Protective Mechanism of Broad Bean Extract on Parkinson’s Disease Model Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Broad Bean Extract
2.3. Determination of the Components of Broad Bean Extract
2.3.1. Determination of L-DA Content
2.3.2. Determination of Total Phenolic Content
2.3.3. Determination of Total Flavonoid Content
2.4. Effects of Broad Bean Extract on PD Model Cell
2.4.1. Cell Culture
2.4.2. Experimental Grouping
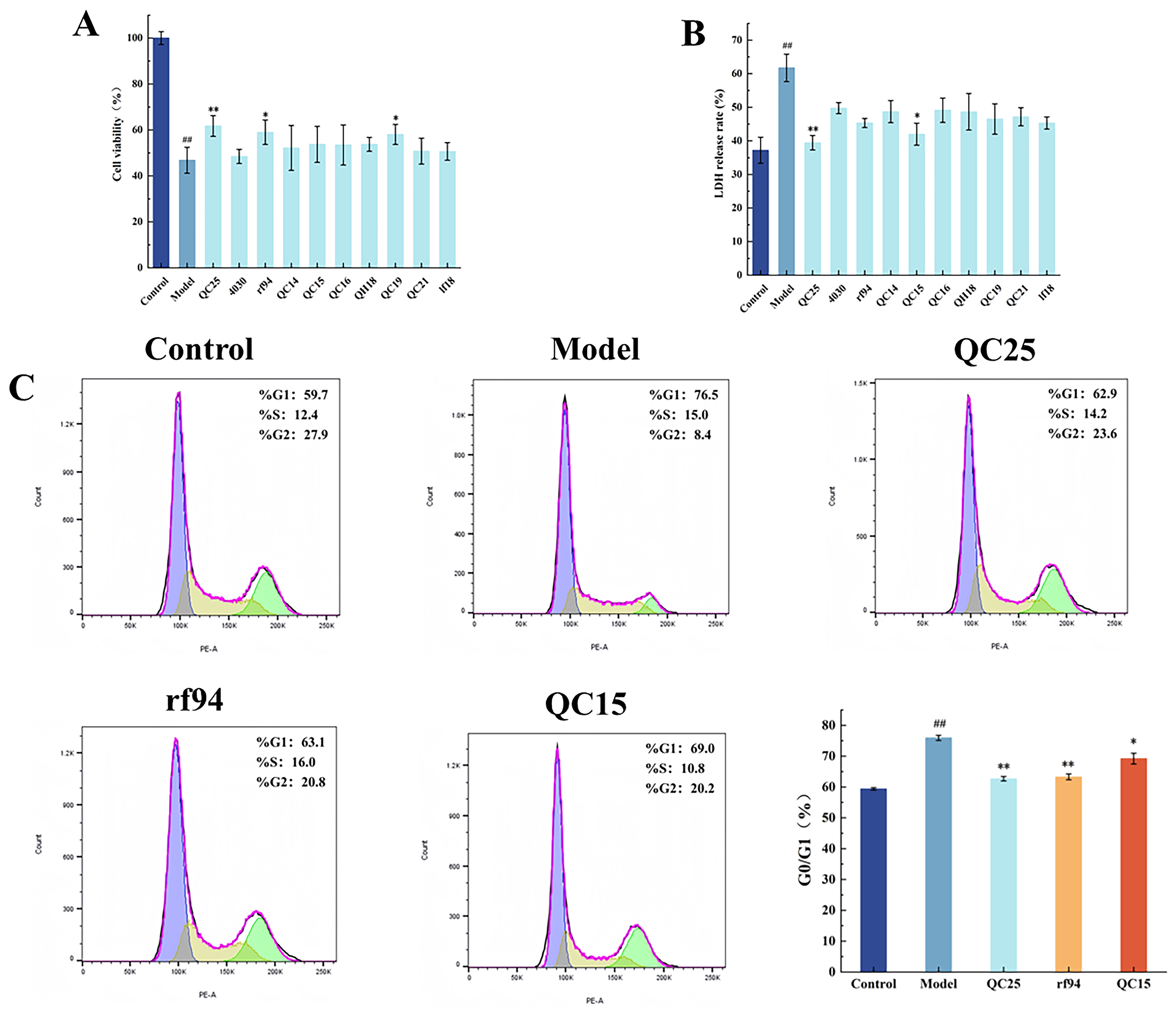
2.4.3. Cell Viability Assay
2.4.4. Measurement of Cell LDH Release
2.4.5. Measurement of Cell Cycle
2.5. Effects of Broad Bean Extract on Mitochondria in PD Model Cell
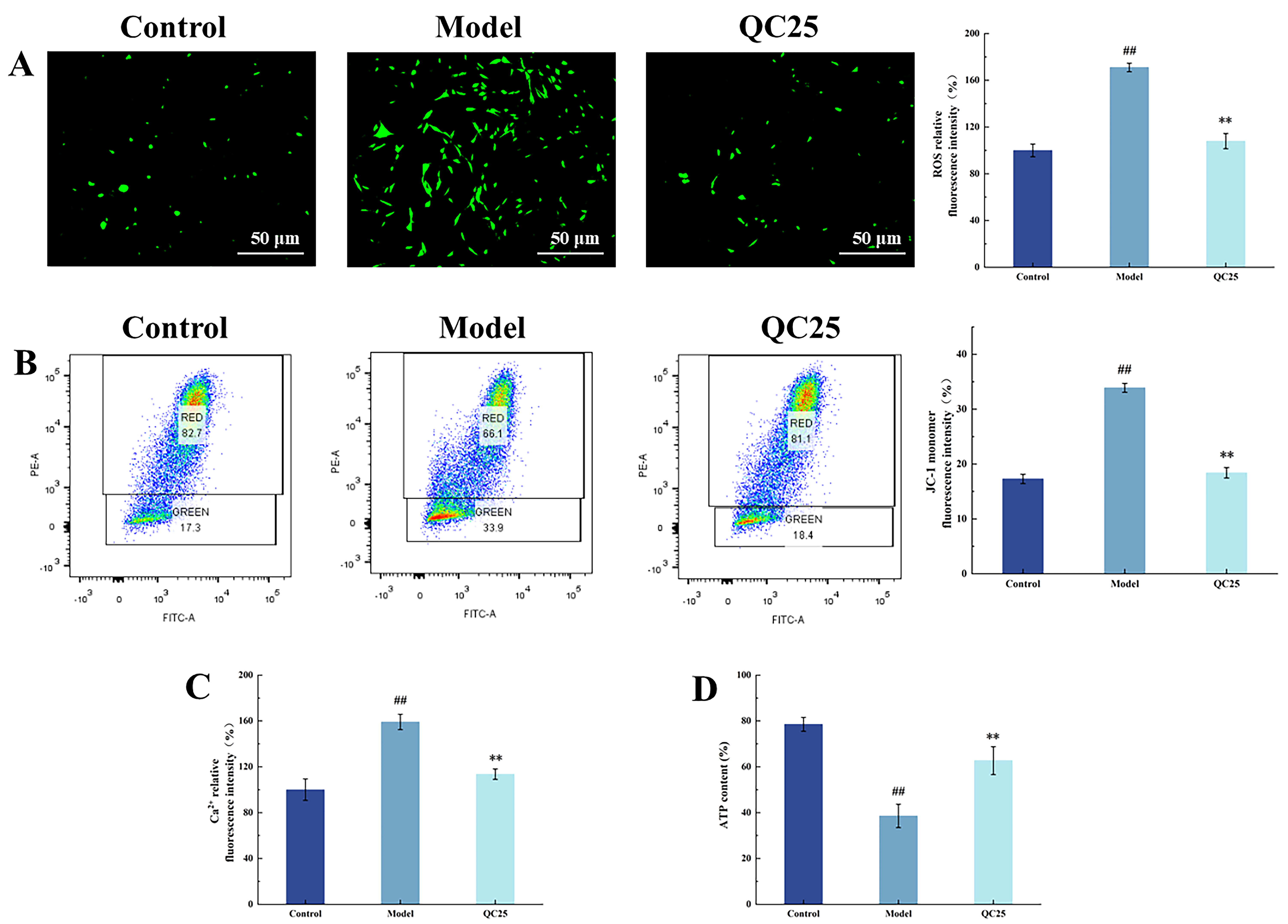
2.5.1. Measurement of ROS Concentration
2.5.2. Mitochondrial Membrane Potential Detection
2.5.3. ATP Content Measurement
2.5.4. Measurement of Ca2+ Concentration
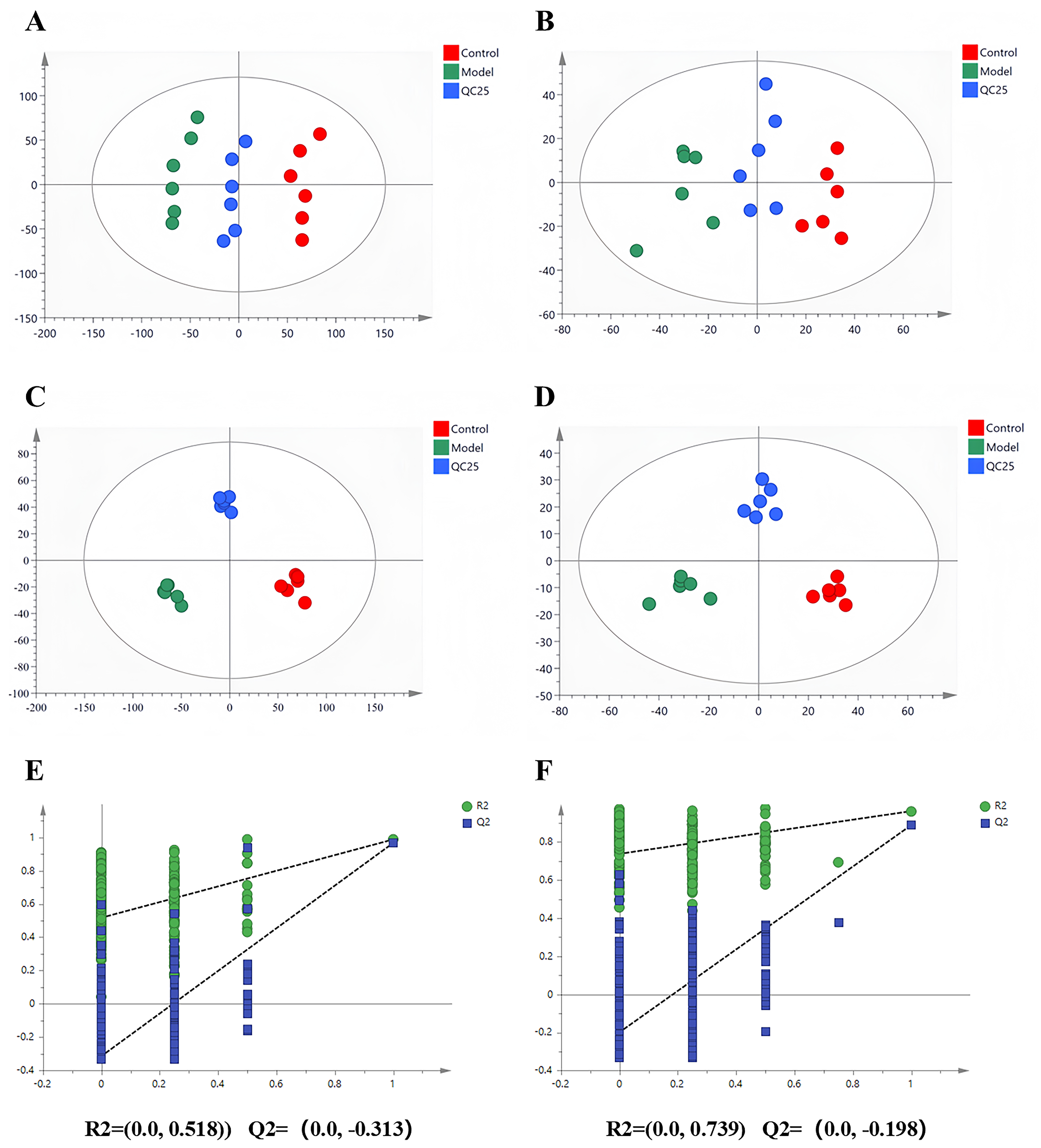
2.6. UPLC-QTOF/MS Quantitative Analysis
2.6.1. Sample Extraction
2.6.2. Instrumental Analysis
2.6.3. Data Processing
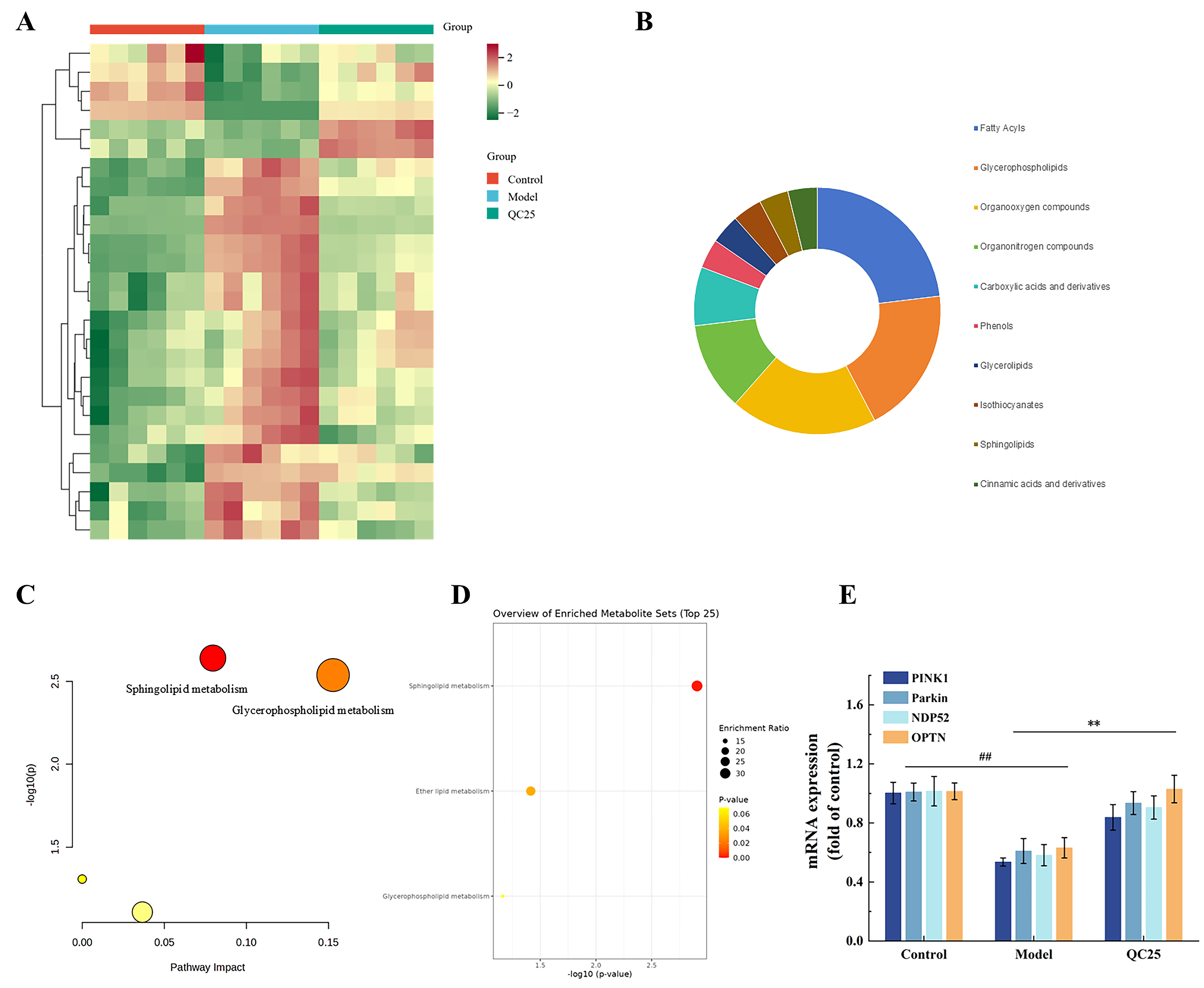
2.7. RT-PCR
2.8. Screening for Levodopa Synergistic Substances
2.9. Effects of 6-Gingerol Combined with Levodopa on PD Model Cell
2.10. Effects of 6-Gingerol Combined with Levodopa on Mitochondria
2.11. Effects of 6-Gingerol Combined with Levodopa on the PINK1/Parkin Pathway
2.12. Statistical Analysis
3. Results and Discussion
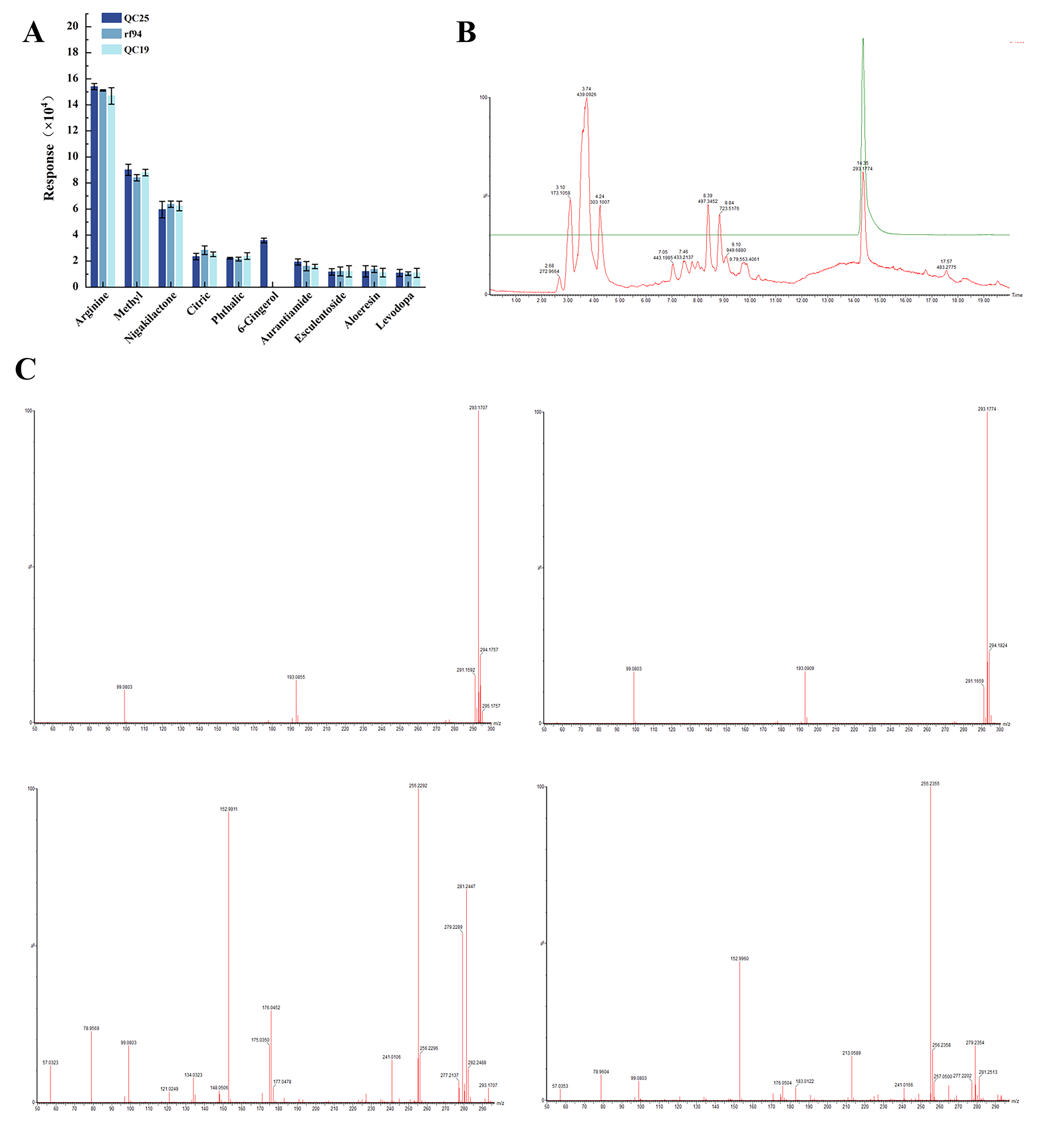
3.1. Determination of Active Components in Broad Bean Extract
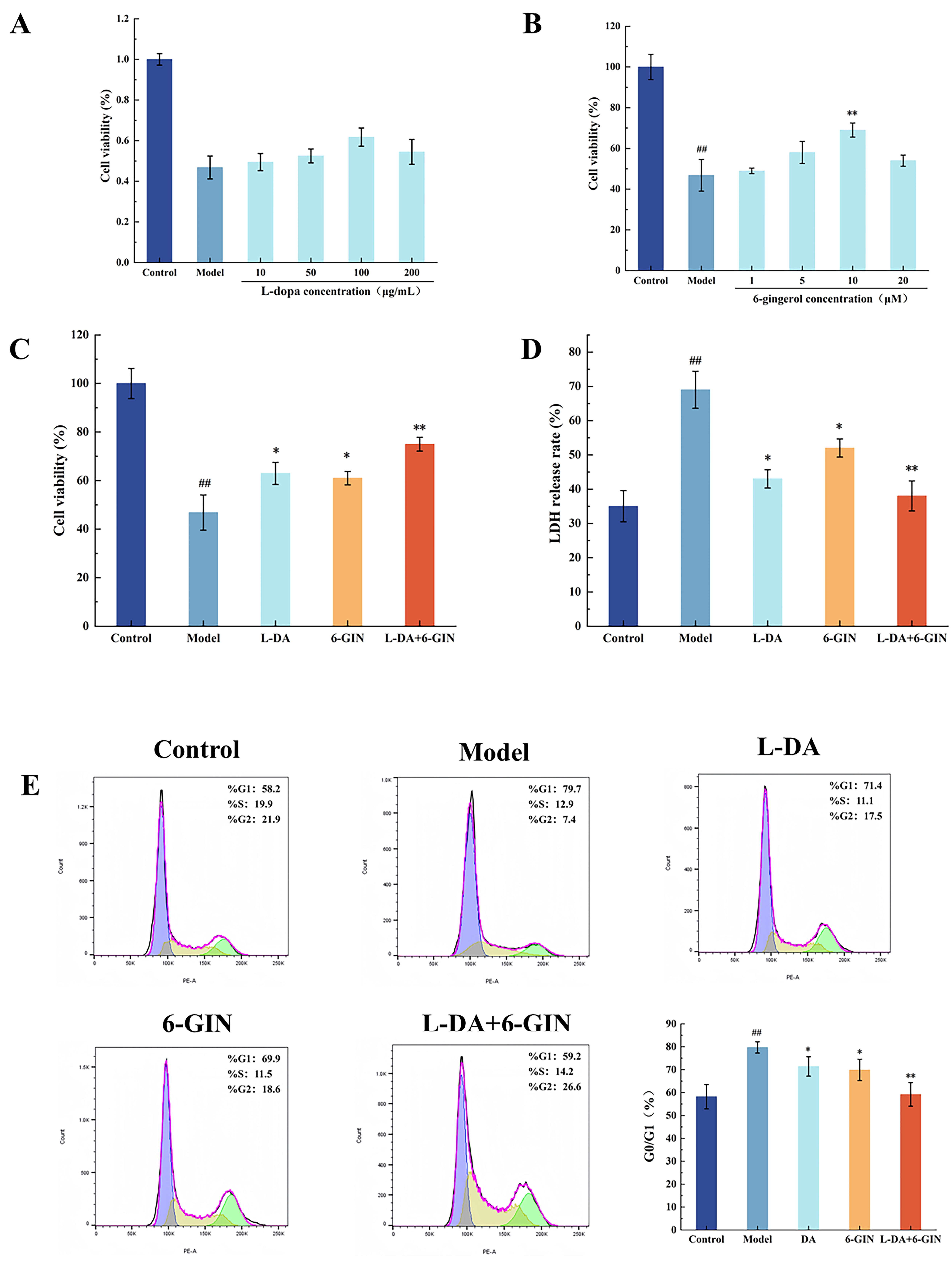
