Uncovering Analytical Patterns for Hazardous Components in Agricultural Production Systems
Abstract
1. Introduction
2. Types of Hazardous Components in Agricultural Products
2.1. Pesticide Residues
2.1.1. Organophosphorus Pesticides
2.1.2. Neonicotinoid Insecticides
2.1.3. Herbicides
2.2. Heavy Metal Contamination
2.2.1. Cd
2.2.2. Pb
2.2.3. As
2.2.4. Cr
2.2.5. Ni
2.2.6. Cu
2.2.7. Zn
2.3. Mycotoxin
2.3.1. Aflatoxin B1
2.3.2. Deoxynivalenol
2.3.3. Fumonisin B1
2.4. Microbial Contamination
2.4.1. Escherichia coli
2.4.2. Salmonella
2.4.3. Listeria monocytogenes
2.5. Antibiotic Residues
2.5.1. Tetracycline Antibiotics
2.5.2. Fluoroquinolone Antibiotics
2.5.3. Macrolide Antibiotics
3. Traditional Detection Methods
3.1. Chromatographic Analysis
3.1.1. Ultra-Performance Liquid Chromatography
3.1.2. Comprehensive Two-Dimensional Gas Chromatography
3.1.3. Ion Chromatography
3.2. Spectroscopic Analysis
3.2.1. Laser-Induced Breakdown Spectroscopy
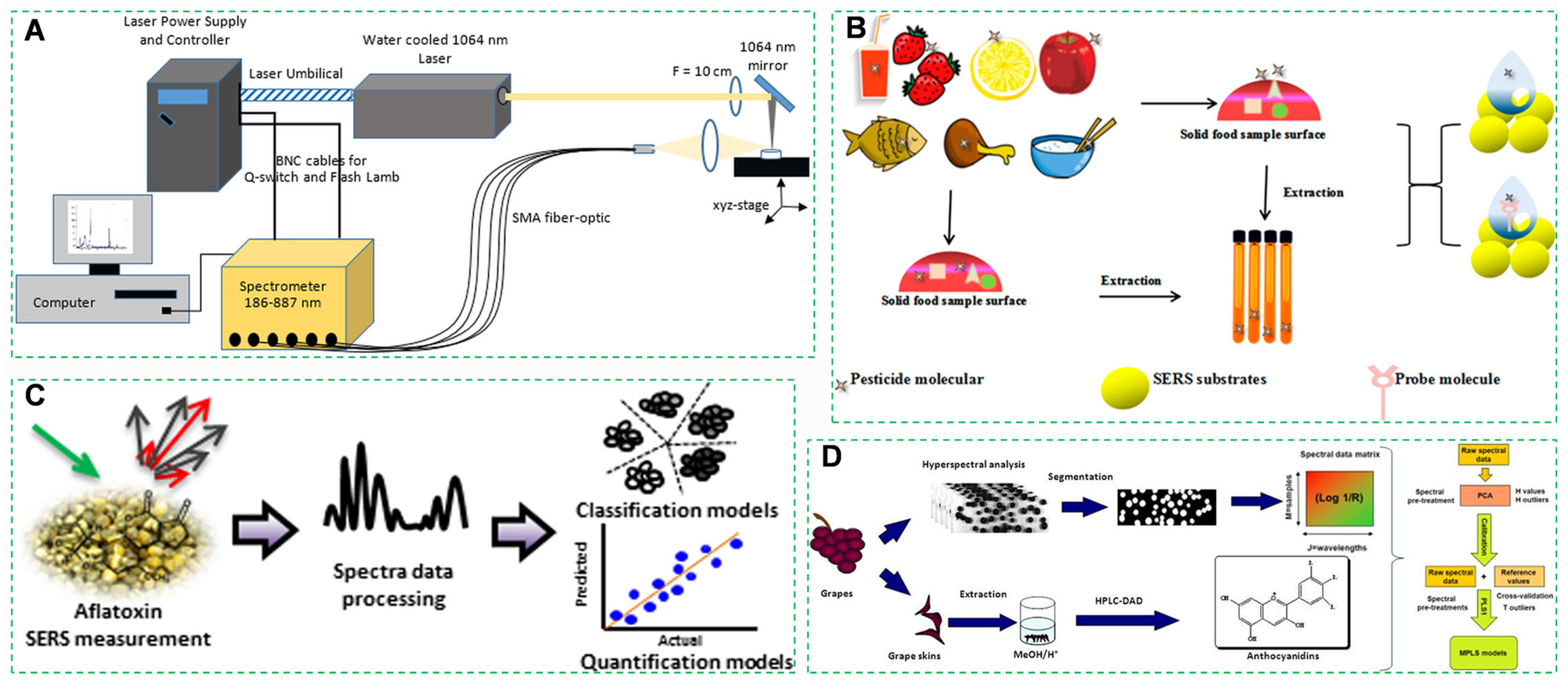
3.2.2. Surface-Enhanced Raman Spectroscopy
3.2.3. Near-Infrared Hyperspectral Imaging
3.3. Electrochemical Methods
3.4. Immunoassays
3.4.1. Quantum Dot-Labeled Immunochromatography
3.4.2. Recombinant Antibody Technology
3.4.3. Enzyme-Linked Immunosorbent Assay (ELISA)
| Technique Category | Typical Methods | Advantages | Disadvantages | SERS |
|---|---|---|---|---|
| Chromatography | HPLC, UPLC, GC × GC, IC | High separation efficiency; accurate qualitative and quantitative analysis; strong capability for multi-residue detection | Complex sample pretreatment; long detection cycle; expensive instrumentation; unsuitable for on-site detection | [255] |
| Spectroscopy | IR, Raman, LIBS, SERS, NIR-HSI | Non-destructive; rapid detection; potential for online/field deployment | Limited sensitivity; strong matrix interference; fluorescence interference in some techniques | [256] |
| Immunoassays | ELISA, colloidal gold test strips, quantum dot immunochromatography | High specificity; easy to operate; suitable for large-scale rapid screening | Susceptible to cross-reactivity; limited sensitivity; antibody preparation is costly | [257] |
3.5. Summary of Strengths and Limitations of Traditional Methods
4. Emerging Detection Technologies
4.1. Biosensor Technology
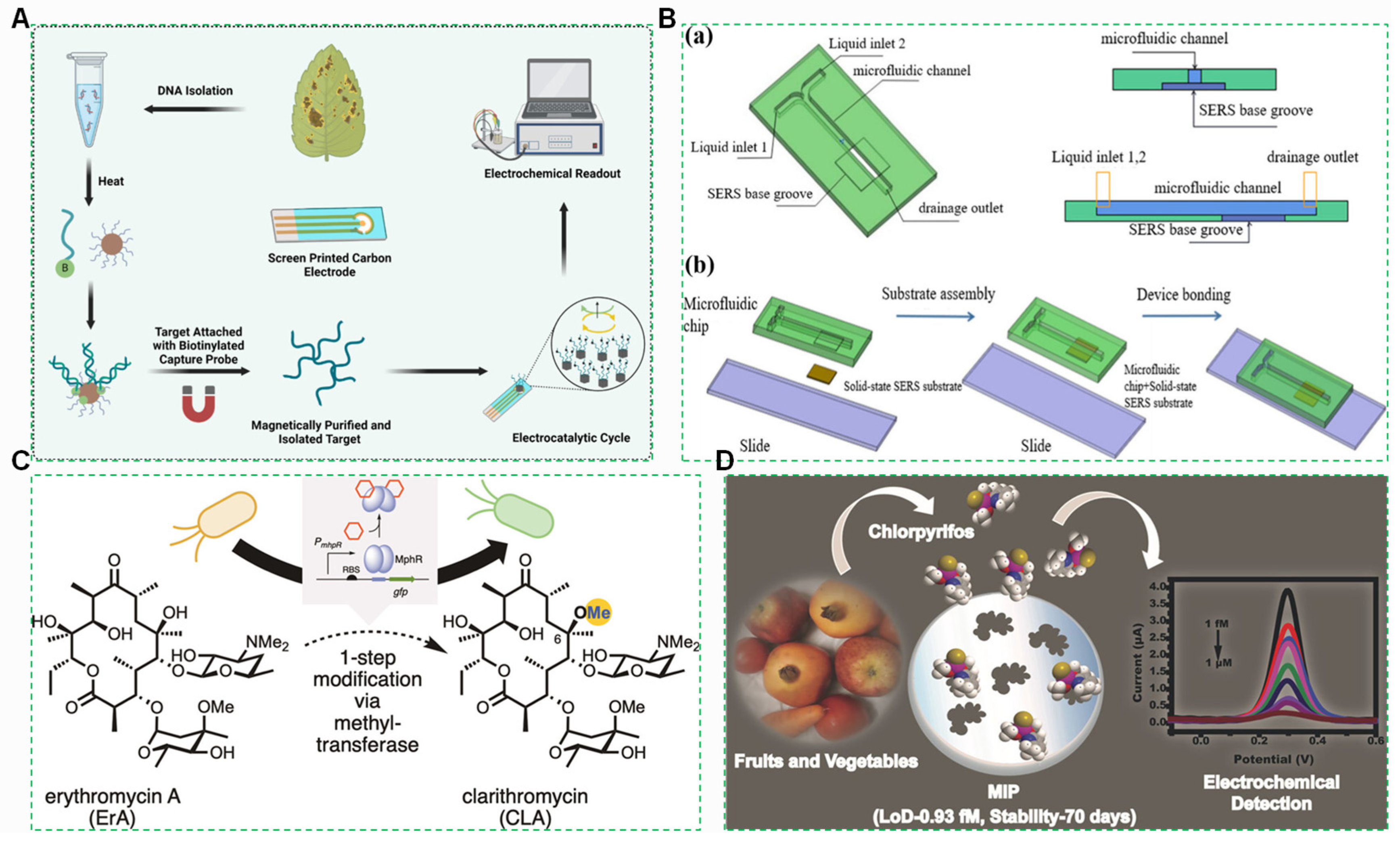
4.1.1. Fully Integrated Microfluidic Biochips
4.1.2. Live Cell Sensor
4.1.3. Molecularly Imprinted Polymer (MIP) Sensors
4.2. Nanomaterial Technology
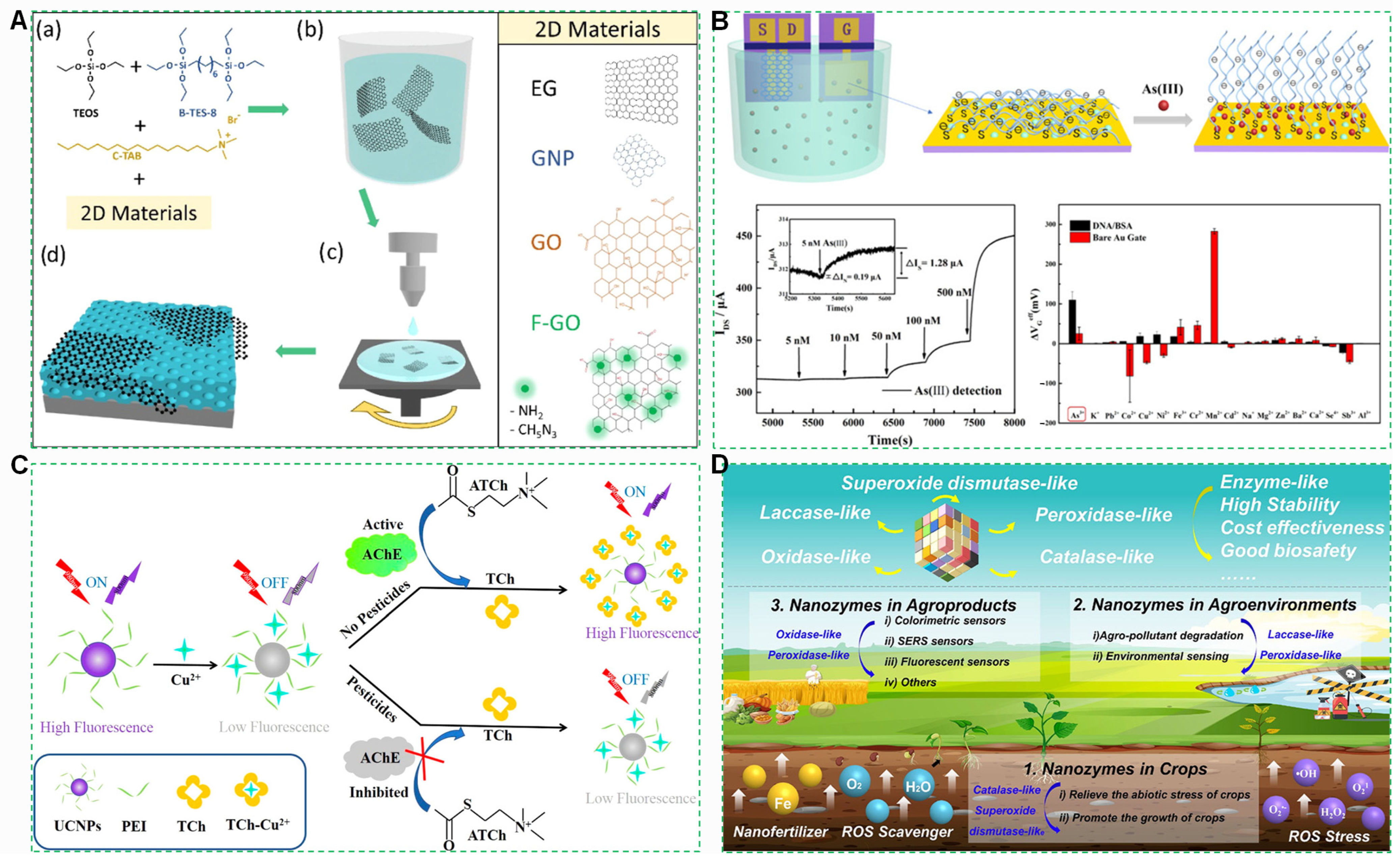
4.2.1. Graphene Field-Effect Transistor (GFET) Sensors
4.2.2. Upconversion Nanoparticles (UCNPs)
4.2.3. Robust Nanozyme Stabilization Breakthrough
4.3. Genome Editing Technologies
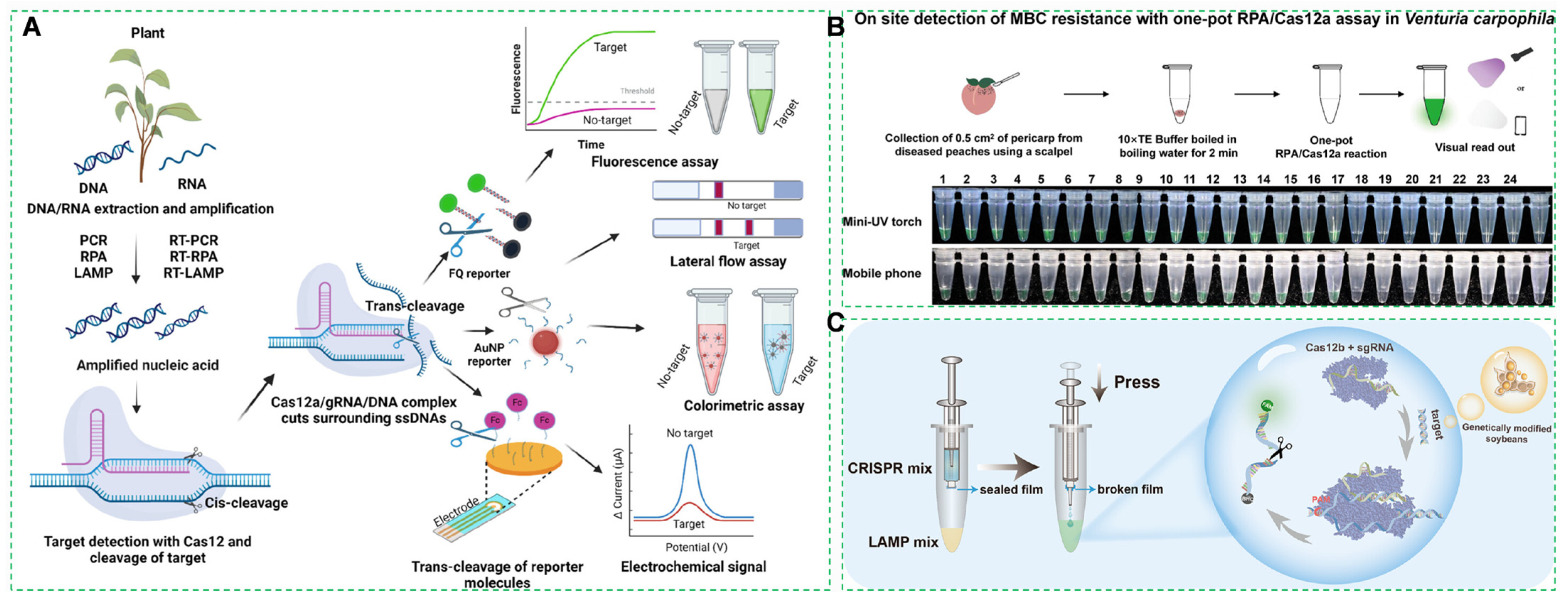
4.3.1. CRISPR-Cas Molecular Diagnostic System
4.3.2. RPA-CRISPR Cascade Amplification System
4.3.3. Genetically Modified (GM) Component Screening
4.4. Artificial Intelligence Technology
4.4.1. Deep Learning-Driven Spectral Intelligence
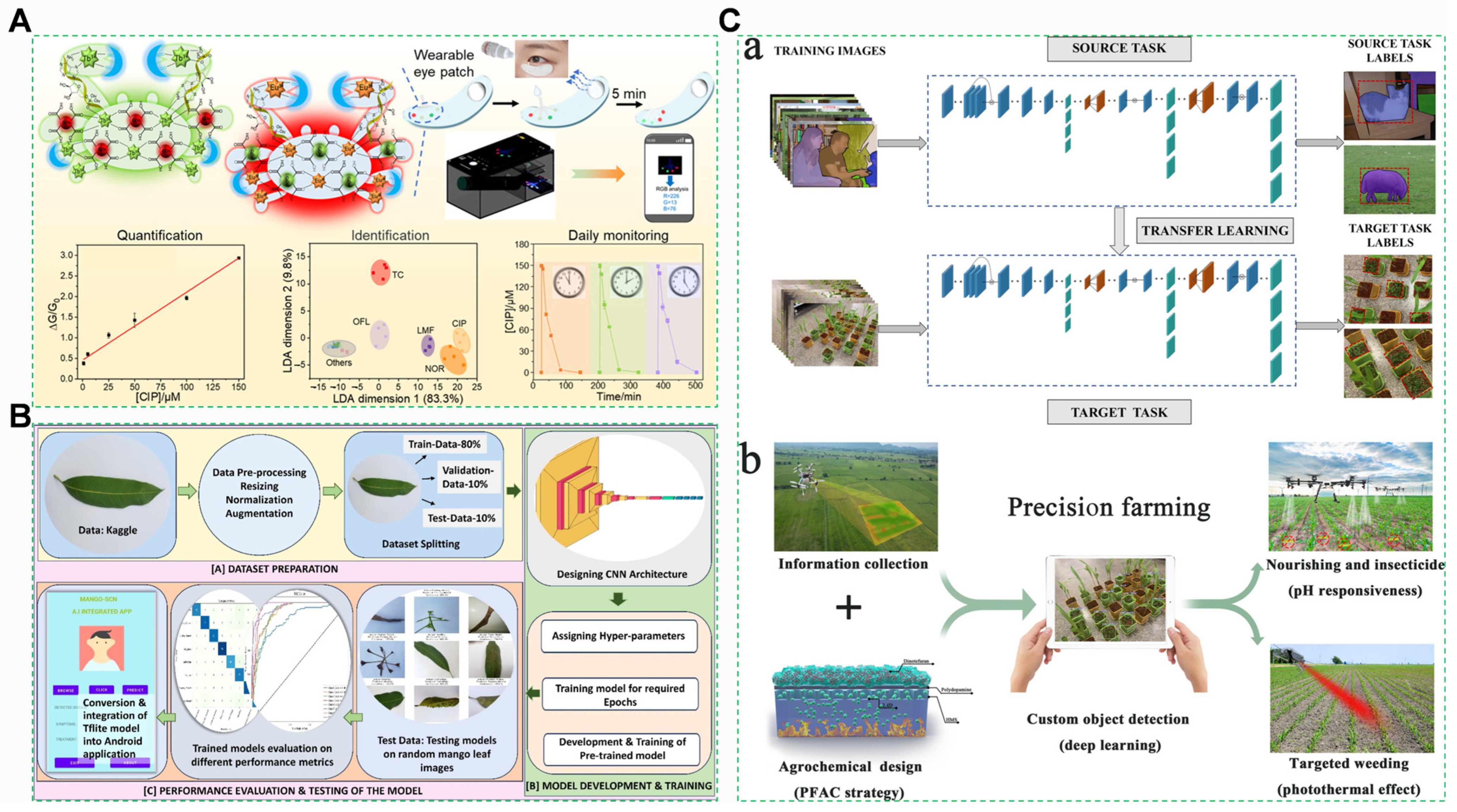
4.4.2. Blockchain-IoT Integrated Traceability System
4.4.3. Digital Twin-Driven Predictive Paradigm Shift for Agricultural Contamination Risks
| Technique Category | Typical Methods | Advantages | Disadvantages | SERS |
|---|---|---|---|---|
| Biosensors | Microfluidic chips, live-cell sensors, MIP sensors | High sensitivity; rapid on-site detection; potential for multiplexed analysis | Limited stability; relatively high cost; lack of standardized protocols | [345] |
| Nanomaterial-based Sensors | GFET, UCNPs, Nanozymes | Fast response; high sensitivity; strong anti-interference ability | Complex material synthesis; limited scalability for routine applications | [346] |
| Gene-based Detection | CRISPR-Cas, RPA-CRISPR | Ultra-high sensitivity and specificity; ideal for GMO and pathogen detection | Requires specific sample pretreatment; some systems rely on costly reagents | [347] |
| AI & Digital Technologies | AI-assisted spectral analysis, Blockchain traceability, Digital Twin systems | Powerful data processing; enables predictive monitoring and smart decision-making | Requires large datasets and computational resources; field application still in early stage | [348] |
4.5. Summary of Strengths and Limitations of Emerging Methods
5. Challenges and Perspectives
5.1. Strategic Pathways for Technical Bottleneck Breakthroughs
5.2. Breakthroughs in China’s Reference Material System for Agri-Food Safety
5.3. Breakthroughs in Microfluidics-Mass Spectrometry Integration
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, H.; Xia, Y.; Jin, J.; Pan, C. The impact of climate change on the efficiency of agricultural production in the world’s main agricultural regions. Environ. Impact Assess. Rev. 2022, 97, 106891. [Google Scholar] [CrossRef]
- Shi, R.; Yao, L.; Zhao, M.; Yan, Z. Low-carbon production performance of agricultural green technological innovation: From multiple innovation subject perspective. Environ. Impact Assess. Rev. 2024, 105, 107424. [Google Scholar] [CrossRef]
- Ali, M.M.; Hashim, N.; Abd Aziz, S.; Lasekan, O. Principles and recent advances in electronic nose for quality inspection of agricultural and food products. Trends Food Sci. Technol. 2020, 99, 1–10. [Google Scholar] [CrossRef]
- Han, Y.; Tian, Y.; Li, Q.; Yao, T.; Yao, J.; Zhang, Z.; Wu, L. Advances in Detection Technologies for Pesticide Residues and Heavy Metals in Rice: A Comprehensive Review of Spectroscopy, Chromatography, and Biosensors. Foods 2025, 14, 1070. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, X.; Mao, H.; Wu, X.; Zhang, X.; Gao, H. Identification of pesticide residue level in lettuce based on hyperspectra and chlorophyll fluorescence spectra. Int. J. Agric. Biol. Eng. 2016, 9, 231–239. [Google Scholar] [CrossRef]
- Meher, A.K.; Zarouri, A. Environmental Applications of Mass Spectrometry for Emerging Contaminants. Molecules 2025, 30, 364. [Google Scholar] [CrossRef]
- Ma, S.; Wang, M.; You, T.; Wang, K. Using Magnetic Multiwalled Carbon Nanotubes as Modified QuEChERS Adsorbent for Simultaneous Determination of Multiple Mycotoxins in Grains by UPLC-MS/MS. J. Agric. Food Chem. 2019, 67, 8035–8044. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, Y.; Li, J.; Lin, G.; Han, X.; Yi, J.; Jayaprada, T.; Zhou, Z.; Ying, Y.; Wang, M. Environmentally persistent microbial contamination in agricultural soils: High risk of pathogenicity and antibiotic resistance. Environ. Int. 2024, 190, 108902. [Google Scholar] [CrossRef]
- Farhan, M.; Awan, N.; Kanwal, A.; Sharif, F.; Hayyat, M.U.; Shahzad, L.; Ghafoor, G.Z. Dairy farmers’ levels of awareness of antibiotic use in livestock farming in Pakistan. Humanit. Soc. Sci. Commun. 2024, 11, 165. [Google Scholar] [CrossRef]
- Jin, T.; Tang, J.; Lyu, H.; Wang, L.; Gillmore, A.B.; Schaeffer, S.M. Activities of Microplastics (MPs) in Agricultural Soil: A Review of MPs Pollution from the Perspective of Agricultural Ecosystems. J. Agric. Food Chem. 2022, 70, 4182–4201. [Google Scholar] [CrossRef]
- Giampieri, F.; Mazzoni, L.; Cianciosi, D.; Alvarez-Suarez, J.M.; Regolo, L.; Sanchez-Gonzalez, C.; Capocasa, F.; Xiao, J.; Mezzetti, B.; Battino, M. Organic vs. conventional plant-based foods: A review. Food Chem. 2022, 383, 132352. [Google Scholar] [CrossRef] [PubMed]
- Torgerson, P.R.; Devleesschauwer, B.; Praet, N.; Speybroeck, N.; Willingham, A.L.; Kasuga, F.; Rokni, M.B.; Zhou, X.-N.; Fevre, E.M.; Sripa, B.; et al. World Health Organization Estimates of the Global and Regional Disease Burden of 11 Foodborne Parasitic Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, e1001920. [Google Scholar] [CrossRef]
- Su, M.; Liu, F.; Luo, Z.; Wu, H.; Zhang, X.; Wang, D.; Zhu, Y.; Sun, Z.; Xu, W.; Miao, Y. The Antibacterial Activity and Mechanism of Chlorogenic Acid Against Foodborne Pathogen Pseudomonas aeruginosa. Foodborne Pathog. Dis. 2019, 16, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Pires, S.M.; Liu, Z.; Ma, X.; Liang, J.; Jiang, Y.; Chen, J.; Liang, J.; Wang, S.; Wang, L.; et al. Surveillance of foodborne disease outbreaks in China, 2003–2017. Food Control 2020, 118, 107359. [Google Scholar] [CrossRef]
- Gao, R.; Liu, X.; Xiong, Z.; Wang, G.; Ai, L. Research progress on detection of foodborne pathogens: The more rapid and accurate answer to food safety. Food Res. Int. 2024, 193, 114767. [Google Scholar] [CrossRef]
- Ahmed, M.W.; Haque, M.A.; Mohibbullah, M.; Khan, M.S.I.; Islam, M.A.; Mondal, M.H.T.; Ahmmed, R. A review on active packaging for quality and safety of foods: Current trends, applications, prospects and challenges. Food Packag. Shelf Life 2022, 33, 100913. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, H.W.; Yin, X.-L.; Geng, T.; Long, W.; Fu, H.; She, Y. Deep leaning in food safety and authenticity detection: An integrative review and future prospects. Trends Food Sci. Technol. 2024, 146, 104396. [Google Scholar] [CrossRef]
- Zareef, M.; Chen, Q.; Ouyang, Q.; Arslan, M.; Hassan, M.M.; Ahmad, W.; Viswadevarayalu, A.; Wang, P.; Wang, A. Rapid screening of phenolic compounds in congou black tea (Camellia sinensis) during in vitro fermentation process using portable spectral analytical system coupled chemometrics. J. Food Process. Preserv. 2019, 43, e13996. [Google Scholar] [CrossRef]
- Liu, J.; Chen, N.; Yang, J.; Yang, B.; Ouyang, Z.; Wu, C.; Yuan, Y.; Wang, W.; Chen, M. An integrated approach combining HPLC, GC/MS, NIRS, and chemometrics for the geographical discrimination and commercial categorization of saffron. Food Chem. 2018, 253, 284–292. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Qiu, Y.; Li, P.; Liu, B.; Yang, L.; Barnych, B.; Hammock, B.D.; Zhang, C. Highly Specific Monoclonal Antibody and Sensitive Quantum Dot Beads-Based Fluorescence Immunochromatographic Test Strip for Tebuconazole Assay in Agricultural Products. J. Agric. Food Chem. 2019, 67, 9096–9103. [Google Scholar] [CrossRef]
- Sun, J.; Cao, Y.; Zhou, X.; Wu, M.; Sun, Y.; Hu, Y. Detection for lead pollution level of lettuce leaves based on deep belief network combined with hyperspectral image technology. J. Food Saf. 2021, 41, e12866. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, F.; Chen, Z.; Wang, J.; Li, W.-Q.; Fan, F.; Tao, Y.; Zhao, L.; Zhong, W.; Zhu, Q.-H.; et al. Intron-targeted gene insertion in rice using CRISPR/Cas9: A case study of the Pi-ta gene. Crop J. 2020, 8, 424–431. [Google Scholar] [CrossRef]
- Guo, D.; Ling, X.; Zhou, X.; Li, X.; Wang, J.; Qu, S.; Yang, Y.; Zhang, B. Evaluation of the Quality of a High-Resistant Starch and Low-Glutelin Rice (Oryza sativa L.) Generated through CRISPR/Cas9-Mediated Targeted Mutagenesis. J. Agric. Food Chem. 2020, 68, 9733–9742. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Huang, A.; He, L.; Cai, C.; You, T. Recent advances in foodborne pathogen detection using photoelectrochemical biosensors: From photoactive material to sensing strategy. Front. Sustain. Food Syst. 2024, 8, 1432555. [Google Scholar] [CrossRef]
- Yao, K.; Sun, J.; Cheng, J.; Xu, M.; Chen, C.; Zhou, X. Monitoring S-ovalbumin content in eggs during storage using portable NIR spectrometer and multivariate analysis. Infrared Phys. Technol. 2023, 131, 104685. [Google Scholar] [CrossRef]
- Wen, T.-T.; Yang, Y.-M.; Zhang, Y.-X.; Liu, M.-Q.; Qian, Z.-Y.; Zhang, Z.-Y.; Dong, C.-H.; Sun, L.; Xu, L.; Sun, W.-J.; et al. CRISPR-Cas9/Safe Harbor-Targeted Overexpression of Glucan Synthase Gene CmGls in Edible Mushroom Cordyceps militaris. J. Agric. Food Chem. 2025, 73, 10456–10469. [Google Scholar] [CrossRef]
- Liu, R.; Ali, S.; Huang, D.; Zhang, Y.; Lu, P.; Chen, Q. A Sensitive Nucleic Acid Detection Platform for Foodborne Pathogens Based on CRISPR-Cas13a System Combined with Polymerase Chain Reaction. Food Anal. Methods 2023, 16, 356–366. [Google Scholar] [CrossRef]
- Wang, H.; Gu, J.; Wang, M. A review on the application of computer vision and machine learning in the tea industry. Front. Sustain. Food Syst. 2023, 7, 1172543. [Google Scholar] [CrossRef]
- Elbeltagi, A.; Srivastava, A.; Deng, J.; Li, Z.; Raza, A.; Khadke, L.; Yu, Z.; El-Rawy, M. Forecasting vapor pressure deficit for agricultural water management using machine learning in semi-arid environments. Agric. Water Manag. 2023, 283, 108302. [Google Scholar] [CrossRef]
