Compositional and Bioactive Differentiation of Opuntia spp. Fruit Varieties by PCA and LDA
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Sample Preparation
2.2. Nutritional Analysis
2.3. Determination of Free Sugars by HPLC
2.4. Elemental Analysis
2.5. Vitamin C by HPLC
2.5.1. Additional Instrumentation and Chromatographic Conditions
2.6. Bioactive Contents and Antioxidant Activity
2.6.1. Extract Preparation
2.6.2. Total Phenolics and Total Flavonoids Contents
2.6.3. Antioxidant Activity
2.7. Statistical Analysis
2.7.1. Principal Components Analysis
2.7.2. Linear Discriminant Analysis
3. Results
3.1. Nutritional Composition
3.2. Mineral Profile
3.3. Bioactive Profile
4. Discussion
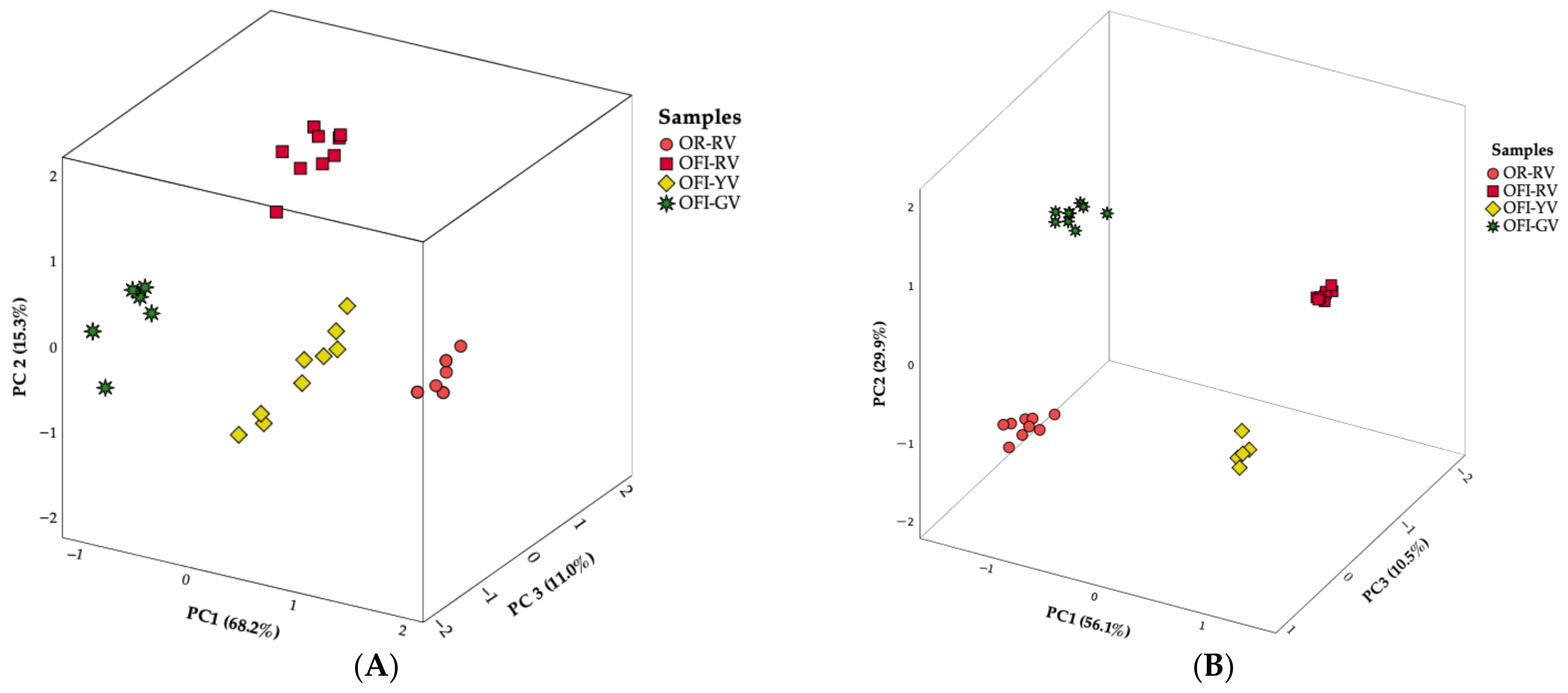
4.1. Principal Components Analysis
4.1.1. Nutritional Parameters
4.1.2. Mineral Profiles
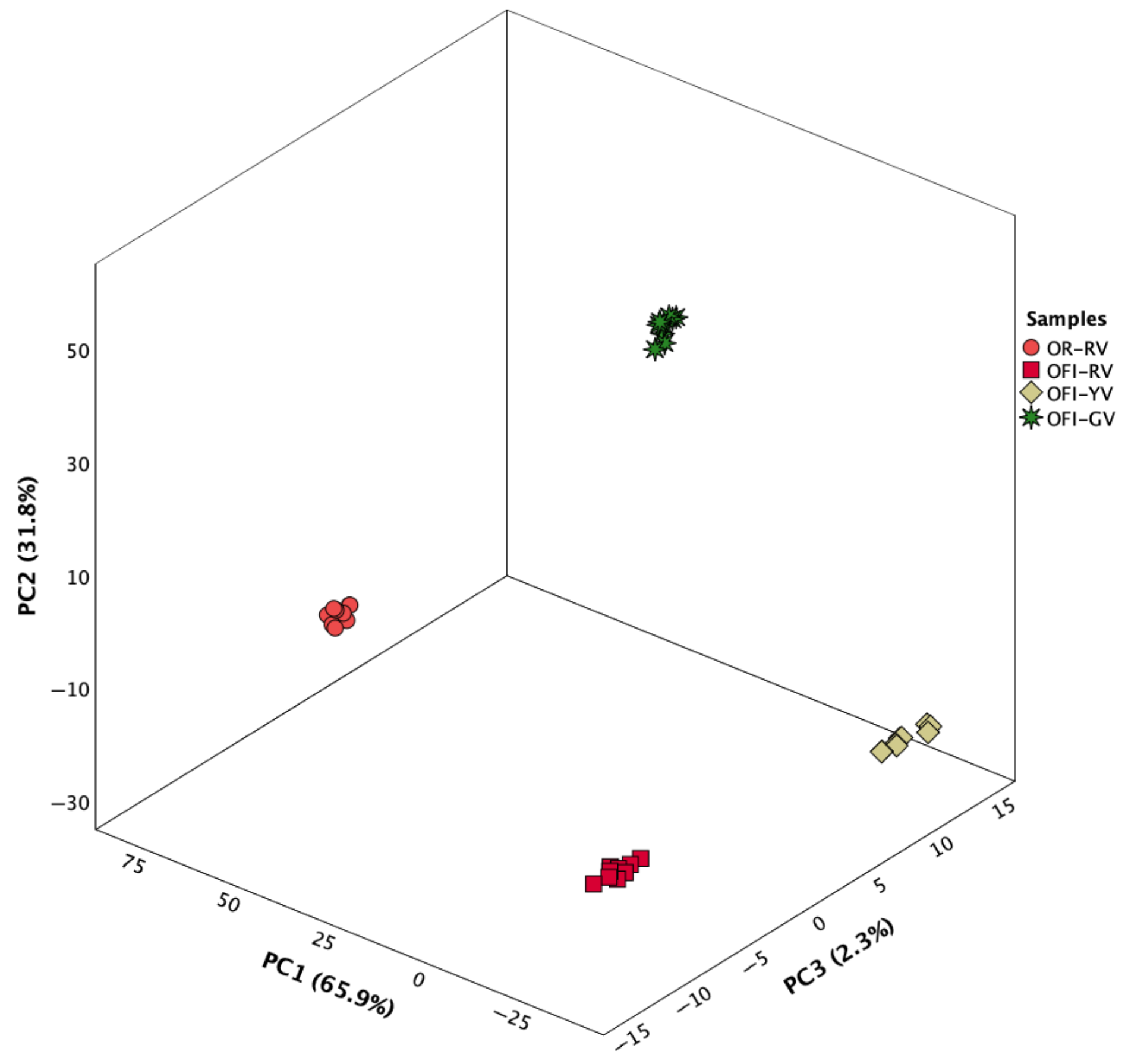
4.1.3. Bioactive Profile
4.2. Linear Discriminant Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aruwa, C.E.; Amoo, S.O.; Kudanga, T. Opuntia (Cactaceae) Plant Compounds, Biological Activities and Prospects—A Comprehensive Review. Food Res. Int. 2018, 112, 328–344. [Google Scholar] [CrossRef]
- Skogberg, M.; Kohonen, K.M.; Lohila, A.; Merbold, L.; Rasanen, M.; Vuorinne, I.; Pellikka, P.; Vesala, T.; Kubert, A. Ecosystem-scale crassulacean acid metabolism (CAM) gas exchange of a sisal (Agave sisalana) plantation. Agric. Ecosyst. Environ. 2025, 381, 109435. [Google Scholar] [CrossRef]
- Arba, M.; Falisse, A.; Choukr-Allah, R.; Sindic, M. Biology, flowering and fruiting of the cactus Opuntia spp.: A review and some observations on three varieties in Morocco. Braz. Arch. Biol. Technol. 2017, 60, e17160568. [Google Scholar] [CrossRef]
- Stavi, I. Ecosystem services related with Opuntia ficus-indica (prickly pear cactus): A review of challenges and opportunities. Agroecol. Sustain. Food Syst. 2022, 46, 815–841. [Google Scholar] [CrossRef]
