Proteins from Edible Mushrooms: Nutritional Role and Contribution to Well-Being
Abstract
1. Introduction
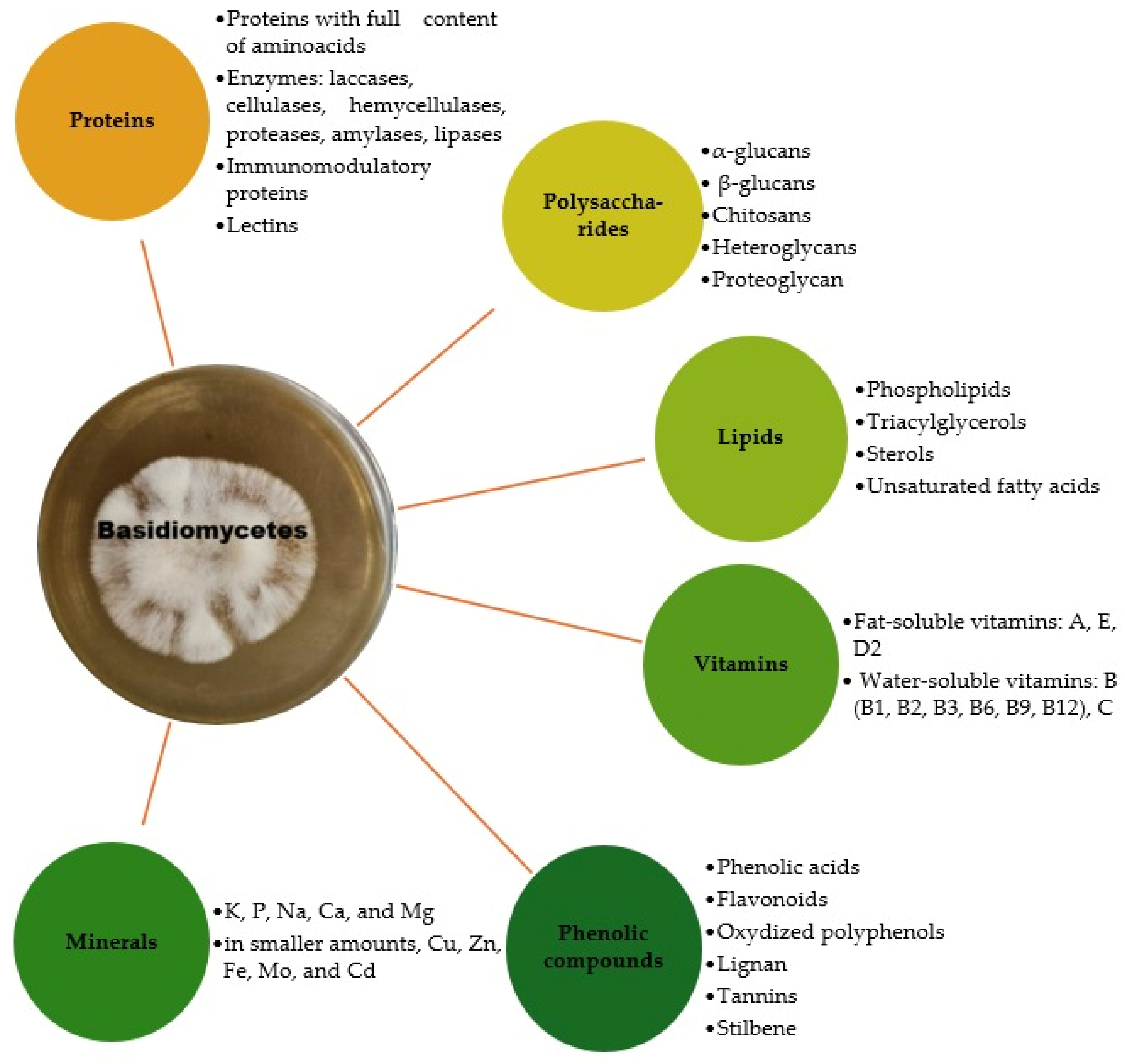
2. The Main Bioactive Compounds in Mushrooms
2.1. Polysaccharides
2.2. Lipids
2.3. Phenolic Compounds
2.4. Terpenes and Terpenoids
2.5. Vitamins and Minerals
3. Mushroom Proteins and Their Properties
3.1. Proteins with Nutritional Value
- The protein-to-energy ratio provides information about the energy value of mushroom protein, which is comparable to that of animal protein and considerably higher than that of vegetable protein (oats and rice) [8]. Thus, according to González et al., species belonging to the Agaricus genus have PER values in the range of 0.7–0.9 g kcal−1, similar to beef jerky [8]. Different species of Pleurotus had PER values of 0.59–0.98 g kcal−1 compared to 0.034 (oats) and 0.018 (white rice) [53,54]. Therefore, the consumption of mushrooms with a low calorie content and a high protein percentage is recommended for people who want to lose weight healthily.
- The Essential Amino Acid Score measures the proportion of each essential amino acid in the protein compared to a standard complete protein. According to Bach et al., almost all essential amino acids in the selected species of Agaricus, Pleurotus, Flamulina, and Lentinus had a score higher than 1 mg in 1 g of protein, meaning that the amino acid requirements are met according to the recommended essential amino acid profile for adults [53].
- Another characteristic of protein is Protein Digestibility, which measures the amount of protein available for absorption after the digestion process and is estimated from dietary, fecal or ileal, and urinary nitrogen values. The amount of ingested protein that is available for absorption represents the Apparent Digestibility and is calculated as the difference between dietary N and fecal N, relative to dietary N. For a more accurate calculation, TPD (True Protein Digestibility) is determined, in which AD is corrected with the mandatory value of fecal N, which is subtracted from fecal N. Some studies have reported the lowest TPD values of approximately 43% for Pleurotus sajor-caju or over 80% for Agaricus macrosporus [55]. Other studies have shown that the TPD of mushrooms ranges from 72% to 83%, similar to that for soybean (74%) and rice (82%) but lower than that for casein (87.49%) [56,57].
- The percentage of amino acids retained by the body after absorption through the intestines is known as Biological Value (BV). Values greater than 60% have been found for protein from Lentinus lepidus, P. sajor-caju, P. ostreatus, and L. edodes, harvested in Thailand [55].
- The Protein-Digestibility-Corrected Amino Acid Score (PDCAAS) is a measure that provides information about the content and profile of amino acids compared to a reference protein, considering TPD as a correction factor [8]. Compared to meat and milk protein, with a TPD of 94% [58], edible mushroom protein typically has TPD values between 30% and 45% [55,57].
3.1.1. Applications in the Food Industry
3.1.2. Mushroom Protein Hydrolysates as Sports Nutrition or Therapeutic Foods
3.1.3. Safety and Allergenicity Assessment of Mushroom Proteins
3.2. Proteins with Functional Roles
3.2.1. Lectins
Applications of Lectins
3.2.2. Enzymes
3.2.3. Ribosome-Inactivating Proteins (RIPs)
3.2.4. Hydrophobins (HPs)
4. Cultivation of Mushrooms from the Basidiomycota Phylum
- The isolation and selection of mushroom species with high production potential in terms of the compound of interest;
- Obtaining and maintaining the laboratory stock culture and choosing a method to preserve the properties of the species;
- Testing the cultivation conditions at the laboratory level (it is necessary to choose the optimal culture medium, temperature, pH, type and quantity of inoculum, aeration and agitation, culture duration, etc.);
- Cultivating the fungus in bioreactors of different capacities (the type of bioreactor and cultivation parameters will be chosen);
- Separation of the product with a high protein content is usually achieved by processing the mycelium and through extraction, centrifugation, precipitation, and other methods [172], along with analyzing the synthesized compounds.
