Fabrication and Anti-Swelling Properties of Gelatin/Sodium Alginate–Carboxymethyl Chitosan-Based Cationic Coordination Hydrogels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Gel/Alg-CMCS-Based Cationic Coordination Hydrogels
2.3. Physicochemical Characterizations
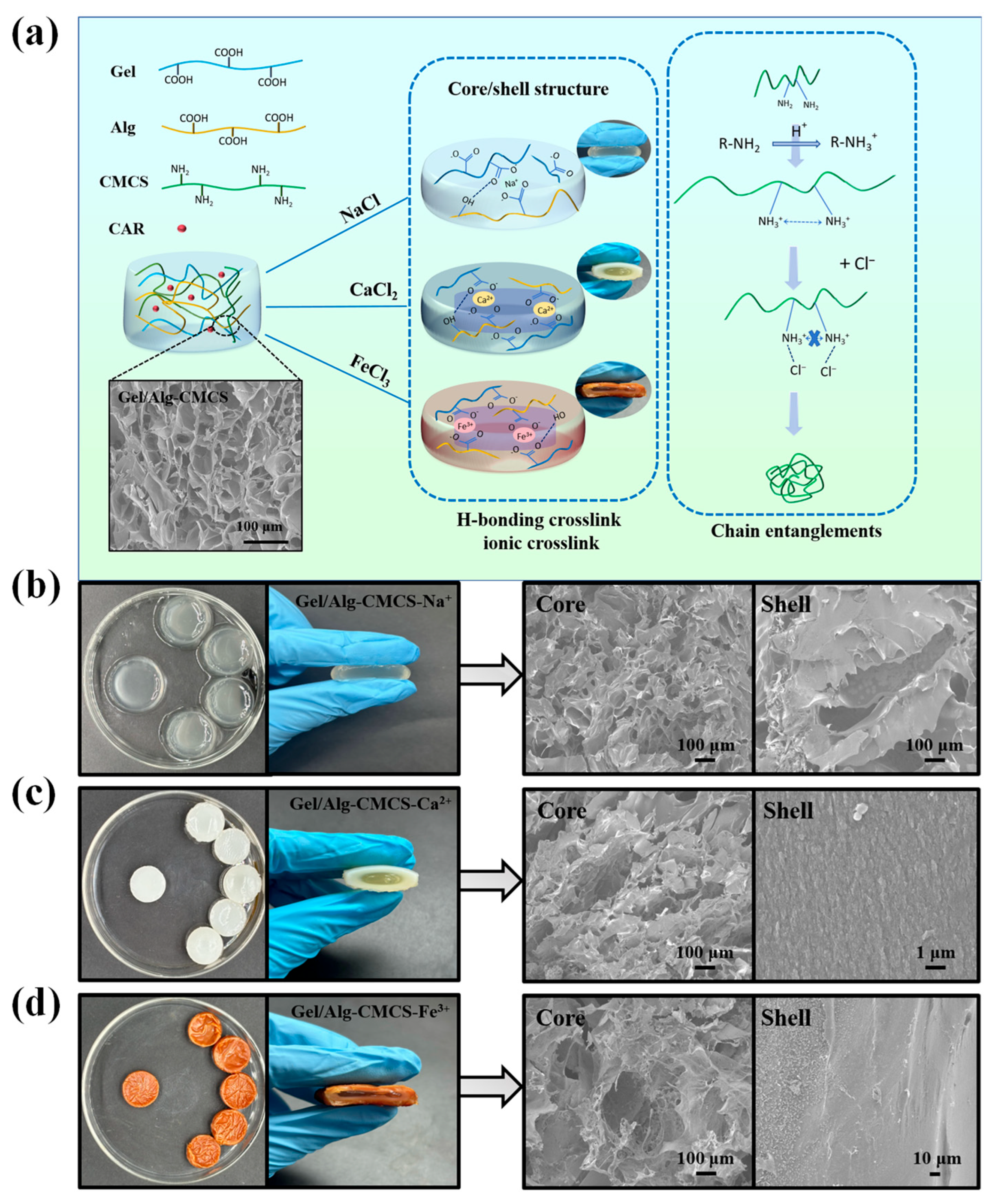
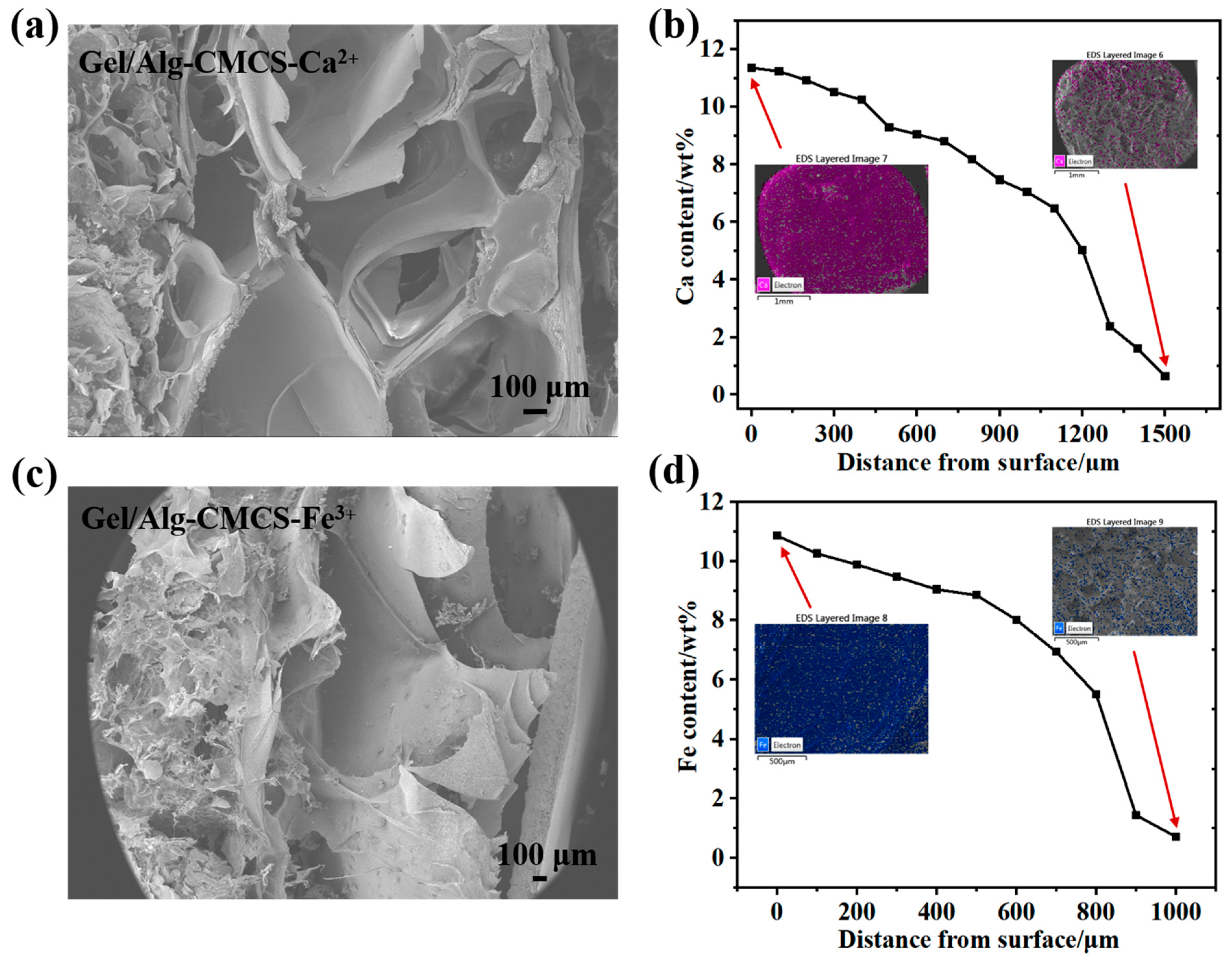
2.3.1. Morphology
2.3.2. FTIR
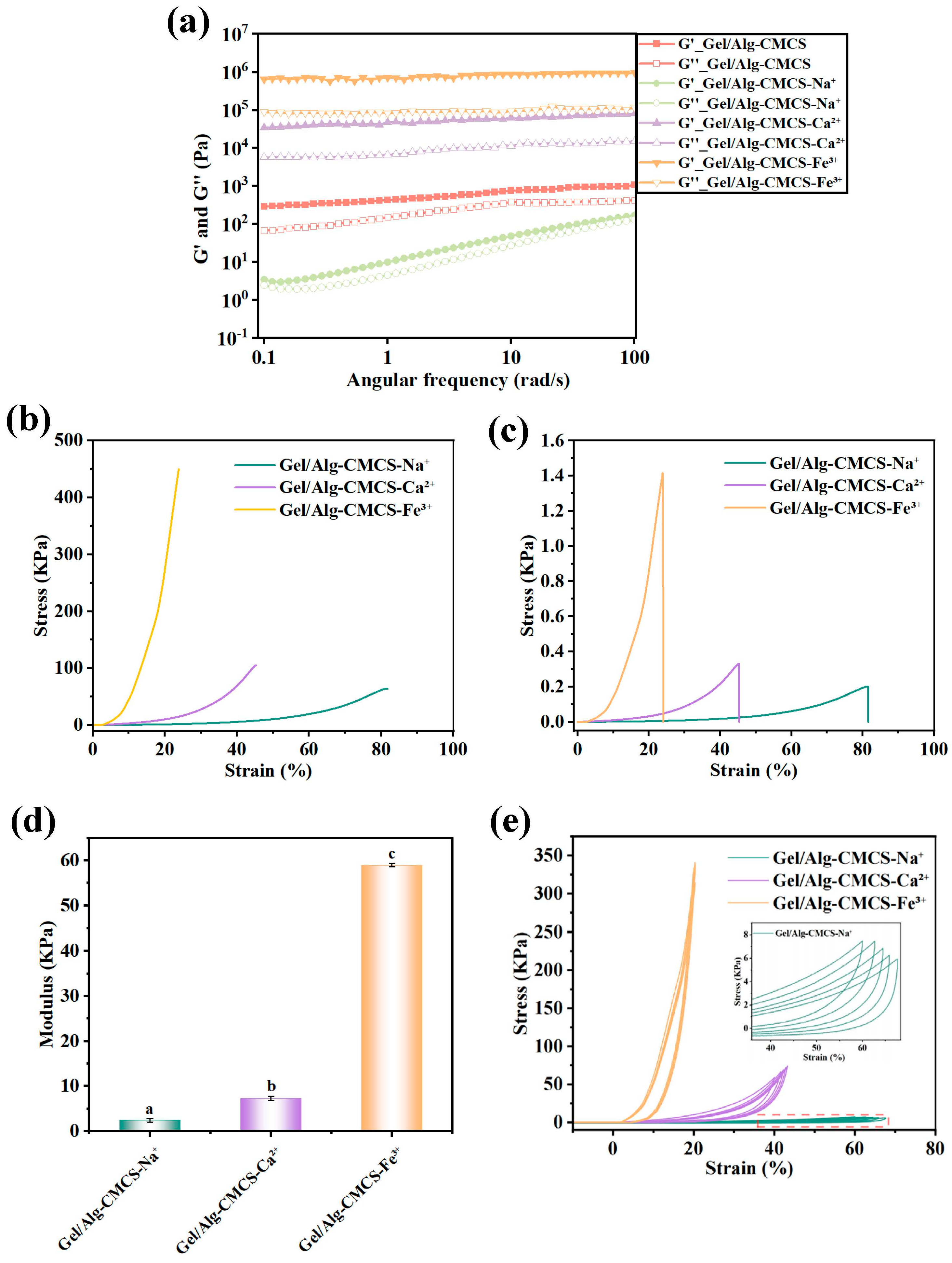
2.4. Rheological Measurements
2.5. Mechanical Property Test
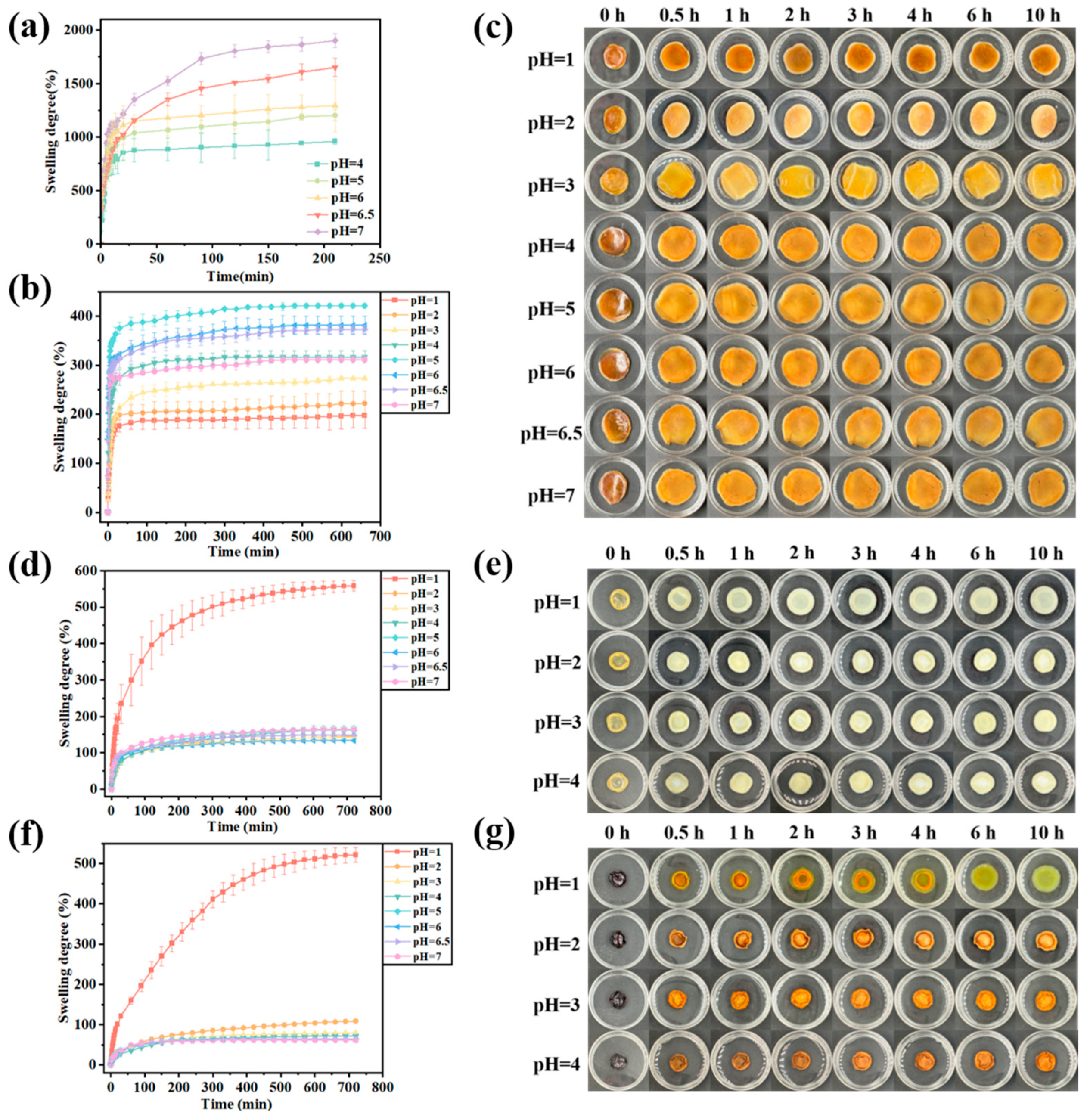
2.6. pH-Responsive Swelling Test
2.7. Anti-Swelling Stability Test
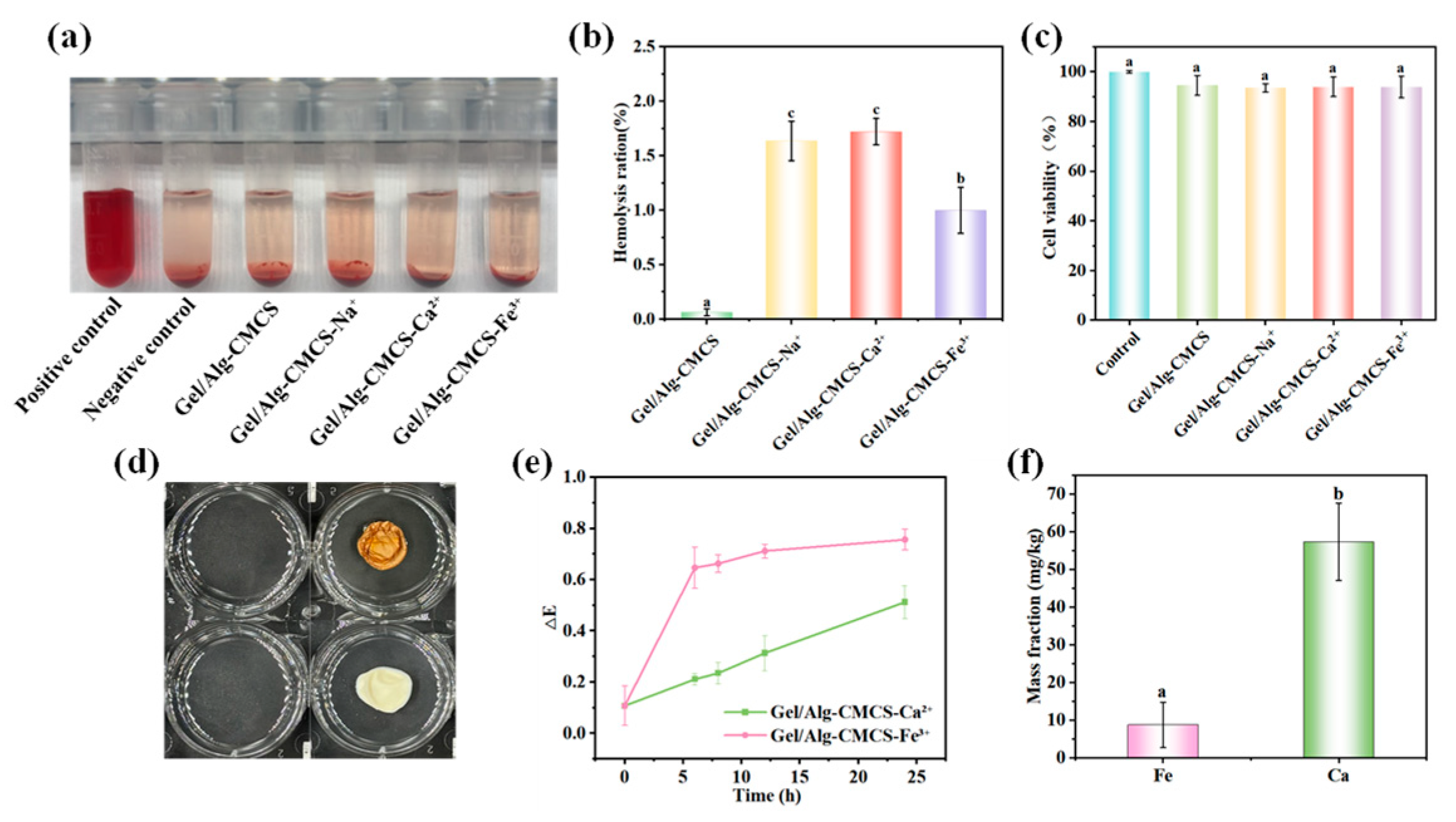
2.8. Hemolysis Assay
2.9. Cytotoxicity Study
2.10. Ion Migration Test
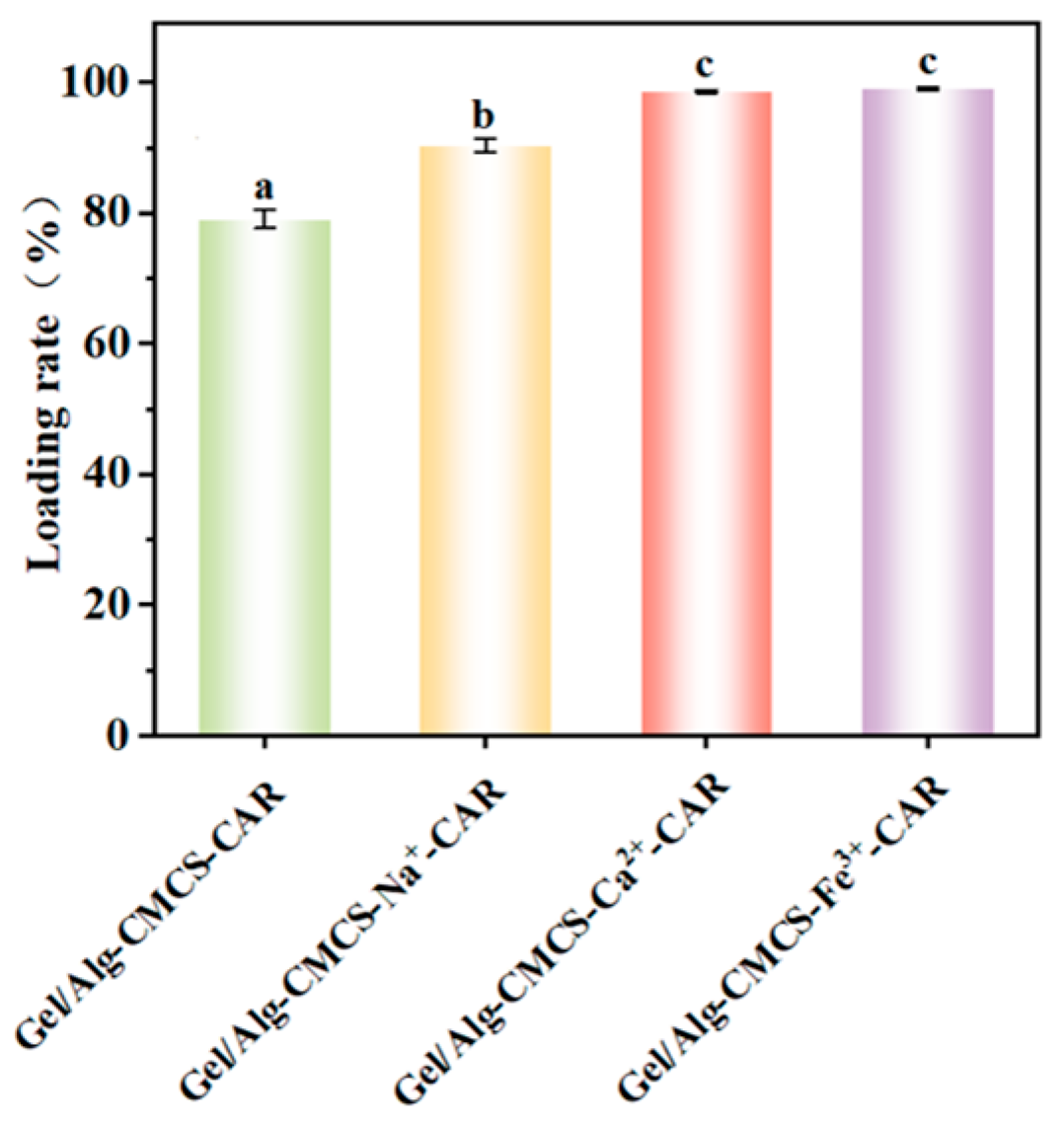
2.11. Loading Efficiency Test
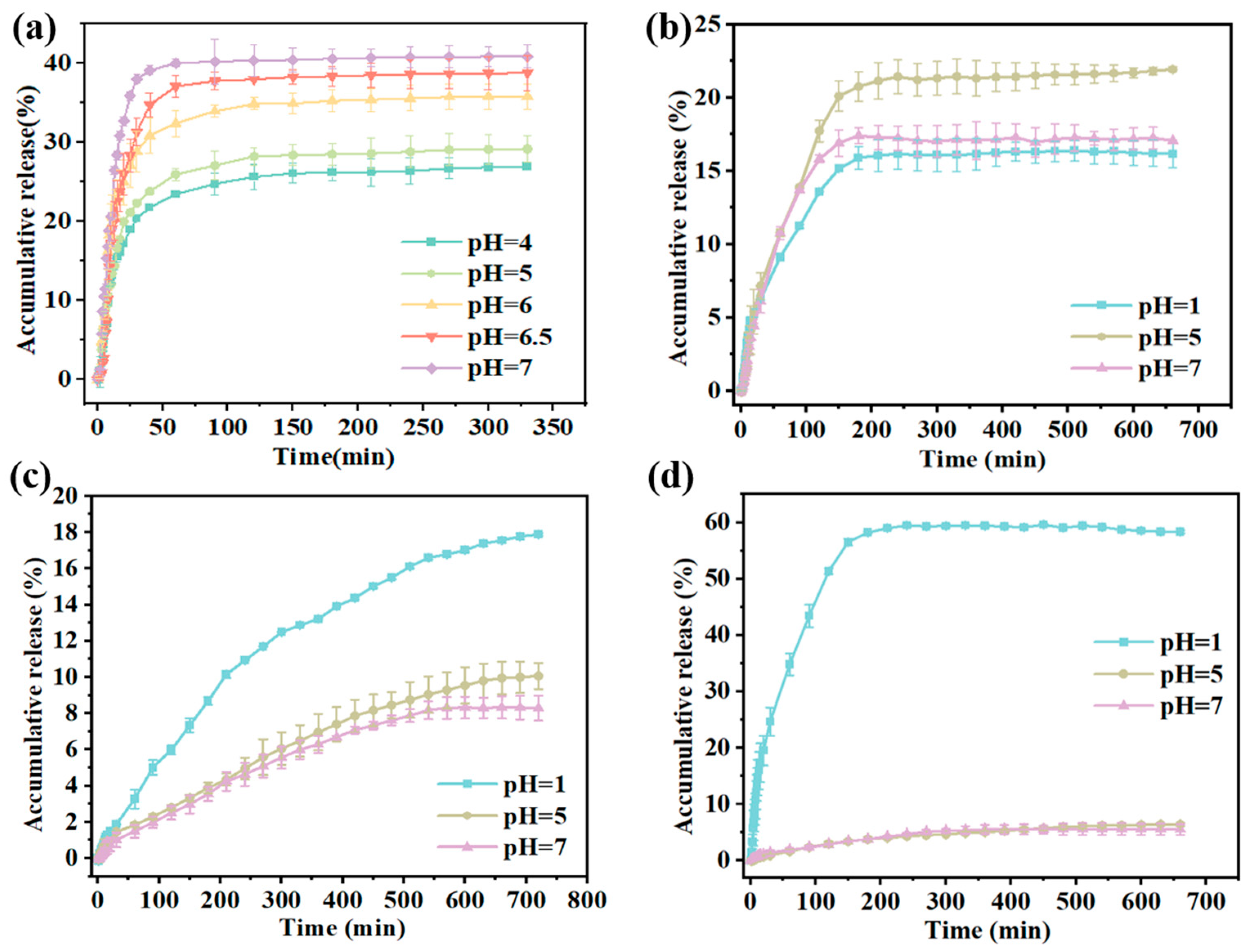
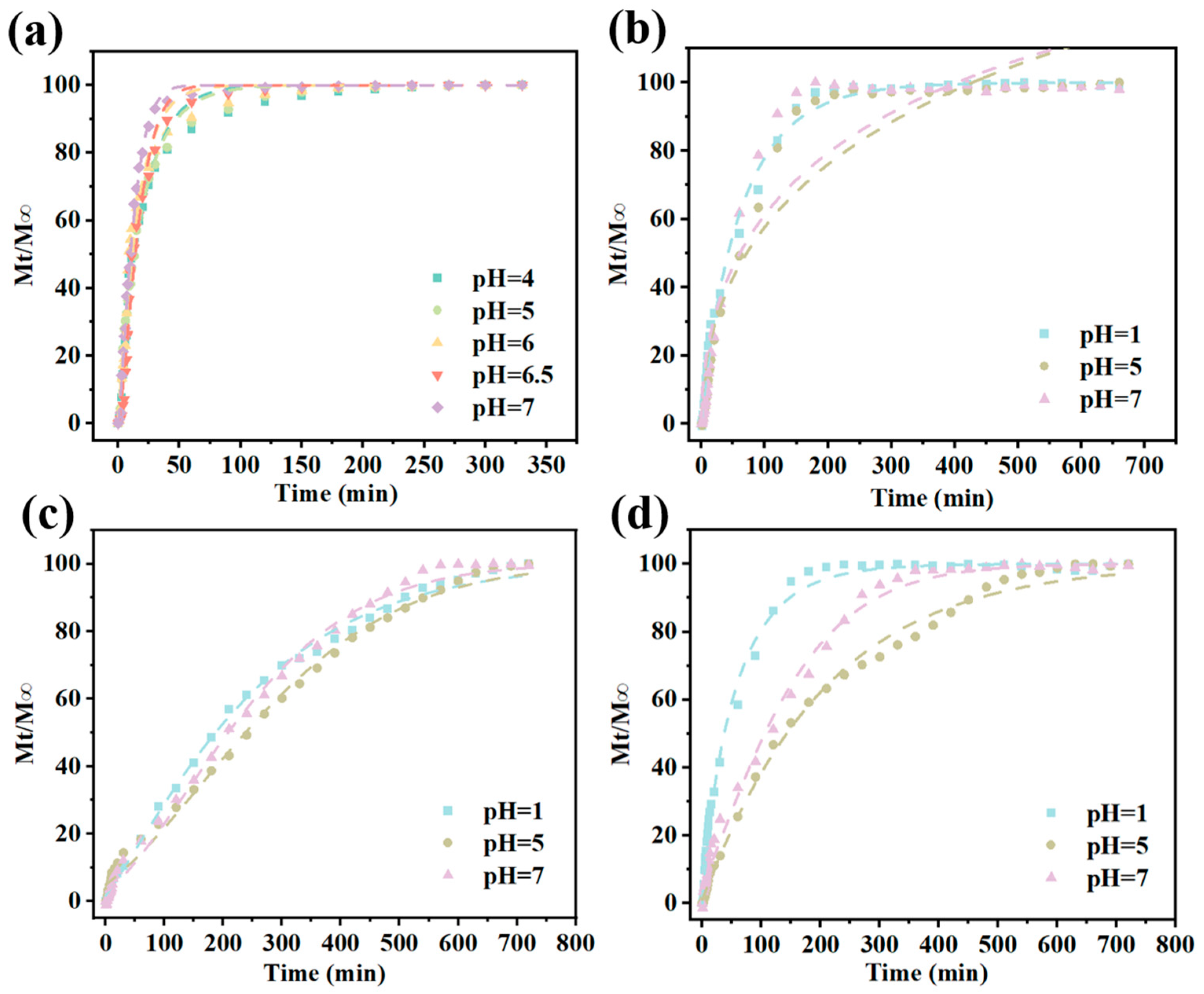
2.12. CAR Release Behavior Test
2.13. Statistical Analysis
3. Results
3.1. Physicochemical Characterizations of Gel/Alg-CMCS-Based Cationic Coordination Hydrogels
3.2. Mechanical Properties of Anti-Swelling Hydrogels
3.3. Anti-Swelling Behavior of Gel/Alg-CMCS-Based Cationic Coordination Hydrogels
3.4. Safety of Gel/Alg-CMCS-Based Cationic Coordination Hydrogels
3.5. Loading Efficiency of Gel/Alg-CMCS-Based Cationic Coordination Hydrogels
