methods that balance growth performance with pork flavor.
Pork flavor originates from three main sources: lipid oxidation, the Maillard reaction and thiamine degradation [
3]. The types and content of volatile flavor compounds (VOCs) directly determine the sensory quality of pork. Factors such as breed, nutrition, feeding methods and processing influence pork flavor. Compared to Yorkshire pigs, Laiwu pigs, a Chinese native breed, have a higher triglyceride content in their muscles, with significantly increased levels of α-ketoglutaric acid, fumaric acid and L-aspartic acid. These play an important role in enhancing the flavor of Laiwu pork [
4]. The Chinese local breed Ningxiang pig has a unique sweet, fruity and floral aroma compared to Berkshire pigs, which originates from higher levels of 2-pentylfuran, pentanal, 2-(E)-octanal and acetic acid [
5]. It is worth noting that dietary nutrition is the most controllable and cost-effective means for regulating pork flavor [
6]. Dietary amino acid composition plays a crucial role in muscle development, fat deposition and the accumulation of metabolic by-products [
7]. For instance, adding 1.5% arginine to a low-protein diet increases the accumulation of arginine in pork by 51.54%, thereby enhancing its overall acceptability [
8]. Additionally, arginine exhibits synergistic effects with other amino acids. Supplementing with 1.0% L-arginine and 1.0% glutamic acid significantly increases IMF accumulation and fatty acid content, generating multiple VOCs derived from fatty acid oxidation. This enhances meat tenderness, juiciness and overall quality [
9]. Concurrently, low dietary protein (14.0%) can also significantly alter the volatile compound profile of pork and increase the abundance of aldehydes, ketones and alcohols. However, these studies rarely, if ever, elucidate and analyze the specific mechanisms influencing flavor, such as the role of precursor substances in flavor formation [
10].
Lysine is an essential amino acid that the body cannot produce itself and must obtain from food. It plays a crucial role in promoting protein synthesis in humans, as well as fatty acid metabolism and calcium absorption. It can even enhance immune function and reduce anxiety [
11,
12,
13]. Lysine is also the first limiting amino acid in pig diets. Previous studies have shown that increasing the level of standard ileal digestible (SID) lysine in diets improves growth performance and linearly increases the hot carcass weight and lean meat percentage in finishing pigs [
10]. Reducing the lysine/protein ratio in diets from 0.046 to 0.035 increased the marbling score of longissimus thoracis (LT) muscle and elevated intramuscular fat (IMF) content by 38.71% [
14]. In finishing pigs weighing 71–123 kg, reducing the dietary ratio of SID lysine to digestible energy from 0.6 to 0.4 increased IMF content by 17.6% and improved tenderness [
15]. Lysine has a sweet taste, and the carnosine formed from lysine could mask bitterness [
16]. Additionally, lysine promotes carnitine synthesis, enhances fatty acid β-oxidation and improves ATP production efficiency, thereby increasing inosine monophosphate (IMP) accumulation [
17], which is a key source of umami flavor in meat. Therefore, dietary lysine level (DLLs) may influence the development of pork flavor. However, studies regarding the effects of DLLs on pork flavor characteristics are currently limited. Furthermore, most studies have used small numbers of pigs, and the impact of DLLs on pork quality and flavor under large-scale commercial farming conditions requires systematic investigation.
Therefore, the objective of this study was to investigate the effects of DLLs on carcass traits, meat quality and VOC composition in pork while meeting the lysine requirements, using 450 finishing pigs. We hypothesized that increasing DLLs would alter pork flavor without negatively affecting growth performance, carcass traits or meat quality under large-scale farming conditions.
2. Materials and Methods
2.1. Experimental Design and Sample Collection
This study obtained the approval of the Institutional Animal Care and Use Committee of China Agricultural University (approval number: AW01202313-1).