5. Future Trends
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Royse, D.J.; Baars, J.; Tan, Q. Current overview of mushroom production in the world. In Edible and Medicinal Mushrooms: Technology and Applications; Zied, D.C., Pardo-Giménez, A., Eds.; John Wiley & Sons: New York, NY, USA, 2017; pp. 5–13. [Google Scholar] [CrossRef]
- Singh, M.; Kamal, S.; Sharma, V.P. Species and region-wise mushroom production in leading mushroom producing countries-China, Japan, USA, Canada and India. Mushroom Res. 2022, 30, 119394. [Google Scholar] [CrossRef]
- Devi, P.V.; Islam, J.; Narzary, P.; Sharma, D.; Sultana, F. Bioactive compounds, nutraceutical values and its application in food product development of oyster mushroom. J. Future Foods 2024, 4, 335–342. [Google Scholar] [CrossRef]
- Marçal, S.; Sousa, A.S.; Taofiq, O.; Antunes, F.; Morais, A.M.M.B.; Freitas, A.C.; Barros, L.; Ferreira, I.C.F.R.; Pintado, M. Impact of postharvest preservation methods on nutritional value and bioactive properties of mushrooms. Trends Food Sci. Technol. 2021, 110, 418–431. [Google Scholar] [CrossRef]
- Giavasis, I. Bioactive fungal polysaccharides as potential functional ingredients in food and nutraceuticals. Curr. Opin. Biotechnol. 2014, 26, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.; Ahmad, A.; Farooq, R.; Ahmed, M.; Shaheryar, M.; Hussain, M. Edible Mushrooms, a Sustainable Source of Nutrition, Biochemically Active Compounds and Its Effect on Human Health. In Current Topics in Functional Food; Shiomi, N., Savitskaya, A., Eds.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Boonsong, S.; Klaypradit, W.; Wilaipun, P. Antioxidant activities of extracts from five edible mushrooms using different extractants. Agric. Nat. Resour. 2016, 50, 89–97. [Google Scholar] [CrossRef]
- González, A.; Cruz, M.; Losoya, C.; Nobre, C.; Loredo, A.; Rodríguez, R.; Contreras, J.; Belmares, R. Edible mushrooms as a novel protein source for functional foods. Food Funct. 2020, 11, 7400–7414. [Google Scholar] [CrossRef]
- Meng, X.; Liang, H.; Luo, L. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res. 2016, 424, 30–41. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, J.; Gao, Z.; Sun, G.; Wang, X.; Jia, L. Characterization and anti-hyperlipidemia effects of enzymatic residue polysaccharides from Pleurotus ostreatus. Int. J. Biol. Macromol. 2019, 129, 316–325. [Google Scholar] [CrossRef]
- Ayimbila, F.; Keawsompong, S. Nutritional Quality and Biological Application of Mushroom Protein as a Novel Protein Alternative. Curr. Nutr. Rep. 2003, 12, 290–307. [Google Scholar] [CrossRef]
- Min, K.; Yoo, Y.J. Amperometric detection of dopamine based on tyrosinase-SWNTs-Ppy composite electrode. Talanta 2009, 80, 1007–1011. [Google Scholar] [CrossRef]
- Yin, H.; Zhou, Y.; Xu, J.; Ai, S.; Cui, L.; Zhu, L. Amperometric biosensor based on tyrosinase immobilized onto multiwalled carbon nanotubes-cobalt phthalocyanine-silk fibroin film and its application to determine bisphenol A. Anal. Chim. Acta 2010, 659, 144–150. [Google Scholar] [CrossRef]
- Bakratsas, G.; Polydera, A.; Katapodis, P.; Stamatis, H. Recent trends in submerged cultivation of mushrooms and their application as a source of nutraceuticals and food additives. Future Foods 2021, 4, 100086. [Google Scholar] [CrossRef]
- Dudekula, U.T.; Doriya, K.; Devarai, S.K. A critical review on submerged pro- duction of mushroom and their bioactive metabolites. 3 Biotech 2020, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Stoica, R.M.; Moscovici, M.; Lakatos, E.S.; Cioca, L.I. Exopolysaccharides of Fungal Origin: Properties and Pharmaceutical Applications. Processes 2023, 11, 335. [Google Scholar] [CrossRef]
- Stylianopoulos, C. Carbohydrates: Chemistry and classification. In Encyclopedia of Human Nutrition, 4th ed.; Caballero, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 114–125. [Google Scholar] [CrossRef]
- Dewi, A.R.; Mukti, Y.P. Immunostimulant Potential of oyster mushroom (Pleourotus ostreatus) nugget. J. Pangan Dan Agroindustri 2022, 10, 78–82. [Google Scholar] [CrossRef]
- Maury, G.L.; Morris-Quevedo, H.J.; Heyker, A.; Lanckacker, E.; Cappoen, D.; Delputte, P.; Berghe, W.V.; Salgueiro, Z.; Cos, P. Differential induction pattern towards classically activated macrophages in response to an Immunomodulatory extract from Pleurotus ostreatus mycelium. J. Fungi 2021, 7, 206. [Google Scholar] [CrossRef]
- Amirullah, N.A.; Abidin, N.Z.; Abdullah, N.; Manickam, S. The ultrasound extract of Pleurotus pulmonarius (Fr.) Quél alleviates metabolic syndromes in hyperlipidaemic Wistar-Kyoto rats fed with a high-fat diet. Biocatal. Agric. Biotechnol. 2021, 34, 102019. [Google Scholar] [CrossRef]
- Agunloye, O.M.; Oboh, G. Blood glucose lowering and effect of oyster (Pleurotus ostreatus)-and shiitake (Lentinus subnudus)-supplemented diet on key enzymes linked diabetes and hypertension in streptozotocin-induced diabetic in rats. Food Front. 2022, 3, 161–171. [Google Scholar] [CrossRef]
- Martinez-Medina, G.A.; Chávez-González, M.L.; Verma, D.K.; Prado-Barragán, L.A.; Martínez-Hernández, J.L.; Flores-Gallegos, A.C.; Thakur, M.; Srivastav, P.P.; Aguilar, C.N. Bio-funcional components in mushrooms, a health opportunity: Ergothionine and huitlacohe as recent trends. J. Funct. Foods 2021, 77, 104326. [Google Scholar] [CrossRef]
- Łysakowska, P.; Sobota, A.; Wirkijowska, A. Medicinal Mushrooms: Their Bioactive Components, Nutritional Value and Application in Functional Food Production—A Review. Molecules 2023, 28, 5393. [Google Scholar] [CrossRef]
- Olson, B.; Marks, D.L.; Grossberg, A.J. Diverging metabolic programmes and behaviours during states of starvation, protein malnutrition, and cachexia. J. Cachexia Sarcopenia Muscle 2020, 11, 1429–1446. [Google Scholar] [CrossRef]
- Bonomi, F.; Iametti, S. Proteins|Foods. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2019; pp. 403–411. [Google Scholar] [CrossRef]
- Lin, J.W.; Jia, J.; Shen, Y.H.; Zhong, M.; Chen, L.J.; Li, H.G.; Ma, H.; Guo, Z.F.; Qi, M.F.; Liu, L.X.; et al. Functional expression of FIP-fve, a fungal immunomodulatory protein from the edible mushroom Flammulina velutipes in Pichia pastoris GS115. J. Biotechnol. 2013, 168, 527–533. [Google Scholar] [CrossRef]
- Barros, L.; Cruz, T.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C.F.R. Wild and commercial mushrooms as source of nutrients and nutraceuticals. Food Chem. Toxicol. 2008, 46, 2742–2747. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthi, R.; Srinivash, M.; Mahalingam, P.U.; Malaikozhundan, B. Dietary nutrients in edible mushroom, Agaricus bisporus and their radical scavenging, antibacterial, and antifungal effects. Process Biochem. 2022, 121, 10–17. [Google Scholar] [CrossRef]
- Zied, D.C.; Pardo, J.E.; Tomaz, R.S.; Miasaki, C.T.; Pardo-Giménez, A. Mycochemical characterization of agaricus subrufescens considering their morphological and physiological stage of maturity on the traceability process. BioMed Res. Int. 2017, 2017, 2713742. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, J.; Shang, X.; Li, Y.; Zhang, L.; Li, Z.; Jiang, N.; Tan, Q.; Yu, H.; Song, C. Characterizing diversity based on the chemical and nutritional composition of shiitake culinary-medicinal mushroom Lentinula edodes (Agaricomycetes) commonly cultivated in China. Int. J. Med. Mushrooms 2021, 23, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Ahlborn, J.; Stephan, A.; Meckel, T.; Maheshwari, G.; Rühl, M.; Zorn, H. Upcycling of food industry side streams by basidiomycetes for production of a vegan protein source. Int. J. Recycl. Org. Waste Agric. 2019, 8, 447–455. [Google Scholar] [CrossRef]