3.6. CAR Release from Gel/Alg-CMCS-Based Cationic Coordination Hydrogels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, W.; Chen, F.; Qiu, Y.; Zhang, L.; Gao, J.; Wu, T.; Wang, P.; Zhang, M.; Zhu, Q. Co-encapsulation of collagen peptide and astaxanthin in WG/OG/W double emulsions-filled alginate hydrogel beads: Fabrication, characterization and digestion behaviors. J. Colloid Interface Sci. 2023, 651, 159–171. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Ren, H.; Shao, W.; Wang, C.; Huang, Y.; Zhang, S.; Han, Y.; Zhang, Y.; Yin, M.; et al. Preparation and characterization of agarose-sodium alginate hydrogel beads for the co-encapsulation of lycopene and resveratrol nanoemulsion. Int. J. Biol. Macromol. 2024, 277, 133753. [Google Scholar] [CrossRef]
- Liu, S.; Fang, Z.; Ng, K. Incorporating inulin and chitosan in alginate-based microspheres for targeted delivery and release of quercetin to colon. Food Res. Int. 2022, 160, 111749. [Google Scholar] [CrossRef]
- Sharpe, L.A.; Ramirez, J.E.V.; Haddadin, O.M.; Ross, K.A.; Narasimhan, B.; Peppas, N.A. pH-responsive microencapsulation systems for the oral delivery of polyanhydride nanoparticles. Biomacromolecules 2018, 19, 793–802. [Google Scholar] [CrossRef]
- Li, Q.; Xue, F.; Qu, J.; Liu, L.; Hu, R.; Liu, C. Nano-in-micro delivery system prepared by co-axial air flow for oral delivery of conjugated linoleic acid. Mar. Drugs 2019, 17, 15. [Google Scholar] [CrossRef]
