Functional Ingredients: From Molecule to Market—AI-Enabled Design, Bioavailability, Consumer Impact, and Clinical Evidence
Abstract
1. Introduction
2. Methodology
3. Classification and Characteristics of Functional Ingredients
3.1. Carbohydrate-Based Functional Ingredients
3.1.1. Dietary Fiber (DF)
3.1.2. Prebiotics
3.2. Protein-Based Functional Ingredients
Proteins and Peptides
3.3. Fat-Based Functional Ingredients
ω-3 Fatty Acids
3.4. Other Functional Ingredients
3.4.1. Probiotics
3.4.2. Plant Polyphenols
3.5. The Interaction of Functional Components
3.6. Bioactivity of Functional Ingredients
4. Innovative Technologies and Applications
4.1. Microencapsulation and Nanotechnology
4.2. Targeted Delivery Systems
4.3. Enzymatic Hydrolysis
4.4. Industrial Feasibility of Emerging Technologies
5. Emerging Research Areas
5.1. Applications of 3D and 4D Printing in Functional Foods
5.2. Molecular Dynamics and Microstructural Modeling
5.3. AI-Assisted Screening and Design of Functional Ingredients
6. Sensory Characteristics of Functional Ingredients and Consumer Acceptance
6.1. Sensory Evaluation Methods
6.2. Regional and Cultural Differences in Acceptance
6.3. Consumer Behavior and Health Consciousness
7. Product Development and Market Trends for Functional Ingredients
7.1. Innovation in Functional Food Products
7.2. Market Trends and Challenges
8. Clinical Research and Safety Evaluation
8.1. The Role of Clinical Trials
8.2. Safety Evaluation
9. Future Research Directions
10. Limitations
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PUFAs | Polyunsaturated fatty acids |
| FAO | Food and Agriculture Organization |
| WHO | The World Health Organization |
| DF | Dietary fiber |
| CVD | Cardiovascular disease |
| SCFAs | Short-chain fatty acids |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| VLDL-C | Very-low-density lipoprotein cholesterol |
| FOS | Fructooligosaccharides |
| GOS | Galactooligosaccha-rides |
| PSG | Polysaccharides |
| BAPs | Bioactive peptides |
| ACEI | Angiotensin-converting enzyme inhibitory |
| SFAs | Saturated fatty acids |
| MUFAs | Monounsaturated fatty acids |
| EPA | Eicosatetraenoic acid |
| DHA | Docosahexaenoic acid |
| ISAPP | International Scientific Association for Probiotics and Prebiotics |
| MH | Manuka honey |
| COX-2 | Cyclooxygenase-2 |
| EGCG | Epigallocatechin gallate |
| LF | Lactoferrin |
| TNBC | Triple-negative breast cancer |
| UAE | Ultrasound-assisted extraction |
| PLE | Pressurized liquid extraction |
| SFE | Supercritical fluid extraction |
| MAE | Microwave-assisted extraction |
| EAE | Enzyme-assisted extraction |
| HHP | High hydrostatic pressure |
| FP | Functional polymers |
| FGG | Functional gels/gums |
| MS | Molecular simulation |
| ADME | Absorption, Distribution, Metabolism, Excretion |
| FFIs | Functional food ingredients |
| PNs | Peptide networks |
| CNNs | Convolutional neural networks |
| VR/AR | Virtual and augmented reality |
| QSARs | Quantitative structure– activity relationships |
| ADF | Antioxidant dietary fiber |
| CAGR | Compound annual growth rate |
| EFSA | The European Food Safety Authority |
| VDLD | Very-low-density lipoprotein |
| ILD | Intermediate-Density Lipoprotein |
| LDL | Low-Density Lipoprotein |
| GLP-1 | Glucagon-Like Peptide-1 |
| HDL | High-density lipoprotein |
| NK | Nattokinase |
| HMF | Hydroxymethylfurfural |
References
- Elma, O.; Brain, K.; Dong, H.J. The Importance of Nutrition as a Lifestyle Factor in Chronic Pain Management: A Narrative Review. J. Clin. Med. 2022, 11, 5950. [Google Scholar] [CrossRef] [PubMed]
