Indigenous Wild Edible Mushrooms: Unveiling the Chemical Compositions and Health Impacts
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Collection and Preparation of Sample
2.3. Morphological and Molecular Identification
2.4. Analysis of Proximate Compositions
2.5. Analysis of Glucan
2.6. Analysis of Total Phenolic Content (TPC)
2.7. Determination of Phenolic Profile by Liquid Chromatography–Mass Spectrometry (LC-MS)
2.8. Analysis of Antioxidant Activities
2.9. Analysis of Key Antioxidant Regulators by Western Blot
2.10. Digestive Enzyme Inhibition Assays
2.11. Cytotoxicity and Hepatotoxicity Prevention Effect
2.12. Statistical Analysis
3. Results and Discussion
3.1. Morphology and Molecular Identification
3.2. Chemical Composition
3.3. Total Phenolic Content and Antioxidant Activity
3.4. Phenolic Profile
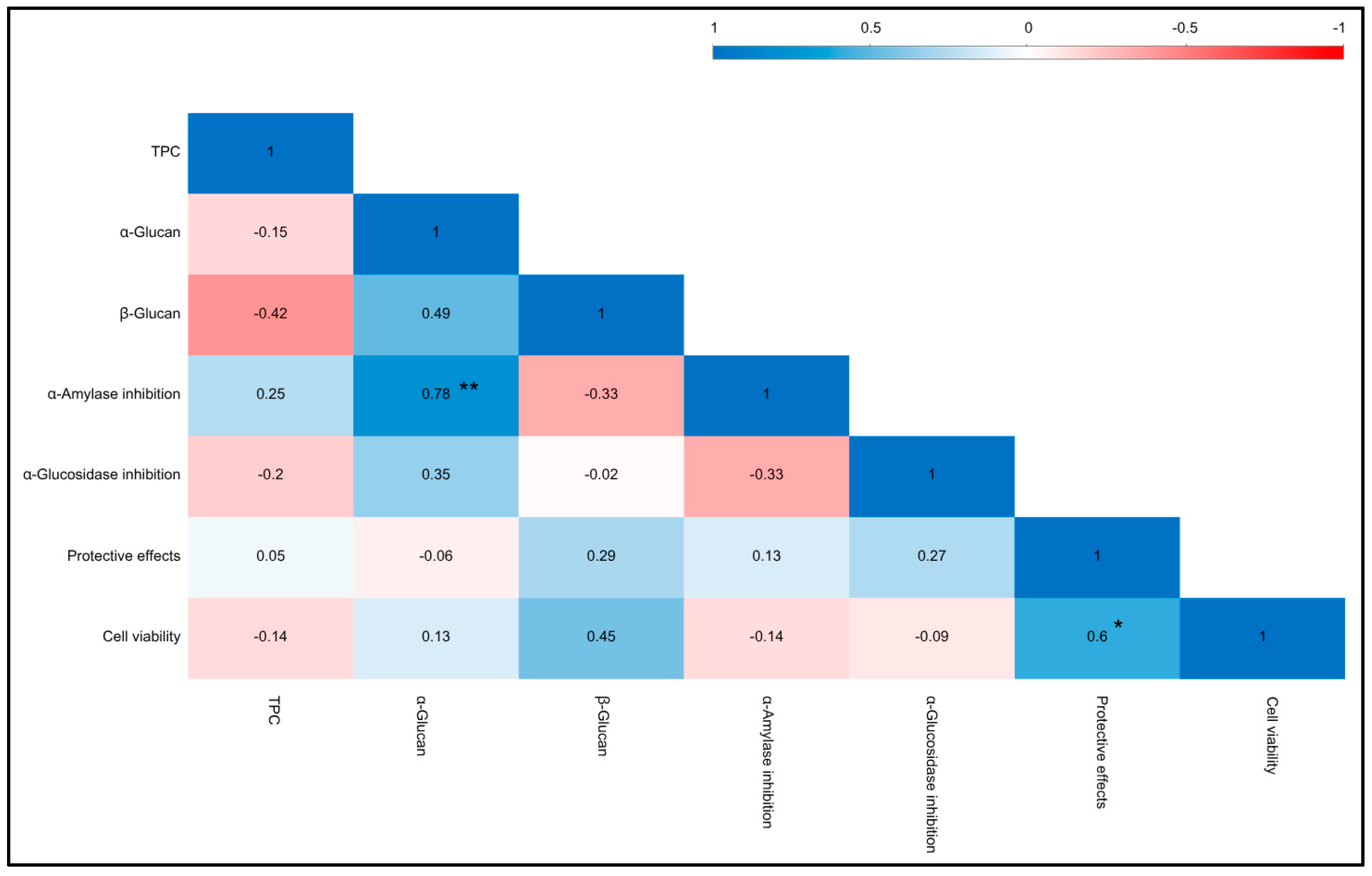
3.5. Important Antioxidant and Anti-Inflammatory Regulators
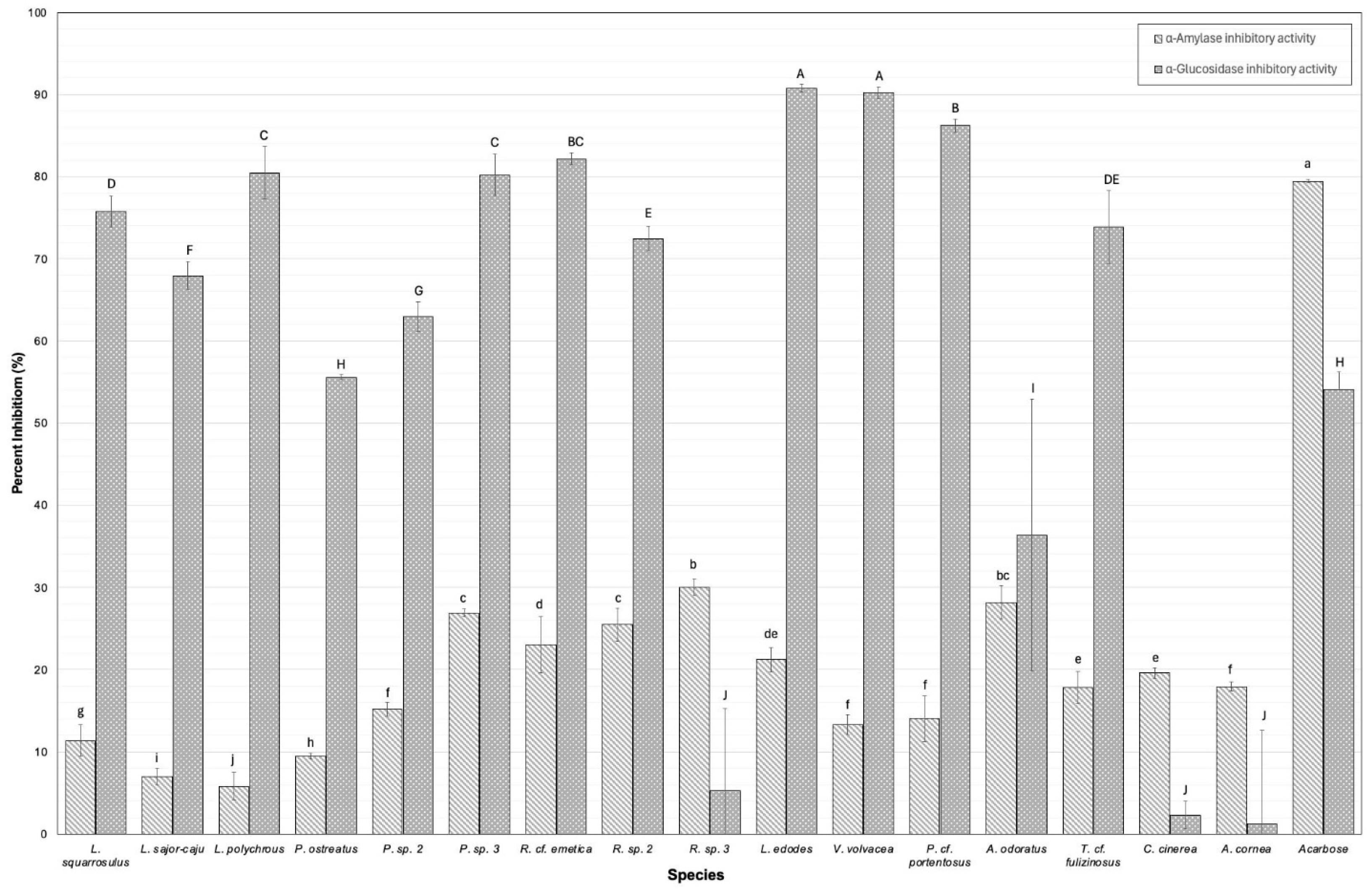
3.6. Digestive Enzyme Inhibition
3.7. Cytotoxicity and Protective Properties of WEMs on HepG2 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thu, Z.M.; Myo, K.K.; Aung, H.T.; Clericuzio, M.; Armijos, C.; Vidari, G. Bioactive phytochemical constituents of wild edible mushrooms from Southeast Asia. Molecules 2020, 25, 1972. [Google Scholar] [CrossRef]
- Rida Oktorida, K.; Nani, M.; Iing Dwi, L. Wild edible mushroom, a potential and valuable source for food security. In Proceedings of the 2nd International Conference for Smart Agriculture, Food, and Environment (ICSAFE 2021); Atlantis Press: Dordrecht, The Netherlands, 2022. [Google Scholar]
- Kumla, J.; Suwannarach, N.; Liu, Y.S.; Tanruean, K.; Lumyong, S. Survey of edible Amanita in Northern Thailand and their nutritional value, total phenolic content, antioxidant and α-glucosidase inhibitory activities. J. Fungi 2023, 9, 343. [Google Scholar] [CrossRef] [PubMed]
- Butkrachang, S.; Boonchieng, E.; Sardsud, U.; Sukchotiratana, M.; Plikomol, A.; Chairote, G.; Narongchai, P. Wild mushroom database of Chiang Mai community forest. Asian J. Biol. Educ. 2007, 3, 65–70. [Google Scholar]
- Bell, V.; Silva, C.; Guina, J.; Fernandes, T. Mushrooms as future generation healthy foods. Front. Nutr. 2022, 9, 1050099. [Google Scholar] [CrossRef] [PubMed]
- Fogarasi, M.; Diaconeasa, Z.M.; Pop, C.R.; Fogarasi, S.; Semeniuc, C.A.; Fărcaş, A.C.; Țibulcă, D.; Sălăgean, C.-D.; Tofană, M.; Socaci, S.A. Elemental Composition, Antioxidant and Antibacterial Properties of Some Wild Edible Mushrooms from Romania. Agronomy 2020, 10, 1972. [Google Scholar] [CrossRef]
- Rahi, D.K.; Malik, D. Diversity of mushrooms and their metabolites of nutraceutical and therapeutic significance. J. Mycol. 2016, 2016, 7654123. [Google Scholar] [CrossRef]
- Samsudin, N.I.P.; Abdullah, N. Edible mushrooms from Malaysia; a literature review on their nutritional and medicinal properties. Int. Food Res. J. 2019, 26, 11–31. [Google Scholar]
- Degreef, J.; Demuynck, L.; Mukandera, A.; Nyirandayambaje, G.; Nzigidahera, B.; De Kesel, A. Wild edible mushrooms, a valuable resource for food security and rural development in Burundi and Rwanda. Base 2016, 20, 441–452. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, X.; Li, J.; Jiang, H.; Shan, X.; Wang, Y.; Ma, W.; Hao, J.; Yu, G. A β-glucan from Durvillaea Antarctica has immunomodulatory effects on RAW264.7 macrophages via toll-like receptor 4. Carbohydr. Polym. 2018, 191, 255–265. [Google Scholar] [CrossRef]