- Guo, Z.; Hou, Y.; Tian, Y.; Tian, J.; Hu, J.; Zhang, Y. Antimicrobial peptide hydrogel with pH-responsive and controllable drug release properties for the efficient treatment of helicobacter pylori infection. ACS Appl. Mater. Interfaces 2024, 16, 51981–51993. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Cui, Y.; Guo, Y.; Zhao, P.; Wang, J.; Zhang, R.; Wang, X. Design of carboxymethyl chitosan-reinforced pH-responsive hydrogels for on-demand release of carvacrol and simulation of release kinetics. Food Chem. 2023, 405, 134856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, W.; Lin, Z.; Tang, Z.; Lin, B. Carboxymethyl chitosan/sodium alginate hydrogel films with good biocompatibility and reproducibility by in situ ultra-fast crosslinking for efficient preservation of strawberry. Carbohydr. Polym. 2023, 316, 121073. [Google Scholar] [CrossRef]
- Khan, A.; Riahi, Z.; Kim, J.T.; Rhim, J.W. Chitosan/gelatin-based multifunctional films integrated with sulfur-functionalized chitin for active packaging applications. Food Hydrocoll. 2024, 149, 109537. [Google Scholar] [CrossRef]
- Xu, C.; Cao, L.; Bilal, M.; Cao, C.; Zhao, P.; Zhang, H.; Huang, Q. Multifunctional manganese-based carboxymethyl chitosan hydrogels for pH-triggered pesticide release and enhanced fungicidal activity. Carbohydr. Polym. 2021, 262, 117933. [Google Scholar] [CrossRef]