3.2. Effect of Broad Bean Extract on the Cell Activity
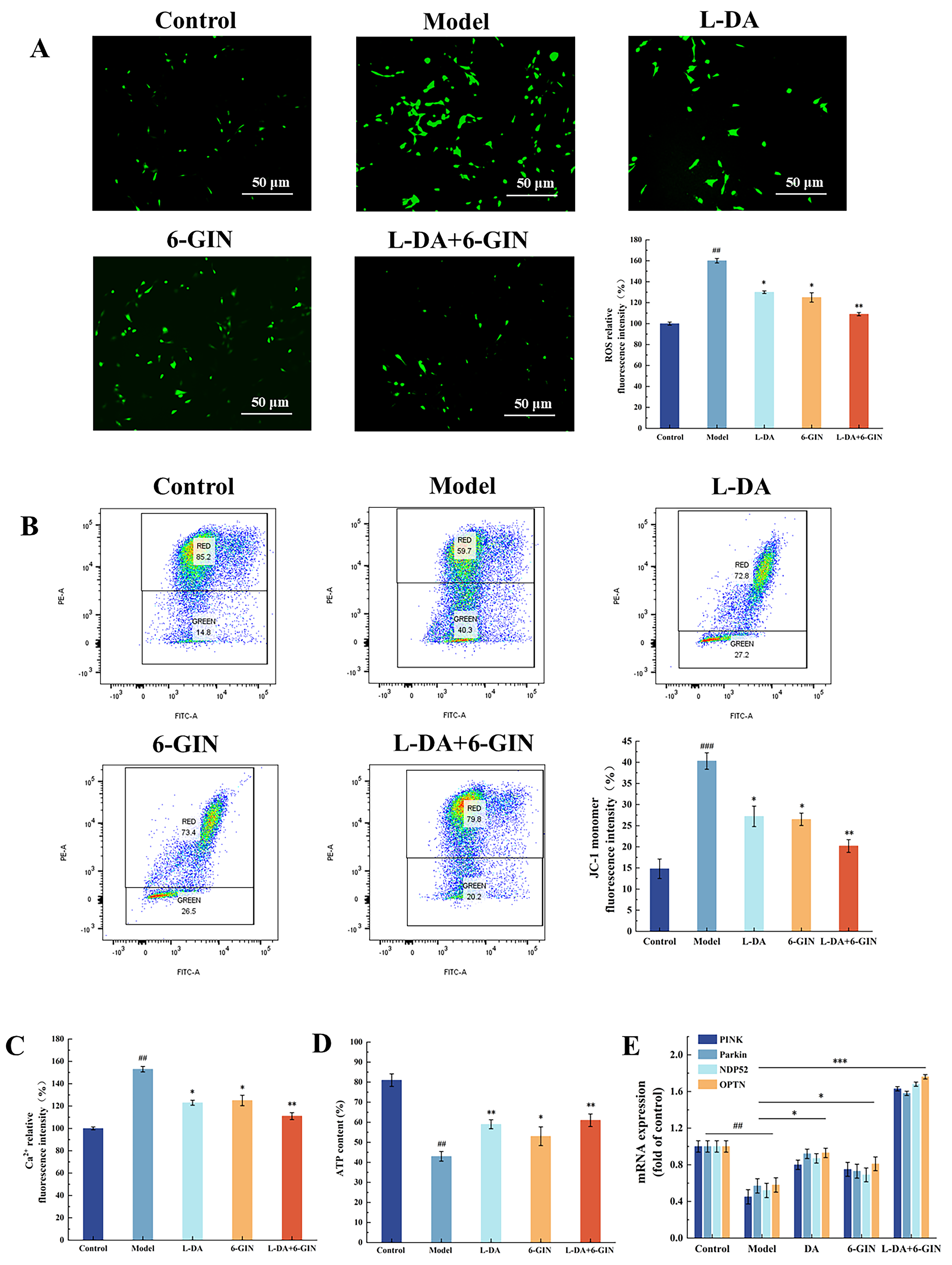
3.3. Effects of Broad Bean Extract on Mitochondrial Function
3.4. Effect of Broad Bean Extract on the PD Models Cell Metabolome by UPLC-QTOF/MS
3.4.1. Metabolomic Profiling Analysis
3.4.2. Metabolic Pathway Analysis
3.5. Effects of Broad Bean Extract on the PINK1/Parkin Pathway
3.6. Screening of Levodopa-Synergizing Substances
3.7. Synergistic Effects of 6-Gingerol and Levodopa in Parkinson’s Model Cells
3.8. Synergistic Effects of 6-Gingerol and Levodopa on Mitochondrial Function
3.9. Regulation of the PINK1/Parkin Pathway by 6-Gingerol and Levodopa Combination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Ebadpour, N.; Mahmoudi, M.; Kheder, R.K.; Abavisani, M.; Baridjavadi, Z.; Abdollahi, N.; Esmaeili, S.-A. From mitochondrial dysfunction to neuroinflammation in Parkinson’s disease: Pathogenesis and mitochondrial therapeutic approaches. Int. Immunopharmacol. 2024, 142, 113015. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-H.; Chen, C.-M. The role of oxidative stress in Parkinson’s disease. Antioxidants 2020, 9, 597. [Google Scholar] [CrossRef]
- Henchcliffe, C.; Beal, M.F. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat. Clin. Pract. Neurol. 2008, 4, 600–609. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, M.; Jiang, J. Mitochondrial dysfunction in neurodegenerative diseases and drug targets via apoptotic signaling. Mitochondrion 2019, 49, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Gu, L.; Smerin, D.; Mao, S.; Xiong, X. The interrelation between reactive oxygen species and autophagy in neurological disorders. Oxidative Med. Cell. Longev. 2017, 2017, 8495160. [Google Scholar] [CrossRef]
- Keane, H. Network Pharmacology of the MPP+ Cellular Model of Parkinson’s Disease; University of Oxford: Oxford, UK, 2015. [Google Scholar]
- Nicklas, W.J.; Youngster, S.K.; Kindt, M.V.; Heikkila, R.E., IV. MPTP, MPP+ and mitochondrial function. Life Sci. 1987, 40, 721–729. [Google Scholar] [CrossRef]
- Xu, X.-J.; Pan, T.; Fan, H.-J.; Wang, X.; Yu, J.-Z.; Zhang, H.-F.; Xiao, B.-G.; Li, Z.-Y.; Zhang, B.; Ma, C.-G. Neuroprotective effect of hyperoside in MPP+/MPTP-induced dopaminergic neurodegeneration. Metab. Brain Dis. 2023, 38, 1035–1050. [Google Scholar]
- Curry, D.W.; Stutz, B.; Andrews, Z.B.; Elsworth, J.D. Targeting AMPK signaling as a neuroprotective strategy in Parkinson’s disease. J. Park. Dis. 2018, 8, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Skinner, T.; Bridges, B.; Weber, J.T. The pathology of Parkinson’s disease and potential benefit of dietary polyphenols. Molecules 2020, 25, 4382. [Google Scholar] [CrossRef]
- Jalouli, M.; Rahman, M.A.; Biswas, P.; Rahman, H.; Harrath, A.H.; Lee, I.-S.; Kang, S.; Choi, J.; Park, M.N.; Kim, B. Targeting natural antioxidant polyphenols to protect neuroinflammation and neurodegenerative diseases: A comprehensive review. Front. Pharmacol. 2025, 16, 1492517. [Google Scholar] [CrossRef]