- Guo, L.Q.; Lin, J.Y.; Lin, J.F. Non-volatile components of several novel species of edible fungi in China. Food Chem. 2007, 100, 643–649. [Google Scholar] [CrossRef]
- Lindequist, U.; Niedermeyer, T.H.; Jülich, W.D. The pharmacological potential of mushrooms. Evid. Based Complement. Altern. Med. 2005, 2, 285–299. [Google Scholar] [CrossRef]
- Bleuler-Martínez, S.; Butschi, A.; Garbani, M.; Wälti, M.A.; Wohlschlager, T.; Potthoff, E.; Sabotiĉ, J.; Pohleven, J.; Lüthy, P.; Hengartner, M.O.; et al. A lectin-mediated resistance of higher fungi against predators and parasites. Mol. Ecol. 2011, 20, 3056–3070. [Google Scholar] [CrossRef]
- Pohleven, J.; Brzin, J.; Vrabec, L.; Leonardi, A.; Cokl, A.; Strukelj, B.; Kos, J.; Sabotič, J. Basidiomycete Clitocybe nebularis is rich in lectins with insecticidal activities. Appl. Microbiol. Biotechnol. 2011, 91, 1141–1148. [Google Scholar] [CrossRef]
- Singh, R.S.; Bhari, R.; Kaur, H.P. Mushroom lectins: Current status and future perspectives. Crit. Rev. Biotechnol. 2010, 30, 99–126. [Google Scholar] [CrossRef]
- Baldrian, P.; Valaskova, V. Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol. Rev. 2008, 32, 501–521. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Walia, A.K.; Kennedy, J.F. Purification and characterization of a mitogenic lectin from Penicillium duclauxii. Int. J. Biol. Macromol. 2018, 116, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Lata, L.; Atri, N.S. Amino acid profile of a basidiomycetous edible mushroom-lentinus sajor-caju. Int. J. Pharm. Pharm. Sci. 2017, 9, 252. [Google Scholar] [CrossRef]
- Shah, A.A.; Totakul, P.; Matra, M.; Cherdthong, A.; Hanboonsong, Y.; Wanapat, M. Nutritional composition of various insects and potential uses as alternative protein sources in animal diets. Anim. Biosci. 2022, 35, 317. [Google Scholar] [CrossRef]
- Hamza, A.; Mylarapu, A.; Krishna, K.V.; Kumar, D.S. An insight into the nutritional and medicinal value of edible mushrooms: A natural treasury for human health. J. Biotechnol. 2024, 381, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, A.; Liu, L.; Tian, G.; Wei, S.; Xu, F. Evaluation of nutritional values of shiitake mushroom (Lentinus edodes) stipes. J. Food Meas. Charact. 2018, 12, 2012–2019. [Google Scholar] [CrossRef]
- Manzi, P.; Gambelli, L.; Marconi, S.; Vivanti, V.; Pizzoferrato, L. Nutrients in edible mushrooms: An inter-species comparative study. Food Chem. 1999, 65, 477–482. [Google Scholar] [CrossRef]
- Yu, Q.; Guo, M.; Zhang, B.; Wu, H.; Zhang, Y.; Zhang, L. Analysis of nutritional composition in 23 kinds of edible fungi. J. Food Qual. 2020, 2020, 8821315. [Google Scholar] [CrossRef]
- Li, I.C.; Chiang, L.H.; Wu, S.Y.; Shih, Y.C.; Chen, C.C. Nutrition profile and animal-tested safety of Morchella esculenta mycelia produced by fermentation in bioreactors. Foods 2022, 11, 1385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, C.; Fan, G.; Li, T.; Wen, X. Characteristics and enhanced antioxidant activity of glycated Morchella esculenta protein isolate. Food Sci. Technol. 2017, 38, 126–133. [Google Scholar] [CrossRef]
- Petrovska, B.B. An evaluation of the protein quality of some macedonian edible Boletaceae mushrooms. Maced. Pharm. Bull. 2001, 47, 21–26. [Google Scholar] [CrossRef]
- Rasalanavho, M.; Moodley, R.; Jonnalagadda, S.B. Elemental bioaccumulation and nutritional value of five species of wild growing mushrooms from South Africa. Food Chem. 2020, 319, 126596. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zeng, N.K.; Xu, B. Chemical profiles and health-promoting effects of porcini mushroom (Boletus edulis): A narrative review. Food Chem. 2022, 390, 133199. [Google Scholar] [CrossRef] [PubMed]
- Saidi, S.; Deratani, A.; Belleville, M.P.; Ben Amar, R. Antioxidant properties of peptide fractions from tuna dark muscle protein by-product hydrolysate produced by membrane fractionation process. Food Res. Int. 2014, 65, 329–336. [Google Scholar] [CrossRef]
- Martínez-Burgosa, W.J.; Ocána, D.; Manzokia, M.C.; Barrosa, R.N.; Vieiraa, R.; Soccol, C.R. Edible macromycetes as an alternative protein source: Advances and trends. Biotechnol. Res. Innov. 2024, 8, e2024002. [Google Scholar] [CrossRef]
- Karnwal, A.; Dohroo, A.; Sharma, S. Analysing the biocontrol attribute of indigenous mushroom concentrates against Pathogenic bacterial spp. Res. J. Pharm. Technol. 2020, 13, 173–177. [Google Scholar] [CrossRef]
- Bach, F.; Helm, C.V.; Bellettini, M.B.; Maciel, G.M.; Windson, C.; Haminiuk, I. Edible mushrooms: A potential source of essential amino acids, glucans and minerals. Int. J. Food Sci. Technol. 2017, 52, 2382–2392. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture, Food Data Central. Available online: https://fdc.nal.usda.gov/ (accessed on 20 August 2025).
- Cuptapun, Y.; Duangchan, H.; Wanpen, M.; Yaieiam, S. Quality and quantity of protein in certain kinds of edible mushroom in Thailand, Kasetsart. J. Nat. Sci. 2010, 44, 664–670. [Google Scholar]
- Wang, B.; Shi, Y.; Lu, H.; Chen, Q. A critical review of fungal proteins: Emerging preparation technology, active efficacy and food application. Trends Food Sci. Technol. 2023, 141, 104178. [Google Scholar] [CrossRef]
- Dabbour, I.R.; Takruri, H.R. Protein digestibility using corrected amino acid score method (PDCAAS) of four types of mushrooms grown in Jordan. Plant Foods Hum. Nutr. 2002, 57, 13–24. [Google Scholar] [CrossRef]
- López, M.M.S.; Kizlansky, A.; López, L.B. Assessment of protein quality in foods by calculating the amino acids score corrected by digestibility. Nutr. Hosp. 2006, 21, 47–51. [Google Scholar]
- Nur-Hidayah, A.; Wan-Mohtar, W.A.A.Q.I.; Khalisanni, K.; Farhana, R.; Abdul-Malek, A.; Nazimah, H.; Razifah, M.R.; Bellere, A.D.; Raseetha, S. Beneficial properties of edible mushrooms and their potential utilisation of mushroom waste in food products. Food Res. 2023, 7 (Suppl. S4), 21–36. [Google Scholar] [CrossRef] [PubMed]
- Schimbator, M.; Culețu, A.; Susman, I.; Duță, D.E. Digestibility of proteins from different sources. Ann. Univ. Dunarea Jos Galati Fascicle VI Food Technol. 2020, 44, 43–50. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, R. A review on nutritional advantages of edible mushrooms and its industrialization development situation in protein meat analogues. J. Future Foods 2023, 3, 1–7. [Google Scholar] [CrossRef]
- Vioque, J.; Clemente, A.; Pedroche, J.; Yust, M.; Millán, F. Obtention and uses of protein hydrolysates. Grasas Aceites 2001, 52, 132–136. [Google Scholar] [CrossRef]
- Clemente, A. Enzymatic protein hydrolysates in human nutrition. Trends Food Sci. Technol. 2000, 11, 254–262. [Google Scholar] [CrossRef]