- Zhang, H.; Gu, Z.; Li, W.; Guo, L.; Wang, L.; Guo, L.; Ma, S.; Han, B.; Chang, J. Ph-sensitive o-carboxymethyl chitosan/sodium alginate nanohydrogel for enhanced oral delivery of insulin. Int. J. Biol. Macromol. 2022, 223, 433–445. [Google Scholar] [CrossRef]
- Li, L.; Scheiger, J.M.; Levkin, P.A. Design and applications of photoresponsive hydrogels. Adv. Mater. 2019, 31, 1807333. [Google Scholar] [CrossRef]
- Kaunisto, E.; Marucci, M.; Borgquist, P.; Axelsson, A. Mechanistic modelling of drug release from polymer-coated and swelling and dissolving polymer matrix systems. Int. J. Pharm. 2011, 418, 54–77. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baharvand, H.; Daemi, H. Chemical crosslinking of biopolymeric scaffolds: Current knowledge and future directions of crosslinked engineered bone scaffolds. Int. J. Biol. Macromol. 2017, 107, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Di, X.; Hou, J.; Yang, M.; Guolin, W.; Sun, P. A bio-inspired, ultra-tough, high-sensitivity, and anti-swelling conductive hydrogel strain sensor for motion detection and information transmission. Mater. Horiz. 2022, 9, 3057–3069. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Gao, S.; Guo, Q.; Wang, C.; Qiao, Y.; Qiu, D. A solvent-exchange strategy to regulate noncovalent interactions for strong and antiswelling hydrogels. Adv. Mater. 2020, 32, 2004579. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hao, D.; Fan, J.; Song, S.; Guo, X.; Song, W.; Liub, M.; Jiang, L. A robust double-network hydrogel with under sea water superoleophobicity fabricated via one-pot, one-step reaction. J. Mater. Chem. B 2016, 4, 4662–4666. [Google Scholar] [CrossRef]
- Zhang, S.; Wan, Y.; Yuan, W.; Zhang, Y.; Zhou, Z.; Zhang, M.; Wang, L.; Wang, R.P. Preparation of PVA–CS/SA–Ca2+ hydrogel with core–shell structure. Polymers 2022, 14, 212. [Google Scholar] [CrossRef]
- Luo, C.; Zhao, Y.; Sun, X.; Luo, F. Fabrication of antiseptic, conductive and robust polyvinyl alcohol/chitosan composite hydrogels. J. Polym. Res. 2020, 27, 269. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, S.; Yang, Y.; He, R.; Lin, J.; Hong, Z.; Yuan, C.; Dai, L. A water state manipulation strategy for ultra-stiff yet highly sensitive hydrogels. Adv. Funct. Mater. 2025, 35, 2424535. [Google Scholar] [CrossRef]
- Sun, F.; Zhao, J.; Shan, P.; Wang, K.; Li, H.; Peng, L. Gelatin-based composite film integrated with nanocellulose and extract-metal complex derived from coffee leaf for sustainable and active food packaging. Food Hydrocoll. 2024, 159, 110610. [Google Scholar] [CrossRef]
