Effects of Thermal Sterilization Conditions on Flavor and Lipid Oxidation of Sauced Duck Necks
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Lipid Oxidation
2.2.1. Peroxide Value
2.2.2. Thiobarbituric Acid-Reactive Substances
2.3. Free Fatty Acid Composition
2.4. Volatile Flavor Compounds
2.5. Sensory Evaluation
2.6. Statistical Analyses
3. Results and Discussion
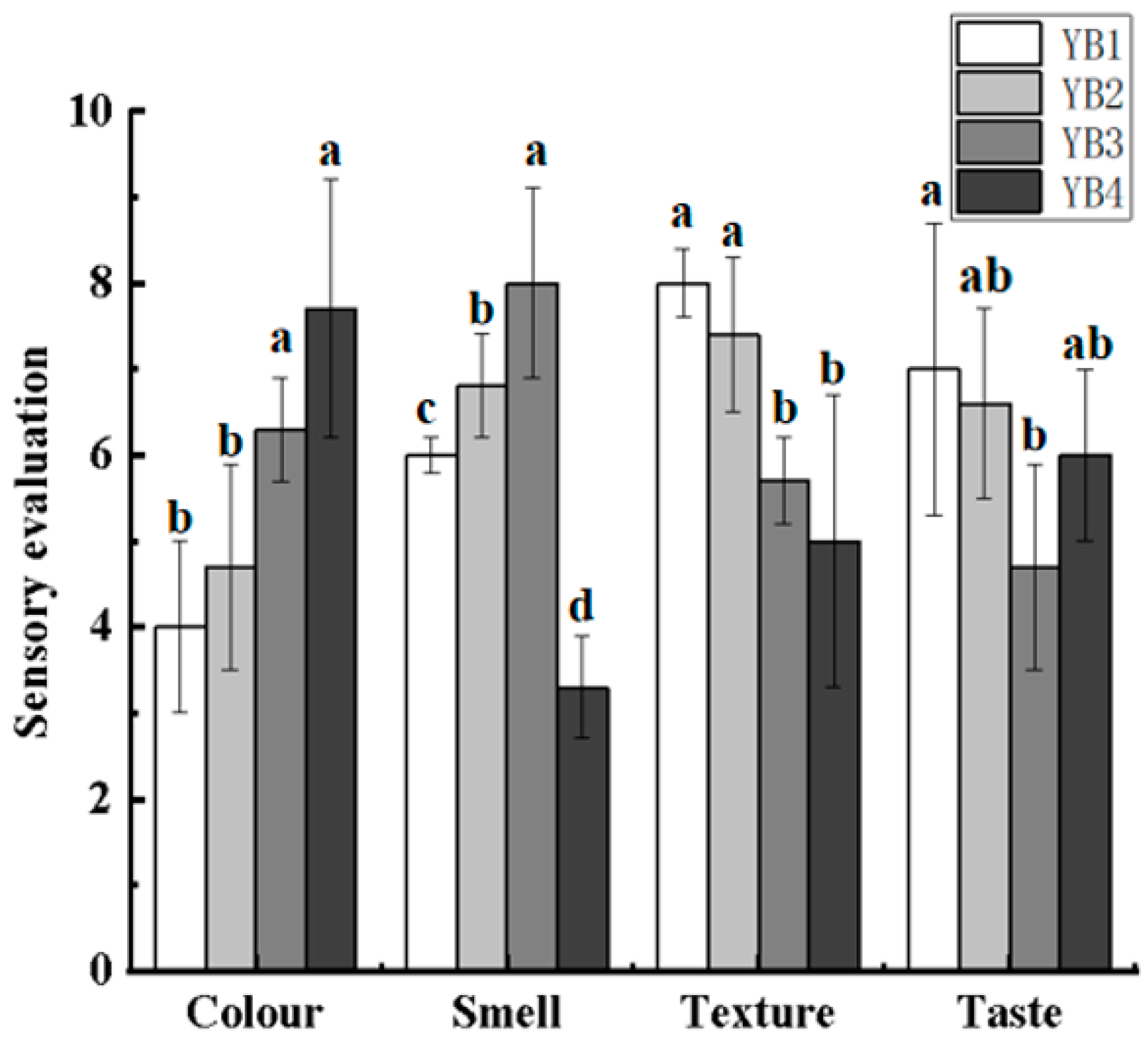
3.1. Changes in the Sensory Evaluation Under Different Thermal Sterilization Conditions
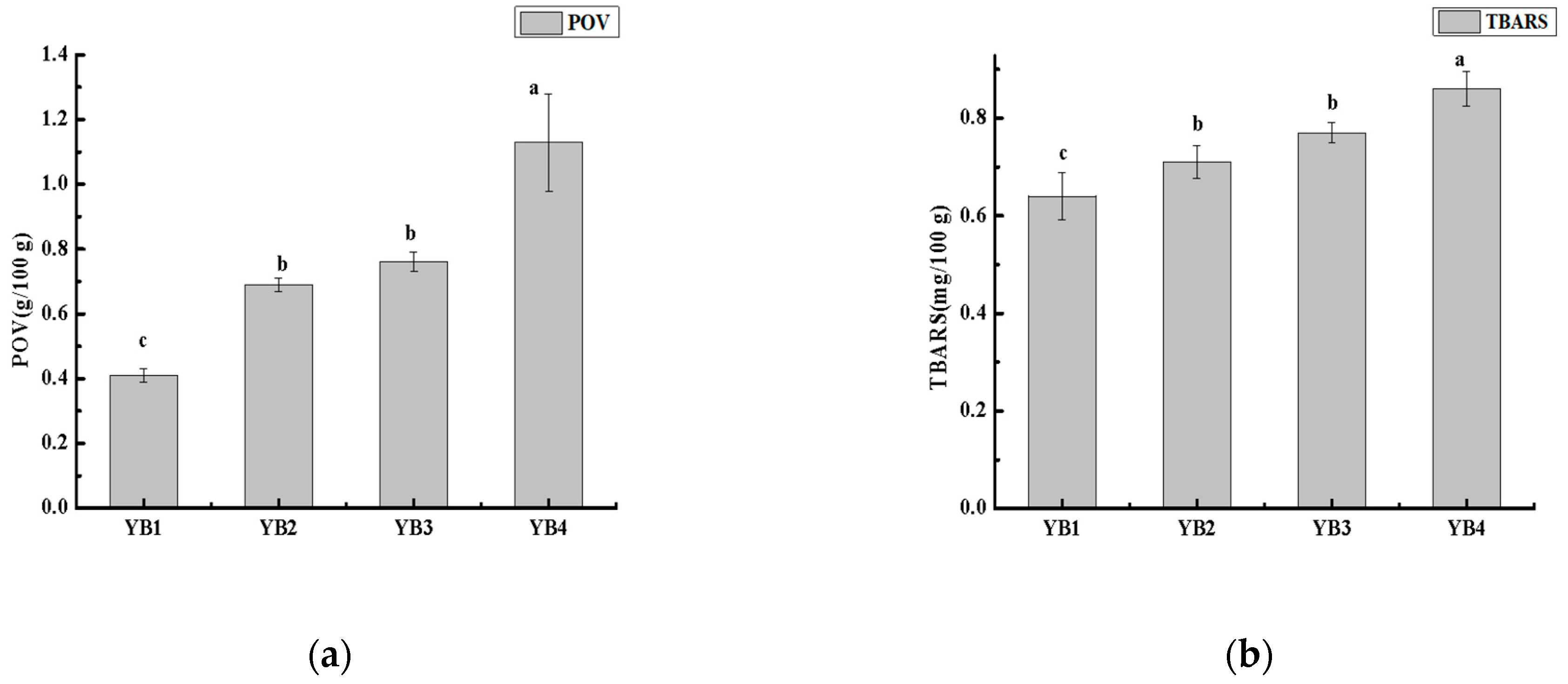
3.2. Changes in Lipid Oxidation Under Different Thermal Sterilization Conditions
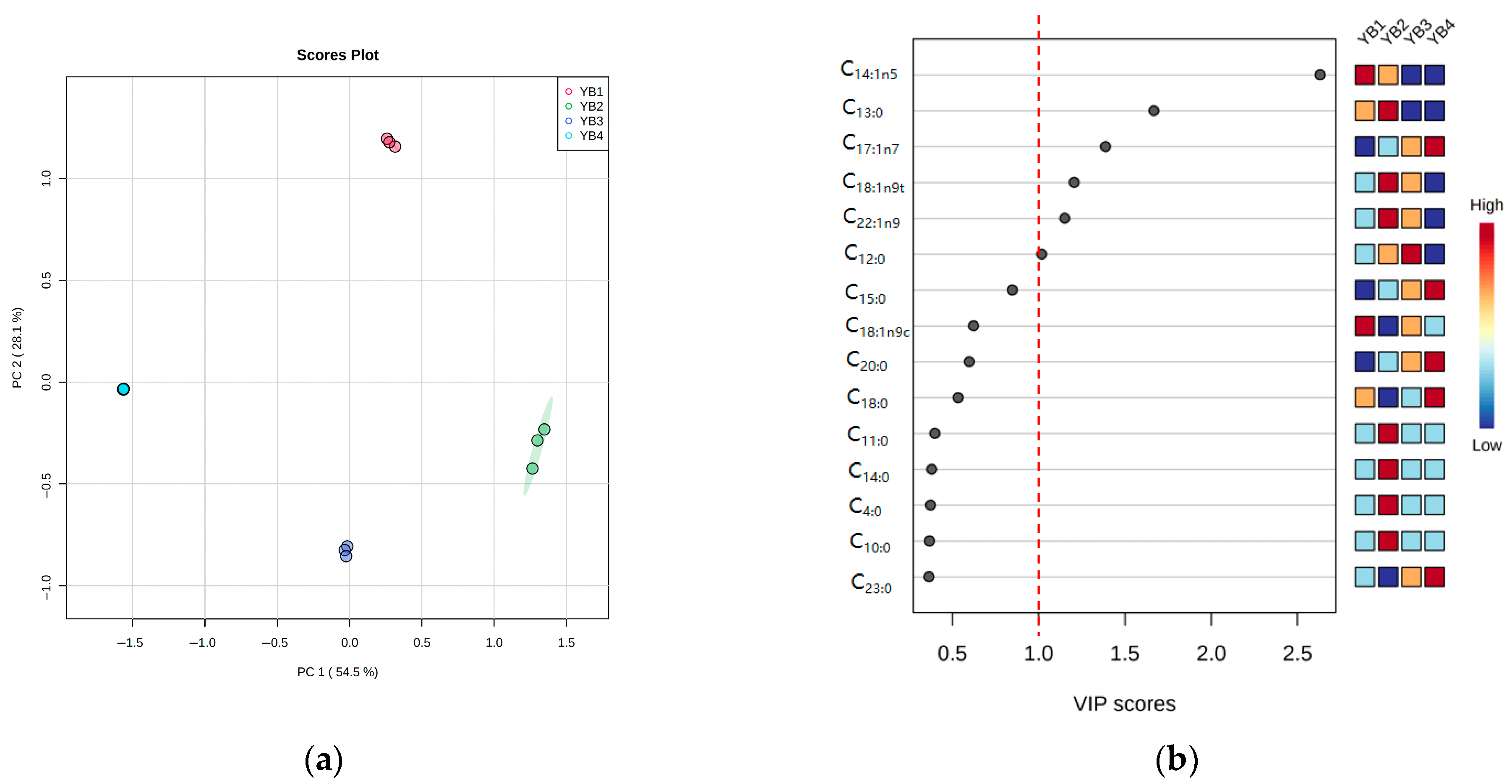
3.3. Changes in Free Fatty Acid Under Different Thermal Sterilization Conditions
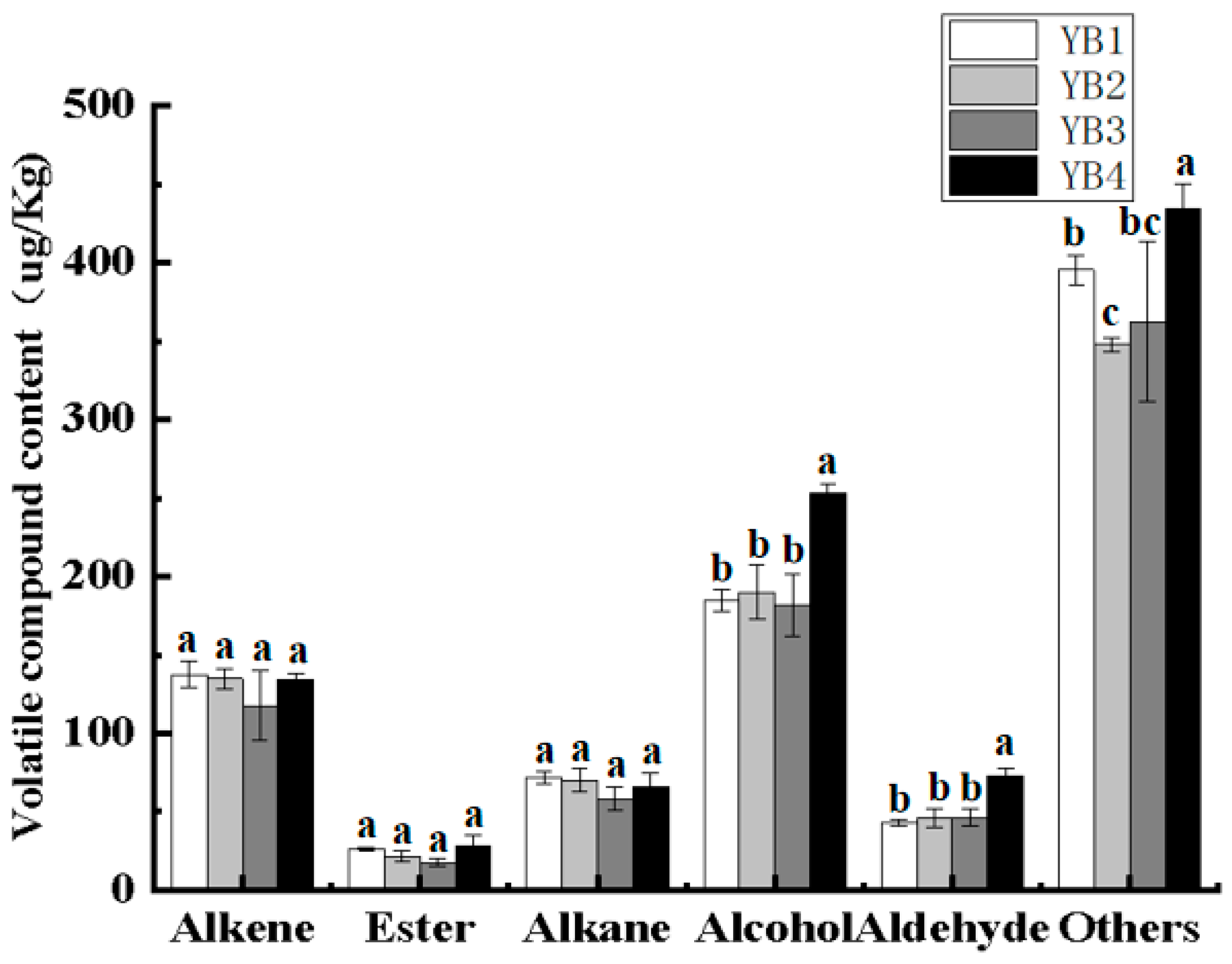
3.4. Changes in Volatile Flavor Components Under Different Thermal Sterilization Conditions
3.5. Correlation Between Free Fatty Acids and Volatile Flavor Substances
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, K.; Cai, J.; Pan, D.; Chen, B.; Fan, J.; Ren, D.; Xiao, Y. The Overall Quality Changes of Chinese Sauced Ducks at Different Stages During Processing and Storage. Foods 2025, 14, 834. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M. Influence of the type of fiber coating and extraction time on foal dry-cured loin volatile compounds extracted by solid-phase microextraction (SPME). Meat Sci. 2014, 96, 179–186. [Google Scholar] [CrossRef]
- Lazárková, Z.; Kratochvílová, A.; Salek, R.N.; Polášek, Z.; Šiška, L.; Pětová, M.; Buňka, F. Influence of Heat Treatment on the Chemical, Physical, Microbiological and Sensorial Properties of Pork Liver Pâté as Affected by Fat Content. Foods 2023, 12, 2423. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.; Gomez, M.; Fonseca, S.; Lorenzo, J.M. Effect of different cooking methods on lipid oxidation and formation of volatile compounds in foal meat. Meat Sci. 2014, 97, 223–230. [Google Scholar] [CrossRef]