- Singar, S.; Nagpal, R.; Arjmandi, B.H.; Akhavan, N.S. Personalized Nutrition: Tailoring Dietary Recommendations Through Genetic Insights. Nutrients 2024, 16, 2673. [Google Scholar] [CrossRef]
- Alongi, M.; Anese, M. Re-thinking functional food development through a holistic approach. J. Funct. Foods 2021, 81, 104466. [Google Scholar] [CrossRef]
- Sun, S.; Liu, Z.; Lin, M.; Gao, N.; Wang, X. Polyphenols in health and food processing: Antibacterial, anti-inflammatory, and antioxidant insights. Front. Nutr. 2024, 11, 1456730. [Google Scholar] [CrossRef]
- Victoria Obayomi, O.; Folakemi Olaniran, A.; Olugbemiga Owa, S. Unveiling the role of functional foods with emphasis on prebiotics and probiotics in human health: A review. J. Funct. Foods 2024, 119, 106337. [Google Scholar] [CrossRef]
- Martirosyan, D.; Ekblad, M. Functional foods classification system: Exemplifying through analysis of bioactive compounds. Funct. Food Sci. 2022, 2, 94–123. [Google Scholar] [CrossRef]
- Duffuler, P.; Bhullar, K.S.; de Campos Zani, S.C.; Wu, J. Bioactive peptides: From basic research to clinical trials and commercialization. J. Agric. Food Chem. 2022, 70, 3585–3595. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J. The Application and Mechanism Analysis of Enteral Nutrition in Clinical Management of Chronic Diseases. Nutrients 2025, 17, 450. [Google Scholar] [CrossRef]
- Zhang, J.H. Effect of Nutritional Management on the Nutritional Status and Quality of Life of Patients with Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obs. Pulmon Dis. 2025, 20, 487–496. [Google Scholar] [CrossRef]
- Misra, S.; Pandey, P.; Mishra, H.N. Novel approaches for co-encapsulation of probiotic bacteria with bioactive compounds, their health benefits and functional food product development: A review. Trends Food Sci. Technol. 2021, 109, 340–351. [Google Scholar] [CrossRef]
- Zhen, S.; Xia, X.; Huang, L.; Cao, Y.; Fu, H.; Ren, Y. Does risk preference matter to consumers’ willingness to pay for functional food: Evidence from lab experiments using the eye-tracking technology. Food Qual. Prefer. 2024, 119, 105197. [Google Scholar] [CrossRef]
- Meldrum, O.W.; Yakubov, G.E. Journey of dietary fiber along the gastrointestinal tract: Role of physical interactions, mucus, and biochemical transformations. Crit. Rev. Food Sci. Nutr. 2024, 65, 4264–4292. [Google Scholar] [CrossRef] [PubMed]
- Bobade, H.; Kaur, H.; Yadav, D.N. Application of Wheat and Its Constituents in Diverse Functional Food Products. In Wheat Science: Nutritional and Anti-Nutritional Properties, Processing, Storage, Bioactivity, and Product Development; Apple Academic Press: Boca Raton, FL, USA, 2023; p. 339. [Google Scholar]
- Głąbska, D.; Guzek, D.; Groele, B.; Gutkowska, K. Fruit and Vegetable Intake and Mental Health in Adults: A Systematic Review. Nutrients 2020, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.; Raji, Z.; Habibi, Y.; Khalloufi, S. A review on the hydration properties of dietary fibers derived from food waste and their interactions with other ingredients: Opportunities and challenges for their application in the food industry. Crit. Rev. Food Sci. Nutr. 2024, 64, 11722–11756. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Xu, X.; Chao, Z.; Jiang, X.; Zheng, L.; Jiang, B. Properties of plant-derived soluble dietary fibers for fiber-enriched foods: A comparative evaluation. Int. J. Biol. Macromol. 2022, 223, 1196–1207. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Raji, V.; Loganathan, C.; Ramesh, T.; Thayumanavan, P. Dual antidiabetic and antihypertensive activity of fucoxanthin isolated from Sargassum wightii Greville in in vivo rat model. Food Sci. Hum. Wellness 2023, 12, 1693–1700. [Google Scholar] [CrossRef]