Animal experiments were carried out in a commercial pig farm (Fuzhiyuan Group, Guiyang, Guizhou, China) and animals were fed ad libitum. A total of approximately 450 Duroc × Landrace × Yorkshire crossbred finishing pigs (103.65 ± 4.28 kg) were randomly divided into four treatment groups, taking into account their initial body weight (BW). There were four replicate pens of 25~30 pigs per replicate in each group. Therefore, a total of approximately 110 pigs were involved in each group. All diets were formulated according to the NRC (2012) [
18]. These groups were named the Lys100, Lys115, Lys130 and Lys145 groups. In brief, in the Lys100 group, SID Lys in the pigs’ diets was at the recommended value. In the Lys115, Lys130 and Lys145 groups, SID Lys in the pigs’ diets was 115%, 130% and 145% of that in the Lys100 group, respectively. The levels of other essential amino acids were determined based on the ideal amino acid ratio (NRC, 2012). Dietary composition and nutrient levels are listed in
Table 1. The finishing pigs had ad libitum access to feed and water during the experimental period of 31 days. The management and immunization procedures were carried out according to the company’s regulations. Feed intake was recorded daily. All pigs were weighed individually at the beginning and end of the experiment. Initial and terminal BW were recorded. The average daily gain (ADG) and ratio of feed to gain were calculated and analyzed for each pen.
After the feeding period, one barrow and one gilt of the average BW per pen were picked up (n = 8, female to male ratio = 1:1). The slaughter weight was approximately 136 kg. After fasting for 12 h with free access to water, the pigs were transported to a designated slaughterhouse near the farm. After resting for at least four hours in holding pens with shower facilities, the pigs were humanely stunned using a high-frequency electrical system. A throat puncture was performed immediately while the animals were unconscious to allow for bleeding. To access meat quality, samples of approximately 100 g were obtained from the left LT of the carcass approximately 30 min after slaughter, located between the 10th and 12th ribs and maintained at 4 °C. Another piece of LT muscle (~100 g) was subjected to chemical composition analysis.
2.2. Carcass Traits
After slaughter, the carcass traits of pigs were measured (
n = 8). The weight of each carcass was measured after exsanguination, evisceration and removal of the head and hooves. Dressing percentage was calculated as follows: Dressing percentage (%) = carcass weight/live weight × 100, where live weight refers to the BW of pigs after 12 h of fasting with free access to water, before slaughter. Backfat thickness was measured at five positions using a vernier caliper with a precision of 0.01 mm: the thickest part of the shoulder, the 6th–7th rib interface, the 10th rib, the last rib and the last lumbar vertebra. The average backfat thickness was calculated using three of these measurements: shoulder backfat depth, backfat depth at the last rib and lumbosacral backfat depth. At the 10th rib, the height and width of the LT muscle (loin eye) were measured using a vernier caliper. The loin eye area was calculated using the formula following:
2.3. Meat Quality Assessment
The meat quality of fresh LT was analyzed at 45 min and 24 h post-slaughter (
n = 8). At 24 h post-slaughter, meat color and marbling were scored according to the National Pork Producer Council criteria: a score of 6.0 denotes a deep purplish-red color, while a score of 1.0 indicates an overly pale white shade. An SPK pH meter (model pH-star, DK2730, Herlev, Denmark) was used to assess pH at 4 °C, with readings taken at both 45 min and 24 h post-slaughter. The pH meter was calibrated beforehand using pre-cooled standard buffers (pH 4.01 and 7.00) at 4 °C, following standardized protocols. After undergoing a 30 min blooming process, the meat color was evaluated using a Konica Minolta CR-410 colorimeter (Konica Minolta, Osaka, Japan) equipped with a 50 mm measuring aperture and illuminated by a standardized xenon lamp. The instrument was set up with a D65 light source and a 2° standard observer to determine lightness (L*), redness (a*) and yellowness (b*) values according to the CIE Lab system. The chroma (c*) and hue angles (h°) were calculated as follows:
,
[
19,
20]. Each piece of meat was measured three times, and the average value was taken. Prior to use, the device was calibrated against a white reference tile.
Drip loss was determined by initially weighing fresh LT samples, sealing them in airtight lock bags and storing them at 4 °C for 24 h. After this period, the samples were weighed again to calculate the drip loss percentage [
21]. To determine cooking loss, pre-weighed muscle samples were steamed in a 70 °C water bath for 30 min in one batch. After cooling to room temperature, the samples were reweighed to calculate the cooking loss percentage [
22]. After calculation, the same piece of meat was cut into 10 cubes for shear force measurement. Shear force was tested on cylindrical cores (1.27 cm diameter) taken from the cooked samples using a C-LM3B digital muscle tenderness meter (Tenovo, Beijing Tianxiang Feiyu Technology Co., Beijing, China), which was fitted with a strain-gauge pressure sensor and operated at 300 mm/min [
23].