- García-Aguilar, A.; Palomino, O.; Benito, M.; Guillén, C. Dietary polyphenols in metabolic and neurodegenerative diseases: Molecular targets in autophagy and biological effects. Antioxidants 2021, 10, 142. [Google Scholar] [CrossRef]
- Poonia, A.; Vikranta, U.; Chaudhary, N.; Dangi, P. Current and potential health claims of faba beans (Vicia faba L.) and its components. In Faba Bean: Chemistry, Properties and Functionality; Springer: Berlin/Heidelberg, Germany, 2022; pp. 331–355. [Google Scholar]
- Baranowska, A. Health-promoting properties of broad beans (Vicia faba L.). Health Probl. Civiliz. 2024, 18, 481–490. [Google Scholar] [CrossRef]
- Kang, K.S.; Yamabe, N.; Wen, Y.; Fukui, M.; Zhu, B.T. Beneficial effects of natural phenolics on levodopa methylation and oxidative neurodegeneration. Brain Res. 2013, 1497, 1–14. [Google Scholar] [CrossRef]
- Box, J. Investigation of the Folin-Ciocalteau phenol reagent for the determination of polyphenolic substances in natural waters. Water Res. 1983, 17, 511–525. [Google Scholar] [CrossRef]
- Guoqing, S.; Qisen, X.; Yichao, F.; Wenen, Z.; Yanqi, L. Optimization of Process on Extracting Flavonoids from Dry Onion Skins by Alkaline Method. Open Chem. Eng. J. 2015, 9, 39–46. [Google Scholar] [CrossRef]
- Bao, B.; Zhang, M.-Q.; Chen, Z.-Y.; Wu, X.-B.; Xia, Z.-B.; Chai, J.-Y.; Yin, X.-P. Sulforaphane prevents PC12 cells from oxidative damage via the Nrf2 pathway. Mol. Med. Rep. 2019, 19, 4890–4896. [Google Scholar] [CrossRef] [PubMed]