- Sá Souza, M.; Júnior, G.N.A.; Souza, L.S.B.; Ferraz Jardim, A.M.R.; da Silva, G.I.N.; Araújo, G.G.L.; Campos, F.S.; Leite, M.L.M.V.; Tabosa, J.N.; Silva, T.G.F. Forage yield, competition and economic benefit of intercropping cactus and millet with mulch in a semi-arid environment. Afr. J. Range Forage Sci. 2022, 40, 219–230. [Google Scholar] [CrossRef]
- Silva, M.A.; Albuquerque, T.G.; Pereira, P.; Ramalho, R.; Vicente, F.; Oliveira, M.B.P.P.; Costa, H.S. Opuntia ficus-indica (L.) Mill.: A multi-benefit potential to be exploited. Molecules 2021, 26, 951. [Google Scholar] [CrossRef]
- Rodrigues, C.; Paula, C.D.D.; Lahbouki, S.; Meddich, A.; Outzourhit, A.; Rashad, M.; Pari, L.; Coelhoso, I.; Fernando, A.L.; Souza, V.G.L. Opuntia spp.: An overview of the bioactive profile and food applications of this versatile crop adapted to arid lands. Foods 2023, 12, 1465. [Google Scholar] [CrossRef]
- Bakar, B.; Çakmak, M.; Ibrahim, M.S.; Özer, D.; Saydam, S.; Karatas, F. Investigation of amounts of vitamins, lycopene, and elements in the fruits of Opuntia ficus-indica subjected to different pretreatments. Biol. Trace Elem. Res. 2020, 198, 315–323. [Google Scholar] [CrossRef]
- Blando, F.; Albano, C.; Jiménez-Martínez, C.; Cardador-Martínez, A. Opuntia ficus-indica [L.] mill. and other species. In Molecular Mechanisms of Functional Food; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2022; pp. 193–237. [Google Scholar] [CrossRef]
- García-Cayuela, T.; Gomez-Maqueo, A.; Guajardo-Flores, D.; Welti-Chanes, J.; Cano, M.P. Characterization and quantification of individual betalain and phenolic compounds in Mexican and Spanish prickly pear (Opuntia ficus-indica L. mill) tissues: A comparative study. J. Food Compos. Anal. 2019, 76, 1–13. [Google Scholar] [CrossRef]
- Astello-García, M.G.; Cervantes, I.; Nair, V.; Santos-Díaz, M.d.S.; Reyes-Agüero, A.; Guéraud, F.; Negre-Salvayre, A.; Rossignol, M.; Cisneros-Zevallos, L.; Barba de la Rosa, A.P. Chemical composition and phenolic compounds profile of cladodes from Opuntia spp. cultivars with different domestication gradient. J. Food Compos. Anal. 2015, 43, 119–130. [Google Scholar] [CrossRef]