- Castro-Alves, V.C.; Gomes, D.; Menolli, N.; Sforça, M.L.; Nascimento, J.R.O. Characterization and immunomodulatory effects of glucans from Pleurotus albidus, a promising species of mushroom for farming and biomass production. Int. J. Biol. Macromol. 2017, 95, 215–223. [Google Scholar] [CrossRef]
- Jing, X.; Mao, D.; Geng, L.; Xu, C. Medium optimization, molecular characterization, and bioactivity of exopolysaccharides from Pleurotus eryngii. Arch. Microbiol. 2013, 195, 749–757. [Google Scholar] [CrossRef]
- Velioğlu, Z.; Öztürk, Ü.R. Biosurfactant production by Pleurotus ostreatus in submerged and solid-state fermentation systems. Turk. J. Biol. 2015, 39, 160–166. [Google Scholar] [CrossRef]
- Das, A.K.; Nanda, P.K.; Dandapat, P.; Bandyopadhyay, S.; Gullón, P.; Sivaraman, G.K.; McClements, D.J.; Gullón, B.; Lorenzo, J.M. Edible Mushrooms as Functional Ingredients for Development of Healthier and More Sustainable Muscle Foods: A Flexitarian Approach. Molecules 2021, 26, 2463. [Google Scholar] [CrossRef] [PubMed]
- Alnoumani, H.; Ataman, Z.A.; Were, L. Lipid and protein antioxidant capacity of dried Agaricus bisporus in salted cooked ground beef. Meat Sci. 2017, 129, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.; Lee, J.; Jo, K.; Jo, C.; Song, M.; Jung, S. Application of winter mushroom powder as an alternative to phosphates in emulsion-type sausages. Meat Sci. 2018, 143, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Jo, K.; Lee, J.; Jung, S. Quality characteristics of low-salt chicken sausage supplemented with a winter mushroom powder. Korean J. Food Sci. Anim. Resour. 2018, 38, 768–779. [Google Scholar] [CrossRef]
- Bao, H.N.D.; Shinomiya, Y.; Ikeda, H.; Ohshima, T. Preventing discoloration and lipid oxidation in dark muscle of yellowtail by feeding an extract prepared from mushroom (Flammulina velutipes) cultured medium. Aquaculture 2009, 295, 243–249. [Google Scholar] [CrossRef]
- Bao, H.N.D.; Ushio, H.; Ohshima, T. Antioxidative activities of mushroom (Flammulina velutipes) extract added to bigeye tuna meat: Dose-dependent efficacy and comparison with other biological antioxidants. J. Food Sci. 2009, 74, C162–C169. [Google Scholar] [CrossRef]
- El-Refai, A.; El-Zeiny, A.R.; Rabo, E.A. Quality attributes of mushroom-beef patties as a functional meat product. J. Hyg. Eng. Des. 2014, 6, 49–62. [Google Scholar]
- Patinho, I.; Saldaña, E.; Selani, M.M.; de Camargo, A.C.; Merlo, T.C.; Menegali, B.S.; de Souza Silva, A.P.; Contreras-Castillo, C.J. Use of Agaricus bisporus mushroom in beef burgers: Antioxidant, flavor enhancer and fat replacing potential. Food Prod. Process. Nutr. 2019, 1, 7. [Google Scholar] [CrossRef]
- Kurt, A.; Gençcelep, H. Enrichment of meat emulsion with mushroom (Agaricus bisporus) powder: Impact on rheological and structural characteristics. J. Food Eng. 2018, 237, 128–136. [Google Scholar] [CrossRef]
- Myrdal Miller, A.; Mills, K.; Wong, T.; Drescher, G.; Lee, S.M.; Sirimuangmoon, C.; Schaefer, S.; Langstaff, S.; Minor, B.; Guinard, J.X. Flavor-enhancing properties of mushrooms in meat-based dishes in which sodium has been reduced and meat has been partially substituted with mushrooms. J. Food Sci. 2014, 79, S1795–S1804. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Brennan, M.; Brennan, C.; Qin, Y.; Cheng, G.; Liu, Y. Physical, chemical, sensorial properties and in vitro digestibility of wheat bread enriched with yunnan commercial and wild edible mushrooms. LWT 2022, 169, 113923. [Google Scholar] [CrossRef]
- Szydłowska-Tutaj, M.; Złotek, U.; Combrzynski, M. Influence of addition of mushroom powder to semolina on proximate composition, physicochemical properties and some safety parameters of material for pasta production. LWT 2021, 151, 112235. [Google Scholar] [CrossRef]
- Panda, J.; Mishra, A.K.; Nath, P.C.; Mahanta, S.; Sharma, M.; Nayak, P.K.; Mohanta, Y.K.; Sridhar, K. Wild edible mushrooms to achieve sustainable development goals: Novel sources for food security, health, and well-being. Food Biosci. 2024, 60, 104277. [Google Scholar] [CrossRef]
- Molitorisova, A.; Monaco, A.; Purnhagen, K.P. An analysis of the regulatory framework applicable to products obtained from mushroom and mycelium. SSRN J. 2021. [Google Scholar] [CrossRef]
- Lopez-Martinez, M.I.; Miguel, M.; Garces-Rimon, M. Protein and sport: Alternative sources and strategies for bioactive and sustainable sports nutrition. Front. Nutr. 2022, 9, 926043. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, E.; Abidin, N.Z.; Aminudin, N.; Abdullah, N. Characterization of cytotoxic proteins from king tuber oyster medicinal mushroom, Pleurotus tuber-regium (Agaricomycetes), sclerotium for human breast MDA-MB-231 cancer cells. Int. J. Med. Mushrooms 2022, 24, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Goswami, B.; Majumdar, S.; Das, A.; Barui, A.; Bhowal, J. Evaluation of bioactive properties of Pleurotus ostreatus mushroom protein hydrolysate of different degree of hydrolysis. LWT 2021, 149, 111768. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Y.; Feng, X.; Liu, Q.; Li, Y.; Hao, J.; Wang, Y.; Dong, Y.; Wang, H.D. Hepatoprotective Effects of Pleurotus ostreatus Protein Hydrolysates Yielded by Pepsin Hydrolysis. Catalysts 2020, 10, 595. [Google Scholar] [CrossRef]
- Krobthong, S.; Choowongkomon, K.; Suphakun, P.; Kuaprasert, B.; Samutrtai, P.; Yingchutrakul, Y. The anti-oxidative effect of Lingzhi protein hydrolysates on lipopolysaccharide-stimulated A549 cells. Food Biosci. 2021, 41, 101093. [Google Scholar] [CrossRef]
- Kheng, Y.S.; Jeng, Y.L.; Nor, M.M.; Jia, G.B. The analysis of Angiotensin-I Converting Enzyme (ACE) by maitake (Grifrola frondosa) mycelia. J. Trop. Resour. Sustain. Sci. 2020, 8, 69–72. [Google Scholar] [CrossRef]
- Wang, R.; Yun, J.; Wu, S.; Bi, Y.; Zhao, F. Optimisation and characterization of novel angiotensin-converting enzyme inhibitory peptides prepared by double enzymatic hydrolysis from Agaricus bisporus Scraps. Foods 2022, 11, 394. [Google Scholar] [CrossRef]
- Betancor, D.; Nuñez-Borque, E.; Cuesta-Herranz, J.; Escudero, C.; Freundt, N.; Pastor-Vargas, C.; Ibañez, M. Porin: A new button mushroom (Agaricus bisporus) allergen. J. Investig. Allergol. Clin. Immunol. 2020, 30, 135–136. [Google Scholar] [CrossRef] [PubMed]
- EFSA NDA Panel. Guidance on the preparation and presentation of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283. EFSA J. 2016, 14, 4594. [Google Scholar] [CrossRef]
- Crevel, R.W.R.; Verhoeckx, K.; Bøgh, K.L.; Buck, N.; Chentouf, A.; Flanagan, S.; Galano, M.; Garthoff, J.A.; Hazebrouck, S.; Yarham, R.; et al. Allergenicity assessment of new or modified protein-containing food sources and ingredients. Food Chem. Toxicol. 2024, 189, 114766. [Google Scholar] [CrossRef]
- The European Parliament and the Council of the European Union. Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on Novel Foods, Amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and Repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001 (Text with EEA Relevance). 2015. Available online: http://data.europa.eu/eli/reg/2015/2283/oj (accessed on 27 August 2025).
- The European Parliament and the Council of the European Union. No 68/2013 of 16 January 2013 on the Catalogue of Feed Materials. 2013. Available online: http://data.europa.eu/eli/reg/2013/68/oj (accessed on 20 August 2025).