- Ruan, W.; Popovich, D.G. Ganoderma lucidum triterpenoid extract induces apoptosis in human colon carcinoma cells (Caco-2). Biomed. Prev. Nutr. 2012, 2, 203–209. [Google Scholar] [CrossRef]
- Khan, A.A.; Yao, F.; Idrees, M.; Lu, L.; Fang, M.; Wang, P.; Jiang, W.-Z.; Zhang, Y.M. A comparative study of growth, biological efficiency, antioxidant activity and molecular structure in wild and commercially cultivated Auricularia cornea strains. Folia Hortic. 2020, 32, 255–264. [Google Scholar] [CrossRef]
- Liu, K.; Wang, J.; Zhao, L.; Wang, Q. Anticancer, antioxidant and antibiotic activities of mushroom Ramaria flava. Food Chem. Toxicol. 2013, 58, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-T.; Sun, J.; Luo, Z.-Y.; Rao, S.-Q.; Su, Y.-J.; Xu, R.-R.; Yang, Y.-J. Chemical composition of five wild edible mushrooms collected from Southwest China and their antihyperglycemic and antioxidant activity. Food Chem. Toxicol. 2012, 50, 1238–1244. [Google Scholar] [CrossRef]
- Fong-in, S.; Khwanchai, P.; Prommajak, T.; Boonsom, S. Physicochemical, nutritional, phytochemical properties and antioxidant activity of edible Astraeus odoratus mushrooms: Effects of different cooking methods. Int. J. Gastron. Food Sci. 2023, 33, 100743. [Google Scholar] [CrossRef]
- Yin, Z.; Liang, Z.; Li, C.; Wang, J.; Ma, C.; Kang, W. Immunomodulatory effects of polysaccharides from edible fungus: A review. Food Sci. Hum. Wellness 2021, 10, 393–400. [Google Scholar] [CrossRef]
- Wu, G.-S.; Guo, J.-J.; Bao, J.-L.; Li, X.-W.; Chen, X.-P.; Lu, J.-J.; Wang, Y.-T. Anti-cancer properties of triterpenoids isolated from Ganoderma lucidum—A review. Expert Opin. Investig. Drugs 2013, 22, 981–992. [Google Scholar] [CrossRef]
- Heleno, S.A.; Barros, L.; Martins, A.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic, polysaccharidic, and lipidic fractions of mushrooms from Northeastern Portugal: Chemical compounds with antioxidant properties. J. Agric. Food Chem. 2012, 60, 4634–4640. [Google Scholar] [CrossRef]
- Largent, D.L. How to Identify Mushrooms to Genus III: Microscopic Features; Mad River Press: Eureka, CA, USA, 1977; Volume 1. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. A Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Horwitz, W.; Latimer, G.W. Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Aumasa, T.; Ogawa, Y.; Singh, J.; Panpipat, W.; Donlao, N. The role of herbal teas in reducing the starch digestibility of cooked rice (Oryza sativa L.): An in vitro co-digestion study. NFS J. 2023, 33, 100154. [Google Scholar] [CrossRef]
- Yi, Y.; Li, T.; Lv, C.; He, W.; Li, W.; Zhou, X.; Qin, S. Proanthocyanidins isolated from lotus seed skin mitigate glycolipid metabolism disorder through the p38/Nrf2/NF-κB signaling pathway. Acta Biochim. Biophys. Sin. (Shanghai) 2024, 56, 1300–1310. [Google Scholar] [CrossRef]
- Johnson, M.H.; Lucius, A.; Meyer, T.; Gonzalez de Mejia, E. Cultivar evaluation and effect of fermentation on antioxidant capacity and in vitro inhibition of α-amylase and α-glucosidase by highbush blueberry (Vaccinium corombosum). J. Agric. Food Chem. 2011, 59, 8923–8930. [Google Scholar] [CrossRef]
- Mir, H.; Elieh Ali Komi, D.; Pouramir, M.; Parsian, H.; Moghadamnia, A.A.; Seyfizadeh, N.; Lakzaei, M. The hepatoprotective effects of Pyrus biossieriana buhse leaf extract on tert-butyl hydroperoxide toxicity in HepG2 cell line. BMC Res Notes 2021, 14, 298. [Google Scholar] [CrossRef]