- Besné-Eseverri, I.; Martín, M.Á.; Lobo, G.; Cano, M.P.; Portillo, M.P.; Trepiana, J. Antioxidant and anti-inflammatory effects of Opuntia extracts on a model of diet-induced steatosis. Antioxidants 2024, 13, 1416. [Google Scholar] [CrossRef]
- Villa-Jaimes, G.S.; Moshage, H.; Avelar-González, F.J.; González-Ponce, H.A.; Buist-Homan, M.; Guevara-Lara, F.; Sánchez-Alemán, E.; Martínez-Hernández, S.L.; Ventura-Juárez, J.; Muñoz-Ortega, M.H.; et al. Molecular and Antioxidant Characterization of Opuntia robusta Fruit Extract and Its Protective Effect Against Diclofenac-Induced Acute Liver Injury in an In Vivo Rat Model. Antioxidants 2023, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Bellumori, M.; Innocenti, M.; Andrenelli, L.; Melani, F.; Cecchi, L.; Pandino, G.; Mauromicale, G.; La Malfa, S.; Mulinacci, N. Composition of discarded Sicilian fruits of Opuntia ficus indica L.: Phenolic content, mineral profile and antioxidant activity in peel, seeds and whole fruit. Food Chem. 2023, 428, 136756. [Google Scholar] [CrossRef] [PubMed]
- Daniloski, D.; D’Cunha, N.M.; Speer, H.; McKune, A.J.; Alexopoulos, N.; Panagiotakos, D.B.; Petkoska, A.T.; Naumovski, N. Recent developments on Opuntia spp., their bioactive composition, nutritional values, and health effects. Food Biosci. 2022, 47, 101665. [Google Scholar] [CrossRef]
- Martins, M.; Ribeiro, M.H.; Almeida, C.M.M. Physicochemical, nutritional, and medicinal properties of Opuntia ficus-indica (L.) Mill. and its main agro-industrial use: A review. Plants 2023, 12, 1512. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Analytical Chemists International; Official Methods; ACOC: Gaithersburg, MD, USA, 2012. [Google Scholar]
- European Parliament and Council of the European Union. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers, 18–61. Available online: https://eur-lex.europa.eu/eli/reg/2011/1169/oj/eng (accessed on 6 August 2025).
- Montesano, D.; Cossigmani, L.; Giua, L.; Urbani, E.; Simonetti, M.S.; Blasi, F. A Simple HPLC-ELSD Method for sugar analysis in goji berry. J. Chem. 2016, 2016, 6271808. [Google Scholar] [CrossRef]