- Peintner, U.; Schwarz, S.; Mešić, A.; Moreau, P.-A.; Moreno, G.; Saviuc, P. Mycophilic or Mycophobic? Legislation and Guidelines on Wild Mushroom Commerce Reveal Different Consumption Behaviour in European Countries. PLoS ONE 2013, 8, e63926. [Google Scholar] [CrossRef]
- Fernandez, A.; Danisman, E.; Taheri Boroujerdi, M.; Kazemi, S.; Moreno, F.J.; Epstein, M.M. Research gaps and future needs for allergen prediction in food safety. Front. Allergy 2024, 5, 1297547. [Google Scholar] [CrossRef]
- Sousa, A.S.; Araújo-Rodrigues, H.; Pintado, M.E. The health-promoting potential of edible mushroom proteins. Curr. Pharm. Des. 2023, 29, 804–823. [Google Scholar] [CrossRef]
- Erjavec, J.; Kos, J.; Ravnikar, M.; Dreo, T.; Sabotič, J. Proteins of higher fungi-from forest to application. Trends Biotechnol. 2012, 30, 259–273. [Google Scholar] [CrossRef]
- Singh, S.S.; Wang, H.; Chan, Y.S.; Pan, W.; Dan, X.; Yin, C.M.; Akkouh, O.; Ng, T.B. Lectins from Edible Mushrooms. Molecules 2015, 20, 446–469. [Google Scholar] [CrossRef]
- Motta, F.; Gershwin, M.E.; Selmi, C. Mushrooms and immunity. J. Autoimmun. 2021, 117, 102576. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ng, T.B.; Ooi, V.E.C. Lectins from mushrooms. Mycol. Res. 1998, 102, 897–906. [Google Scholar] [CrossRef]
- Mikiashvili, N.A.; Elisashvili, V.I.; Wasser, S.P.; Nevo, E. Comparative study of lectin activity of higher Basidiomycetes. Int. J. Med. Mushrooms 2006, 8, 31–38. [Google Scholar] [CrossRef]
- Khan, F.; Khan, M.I. Fungal lectins: Current molecular and biochemical perspectives. Int. J. Biol. Chem. 2011, 5, 1–20. [Google Scholar] [CrossRef]
- Goldstein, I.J.; Winter, H.C. Mushroom lectins. In Comprehensive Glycoscience: From Chemistry to Systems Biology; Kamerling, J.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Varrot, A.; Basheer, S.M.; Imberty, A. Fungal lectins: Structure, function and potential applications. Curr. Opin. Struct. Biol. 2013, 23, 678–685. [Google Scholar] [CrossRef]
- Li, Y.R.; Liu, Q.H.; Wang, H.X.; Ng, T.B. A novel lectin with potent antitumor, mitogenic and HIV-1 reverse transcriptase inhibitory activities from the edible mushroom Pleurotus citrinopileatus. Biochim. Biophys. Acta 2008, 1780, 51–57. [Google Scholar] [CrossRef]
- Singh, R.S.; Walia, A.K.; Kennedy, J.F. Mushroom lectins in biomedical research and development. Int. J. Biol. Macromol. 2020, 151, 1340–1350. [Google Scholar] [CrossRef]
- da Silva, R.R. Enzyme technology in food preservation: A promising and sustainable strategy for biocontrol of post-harvest fungal pathogens. Food Chem. 2019, 277, 531–532. [Google Scholar] [CrossRef]
- Yamasaki, K.; Kubota, T.; Yamasaki, T.; Nagashima, I.; Shimizu, H.; Terada, R.I.; Nishigami, H.; Kang, J.; Tateno, M.; Tateno, H. Structural basis for specific recognition of core fucosylation in N-glycans by Pholiota squarrosa lectin (PhoSL). Glycobiology 2019, 29, 576–587. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, H.; Tong, X.; Qi, Y. An antitumour lectin from the edible mushroom Agrocybe aegerita. Biochem. J. 2003, 374, 321–327. [Google Scholar] [CrossRef]
- El Enshasy, H.A.; Hatti-Kaul, R. Mushroom immunomodulators: Unique molecules with unlimited applications. Trends Biotechnol. 2013, 31, 668–677. [Google Scholar] [CrossRef]
- Sze, S.C.; Ho, J.C.; Liu, W.K. Volvariella volvacea lectin activates mouse T lymphocytes by a calcium dependent pathway. J. Cell. Biochem. 2004, 92, 1193–1202. [Google Scholar] [CrossRef]
- Wang, H.X.; Ng, T.B.; Ooi, V.E.; Liu, W.K.; Chang, S.T. Actions of lectins from the mushroom Tricholoma mongolicum on macrophages, splenocytes and life-span in sarcoma-bearing mice. Anticancer Res. 1997, 17, 419–424. [Google Scholar]
- Tanaka, S.; Ko, K.; Kino, K.; Tsuchiya, K.; Yamashita, A.; Murasugi, A.; Sakuma, S.; Tsunoo, H. Complete amino acid sequence of an immunomodulatory protein, ling zhi-8 (LZ-8) an immunomodulator from a fungus Ganoderma lucidum, having similarity to immunoglobulin variable regions. J. Biol. Chem. 1989, 464, 16372–16377. [Google Scholar] [CrossRef]
- Wang, P.H.; Hsu, C.I.; Tang, S.C.; Huang, Y.L.; Lin, J.Y.; Ko, J.L. Immunomodulatory protein from Flammulina velutipes inducesinterferon-g-productionthroughp38mitogen-activated protein kinase signaling pathway. Agric. Food Chem. 2004, 52, 2721–2725. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.M.; Huang, Y.L.; Tang, S.C.; Shieh, G.J.; Lai, J.Y.; Wang, P.H.; Ying, T.H.; Ko, J.L. Effect of a fungal immunomodulatory protein from Ganoderma tsugae on cell cycle and interferon-gamma production through phosphatidylinositol 3-kinase signal pathway. Process Biochem. 2008, 43, 423–430. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Chen, Y.; Lin, J.; Zhou, X. Cytokines expression induced by Ganoderma sinensis fungal immunomodulatory proteins (FIP-gsi) in mouse spleen cells. Appl. Biochem. Biotechnol. 2010, 162, 1403–1413. [Google Scholar] [CrossRef]
- Chang, H.H.; Sheu, F. A novel fungal immunomodulatory protein (PCP) isolated from Poria cocos activates mouse peritoneal macrophage involved in toll-like receptor 4. FASEB J. 2007, 21, 702–715. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Hua, K.F.; Wu, W.C.; Hsu, J.; Weng, S.T.; Lin, T.L.; Liu, C.Y.; Hseu, R.S.; Huang, C.T. Reishi immuno-modulation protein induces interleukin-2 expression via protein kinase-dependent signaling pathways within human T-cells. J. Cell. Physiol. 2008, 215, 15–26. [Google Scholar] [CrossRef]
- Sheu, F.; Chien, P.J.; Hsieh, K.Y.; Chin, K.L.; Huang, W.T.; Tsao, C.Y.; Chen, Y.F.; Cheng, H.C.; Chang, H.H. Purification, cloning, and functional characterization of a novel immunomodulatory protein from Antroida camphorate (Bitter mushroom) that exhibits TLR2- dependent NF-kB activation and M1 polarization within murine macrophages. J. Agric. Food Chem. 2009, 57, 4130–4141. [Google Scholar] [CrossRef]
- Lin, C.H.; Hsiao, Y.M.; Ou, C.C.; Lin, Y.W.; Chiu, Y.L.; Lue, K.H.; Chang, J.G.; Ko, J.L. GMI, a Ganoderma immunomodulatory protein, down-regulates tumor necrosis factor a-induced expression of matrix metalloproteinase 9 via NF-kB pathway in human alveolar empithelial A549 cells. J. Agric. Food Chem. 2010, 58, 12014–12021. [Google Scholar] [CrossRef]
- Li, F.; Wen, H.; Zhang, Y.; Aa, M.; Liu, X.Z. Purification and characterization of a novel immunomodulatory protein from the medicinal mushroom Trametes versicolor. Sci. China Life Sci. 2011, 54, 379–385. [Google Scholar] [CrossRef]
- Li, Q.Z.; Wang, X.F.; Bao, T.W.; Ran, L.; Lin, J.; Zhou, X.W. In vitro synthesis of a recombinant fungal immunomodulatory protein from Linghzi or Reishi medicinal mushroom, Ganoderma lucidum (W. Curt.: Fr.) P. Karst. (Aphyllophoromycetideae) and analysis of its immunomodulatory activity. Int. J. Med. Mushrooms 2010, 12, 347–358. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Zhou, X. Recent status and prospects of the fungal immunomodulatory protein family. Crit. Rev. Biotechnol. 2011, 31, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Li, X.W.; Zheng, Q.Z.; Zhou, Y.Z. Fungal immunomodulatory proteins: Characteristic, potential antitumor activities and their molecular mechanisms. Drug Discov. Today 2019, 24, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Chien, P.J.; Tong, M.H.; Sheu, F. Mushroom immunomodulatory proteins possess potential thermal/freezing resistance, acid/alkali tolerance and dehydration stability. Food Chem. 2007, 105, 597–605. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Shao, J.; Wu, B.; Li, B. Potential immunomodulatory activities of a lectin from the mushroom Latiporus sulphureus. Int. J. Biol. Macromol. 2019, 130, 399–406. [Google Scholar] [CrossRef]