- Mao, Y.; Tan, H.; Wang, M.; Jiang, T.; Wei, H.; Xu, W.; Jiang, Q.; Bao, H.; Ding, Y.; Wang, F.; et al. Research Progress of Soil Microorganisms in Response to Heavy Metals in Rice. J. Agric. Food Chem. 2022, 70, 8513–8522. [Google Scholar] [CrossRef]
- Gu, R.; Duan, Y.; Li, Y.; Luo, Z. Fiber-Optic-Based Biosensor as an Innovative Technology for Point-of-Care Testing Detection of Foodborne Pathogenic Bacteria To Defend Food and Agricultural Product Safety. J. Agric. Food Chem. 2023, 71, 10982–10988. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Baek, E.J.; Na, T.W.; Sim, K.S.; Kim, H.; Kim, H.J. LC–MS/MS and GC–MS/MS Cross-Checking Analysis Method for 426 Pesticide Residues in Agricultural Products: A Method Validation and Measurement of Uncertainty. J. Agric. Food Chem. 2024, 72, 22814–22821. [Google Scholar] [CrossRef]
- Liu, J.; Sun, J.; Wang, Y.; Liu, X.; Zhang, Y.; Fu, H. Non-Destructive Detection of Fruit Quality: Technologies, Applications and Prospects. Foods 2025, 14, 2137. [Google Scholar] [CrossRef]
- Bonah, E.; Huang, X.; Aheto, J.H.; Osae, R. Application of electronic nose as a non-invasive technique for odor fingerprinting and detection of bacterial foodborne pathogens: A review. J. Food Sci. Technol.-Mysore 2020, 57, 1977–1990. [Google Scholar] [CrossRef]
- Umapathi, R.; Sonwal, S.; Lee, M.J.; Rani, G.M.; Lee, E.-S.; Jeon, T.-J.; Kang, S.-M.; Oh, M.-H.; Huh, Y.S. Colorimetric based on-site sensing strategies for the rapid detection of pesticides in agricultural foods: New horizons, perspectives, and challenges. Coord. Chem. Rev. 2021, 446, 214061. [Google Scholar] [CrossRef]
- Azam, S.M.R.; Ma, H.; Xu, B.; Devi, S.; Siddique, M.A.B.; Stanley, S.L.; Bhandari, B.; Zhu, J. Efficacy of ultrasound treatment in the removal of pesticide residues from fresh vegetables: A review. Trends Food Sci. Technol. 2020, 97, 417–432. [Google Scholar] [CrossRef]
- Hassan, M.M.; Zareef, M.; Jiao, T.; Liu, S.; Xu, Y.; Viswadevarayalu, A.; Li, H.; Chen, Q. Signal optimized rough silver nanoparticle for rapid SERS sensing of pesticide residues in tea. Food Chem. 2021, 338, 127796. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ge, X.; Wu, X.; Dai, C.; Yang, N. Identification of pesticide residues in lettuce leaves based on near infrared transmission spectroscopy. J. Food Process Eng. 2018, 41, e12816. [Google Scholar] [CrossRef]
- Yang, N.; Wang, P.; Xue, C.-Y.; Sun, J.; Mao, H.-P.; Oppong, P.K. A portable detection method for organophosphorus and carbamates pesticide residues based on multilayer paper chip. J. Food Process Eng. 2018, 41, e12867. [Google Scholar] [CrossRef]
- Gama, J.; Neves, B.; Pereira, A. Chronic Effects of Dietary Pesticides on the Gut Microbiome and Neurodevelopment. Front. Microbiol. 2022, 13, 931440. [Google Scholar] [CrossRef]
- Li, C.; Zhu, H.; Li, C.; Qian, H.; Yao, W.; Guo, Y. The present situation of pesticide residues in China and their removal and transformation during food processing. Food Chem. 2021, 354, 129552. [Google Scholar] [CrossRef]
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils—A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef]
- Chen, H.; Li, W.; Zhu, H.; Weng, H.; Shen, S. Insights on Degradation, Processing Factors, and Risk Assessment of Pesticide Pymetrozine, Spirotetramat, and Its Four Metabolites on Goji Berry: “Third Pole” Medicine and Food Homologous Crop. J. Agric. Food Chem. 2025, 73, 7423–7431. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Li, Q.; Deng, F.; He, J.; Zhou, J.; Sun, L.; Yuan, J.; Tan, L. Serial Cross-Sectional Human Biomonitoring Analysis of Pesticide Exposure Patterns and Their Association with Lipid Metabolism Biomarkers: The Mediating Role of Liver Function. Environ. Health 2025, 3, 818–830. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wu, F.; Cheng, L.; Ji, T.; Zheng, C.; Ma, Y.; Jin, Y.; Dong, J.; Jin, Y.; Fang, B. Chlorpyrifos Inhibits Intestinal Stem Cell Proliferation and Differentiation at the Acceptable Daily Intake and Disrupts Immune Responses at High Doses. J. Agric. Food Chem. 2025, 73, 12455–12464. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Luo, L.; Ning, F.; Li, J. Precision of in Vivo Pesticide Toxicology Research Can Be Promoted by Mass Spectrometry Imaging Technology. J. Agric. Food Chem. 2025, 73, 8113–8128. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, L.; Li, M.; Wang, M.; Liu, G.; Ping, J. Nanozyme-based biosensor for organophosphorus pesticide monitoring: Functional design, biosensing strategy, and detection application. TrAC-Trends Anal. Chem. 2023, 165, 117152. [Google Scholar] [CrossRef]
- Romero-Marquez, J.M.; Navarro-Hortal, M.D.; Forbes-Hernandez, T.Y.; Varela-Lopez, A.; Puentes, J.G.; Sanchez-Gonzalez, C.; Sumalla-Cano, S.; Battino, M.; Garcia-Ruiz, R.; Sanchez, S.; et al. Effect of olive leaf phytochemicals on the anti-acetylcholinesterase, anti-cyclooxygenase-2 and ferric reducing antioxidant capacity. Food Chem. 2024, 444, 138516. [Google Scholar] [CrossRef]
- Songa, E.A.; Okonkwo, J.O. Recent approaches to improving selectivity and sensitivity of enzyme-based biosensors for organophosphorus pesticides: A review. Talanta 2016, 155, 289–304. [Google Scholar] [CrossRef]
- Pundir, C.S.; Malik, A.; Preety. Bio-sensing of organophosphorus pesticides: A review. Biosens. Bioelectron. 2019, 140, 5–17. [Google Scholar] [CrossRef]
- Tang, C.Y.; He, Y.; Yuan, B.Z.; Li, L.B.; Luo, L.J.; You, T.Y. Simultaneous detection of multiple mycotoxins in agricultural products: Recent advances in optical and electrochemical sensing methods. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70062. [Google Scholar] [CrossRef]
- Marimuthu, M.; Xu, K.; Song, W.; Chen, Q.; Wen, H. Safeguarding food safety: Nanomaterials-based fluorescent sensors for pesticide tracing. Food Chem. 2025, 463, 141288. [Google Scholar] [CrossRef]
- Kaushal, J.; Khatri, M.; Arya, S.K. A treatise on Organophosphate pesticide pollution: Current strategies and advancements in their environmental degradation and elimination. Ecotoxicol. Environ. Saf. 2021, 207, 111483. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, G.; Dar, M.A.; Nimesh, S.; Lopez-Chuken, U.J.; Villarreal-Chiu, J.F. Microbial Degradation of Organophosphate Pesticides: A Review. Pedosphere 2018, 28, 190–208. [Google Scholar] [CrossRef]
- Mali, H.; Shah, C.; Raghunandan, B.H.; Prajapati, A.S.; Patel, D.H.; Trivedi, U.; Subramanian, R.B. Organophosphate pesticides an emerging environmental contaminant: Pollution, toxicity, bioremediation progress, and remaining challenges. J. Environ. Sci. 2023, 127, 234–250. [Google Scholar] [CrossRef]
- Sidhu, G.K.; Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Singh, J. Toxicity, monitoring and biodegradation of organophosphate pesticides: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1135–1187. [Google Scholar] [CrossRef]
- Neff, M.J.; Reddy, D.S. Long-Term Neuropsychiatric Developmental Defects after Neonatal Organophosphate Exposure: Mitigation by Synthetic Neurosteroids. J. Pharmacol. Exp. Ther. 2024, 388, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.; Barnych, B.; Huo, J.; Wan, D.; Vasylieva, N.; Xu, J.; Li, P.; Liu, B.; Zhang, C.; et al. Investigation of the Small Size of Nanobodies for a Sensitive Fluorescence Polarization Immunoassay for Small Molecules: 3-Phenoxybenzoic Acid, an Exposure Biomarker of Pyrethroid Insecticides as a Model. J. Agric. Food Chem. 2019, 67, 11536–11541. [Google Scholar] [CrossRef] [PubMed]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef]
- Hladik, M.L.; Main, A.R.; Goulson, D. Environmental Risks and Challenges Associated with Neonicotinoid Insecticides. Environ. Sci. Technol. 2018, 52, 3329–3335. [Google Scholar] [CrossRef]
- Pisa, L.W.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Downs, C.A.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; McField, M.; et al. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ. Sci. Pollut. Res. 2015, 22, 68–102. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, N.; Samson-Robert, O.; Sood, K.; Patel, H.S.; Malena, D.A.; Gajiwala, P.H.; Maciukiewicz, P.; Fournier, V.; Zayed, A. Chronic exposure to neonicotinoids reduces honey bee health near corn crops. Science 2017, 356, 1395–1397. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Anadon, A.; Wu, Q.; Qiao, F.; Ares, I.; Martinez-Larranaga, M.-R.; Yuan, Z.; Martinez, M.-A. Mechanism of Neonicotinoid Toxicity: Impact on Oxidative Stress and Metabolism. In Annual Review of Pharmacology and Toxicology; Insel, P.A., Ed.; Annual Reviews: San Mateo, CA, USA, 2018; Volume 58, pp. 471–507. [Google Scholar]
- Williams, G.R.; Troxler, A.; Retschnig, G.; Roth, K.; Yanez, O.; Shutler, D.; Neumann, P.; Gauthier, L. Neonicotinoid pesticides severely affect honey bee queens. Sci. Rep. 2015, 5, 14621. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, B.A.; Isaac, N.J.B.; Bullock, J.M.; Roy, D.B.; Garthwaite, D.G.; Crowe, A.; Pywell, R.F. Impacts of neonicotinoid use on long-term population changes in wild bees in England. Nat. Commun. 2016, 7, 12459. [Google Scholar] [CrossRef]
- Mei, X.-Y.; Hong, Y.-Q.; Chen, G.-H. Review on Analysis Methodology of Phenoxy Acid Herbicide Residues. Food Anal. Methods 2016, 9, 1532–1561. [Google Scholar] [CrossRef]
- Dayan, F.E. Current Status and Future Prospects in Herbicide Discovery. Plants 2019, 8, 341. [Google Scholar] [CrossRef]
- Li, H.; Travlos, I.; Qi, L.; Kanatas, P.; Wang, P. Optimization of Herbicide Use: Study on Spreading and Evaporation Characteristics of Glyphosate-Organic Silicone Mixture Droplets on Weed Leaves. Agronomy 2019, 9, 547. [Google Scholar] [CrossRef]
- Wu, M.; Ou, M.; Zhang, Y.; Jia, W.; Dai, S.; Wang, M.; Dong, X.; Wang, X.; Jiang, L. Development and Evaluation of a Monodisperse Droplet-Generation System for Precision Herbicide Application. Agriculture 2024, 14, 1885. [Google Scholar] [CrossRef]
- Qi, S.; Chen, D.; Yan, M.; Huang, Z.; Yu, H.; Ren, G.; Xiong, H.a.; Fu, W.; Zhao, B.; Dai, Z.; et al. Arbuscular mycorrhizal fungi enhance glyphosate resistance in an invasive weed: Implications for eco-environmental risks. Appl. Soil Ecol. 2025, 212, 106203. [Google Scholar] [CrossRef]
- Lu, X.Y.; Jayakumar, K.; Wen, Y.P.; Hojjati-Najafabadi, A.; Duan, X.M.; Xu, J.K. Recent advances in metal-organic framework (MOF)-based agricultural sensors for metal ions: A review. Microchim. Acta 2024, 191, 24. [Google Scholar] [CrossRef]
- Hossain, M.M.; Tripty, S.J.; Shishir, M.Z.A.; Wang, S.; Hossain, I.; Geng, A.; Han, S.; Zhu, D. Malondialdehyde and heavy metal contents in Piper betel: Possible risks of heavy metals in human health. J. Food Compos. Anal. 2024, 134, 106540. [Google Scholar] [CrossRef]
- El-Sharkawy, M.; Li, J.; Kamal, N.; Mahmoud, E.; El-Dein Omara, A.; Du, D. Assessing and Predicting Soil Quality in Heavy Metal-Contaminated Soils: Statistical and ANN-Based Techniques. J. Soil Sci. Plant Nutr. 2023, 23, 6510–6526. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, R.B.; Zhang, Y.C.; Li, G.X.; Liang, Q.F. Estimating freshness of carp based on EIS morphological characteristic. J. Food Eng. 2017, 193, 58–67. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, X.; Hou, B.; Hao, C.; Li, X.; Wu, J. Construction of a Lignosulfonate-Lysine Hydrogel for the Adsorption of Heavy Metal Ions. J. Agric. Food Chem. 2020, 68, 3050–3060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, Y.; Zou, X. Rapid determination of cadmium in rice using an all-solid RGO-enhanced light addressable potentiometric sensor. Food Chem. 2018, 261, 1–7. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, P.; Yosri, N.; Chen, Q.; Elseedi, H.R.; Zou, X.; Yang, H. Detection of Heavy Metals in Food and Agricultural Products by Surface-enhanced Raman Spectroscopy. Food Rev. Int. 2023, 39, 1440–1461. [Google Scholar] [CrossRef]
- Rajendran, S.; Priya, T.A.K.; Khoo, K.S.; Hoang, T.K.A.; Ng, H.-S.; Munawaroh, H.S.H.; Karaman, C.; Orooji, Y.; Show, P.L. A critical review on various remediation approaches for heavy metal contaminants removal from contaminated soils. Chemosphere 2022, 287, 132369. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, M.K.; Habibullah-Al-Mamun, M. Heavy Metals in Cereals and Pulses: Health Implications in Bangladesh. J. Agric. Food Chem. 2014, 62, 10828–10835. [Google Scholar] [CrossRef]