- Pinto, E.; Almeida, A.A.; Aguiar, A.A.R.M.; Ferreira, I.M.P.L.V.O. Changes in macrominerals, trace elements and pigments content during lettuce (Lactuca sativa L.) growth: Influence of soil composition. Food Chem. 2014, 152, 603–611. [Google Scholar] [CrossRef]
- Valente, A.; Albuquerque, T.G.; Sanches-Silva, A.; Costa, H.S. Ascorbic acid content in exotic fruits: A contribution to produce quality data for food composition databases. Food Res. Int. 2011, 44, 2237–2242. [Google Scholar] [CrossRef]
- Vinha, A.F.; Costa, A.S.G.; Espírito Santo, L.; Ferreira, D.M.; Sousa, C.; Pinto, E.; Almeida, A.; Oliveira, M.B.P.P. High-value compounds in papaya by-products (Carica papaya L. var. Formosa and Aliança): Potential sustainable use and exploitation. Plants 2024, 13, 1009. [Google Scholar] [CrossRef]
- Costa, A.S.G.; Alves, R.C.; Vinha, A.F.; Barreira, S.V.P.; Nunes, M.A.; Cunha, L.M.; Oliveira, M.B.P.P. Optimization of antioxidants extraction from coffee silverskin, a roasting by-product, having in view a sustainable process. Ind. Crop. Prod. 2014, 53, 350–357. [Google Scholar] [CrossRef]
- Sauerbrei, W.; Perperoglou, A.; Schmid, M.; Abrahamowicz, M.; Becher, H.; Binder, H.; Dunkler, D.; Harrell, F.E.; Royston, P.; Heinze, G. State of the art in selection of variables and functional forms in multivariable analysis—Outstanding issues. Diagn. Progn. Res. 2020, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Albergamo, A.; Potortí, A.G.; Di Bella, G.; Amor, N.B.; Vecchio, G.L.; Nava, V.; Rando, R.; Mansour, H.B.; Turco, V.L. Chemical Characterization of Different Products from the Tunisian Opuntia ficus-indica (L.) Mill. Foods 2022, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Dubeux, J.C.B.; Santos, M.V.F.; Cunha, M.V.; Santos, D.C.; Souza, R.T.A.; Mello, A.C.L.; Souza, T.C. Cactus (Opuntia and Nopalea) nutritive value: A review. Anim. Feed. Sci. Technol. 2021, 275, 114890. [Google Scholar] [CrossRef]