2.4. Chemical Composition of Meat
Approximately 10 g of LT muscle samples (
n = 8) were accurately weighed. Moisture content was determined by vacuum freeze-drying the samples at −50 °C under 0.1 mbar for 48 h (Christ Alpha 1–4 LDplus, Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany), followed by reweighing. Moisture content was calculated as follows:
. For crude protein (CP) determination, freeze-dried muscle samples were ground into a homogeneous powder and then analyzed via the Kjeldahl method [
24] using a Kjeltec 8400 Auto Analyzer (FOSS Analytical A/S, Hillerød, Denmark). CP content was calculated by multiplying the nitrogen content by a conversion factor of 6.25. IMF was extracted using a Budwi Extraction System B-11 (Budwi, Lausanne, Switzerland) based on the Soxhlet extraction principle [
25]. Ground freeze-dried samples were wrapped in filter paper thimbles extracted with petroleum ether (boiling range 30–60 °C) for 6 h, then dried at 105 °C for 1 h and cooled in a desiccator before weighing. IMF content was expressed as a percentage relative to the initial fresh weight of the muscle.
2.5. Analysis of Amino Acid Composition
The amino acid composition of the LT muscle was measured using the AOAC (2007) standard method [
26] (
n = 8). First, approximately 0.3 g of freeze-dried muscle was weighed and pulverized. This was then mixed with 5 mL of 20% methanol and 8 μL of internal standard (D-phenylalanine) by vortexing. This mixture was sonicated at room temperature for 5 min, left to stand at room temperature for 1 min and then vortexed; this cycle was repeated six times until the total sonication time reached 30 min. After standing for 2 h, the mixture was centrifuged at 13,000 rpm for 10 min at 4 °C. Subsequently, 400 μL of the supernatant was transferred to a 1.5 mL centrifuge tube and vacuum-dried at 45 °C for 3 h using a rotary evaporator. Finally, 100 μL of borate buffer solution and 20 μL of AccQ Tag derivatizing reagent (Kairos,
Los Angeles,USA) were added. Analysis of free amino acids in muscle samples was conducted via ion-exchange chromatography using an Amino Acid Analyzer L-8900 (Hitachi, Tokyo, Japan). Tryptophan was quantified separately via high-performance liquid chromatography (HPLC 1200 Series, Agilent, Santa Clara, CA, USA) using alkaline hydrolysis.
2.6. Determination of Volatile Compounds
VOCs were extracted using solid-phase microextraction (SPME) (
n = 7–8) [
27]. A 500 mg sample of LT muscle was placed into a headspace vial along with 10 μL of 1 mg/L deuterated n-hexanol-d13 (internal standard). The sample was then incubated at 80 °C for 10 min. The SPME fiber was conditioned at 270 °C for 10 min before being transferred to the sample vial. Adsorption proceeded for 25 min at 80 °C. The loaded SPME fiber was then transferred to the gas chromatography injection port and desorbed at 250 °C for 5 min. The conditioning step was then repeated. Finally, 10 μL of n-alkanes were added to the vial to complete the extraction process.
Subsequent analysis was performed using comprehensive two-dimensional gas chromatography coupled with time-of-flight mass spectrometry (GC × GC-TOFMS). Separation was achieved using a gas chromatograph from Agilent Technologies (Palo Alto, CA, USA) equipped with primary and secondary columns. High-purity helium carrier gas flowed at a rate of 1.0 mL/min. The temperature program was as follows: the primary column was held at 50 °C for 2 min, then ramped to 230 °C at 5 °C/min and held for a further 5 min. The secondary column and modulator temperature programs were maintained at temperatures 5 °C and 20 °C higher than the primary column, respectively.