- Parvez, M.S.; Nawshin, S.; Sultana, S.; Hossain, M.S.; Rashid Khan, M.H.; Habib, M.A.; Nijhum, Z.T.; Khan, R. Evaluation of Heavy Metal Contamination in Soil Samples around Rampal, Bangladesh. ACS Omega 2023, 8, 15990–15999. [Google Scholar] [CrossRef]
- He, C.-T.; Wang, X.-S.; Hu, X.-X.; Yuan, J.; Zhang, Q.-H.; Tan, X.-T.; Wang, Y.-F.; Tan, X.; Yang, Z.-Y. Phytochelatin-Mediated Cultivar-Dependent Cd Accumulations of Lactuca sativa and Implication for Cd Pollution-Safe Cultivars Screening. J. Agric. Food Chem. 2024, 72, 715–725. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Schaufelberger, M.; Li, X.; Cao, Q.; Xiao, H.; Ren, Z. Role of Flavonol Synthesized by Nucleus FLS1 in Arabidopsis Resistance to Pb Stress. J. Agric. Food Chem. 2020, 68, 9646–9653. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, Z.; Deng, J.; Ding, Z.; Chen, Q. Quantitative detection of heavy metal Cd in vegetable oils: A nondestructive method based on Raman spectroscopy combined with chemometrics. J. Food Sci. 2024, 89, 8054–8065. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wei, M.; Wu, B.; Cheng, H.; Wang, C. Combined nitrogen deposition and Cd stress antagonistically affect the allelopathy of invasive alien species Canada goldenrod on the cultivated crop lettuce. Sci. Hortic. 2020, 261, 108955. [Google Scholar] [CrossRef]
- Wang, R.; Sang, P.; Guo, Y.; Jin, P.; Cheng, Y.; Yu, H.; Xie, Y.; Yao, W.; Qian, H. Cadmium in food: Source, distribution and removal. Food Chem. 2023, 405, 134666. [Google Scholar] [CrossRef]
- Alenyorege, E.A.; Ma, H.; Ayim, I.; Zhou, C. Ultrasound decontamination of pesticides and microorganisms in fruits and vegetables: A review. J. Food Saf. Food Qual.-Arch. Leb. 2018, 69, 80–91. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Wu, B.; Wang, S.; Wei, M.; Du, D.; Wang, C. Allelopathy of three Compositae invasive alien species on indigenous Lactuca sativa L. enhanced under Cu and Pb pollution. Sci. Hortic. 2020, 267, 109323. [Google Scholar] [CrossRef]
- Wang, C.; Wu, B.; Jiang, K.; Wei, M.; Wang, S. Effects of different concentrations and types of Cu and Pb on soil N-fixing bacterial communities in the wheat rhizosphere. Appl. Soil Ecol. 2019, 144, 51–59. [Google Scholar] [CrossRef]
- Rouhani, A.; Gutierrez, M.; Newton, R.A.; Al Souki, K.S. An overview of potentially toxic element pollution in soil around lead-zinc mining areas. Environ. Rev. 2025, 33, 1–16. [Google Scholar] [CrossRef]
- Alka, S.; Shahir, S.; Ibrahim, N.; Ndejiko, M.J.; Vo, D.-V.N.; Abd Manan, F. Arsenic removal technologies and future trends: A mini review. J. Clean. Prod. 2021, 278, 123805. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Costa, M. Arsenic: A Global Environmental Challenge. In Annual Review of Pharmacology and Toxicology; Insel, P.A., Ed.; Annual Reviews: San Mateo, CA, USA, 2021; Volume 61, pp. 47–63. [Google Scholar]
- Shaji, E.; Santosh, M.; Sarath, K.V.; Prakash, P.; Deepchand, V.; Divya, B.V. Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula. Geosci. Front. 2021, 12, 101079. [Google Scholar] [CrossRef]
- Cao, Y.; Huang, Y.; Zheng, J.; Chen, J.; Zeng, B.; Cheng, X.; Wu, C.; Wang, J.; Tang, J. Bipolar Photoelectrochemistry for Phase-Modulated Optoelectronic Hybrid Nanomotor. J. Am. Chem. Soc. 2024, 146, 17931–17939. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Q.; Jia, W.; Luan, Y. Synthesis and modification of MIL-101(Cr) and its applications in the adsorption of food hazard factors: A review. J. Food Compos. Anal. 2025, 146, 107871. [Google Scholar] [CrossRef]
- Hu, J.; Yun, X.; Zheng, Y.; Sun, T.; Song, L.; Pan, P.; Dong, T. Development of ultra-thin poly(L-lactic acid)-based films integrating toughness, barrier properties, and gas selectivity: Towards gas-permeation controllable green food packaging. Food Chem. 2024, 449, 139218. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, X.; Li, D.; Luan, G.; Hu, X.; Zhao, Z.; Fang, L. Multifunction hydrogen-bonded organic framework aerogel platform for detection and removal of heavy metal ions in pear juice. Food Chem. 2025, 485, 144483. [Google Scholar] [CrossRef]
- Fakayode, S.O.; Walgama, C.; Narcisse, V.E.F.; Grant, C. Electrochemical and Colorimetric Nanosensors for Detection of Heavy Metal Ions: A Review. Sensors 2023, 23, 9080. [Google Scholar] [CrossRef]
- Zhai, W.; You, T.; Ouyang, X.; Wang, M. Recent progress in mycotoxins detection based on surface-enhanced Raman spectroscopy. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1887–1909. [Google Scholar] [CrossRef]
- Yang, Y.; Li, G.; Wu, D.; Liu, J.; Li, X.; Luo, P.; Hu, N.; Wang, H.; Wu, Y. Recent advances on toxicity and determination methods of mycotoxins in foodstuffs. Trends Food Sci. Technol. 2020, 96, 233–252. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y.; Hu, J.; Chen, X.; Qiu, Y.; Shi, J.; Wang, G.; Xu, J. Isolation and characterization of Bacillus amyloliquefaciens MQ01, a bifunctional biocontrol bacterium with antagonistic activity against Fusarium graminearum and biodegradation capacity of zearalenone. Food Control 2021, 130, 108259. [Google Scholar] [CrossRef]
- Zhu, Q.; Fei, Y.-J.; Wu, Y.-B.; Luo, D.-L.; Chen, M.; Sun, K.; Zhang, W.; Dai, C.-C. Endophytic Fungus Reshapes Spikelet Microbiome to Reduce Mycotoxin Produced by Fusarium proliferatum through Altering Rice Metabolites. J. Agric. Food Chem. 2023, 71, 11350–11364. [Google Scholar] [CrossRef]
- Sobolev, V.; Walk, T.; Arias, R.; Massa, A.; Lamb, M. Inhibition of Aflatoxin Formation in Aspergillus Species by Peanut (Arachis hypogaea) Seed Stilbenoids in the Course of Peanut–Fungus Interaction. J. Agric. Food Chem. 2019, 67, 6212–6221. [Google Scholar] [CrossRef]
- Zachariasova, M.; Vaclavikova, M.; Lacina, O.; Vaclavik, L.; Hajslova, J. Deoxynivalenol Oligoglycosides: New “Masked” Fusarium Toxins Occurring in Malt, Beer, and Breadstuff. J. Agric. Food Chem. 2012, 60, 9280–9291. [Google Scholar] [CrossRef]
- Uegaki, R.; Tohno, M.; Yamamura, K.; Tsukiboshi, T. Changes in the Concentration of Fumonisins in Forage Rice during the Growing Period, Differences among Cultivars and Sites, and Identification of the Causal Fungus. J. Agric. Food Chem. 2014, 62, 3356–3362. [Google Scholar] [CrossRef]
- Wang, B.; Mahoney, N.E.; Pan, Z.; Khir, R.; Wu, B.; Ma, H.; Zhao, L. Effectiveness of pulsed light treatment for degradation and detoxification of aflatoxin B1 and B2 in rough rice and rice bran. Food Control 2016, 59, 461–467. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Z.; An, W.; Gao, B.; Li, C.; Han, B.; Tao, H.; Wang, J.; Wang, X.; Li, H. Bacillus subtilis Simultaneously Detoxified Aflatoxin B1 and Zearalenone. Appl. Sci. 2024, 14, 1589. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Cui, Y.; Hong, X.; Du, D. Using of Tyramine Signal Amplification to Improve the Sensitivity of ELISA for Aflatoxin B1 in Edible Oil Samples. Food Anal. Methods 2018, 11, 2553–2560. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, Y.; Li, M.; Garba, B.; Zhang, Q.; Wang, Y.; Zhang, H.; Li, P. Isolation and characterization of a Bacillus subtilis strain with aflatoxin B1 biodegradation capability. Food Control 2017, 75, 92–98. [Google Scholar] [CrossRef]
- Xie, G.; Zhu, M.; Liu, Z.; Zhang, B.; Shi, M.; Wang, S. Development and evaluation of the magnetic particle-based chemiluminescence immunoassay for rapid and quantitative detection of Aflatoxin B1 in foodstuff. Food Agric. Immunol. 2018, 29, 564–576. [Google Scholar] [CrossRef]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- You, F.; Wen, Z.; Yuan, R.; Qian, J.; Long, L.; Wang, K. Sensitive and stable detection of deoxynivalenol based on electrochemiluminescence aptasensor enhanced by 0D/2D homojunction effect in food analysis. Food Chem. 2023, 403, 134397. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, X.; Wang, J.; Wang, Q.; Meng, H.; Wang, Z. One-Step Core/Multishell Quantum Dots-Based Fluoroimmunoassay for Screening of Deoxynivalenol in Maize. Food Anal. Methods 2018, 11, 2569–2578. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, Y.; Hamadou, A.H.; Li, B.; Xu, B. Gentle debranning as a technology to reduce microbial and deoxynivalenol levels in common wheat (Triticum aestivum L.) and its application in milling industry. J. Cereal Sci. 2022, 107, 103518. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Mohan, K.; Rajan, D.K.; Pillay, A.A.; Palanisami, T.; Sathishkumar, P.; Conterno, L. Distribution, toxicity, interactive effects, and detection of ochratoxin and deoxynivalenol in food: A review. Food Chem. 2022, 378, 131978. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhao, L.; Gong, G.; Zhang, L.; Shi, L.; Dai, J.; Han, Y.; Wu, Y.; Khalil, M.M.; Sun, L. Invited review: Remediation strategies for mycotoxin control in feed. J. Anim. Sci. Biotechnol. 2022, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Gu, H.; Wang, S.; Ji, F.; He, C.; Jiang, C.; Shi, J.; Liu, X.; Shen, G.; Lee, Y.-W.; et al. A diverse Fusarium community is responsible for contamination of rice with a variety of Fusarium toxins. Food Res. Int. 2024, 195, 114987. [Google Scholar] [CrossRef]
- Hu, J.; Lv, H.; Hou, M.; Wang, G.; Lee, Y.-W.; Shi, J.; Gu, Z.; Xu, J. Preparative isolation and purification of B-type fumonisins by using macroporous resin column and high-speed countercurrent chromatography. Food Addit. Contam. Part A-Chem. Anal. Control Expo Risk Assess. 2020, 37, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Kang, X.; Su, J.; Qiu, J.; Liu, X.; Xu, J.; Shi, J.; Mohamed, S.R. Rapid detection of fumonisin B1 and B2 in ground corn samples using smartphone-controlled portable near-infrared spectrometry and chemometrics. Food Chem. 2022, 384, 132487. [Google Scholar] [CrossRef]
- Neme, K.; Mohammed, A. Mycotoxin occurrence in grains and the role of postharvest management as a mitigation strategies. A review. Food Control 2017, 78, 412–425. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens-the IARC Monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- Aladhadh, M. A Review of Modern Methods for the Detection of Foodborne Pathogens. Microorganisms 2023, 11, 1111. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Yuan, L.; Li, W.; Lin, L. Edible film incorporated with chitosan and Artemisia annua oil nanoliposomes for inactivation of Escherichia coli O157:H7 on cherry tomato. Int. J. Food Sci. Technol. 2017, 52, 687–698. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Rashed, M.M.A.; Lin, L. The antibacterial activity of clove oil/chitosan nanoparticles embedded gelatin nanofibers against Escherichia coli O157:H7 biofilms on cucumber. Int. J. Food Microbiol. 2018, 266, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Mi, F.; Hu, C.; Wang, Y.; Wang, L.; Peng, F.; Geng, P.; Guan, M. Recent advancements in microfluidic chip biosensor detection of foodborne pathogenic bacteria: A review. Anal. Bioanal. Chem. 2022, 414, 2883–2902. [Google Scholar] [CrossRef]
- Duan, N.; Chang, B.; Zhang, H.; Wang, Z.; Wu, S. Salmonella typhimurium detection using a surface-enhanced Raman scattering-based aptasensor. Int. J. Food Microbiol. 2016, 218, 38–43. [Google Scholar] [CrossRef]
- Xie, S.; Tao, X. Research progress of metal-organic framework-based fluorescence sensor in detection of veterinary antibiotics residues in food. Food Ferment. Ind. 2024, 50, 334–342,355. [Google Scholar]
- Välimaa, A.-L.; Tilsala-Timisjärvi, A.; Virtanen, E. Rapid detection and identification methods for Listeria monocytogenes in the food chain—A review. Food Control 2015, 55, 103–114. [Google Scholar] [CrossRef]
- Kumar Awasthi, M.; Chen, H.; Duan, Y.; Liu, T.; Kumar Awasthi, S.; Wang, Q.; Pandey, A.; Zhang, Z. An assessment of the persistence of pathogenic bacteria removal in chicken manure compost employing clay as additive via meta-genomic analysis. J. Hazard. Mater. 2019, 366, 184–191. [Google Scholar] [CrossRef]