- Wu, J.-G.; Kan, Y.-J.; Wu, Y.-B.; Yi, J.; Chen, T.-Q.; Wu, J.-Z. Hepatoprotective effect of ganoderma triterpenoids against oxidative damage induced by tert-butyl hydroperoxide in human hepatic HepG2 cells. Pharm. Biol. 2016, 54, 919–929. [Google Scholar] [CrossRef]
- Wisitrassameewong, K.; Looney, B.P.; Le, H.T.; De Crop, E.; Das, K.; Van de Putte, K.; Eberhardt, U.; Jiayu, G.; Stubbe, D.; Hyde, K.D.; et al. Lactarius subgenus Russularia (Basidiomycota, Russulales): Novel Asian species, worldwide phylogeny and evolutionary relationships. Fungal Biol. 2016, 120, 1554–1581. [Google Scholar] [CrossRef] [PubMed]
- Sanmee, R.; Dell, B.; Lumyong, P.; Izumori, K.; Lumyong, S. Nutritive value of popular wild edible mushrooms from northern Thailand. Food Chem. 2003, 82, 527–532. [Google Scholar] [CrossRef]
- Hyde, K.D.; Norphanphoun, C.; Chen, J.; Dissanayake, A.J.; Doilom, M.; Hongsanan, S.; Jayawardena, R.S.; Jeewon, R.; Perera, R.H.; Thongbai, B.; et al. Thailand’s amazing diversity: Up to 96% of fungi in northern Thailand may be novel. Fungal Divers. 2018, 93, 215–239. [Google Scholar] [CrossRef]
- Naksuwankul, K.; Thongbor, A.; Chantharasena, C.; Khottawong, W.; Parnmen, S.; Nooron, N.; Sikaphan, S.; Leudang, S.; Kongdang, P.; Khamenkhetkarn, M. Identification by morphological and local wisdom and distribution of poisonous and edible mushroom in Thailand. Burapha Sci. J. 2022, 27, 66–84. [Google Scholar]
- Effiong, M.E.; Umeokwochi, C.P.; Afolabi, I.S.; Chinedu, S.N. Assessing the nutritional quality of Pleurotus ostreatus (oyster mushroom). Front. Nutr. 2024, 10, 1279208. [Google Scholar] [CrossRef]
- Sousa, A.S.; Araújo-Rodrigues, H.; Pintado, M.E. The Health-promoting potential of edible mushroom proteins. Curr. Pharm. Des. 2023, 29, 804–823. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.; Ferreira, I.C. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Witkowska, A.M. Evaluation of Polish wild mushrooms as beta-glucan sources. Int. J. Environ. Res. Public Health 2020, 17, 7299. [Google Scholar] [CrossRef]
- Nowacka, N.; Nowak, R.; Drozd, M.; Olech, M.; Los, R.; Malm, A. Antibacterial, antiradical potential and phenolic compounds of thirty-one polish mushrooms. PLoS ONE 2015, 10, e0140355. [Google Scholar] [CrossRef]
- Singdevsachan, S.K.; Auroshree, P.; Mishra, J.; Baliyarsingh, B.; Tayung, K.; Thatoi, H. Mushroom polysaccharides as potential prebiotics with their antitumor and immunomodulating properties: A review. Bioact. Carbohydr. Diet. Fibre 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Cheung, P.C. Mushrooms as Functional Foods; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Wunjuntuk, K.; Ahmad, M.; Techakriengkrai, T.; Chunhom, R.; Jaraspermsuk, E.; Chaisri, A.; Kiwwongngam, R.; Wuttimongkolkul, S.; Charoenkiatkul, S. Proximate composition, dietary fibre, beta-glucan content, and inhibition of key enzymes linked to diabetes and obesity in cultivated and wild mushrooms. J. Food Compos. Anal. 2022, 105, 104226. [Google Scholar] [CrossRef]
- Yusran, Y.; Erniwati, E.; Maksum, H.; Khumaidi, A.; Setiarto, R.H.B. Effect of cooking on the proximate composition and minerals content of wild edible macro fungi from Lore Lindu National Park, Central Sulawesi, Indonesia. Afr. J. Food Agric. Nutr. Dev. 2022, 22, 20523–20541. [Google Scholar] [CrossRef]