- Wu, W.; Gao, P.; Jiang, Q.; Yang, F.; Yu, D.; Yu, P.; Xia, W.; Yu, D. Integrated GC-MS and proteomics reveal the changes and correlations between flavor compounds and the proteome in surimi gels treated with high-temperature sterilization. Food Biosci. 2024, 62, 105030. [Google Scholar] [CrossRef]
- Rakotondramavo, A.; Ribourg, L.; Meynier, A.; Guyon, C.; de Lamballerie, M.; Pottier, L. Monitoring oxidation during the storage of pressure-treated cooked ham and impact on technological attributes. Heliyon 2019, 5, e02285. [Google Scholar] [CrossRef]
- Jo, Y.; An, K.-A.; Arshad, M.S.; Kwon, J.-H. Effects of e-beam irradiation on amino acids, fatty acids, and volatiles of smoked duck meat during storage. Innov. Food Sci. Emerg. Technol. 2018, 47, 101–109. [Google Scholar] [CrossRef]
- White, S.; Jackson-Davis, A.; Gordon, K.; Morris, K.; Dudley, A.; Abdallah-Ruiz, A.; Allgaier, K.; Sharpe, K.; Yenduri, A.K.; Green, K.; et al. A Review of Non-thermal Interventions in Food Processing Technologies. J. Food Prot. 2025, 88, 100508. [Google Scholar] [CrossRef]
- Li, P.; Zhang, H.; Tian, C.; Zou, H. Experimental Investigation of Bacterial Inactivation of Beef Using Indirect Cold Plasma in Cold Chain and at Room Temperature. Foods 2024, 13, 2846. [Google Scholar] [CrossRef]
- Shi, J.; Nian, Y.; Da, D.; Xu, X.; Zhou, G.; Zhao, D.; Li, C. Characterization of flavor volatile compounds in sauce spareribs by gas chromatography-mass spectrometry and electronic nose. LWT Food Sci. Technol. 2020, 124, 109182. [Google Scholar] [CrossRef]
- Barbosa-Canovas, G.V.; Medina-Meza, I.; Candogan, K.; Bermudez-Aguirre, D. Advanced retorting, microwave assisted thermal sterilization (MATS), and pressure assisted thermal sterilization (PATS) to process meat products. Meat Sci. 2014, 98, 420–434. [Google Scholar] [CrossRef]