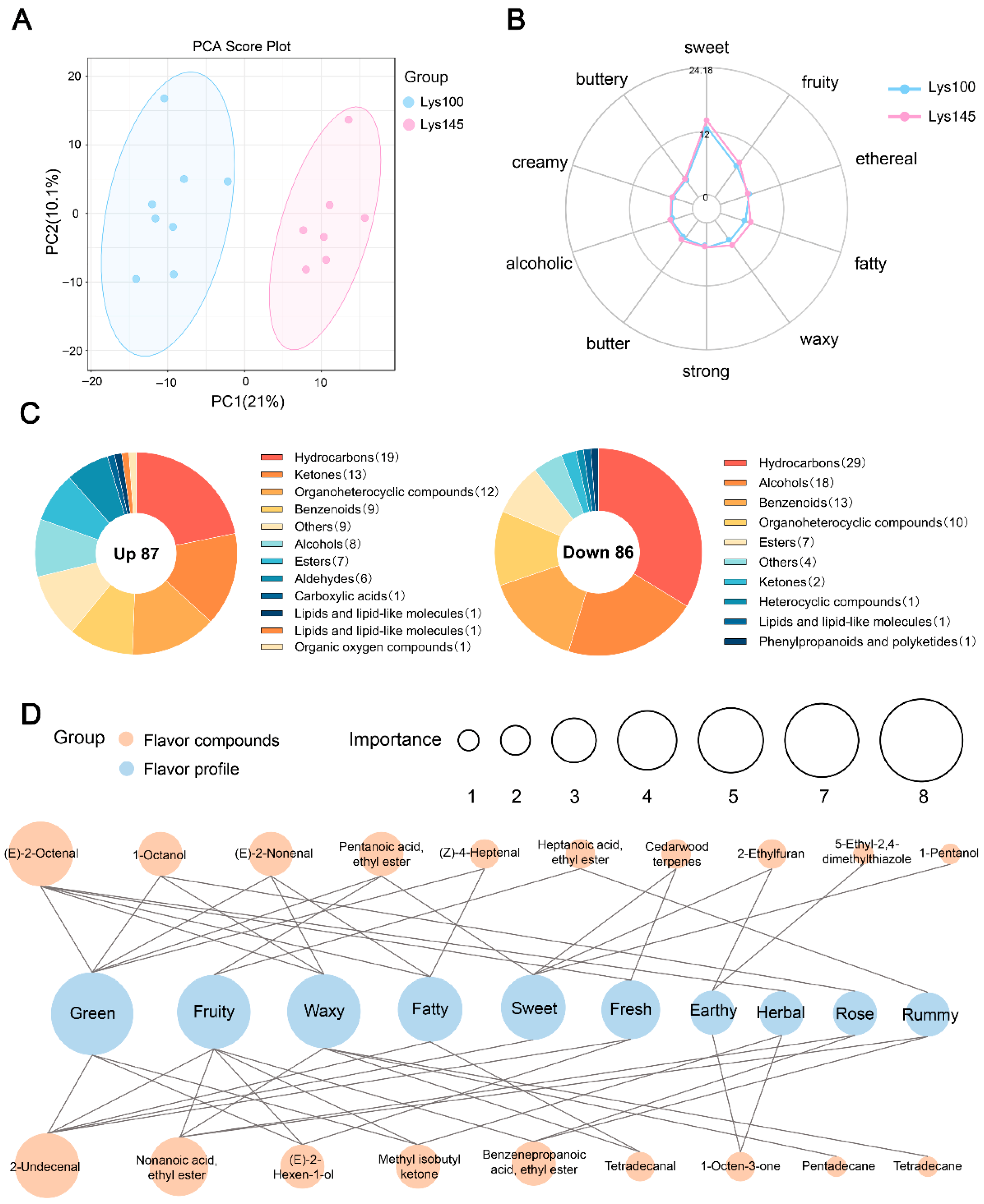
Mass spectra were acquired using a LECO Pegasus BT 4D mass spectrometer (LECO, St. Joseph, MI, USA) with the following parameters: transfer line temperature 250 °C, ion source temperature 250 °C, acquisition rate 200 spectra/s, electron ionization energy 70 eV and detector voltage 1960 V. Compound annotation was performed using Chroma TOF (version 5.0) software to identify compound names, retention times and peak areas. Unsupervised principal component analysis (PCA) was performed using the ropls package in R (version 4.2.0) to visualize natural clustering of samples from the Lys100 and Lys145 groups. Relative contents of VOCs were calculated using the semi-quantitative method with an internal standard. Compounds meeting the criteria of p < 0.05 and VIP > 1 were defined as differential VOCs. The relative odor activity value (ROAV) was calculated as follows:
. Where A represents the target compound and stan represents the compound with the highest relative content/odor threshold value. Sensory characteristics of all compounds were analyzed based on the FlavorDB database. A sensory flavor radar chart was generated for the top 10 sensory attributes ranked by content.