- Elkanah, F.A.; Oke, M.A.; Adebayo, E.A. Substrate composition effect on the nutritional quality of Pleurotus ostreatus (MK751847) fruiting body. Heliyon 2022, 8, e11841. [Google Scholar] [CrossRef] [PubMed]
- Irshad, A.; Tahir, A.; Sharif, S.; Khalid, A.; Ali, S.; Naz, A.; Sadia, H.; Ameen, A. Determination of Nutritional and Biochemical Composition of Selected Pleurotus spps. BioMed Res. Int. 2023, 2023, 8150909. [Google Scholar] [CrossRef]
- Ali, A.A.H. Overview of the vital roles of macro minerals in the human body. J. Trace Elem. Miner. 2023, 4, 100076. [Google Scholar] [CrossRef]
- Okwulehie, I.C.; Nwosu, C.; Johnpaul, O. Pharmaceutical and nutritional prospects of two wild macro-fungi found in Nigeria. Biotechnology 2007, 6, 567–572. [Google Scholar] [CrossRef]
- Akram, K.; Shahbaz, H.M.; Kim, G.-R.; Farooq, U.; Kwon, J.-H. Improved extraction and quality characterization of water-soluble polysaccharide from gamma-irradiated Lentinus edodes. J. Food Sci. 2017, 82, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Bristy, A.T.; Islam, T.; Ahmed, R.; Hossain, J.; Reza, H.M.; Jain, P. Evaluation of total phenolic content, HPLC analysis, and antioxidant potential of three local varieties of mushroom: A comparative study. Int. J. Food Sci. 2022, 2022, 3834936. [Google Scholar] [CrossRef] [PubMed]
- Kaewnarin, K.; Suwannarach, N.; Kumla, J.; Lumyong, S. Phenolic profile of various wild edible mushroom extracts from Thailand and their antioxidant properties, anti-tyrosinase and hyperglycaemic inhibitory activities. J. Funct. Foods 2016, 27, 352–364. [Google Scholar] [CrossRef]
- Kolayli, S.; Sahin, H.; Aliyazicioglu, R.; Sesli, E. Phenolic components and antioxidant activity of three edible wild mushrooms from Trabzon, Turkey. Chem. Nat. Compd. 2012, 48, 137–140. [Google Scholar] [CrossRef]
- Sunthudlakhar, P.; Sithisarn, P.; Rojsanga, P.; Jarikasem, S. HPLC quantitative analysis of protocatechuic acid contents in 11 Phellinus mushroom species collected in Thailand. Braz. J. Pharm. Sci. 2022, 58, e20656. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and oxidative stress: A general overview of mechanisms and implications in human disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Kokubo, T.; Taniguchi, Y.; Kanayama, M.; Shimura, M.; Konishi, Y.; Kawagishi, H.; Yamamoto, M.; Shindo, K.; Yoshida, A. Extract of the mushroom Mycoleptodonoides aitchisonii induces a series of anti-oxidative and phase II detoxifying enzymes through activation of the transcription factor Nrf2. Food Chem. 2011, 129, 92–99. [Google Scholar] [CrossRef]
- Yang, H.L.; Lin, S.W.; Lee, C.C.; Lin, K.Y.; Liao, C.H.; Yang, T.Y.; Wang, H.M.; Huang, H.C.; Wu, C.R.; Hseu, Y.C. Induction of Nrf2-mediated genes by Antrodia salmonea inhibits ROS generation and inflammatory effects in lipopolysaccharide-stimulated RAW264.7 macrophages. Food Funct. 2015, 6, 230–241. [Google Scholar] [CrossRef]
- Yang, T.; Fang, H.; Lin, D.; Yang, S.; Luo, H.; Wang, L.; Yang, B. Ganoderma Lucidum polysaccharide peptide (GL-PP2): A potential therapeutic agent against sepsis-induced organ injury by modulating Nrf2/NF-κB pathways. Int. J. Biol. Macromol. 2025, 285, 138378. [Google Scholar] [CrossRef]
- Li, Y.-M.; Zhong, R.-f.; Chen, J.; Luo, Z.-G. Structural characterization, anticancer, hypoglycemia and immune activities of polysaccharides from Russula virescens. Int. J. Biol. Macromol. 2021, 184, 380–392. [Google Scholar] [CrossRef]