- Selma, M.V.; Larrosa, M.; Beltrán, D.; Lucas, R.; Morales, J.C.; Tomás-Barberán, F.; Espín, J.C. Resveratrol and Some Glucosyl, Glucosylacyl, and Glucuronide Derivatives Reduce Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes Scott A Adhesion to Colonic Epithelial Cell Lines. J. Agric. Food Chem. 2012, 60, 7367–7374. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Fang, H.; Chang, S.; Ren, G.; Cheng, X.; Pan, Y.; Wu, R.; Liu, H.; Wu, J. Construction of Probiotic Double-Layered Multinucleated Microcapsules Based on Sulfhydryl-Modified Carboxymethyl Cellulose Sodium for Increased Intestinal Adhesion of Probiotics and Therapy for Intestinal Inflammation Induced by Escherichia coli O157:H7. ACS Appl. Mater. Interfaces 2023, 15, 18569–18589. [Google Scholar] [CrossRef]
- Chen, X.; Chang, Y.; Ye, M.; Wang, Z.; Wu, S.; Duan, N. Rational Design of a Robust G-Quadruplex Aptamer as an Inhibitor to Alleviate Listeria monocytogenes Infection. ACS Appl. Mater. Interfaces 2024, 16, 15946–15958. [Google Scholar] [CrossRef]
- Liu, D.; Abdellah, Y.A.Y.; Dou, T.; Keiblinger, K.M.; Zhou, Z.; Bhople, P.; Jiang, J.; Shi, X.; Zhang, F.; Yu, F.; et al. Livestock–Crop–Mushroom (LCM) Circular System: An Eco-Friendly Approach for Enhancing Plant Performance and Mitigating Microbiological Risks. Environ. Sci. Technol. 2025, 59, 8541–8554. [Google Scholar] [CrossRef]
- Zheng, L.; Cai, G.; Wang, S.; Liao, M.; Li, Y.; Lin, J. A microfluidic colorimetric biosensor for rapid detection of Escherichia coli 0157:H7 using gold nanoparticle aggregation and smart phone imaging. Biosens. Bioelectron. 2019, 124, 143–149. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, P.; Zhao, Y.; Ma, X. Enterotoxigenic Escherichia coli: Intestinal pathogenesis mechanisms and colonization resistance by gut microbiota. Gut Microbes 2022, 14, 2055943. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, Z.; Zheng, L.; Gong, Z.; Li, Y.; Jin, Y.; Huang, Y.; Chi, M. Urinary Tract Infections Caused by Uropathogenic Escherichia coli: Mechanisms of Infection and Treatment Options. Int. J. Mol. Sci. 2023, 24, 10537. [Google Scholar] [CrossRef] [PubMed]
- Eng, S.-K.; Pusparajah, P.; Ab Mutalib, N.-S.; Ser, H.-L.; Chan, K.-G.; Lee, L.-H. Salmonella: A review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci. 2015, 8, 284–293. [Google Scholar] [CrossRef]
- Liu, H.; Whitehouse, C.A.; Lis, B. Presence and Persistence of Salmonella in Water: The Impact on Microbial Quality of Water and Food Safety. Front. Public Health 2018, 6, 159. [Google Scholar] [CrossRef]
- Nair, D.V.T.; Venkitanarayanan, K.; Johny, A.K. Antibiotic-Resistant Salmonella in the Food Supply and the Potential Role of Antibiotic Alternatives for Control. Foods 2018, 7, 167. [Google Scholar] [CrossRef]
- Teklemariam, A.D.; Al-Hindi, R.R.; Albiheyri, R.S.; Alharbi, M.G.; Alghamdi, M.A.; Filimban, A.A.R.; Al Mutiri, A.S.; Al-Alyani, A.M.; Alseghayer, M.S.; Almaneea, A.M.; et al. Human Salmonellosis: A Continuous Global Threat in the Farm-to-Fork Food Safety Continuum. Foods 2023, 12, 1756. [Google Scholar] [CrossRef]
- Matle, I.; Mbatha, K.R.; Madoroba, E. A review of Listeria monocytogenes from meat and meat products: Epidemiology, virulence factors, antimicrobial resistance and diagnosis. Onderstepoort J. Vet. Res. 2020, 87, 20. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antimicrobial mechanism of clove oil on Listeria monocytogenes. Food Control 2018, 94, 140–146. [Google Scholar] [CrossRef]
- Zhu, Q.; Gooneratne, R.; Hussain, M.A. Listeria monocytogenes in Fresh Produce: Outbreaks, Prevalence and Contamination Levels. Foods 2017, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.Y.; Wu, J.; Lin, L. Inhibitory effect of liposome-entrapped lemongrass oil on the growth of Listeria monocytogenes in cheese. J. Dairy Sci. 2016, 99, 6097–6104. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Bacanli, M.G. The two faces of antibiotics: An overview of the effects of antibiotic residues in foodstuffs. Arch. Toxicol. 2024, 98, 1717–1725. [Google Scholar] [CrossRef]
- Chen, J.; Ying, G.-G.; Deng, W.-J. Antibiotic Residues in Food: Extraction, Analysis, and Human Health Concerns. J. Agric. Food Chem. 2019, 67, 7569–7586. [Google Scholar] [CrossRef]
- Ghimpeteanu, O.M.; Pogurschi, E.N.; Popa, D.C.; Dragomir, N.; Dragotoiu, T.; Mihai, O.D.; Petcu, C.D. Antibiotic Use in Livestock and Residues in Food-A Public Health Threat: A Review. Foods 2022, 11, 1430. [Google Scholar] [CrossRef]
- Gan, Z.; Hu, X.; Xu, X.; Zhang, W.; Zou, X.; Shi, J.; Zheng, K.; Arslan, M. A portable test strip based on fluorescent europium-based metal-organic framework for rapid and visual detection of tetracycline in food samples. Food Chem. 2021, 354, 129501. [Google Scholar] [CrossRef]
- Yang, W.; Cao, L.; Lu, H.; Huang, Y.; Yang, W.; Cai, Y.; Li, S.; Li, S.; Zhao, J.; Xu, W. Custom-printed microfluidic chips using simultaneous ratiometric fluorescence with “Green” carbon dots for detection of multiple antibiotic residues in pork and water samples. J. Food Sci. 2024, 89, 5980–5992. [Google Scholar] [CrossRef]
- Pal, S. A journey across the sequential development of macrolides and ketolides related to erythromycin. Tetrahedron 2006, 62, 3171–3200. [Google Scholar] [CrossRef]
- Hong, Y.-Q.; Guo, X.; Chen, G.-H.; Zhou, J.-W.; Zou, X.-M.; Liao, X.; Hou, T. Determination of five macrolide antibiotic residues in milk by micellar electrokinetic capillary chromatography with field amplified sample stacking. J. Food Saf. 2018, 38, e12382. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, Y.; Guo, L.; Xiao, Y.; Li, H.; Yang, P.; Xia, L.; Liu, X.; Chen, Z.; Li, L.; et al. Microbial Degradation of Tetracycline Antibiotics: Mechanisms and Environmental Implications. J. Agric. Food Chem. 2024, 72, 13523–13536. [Google Scholar] [CrossRef]
- Yang, Q.; Kaw, H.Y.; Yu, J.; Ma, X.; Yang, K.; Zhu, L.; Wang, W. Basic Nitrogenous Heterocyclic Rings at the 7-Position of Fluoroquinolones Foster Their Induction of Antibiotic Resistance in Escherichia coli. Environ. Sci. Technol. 2025, 59, 6787–6798. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Lian, L.; Yan, S.; Song, Y.; Nie, J.; Zhu, X.; Song, W. Comprehensive Understanding of the Phototransformation Process of Macrolide Antibiotics in Simulated Natural Waters. ACS ES&T Water 2021, 1, 938–948. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Y.; Ke, Y.; Chen, C.; Xie, S. A comprehensive review on biodegradation of tetracyclines: Current research progress and prospect. Sci. Total Environ. 2022, 814, 152852. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, H.; Xiong, P.; Zhu, Q.; Liao, C.; Jiang, G. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Sci. Total Environ. 2021, 753, 141975. [Google Scholar] [CrossRef]
- Bhatt, S.; Chatterjee, S. Fluoroquinolone antibiotics: Occurrence, mode of action, resistance, environmental detection, and remediation-A comprehensive review. Environ. Pollut. 2022, 315, 120440. [Google Scholar] [CrossRef]
- Bush, N.G.; Diez-Santos, I.; Abbott, L.R.; Maxwell, A. Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance. Molecules 2020, 25, 5662. [Google Scholar] [CrossRef]
- Dinos, G.P. The macrolide antibiotic renaissance. Br. J. Pharmacol. 2017, 174, 2967–2983. [Google Scholar] [CrossRef]
- Li, C.; Zhang, W.; Xu, X.; Zhou, L. Applications and Challenges of Fluorescent Probes for the Detection of Pesticide Residues in Food. J. Agric. Food Chem. 2025, 73, 4982–4997. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, J.; Yang, W.; Qiu, Q.; Liu, Q.; Li, J.; Wen, J.; Cheng, W.; Xia, X. A rapid, multiplexed, and naked-eye-readable paper assay for detecting heavy metal pollution in food using a catalytic colorimetric reaction. J. Dairy Sci. 2025, 108, 3172–3180. [Google Scholar] [CrossRef] [PubMed]
- Ayelign, A.; De Saeger, S. Mycotoxins in Ethiopia: Current status, implications to food safety and mitigation strategies. Food Control 2020, 113, 107163. [Google Scholar] [CrossRef]
- Calderon-Franco, D.; Corbera-Rubio, F.; Cuesta-Sanz, M.; Pieterse, B.; de Ridder, D.; van Loosdrecht, M.C.M.; van Halem, D.; Laureni, M.; Weissbrodt, D.G. Microbiome, resistome and mobilome of chlorine-free drinking water treatment systems. Water Res. 2023, 235, 119905. [Google Scholar] [CrossRef]
- Han, X.; Bai, L.; Luo, X.; Zhang, J.; Song, Y.; Liu, Z.; Li, N. Safety management status for genetically modified microorganism and related products used for food industry. Chin. J. Food Hyg. 2024, 36, 239–245. [Google Scholar]
- Hong, E.; Lee, S.Y.; Jeong, J.Y.; Park, J.M.; Kim, B.H.; Kwon, K.; Chun, H.S. Modern analytical methods for the detection of food fraud and adulteration by food category. J. Sci. Food Agric. 2017, 97, 3877–3896. [Google Scholar] [CrossRef]
- Yang, N.; Xie, L.-L.; Pan, C.; Yuan, M.-F.; Tao, Z.-H.; Mao, H.-P. A novel on-chip solution enabling rapid analysis of melamine and chloramphenicol in milk by smartphones. J. Food Process Eng. 2019, 42, e12976. [Google Scholar] [CrossRef]
- Tahir, H.E.; Zou, X.; Huang, X.; Shi, J.; Mariod, A.A. Discrimination of honeys using colorimetric sensor arrays, sensory analysis and gas chromatography techniques. Food Chem. 2016, 206, 37–43. [Google Scholar] [CrossRef]
- Moudgil, P.; Bedi, J.S.; Aulakh, R.S.; Gill, J.P.S.; Kumar, A. Validation of HPLC Multi-residue Method for Determination of Fluoroquinolones, Tetracycline, Sulphonamides and Chloramphenicol Residues in Bovine Milk. Food Anal. Methods 2019, 12, 338–346. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Angeloni, S.; Abouelenein, D.; Acquaticci, L.; Xiao, J.; Sagratini, G.; Maggi, F.; Vittori, S.; Caprioli, G. A new HPLC-MS/MS method for the simultaneous determination of 36 polyphenols in blueberry, strawberry and their commercial products and determination of antioxidant activity. Food Chem. 2022, 367, 130743. [Google Scholar] [CrossRef]
- Adunphatcharaphon, S.; Kolawole, O.; Sooksimuang, T.; Panchan, W.; Wasuthep, W.; Petdum, A.; Pichayawaytin, G.; Jintamethasawat, R.; Doljirapisit, N.; Somboonkaew, A.; et al. A multiplex microarray lateral flow immunoassay device for simultaneous determination of five mycotoxins in rice. npj Sci. Food 2024, 8, 116. [Google Scholar] [CrossRef]
- Gago-Ferrero, P.; Bletsou, A.A.; Damalas, D.E.; Aalizadeh, R.; Alygizakis, N.A.; Singer, H.P.; Hollender, J.; Thomaidis, N.S. Wide-scope target screening of >2000 emerging contaminants in wastewater samples with UPLC-Q-ToF-HRIVIS/MS and smart evaluation of its performance through the validation of 195 selected representative analytes. J. Hazard. Mater. 2020, 387, 121712. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, W.; Liu, Z.; Fu, X.; Du, D. High-performance liquid chromatography for the sensitive zearalenone determination by the automated immunomagnetic beads purifier for one-step sample pre-treatment. Eur. Food Res. Technol. 2022, 248, 109–117. [Google Scholar] [CrossRef]
- Scheijen, J.L.J.M.; Clevers, E.; Engelen, L.; Dagnelie, P.C.; Brouns, F.; Stehouwer, C.D.A.; Schalkwijk, C.G. Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: Presentation of a dietary AGE database. Food Chem. 2016, 190, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qin, D.; Wu, Z.; Sun, B.; Sun, X.; Huang, M.; Sun, J.; Zheng, F. Characterization of key aroma compounds in Chinese Guojing sesame-flavor Baijiu by means of molecular sensory science. Food Chem. 2019, 284, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Qi, X.; Lu, D.; Chen, B. Gas chromatography-ion mobility spectrometric classification of vegetable oils based on digital image processing. J. Food Meas. Charact. 2019, 13, 1973–1979. [Google Scholar] [CrossRef]
- Chen, T.; Liu, C.; Meng, L.; Lu, D.; Chen, B.; Cheng, Q. Early warning of rice mildew based on gas chromatography-ion mobility spectrometry technology and chemometrics. J. Food Meas. Charact. 2021, 15, 1939–1948. [Google Scholar] [CrossRef]
- Chen, T.; Li, H.; Chen, X.; Wang, Y.; Cheng, Q.; Qi, X. Construction and application of exclusive flavour fingerprints from fragrant rice based on gas chromatography—Ion mobility spectrometry (GC-IMS). Flavour Fragr. J. 2022, 37, 345–353. [Google Scholar] [CrossRef]
- Guo, X.; Schwab, W.; Ho, C.-T.; Song, C.; Wan, X. Characterization of the aroma profiles of oolong tea made from three tea cultivars by both GC-MS and GC-IMS. Food Chem. 2022, 376, 131933. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, Z.; Zhao, S.; Liu, J.; Gao, R.; Jiang, P. Characterization of volatile organic compounds of different pigmented rice after puffing based on gas chromatography-ion migration spectrometry and chemometrics. Food Res. Int. 2023, 169, 112879. [Google Scholar] [CrossRef]