2.7. Statistical Analysis
Data are expressed as mean ± SEM. Unpaired two-tailed Student’s t-test and one-way ANOVA procedures were conducted using SAS (v.9.2, SAS Institute, Cary, NC, USA) to compare differences between two or four groups, respectively. The differences between the four groups was analyzed using a mixed linear model in SAS as follows: Yij = μ + Ti + uij + eij, where Yij is the dependent variable; μ is the overall mean; Ti is the fixed treatment effect; uij is the random effect of the pen within the dietary treatment; and eij is random error. Linear and quadratic regression analyses were performed to assess the effect of expository dose. Means were considered significantly different when p < 0.05 and as a trend when 0.05 ≤ p ≤ 0.10. Data visualization was implemented using R (version 4.0.3) and GraphPad Prism software (version 8.0).
4. Discussion
Current research mainly focuses on ensuring the growth performance of pigs through the balance of dietary amino acids [
28,
29,
30]. However, studies on the effect of DLLs on pork flavor characteristics are limited. Recently, Pan et al. found that increasing DLLs linearly increased the content of sweet amino acids in soleus muscles of growing-finishing pigs (33–125 kg) and reduced the content of bitter amino acids, while also increasing the content of IMP. When DLLs were at 90% of the recommended value, it was beneficial to flavor formation and improved meat quality [
31]. Nevertheless, there is still a lack of clear description of the flavor characteristics of pork. It should also be noted that a DLL below the recommended level may negatively affect the growth performance of pigs. In this study, it was found that increasing DLLs had no significant effect on ADG, ADFI, or the ratio of feed to gain when the lysine requirements of finishing pigs were met and the ideal ratio of other essential amino acids to lysine was maintained.
By contrast, carcass traits were more sensitive to changes in lysine levels. Loin eye area increased linearly with the increasing lysine levels. Lysine is a core driver of protein synthesis, promoting satellite cell differentiation by activating the mTORC1 signaling pathway and increasing skeletal muscle mass [
32]. Furthermore, lysine has been reported to induce satellite cell migration by activating the FAK pathway [
33] and act as a ligand for Fizzled, thereby activating the classical Wnt/β-catenin signaling pathway and promoting satellite cell proliferation and skeletal muscle growth [
34]. Interestingly, the backfat thickness at the 10th rib decreased with increasing lysine levels. AMPK is an energy sensor whose activation promotes fatty acid oxidation and inhibits fat synthesis [
35]. The expression levels of AMPKα1, SIRT1 and PGC-1α were significantly reduced under lysine restriction, leading to inhibited lipolysis [
36]. Conversely, a high-lysine diet enhanced the activity of antioxidant enzymes in the intestine of grass carp by activating the mTOR signaling pathway and inhibiting the p38 MAPK signaling pathway [
37]. However, in mice with obesity induced by a high-fat diet, adding lysine to the diet had no effect on BW or white adipose tissue weight [
38], suggesting that the impact of lysine on fat deposition may depend on the species and type of diet. In this study, the 10th rib fat thickness in finishing pigs showed a linear decrease with increasing lysine levels. This fills the gap between basic lysine research in other animals and applied flavor optimization in swine production. It should be noted that backfat thickness showed strong decreasing trends that did not reach strict statistical significance. This may be due to the inherent variability in commercial populations and the sample size, which may have limited our ability to detect more subtle effects on all measured parameters.
Previous studies have shown that dietary lysine restriction increases IMF deposition in lean-type pigs, but also reduces feed conversion efficiency and lean meat percentage [
39,
40]. In this study, however, there were no significant differences in meat quality parameters, including IMF content, among the four groups. Therefore, while meeting the lysine requirements of finishing pigs, DLLs do not affect IMF content. Additionally, CP content first decreased and then increased with increasing DLLs, with the lowest value observed in the Lys130 group. This suggests that the effect of lysine levels on muscle protein synthesis may exhibit a ‘threshold effect’. Once this threshold is exceeded, protein synthesis signaling pathways are activated by a sufficient supply of substrates [
41], leading to enhanced muscle protein deposition efficiency.