- Teklu, G.W.; Ayimut, K.M.; Abera, F.A.; Egziabher, Y.G.; Fitiwi, I. Nutritive and Chemical Composition and In Vitro Digestibility of Cladodes of the Opuntia Species. Sustainability 2023, 15, 6624. [Google Scholar] [CrossRef]
- Razzak, S.; Aouji, M.; Zirari, M.; Benchehida, H.; Taibi, M.; Bengueddour, R.; Wondmie, G.F.; Ibenmoussa, S.; Bin Jardan, Y.A.; Taboz, Y. Nutritional composition, functional and chemical characterization of Moroccan Opuntia ficus-indica cladode powder. Int. J. Food Prop. 2024, 27, 1167–1179. [Google Scholar] [CrossRef]
- European Chemicals Agency (ECHA). The Facts on Beryllium Compounds. 2025. Available online: https://stopcarcinogensatwork.eu/fact/beryllium-compounds/ (accessed on 6 August 2025).
- Commission Regulation (EU) 2023/915, of April 25. Maximum Levels for Certain Contaminants in Food. Available online: https://eur-lex.europa.eu/eli/reg/2023/915/oj (accessed on 6 August 2025).
- European Food Safety Authority (EFSA). Statement on tolerable weekly intake for cadmium. EFSA J. 2011, 9, 1975. [Google Scholar] [CrossRef]
- Giraldo-Silva, L.; Ferreira, B.; Rosa, E.; Dias, A.C.P. Opuntia ficus-indica Fruit: A Systematic Review of Its Phytochemicals and Pharmacological Activities. Plants 2023, 12, 543. [Google Scholar] [CrossRef]
- Maiuolo, J.; Nucera, S.; Serra, M.; Caminiti, R.; Oppedisano, F.; Macrì, R.; Scarano, F.; Ragusa, S.; Muscoli, C.; Palma, E.; et al. Cladodes of Opuntia ficus-indica (L.) Mill. Possess Important Beneficial Properties Dependent on Their Different Stages of Maturity. Plants 2024, 13, 1365. [Google Scholar] [CrossRef]

| Opuntia Variety 1 | Levene’s Test p-Value (n = 36) | ||||
|---|---|---|---|---|---|
| OR-RV | OFI-RV | OFI-YV | OFI-GV | ||
| Moisture | 86.8 ± 0.4 a | 82.1 ± 0.5 b | 81.7 ± 0.5 b | 80.3 ± 0.5 c | 0.766 |
| Fat | 1.7 ± 0.1 a | 1.5 ± 0.1 b | 1.3 ± 0.1 c | 1.2 ± 0.1 d | 0.004 |
| Protein | 6.3 ± 0.1 a | 6.4 ± 0.2 a | 4.9 ± 0.2 c | 5.4 ± 0.2 b | 0.286 |
| Non-fibre carbohydrates | 64.7 ± 0.2 a | 59.5 ± 0.5 c | 61.8 ± 0.5 b | 58.2 ± 0.5 d | 0.003 |