- Yao, Y.; Huang, M.; Liao, Q.; Wang, X.; Yu, J.; Hayat, K.; Zhang, X.; Ho, C.T. Unravelling the formation of characteristic aroma of traditional braised pork through untargeted and targeted flavoromics. Food Chem. 2025, 464, 141629. [Google Scholar] [CrossRef]
- Tan, C.; Li, X.; Yu, Y.; Nie, S.; Wen, Q.; Tu, Z.; Zhang, L. Effects of five thermal processing methods on the physicochemical properties and flavor characteristics of grass carp meat. LWT Food Sci. Technol. 2024, 206, 116599. [Google Scholar] [CrossRef]
- Zhan, H.; Jiang, Y.; Xu, M.; Cao, D.; Lu, W.; Xin, W.; Shen, Q.; Diao, Q. The development of chicken meat flavor from the interaction between Maillard reaction intermediates and enzymatically hydrolyzed-oxidized chicken fat. Food Res. Int. 2025, 218, 116927. [Google Scholar] [CrossRef]
- Song, S.; Zhang, X.; Hayat, K.; Liu, P.; Jia, C.; Xia, S.; Xiao, Z.; Tian, H.; Niu, Y. Formation of the beef flavour precursors and their correlation with chemical parameters during the controlled thermal oxidation of tallow. Food Chem. 2011, 124, 203–209. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, L.; Huang, F.; Wei, W.; Barbut, S.; Erasmus, S.; Zhang, C. Lipid-derived odour-active volatile compound formation pathways in Tibetan pork across different cooking methods: Insights from iron properties, lipid oxidation, and lipidomics analysis. Food Chem. 2025, 491, 145256. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; He, Y.; Cheng, S.; He, J.; Pan, D.; Cao, J.; Sun, Y. Free fatty acids responsible for characteristic aroma in various sauced-ducks. Food Chem. 2021, 343, 128493. [Google Scholar] [CrossRef] [PubMed]
- GB 5009.227-2023; National Food Safety Standard—Determination of Peroxide Value in Food. Standards Press of China: Beijing, China, 2023.