The formation of pork flavor is a complex process involving the metabolic conversion of flavor precursors such as amino acids and lipids into VOCs, with the final flavor being determined by the synergistic effects of multiple VOCs [
42,
43]. The composition of free amino acids in muscle directly influences the development of sensory flavors such as umami and sweetness [
44]. In this study, the levels of methionine and glycine in muscle were highest in the Lys145 group. Methyl mercaptan and dimethyl sulfide, the metabolic intermediates of methionine, are important contributors to flavor characteristics [
45]. Glycine, which contributes to sweetness, exhibited a linear increase. For finishing pigs, adding 0.57% glycine to a low-protein diet can increase muscle levels of glycine, tyrosine and glutamic acid and improve flavor scores [
46]. In this study, the increase in muscle glycine content may be related to the synergistic regulation of amino acid metabolism. Lysine levels may indirectly affect glycine accumulation by influencing transamination or carbon skeleton allocation [
47]. However, the direct contribution of increased methionine/glycine to flavor should also be investigated in the future.
By analyzing the VOCs in pork, we found that the proportions of nitrogen compounds, ethers and aldehydes were significantly higher in the Lys145 group than in the Lys100 group. Nitrogen compounds primarily originate from the Maillard reaction, protein degradation, and biosynthesis. These compounds have high flavor intensity and low thresholds, exhibiting flavor characteristics such as roasted and burnt aromas [
48]. Ethers are commonly used as flavor enhancers, capable of intensifying sweet, fruity and floral aromas [
49]. Aldehydes are products of fat oxidation or amino acid degradation with extremely low thresholds and diverse flavor characteristics. For example, short-chain saturated aldehydes and unsaturated aldehydes contribute a fresh grassy aroma; long-chain saturated aldehydes primarily exhibit fatty and waxy aromas; and aromatic aldehydes present sweet and fruity aromas [
50,
51]. Notably, the differential VOCs include six aldehydes, all of which are upregulated in the Lys145 group. Consistent with the changes in VOCs, the Lys145 group exhibits stronger flavor characteristics, such as sweet, fruity, waxy and fatty aroma, compared to the Lys100 group. ROAV can be used to assess the extent to which VOCs influence the overall flavor, with compounds having ROAV ≥ 1 being considered key flavor components [
52]. In this study, 1-Octen-3-one, (E)-2-Nonenal and (E)-2-Octenal all had ROAVs greater than 1 and were upregulated in the Lys145 group. 1-Octen-3-one primarily exhibits mushroom and earthy flavors. Both 1-Octen-3-one and (E)-2-Octenal are key aromatic compounds in pork broth [
53]. (E)-2-Octenal is typically associated with fatty, grassy and meat aromas. Compared to Berkshire pork, Ningxiang pork has a higher ROAV for (E)-2-octenal, which is considered a key aromatic marker that distinguishes Ningxiang pork from Berkshire pork [
5]. It is important to note that linoleic acid is cleaved by 13-hydroperoxide to produce 1-Octen-3-one [
54]. (E)-2-Nonenal has an extremely low threshold and is a specific product of oleic acid oxidation [
55], while (E)-2-Octenal is generated from the oxidation of linoleic acid [
56]. Therefore, all three of these compounds may be related to enhanced lipid oxidation, and oxidation markers should be measured in future research. Furthermore, the VOC analysis was limited to the Lys100 and Lys145 groups due to high cost. Including all groups would provide a more complete dose–response relationship. In this study, a 45% increase in lysine may raise feed costs, so an economic analysis is needed to balance flavor improvement with production costs. Its application requires large-scale trials to validate economic benefits. The pros and cons of increasing the lysine levels in the pigs’ diets are summarized in
Table S3. Moreover, our results are based on Duroc × Landrace × Yorkshire pigs, which are widely used in commercial production. However, responses may differ in local breeds due to genetic variations in metabolism and fat deposition.