- Choi, E.J.; Ahn, H.W.; Kim, W.J. Effect of α-acetolactate decarboxylase on diacetyl content of beer. Food Sci. Biotechnol. 2015, 24, 1373–1380. [Google Scholar] [CrossRef]
- Ho, J.C.K.; Sze, S.C.W.; Shen, W.Z.; Liu, W.K. Mitogenic activity of edible mushroom lectins. Biochim. Biophys. Acta 2004, 1671, 9–17. [Google Scholar] [CrossRef]
- El-Maradny, Y.A.; El-Fakharany, E.M.; Abu-Serie, M.M.; Hashish, M.H.; Selim, H.S. Lectins purified from medicinal and edible mushrooms: Insights into their antiviral activity against pathogenic viruses. Int. J. Biol. Macromol. 2021, 179, 239–258. [Google Scholar] [CrossRef]
- Seo, D.J.; Choi, C. Antiviral bioactive compounds of mushrooms and their antiviral mechanisms: A review. Viruses 2021, 13, 350. [Google Scholar] [CrossRef]
- Zhao, S.; Guo, Y.X.; Liu, Q.H.; Wang, H.X.; Ng, T.B. Lectins but not antifungal proteins exhibit antinematode activity. Environ. Toxicol. Pharmacol. 2009, 28, 265–268. [Google Scholar] [CrossRef]
- Qi, W.; Zhang, Y.; Yan, Y.B.; Lei, W.; Wu, Z.X.; Liu, N.; Liu, S.; Shi, L.; Fan, Y. The Protective Effect of Cordymin, a Peptide Purified from the Medicinal Mushroom Cordyceps sinensis, on Diabetic Osteopenia in Alloxan-Induced Diabetic Rats. Evid. Based Complement. Altern. Med. 2013, 2013, 985636. [Google Scholar] [CrossRef] [PubMed]
- Debnath, R.; Saha, T. An insight into the production strategies and applications of the ligninolytic enzyme laccase from bacteria and fungi. Biocatal. Agric. Biotechnol. 2020, 26, 101645. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Swiderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczynski, A. Laccase Properties, Physiological Functions, and Evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Deng, W.; Shen, M.; Yan, G.; Zhao, W.; Yang, Y. A laccase Gl-LAC-4 purified from white-rot fungus Ganoderma lucidum had a strong ability to degrade and detoxify the alkylphenol pollutants 4-n-octylphenol and 2-phenylphenol. J. Hazard. Mater. 2021, 408, 124775. [Google Scholar] [CrossRef]
- Dana, M.; Khaniki, G.B.; Mokhtarieh, A.A.; Davarpanah, S.J. Biotechnological and Industrial Applications of Laccase: A Review. J. Appl. Biotechnol. Rep. 2017, 4, 675–679. [Google Scholar]
- Hatakka, A.; Hammel, K.E. Fungal biodegradation of lignocellulose. In Industrial Applications. The Mycota; Hofrichter, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 10, pp. 319–340. [Google Scholar] [CrossRef]
- Halaouli, S.; Asther, M.; Sigoillot, J.C.; Hamdi, M.; Lomascolo, A. Fungal tyrosinases: New prospects in molecular characteristics, bioengineering and biotechnological applications. J. Appl. Microbiol. 2006, 100, 219–232. [Google Scholar] [CrossRef]
- Jeon, J.R.; Baldrian, P.; Murugesan, K.; Chang, Y.S. Laccase-catalysed oxidations of naturally occurring phenols: From in vivo biosynthetic pathways to green synthetic applications. Microb. Biotechnol. 2012, 5, 318–332. [Google Scholar] [CrossRef]
- Kunamneni, A.; Camarero, S.; García-Burgos, C.; Plou, F.J.; Ballesteros, A.; Alcalde, M. Engineering and applications of fungal laccases for organic synthesis. Microb. Cell Fact. 2008, 7, 32. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Tjandrawinata, R.R.; Rachmawati, H. Lectins from the edible mushroom Agaricus bisporus and their therapeutic potentials. Molecules 2020, 25, 2368. [Google Scholar] [CrossRef]
- Rao, D.E.; Rao, K.V.; Reddy, T.P.; Reddy, V.D. Molecular characterization, physicochemical properties, known and potential applications of phytases: An overview. Crit. Rev. Biotechnol. 2009, 29, 182–198. [Google Scholar] [CrossRef]
- Sabotic, J.; Trcek, T.; Popovic, T.; Brzin, J. Basidiomycetes harbour a hidden treasure of proteolytic diversity. J. Biotechnol. 2007, 128, 297–307. [Google Scholar] [CrossRef]
- Sabotic, J.; Galesa, K.; Popovic, T.; Leonardi, A.; Brzin, J. Comparison of natural and recombinant clitocypins, the fungal cysteine protease inhibitors. Protein Expr. Purif. 2007, 53, 104–111. [Google Scholar] [CrossRef]
- Sabotic, J.; Popovic, T.; Puizdar, V.; Brzin, J. Macrocypins, a family of cysteine protease inhibitors from the basidiomycete Macrolepiota procera. FEBS J. 2009, 276, 4334–4345. [Google Scholar] [CrossRef]
- Avanzo, P.; Sabotič, J.; Anžlovar, S.; Popovič, T.; Leonardi, A.; Pain, R.H.; Kos, J.; Brzin, J. Trypsin-specific inhibitors from the basidiomycete Clitocybe nebularis with regulatory and defensive functions. Microbiology 2009, 155, 3971–3981. [Google Scholar] [CrossRef] [PubMed]
- Sabotic, J.; Bleuler-Martinez, S.; Renko, M.; Avanzo Caglič, P.; Kallert, S.; Štrukelj, B.; Turk, D.; Aebi, M.; Kos, J.; Künzler, M. Structural basis of trypsin inhibition and entomotoxicity of cospin, a serine protease inhibitor involved in defence of Coprinopsis cinerea fruiting bodies. J. Biol. Chem. 2012, 287, 3898–3907. [Google Scholar] [CrossRef]
- Fang, E.F.; Ng, T.B. Ribonucleases of different origins with a wide spectrum of medicinal applications. Biochim. Biophys. Acta 2011, 1815, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Liu, Z.K.; Zhang, Y.N.; Wong, J.H.; Ng, T.B.; Liu, F. Research progress of bioactive proteins from the edible and medicinal mushrooms. Curr. Protein Pept. Sci. 2019, 20, 196–219. [Google Scholar] [CrossRef]
- Pan, W.L.; Wong, J.H.; Fang, E.F.; Chan, Y.S.; Ye, X.J.; Ng, T.B. Differential inhibitory potencies and mechanisms of the type I ribosome inactivating protein marmorin on estrogen receptor (ER)-positive and ER-negative breast cancer cells. Biochim. Biophys. Acta 2013, 1833, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.B.; Szilvay, G.R.; Nakari-Setälä, T.; Penttilä, M.E. Hydrophobin: The protein-amphiphiles of filamentous fungi. FEMS Microbiol. 2005, 29, 877–896. [Google Scholar] [CrossRef]
- Kulkarni, S.; Nene, S.; Joshi, K. Production of Hydrophobins from fungi. Process Biochem. 2017, 61, 1–11. [Google Scholar] [CrossRef]
- Lugones, L.G.; Bosscher, J.S.; Scholtmeijer, K.; de Vries, O.M.H.; Wessels, J.G.H. An abundant Hydrophobin (ABH1) forms hydrophobic rodlet layers in Agaricus bisporus fruiting bodies. Microbiology 1996, 142, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Lugones, L.G.; Wösten, H.A.B.; Wessels, J.G.H. A Hydrophobin (ABH3) secreted by the substrate mycelium of Agaricus bisporus (common white button mushroom). Microbiology 1998, 144, 2345–2353. [Google Scholar] [CrossRef]
- de Groot, P.W.J.; Schaap, P.J.; Sonnenberg, A.S.M.; Visser, J.; Van Griensven, L.J. The Agaricus bisporus hypA gene encodes a Hydrophobin and specifically accumulates in peel tissue of mushroom caps during fruit body development. J. Mol. Biol. 1996, 257, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Ásgeirsdóttir, S.A.; de Vries, O.M.H.; Wessels, J.G.H. Identification of three differentially expressed Hydrophobins in Pleurotus ostreatus (oyster mushroom). Microbiology 1998, 144, 2961–2969. [Google Scholar] [CrossRef]
- Martínez, A.T.; Camarero, S.; GuiMn, F.; Gutibrrez, A.; Mufioz, C.; Varela, E.; Martinez, M.J.; Barrasal, M.; Ruel, K.; Pelayo, J.M. Progress in biopulping of nonwoody materials: Chemical, enzymatic and ultrastructural aspects of wheat straw delignification with ligninolytic fungi from the genus Pleurotus. FEMS Microbiol. Rev. 1994, 13, 265–274. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Hu, D.; Gu, G.J.; Zhang, J.X.; Goodwin, P.H.; Hu, Q.X. Purification of a novel Hydrophobin PN1 involved in antibacterial activity from an edible mushroom Pleurotus nebrodensis. Eur. J. Plant Pathol. 2015, 143, 823–831. [Google Scholar] [CrossRef]