- Bertero, E.; Maack, C. Calcium signaling and reactive oxygen species in mitochondria. Circ. Res. 2018, 122, 1460–1478. [Google Scholar] [CrossRef]
- Komary, Z.; Tretter, L.; Adam-Vizi, V. Membrane potential-related effect of calcium on reactive oxygen species generation in isolated brain mitochondria. Biochim. Biophys. Acta (BBA)-Bioenerg. 2010, 1797, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, J.; Eckert, S.H.; Leuner, K.; Muller, W.E.; Eckert, G.P. Mitochondria: Mitochondrial membranes in brain ageing and neurodegeneration. Int. J. Biochem. Cell Biol. 2013, 45, 76–80. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, J.; Sun, L.; Zheng, Q.; Cao, C.; Ding, W.; Yang, S.; Ma, L.; Zhang, W. GALNT9 enrichment attenuates MPP+-induced cytotoxicity by ameliorating protein aggregations containing α-synuclein and mitochondrial dysfunction. Biol. Direct 2024, 19, 77. [Google Scholar] [CrossRef]
- Zündorf, G.; Reiser, G. Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antioxid. Redox Signal. 2011, 14, 1275–1288. [Google Scholar] [CrossRef]
- Richter, C.; Frei, B. Ca2+ release from mitochondria induced by prooxidants. Free Radic. Biol. Med. 1988, 4, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S. Mitochondria. Cold Spring Harb. Perspect. Biol. 2021, 13, a040543. [Google Scholar] [CrossRef]
- Choi, E.-H.; Kim, M.-H.; Park, S.-J. Targeting mitochondrial dysfunction and reactive oxygen species for neurodegenerative disease treatment. Int. J. Mol. Sci. 2024, 25, 7952. [Google Scholar] [CrossRef]