- Liu, D.; Bai, L.; Feng, X.; Chen, Y.P.; Zhang, D.; Yao, W.; Zhang, H.; Chen, G.; Liu, Y. Characterization of Jinhua ham aroma profiles in specific to aging time by gas chromatography-ion mobility spectrometry (GC-IMS). Meat Sci. 2020, 168, 108178. [Google Scholar] [CrossRef]
- Wang, F.; Gao, Y.; Wang, H.; Xi, B.; He, X.; Yang, X.; Li, W. Analysis of volatile compounds and flavor fingerprint in Jingyuan lamb of different ages using gas chromatography-ion mobility spectrometry (GC-IMS). Meat Sci. 2021, 175, 108449. [Google Scholar] [CrossRef]
- Chen, M.; Chen, T.; Qi, X.; Lu, D.; Chen, B. Analyzing changes of volatile components in dried pork slice by gas chromatography-ion mobility spectroscopy. CyTA-J. Food 2020, 18, 328–335. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography-ion mobility spectrometry (GC-IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Cai, W.; Zhao, S.; Gao, R.; Jiang, P. Uncovering the differences in flavor volatiles of different colored foxtail millets based on gas chromatography-ion migration spectrometry and chemometrics. Curr. Res. Food Sci. 2023, 7, 100585. [Google Scholar] [CrossRef] [PubMed]
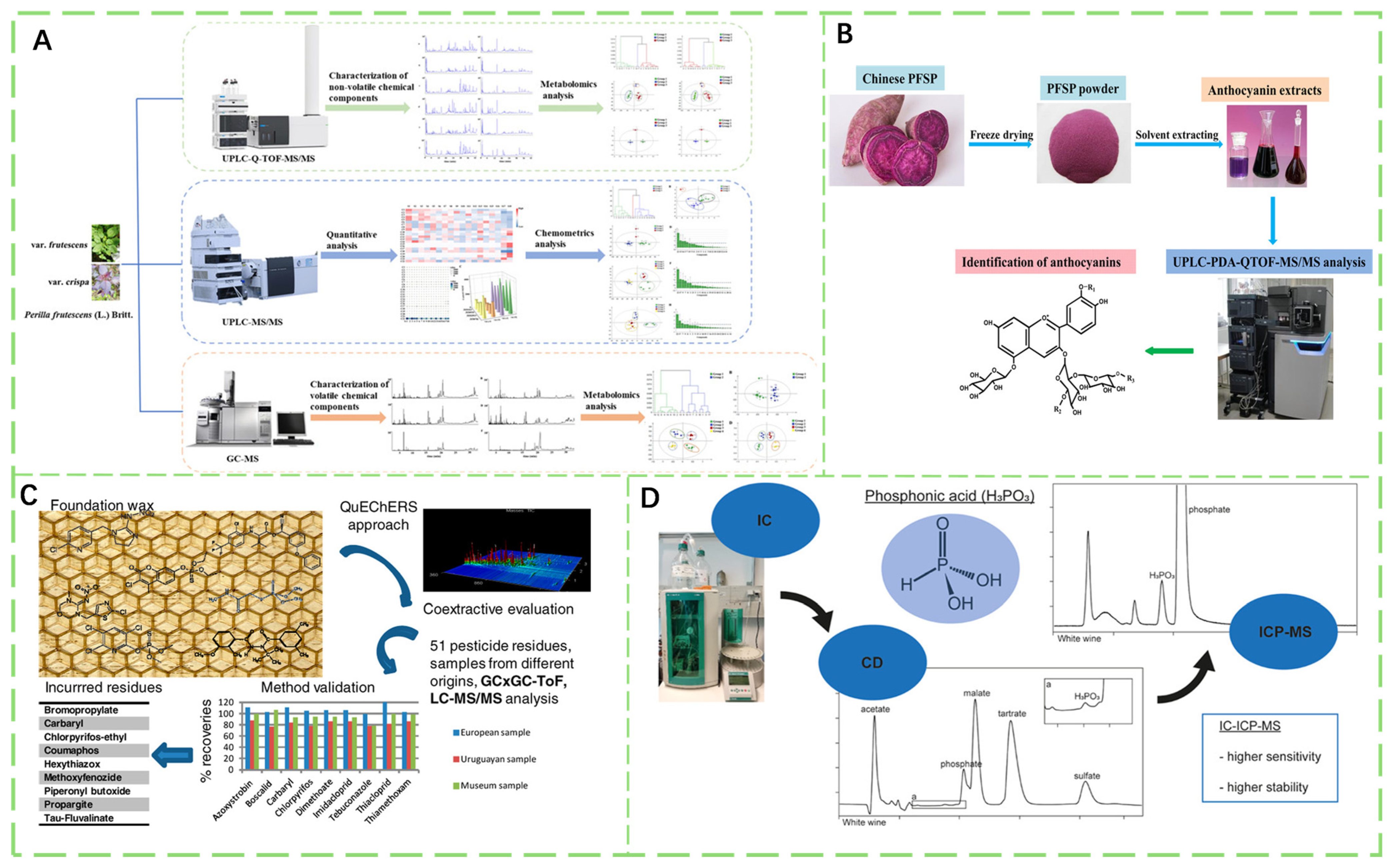
- Fan, Y.; Cao, X.; Zhang, M.; Wei, S.; Zhu, Y.; Ouyang, H.; He, J. Quantitative Comparison and Chemical Profile Analysis of Different Medicinal Parts of Perilla frutescens (L.) Britt. from Different Varieties and Harvest Periods. J. Agric. Food Chem. 2022, 70, 8838–8853. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zeng, M.; Chen, J.; Jiao, Y.; Niu, F.; Tao, G.; Zhang, S.; Qin, F.; He, Z. Identification and Quantitation of Anthocyanins in Purple-Fleshed Sweet Potatoes Cultivated in China by UPLC-PDA and UPLC-QTOF-MS/MS. J. Agric. Food Chem. 2016, 64, 171–177. [Google Scholar] [CrossRef]
- Niell, S.; Cesio, V.; Hepperle, J.; Doerk, D.; Kirsch, L.; Kolberg, D.; Scherbaum, E.; Anastassiades, M.; Heinzen, H. QuEChERS-Based Method for the Multiresidue Analysis of Pesticides in Beeswax by LC-MS/MS and GC×GC-TOF. J. Agric. Food Chem. 2014, 62, 3675–3683. [Google Scholar] [CrossRef]
- Otto, S.; May, B.; Schweiggert, R. Comparison of Ion Chromatography Conductivity Detection (IC-CD) and Ion Chromatography Inductively Coupled Plasma Mass Spectrometry (IC-ICP-MS) for the Determination of Phosphonic Acid in Grapevine Plant Parts, Wine, and Soil. J. Agric. Food Chem. 2022, 70, 10349–10358. [Google Scholar] [CrossRef]
- Guo, M.; Wang, K.; Lin, H.; Wang, L.; Cao, L.; Sui, J. Spectral data fusion in nondestructive detection of food products: Strategies, recent applications, and future perspectives. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13301. [Google Scholar] [CrossRef]
- Jiang, S.; Sun, J.; Xin, Z.; Mao, H.; Wu, X.; Li, Q. Visualizing distribution of pesticide residues in mulberry leaves using NIR hyperspectral imaging. J. Food Process Eng. 2017, 40, e12510. [Google Scholar] [CrossRef]
- Wu, X.; Liang, X.; Wang, Y.; Wu, B.; Sun, J. Non-Destructive Techniques for the Analysis and Evaluation of Meat Quality and Safety: A Review. Foods 2022, 11, 3713. [Google Scholar] [CrossRef]
- Zareef, M.; Arslan, M.; Hassan, M.M.; Ali, S.; Ouyang, Q.; Li, H.; Wu, X.; Hashim, M.M.; Javaria, S.; Chen, Q. Application of benchtop NIR spectroscopy coupled with multivariate analysis for rapid prediction of antioxidant properties of walnut (Juglans regia). Food Chem. 2021, 359, 129928. [Google Scholar] [CrossRef] [PubMed]
- Arslan, M.; Xiaobo, Z.; Shi, J.; Tahir, H.E.; Zareef, M.; Rakha, A.; Bilal, M. In situ prediction of phenolic compounds in puff dried Ziziphus jujuba Mill. using hand-held spectral analytical system. Food Chem. 2020, 331, 127361. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Lin, H.; Xu, S.; He, L. Recent Advances in Spectroscopic Techniques for the Analysis of Microplastics in Food. J. Agric. Food Chem. 2022, 70, 1410–1422. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Agyekum, A.A.; Kutsanedzie, F.Y.H.; Li, H.; Chen, Q.; Ouyang, Q.; Jiang, H. Qualitative and quantitative analysis of chlorpyrifos residues in tea by surface-enhanced Raman spectroscopy (SERS) combined with chemometric models. LWT-Food Sci. Technol. 2018, 97, 760–769. [Google Scholar] [CrossRef]
- Liu, R.; Ali, S.; Haruna, S.A.; Ouyang, Q.; Li, H.; Chen, Q. Development of a fluorescence sensing platform for specific and sensitive detection of pathogenic bacteria in food samples. Food Control 2022, 131, 108419. [Google Scholar] [CrossRef]
- Kurouski, D.; Van Duyne, R.P. In Situ Detection and Identification of Hair Dyes Using Surface-Enhanced Raman Spectroscopy (SERS). Anal. Chem. 2015, 87, 2901–2906. [Google Scholar] [CrossRef]
- Tahir, H.E.; Zou, X.; Li, Z.; Shi, J.; Xiaodong, Z.; Sheng, W.; Mariod, A.A. Rapid prediction of phenolic compounds and antioxidant activity of Sudanese honey using Raman and Fourier transform infrared (FT-IR) spectroscopy. Food Chem. 2017, 226, 202–211. [Google Scholar] [CrossRef]
- Chen, H.; Geng, D.; Chen, T.; Lu, D.; Chen, B. Second-derivative laser-induced fluorescence spectroscopy combined with chemometrics for authentication of the adulteration of camellia oil. Cyta-J. Food 2018, 16, 747–754. [Google Scholar] [CrossRef]
- Zhou, X.; Jun, S.; Yan, T.; Bing, L.; Hang, Y.; Quansheng, C. Hyperspectral technique combined with deep learning algorithm for detection of compound heavy metals in lettuce. Food Chem. 2020, 321, 126503. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, J.; Zhang, Y.; Tian, Y.; Yao, K.; Xu, M. Visualization of heavy metal cadmium in lettuce leaves based on wavelet support vector machine regression model and visible-near infrared hyperspectral imaging. J. Food Process Eng. 2021, 44, e13897. [Google Scholar] [CrossRef]
- Ma, L.; Yang, X.; Xue, S.; Zhou, R.; Wang, C.; Guo, Z.; Wang, Y.; Cai, J. “Raman plus X” dual-modal spectroscopy technology for food analysis: A review. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70102. [Google Scholar] [CrossRef]
- Jiang, L.; Hassan, M.M.; Ali, S.; Li, H.; Sheng, R.; Chen, Q. Evolving trends in SERS-based techniques for food quality and safety: A review. Trends Food Sci. Technol. 2021, 112, 225–240. [Google Scholar] [CrossRef]
- Abu Bakar, N.; Fronzi, M.; Shapter, J.G. Surface-Enhanced Raman Spectroscopy Using a Silver Nanostar Substrate for Neonicotinoid Pesticides Detection. Sensors 2024, 24, 373. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wang, M.; Barimah, A.O.; Chen, Q.; Li, H.; Shi, J.; El-Seedi, H.R.; Zou, X. Label-free surface enhanced Raman scattering spectroscopy for discrimination and detection of dominant apple spoilage fungus. Int. J. Food Microbiol. 2021, 338, 108990. [Google Scholar] [CrossRef] [PubMed]
- Jiao, T.; Hassan, M.M.; Zhu, J.; Ali, S.; Ahmad, W.; Wang, J.; Lv, C.; Chen, Q.; Li, H. Quantification of deltamethrin residues in wheat by Ag@ZnO NFs-based surface-enhanced Raman spectroscopy coupling chemometric models. Food Chem. 2021, 337, 127652. [Google Scholar] [CrossRef]
- Hu, X.; Shi, J.; Zhang, F.; Zou, X.; Holmes, M.; Zhang, W.; Huang, X.; Cui, X.; Xue, J. Determination of Retrogradation Degree in Starch by Mid-infrared and Raman Spectroscopy during Storage. Food Anal. Methods 2017, 10, 3694–3705. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, M.; Wu, J.; Tao, F.; Chen, Q.; Wang, Q.; Ouyang, Q.; Shi, J.; Zou, X. Quantitative assessment of zearalenone in maize using multivariate algorithms coupled to Raman spectroscopy. Food Chem. 2019, 286, 282–288. [Google Scholar] [CrossRef]
- Wu, Z.; Pu, H.; Sun, D.-W. Fingerprinting and tagging detection of mycotoxins in agri-food products by surface-enhanced Raman spectroscopy: Principles and recent applications. Trends Food Sci. Technol. 2021, 110, 393–404. [Google Scholar] [CrossRef]
- Zhu, A.; Ali, S.; Jiao, T.; Wang, Z.; Ouyang, Q.; Chen, Q. Advances in surface-enhanced Raman spectroscopy technology for detection of foodborne pathogens. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1466–1494. [Google Scholar] [CrossRef]
- Yang, T.; Zhao, B.; Kinchla, A.J.; Clark, J.M.; He, L. Investigation of Pesticide Penetration and Persistence on Harvested and Live Basil Leaves Using Surface-Enhanced Raman Scattering Mapping. J. Agric. Food Chem. 2017, 65, 3541–3550. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, H.; Zou, X.; Meng, G.; Wu, N. Raman spectroscopy for food quality assurance and safety monitoring: A review. Curr. Opin. Food Sci. 2022, 47, 100910. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Y.; Zhao, J.; Hui, Z. Nondestructive measurement of total volatile basic nitrogen (TVB-N) content in salted pork in jelly using a hyperspectral imaging technique combined with efficient hypercube processing algorithms. Anal. Methods 2013, 5, 6382–6388. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, S.; Mao, H.; Wu, X.; Li, Q. Classification of Black Beans Using Visible and Near Infrared Hyperspectral Imaging. Int. J. Food Prop. 2016, 19, 1687–1695. [Google Scholar] [CrossRef]
- Shen, G.; Cao, Y.; Yin, X.; Dong, F.; Xu, J.; Shi, J.; Lee, Y.-W. Rapid and nondestructive quantification of deoxynivalenol in individual wheat kernels using near-infrared hyperspectral imaging and chemometrics. Food Control 2022, 131, 108420. [Google Scholar] [CrossRef]
- Sun, J.; Nirere, A.; Dusabe, K.D.; Zhong, Y.; Adrien, G. Rapid and nondestructive watermelon (Citrullus lanatus) seed viability detection based on visible near-infrared hyperspectral imaging technology and machine learning algorithms. J. Food Sci. 2024, 89, 4403–4418. [Google Scholar] [CrossRef]
- Cao, Y.; Li, H.; Sun, J.; Zhou, X.; Yao, K.; Nirere, A. Nondestructive determination of the total mold colony count in green tea by hyperspectral imaging technology. J. Food Process Eng. 2020, 43, e13570. [Google Scholar] [CrossRef]
- Fu, L.; Sun, J.; Wang, S.; Xu, M.; Yao, K.; Cao, Y.; Tang, N. Identification of maize seed varieties based on stacked sparse autoencoder and near-infrared hyperspectral imaging technology. J. Food Process Eng. 2022, 45, e14120. [Google Scholar] [CrossRef]
- Sezer, B.; Bilge, G.; Boyaci, I.H. Laser-Induced Breakdown Spectroscopy Based Protein Assay for Cereal Samples. J. Agric. Food Chem. 2016, 64, 9459–9463. [Google Scholar] [CrossRef]
- Xu, M.-L.; Gao, Y.; Han, X.X.; Zhao, B. Detection of Pesticide Residues in Food Using Surface-Enhanced Raman Spectroscopy: A Review. J. Agric. Food Chem. 2017, 65, 6719–6726. [Google Scholar] [CrossRef]
- Lee, K.-M.; Herrman, T.J.; Bisrat, Y.; Murray, S.C. Feasibility of Surface-Enhanced Raman Spectroscopy for Rapid Detection of Aflatoxins in Maize. J. Agric. Food Chem. 2014, 62, 4466–4474. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hierro, J.M.; Nogales-Bueno, J.; Rodríguez-Pulido, F.J.; Heredia, F.J. Feasibility Study on the Use of Near-Infrared Hyperspectral Imaging for the Screening of Anthocyanins in Intact Grapes during Ripening. J. Agric. Food Chem. 2013, 61, 9804–9809. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Tan, J.; Li, Y. Application of Electrospun Nanofiber-Based Electrochemical Sensors in Food Safety. Molecules 2024, 29, 4412. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Wei, W.; Jiang, L.; Zhu, F.; Du, D. Use of Carbon Nanotubes as a Solid Support To Establish Quantitative (Centrifugation) and Qualitative (Filtration) Immunoassays To Detect Gentamicin Contamination in Commercial Milk. J. Agric. Food Chem. 2016, 64, 7874–7881. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Penalver-Soler, R.; Campillo, N.; Vinas, P. Dispersive Solid-Phase Extraction Using Magnetic Carbon Nanotube Composite for the Determination of Emergent Mycotoxins in Urine Samples. Toxins 2020, 12, 51. [Google Scholar] [CrossRef]
- Song, S.-H.; Gao, Z.-F.; Guo, X.; Chen, G.-H. Aptamer-Based Detection Methodology Studies in Food Safety. Food Anal. Methods 2019, 12, 966–990. [Google Scholar] [CrossRef]
- Lu, C.; Luo, S.; Wang, X.; Li, J.; Li, Y.; Shen, Y.; Wang, J. Illuminating the nanomaterials triggered signal amplification in electrochemiluminescence biosensors for food safety: Mechanism and future perspectives. Coord. Chem. Rev. 2024, 501, 215571. [Google Scholar] [CrossRef]