- Trembley, M.L.; Ringli, C.; Honegger, R. Differential expression of Hydrophobins DGH1, DGH2, DGH3 and immunolocalization of DGH1 in strata of the lichenized basidiocarp of Dictyonema glabratum. New Phytol. 2002, 154, 185–195. [Google Scholar] [CrossRef]
- Mankel, A.; Krause, K.; Kothe, E. Identification of a hydrophobin gene that is developmentally regulated in the ectomycorrhizal fungus Tricholoma terreum. Appl. Environ. Microbiol. 2002, 68, 1408–1413. [Google Scholar] [CrossRef]
- Bayry, J.; Aimanianda, V.; Iñaki Guijarro, J.; Sunde, M.; Latge, J.P. Hydrophobinsunique fungal proteins. PLoS Pathog. 2012, 8, e1002700. [Google Scholar] [CrossRef]
- Wösten, H.A.B.; van Wetter, M.A.; Lugones, L.G.; van der Mei, H.C.; Busscher, H.J.; Wessels, J.G.H. How a fungus escapes the water to grow into the air. Curr. Biol. 1999, 9, 85–88. [Google Scholar] [CrossRef]
- Pérez-Chávez, A.M.; Mayer, L.; Albertó, E. Mushroom cultivation and biogas pro- duction: A sustainable reuse of organic resources. Energy Sustain. Dev. 2019, 50, 50–60. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; Brugnari, T.; Bracht, A.; Peralta, R.M.; Ferreira, I.C.F.R. Biotechno- logical, nutritional and therapeutic uses of Pleurotus spp. (Oyster mushroom) related with its chemical composition: A review on the past decade findings. Trends Food Sci. Technol. 2016, 50, 103–117. [Google Scholar] [CrossRef]
- Elisashvili, V. Submerged cultivation of medicinal mushrooms: Bioprocesses and products. Int. J. Med. Mushrooms 2012, 14, 211–239. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Gani, A.; Khanday, F.A.; Masoodi, F.A. Biological and pharmaceutical activities of mushroom β-glucan discussed as a potential functional food ingredient. Bioact. Carbohydr. Diet. Fibre 2018, 16, 1–13. [Google Scholar] [CrossRef]
- Petre, M.; Petre, V. Biotechnology of mushroom growth through submerged cultivation. In Mushroom Biotechnology: Developments and Applications; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2016; pp. 1–18. [Google Scholar] [CrossRef]
- Sandargo, B.; Chepkirui, C.; Cheng, T.; Chaverra-Muñoz, L.; Thongbai, B.; Stadler, M.; Hüttel, S. Biological and chemical diversity go hand in hand: Basidiomycota as source of new pharmaceuticals and agrochemicals. Biotechnol. Adv. 2019, 37, 107344. [Google Scholar] [CrossRef]
- Bentil, J.A.; Thygesen, A.; Mensah, M.; Lange, L.; Meyer, A.S. Cellulase production by white-rot basidiomycetous fungi: Solid-state versus submerged cultivation. Appl. Microbiol. Biotechnol. 2018, 102, 5827–5839. [Google Scholar] [CrossRef]
- Kim, S.W.; Hwang, H.J.; Lee, B.C.; Yun, J.W. Submerged production and characterization of Grifola frondosa polysaccharides—A new application to cosmeceuticals. Food Technol. Biotechnol. 2007, 45, 295–305. [Google Scholar]
- Shih, I.L.; Chou, B.W.; Chen, C.C.; Wu, J.Y.; Hsieh, C. Study of mycelial growth and bioactive polysaccharide production in batch and fed-batch culture of Grifola frondosa. Bioresour. Technol. 2008, 99, 785–793. [Google Scholar] [CrossRef]
- Turlo, J. The biotechnology of higher fungi—Current state and perspectives. Folia Biol. Oecol. 2014, 10, 49–65. [Google Scholar] [CrossRef]
- Berger, R.G.; Bordewick, S.; Krahe, N.-K.; Ersoy, F. Mycelium vs. Fruiting Bodies of Edible Fungi—A Comparison of Metabolites. Microorganisms 2022, 10, 1379. [Google Scholar] [CrossRef]
- Bellettini, M.B.; Fiorda, F.A.; Maieves, H.A.; Teixeira, G.L.; Ávila, S.; Hornung, P.S.; Júnior, A.M.; Ribani, R.H. Factors affecting mushroom Pleurotus spp. Saudi J. Biol. Sci. 2019, 26, 633–646. [Google Scholar] [CrossRef]
- Hoa, H.T.; Wang, C.L. The effects of temperature and nutritional conditions on mycelium growth of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2015, 43, 14–23. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Zhang, F.; Linhardt, R.J.; Zeng, G.; Zhang, A. Extraction, structure and bioactivities of the polysaccharides from Pleurotus eryngii: A review. Int. J. Biol. Macromol. 2020, 150, 1342–1347. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Papanikolaou, S.; Komaitis, M.; Aggelis, G.; Philippoussis, A. Patterns of major metabolites biosynthesis by different mushroom fungi grown on glucose-based submerged cultures. Bioprocess Biosyst. Eng. 2014, 37, 1385–1400. [Google Scholar] [CrossRef]
- Smiderle, F.R.; Olsen, L.M.; Ruthes, A.C.; Czelusniak, P.A.; Santana-Filho, A.P.; Sassaki, G.L.; Gorin, P.A.J.; Iacomini, M. Exopolysaccharides, proteins and lipids in Pleurotus pulmonarius submerged culture using different carbon sources. Carbohydr. Polym. 2012, 87, 368–376. [Google Scholar] [CrossRef]
- Assis, I.S.; Chaves, M.B.; Silveira, M.L.L.; Gern, R.M.M.; Wisbeck, E.; Júnior, A.F.; Furlan, S.A. Production of bioactive compounds with antitumor activity against sarcoma 180 by Pleurotus sajor-caju. J. Med. Food 2013, 16, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Confortin, F.G.; Marchetto, R.; Bettin, F.; Camassola, M.; Salvador, M.; Dillon, A.J.P. Production of Pleurotus sajor-caju strain PS-2001 biomass in submerged culture. J. Ind. Microbiol. Biotechnol. 2008, 35, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R.; Chatterjee, S.; Chatterjee, B.P.; Guha, A.K. Enhance- ment of biomass production of edible mushroom Pleurotus sajor-caju grown in whey by plant growth hormones. Process Biochem. 2005, 40, 1241–1244. [Google Scholar] [CrossRef]
- Chahal, D.S. Production of protein-rich mycelial biomass of a mushroom, Pleurotus sajor-caju, on corn stover. J. Ferment. Bioeng. 1989, 68, 334–338. [Google Scholar] [CrossRef]
- Elisashvili, V.; Penninckx, M.; Kachlishvili, E.; Tsiklauri, N.; Metreveli, E.; Kharziani, T.; Kvesitadze, G. Lentinus edodes and Pleurotus species lignocellulolytic enzymes activity in submerged and solid-state fermentation of lignocellulosic wastes of different composition. Bioresour. Technol. 2008, 99, 457–462. [Google Scholar] [CrossRef]
- Bettin, F.; Montanari, Q.; Calloni, R.; Gaio, T.A.; Silveira, M.M.; Dillon, A.J.P. Additive effects of CuSO4 and aromatic compounds on laccase production by Pleurotus sajor-caju PS-2001 using sucrose as a carbon source. Braz. J. Chem. Eng. 2014, 31, 335–346. [Google Scholar] [CrossRef]
- do Valle, J.S.; de Souza Vandenberghe, L.P.; Santana, T.T.; Linde, G.A.; Colauto, N.B.; Soccol, C.R. Optimization of Agaricus blazei laccase production by submerged cultivation with sugarcane molasses. Afr. J. Microbiol. Res. 2014, 8, 939–946. [Google Scholar] [CrossRef]
- Li, J.; Yang, W.; Ren, J.; Cao, B.; Zhu, X.; Lin, L.; Ye, W.; Zhao, R. A New Species Agrocybe striatipes, also a Newly Commercially Cultivated Mushroom with Highly Nutritional and Healthy Values. J. Fungi 2023, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Peiris, T.M.; Perera, M.; Munasinghe, H.H.; Thambugala, K.M.; Dharmasena, B.P.; Suttiprapan, P.; Cheewangkoon, R. The treasured giants: A current overview on agricultural, nutritional, bioactive, and economic potential of Macrocybe Species (Agaricales, Basidiomycota). Front. Cell. Infect. Microbiol. 2024, 14, 1493532. [Google Scholar] [CrossRef]
- Klomklung, N.; Karunarathna, S.C.; Chukeatirote, E.; Hyde, K.D. Domestication of wild strain of Pleurotus giganteus. Sydowia 2012, 60, 39–53. [Google Scholar]
- Kwon, H.; Thatithatgoon, S. Mushroom Growing for a Living Worldwide: Mushroom Growing in Northern Thailand. In Mushroom Growers’ Handbook1: Oyster mushroom Cultivation; Gush, R., Ed.; Mush World-Heineart Inc.: Seoul, Korea, 2004. [Google Scholar]