- Gonzalez-Baro, M.R.; Coleman, R.A. Mitochondrial acyltransferases and glycerophospholipid metabolism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 49–55. [Google Scholar] [CrossRef]
- Mejia, E.M.; Hatch, G.M. Mitochondrial phospholipids: Role in mitochondrial function. J. Bioenerg. Biomembr. 2016, 48, 99–112. [Google Scholar] [CrossRef]
- Kazlauskaite, A.; Muqit, M.M. PINK 1 and Parkin–mitochondrial interplay between phosphorylation and ubiquitylation in Parkinson’s disease. FEBS J. 2015, 282, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Vainshtein, A.; Grumati, P. Selective autophagy by close encounters of the ubiquitin kind. Cells 2020, 9, 2349. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Zeng, Y.; Zeng, X.; Tan, Q.; He, Q.; Wang, S.; Wang, J. 6-Gingerol improves lipid metabolism disorders in skeletal muscle by regulating AdipoR1/AMPK signaling pathway. Biomed. Pharmacother. 2024, 180, 117462. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Shima, S.; Kawabata, K.; Mizutani, Y.; Ueda, A.; Ito, M. Brain network and energy imbalance in Parkinson’s disease: Linking ATP reduction and α-synuclein pathology. Front. Mol. Neurosci. 2025, 17, 1507033. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, X.; Jiang, L.; Liu, X.; Chen, M.; Yao, X.; Sun, Q.; Yang, G. 6-Gingerol induces autophagy to protect HUVECs survival from apoptosis. Chem.-Biol. Interact. 2016, 256, 249–256. [Google Scholar] [CrossRef] [PubMed]
| Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| PINK1 | TGACCCACTGGACACACGAC | GCTCCCTTTGAGACGACATC |
| PARKIN | ACGCTCAACTTGGCTACTCC | CACTCCTCGGCACCATACTG |
| OPTN | GGACAGCCCTTGTGAGACCC | TCAAATCGCCCTTTCATAGC |
| NDP52 | CGCTACCAGTTCTGCTATGTGGA | TCATGGAGGTCAGCGTACTTGTC |
| GAPDH | GACATGCCGCCTGGAGAAAC | AGCCCAGGATGCCCTTTAGT |
| Content | QC25 | 4030 | rf94 | QC14 | QC15 | QC16 | QC18 | QC19 | QC21 | lf18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Breed | |||||||||||
| Levodopa | 14.89 ± 0.87% | 10.01 ± 0.82% | 15.24 ± 1.42% | 10.53 ± 0.60% | 5.59 ± 0.53% | 6.38 ± 1.78% | 9.94 ± 0.44% | 8.47 ± 0.17% | 9.7 ± 0.80% | 5.33 ± 0.08% | |
| Total phenol (mg GAE/g) | 27.52 ± 0.20% | 19.64 ± 0.28% | 13.71 ± 0.23% | 11.42 ± 0.08% | 6.44 ± 0.19% | 5.50 ± 0.06% | 10.18 ± 0.09% | 8.93 ± 0.07% | 13.16 ± 0.09% | 8.69 ± 0.14% | |
| Total flavone (mg RE/g) | 3.32 ± 0.08% | 1.45 ± 0.03% | 2.78 ± 0.04% | 1.22 ± 0.03% | 1.55 ± 0.03% | 1.65 ± 0.04% | 0.73 ± 0.03% | 0.84 ± 0.04% | 0.92 ± 0.05% | 0.73 ± 0.02% | |
| Mode | No. | Component | RT | Observed m/z | Molecular Formulas | Fragment Ions (m/z) |
|---|---|---|---|---|---|---|
| ESI+ | 1 | Arginine | 0.95 | 175.1178 | C6H14N4O2 | 70.0664, 1052.0 |
| ESI- | 2 | Methyl lucidenate Q | 19.06 | 473.2906 | C28H42O6 | 473.29012, 208.00186 |
| ESI+ | 3 | Nigakilactone H | 15.84 | 425.2147 | C22H32O8 | 129.01709, 365.19426 |
| ESI- | 4 | Citric acid_1 | 2.15 | 191.0211 | C6H8O7 | 111.1, 100.0 |