- Angeletti, B.; Trinh, D.T.; Dia, V.; Burns, S.; Chester, M.A.; Bergee, R.E.; Wang, T. Hempseed Hydrolysates Exhibit Antioxidant Activity in Meat Systems. Foods 2025, 14, 1728. [Google Scholar] [CrossRef]
- Ying, W.; Jiang, Y.T.; Cao, J.X.; Chen, Y.J.; Gan, N. Study on lipolysis-oxidation and volatile flavour compounds of dry-cured goose with different curing salt content during production. Food Chem. 2016, 190, 33–40. [Google Scholar] [CrossRef]
- Zhou, J.; Han, Y.; Zhuang, H.; Feng, T.; Xu, B. Influence of the Type of Extraction Conditions and Fiber Coating on the Meat of Sauced Duck Necks Volatile Compounds Extracted by Solid-Phase Microextraction (SPME). Food Anal. Method 2014, 8, 1661–1672. [Google Scholar] [CrossRef]
- Huang, L.; Xiong, Y.L.; Kong, B.; Huang, X.; Li, J. Influence of storage temperature and duration on lipid and protein oxidation and flavour changes in frozen pork dumpling filler. Meat Sci. 2013, 95, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-H.; Qi, L.-B.; Fu, B.-S.; Chen, Z.-H.; Zhang, Y.-Y.; Du, M.; Dong, X.-P.; Zhu, B.-W.; Qin, L. Flavor formation in different production steps during the processing of cold-smoked Spanish mackerel. Food Chem. 2019, 286, 241–249. [Google Scholar] [CrossRef]
- Chong, J.; Othman, S.; Li, C.; Iurie, C.; Li, S.; Guillaume, B.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, X.; Huang, Q.; Zhuo, Y.; Xu, B.; Wang, Z. Changes in the phospholipid molecular species in water-boiled salted duck during processing based on shotgun lipidomics. Food Res. Int. 2020, 132, 109064. [Google Scholar] [CrossRef] [PubMed]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, M.; Cao, P.; Adhikari, B. Effect of ZnO nanoparticles combined radio frequency pasteurization on the protein structure and water state of chicken thigh meat. LWT Food Sci. Technol. 2020, 134, 110168. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Nam, K.; Ahn, D.U. Analytical Methods for Lipid Oxidation and Antioxidant Capacity in Food Systems. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef]
- Manessis, G.; Kalogianni, A.I.; Lazou, T.; Moschovas, M.; Bossis, I.; Gelasakis, A.I. Plant-Derived Natural Antioxidants in Meat and Meat Products. Antioxidants 2020, 9, 1215. [Google Scholar] [CrossRef]
- Rasinska, E.; Rutkowska, J.; Czarniecka-Skubina, E.; Tambor, K. Effects of cooking methods on changes in fatty acids contents, lipid oxidation and volatile compounds of rabbit meat. LWT Food Sci. Technol. 2019, 110, 64–70. [Google Scholar] [CrossRef]
- Rui, G.; Estévez, M.; Morcuende, D. Suitability of the TBA method for assessing lipid oxidation in a meat system with added phenolic-rich materials. Food Chem. 2011, 126, 772–778. [Google Scholar] [CrossRef]
- Chen, X.; Luo, J.; Lou, A.; Wang, Y.; Yang, D.; Shen, Q.W. Duck breast muscle proteins, free fatty acids and volatile compounds as affected by curing methods. Food Chem. 2021, 338, 128138. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Zhu, Y.Z.; Xu, W.M. Composition of intramuscular phospholipids and free fatty acids in three kinds of traditional Chinese duck meat products. Poult. Sci. J. Anim. Sci. 2009, 88, 221–226. [Google Scholar] [CrossRef]