- Abd El-Ghaffar, M.A.; Hashem, M.S.; El-Awady, M.K.; Rabie, A.M. Ph-sensitive sodium alginate hydrogels for riboflavin controlled release. Carbohydr. Polym. 2012, 89, 667–675. [Google Scholar] [CrossRef]
- Hu, X.; Yan, L.; Wang, Y.; Xu, M. Self-assembly of binary oppositely charged polysaccharides into polyelectrolyte complex hydrogel film for facile and efficient Pb2+ removal. Chem. Eng. J. 2020, 388, 124189. [Google Scholar] [CrossRef]
- Nadira, P.; Mujeeb, V.; Rahman, P.; Muraleedharan, K. Effects of cashew leaf extract on physicochemical, antioxidant, and antimicrobial properties of N, O–carboxymethyl chitosan films. Carbohydr. Polym. Technol. Appl. 2022, 3, 100191. [Google Scholar] [CrossRef]
- Teng, X.N.; Wang, S.C.; Zeb, L.; Xiu, Z.L. Effects of carboxymethyl chitosan adsorption on bioactive components of antarctic krill oil. Food Chem. 2022, 388, 132995. [Google Scholar] [CrossRef] [PubMed]
- Bi, F.; Zhang, X.; Liu, J.; Yong, H.; Gao, L.; Liu, J. Development of antioxidant and antimicrobial packaging films based on chitosan, D-α-tocopheryl polyethylene glycol 1000 succinate and silicon dioxide nanoparticles. Food Packag. Shelf Life 2020, 24, 100503. [Google Scholar] [CrossRef]