- Lyu, B.; Wang, H.; Swallah, M.S.; Fu, H.; Shen, Y.; Guo, Z.; Tong, X.; Li, Y.; Yu, H.; Jiang, L. Structure, properties and potential bioactivities of high-purity insoluble fibre from soybean dregs (Okara). Food Chem. 2021, 364, 130402. [Google Scholar] [CrossRef]
- Mondal, P.; Meeran, S.M. The emerging role of the gut microbiome in cancer cell plasticity and therapeutic resistance. Cancer Metastasis Rev. 2023, 43, 135–154. [Google Scholar] [CrossRef]
- Li, L.; Ma, L.; Wen, Y.; Xie, J.; Yan, L.; Ji, A.; Zeng, Y.; Tian, Y.; Sheng, J. Crude Polysaccharide Extracted From Moringa oleifera Leaves Prevents Obesity in Association With Modulating Gut Microbiota in High-Fat Diet-Fed Mice. Front. Nutr. 2022, 9, 861588. [Google Scholar] [CrossRef]
- Liu, G.; Feng, S.; Yan, J.; Luan, D.; Sun, P.; Shao, P. Antidiabetic potential of polysaccharides from Brasenia schreberi regulating insulin signaling pathway and gut microbiota in type 2 diabetic mice. Curr. Res. Food Sci. 2022, 5, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.S.; Deshmukh, K.N.; Dey, A.; Ranjan, D.; Goyal, A.; Jachak, S.M. An alkaloid enriched fraction from Murraya koenigii (L.) Spreng. Leaves ameliorate HFD-induced obesity and metabolic complexities in C57BL/6J mice. J. Ethnopharmacol. 2024, 333, 118423. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ren, Y.W.; Li, X.L.; Li, Z.N. A Review of the Associations between Dietary Fiber Intake and Cancer Prevention or Prognosis. J. Nutr. Oncol. 2020, 5, 123–131. [Google Scholar] [CrossRef]
- Hu, J.; Wang, J.; Li, Y.; Xue, K.; Kan, J. Use of Dietary Fibers in Reducing the Risk of Several Cancer Types: An Umbrella Review. Nutrients 2023, 15, 2545. [Google Scholar] [CrossRef]
- Huang, C.-H.; Hsu, H.-S.; Chiang, M.-T. Influence of Varied Dietary Cholesterol Levels on Lipid Metabolism in Hamsters. Nutrients 2024, 16, 2472. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Zhang, L.; An, L.; Guo, L.; Huang, L.; Gao, W. Recent progress in plant-derived polysaccharides with prebiotic potential for intestinal health by targeting gut microbiota: A review. Crit. Rev. Food Sci. Nutr. 2023, 64, 12242–12271. [Google Scholar] [CrossRef]
- Srıvastava, S. Functional foods and spices in the management of metabolic syndrome. In Nutraceuticals in Obesity Management and Control; Keservani, R.K., Lohani, A., Eds.; Apple Academic Press: New York, NY, USA, 2025; Volume 3, p. 73. [Google Scholar]
- So, S.Y.; Badu, S.; Wu, Q.; Yalcinkaya, N.; Mirabile, Y.; Castaneda, R.; Musaad, S.; Heitkemper, M.; Savidge, T.C.; Shulman, R.J. Sex-Dependent Efficacy of Dietary Fiber in Pediatric Functional Abdominal Pain. Gastroenterology 2024, 166, 645–657.e14. [Google Scholar] [CrossRef]
- Kumari, A.; Rashmi, K.G.; Sudhakaran, V.A.; Warrier, A.S.; Singh, N.K. Unveiling the Health Benefits of Prebiotics: A Comprehensive Review. Indian J. Microbiol. 2024, 64, 376–388. [Google Scholar] [CrossRef]
- Kumari, T.; Das, A.J.; Das, A.B.; Reddy, C.K.; Deka, S.C. Prebiotic activity of enzymatically modified pea peel dietary fiber: An in vitro study. Bioact. Carbohydr. Diet. Fibre 2024, 32, 100452. [Google Scholar] [CrossRef]