- Zeng, W.; Wen, W.; Yue, D.; Tian, Y.; Sun, Q.J. Chinese ethnic meat products: Continuity and development. Meat Sci. 2016, 120, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.; Smith, D.M. Use of Volatiles as Indicators of Lipid Oxidation in Muscle Foods. Compr. Rev. Food Sci. Food Saf. 2006, 5, 18–25. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Singhal, R.S.; Gholap, A.S.; Variyar, P.S.; Bongirwar, D.R. Hydrocarbons as marker compounds for irradiated cashew nuts. Food Chem. 2003, 80, 151–157. [Google Scholar] [CrossRef]
- Morrill, J.C.; Sawyer, J.E.; Smith, S.B.; Miller, R.K.; Johnson, M.D.; Wickersham, T.A. Post-extraction algal residue in beef steer finishing diets: II. Beef flavor, fatty acid composition, and tenderness. Algal Res. 2017, 25, 578–583. [Google Scholar] [CrossRef]
- Xu, L.; Xia, Q.; Cao, J.; He, J.; Zhou, C.; Guo, Y.; Pan, D. Ultrasonic effects on the headspace volatilome and protein isolate microstructure of duck liver, as well as their potential correlation mechanism. Ultrason. Sonochem. 2021, 71, 105358. [Google Scholar] [CrossRef]
- Muriel, E.; Antequera, T.; Petron, M.J.; Andres, A.I.; Ruiz, J. Volatile compounds in Iberian dry-cured loin. Meat Sci. 2004, 68, 391–400. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Y.; Pan, D.; Wang, Y.; Cao, J. Effects of high pressure treatment on lipolysis-oxidation and volatiles of marinated pork meat in soy sauce. Meat Sci. 2018, 145, 186–194. [Google Scholar] [CrossRef]
- Bueno, M.; Campo, M.M.; Cacho, J.; Ferreira, V.; Escudero, A. A model explaining and predicting lamb flavour from the aroma-active chemical compounds released upon grilling light lamb loins. Meat Sci. 2014, 98, 622–628. [Google Scholar] [CrossRef]
- Ba, H.V.; Seo, H.W.; Seong, P.N.; Cho, S.H.; Kang, S.M.; Kim, Y.S.; Moon, S.S.; Choi, Y.M.; Kim, J.H. Live weights at slaughter significantly affect the meat quality and flavor components of pork meat. Anim. Sci. J. 2019, 90, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Dransfield, E. The taste of fat. Meat Sci. 2008, 80, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Fu, M.; Li, Z.; Peng, X. Evaluation of Freshness in Determination of Volatile Organic Compounds Released from Pork by HS-SPME-GC-MS. Food Anal. Method 2018, 11, 1321–1329. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
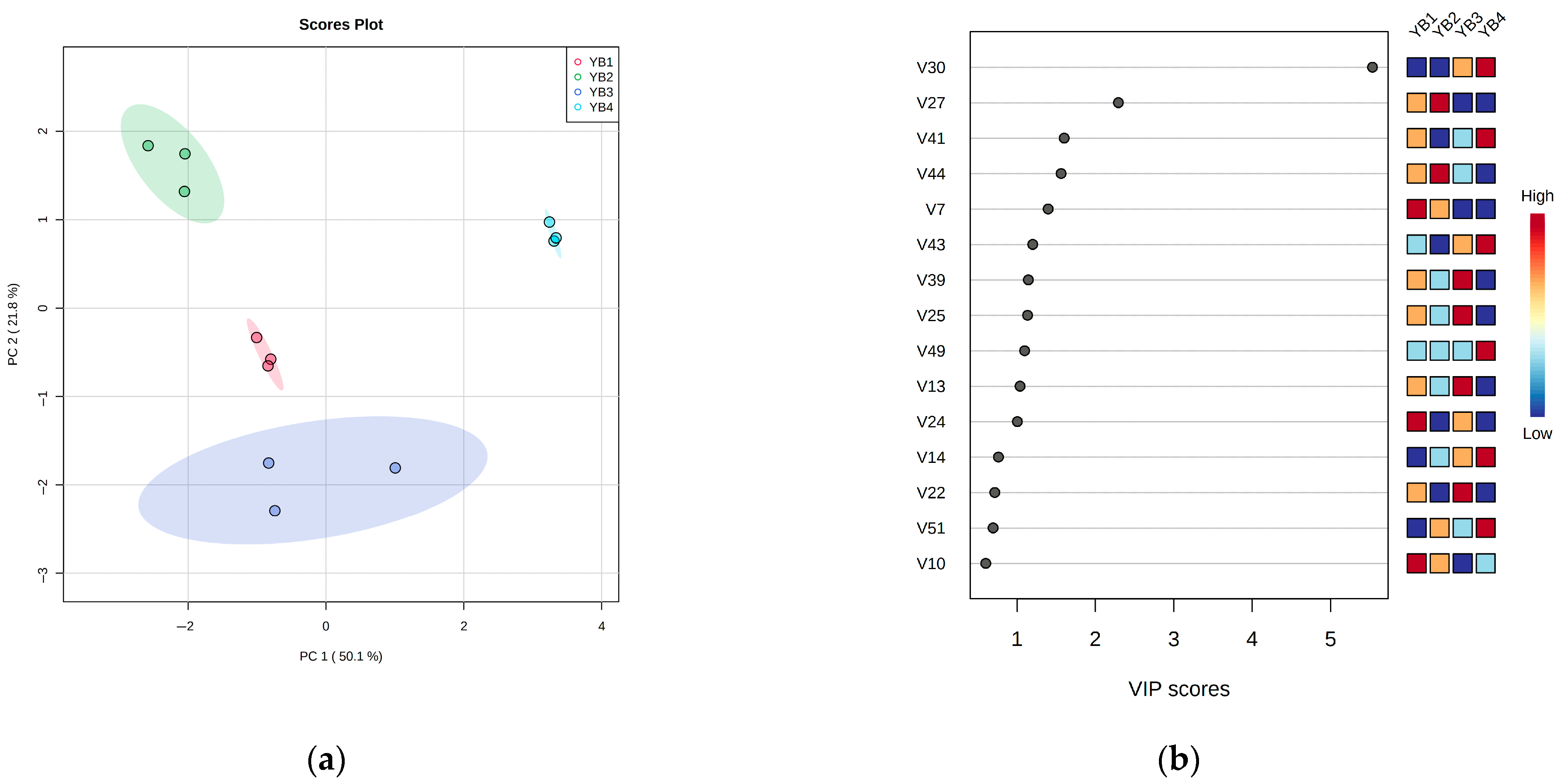
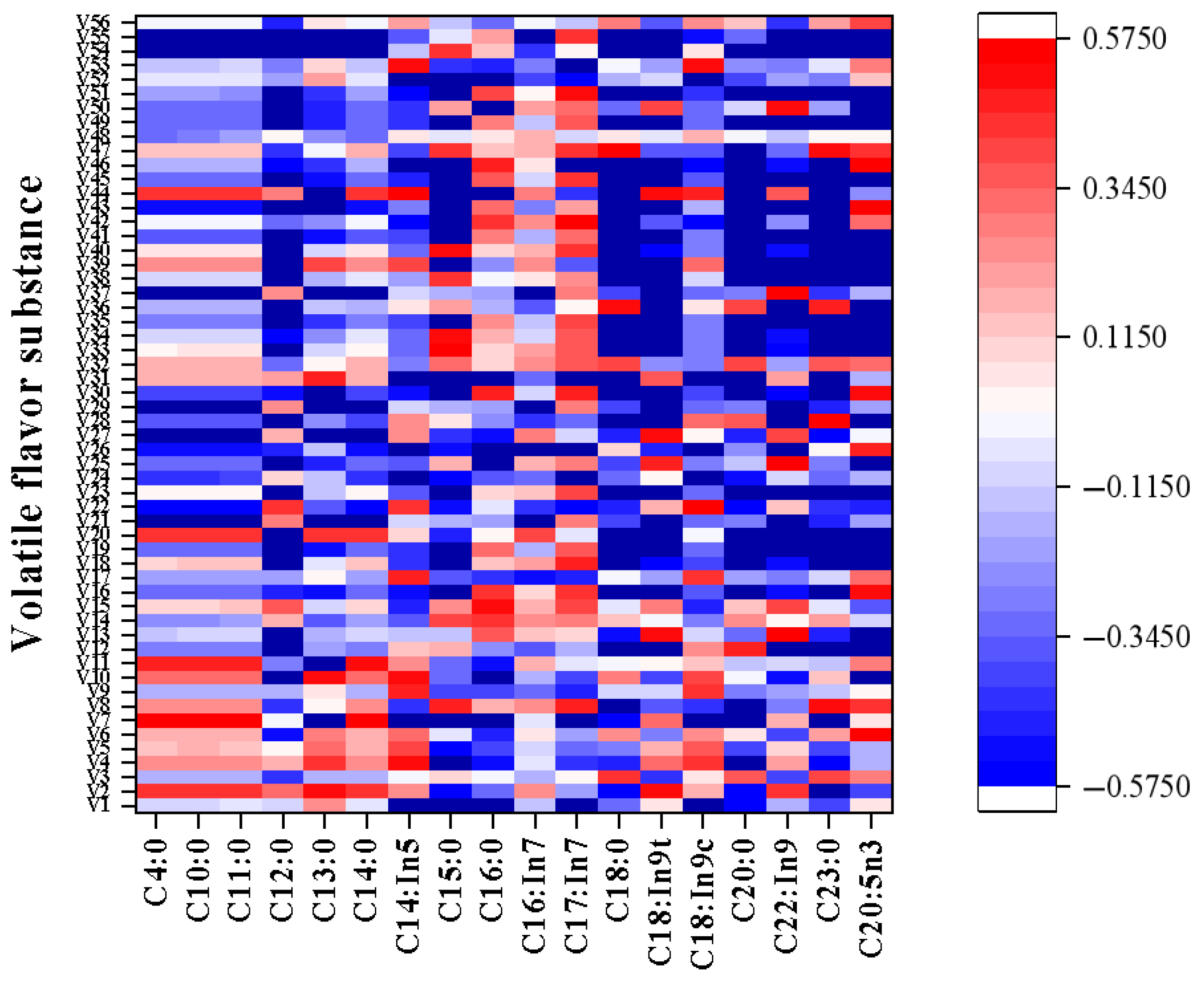
| YB1 (mg/100 g) | YB2 (mg/100 g) | YB3 (mg/100 g) | YB4 (mg/100 g) | ||
|---|---|---|---|---|---|
| 1 | C4:0 | 0 ± 0.00 | 1.66 ± 0.06 | 0 ± 0.00 | 0 ± 0.00 |
| 2 | C10:0 | 0 ± 0.00 | 14.86 ± 0.14 | 0 ± 0.00 | 0 ± 0.00 |
| 3 | C11:0 | 0 ± 0.00 | 7.35 ± 0.87 | 0 ± 0.00 | 0 ± 0.00 |
| 4 | C12:0 | 19.23 ± 0.23 c | 41.99 ± 0.26 b | 62.54 ± 0.42 a | 0 ± 0.00 c |