- Li, Y.; Luo, S.; Sun, L.; Kong, D.; Sheng, J.; Wang, K.; Dong, C. A Green, Simple, and Rapid Detection for Amaranth in Candy Samples Based on the Fluorescence Quenching of Nitrogen-Doped Graphene Quantum Dots. Food Anal. Methods 2019, 12, 1658–1665. [Google Scholar] [CrossRef]
- Hosseini, H.A.; Sadat-Barati, M.; Feizy, J. Synthesis of GO-SiO2/ZnO/Fe3O4 nano adsorbent for preconcentration of aflatoxins in food samples using SPE-HPLC-FLD method. Food Chem. 2025, 470, 142264. [Google Scholar] [CrossRef]
- Li, C.; Zhu, L.; Yang, W.; He, X.; Zhao, S.; Zhang, X.; Tang, W.; Wang, J.; Yue, T.; Li, Z. Amino-Functionalized Al-MOF for Fluorescent Detection of Tetracyclines in Milk. J. Agric. Food Chem. 2019, 67, 1277–1283. [Google Scholar] [CrossRef]
- Zhou, J.-W.; Zou, X.-M.; Song, S.-H.; Chen, G.-H. Quantum Dots Applied to Methodology on Detection of Pesticide and Veterinary Drug Residues. J. Agric. Food Chem. 2018, 66, 1307–1319. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Li, Q.; Mao, S. Fluorescence analysis of antibiotics and antibiotic-resistance genes in the environment: A mini review. Chin. Chem. Lett. 2024, 35, 109541. [Google Scholar] [CrossRef]
- Zhou, X.; Pan, W.; Li, N.; Salah, M.; Guan, S.; Li, X.; Wang, Y. Development of a Sensitive Monoclonal Antibody-Based Colloidal Gold Immunochromatographic Strip for Lomefloxacin Detection in Meat Products. Foods 2024, 13, 2550. [Google Scholar] [CrossRef] [PubMed]
- Jansing, J.; Sack, M.; Augustine, S.M.; Fischer, R.; Bortesi, L. CRISPR/Cas9-mediated knockout of six glycosyltransferase genes in Nicotiana benthamiana for the production of recombinant proteins lacking β-1,2-xylose and core α-1,3-fucose. Plant Biotechnol. J. 2019, 17, 350–361. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, P.; Liu, B.; Liu, Y.; Wang, Y.; Tao, T.; Xu, J.; Hammock, B.D.; Liu, X.; Guan, R.; et al. Phage-displayed nanobody based double antibody sandwich chemiluminescent immunoassay for the detection of Cry2A toxin in cereals. Food Agric. Immunol. 2019, 30, 924–936. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, H.; Han, Y.; Yu, F.; Shi, X. Development of a biomimetic enzyme-linked immunosorbent assay based on molecularly imprinted polymers on paper for the detection of carbaryl. Food Chem. 2018, 240, 893–897. [Google Scholar] [CrossRef]
- Jiao, S.; Xie, X.; He, Z.; Sun, Z.; Wang, Z.; Zhang, S.; Cao, H.; Hammock, B.D.; Liu, X. Lateral Flow Immunochromatographic Assay for Competitive Detection of Crustacean Allergen Tropomyosin Using Phage-Displayed Shark Single-Domain Antibody. J. Agric. Food Chem. 2024, 72, 1811–1821. [Google Scholar] [CrossRef]
- Verhoeckx, K.C.M.; Vissers, Y.M.; Baumert, J.L.; Faludi, R.; Feys, M.; Flanagan, S.; Herouet-Guicheney, C.; Holzhauser, T.; Shimojo, R.; van der Bolt, N.; et al. Food processing and allergenicity. Food Chem. Toxicol. 2015, 80, 223–240. [Google Scholar] [CrossRef]
- Xu, L.; Abd El-Aty, A.M.; Eun, J.-B.; Shim, J.-H.; Zhao, J.; Lei, X.; Gao, S.; She, Y.; Jin, F.; Wang, J.; et al. Recent Advances in Rapid Detection Techniques for Pesticide Residue: A Review. J. Agric. Food Chem. 2022, 70, 13093–13117. [Google Scholar] [CrossRef]
- Centeno, E.R.; Johnson, W.J.; Sehon, A.H. Antibodies to two common pesticides, DDT and malathion. Int. Arch. Allergy Appl. Immunol. 1970, 37, 1–13. [Google Scholar] [CrossRef]
- Kaufman, B.M.; Clower, M., Jr. Immunoassay of pesticides. J.-Assoc. Off. Anal. Chem. 1991, 74, 239–247. [Google Scholar] [CrossRef]
- Schneider, P.; Hammock, B.D. Influence of the ELISA format and the hapten-enzyme conjugate on the sensitivity of an immunoassay for S-triazine herbicides using monoclonal antibodies. J. Agric. Food Chem. 1992, 40, 525–530. [Google Scholar] [CrossRef]
- Van Emon, J.M.; Lopez-Avila, V. Immunochemical methods for environmental analysis. Anal. Chem. 1992, 64, 78A–88A. [Google Scholar] [CrossRef]
- Engvall, E.; Perlmann, P. Enzyme-linked immunosorbent assay (ELISA) quantitative assay of immunoglobulin G. Immunochemistry 1971, 8, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Soini, E.; Kojola, H. Time-resolved fluorometer for lanthanide chelates—A new generation of nonisotopic immunoassays. Clin. Chem. 1983, 29, 65–68. [Google Scholar] [CrossRef]
- Dandliker, W.B.; Halbert, S.P.; Florin, M.C.; Alonso, R.; Schapiro, H.C. Study of penicillin antibodies by fluorescence polarization and immunodiffusion. J. Exp. Med. 1965, 122, 1029–1048. [Google Scholar] [CrossRef]
- Bruchez, M., Jr.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor nanocrystals as fluorescent biological labels. Science 1998, 281, 2013–2016. [Google Scholar] [CrossRef]
- Chan, W.C.; Nie, S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 1998, 281, 2016–2018. [Google Scholar] [CrossRef]
- Nam, J.M.; Thaxton, C.S.; Mirkin, C.A. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science 2003, 301, 1884–1886. [Google Scholar] [CrossRef]
- Cao, J.; Wang, M.; Yu, H.; She, Y.; Cao, Z.; Ye, J.; Abd El-Aty, A.M.; Hacımüftüoğlu, A.; Wang, J.; Lao, S. An Overview on the Mechanisms and Applications of Enzyme Inhibition-Based Methods for Determination of Organophosphate and Carbamate Pesticides. J. Agric. Food Chem. 2020, 68, 7298–7315. [Google Scholar] [CrossRef]
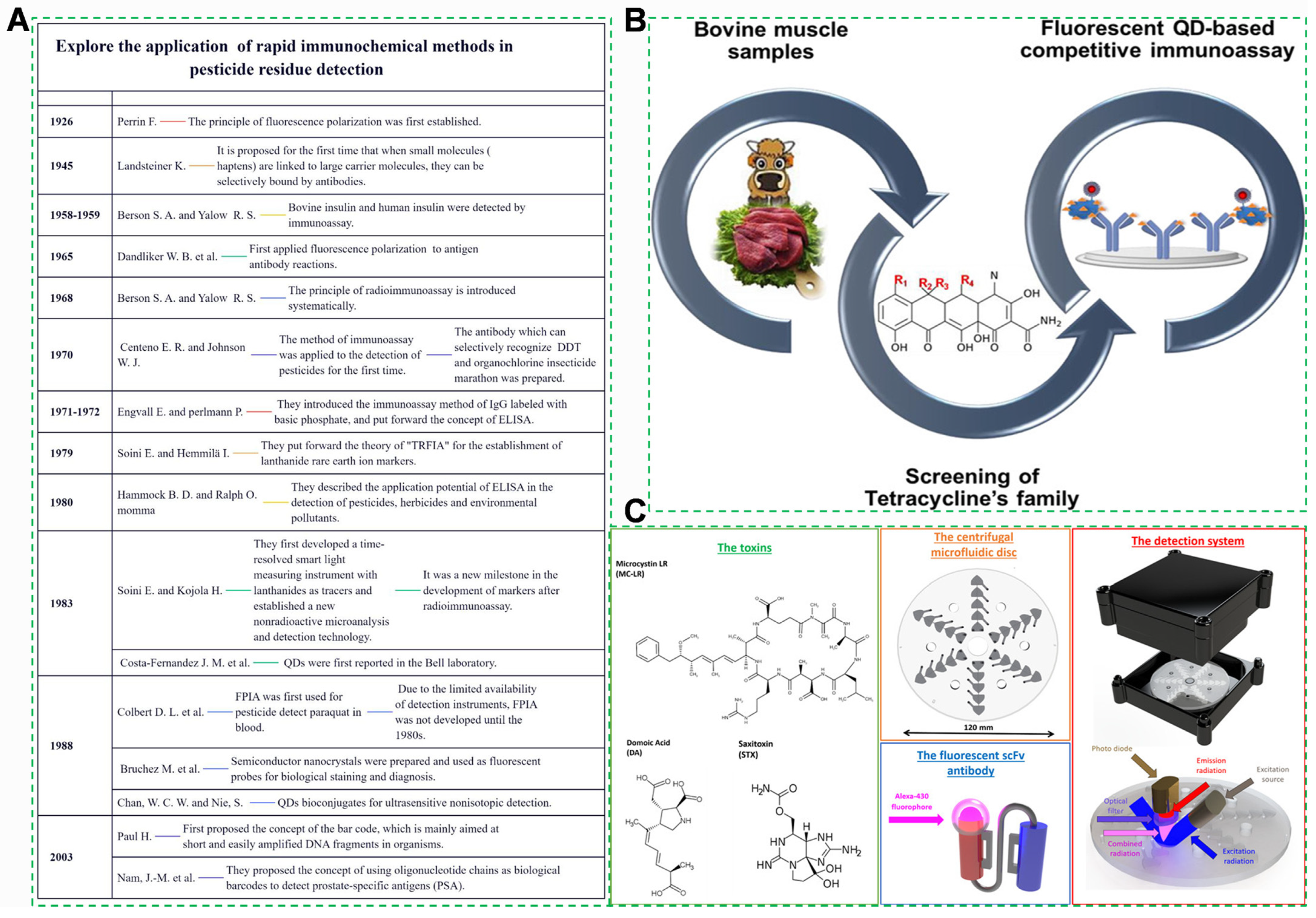
- García-Fernández, J.; Trapiella-Alfonso, L.; Costa-Fernández, J.M.; Pereiro, R.; Sanz-Medel, A. A Quantum Dot-Based Immunoassay for Screening of Tetracyclines in Bovine Muscle. J. Agric. Food Chem. 2014, 62, 1733–1740. [Google Scholar] [CrossRef] [PubMed]
- Maguire, I.; Fitzgerald, J.; Heery, B.; Nwankire, C.; O’Kennedy, R.; Ducrée, J.; Regan, F. Novel Microfluidic Analytical Sensing Platform for the Simultaneous Detection of Three Algal Toxins in Water. ACS Omega 2018, 3, 6624–6634. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.-Y.; Yu, C.-Y.; Ku, C.-A.; Chung, C.-K. Design, Fabrication, and Applications of SERS Substrates for Food Safety Detection: Review. Micromachines 2023, 14, 1343. [Google Scholar] [CrossRef] [PubMed]
- Pandiselvam, R.; Aydar, A.Y.; Ozbek, Z.A.; Atik, D.S.; Sufer, O.; Taskin, B.; Olum, E.; Ramniwas, S.; Rustagi, S.; Cozzolino, D. Farm to fork applications: How vibrational spectroscopy can be used along the whole value chain? Crit. Rev. Biotechnol. 2025, 45, 938–981. [Google Scholar] [CrossRef]
- Li, W.; Xu, Z.; He, Q.; Pan, J.; Zhang, Y.; El-Sheikh, E.-S.A.; Hammock, B.D.; Li, D. Nanobody-Based Immunoassays for the Detection of Food Hazards-A Review. Biosensors 2025, 15, 183. [Google Scholar] [CrossRef]
- Qu, L.; Zhang, X.; Chu, Y.; Zhang, Y.; Lin, Z.; Kong, F.; Ni, X.; Zhao, Y.; Lu, Q.; Zou, B. Research Progress on Nanotechnology-Driven Enzyme Biosensors for Electrochemical Detection of Biological Pollution and Food Contaminants. Foods 2025, 14, 1254. [Google Scholar] [CrossRef]
- Sun, J.; Lu, X.; Mao, H.; Wu, X.; Gao, H. Quantitative Determination of Rice Moisture Based on Hyperspectral Imaging Technology and BCC-LS-SVR Algorithm. J. Food Process Eng. 2017, 40, e12446. [Google Scholar] [CrossRef]
- Yang, N.; Zhou, X.; Yu, D.; Jiao, S.; Han, X.; Zhang, S.; Yin, H.; Mao, H. Pesticide residues identification by impedance time-sequence spectrum of enzyme inhibition on multilayer paper-based microfluidic chip. J. Food Process Eng. 2020, 43, e13544. [Google Scholar] [CrossRef]
- Cai, Y.; Cao, L.; Cai, H.; Yang, W.; Lu, H.; Adila, A.; Zhang, B.; Cao, Y.; Huang, W.; Xu, W.; et al. A rapid microfluidic paper-based chip sensor using ratiometric fluorescence and molecularly imprinted polymers for visual detection of sulfadiazine in actual samples. J. Food Compos. Anal. 2025, 139, 107108. [Google Scholar] [CrossRef]
- Qin, P.; Park, M.; Alfson, K.J.; Tamhankar, M.; Carrion, R.; Patterson, J.L.; Griffiths, A.; He, Q.; Yildiz, A.; Mathies, R.; et al. Rapid and Fully Microfluidic Ebola Virus Detection with CRISPR-Cas13a. Acs Sens. 2019, 4, 1048–1054. [Google Scholar] [CrossRef]
- Liu, D.; Liu, C.; Yuan, Y.; Zhang, X.; Huang, Y.; Yan, S. Microfluidic Transport of Hybrid Optoplasmonic Particles for Repeatable SERS Detection. Anal. Chem. 2021, 93, 10672–10678. [Google Scholar] [CrossRef]
- Wang, Y.; Salazar, J.K. Culture-Independent Rapid Detection Methods for Bacterial Pathogens and Toxins in Food Matrices. Compr. Rev. Food Sci. Food Saf. 2016, 15, 183–205. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Zhang, W.; Li, N.; Pu, Q.; Lin, J.-M. Recent progress on microfluidic biosensors for rapid detection of pathogenic bacteria. Chin. Chem. Lett. 2022, 33, 1743–1751. [Google Scholar] [CrossRef]
- Lee, W.; Kim, H.; Kang, Y.; Lee, Y.; Yoon, Y. A Biosensor Platform for Metal Detection Based on Enhanced Green Fluorescent Protein. Sensors 2019, 19, 1846. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Nam, S.; Jeung, K.; Noh, M.H.; Jung, G.Y. Biosensor-Assisted Engineering for Diverse Microbial Cellular Physiologies. J. Agric. Food Chem. 2024, 72, 18321–18334. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Deng, H.; Zhou, S.; Wu, Y.; Li, Y. Fast and bioluminescent detection of antibiotic contaminants by on-demand transcription of RNA scaffold arrays. Anal. Chim. Acta 2023, 1273, 341538. [Google Scholar] [CrossRef]
- Qiu, H.; Gao, L.; Wang, J.; Pan, J.; Yan, Y.; Zhang, X. A precise and efficient detection of Seta-Cyfluthrin via fluorescent molecularly imprinted polymers with ally fluorescein as functional monomer in agricultural products. Food Chem. 2017, 217, 620–627. [Google Scholar] [CrossRef]
- Li, G.; Qi, X.; Wu, J.; Wan, X.; Wang, T.; Liu, Y.; Chen, Y.; Xia, Y. Highly stable electrochemical sensing platform for the selective determination of pefloxacin in food samples based on a molecularly imprinted-polymer-coated gold nanoparticle/black phosphorus nanocomposite. Food Chem. 2024, 436, 137753. [Google Scholar] [CrossRef]
- Ozdemir, N.; Karslioglu, B.; Yola, B.B.; Atar, N.; Yola, M.L. A Novel Molecularly Imprinted Quartz Crystal Microbalance Sensor Based on Erbium Molybdate Incorporating Sulfur-Doped Graphitic Carbon Nitride for Dimethoate Determination in Apple Juice Samples. Foods 2024, 13, 810. [Google Scholar] [CrossRef]
- Pan, M.; Sun, J.; Wang, Y.; Yang, J.; Wang, Z.; Li, L.; Wang, S. Carbon-dots encapsulated luminescent metal-organic frameworks@surface molecularly imprinted polymer: A facile fluorescent probe for the determination of chloramphenicol. Food Chem. 2024, 442, 138461. [Google Scholar] [CrossRef]
- Yu, X.; Yang, Y.; Shen, Q.; Sun, Y.; Kang, Q.; Shen, D. A novel differential ratiometric molecularly imprinted electrochemical sensor for determination of sulfadiazine in food samples. Food Chem. 2024, 434, 137461. [Google Scholar] [CrossRef] [PubMed]
- Sambasivam, P.; Bilkiss, M.; Soda, N.; Bar, I.; Shiddiky, M.J.A.; Ford, R. A Rapid Electrochemical Biosensor Diagnostic for Botrytis ssp. Causing Botrytis Gray Mold of Temperate Legumes. ACS Agric. Sci. Technol. 2024, 4, 1184–1193. [Google Scholar] [CrossRef]
- Peng, W.; Yi, C.; Wang, L.; Zhang, Y.; Liao, Q. 3D Porous Silicon Carbide SERS Microfluidic Chip for Pesticide Residue Detection. ACS Agric. Sci. Technol. 2024, 4, 818–826. [Google Scholar] [CrossRef]
- Li, Y.; Reed, M.; Wright, H.T.; Cropp, T.A.; Williams, G.J. Development of Genetically Encoded Biosensors for Reporting the Methyltransferase-Dependent Biosynthesis of Semisynthetic Macrolide Antibiotics. ACS Synth. Biol. 2021, 10, 2520–2531. [Google Scholar] [CrossRef]
- Nagabooshanam, S.; Roy, S.; Deshmukh, S.; Wadhwa, S.; Sulania, I.; Mathur, A.; Krishnamurthy, S.; Bharadwaj, L.M.; Roy, S.S. Microfluidic Affinity Sensor Based on a Molecularly Imprinted Polymer for Ultrasensitive Detection of Chlorpyrifos. ACS Omega 2020, 5, 31765–31773. [Google Scholar] [CrossRef]
- Xu, Y.; Hassan, M.M.; Sharma, A.S.; Li, H.; Chen, Q. Recent advancement in nano-optical strategies for detection of pathogenic bacteria and their metabolites in food safety. Crit. Rev. Food Sci. Nutr. 2023, 63, 486–504. [Google Scholar] [CrossRef]
- Zou, Y.; Shi, Y.; Wang, T.; Ji, S.; Zhang, X.; Shen, T.; Huang, X.; Xiao, J.; Farag, M.A.; Shi, J.; et al. Quantum dots as advanced nanomaterials for food quality and safety applications: A comprehensive review and future perspectives. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13339. [Google Scholar] [CrossRef]