- Motolinia-Alcántara, E.A.; Franco-Vásquez, A.M.; Nieto-Camacho, A.; Arreguín-Espinosa, R.; Rodríguez-Monroy, M.; Cruz-Sosa, F.; Román-Guerrero, A. Phenolic Compounds from Wild Plant and In Vitro Cultures of Ageratina pichichensis and Evaluation of Their Antioxidant Activity. Plants 2023, 12, 1107. [Google Scholar] [CrossRef] [PubMed]


| No. | Family | Species | Thai Common Name | DNA Sequence | Number of Bases |
|---|---|---|---|---|---|
| 1 | Polyporaceae | Lentinus squarrosulus | Hed lom | CTTTGGGTATGTGCACATCCTCCTCCGATTTCTATTCATCCACCTGTGCACTTTTTGTAGAGTTCTTTCATCGGGTTTTTGAAGGTGCTCATTATGAGTTACTTGAAAAGACTAGTTGACAAGGCTTCTATGTTCTTATAAACCATTGAAGTATGTTATAGAATGATCTTGTTATTGGGACTTTATTGACCCTTTAAACTTAATACAACTTTCAGCAACGGATCTCTTGGCTCTCCCATCGATGAAGAACGCAGCGAAATGCGATAAGTAATGTGAATTGCAGAATTCAGTGAATCATCGAATCTTTGAACGCACCTTGCGCCCTCTGGTATTCCGGAGGGCATGCCTGTTTGAGTGTCATTAAATTCTCAACTTTATAAGTTTTT | 387 |
| 2 | Polyporaceae | Lentinus sajor-caju | Hed lom | GAAGTAAAAGTCGTAACAAGGTTTCCGTAGGTGAACCTGCGGAAGGATCATTATCGAGTTATTGAAACGGGTTGTAGCTGGCCTTACGAGGCATGTGCACGCCCTGCTCATCCACTCTACACCTGTGCACTTACTGTGGGTTTCAGGAGCTTCGAAAGCGGAGGGCCTTTGCGGGCTTTTCGTTATTAGTTGTGACTGGGCTCATGTCCACTACACACTCTTATAAAGTAACAGAATGTGTATTGCGATGTAACGCATCTATATACAACTTTCAGCAACGGATCTCTTGGCTCTCGCATCGATGAAGAACGCAGCGAAATGCGATAAGTAATGTGAATTGCAGAATTCAGTGAATCATCGAATCTTTGAACGCACCTTGCGCTCCTTGGTATTCCGAGGAGCATGCCTGTTTGAGTGTCATGAAATTCTCAACCTGACGGGTTCTTAACGGAGCTTGGTTCAGGCTTGGACTTGGAGGCTTGTCGGCTTGCTTTGTCGAGTCGGCTCCTCTCAAATGCATTAGCTTGGTTCTTTGCGGATCGGCTCACGGTGTGATAATTGTCTACGCCGCGACCGTTGAAGCGTTTGAATGGGCCAGCTTATAGTCGTCTCCATCGCGAGACAACATTTCATCGAACTCTGACCTCAAATCAGGTAGGACTACCCGCTGAACTTAAGCATATCATAA | 445 |
| 3 | Polyporaceae | Lentinus polychrous | Hed lom | CCTGCGGAAGGATCATTATCGAGTCTTGAAACGGGTTGTAGCTGGCCTTCCGAGGCATGTGCACGCCCTGCTCATCCACTCTACACCTGTGCACTTACTGTGGGTTTCAGGAGCTTCGAAAGCGAGAAGGGCCCCTYGGGGGGTCAGTCTCGTCGTAGTAGTGACTGGGCCCACGTTTACTATAAACTCTTAAAAGTATCAGAATGTGTATTGCGATGTAACGCATCTATATACAACTTTCAGCAACGGATCTCTTGGCTCTCGCATCGATGAAGAACGCAGCGAAATGCGATAAGTAATGTGAATTGCAGAATTCAGTGAATCATCGAATCTTTGAACGCACCTTGCGCTCCTTGGTATTCCGAGGAGCATGCCTGTTTGAGTGTCATGAAATTCTCAACCTAGCGGGTTCTTAACCGGACTTGCTTAGGCTTGGACTTGGAGGCTTTGTCGGCTTGCTTATGTCGAGTCGGCTCCTCTCAAATGCATTAGCTTGGTTCCTTTGCGGATCGGCTCACGGTGTGATAATTGTCTACGCCGCGACCGTTGAAGCGTTTTAATGGCCAGCTTCTAATCGTCTCTTGCGAGACAACATTCATCGAACTCCTGACC | 612 |
| 4 | Pleurotaceae | Pleurotus ostreatus | Hed nang fah | Identified by morphology | |
| 5 | Pleurotaceae | Pleurotus sp. 2 | Hed nang fah | Identified by morphology | |
| 6 | Pleurotaceae | Pleurotus sp. 3 | Hed nang fah | Identified by morphology | |
| 7 | Russulaceae | Russula cf. emetica | Hed lom krakeaw | Identified by morphology | |
| 8 | Russulaceae | Russula sp. 2 | Hed deang nhammak | GGGCTGTCGCTGACCTTTGCGGGTCGTGCACGCTCAAAGTGCTCTCTCTCATATCCAACTCACCCCTTTGTGCATCACCGCGTGGGCCCCACCCTCTTGGGATCGGTTCACGTTTTTCTACAAACACCTTTCTTTTAATGCATGTGTAGAATGTCTTCCTTTTTTGCGATCACGCGCAATCAATACAACTTTCAACAACGGATCTCTTGGCTCTCGCATCGATGAARAACGCAGCGAAATGCGATACGTAATGTGAATTGCAGAATTCAGTGAATCATCGAATCTTTGAACGCACCTTGCGCCCCTTGGTATTCCGAGGGGCACACCCGTTTGAGTGTCGTGAACATCCTCAACCTTTTTGGTTTCTTGACCAAGAAGGCTTGGACTTTGGAGGTTTATCCTTGCTGGCTTACCTTTGAAGCCAGCTCCTCCTAAATGAATTAKTGGGGTCCCCTTTGCCGATCCTTGATGTGATAAGTATTGCTTCTACGTCTTGGGTCTCGCACTGTTGCCCTGGAACCCCSCYTYCMACMRKCYTTCTTTYCAAAAASAATGTYGAAKTGGMTTGGTACTCCAMTCTCTACCAACTTGAACTCCAAWYSGGKGARAATAACCGCTGGAMTTWAKCATAATCAAA | 637 |