| 5 | C13:0 | 53.75 ± 0.59 b | 124.52 ± 0.58 a | 0 ± 0.00 c | 0 ± 0.00 c |
| 6 | C14:0 | 0 ± 0.00 | 2.11 ± 0.12 | 0 ± 0.00 | 0 ± 0.00 |
| 7 | C14:1n5 | 97.51 ± 1.87 a | 23.68 ± 4.07 b | 0 ± 0.00 c | 0 ± 0.00 c |
| 8 | C15:0 | 8.03 ± 0.37 d | 14.82 ± 0.62 c | 19.36 ± 2.17 b | 25.44 ± 1.57 a |
| 9 | C16:0 | 73.87 ± 0.61 d | 88.37 ± 1.25 c | 111.90 ± 0.58 b | 101.64 ± 0.68 a |
| 10 | C16:1n7 | 15.22 ± 4.46 a | 27.34 ± 5.31 b | 22.99 ± 3.34 b | 19.95 ± 0.25 ab |
| 11 | C17:1n7 | 0 ± 0.00 b | 16.93 ± 4.02 a | 18.07 ± 0.95 a | 18.76 ± 0.42 a |
| 12 | C18:0 | 47.89 ± 0.65 b | 38.88 ± 1.72 d | 42.10 ± 0.37 c | 101.80 ± 0.46 a |
| 13 | C18:1n9t | 8.81 ± 0.92 c | 20.28 ± 1.31 a | 17.56 ± 0.58 b | 0 ± 0.00 d |
| 14 | C18:1n9c | 138.52 ± 12.10 a | 53.99 ± 0.31 b | 55.62 ± 0.90 b | 54.91 ± 0.16 b |
| 15 | C20:0 | 32.86 ± 0.42 d | 41.14 ± 0.24 c | 46.69 ± 0.63 b | 75.60 ± 0.16 a |
| 16 | C22:1n9 | 2.20 ± 0.13 b | 8.63 ± 0.70 a | 8.13 ± 0.47 a | 0 ± 0.00 c |
| 17 | C23:0 | 24.93 ± 0.53 b | 22.62 ± 0.50 c | 25.73 ± 1.13 b | 40.76 ± 0.23 a |
| 18 | C20:5n3 | 8.18 ± 0.28 b | 7.70 ± 0.22 c | 7.05 ± 0.10 d | 8.74 ± 0.11 a |
| SFA | 260.56 ± 3.40 | 398.32 ± 6.36 | 308.32 ± 5.30 | 345.24 ± 3.10 | |
| UFA | 270.44 ± 19.76 | 158.55 ± 15.94 | 129.42 ± 6.34 | 102.36 ± 0.94 | |
| ∑FFA | 531 ± 23.16 | 556.87 ± 22.30 | 437.74 ± 11.64 | 447.60 ± 4.04 | |
| SFA/UFA | 0.96 | 2.52 | 2.39 | 3.38 |
| Order | Retention Time | Components | Threshold Value (µg/kg) | YB1 | OAV | YB2 | OAV | YB3 | OAV | YB4 | OAV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| V1 | 7.04 | α-Pinene | ND | 7.03 ± 0.93 a | 5.36 ± 1.39 ab | 5.06 ± 0.89 b | 4.73 ± 0.36 b | ||||
| V2 | 8.61 | β-Pinene | 6 | 46.81 ± 4.71 a | 7.80 | 49.04 ± 0.78 a | 8.17 | 45.28 ± 7.11 a | 7.55 | 41.26 ± 1.70 a | 6.87 |
| V3 | 9.26 | (+)-2-Carene | ND | 3.15 ± 0.41 a | 3.04 ± 0.07 a | 2.99 ± 0.50 a | 3.40 ± 0.29 a | ||||
| V4 | 10.03 | trans-β-Ocimene | 34 | 10.52 ± 0.15 a | 0.31 | 10.22 ± 0.22 a | 0.30 | 9.60 ± 1.64 a | 0.28 | 9.00 ± 0.62 a | 0.26 |
| V5 | 10.31 | 3-Carene | 5 | 9.91 ± 0.83 a | 1.98 | 9.62 ± 0.37 a | 1.92 | 9.11 ± 1.51 a | 1.82 | 8.89 ± 0.78 a | 1.78 |
| V6 | 11.05 | 2-Carene | ND | 4.68 ± 0.30 a | 4.52 ± 0.22 a | 2.65 ± 2.25 a | 4.64 ± 0.48 a | ||||
| V7 | 12.07 | Cyclohexene, 1-methyl-4-(1-methylethylidene)- | ND | 1.94 ± 0.05 a | 1.84 ± 0.06 b | ND | ND | ||||
| V8 | 16.97 | γ-Elemene | ND | 3.51 ± 0.11 b | 4.01 ± 0.23 ab | 3.56 ± 0.53 b | 4.29 ± 0.10 a | ||||
| V9 | 17.84 | alfa.-Copaene | ND | 3.04 ± 0.74 a | 0.42 ± 0.07 a | 0.31 ± 0.10 a | 0.49 ± 0.02 a | ||||
| V10 | 18.81 | Caryophyllene | 64 | 18.82 ± 0.53 a | 0.29 | 17.86 ± 0.97 ab | 0.27 | 16.63 ± 2.43 c | 0.26 | 16.93 ± 0.31 b | 0.26 |
| V11 | 19.08 | trans-α-Bergamotene | ND | 0.78 ± 0.05 ab | 0.85 ± 0.13 a | 0.63 ± 0.08 b | 0.75 ± 0.10 ab | ||||
| V12 | 19.52 | Humulene | ND | 1.23 ± 0.18 ab | 1.06 ± 0.03 b | 0.96 ± 0.10 b | 1.39 ± 0.15 a | ||||