- Thongklang, N.; Sysouphanthong, P.; Hyde, K.D.; Moinard, M.; Hoang, E.; Rodriguez, A.; Kerrigan, R.W.; Foulongne-Oriol, M.; Callac, P. Evidence for diverse amphithallic lifestyles and broad geographical hybridization potential among Agaricus subrufescens isolates from Brazil, France and Thailand. Fungal Biol. 2014, 118, 1013–1023. [Google Scholar] [CrossRef]
- Thawthong, A.; Karunarathna, S.C.; Thongklang, N.; Chukeatirote, E.; Kakumyan, P.; Chamyuang, S.; Rizal, L.M.; Mortimer, P.E.; Xu, J.; Callac, P.; et al. Discovering and Domesticating Wild Tropical Cultivatable Mushrooms. Chiang Mai J. Sci. 2014, 41, 731–764. [Google Scholar]
- Dey, A.; Patel, S.; Rashid, M.M. Small-scale mushroom production unit for the upliftment of rural economy and women empowerment in India: A review. Curr. Appl. Sci. Technol. 2020, 39, 38–46. [Google Scholar] [CrossRef]
- Dulay, R.M.R.; Cabrera, E.C.; Kalaw, S.P.; Reyes, R.G.; Hou, C.T. Nutritional requirements for mycelial growth of three Lentinus species from the Philippines. Biocatal. Agric. Biotechnol. 2020, 23, 101506. [Google Scholar] [CrossRef]
- Lau, B.F.; Abdullah, N. Bioprospecting of Lentinus squarrosulus Mont., an underutilized wild edible mushroom, as a potential source of functional ingredients: A review. Trends Food Sci. Technol. 2017, 61, 116–131. [Google Scholar] [CrossRef]
- Rathore, H.; Prasad, S.; Kapri, M.; Tiwari, A.; Sharma, S. Medicinal importance of mushroom mycelium: Mechanisms and applications. J. Funct. Foods 2019, 56, 182–193. [Google Scholar] [CrossRef]
- Aditya; Neeraj; Jarial, R.S.; Jarial, K.; Bhatia, J.N. Comprehensive review on oyster mushroom species (Agaricomycetes): Morphology, nutrition, cultivation and future aspects. Heliyon 2024, 10, e26539. [Google Scholar] [CrossRef]
- Rachmayati, R.; Agustriana, E.; Rahman, D.Y. UV mutagenesis as a strategy to enhance growth and lipid productivity of chlorella sp. 042. J. Trop. Biodivers. Biotechnol. 2020, 5, 218. [Google Scholar] [CrossRef]
- Hidayati, F.L.N.; Sardjono, G.; Cahyanto, M.N. Enhancement of indigenous fungal cellulase production by gamma rays. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1192, 012004. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.L.; Xu, H. The effects of N+ ion implantation mutagenesis on the laccase production of Ceriporiopsis subvermispora. Biotechnol. Bioproc. Eng. 2012, 17, 946–951. [Google Scholar] [CrossRef]
- Azi, F.; Wang, Z.; Chen, W.; Lin, D.; Xu, P. Developing Ganoderma lucidum as a next-generation cell factory for food and nutraceuticals. Trends Biotechnol. 2024, 42, 197–211. [Google Scholar] [CrossRef]
- Moon, S.; An, J.Y.; Choi, Y.J.; Oh, Y.L.; Ro, H.S.; Ryu, H. Construction of a CRISPR/Cas9-Mediated genome editing system in Lentinula edodes. Mycobiology 2021, 49, 599–603. [Google Scholar] [CrossRef]
- Wang, T.; Yue, S.; Jin, Y.; Wei, H.; Lu, L. Advances allowing feasible pyrG gene editing by a CRISPR-Cas9 system for the edible mushroom Pleurotus eryngii. Fungal Genet. Biol. 2021, 147, 103509. [Google Scholar] [CrossRef]
- Dong, Y.; Miao, R.; Feng, R.; Wang, T.; Yan, J.; Zhao, X.; Han, X.; Gan, Y.; Lin, J.; Li, Y.; et al. Edible and medicinal fungi breeding techniques, a review: Current status and future prospects. Curr. Res. Food Sci. 2022, 5, 2070–2080. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, N.; Li, W.; Li, J.; Li, Z.; Wang, J.; Tang, X. Laser mutagenesis of Phellinus igniarius protoplasts for the selective breeding of strains with high laccase activity. Appl. Biochem. Biotechnol. 2020, 190, 584–600. [Google Scholar] [CrossRef]
- Drori, A.; Shabat, Y.; Ben, Y.A.; Danay, O.; Levanon, D.; Zolotarov, L.; Ilan, Y. Extracts from Lentinula edodes (Shiitake) edible mushrooms enriched with vitamin D exert an anti-inflammatory hepatoprotective effect. J. Med. Food 2016, 19, 383–389. [Google Scholar] [CrossRef]
- Hereher, F.; ElFallal, A.; Toson, E.; Abou-Dobara, M.; Abdelaziz, M. Pilot study: Tumor suppressive effect of crude polysaccharide substances extracted from some selected mushroom. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 767–775. [Google Scholar] [CrossRef]
- Ruthes, A.C.; Smiderle, F.R.; Iacomini, M. Mushroom heteropolysaccharides: A review on their sources, structure and biological effects. Carbohydr. Polym. 2016, 136, 358–375. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Gonzalez, M.L.; Sepulveda, L.; Verma, D.K.; Luna-García, H.A.; RodríguezDuran, L.V.; Ilina, A.; Aguilar, C.N. Conventional and emerging extraction processes of flavonoids. Processes 2020, 8, 434. [Google Scholar] [CrossRef]
- Gupta, S.; Summuna, B.; Gupta, M.; Annepu, S.K. Edible mushrooms: Cultivation, bioactive molecules, and health benefits. In Bioactive Molecules in Food; Merillon, J.M., Ramawat, K.G., Eds.; Springer Nature: Berlin, Germany, 2019; pp. 1815–1847. [Google Scholar] [CrossRef]
- Lu, X.; Brennan, M.A.; Serventi, L.; Liu, J.; Guan, W.; Brennan, C.S. Addition of mushroom powder to pasta enhances the antioxidant content and modulates the predictive glyacemic response of pasta. Food Chem. 2018, 264, 199–209. [Google Scholar] [CrossRef]
- Trigo, J.P.; Alexandre, E.M.C.; Saraiva, J.A.; Pintado, M.E. High value-added compounds from fruit and vegetable by-products—Characterization, bioactivities, and application in the development of novel food products. Crit. Rev. Food Sci. Nutr. 2019, 60, 1388–1416. [Google Scholar] [CrossRef]
- Verma, D.K.; Srivastav, P.P. Bioactive compounds of rice (Oryza sativa L.): Review on paradigm and its potential benefit in human health. Trends Food Sci. Technol. 2020, 97, 355–365. [Google Scholar] [CrossRef]
- Elnahas, M.O.; Elkhateeb, W.A.; Daba, G.M. Nutritive profile, pharmaceutical potentials, and structural analysis of multifunctional bioactive fungal polysaccharides—A review. Int. J. Biol. Macromol. 2024, 266, 130893. [Google Scholar] [CrossRef]
- Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.-I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of vitamin D2 mushroom powder as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2020, 18, e05948. [Google Scholar] [CrossRef]
- Lahteenmaki-Uutela, A.; Rahikainen, M.; Lonkila, A.; Yang, B. Alternative proteins and EU food law. Food Control 2021, 130, 108336. [Google Scholar] [CrossRef]
- Hildebrand, G.; Poojary, M.M.; O’Donnell, C.; Lund, M.; Garcia-Vaquero, M.; Tiwari, B. Ultrasound-assisted processing of Chlorella vulgaris for enhanced protein extraction. J. Appl. Phycol. 2020, 32, 1709–1718. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lamsal, B.P. Ultrasound-assisted extraction and modification of plant-based proteins: Impact on physicochemical, functional, and nutritional properties. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1457–1480. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionescu, M.; Dincă, M.-N.; Ferdeș, M.; Zăbavă, B.-Ș.; Paraschiv, G.; Moiceanu, G. Proteins from Edible Mushrooms: Nutritional Role and Contribution to Well-Being. Foods 2025, 14, 3201. https://doi.org/10.3390/foods14183201
Ionescu M, Dincă M-N, Ferdeș M, Zăbavă B-Ș, Paraschiv G, Moiceanu G. Proteins from Edible Mushrooms: Nutritional Role and Contribution to Well-Being. Foods. 2025; 14(18):3201. https://doi.org/10.3390/foods14183201
Chicago/Turabian StyleIonescu, Mariana, Mirela-Nicoleta Dincă, Mariana Ferdeș, Bianca-Ștefania Zăbavă, Gigel Paraschiv, and Georgiana Moiceanu. 2025. "Proteins from Edible Mushrooms: Nutritional Role and Contribution to Well-Being" Foods 14, no. 18: 3201. https://doi.org/10.3390/foods14183201
APA StyleIonescu, M., Dincă, M.-N., Ferdeș, M., Zăbavă, B.-Ș., Paraschiv, G., & Moiceanu, G. (2025). Proteins from Edible Mushrooms: Nutritional Role and Contribution to Well-Being. Foods, 14(18), 3201. https://doi.org/10.3390/foods14183201