| 9 | Russulaceae | Russula sp. 3 | Hed nha meung | GCTGTCGCTGACCTTTGCGGGTCGTGCACGCTCAAAGTGCTCTCTCTCATATCCAACTCACCCCTTTGTGCATCACCGCGTGGGCCCCACCCTCTTGGGATCGGTTCACGTTTTTCTACAAACACCTTTCTTTTAATGCATGTGTAGAATGTCTTCCTTTTTTGCGATCACGCGCAATCAATACAACTTTCAACAACGGATCTCTTGGCTCTCGCATCGATGAARAACGCAGCGAAATGCGATACGTAATGTGAATTGCAGAATTCAGTGAATCATCGAATCTTTGAACGCACCTTGCGCCCCTTGGTATTCCGAGGGGCACACCCGTTTGAGTGTCGTGAACATCCTCAACCTTTTTGGTTTCTTGACCAAGAAGGCTTGGACTTTGGAGGTTTATCCTTGCTGGCTTACCTTTGAAGCCAGCTCCTCCTAAATGAATTAKTGGGGTCCCCTTTGCCGATCCTTGATGTGATAAGTATTGCTTCTACGTCTTGGGTCTCGCACTGTTGCCCTGGAACCCCSCYTYCMACMRKCYTTCTTTYCAAAAASAATGTYGAAKTGGMTTGGTACTCCAMTCTCTACCAACTTGAACTCCAA | 597 |
| 10 | Marasmiaceae | Lentinula edodes | ATTGTTGCTGGCCTTTGGGTATGTGCACATCCTCCTCCGATTTCTATTCATCCACCTGTGCACTTTTTGTAGGAGTTCTTTCATCGGGTTTTTGAAGGTGCTCATTATGAGTTACTTGAAAAGACTAGTTGACAAGGCTTCTATGTTCTTATAAACCATTGAAGTATGTTATAGAATGATCTTGTTATTGGGACTTTATTGACCCTTTAAACTTAATACAACTTTCAGCAACGGATCTCTTGGCTCTCCCATCGATGAAGAACGCAGCGAAATGCGATAAGTAATGTGAATTGCAGAATTCAGTGAATCATCGAATCTTTGAACGCACCTTGCGCCCTCTGGTATTCCGGAGGGCATGCCTGTTTGAGTGTCATTAAATTCTCAACTTTATAAGTTTTTAMTTATTAAARSTTGGAAGGTGGAAGSTTGGMRGGSKTTGGCMRCTCCTCCTAAATTTATTAAKGGGAACCCTGGTTTGGTARTTCYAACCTTGGKGGGAAAATTATCTAC | 510 | |
| 11 | Pluteaceae | Volvariella volvacea | Hed fang | TGCTGGCTCCTCGGAGCAGGTGCACGCCCTCCCCGACGCCTTCCATTCTCCACGTCCCCACCTGTGCACCTTCTGTAGGCCGTGAAGCCGCCTCGTTCGGCTCCCTCGGCTCTACGAGATCTTTTGTACACCCTTGAGAAAAACGTGTTGCAGAGTGTTCTTGTACGACCGGGGACCCCTCGTCGGCCCCATAGACATACCAATACAACTTTCAACAACGGATCTCTTGGCTCTCGCATCGATGAAGAACGCAGCGAAATGCGATAAGTAATGTGAATTGCAGAATTCAGTGAATCATCGAATCTTTGAACGCACCTTGCGCTCTTTGGCCATTCCGAAGAGCATGCCTGTTTGAGTGTCATCGAATCCTCAAGCCCAGCCCGGCTTCTCCCCGGGCTTTTGGGGGCTTGGAGTTGGGAGCTGTGCGGGTCGCTAGCCTTCGCGATCCGCTCTCCTCAAAGGCATCAGCAGGGCCCAGTCGCAGTCGGCCTCGTGGCGTTGATAGTCCATCTACGCCCCCCCGCGGCCGCACTCAGCGTGGCTCGGCTTCGAACCGTCCGGAC | 563 |
| 12 | Boletinellaceae | Phlebopus cf. portentosus | Identified by morphology | ||
| 13 | Diplocystaceae | Astraeus odoratus | AAAGATTTCCGGCCCCGGGATTAACAACCCCGGCGCGGCGCGGCAACATGGTTGGAAGCATGAGACACGTCGATCCAAGCACTTCCAGCCCACGACGATCACTACGACGTCGAACAGGCCGTGCCGTGCAAAGGCTCGAAGCCCACCGCTAATGCATTTGAGGAGAGCCGGCGTCCCGAGCAAAGTCGGGTCGCCCGCAGACTCCCAAAGTCCAAGTCCGAGCTCGCTCCGAGTCGGCGACCGAAGCAAAGCTTAGGATTTGAGATTTCGATGACACTCAAACAGGCATGCTCCTCGGAATACCAAGGAGCGCAAGGTGCGTTCAAAGATTCGATGATTCACGGAAAATCTGCAATTCACATTACTTATCGCGATTCGCTGCGTTCTTCATCGATGCGAGAGCCAAGAGATCCATTGCTGAAAGTTATATATATGTTTATATGACATGTTTGTCAAAAGACAACGTTCTGTATACATGCAGAGAGCTTTATAAAAA | 496 | |
| 14 | Lyophyllaceae | Termitomyces cf. fulizinosus | Hed kone | Identified by morphology | |
| 15 | Psathyrellaceae | Coprinopsis cinerea | Hed hu noo | Identified by morphology | |
| 16 | Auriculariaceae | Auricularia cornea | CTTGGTCATTTAGAGGAAGTAAAAGTCGTAACAAGGTTTCCGTAGGTGAACCTGCGGAAGGATCATTAAAGATTTTGGGCTTTTAacCCGATCGTTCAGCTGTGCGCCCTTCACAGGGCTGCACGCTGGAGCAAGACCCCACACCTGTGCACCTTTTCGGTTGCGGCTTCGGTCGCTGCCGCTTTCAAATGCAACAACTCAGTCTCGAATGTTAACAAAACCATAAAAAGTAACAACTTTCAACAACGGATCTCTTGGCTCTCGCATCGATGAAGAACGCAGCGAAATGCGATAAGTAATGTGAATTGCAGAATTCAGTGAATCATCGAATCTTTGAACGCATCTTGCGCTCCTTGGTATTCCATGGAGCATGCCTGTTTGAGTGTCACGTAAACCCTCACCCTTGCGATGTAACAGTCGCCCGTGGTGGACTTGGACTGTGCCGTAACCGGCTCGTCTTGAAATGCATTAGCTGGCGCTTTTAGAGTGCTGGGCGACGGTGTGATAATTATCTGCGCCAATGCCTTAGGCCTCTTCAGCGGTGCTGCTTACAGCCGTCCCTCTGTGGACACATTATTTTTAAAGCTTTGGCCTCAAATCAGGTAGGACTACCCGCTGAACTTAAGCATATCAATAAGCGGAGGAA | 646 |
| Species | Crude Fat A | Ash A | Crude Fiber A | Protein A | β-Glucan B | α-Glucan B | Total Glucan B |
|---|---|---|---|---|---|---|---|