| Fructose | 19.6 ± 0.3 c | 32.4 ± 0.4 a | 32.5 ± 0.5 a | 30.7 ± 0.4 b | 0.121 |
| Glucose | 45 ± 1 ab | 46 ± 1 a | 43 ± 2 b | 41 ± 1 c | 0.001 |
| Soluble fibre | 1.2 ± 0.1 c | 7.7 ± 0.5 b | 9.1 ± 0.4 a | 9.4 ± 0.5 a | <0.001 |
| Insoluble fibre | 19.1 ± 0.1 c | 21.0 ± 0.2 b | 18.7 ± 0.4 d | 21.9 ± 0.2 a | <0.001 |
| Ash | 6.9 ± 0.1 a | 3.9 ± 0.1 c | 4.2 ± 0.1 b | 3.9 ± 0.1 c | 0.008 |
| Energy | 340 ± 1 a | 334 ± 1 b | 334 ± 1 b | 328 ± 1 c | 0.011 |
| Opuntia Variety | Levene’s Test p-Value (n = 36) | ||||
|---|---|---|---|---|---|
| Minerals | OR-RV | OFI-RV | OFI-YV | OFI-GV | |
| Macro elements (g/kg) | |||||
| Mg | 1.00 ± 0.03 c | 1.24 ± 0.05 b | 1.35 ± 0.03 b | 2.63 ± 0.05 a | <0.001 |
| K | 25 ± 2 a | 12.7 ± 0.4 b | 13.7 ± 0.2 b | 10.9 ± 0.1 c | <0.001 |
| Ca | 1.8 ± 0.1 d | 4.5 ± 0.3 b | 5.7 ± 0.2 a | 4.2 ± 0.1 c | 0.009 |
| Micro elements (mg/kg) | |||||
| B | 34 ± 2 a | 9.4 ± 0.5 d | 14.3 ± 0.2 b | 11.6 ± 0.5 c | <0.001 |
| Na | 2.3 ± 0.3 c | 3.9 ± 0.4 b | 5.8 ± 0.3 a | 2.7 ± 0.3 c | 0.621 |
| Al | 1.3 ± 0.1 b | nd | 3.4 ± 0.1 a | 1.4 ± 0.2 b | <0.001 |
| Ti | 1.2 ± 0.1 d | 3.2 ± 0.2 b | 4.2 ± 0.1 a | 2.9 ± 0.1 c | 0.009 |
| Cr | nd | 66 ± 5 | 147 ± 22 | nd | <0.001 |
| Mn | 46 ± 2 b | 5.4 ± 0.1 c | 4.1 ± 0.4 c | 63 ± 2 a | <0.001 |
| Fe | 10.9 ± 0.5 b | 9.5 ± 0.5 c | 8.4 ± 0.2 d | 13.1 ± 0.3 a | 0.046 |
| Ni | 3.2 ± 0.1 a | 0.50 ± 0.04 c | 0.40 ± 0.05 c | 1.3 ± 0.1 b | 0.028 |
| Cu | 8.7 ± 0.3 a | 2.5 ± 0.1 b | 2.0 ± 0.2 d | 2.2 ± 0.1 c | <0.001 |
| Zn | 12.3 ± 0.4 b | 7.3 ± 0.5 c | 6.3 ± 0.5 d | 14.1 ± 0.1 a | <0.001 |
| Rb | 29 ± 3 a | 7.2 ± 0.5 c | 6.9 ± 0.2 c | 22 ± 1 b | <0.001 |
| Sr | 1.8 ± 0.1 b | 0.9 ± 0.1 c | 0.8 ± 0.1 c | 4.5 ± 0.2 a | 0.019 |
| Mo | 1.0 ± 0.1 | nd | nd | nd | - |
| Ba | 0.8 ± 0.1 c | 1.2 ± 0.1 b | 1.2 ± 0.1 b | 2.1 ± 0.1 a | 0.061 |
| Trace elements (μg/kg) | |||||
| Be | 3.2 ± 0.4 | nd | nd | 20 ± 2 | 0.012 |
| Co | 113 ± 4 b | 20 ± 3 c | 30 ± 3 c | 178 ± 16 a | <0.001 |
| Zr | 3 ± 1 b | 4 ± 1 b | 43 ± 4 a | 5 ± 1 b | <0.001 |