- Li, H.; Sheng, W.; Haruna, S.A.; Hassan, M.M.; Chen, Q. Recent advances in rare earth ion-doped upconversion nanomaterials: From design to their applications in food safety analysis. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3732–3764. [Google Scholar] [CrossRef]
- Sharma, A.S.; Ali, S.; Sabarinathan, D.; Murugavelu, M.; Li, H.; Chen, Q. Recent progress on graphene quantum dots-based fluorescence sensors for food safety and quality assessment applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5765–5801. [Google Scholar] [CrossRef]
- Han, B.; Rupam, T.H.; Chakraborty, A.; Saha, B.B. A comprehensive review on VOCs sensing using different functional materials: Mechanisms, modifications, challenges and opportunities. Renew. Sustain. Energy Rev. 2024, 196, 114365. [Google Scholar] [CrossRef]
- Wongkaew, N.; Simsek, M.; Griesche, C.; Baeumner, A.J. Functional Nanomaterials and Nanostructures Enhancing Electrochemical Biosensors and Lab-on-a-Chip Performances: Recent Progress, Applications, and Future Perspective. Chem. Rev. 2019, 119, 120–194. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ouyang, Q.; Li, H.; Chen, M.; Zhan, Z.; Chen, Q. Turn-On Fluoresence Sensor for Hg2+ in Food Based on FRET between Aptamers-Functionalized Upconversion Nanoparticles and Gold Nanoparticles. J. Agric. Food Chem. 2018, 66, 6188–6195. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Feng, J.; Gao, X.; Zhang, H. Nanocomposites based on lanthanide-doped upconversion nanoparticles: Diverse designs and applications. Light-Sci. Appl. 2022, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ahmad, W.; Rong, Y.; Chen, Q.; Zuo, M.; Ouyang, Q.; Guo, Z. Designing an aptamer based magnetic and upconversion nanoparticles conjugated fluorescence sensor for screening Escherichia coli in food. Food Control 2020, 107, 106761. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zhang, D.; Tan, W.; Shi, J.; Li, Z.; Liu, H.; Yu, Y.; Yang, L.; Wang, X.; et al. A fluorescence resonance energy transfer probe based on functionalized graphene oxide and upconversion nanoparticles for sensitive and rapid detection of zearalenone. LWT-Food Sci. Technol. 2021, 147, 111541. [Google Scholar] [CrossRef]
- Li, X.; Zhang, F.; Zhao, D. Lab on upconversion nanoparticles: Optical properties and applications engineering via designed nanostructure. Chem. Soc. Rev. 2015, 44, 1346–1378. [Google Scholar] [CrossRef]
- Rong, Y.; Ali, S.; Ouyang, Q.; Wang, L.; Li, H.; Chen, Q. Development of a bimodal sensor based on upconversion nanoparticles and surface-enhanced Raman for the sensitive determination of dibutyl phthalate in food. J. Food Compos. Anal. 2021, 100, 103929. [Google Scholar] [CrossRef]
- Ouyang, Q.; Wang, L.; Ahmad, W.; Rong, Y.; Li, H.; Hu, Y.; Chen, Q. A highly sensitive detection of carbendazim pesticide in food based on the upconversion-MnO2 luminescent resonance energy transfer biosensor. Food Chem. 2021, 349, 129157. [Google Scholar] [CrossRef]
- Yin, L.; Hu, X.; Hao, M.; Shi, J.; Zou, X.; Dusabe, K.D. Upconversion nanoparticles-based background-free selective fluorescence sensor developed for immunoassay of fipronil pesticide. J. Food Meas. Charact. 2023, 17, 3125–3133. [Google Scholar] [CrossRef]
- Zhang, B.; Li, H.; Pan, W.; Chen, Q.; Ouyang, Q.; Zhao, J. Dual-Color Upconversion Nanoparticles (UCNPs)-Based Fluorescent Immunoassay Probes for Sensitive Sensing Foodborne Pathogens. Food Anal. Methods 2017, 10, 2036–2045. [Google Scholar] [CrossRef]
- Li, S.; Wu, J.; Zhang, S.; Jiao, T.; Wei, J.; Chen, X.; Chen, Q.; Chen, Q. Inner filter effect-based upconversion nanosensor for rapid detection of thiram pesticides using upconversion nanoparticles and dithizone-cadmium—Cadmium complexes. Food Chem. 2024, 434, 137438. [Google Scholar] [CrossRef]
- Wang, X.; Valiev, R.R.; Ohulchanskyy, T.Y.; Agren, H.; Yang, C.; Chen, G. Dye-sensitized lanthanide-doped upconversion nanoparticles. Chem. Soc. Rev. 2017, 46, 4150–4167. [Google Scholar] [CrossRef]
- Zhang, Y.; Hassan, M.M.; Rong, Y.; Liu, R.; Li, H.; Ouyang, Q.; Chen, Q. An upconversion nanosensor for rapid and sensitive detection of tetracycline in food based on magnetic-field-assisted separation. Food Chem. 2022, 373, 131497. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Yin, X.; Wang, S.; Tian, Y.; Shu, R.; Jia, C.; Chen, Y.; Sun, J.; Zhang, D.; et al. Nature-inspired nanozymes as signal markers for in-situ signal amplification strategy: A portable dual-colorimetric immunochromatographic analysis based on smartphone. Biosens. Bioelectron. 2022, 210, 114289. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Zhao, Y.; Zhou, Y.; Yi, Y.; Weng, W.; Zhu, G. Colorimetric detection of organophosphorus pesticides based on Nb2CTx MXene self-reducing PdPt nanozyme integrated with hydrogel and smartphone. J. Food Meas. Charact. 2024, 18, 9223–9232. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, R.; Fan, K.; Gao, L.; Yan, X. Exploring the Specificity of Nanozymes. ACS Nano 2024, 18, 2533–2540. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, S.; De Luca, L.; Gaspa, S.; Mariani, A.; Garroni, S.; Iacomini, A.; Stagi, L.; Innocenzi, P.; Malfatti, L. Comparative Evaluation of Graphene Nanostructures in GERS Platforms for Pesticide Detection. ACS Omega 2022, 7, 5670–5678. [Google Scholar] [CrossRef]
- Wang, Y.; Bi, Y.; Wang, R.; Wang, L.; Qu, H.; Zheng, L. DNA-Gated Graphene Field-Effect Transistors for Specific Detection of Arsenic(III) in Rice. J. Agric. Food Chem. 2021, 69, 1398–1404. [Google Scholar] [CrossRef]
- Wang, P.; Li, H.; Hassan, M.M.; Guo, Z.; Zhang, Z.-Z.; Chen, Q. Fabricating an Acetylcholinesterase Modulated UCNPs-Cu2+ Fluorescence Biosensor for Ultrasensitive Detection of Organophosphorus Pesticides-Diazinon in Food. J. Agric. Food Chem. 2019, 67, 4071–4079. [Google Scholar] [CrossRef]
- Cui, Z.; Li, Y.; Zhang, H.; Qin, P.; Hu, X.; Wang, J.; Wei, G.; Chen, C. Lighting Up Agricultural Sustainability in the New Era through Nanozymology: An Overview of Classifications and Their Agricultural Applications. J. Agric. Food Chem. 2022, 70, 13445–13463. [Google Scholar] [CrossRef]
- Fokum, E.; Zabed, H.M.; Guo, Q.; Yun, J.; Yang, M.; Pang, H.; An, Y.; Li, W.; Qi, X. Metabolic engineering of bacterial strains using CRISPR/Cas9 systems for biosynthesis of value-added products. Food Biosci. 2019, 28, 125–132. [Google Scholar] [CrossRef]
- Yang, H.; Chen, J.; Yang, S.; Zhang, T.; Xia, X.; Zhang, K.; Deng, S.; He, G.; Gao, H.; He, Q.; et al. CRISPR/Cas14a-Based Isothermal Amplification for Profiling Plant MicroRNAs. Anal. Chem. 2021, 93, 12602–12608. [Google Scholar] [CrossRef] [PubMed]
- Knott, G.J.; Doudna, J.A. CRISPR-Cas guides the future of genetic engineering. Science 2018, 361, 866–869. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xie, Y.; Chen, F.; Bai, H.; Xiu, L.; Zhou, X.; Guo, X.; Hu, Q.; Yin, K. Amplification-free CRISPR/Cas detection technology: Challenges, strategies, and perspectives. Chem. Soc. Rev. 2023, 52, 361–382. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Man, S.; Ye, S.; Liu, G.; Ma, L. CRISPR-Cas-based detection for food safety problems: Current status, challenges, and opportunities. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3770–3798. [Google Scholar] [CrossRef]
- Shmakov, S.; Smargon, A.; Scott, D.; Cox, D.; Pyzocha, N.; Yan, W.; Abudayyeh, O.O.; Gootenberg, J.S.; Makarova, K.S.; Wolf, Y.I.; et al. Diversity and evolution of class 2 CRISPR-Cas systems. Nat. Rev. Microbiol. 2017, 15, 169–182. [Google Scholar] [CrossRef]
- Yin, L.; Man, S.; Ye, S.; Liu, G.; Ma, L. CRISPR-Cas based virus detection: Recent advances and perspectives. Biosens. Bioelectron. 2021, 193, 113541. [Google Scholar] [CrossRef]
- Zhu, H.; Li, C.; Gao, C. Applications of CRISPR-Cas in agriculture and plant biotechnology. Nat. Rev. Mol. Cell Biol. 2020, 21, 661–677. [Google Scholar] [CrossRef]
- Koonin, E.V.; Makarova, K.S. Origins and evolution of CRISPR-Cas systems. Philos. Trans. R. Soc. B-Biol. Sci. 2019, 374, 20180087. [Google Scholar] [CrossRef]
- Chen, F.; Chen, L.; Yan, Z.; Xu, J.; Feng, L.; He, N.; Guo, M.; Zhao, J.; Chen, Z.; Chen, H.; et al. Recent advances of CRISPR-based genome editing for enhancing staple crops. Front. Plant Sci. 2024, 15, 1478398. [Google Scholar] [CrossRef]
- Guo, W.; Guo, Y.; Xu, H.; Li, C.; Zhang, X.; Zou, X.; Sun, Z. Ultrasensitive “On-Off” Ratiometric Fluorescence Biosensor Based on RPA-CRISPR/Cas12a for Detection of Staphylococcus aureus. J. Agric. Food Chem. 2025, 73, 2167–2173. [Google Scholar] [CrossRef]
- Kang, T.; Lu, J.; Yu, T.; Long, Y.; Liu, G. Advances in nucleic acid amplification techniques (NAATs): COVID-19 point-of-care diagnostics as an example. Biosens. Bioelectron. 2022, 206, 114109. [Google Scholar] [CrossRef]
- Lin, M.; Yue, H.; Tian, T.; Xiong, E.; Zhu, D.; Jiang, Y.; Zhou, X. Glycerol Additive Boosts 100-fold Sensitivity Enhancement for One-Pot RPA-CRISPR/Cas12a Assay. Anal. Chem. 2022, 94, 8277–8284. [Google Scholar] [CrossRef]
- Deng, H.; Gao, Z. Bioanalytical applications of isothermal nucleic acid amplification techniques. Anal. Chim. Acta 2015, 853, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, H.; Lin, K.; Han, Y.; Gu, Z.; Wei, H.; Mu, K.; Wang, D.; Liu, L.; Jin, R.; et al. Ultrasensitive single-step CRISPR detection of monkeypox virus in minutes with a vest-pocket diagnostic device. Nat. Commun. 2024, 15, 3279. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; Zeng, H.; Liu, X.; Jiang, W.; Wang, Y.; Ouyang, W.; Tang, X. RPA-Cas12a-FS: A frontline nucleic acid rapid detection system for food safety based on CRISPR-Cas12a combined with recombinase polymerase amplification. Food Chem. 2021, 334, 127608. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Liang, M.; Song, A.; Zhang, M.; Lei, Y.; Huang, Z.; Xiao, J. Quantitative Detection of Genetically Modified Maize (Zea mays) Bt11 Strain Based on Duplex Droplet Digital PCR. J. Agric. Biotechnol. 2020, 28, 543–552. [Google Scholar]
- Pan, Z.; Zuo, C.; Wu, Q.; Qian, C.; Yin, L.; Liu, Y.; Li, X. Duplex Real-time Quantitative PCR Method for Detection of Genetically Modified Cotton (Gossypium hirsutum) Event COT102. J. Agric. Biotechnol. 2021, 29, 2248–2258. [Google Scholar]
- Qaim, M. Role of New Plant Breeding Technologies for Food Security and Sustainable Agricultural Development. Appl. Econ. Perspect. Policy 2020, 42, 129–150. [Google Scholar] [CrossRef]
- Tanny, T.; Sallam, M.; Soda, N.; Nguyen, N.-T.; Alam, M.; Shiddiky, M.J.A. CRISPR/Cas-Based Diagnostics in Agricultural Applications. J. Agric. Food Chem. 2023, 71, 11765–11788. [Google Scholar] [CrossRef]
- Hu, J.-J.; Liu, D.; Cai, M.-Z.; Zhou, Y.; Yin, W.-X.; Luo, C.-X. One-Pot Assay for Rapid Detection of Benzimidazole Resistance in Venturia carpophila by Combining RPA and CRISPR/Cas12a. J. Agric. Food Chem. 2023, 71, 1381–1390. [Google Scholar] [CrossRef]
- Han, X.; Lu, M.; Zhang, Y.; Liu, X.; Zhang, Q.; Bai, X.; Man, S.; Zhao, L.; Ma, L. A Thermostable Cas12b-Powered Bioassay Coupled with Loop-Mediated Isothermal Amplification in a Customized “One-Pot” Vessel for Visual, Rapid, Sensitive, and On-Site Detection of Genetically Modified Crops. J. Agric. Food Chem. 2024, 72, 11195–11204. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Wang, J.; Sun, P.; Ran, W.; Li, Y. Mango variety classification based on convolutional neural network with attention mechanism and near-infrared spectroscopy. J. Food Meas. Charact. 2024, 18, 2237–2247. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.; Ju, C.; Son, H.I. Unmanned Aerial Vehicles in Agriculture: A Review of Perspective of Platform, Control, and Applications. IEEE Access 2019, 7, 105100–105115. [Google Scholar] [CrossRef]
- Pathak, A.K.; Saikia, P.; Dutta, S.; Sinha, S.; Ghosh, S. Development of a Robust CNN Model for Mango Leaf Disease Detection and Classification: A Precision Agriculture Approach. ACS Agric. Sci. Technol. 2024, 4, 806–817. [Google Scholar] [CrossRef]
- Liu, C.; Xu, D.; Dong, X.; Huang, Q. A review: Research progress of SERS-based sensors for agricultural applications. Trends Food Sci. Technol. 2022, 128, 90–101. [Google Scholar] [CrossRef]
- Wang, C.; Liu, B.; Liu, L.; Zhu, Y.; Hou, J.; Liu, P.; Li, X. A review of deep learning used in the hyperspectral image analysis for agriculture. Artif. Intell. Rev. 2021, 54, 5205–5253. [Google Scholar] [CrossRef]