- Lu, W.; Chen, M.; Cheng, M.; Yan, X.; Zhang, R.; Kong, R.; Wang, J.; Wang, X. Development of antioxidant and antimicrobial bioactive films based on Oregano essential oil/mesoporous nano-silica/sodium alginate. Food Packag. Shelf Life 2021, 29, 100691. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, W.; Liu, Y.; Zhao, Y.; Zhang, J.; Hou, M. Hygroscopicity modulation of hydrogels based on carboxymethyl chitosan/Alginate polyelectrolyte complexes and its application as pH-sensitive delivery system. Carbohydr. Polym. 2018, 198, 86–93. [Google Scholar] [CrossRef]
- Dou, X.; Wang, H.; Yang, F.; Shen, H.; Wang, X.; Wu, D. One-step soaking strategy toward anti-swelling hydrogels with a stiff “armor”. Adv. Sci. 2023, 10, 2206242. [Google Scholar] [CrossRef]
- Xu, J.; Jin, R.; Ren, X.; Gao, G. Cartilage-inspired hydrogel strain sensors with ultrahigh toughness, good self-recovery and stable anti-swelling properties†. J. Mater. Chem. A 2019, 7, 25441–25448. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Wang, D.; Sun, Z.; Liu, F.; Zhang, D.; Wang, D. Development of a food packaging antibacterial hydrogel based on gelatin, chitosan, and 3-phenyllactic acid for the shelf-life extension of chilled chicken. Food Hydrocoll. 2022, 127, 107546. [Google Scholar] [CrossRef]
- GB 9685-2016; Standard for Use of Additives in Food Contact Materials and Products. National Health Commission of the People’s Republic of China: Beijing, China, 2016.