| V13 | 19.97 | γ-Muurolene | ND | 2.71 ± 0.17 b | 2.69 ± 0.36 b | 8.48 ± 5.76 a | — | ||||
| V14 | 19.71 | β-copaene | ND | 0.30 ± 0.01 a | 0.39 ± 0.09 a | 1.28 ± 0.78 a | 0.93 ± 0.19 a | ||||
| V15 | 20.21 | α-Guaiene | ND | 4.32 ± 0.20 a | 5.76 ± 1.63 a | 6.49 ± 2.34 a | 5.43 ± 0.14 a | ||||
| V16 | 20.32 | β-curcumene | ND | 9.72 ± 0.96 b | 10.51 ± 1.59 b | 13.29 ± 8.24 b | 23.15 ± 2.86 a | ||||
| V17 | 20.58 | β-Bisabolene | ND | 7.00 ± 3.05 a | 5.03 ± 0.52 a | 4.97 ± 0.56 a | 5.34 ± 1.26 a | ||||
| V18 | 21.35 | α-Calacorene | ND | 1.79 ± 0.30 c | 2.70 ± 0.65 b | 1.60 ± 0.14 c | 3.93 ± 0.47 a | ||||
| V19 | 11.92 | Octanoic acid, methyl ester | ND | 1.64 ± 0.06 b | 1.64 ± 0.06 b | 1.66 ± 0.36 b | 3.60 ± 0.27 a | ||||
| V20 | 16.62 | Decanoic acid, methyl ester | ND | 2.10 ± 0.08 b | 2.77 ± 0.09 a | 2.85 ± 0.46 a | 0.87 ± 0.04 c | ||||
| V21 | 17.5 | (R)-lavandulyl acetate | ND | ND | 4.43 ± 0.45 | ND | ND | ||||
| V22 | 18.16 | Decanoic acid, ethyl ester | 5 | 0.87 ± 0.38 a | 0.17 | ND | 0.88 ± 0.14 a | ND | |||
| V23 | 21.46 | Geranyl isovalerate | ND | 0.60 ± 0.18 b | 0.88 ± 0.27 b | 0.45 ± 0.05 b | 1.54 ± 0.26 a | ||||
| V24 | 22.2 | Dodecanoic acid, ethyl ester | ND | 3.75 ± 2.84 a | ND | 1.95 ± 0.21 ab | ND | ||||
| V25 | 24.64 | Methyl tetradecanoate | ND | 0.41 ± 0.06 b | 0.28 ± 0.15 b | 5.33 ± 0.24 a | ND | ||||
| V26 | 25.65 | Ethyl 9-tetradecenoate | ND | 1.81 ± 0.68 a | 0.0089 ± 0.004 b | 0.23 ± 0.027 b | 0.63 ± 0.01 b | ||||
| V27 | 25.86 | Tetradecanoic acid, ethyl ester | ND | 2.52 ± 0.92 b | 7.57 ± 1.03 a | ND | ND | ||||
| V28 | 26.94 | Hexadecanoic acid, ethyl ester | 2000 | 12.83 ± 3.80 ab | <0.01 | 4.61 ± 1.18 bc | <0.01 | 2.80 ± 1.87 c | <0.01 | 15.72 ± 7.38 a | <0.01 |
| V29 | 31.86 | Ethyl Oleate | ND | ND | 0.23 ± 0.04 | ND | ND | ||||
| V30 | 28.86 | Ethyl 9-hexadecenoate | ND | ND | ND | 1.39 ± 0.38 b | 5.27 ± 0.23 a | ||||
| V31 | 8.16 | Bicyclo [3.1.0]hexane, 4-methylene-1-(1-methylethyl)- | ND | 62.87 ± 2.56 a | 55.37 ± 1.54 ab | 51.04 ± 7.56 b | 43.37 ± 0.42 c | ||||
| V32 | 21.56 | Tetradecane, 2,6,10-trimethyl- | ND | 4.46 ± 1.61 b | 7.36 ± 1.75 a | 4.51 ± 0.04 b | 9.01 ± 1.00 a | ||||
| V33 | 22.27 | Nonadecane | ND | 4.55 ± 2.19 b | 7.43 ± 4.37 b | 2.66 ± 0.41 b | 13.88 ± 2.07 a | ||||
| V34 | 11.44 | 1,6-Octadien-3-ol, 3,7-dimethyl- | ND | 162.42 ± 6.77 b | 167.56 ± 14.28 ab | 161.28 ± 18.32 b | 188.62 ± 2.80 a | ||||
| V35 | 13.27 | Terpinen-4-ol | 340 | 7.32 ± 0.28 b | 0.02 | 7.37 ± 0.87 b | 0.02 | 7.28 ± 0.76 b | 0.02 | 10.06 ± 0.07 a | 0.03 |
| V36 | 13.6 | α-Terpineol | ND | 1.66 ± 0.45 a | 1.54 ± 0.20 a | 1.2 ± 0.14 a | 1.86 ± 0.08 a | ||||
| V37 | 19.23 | α-acorenol | ND | ND | 1.64 ± 0.61 | ND | ND | ||||
| V38 | 21.67 | 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl-, (E)- | ND | 5.34 ± 0.70 b | 5.44 ± 0.87 b | 4.77 ± 0.32 b | 6.75 ± 0.45 a | ||||
| V39 | 21.88 | Behenic alcohol | ND | 0.54 ± 0.05 a | 0.51 ± 0.10 a | 0.56 ± 0.16 a | ND | ||||