| ESI+ | 5 | Phthalic anhydride | 14.78 | 149.022 | C9H6O3 | 163.038971, 99.999999 |
| ESI- | 6 | 6-Gingerol | 11.23 | 293.1797 | C17H26O4 | 99.0815, 193.090600 |
| ESI- | 7 | Aurantiamide acetate | 4.04 | 443.2005 | C27H28N2O4 | 194.1176, 21818032.0 |
| ESI+ | 8 | Esculentoside A | 15.84 | 827.4432 | C24H66O16 | 425.21474, 129.01709 |
| ESI- | 9 | Aloeresin C | 1.41 | 701.2056 | C34H38O16 | 383.12500, 89.02377 |
| ESI+ | 10 | Levodopa | 2.39 | 198.075 | C9H11NO4 | 152.0, 64632.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Gao, Q.; Li, T.; Zhao, J.; Liu, Y.; Wang, X.; Fan, M.; Qian, H.; Li, Y.; Wang, L. Protective Mechanism of Broad Bean Extract on Parkinson’s Disease Model Cells. Foods 2025, 14, 3244. https://doi.org/10.3390/foods14183244
Chen X, Gao Q, Li T, Zhao J, Liu Y, Wang X, Fan M, Qian H, Li Y, Wang L. Protective Mechanism of Broad Bean Extract on Parkinson’s Disease Model Cells. Foods. 2025; 14(18):3244. https://doi.org/10.3390/foods14183244
Chicago/Turabian StyleChen, Xuhao, Qiang Gao, Tingting Li, Jiajia Zhao, Yujiao Liu, Xuejun Wang, Mingcong Fan, Haifeng Qian, Yan Li, and Li Wang. 2025. "Protective Mechanism of Broad Bean Extract on Parkinson’s Disease Model Cells" Foods 14, no. 18: 3244. https://doi.org/10.3390/foods14183244
APA StyleChen, X., Gao, Q., Li, T., Zhao, J., Liu, Y., Wang, X., Fan, M., Qian, H., Li, Y., & Wang, L. (2025). Protective Mechanism of Broad Bean Extract on Parkinson’s Disease Model Cells. Foods, 14(18), 3244. https://doi.org/10.3390/foods14183244