| L. squarrosulus | 1.16 ± 0.04 c | 8.56 ± 0.03 abc | 21.26 ± 0.86 f | 31.96 ± 0.54 bc | 5.00 ± 0.97 cd | 2.41 ± 0.09 cdef | 7.41 ± 0.99 efgh |
| L. sajor-caju | 1.31 ± 0.05 c | 5.41 ± 0.85 def | 23.42 ± 0.58 e | 19.84 ± 0.38 f | 16.69 ± 0.89 b | 8.40 ± 1.36 a | 25.08 ± 0.99 b |
| L. polychrous | 0.83 ± 0.12 c | 5.41 ± 0.11 def | 30.08 ± 1.34 b | 28.42 ± 0.34 c | 5.06 ± 1.42 cd | 6.34 ± 0.44 b | 11.40 ± 0.99 de |
| P. ostreatus | 1.12 ± 0.05 c | 5.81 ± 0.09 cde | 23.68 ± 0.94 d | 22.10 ± 0.17 e | 15.64 ± 2.12 b | 7.74 ± 1.35 a | 23.37 ± 0.99 b |
| P. sp. 2 | 1.04 ± 0.11 c | 4.66 ± 0.08 def | 18.49 ± 1.12 j | 26.74 ± 0.26 d | 5.23 ± 1.01 cd | 2.18 ± 0.07 def | 7.41 ± 0.99 efgh |
| P. sp. 3 | 0.91 ± 0.05 c | 3.25 ± 0.03 ef | 23.26 ± 2.29 e | 23.74 ± 0.26 e | 9.19 ± 0.94 c | 1.64 ± 0.06 defg | 10.83 ± 0.99d ef |
| R. cf. emetica | 1.38 ± 0.06 c | 9.32 ± 0.26 ab | 19.29 ± 1.61 i | 31.74 ± 2.33 bc | 9.64 ± 4.55 c | 0.62 ± 0.05 g | 10.26 ± 4.52 defg |
| R. sp. 2 | 2.38 ± 0.29 b | 8.62 ± 0.10 abc | 17.87 ± 1.02 j | 30.15 ± 0.39 c | 6.41 ± 1.64 cd | 0.43 ± 0.09 g | 6.84 ± 1.71 fgh |
| R. sp. 3 | 4.71 ± 0.03 a | 9.21 ± 0.03 ab | 20.27 ± 1.21 h | 30.93 ± 0.23 c | 6.33 ± 1.14 cd | 1.53 ± 0.07 defg | 7.86 ± 1.20 efgh |
| L. edodes | 0.78 ± 0.04 c | 6.16 ± 0.12 cde | 17.65 ± 0.58 j | 21.99 ± 0.79 e | 9.45 ± 0.25 c | 3.66 ± 0.74 c | 13.11 ± 0.99 d |
| V. volvacea | 1.50 ± 0.12 b | 5.54 ± 0.40 cde | 20.53 ± 1.47 g | 30.85 ± 0.59 c | 23.86 ± 7.66 a | 6.35 ± 1.82 b | 30.21 ± 6.01 a |
| P. cf. portentosus | 0.52 ± 0.01 d | 9.19 ± 0.07 ab | 11.75 ± 0.91 k | 33.89 ± 1.41 b | 7.94 ± 0.43 c | 5.74 ± 0.43 b | 13.68 ± 0.00 d |
| A. odoratus | 2.05 ± 0.16 b | 11.49 ± 1.13 a | 31.14 ± 3.67 a | 35.11 ± 2.82 b | 2.21 ± 1.00 d | 1.78 ± 0.07 defg | 3.99 ± 0.99 h |
| T. cf. fulizinosus | 0.91 ± 0.05 c | 3.25 ± 0.03 ef | 0.91 ± 0.05 c | 23.74 ± 0.26 e | 4.64 ± 0.99 cd | 1.63 ± 0.06 defg | 6.27 ± 0.99 gh |
| C. cinerea | 1.34 ± 0.02 c | 7.41 ± 0.08 bcd | 1.34 ± 0.02 c | 51.87 ± 1.05 a | 9.30 ± 6.20 c | 1.53 ± 1.55 defg | 10.83 ± 5.50 def |
| A. cornea | 0.76 ± 0.01 c | 2.52 ± 0.04 f | 28.94 ± 1.35 c | 12.02 ± 0.26 g | 17.56 ± 2.09 b | 1.26 ± 1.25 efg | 18.81 ± 1.71 c |
| Species | P-Coumaric Acid | O-Coumaric Acid | Quercetin | Rutin | Ethyl Gallate | DOPAC | Protocate Chuic Acid | Total Catechin |
|---|---|---|---|---|---|---|---|---|
| L. squarrosulus | 0.05 ± 0.02 b | 0.32 ± 0.02 a | 1.85 ± 0.03 a | ND | ND | ND | 4.80 ± 2.54 d | 2.87 ± 0.93 b |
| L. sajor-caju | 0.05 ± 0.01 b | 0.32 ± 0.01 a | 1.88 ± 0.01 a | 0.51 ± 0.00 a | ND | 32.32 ± 0.05 d | ND | 1.78 ± 0.17 cd |
| L. polychrous | 0.05 ± 0.00 b | 0.32 ± 0.01 a | 1.88 ± 0.00 a | 0.51 ± 0.00 a | 2.17 ± 0.00 c | 25.81 ± 0.00 e | ND | 1.85 ± 0.05 c |
| P. ostreatus | 0.06 ± 0.00 b | 0.33 ± 0.01 a | 1.88 ± 0.00 a | 0.51 ± 0.00 a | ND | ND | ND | 1.64 ± 1.17 d |
| P. sp. 2 | 0.07 ± 0.01 b | 0.34 ± 0.01 a | 1.88 ± 0.00 a | 0.52 ± 0.01 a | ND | ND | 6.15 ± 0.03 d | ND |
| P. sp. 3 | 0.04 ± 0.00 b | 0.31 ± 0.02 a | 1.88 ± 0.00 a | 0.52 ± 0.03 a | ND | ND | 58.08 ± 0.05 c | 1.84 ± 0.02 c |
| R. cf. emetica | 0.05 ± 0.01 b | 0.31 ± 0.00 a | 1.86 ± 0.01 a | ND | ND | 68.84 ± 0.15 a | ND | 1.56 ± 1.35 e |
| R. sp. 2 | 0.05 ± 0.01 b | 0.32 ± 0.00 a | 1.88 ± 0.01 a | 0.52 ± 0.01 a | ND | 64.97 ± 0.01 b | 77.42 ± 58.54 b | 0.39 ± 0.01 h |
| R. sp. 3 | 0.05 ± 0.00 b | 0.31 ± 0.01 a | 1.89 ± 0.00 a | ND | 9.80 ± 0.01 b | ND | 56.31 ± 24.88 bc | 0.80 ± 0.67 f |