| Sample | pH | 0 d | 120 d |
|---|---|---|---|
| Gel/Alg-CMCS-Na+ | 1.0 | 197.23 ± 25.66% a | 192.81 ± 20.77% a |
| 5.0 | 421.21 ± 5.05% a | 417.16 ± 2.92% a | |
| 7.0 | 311.23 ± 7.04% a | 307.26 ± 6.19% a | |
| Gel/Alg-CMCS-Ca2+ | 1.0 | 556.06 ± 19.21% a | 550.28 ± 15.45% a |
| 5.0 | 161.85 ± 2.49% a | 157.73 ± 1.45% a | |
| 7.0 | 163.30 ± 1.95% a | 156.62 ± 1.62% a | |
| Gel/Alg-CMCS-Fe3+ | 1.0 | 518.38 ± 20.27% a | 513.50 ± 11.22% a |
| 5.0 | 64.66 ± 2.49% a | 60.54 ± 1.05% a | |
| 7.0 | 61.42 ± 0.61% a | 59.34 ± 0.64% a |
| Sample | pH | R2 | |||
|---|---|---|---|---|---|
| Higuchi Model | Korsmeyer–Peppas Model | Peppas–Sahlin Model | Weibull Model | ||
| Gel/Alg-CMCS-CAR | 4.0 | 0.6239 | 0.7167 | 0.9410 | 0.9833 |
| 5.0 | 0.5854 | 0.6953 | −5.9632 | 0.9928 | |
| 6.0 | 0.4757 | 0.6421 | −5.8949 | 0.9796 | |
| 6.5 | 0.6359 | 0.6867 | −4.4557 | 0.9853 | |
| 7.0 | 0.3653 | 0.6269 | −5.6786 | 0.9968 | |
| Gel/Alg-CMCS-Na+-CAR | 1.0 | 0.8473 | 0.8541 | 0.9867 | 0.9956 |
| 5.0 | 0.8885 | 0.8912 | 0.9284 | 0.9377 | |
| 7.0 | 0.8614 | 0.8575 | 0.9106 | 0.9194 | |
| Gel/Alg-CMCS-Ca2+-CAR | 1.0 | 0.9798 | 0.9504 | 0.9952 | 0.9977 |
| 5.0 | 0.9744 | 0.9380 | 0.9933 | 0.9948 | |
| 7.0 | 0.9685 | 0.9343 | 0.9912 | 0.9960 | |
| Gel/Alg-CMCS-Fe3+-CAR | 1.0 | 0.8344 | 0.7506 | 0.9889 | 0.9965 |
| 5.0 | 0.9849 | 0.9231 | 0.9955 | 0.9960 | |
| 7.0 | 0.9628 | 0.9720 | 0.9792 | 0.9945 | |
| Sample | pH | R2 | b |
|---|---|---|---|
| Gel/Alg-CMCS-CAR | 4.0 | 0.9833 | 1.0029 ± 0.0921 |
| 5.0 | 0.9928 | 1.0199 ± 0.0369 | |
| 6.0 | 0.9796 | 1.0393 ± 0.1245 | |
| 6.5 | 0.9853 | 1.3592 ± 0.1987 | |
| 7.0 | 0.9968 | 1.2785 ± 0.0792 | |
| Gel/Alg-CMCS-Na+-CAR | 1.0 | 0.9956 | 0.9027 ± 0.0270 |
| 5.0 | 0.9284 | 0.4999 ± 0.1006 | |
| 7.0 | 0.9106 | 0.4998 ± 0.1061 | |
| Gel/Alg-CMCS-Ca2+-CAR | 1.0 | 0.9977 | 1.2075 ± 0.0380 |
| 5.0 | 0.9948 | 1.7150 ± 0.1158 | |
| 7.0 | 0.9960 | 1.6359 ± 0.0945 | |
| Gel/Alg-CMCS-Fe3+-CAR | 1.0 | 0.9965 | 0.8700 ± 0.0224 |
| 5.0 | 0.9960 | 1.0221 ± 0.0400 | |
| 7.0 | 0.9945 | 1.2796 ± 0.0989 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Li, J.; Cong, S.; Hou, H.; Zhang, G.; Bi, J. Fabrication and Anti-Swelling Properties of Gelatin/Sodium Alginate–Carboxymethyl Chitosan-Based Cationic Coordination Hydrogels. Foods 2025, 14, 3149. https://doi.org/10.3390/foods14183149
Zhao H, Li J, Cong S, Hou H, Zhang G, Bi J. Fabrication and Anti-Swelling Properties of Gelatin/Sodium Alginate–Carboxymethyl Chitosan-Based Cationic Coordination Hydrogels. Foods. 2025; 14(18):3149. https://doi.org/10.3390/foods14183149
Chicago/Turabian StyleZhao, Haixin, Jinlong Li, Shuang Cong, Hongman Hou, Gongliang Zhang, and Jingran Bi. 2025. "Fabrication and Anti-Swelling Properties of Gelatin/Sodium Alginate–Carboxymethyl Chitosan-Based Cationic Coordination Hydrogels" Foods 14, no. 18: 3149. https://doi.org/10.3390/foods14183149
APA StyleZhao, H., Li, J., Cong, S., Hou, H., Zhang, G., & Bi, J. (2025). Fabrication and Anti-Swelling Properties of Gelatin/Sodium Alginate–Carboxymethyl Chitosan-Based Cationic Coordination Hydrogels. Foods, 14(18), 3149. https://doi.org/10.3390/foods14183149