| Cd | 2.8 ± 0.2 a | 1.7 ± 0.1 c | 1.5 ± 0.2 c | 2.5 ± 0.1 b | 0.159 |
| Sn | nd | nd | 53 ± 5 | nd | - |
| Cs | 19 ± 2 b | 5.6 ± 0.5 c | 5.5 ± 0.4 c | 73 ± 3 a | <0.001 |
| W | 10.9 ± 0.1 | nd | nd | nd | - |
| Pb | 28 ± 4 a | nd | 6.6 ± 0.3 b | 28 ± 2 a | <0.001 |
| Opuntia Variety 1 | Levene’s Test p-Value (n = 36) | ||||
|---|---|---|---|---|---|
| OR-RV | OFI-RV | OFI-YV | OFI-GV | ||
| Phenols (mg GAE) | 750 ± 20 a | 760 ± 32 a | 679 ± 30 b | 606 ± 37 c | 0.185 |
| Flavonoids (mg CE) | 136 ± 11 a | 68 ± 4 b | 50 ± 3 c | 36 ± 4 d | 0.002 |
| FRAP (μmol SFE) | 11,622 ± 792 a | 4066 ± 274 b | 3741 ± 352 b | 2892 ± 122 c | 0.006 |
| DPPH (mg TE) | 192 ± 15 a | 98 ± 5 b | 80 ± 10 c | 51 ± 4 d | 0.018 |
| Dehydroascorbic acid (mg) | 31 ± 1 a | 26 ± 1 b | 28 ± 1 b | 32 ± 2 a | 0.082 |
| Ascorbic acid (mg) | 304 ± 4 a | 108 ± 2 c | 123 ± 1 b | 110 ± 3 c | 0.120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santo, L.E.; Pereira, C.S.G.P.; Costa, A.S.G.; Almeida, A.; Barreira, J.C.M.; Oliveira, M.B.P.P.; Vinha, A.F. Compositional and Bioactive Differentiation of Opuntia spp. Fruit Varieties by PCA and LDA. Foods 2025, 14, 3170. https://doi.org/10.3390/foods14183170
Santo LE, Pereira CSGP, Costa ASG, Almeida A, Barreira JCM, Oliveira MBPP, Vinha AF. Compositional and Bioactive Differentiation of Opuntia spp. Fruit Varieties by PCA and LDA. Foods. 2025; 14(18):3170. https://doi.org/10.3390/foods14183170
Chicago/Turabian StyleSanto, Liliana Espírito, Cláudia S. G. P. Pereira, Anabela S. G. Costa, Agostinho Almeida, João C. M. Barreira, Maria Beatriz P. P. Oliveira, and Ana F. Vinha. 2025. "Compositional and Bioactive Differentiation of Opuntia spp. Fruit Varieties by PCA and LDA" Foods 14, no. 18: 3170. https://doi.org/10.3390/foods14183170
APA StyleSanto, L. E., Pereira, C. S. G. P., Costa, A. S. G., Almeida, A., Barreira, J. C. M., Oliveira, M. B. P. P., & Vinha, A. F. (2025). Compositional and Bioactive Differentiation of Opuntia spp. Fruit Varieties by PCA and LDA. Foods, 14(18), 3170. https://doi.org/10.3390/foods14183170