| L. edodes | 0.09 ± 0.35 ab | 0.31 ± 0.02 a | 1.57 ± 0.20 b | 0.53 ± 0.01 a | ND | ND | ND | 3.37 ± 0.13 ab |
| V. volvacea | 0.09 ± 0.02 a | 0.35 ± 0.02 a | 1.83 ± 0.01 a | 0.52 ± 0.02 a | ND | ND | ND | 0.71 ± 0.14 g |
| P. cf. portentosus | ND | 0.31 ± 0.01 a | 1.88 ± 0.01 a | 0.52 ± 0.01 a | ND | ND | 7.08 ± 0.01 | 2.39 ± 0.08 b |
| A. odoratus | 0.95 ± 0.00 a | 0.32 ± 0.01 a | 1.88 ± 0.00 a | 0.51 ± 0.00 a | 11.17 ± 0.01 a | ND | 90.52 ± 0.02 a | 1.34 ± 0.90 ed |
| T. cf. fulizinosus | 0.05 ± 0.01 b | 0.32 ± 0.00 a | 1.88 ± 0.01 a | 0.51 ± 0.00 a | ND | ND | ND | 0.41 ± 0.01 h |
| C. cinerea | 0.07 ± 0.01 b | 0.34 ± 0.00 a | 1.88 ± 0.00 a | 0.51 ± 0.01 a | ND | 50.35 ± 0.02 c | ND | ND |
| A. cornea | 0.05 ± 0.00 b | 0.32 ± 0.01 a | 1.88 ± 0.00 a | 0.51 ± 0.01 a | ND | 13.50 ± 14.67 f | ND | 3.81 ± 0.28 a |
| Species | Cell Viability (%) | Protective Effects on HepG2 Cells (mg/mL) | |||
|---|---|---|---|---|---|
| Concentration of Mushroom Extract (mg/mL) | |||||
| 0.02 | 0.1 | 0.5 | 1 | ||
| Model A | NA | NA | NA | NA | 44.54 ± 6.20 |
| Positive B | NA | NA | NA | NA | 88.20 ± 3.35 |
| L. squarrosulus | 95.26 ± 5.54 | 94.73 ± 8.32 | 95.37 ± 4.46 | 92.28 ± 7.14 | 50.86 ± 3.37 |
| L. sajor-caju | 100.63 ± 9.93 | 103.08 ± 8.93 | 96.44 ± 10.43 | 92.72 ± 5.43 | 59.04 ± 12.54 |
| L. polychrous | 95.26 ± 5.54 | 94.73 ± 8.32 | 95.37 ± 4.46 | 92.28 ± 7.14 | 50.86 ± 3.37 |
| P. ostreatus | 101.43 ± 3.70 | 103.07 ± 0.58 | 104.98 ± 10.27 | 104.06 ± 8.32 | 50.36 ± 5.12 |
| P. sp. 2 | 104.46 ± 6.23 | 108.91 ± 10.59 | 109.51 ± 14.21 | 107.55 ± 10.55 | 58.99 ± 3.29 |
| P. sp. 3 | 95.03 ± 9.48 | 102.46 ± 7.68 | 102.10 ± 12.48 | 93.19 ± 8.77 | 51.21 ± 2.60 |
| R. cf. emetica | 97.83 ± 5.47 | 97.90 ± 6.90 | 103.15 ± 8.15 | 104.01 ± 14.84 | 86.51 ± 5.96 |
| R. sp. 2 | 99.09 ± 6.57 | 93.72 ± 11.32 | 95.74 ± 9.74 | 94.33 ± 9.67 | 62.86 ± 11.14 |
| R. sp. 3 | 99.72 ± 4.38 | 100.67 ± 1.04 | 102.79 ± 4.69 | 100.58 ± 1.44 | 61.98 ± 10.56 |
| L. edodes | 97.69 ± 3.13 | 102.20 ± 14.25 | 94.79 ± 14.59 | 89.15 ± 19.88 | 52.84 ± 8.26 |
| V. volvacea | 105.49 ± 6.13 | 103.73 ± 5.23 | 99.72 ± 3.99 | 107.54 ± 7.81 | 77.39 ± 8.15 |
| P. cf. portentosus | 100.58 ± 3.26 | 104.95 ± 11.58 | 97.85 ± 9.93 | 98.35 ± 10.41 | 60.37 ± 6.36 |
| A. odoratus | 96.98 ± 13.06 | 94.61 ± 9.86 | 90.51 ± 11.55 | 86.64 ± 8.39 | 50.36 ± 11.78 |
| T. cf. fulizinosus | 97.53 ± 8.89 | 97.16 ± 5.24 | 87.17 ± 13.13 | 87.20 ± 11.03 | 51.71 ± 11.43 |
| C. cinerea | 106.50 ± 11.23 | 108.37 ± 7.25 | 103.63 ± 9.03 | 102.23 ± 3.48 | 58.61 ± 6.55 |
| A. cornea | 98.31 ± 7.44 | 96.17 ± 6.17 | 96.35 ± 5.21 | 94.69 ± 5.29 | 46.41 ± 2.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konsue, N.; Ketnawa, S.; Qin, S. Indigenous Wild Edible Mushrooms: Unveiling the Chemical Compositions and Health Impacts. Foods 2025, 14, 2331. https://doi.org/10.3390/foods14132331
Konsue N, Ketnawa S, Qin S. Indigenous Wild Edible Mushrooms: Unveiling the Chemical Compositions and Health Impacts. Foods. 2025; 14(13):2331. https://doi.org/10.3390/foods14132331
Chicago/Turabian StyleKonsue, Nattaya, Sunantha Ketnawa, and Si Qin. 2025. "Indigenous Wild Edible Mushrooms: Unveiling the Chemical Compositions and Health Impacts" Foods 14, no. 13: 2331. https://doi.org/10.3390/foods14132331
APA StyleKonsue, N., Ketnawa, S., & Qin, S. (2025). Indigenous Wild Edible Mushrooms: Unveiling the Chemical Compositions and Health Impacts. Foods, 14(13), 2331. https://doi.org/10.3390/foods14132331