- Yin, S.; Chen, X.; Li, R.; Sun, L.; Yao, C.; Li, Z. Wearable, Biocompatible, and Dual-Emission Ocular Multisensor Patch for Continuous Profiling of Fluoroquinolone Antibiotics in Tears. ACS Nano 2024, 18, 18522–18533. [Google Scholar] [CrossRef]
- Ji, Y.; Ma, S.; Lv, S.; Wang, Y.; Lü, S.; Liu, M. Nanomaterials for Targeted Delivery of Agrochemicals by an All-in-One Combination Strategy and Deep Learning. ACS Appl. Mater. Interfaces 2021, 13, 43374–43386. [Google Scholar] [CrossRef]
- Chandra, S.; Verma, S.; Lim, W.M.; Kumar, S.; Donthu, N. Personalization in personalized marketing: Trends and ways forward. Psychol. Mark. 2022, 39, 1529–1562. [Google Scholar] [CrossRef]
- Saurabh, S.; Dey, K. Blockchain technology adoption, architecture, and sustainable agri-food supply chains. J. Clean. Prod. 2021, 284, 124731. [Google Scholar] [CrossRef]
- Varriale, V.; Cammarano, A.; Michelino, F.; Caputo, M. The role of digital technologies in production systems for achieving sustainable development goals. Sustain. Prod. Consum. 2024, 47, 87–104. [Google Scholar] [CrossRef]
- Venkatesh, V.G.; Kang, K.; Wang, B.; Zhong, R.Y.; Zhang, A. System architecture for blockchain based transparency of supply chain social sustainability. Robot. Comput.-Integr. Manuf. 2020, 63, 101896. [Google Scholar] [CrossRef]
- Haggerty, R.; Sun, J.; Yu, H.; Li, Y. Application of machine learning in groundwater quality modeling—A comprehensive review. Water Res. 2023, 233, 119745. [Google Scholar] [CrossRef]
- Chen, H.; Chen, A.; Xu, L.; Xie, H.; Qiao, H.; Lin, Q.; Cai, K. A deep learning CNN architecture applied in smart near-infrared analysis of water pollution for agricultural irrigation resources. Agric. Water Manag. 2020, 240, 106303. [Google Scholar] [CrossRef]
- Kowalska, J.B.; Mazurek, R.; Gasiorek, M.; Zaleski, T. Pollution indices as useful tools for the comprehensive evaluation of the degree of soil contamination-A review. Environ. Geochem. Health 2018, 40, 2395–2420. [Google Scholar] [CrossRef] [PubMed]
- Singha, S.; Pasupuleti, S.; Singha, S.S.; Singh, R.; Kumar, S. Prediction of groundwater quality using efficient machine learning technique. Chemosphere 2021, 276, 130265. [Google Scholar] [CrossRef] [PubMed]
- Hajjaji, Y.; Boulila, W.; Farah, I.R.; Romdhani, I.; Hussain, A. Big data and IoT-based applications in smart environments: A systematic review. Comput. Sci. Rev. 2021, 39, 100318. [Google Scholar] [CrossRef]
- Malik, P.K.; Sharma, R.; Singh, R.; Gehlot, A.; Satapathy, S.C.; Alnumay, W.S.; Pelusi, D.; Ghosh, U.; Nayak, J. Industrial Internet of Things and its Applications in Industry 4.0: State of The Art. Comput. Commun. 2021, 166, 125–139. [Google Scholar] [CrossRef]
- Tokognon, C.J.A.; Gao, B.; Tian, G.Y.; Yan, Y. Structural Health Monitoring Framework Based on Internet of Things: A Survey. IEEE Internet Things J. 2017, 4, 619–635. [Google Scholar] [CrossRef]
- Ullo, S.L.; Sinha, G.R. Advances in Smart Environment Monitoring Systems Using IoT and Sensors. Sensors 2020, 20, 3113. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Xu, Y.; Sayada, J.; Zareef, M.; Shoaib, M.; Chen, X.; Li, H.; Chen, Q. Progress of machine learning-based biosensors for the monitoring of food safety: A review. Biosens. Bioelectron. 2025, 267, 116782. [Google Scholar] [CrossRef] [PubMed]
- Bahlol, H.S.; Li, J.; Deng, J.; Foda, M.F.; Han, H. Recent Progress in Nanomaterial-Based Surface-Enhanced Raman Spectroscopy for Food Safety Detection. Nanomaterials 2024, 14, 1750. [Google Scholar] [CrossRef] [PubMed]
- Dmitric, M.; Vidanovic, D.; Matovic, K.; Tesovic, B.; Sekler, M.; Vicic, I.; Karabasil, N. Development of a novel invA gene-based real-time PCR assay for the detection of Salmonella in food. Czech J. Food Sci. 2023, 41, 287–294. [Google Scholar] [CrossRef]
- Reem, C.S.A.; Chowdhury, M.A.H.; Ashrafudoulla, M.; Ha, S.-D. Leveraging Blockchain and AI for Biofilm Control in Food Processing Environments. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70261. [Google Scholar] [CrossRef]
- Han, J.; Wang, L.; Wang, L.; Li, C.; Mao, Y.; Wang, Y. Fabrication of a core-shell-shell magnetic polymeric microsphere with excellent performance for separation and purification of bromelain. Food Chem. 2019, 283, 1–10. [Google Scholar] [CrossRef]
- Song, H.; Liu, J. GC-O-MS technique and its applications in food flavor analysis. Food Res. Int. 2018, 114, 187–198. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Guo, W.; Guo, Y.; Zou, X.; Sun, Z. Recyclable magnetic HNTs@MIPs-Based SERS sensors for selective, sensitive, and reliable detection of capsaicin for gutter oil discrimination. Food Biosci. 2025, 66, 106179. [Google Scholar] [CrossRef]
- Kamble, S.; Agrawal, S.; Cherumukkil, S.; Sharma, V.; Jasra, R.V.; Munshi, P. Revisiting Zeta Potential, the Key Feature of Interfacial Phenomena, with Applications and Recent Advancements. Chemistryselect 2022, 7, e202103084. [Google Scholar] [CrossRef]
- Gu, J.; Duan, F.; Liu, S.; Cha, W.; Lu, J. Phase Engineering of Nanostructural Metallic Materials: Classification, Structures, and Applications. Chem. Rev. 2024, 124, 1247–1287. [Google Scholar] [CrossRef]
- Borsini, A.; Nicolaou, A.; Camacho-Munoz, D.; Kendall, A.C.; Di Benedetto, M.G.; Giacobbe, J.; Su, K.-P.; Pariante, C.M. Omega-3 polyunsaturated fatty acids protect against inflammation through production of LOX and CYP450 lipid mediators: Relevance for major depression and for human hippocampal neurogenesis. Mol. Psychiatry 2021, 26, 6773–6788. [Google Scholar] [CrossRef]
- Li, Y.; Wei, K. Comparative functional genomics analysis of cytochrome P450 gene superfamily in wheat and maize. BMC Plant Biol. 2020, 20, 93. [Google Scholar] [CrossRef]
- Bashir, M.; Batool, M.; Arif, N.; Tayyab, M.; Zeng, Y.-J.; Zafar, M.N. Strontium-based nanomaterials for the removal of organic/inorganic contaminants from water: A review. Coord. Chem. Rev. 2023, 492, 215286. [Google Scholar] [CrossRef]
- Li, L.; Zou, D.; Xiao, Z.; Zeng, X.; Zhang, L.; Jiang, L.; Wang, A.; Ge, D.; Zhang, G.; Liu, F. Biochar as a sorbent for emerging contaminants enables improvements in waste management and sustainable resource use. J. Clean. Prod. 2019, 210, 1324–1342. [Google Scholar] [CrossRef]
- Wang, J.; Wu, W.; Yang, M.; Gao, Y.; Shao, J.; Yang, W.; Ma, G.; Yu, F.; Yao, N.; Jiang, H. Exploring the complex trade-offs and synergies of global ecosystem services. Environ. Sci. Ecotechnol. 2024, 21, 100391. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, B.; Tassielli, G.; Renzulli, P.A.; Castellani, V.; Sala, S. Environmental impacts of food consumption in Europe. J. Clean. Prod. 2017, 140, 753–765. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Li, J.; Wang, Z. Green process innovation, green product innovation and its economic performance improvement paths: A survey and structural model. J. Environ. Manag. 2021, 297, 113282. [Google Scholar] [CrossRef]
- Nie, Y.; Yang, J.; Wen, H.; Gao, L.; Xu, J. Light weight detection of mango surface defects based on machine vision. Food Mach. 2023, 39, 91–95, 240. [Google Scholar]
- Yang, J.; Liu, X.; Fu, Y.; Song, Y. Recent advances of microneedles for biomedical applications: Drug delivery and beyond. Acta Pharm. Sin. B 2019, 9, 469–483. [Google Scholar] [CrossRef]
- Liu, C.; Ji, Y.; Jiang, X.; Yuan, X.; Zhang, X.; Zhao, L. The determination of pesticides in tea samples followed by magnetic multiwalled carbon nanotube-based magnetic solid-phase extraction and ultra-high performance liquid chromatography-tandem mass spectrometry. New J. Chem. 2019, 43, 5395–5403. [Google Scholar] [CrossRef]
- Ma, S.; Pan, L.g.; You, T.; Wang, K. g-C3N4/Fe3O4 Nanocomposites as Adsorbents Analyzed by UPLC-MS/MS for Highly Sensitive Simultaneous Determination of 27 Mycotoxins in Maize: Aiming at Increasing Purification Efficiency and Reducing Time. J. Agric. Food Chem. 2021, 69, 4874–4882. [Google Scholar] [CrossRef] [PubMed]
- Weise, C.; Fischer, J.; Belder, D. Mass spectrometry coupling of chip-based supercritical fluid chromatography enabled by make-up flow-assisted backpressure regulation. Anal. Bioanal. Chem. 2024, 416, 4447–4456. [Google Scholar] [CrossRef] [PubMed]
- Hamadou, A.H.; Zhang, J.; Li, H.; Chen, C.; Xu, B. Modulating the glycemic response of starch-based foods using organic nanomaterials: Strategies and opportunities. Crit. Rev. Food Sci. Nutr. 2023, 63, 11942–11966. [Google Scholar] [CrossRef]
- Fang, X.; Wang, Y.; Wang, S.; Liu, B. Nanomaterials assisted exosomes isolation and analysis towards liquid biopsy. Mater. Today Bio 2022, 16, 100371. [Google Scholar] [CrossRef]
- Gebreyesus, S.T.; Siyal, A.A.; Kitata, R.B.; Chen, E.S.-W.; Enkhbayar, B.; Angata, T.; Lin, K.-I.; Chen, Y.-J.; Tu, H.-L. Streamlined single-cell proteomics by an integrated microfluidic chip and data-independent acquisition mass spectrometry. Nat. Commun. 2022, 13, 37. [Google Scholar] [CrossRef]
- Samiei, E.; Tabrizian, M.; Hoorfar, M. A review of digital microfluidics as portable platforms for lab-on a-chip applications. Lab Chip 2016, 16, 2376–2396. [Google Scholar] [CrossRef]
- Xia, Y.; Si, J.; Li, Z. Fabrication techniques for microfluidic paper-based analytical devices and their applications for biological testing: A review. Biosens. Bioelectron. 2016, 77, 774–789. [Google Scholar] [CrossRef]
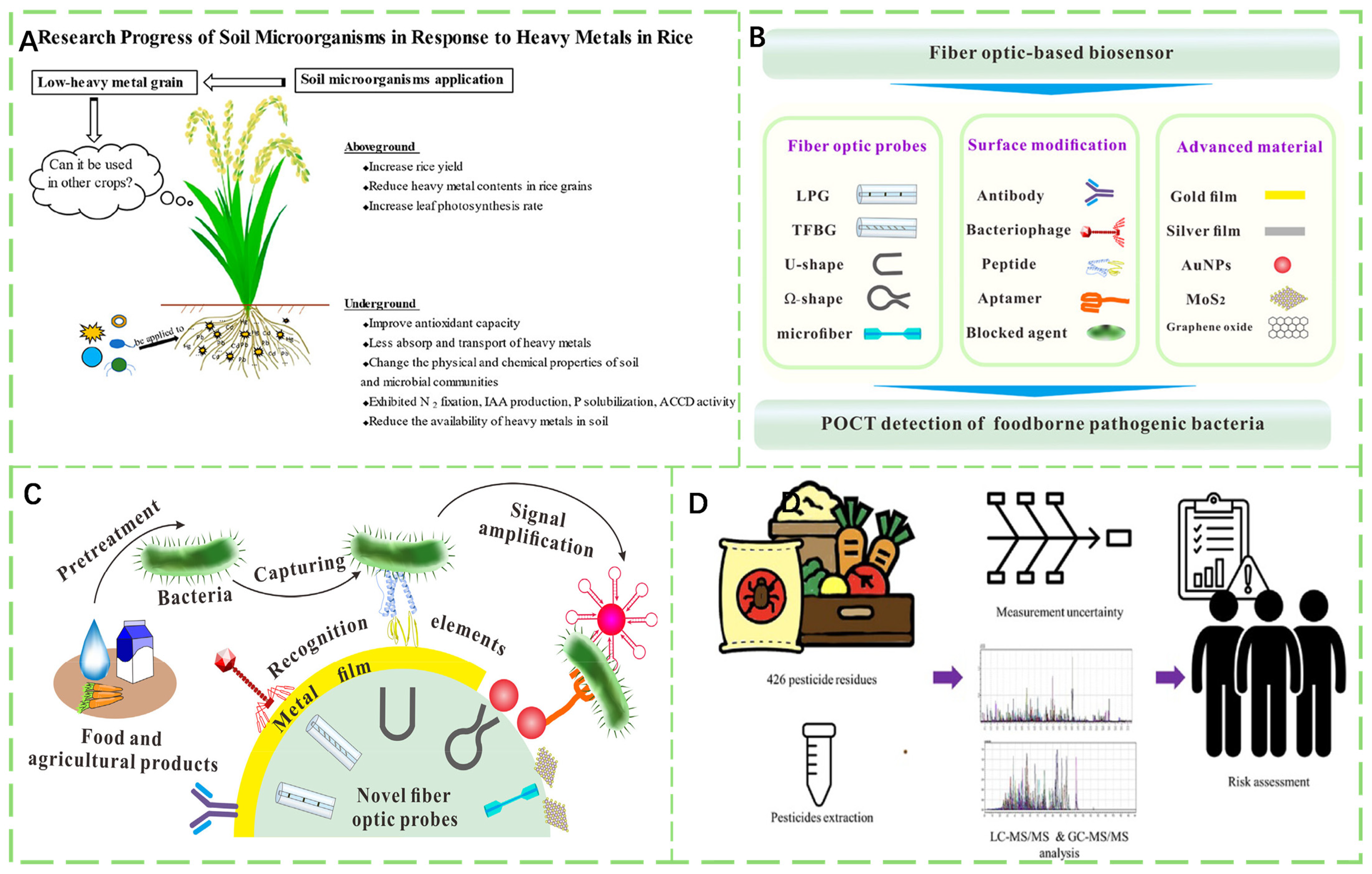
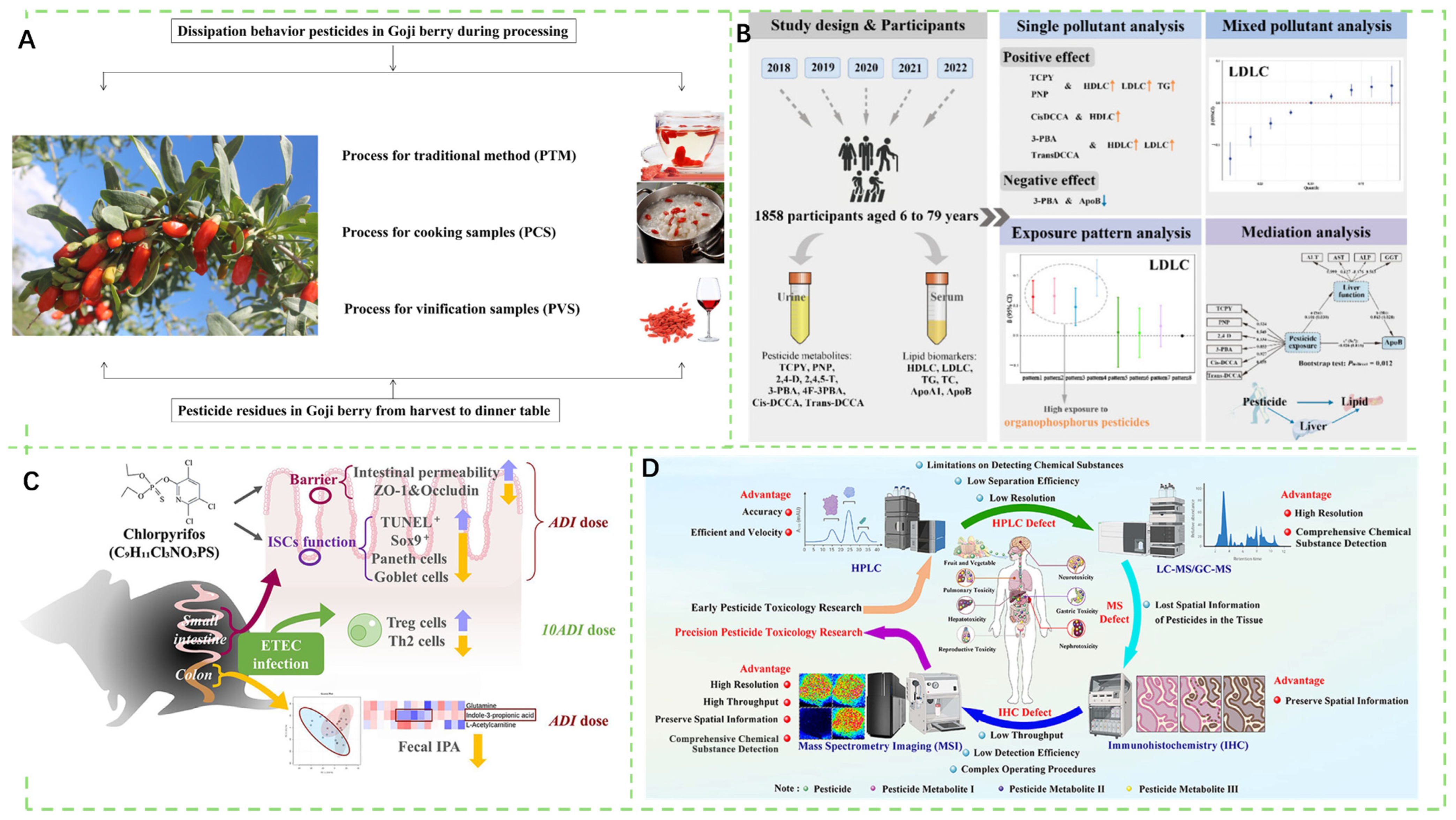

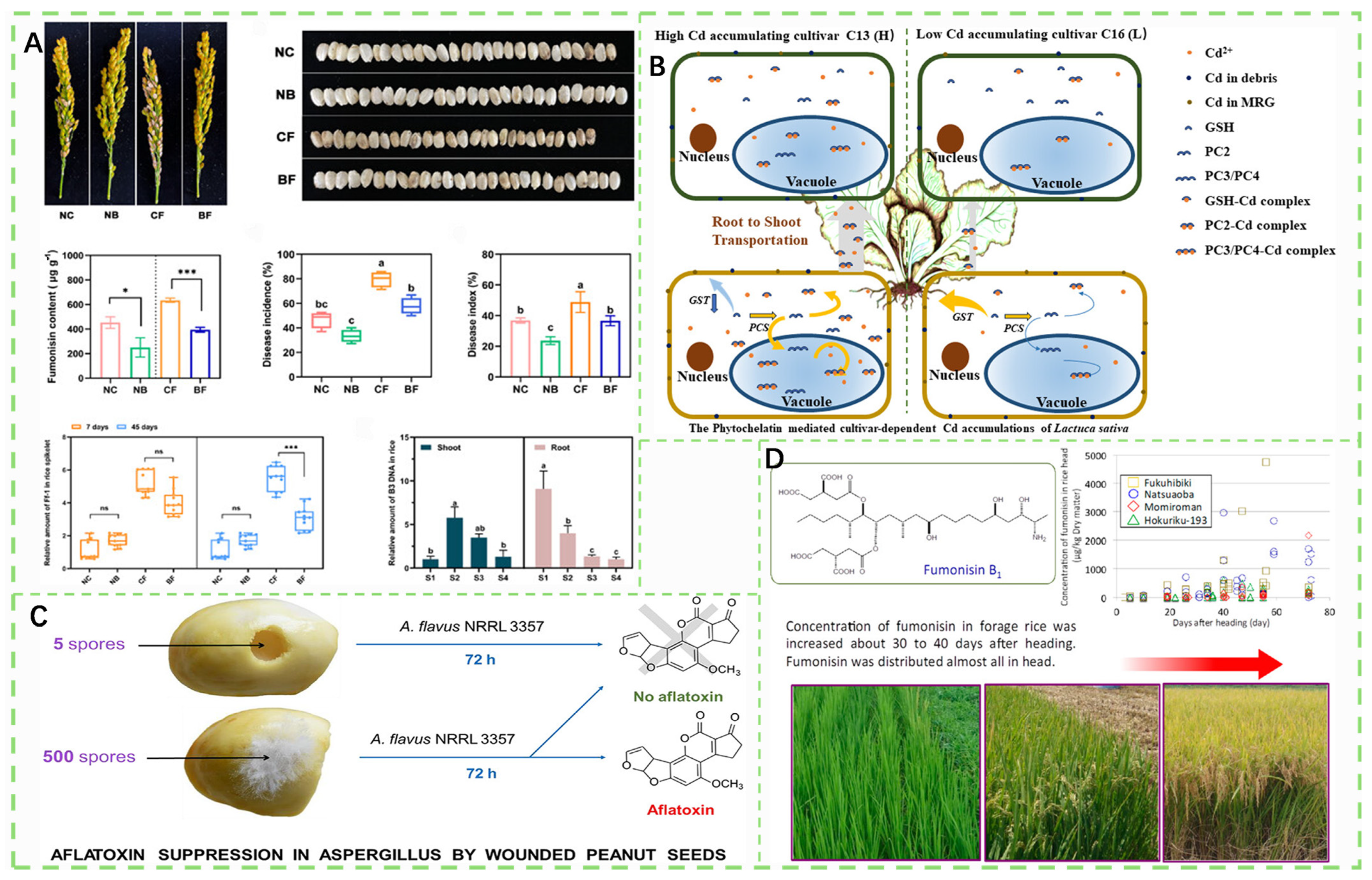
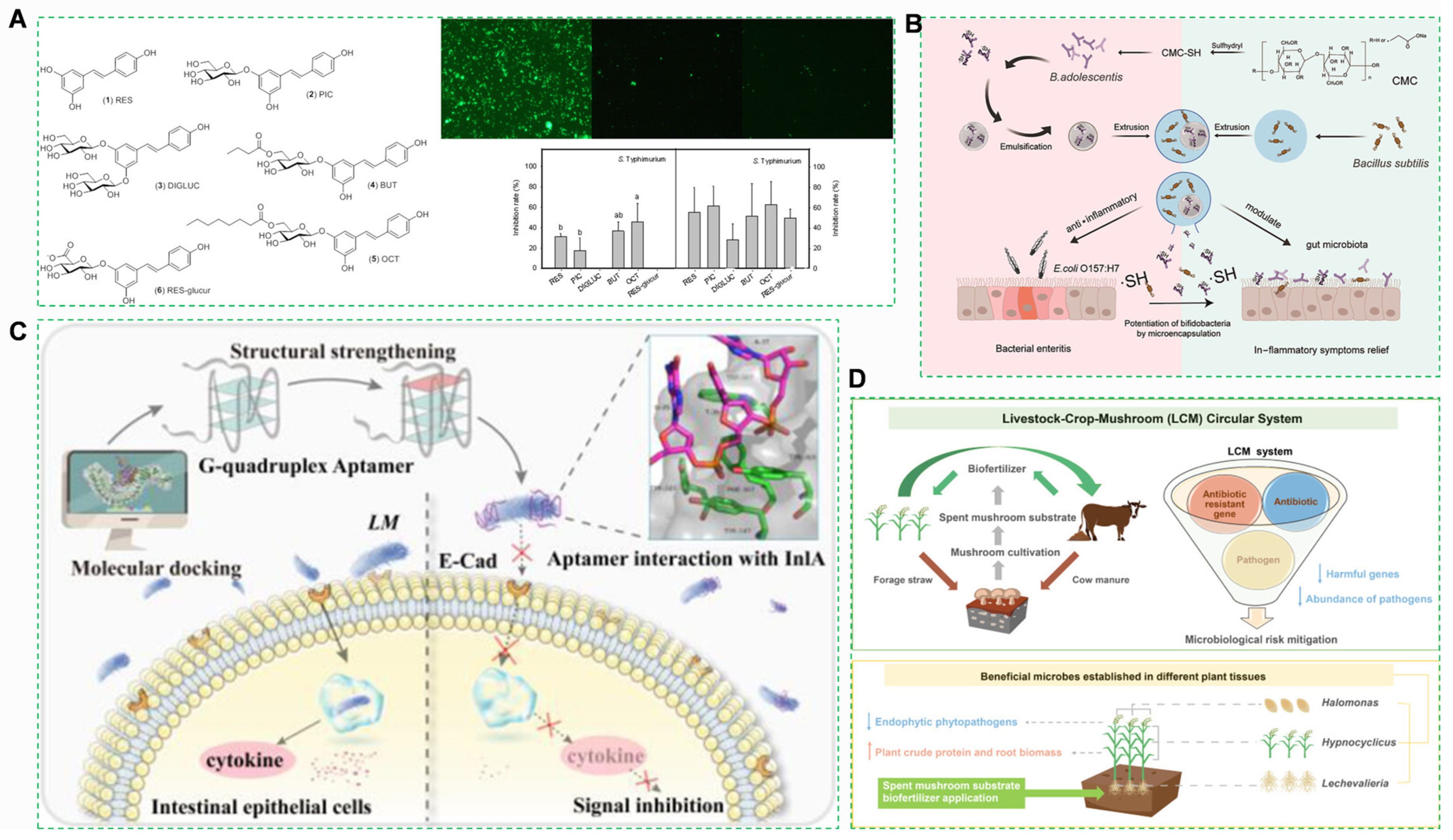
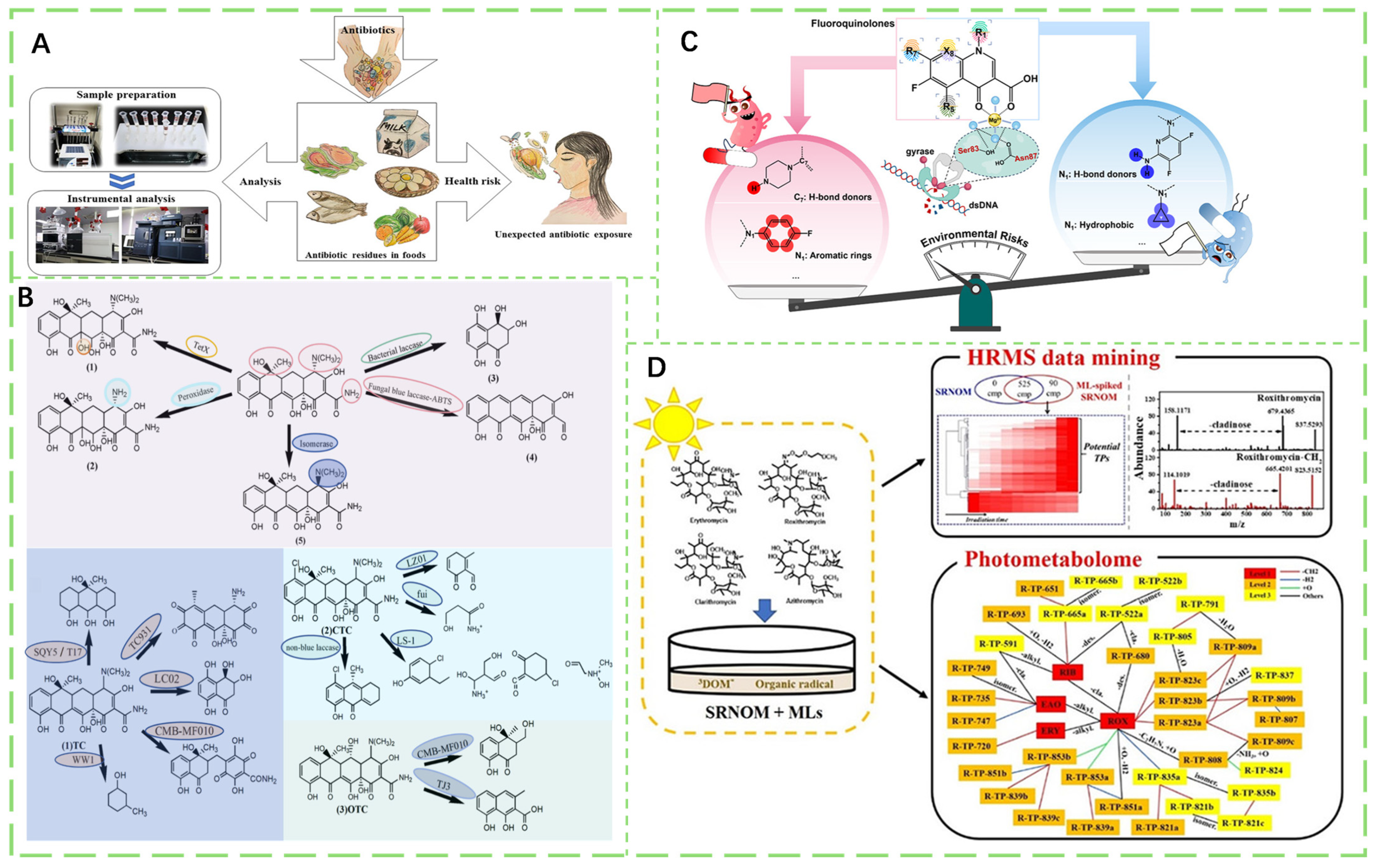
| Hazardous Component | Representative Examples | Traditional Methods | Emerging Methods | Strengths | Limitations | SERS |
|---|---|---|---|---|---|---|
| Pesticide Residues | Organophosphates, Neonicotinoids, Herbicides | HPLC, GC–MS/MS | Fluorescent probes, Biosensors (electrochemical, microfluidic) | High sensitivity; standardized protocols; real-time potential with biosensors | Chromatography is costly and slow; biosensors face stability and standardization issues | [162] |
| Heavy Metals | Cd, Pb, As | AAS, ICP-MS | LIBS, Graphene FET nanosensors | Accurate trace-level quantification; nanosensors allow portability | Lab-based methods are expensive; nanosensors not yet widely commercialized | [163] |
| Mycotoxins | Aflatoxin B1, DON, Fumonisin B1 | LC–MS/MS, ELISA | SERS, Quantum dot immunoassays | High specificity; trace-level detection; rapid immunoassays | Antibody-based methods may cross-react; some need cold-chain storage | [164] |
| Microbial Contaminants | E. coli O157:H7, Salmonella, Listeria monocytogenes | Culture methods, PCR | CRISPR-Cas assays, Biosensors (fiber-optic, nanomaterials) | Molecular methods highly specific; rapid detection possible | Culture-based methods are slow; molecular assays still costly | [165] |
| Antibiotic Residues | Tetracyclines, Fluoroquinolones, Macrolides | HPLC, ELISA | Live-cell biosensors, Fluorescent probes | Sensitive detection; biosensors provide real-time monitoring | Traditional methods are time-intensive; biosensors require further validation | [149] |
| Genetically Modified Material | GMO maize, soybean | PCR, qPCR | CRISPR-Cas, Next-generation sequencing (NGS) | Genome-level specificity; high accuracy | Requires DNA extraction and specialized instruments | [166] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, S.; Wu, X.; Shi, Y.; El-Mesery, H.S.; Zhang, X. Uncovering Analytical Patterns for Hazardous Components in Agricultural Production Systems. Foods 2025, 14, 3261. https://doi.org/10.3390/foods14183261
Deng S, Wu X, Shi Y, El-Mesery HS, Zhang X. Uncovering Analytical Patterns for Hazardous Components in Agricultural Production Systems. Foods. 2025; 14(18):3261. https://doi.org/10.3390/foods14183261
Chicago/Turabian StyleDeng, Shiyu, Xinxin Wu, Yongqiang Shi, Hany S. El-Mesery, and Xinai Zhang. 2025. "Uncovering Analytical Patterns for Hazardous Components in Agricultural Production Systems" Foods 14, no. 18: 3261. https://doi.org/10.3390/foods14183261
APA StyleDeng, S., Wu, X., Shi, Y., El-Mesery, H. S., & Zhang, X. (2025). Uncovering Analytical Patterns for Hazardous Components in Agricultural Production Systems. Foods, 14(18), 3261. https://doi.org/10.3390/foods14183261