| V40 | 22.07 | (-)-Spathulenol | ND | 1.28 ± 0.22 b | 1.50 ± 0.31 ab | 1.19 ± 0.15 b | 1.91 ± 0.21 a | ||||
| V41 | 22.43 | tert-Hexadecanethiol | ND | 2.92 ± 0.99 b | 1.17 ± 0.58 b | 1.19 ± 0.08 b | 36.12 ± 3.49 a | ||||
| V42 | 22.55 | 12-Methyl-E,E-2,13-octadecadien-1-ol | ND | 1.51 ± 0.52 b | 2.54 ± 0.48 ab | 2.39 ± 0.80 ab | 3.86 ± 1.60 a | ||||
| V43 | 23.26 | Epiglobulol | ND | 1.01 ± 0.54 b | ND | 1.15 ± 0.49 b | 3.58 ± 2.01 a | ||||
| V44 | 23.47 | 1-Hexadecanol, 2-methyl- | ND | 0.71 ± 0.48 a | 0.71 ± 0.23 a | 0.33 ± 0.01 ab | ND | ||||
| V45 | 4.3 | Hexanal | 4.5 | 4.50 ± 0.93 b | 1 | 4.58 ± 1.42 b | 1.01 | 5.08 ± 0.67 b | 1.13 | 10.90 ± 1.59 a | 2.42 |
| V46 | 7.9 | Benzaldehyde | 350 | 4.16 ± 0.24 c | 0.01 | 5.67 ± 0.33 b | 0.02 | 5.64 ± 0.90 b | 0.02 | 10.11 ± 0.64 a | 0.03 |
| V47 | 14.77 | Benzaldehyde, 4-(1-methylethyl)- | 24 | 25.35 ± 0.20 a | 1.05 | 27.41 ± 2.05 a | 1.14 | 25.48 ± 3.09 a | 1.06 | 29.23 ± 1.23 a | 1.21 |
| V48 | 15.58 | 2-Propenal, 3-phenyl- | 15.1 | 5.86 ± 1.50 a | 0.38 | 4.43 ± 3.93 a | 0.29 | 5.96 ± 0.73 a | 0.39 | 5.37 ± 0.99 a | 0.35 |
| V49 | 16.48 | 2,4-Decadienal, (E,E)- | 0.07 | ND | ND | ND | 8.64 ± 1.80 | 123.42 | |||
| V50 | 24.64 | Methyl tetradecanoate | ND | ND | ND | 0.35 ± 0.06 | ND | ||||
| V51 | 26.26 | Tetradecanal | 14 | 2.74 ± 0.40 b | 0.20 | 3.70 ± 0.61 b | 0.26 | 3.57 ± 0.33 b | 0.26 | 8.45 ± 1.04 a | 0.60 |
| V52 | 11.78 | Thujone | 360 | 3.47 ± 0.03 a | <0.01 | 3.17 ± 0.13 a | <0.01 | 3.05 ± 0.49 a | <0.01 | 3.09 ± 0.06 a | <0.01 |
| V53 | 13.77 | Estragole | 16 | 78.33 ± 2.12 a | 4.89 | 65.61 ± 10.16 a | 4.10 | 62.16 ± 16.71 a | 3.89 | 66.93 ± 12.97 a | 1.43 |
| V54 | 15.91 | Anethole | 15 | 285.77 ± 5.41 b | 19.05 | 244.21 ± 5.71 b | 16.28 | 267.31 ± 36.41 b | 17.82 | 336.62 ± 20.47 a | 22.44 |
| V55 | 20.06 | Benzene, 1-(1,5-dimethyl-4-hexenyl)-4-methyl- | ND | ND | 8.14 ± 0.60 a | 4.96 ± 2.33 b | ND | ||||
| V56 | 20.39 | Naphthalene | 6.8 | 27.78 ± 1.82 a | 4.08 | 26.71 ± 2.66 a | 3.92 | 24.88 ± 2.97 a | 3.65 | 27.94 ± 2.44 a | 4.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, B.; Zhang, C.; Song, Y.; Zhou, H.; Chen, X.; Gu, Q. Effects of Thermal Sterilization Conditions on Flavor and Lipid Oxidation of Sauced Duck Necks. Foods 2025, 14, 3136. https://doi.org/10.3390/foods14173136
Chu B, Zhang C, Song Y, Zhou H, Chen X, Gu Q. Effects of Thermal Sterilization Conditions on Flavor and Lipid Oxidation of Sauced Duck Necks. Foods. 2025; 14(17):3136. https://doi.org/10.3390/foods14173136
Chicago/Turabian StyleChu, Beibei, Chao Zhang, Yushen Song, Hui Zhou, Xingguang Chen, and Qianhui Gu. 2025. "Effects of Thermal Sterilization Conditions on Flavor and Lipid Oxidation of Sauced Duck Necks" Foods 14, no. 17: 3136. https://doi.org/10.3390/foods14173136
APA StyleChu, B., Zhang, C., Song, Y., Zhou, H., Chen, X., & Gu, Q. (2025). Effects of Thermal Sterilization Conditions on Flavor and Lipid Oxidation of Sauced Duck Necks. Foods, 14(17), 3136. https://doi.org/10.3390/foods14173136