- Cantu-Jungles, T.; Hall, D.; Goetz, C.; Keshavarzian, A.; Hamaker, B. Designed Dietary Fiber Prebiotics for Gut-Brain Axis Health. Curr. Dev. Nutr. 2024, 8, 103435. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Cai, T.; Zhang, J.; He, L.; Cai, R.; Zhu, C.; Shi, H.; Chu, Z.; Shen, X. Energy storage enabled by cross-linked multilayer films using block copolymer-modified nanocapsules and chitosan biopolymers. Macromol. Res. 2024, 32, 453–465. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Shinohara, K.; Kitamura, N.; Nakamura, A.; Onoue, A.; Tanaka, K.; Hirayama, A.; Aw, W.; Nakamura, S.; Ogawa, Y.; et al. Metabolic Effects of Bee Larva-Derived Protein in Mice: Assessment of an Alternative Protein Source. Foods 2021, 10, 2642. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Yang, J.; Suo, H.; Song, J. Tenebrio molitor proteins and peptides: Cutting-edge insights into bioactivity and expanded food applications. Food Biosci. 2025, 68, 106369. [Google Scholar] [CrossRef]
- Fatima, N.; Emambux, M.N.; Olaimat, A.N.; Stratakos, A.C.; Nawaz, A.; Wahyono, A.; Gul, K.; Park, J.; Shahbaz, H.M. Recent advances in microalgae, insects, and cultured meat as sustainable alternative protein sources. Food Humanit. 2023, 1, 731–741. [Google Scholar] [CrossRef]
- Echave, J.; Otero, P.; Garcia-Oliveira, P.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M.; Simal-Gandara, J.; Prieto, M.A. Seaweed-Derived Proteins and Peptides: Promising Marine Bioactives. Antioxidants 2022, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Lara-Parra, A.I.; Hernández-Hernández, A.A.; Jaguey-Hernández, Y.; Jiménez-Osorio, A.S.; Castañeda-Ovando, A.; Aguilar-Arteaga, K.; Añorve-Morga, J. Exploring alternative sources of protein in food: Trends in nutrient and functional features. Food Res. Int. 2025, 208, 116224. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Wu, J. Food-derived bioactive peptides: The gateway to reach the full potential of food proteins for human health. Trends Food Sci. Technol. 2025, 157, 104896. [Google Scholar] [CrossRef]
- Minj, S.; Anand, S. Whey Proteins and Its Derivatives: Bioactivity, Functionality, and Current Applications. Dairy 2020, 1, 233–258. [Google Scholar] [CrossRef]
- Fan, H.; Liu, H.; Zhang, Y.; Zhang, S.; Liu, T.; Wang, D. Review on plant-derived bioactive peptides: Biological activities, mechanism of action and utilizations in food development. J. Future Foods 2022, 2, 143–159. [Google Scholar] [CrossRef]
- Fadimu, G.J.; Le, T.T.; Gill, H.; Farahnaky, A.; Olatunde, O.O.; Truong, T. Enhancing the Biological Activities of Food Protein-Derived Peptides Using Non-Thermal Technologies: A Review. Foods 2022, 11, 1823. [Google Scholar] [CrossRef]
- Mora, L.; Toldrá, F. Advanced enzymatic hydrolysis of food proteins for the production of bioactive peptides. Curr. Opin. Food Sci. 2023, 49, 100973. [Google Scholar] [CrossRef]
- Lund, J.; Rustan, A.C. Fatty acids: Structures and properties. In Encyclopedia of Life Sciences; Wiley-Blackwell: Hoboken, NJ, USA, 2020; pp. 283–292. [Google Scholar]
- Banaszak, M.; Dobrzyńska, M.; Kawka, A.; Górna, I.; Woźniak, D.; Przysławski, J.; Drzymała-Czyż, S. Role of Omega-3 fatty acids eicosapentaenoic (EPA) and docosahexaenoic (DHA) as modulatory and anti-inflammatory agents in noncommunicable diet-related diseases—Reports from the last 10 years. Clin. Nutr. ESPEN 2024, 63, 240–258. [Google Scholar] [CrossRef]
- O’Keefe, E.L.; O’Keefe, J.H.; Abuissa, H.; Metzinger, M.; Murray, E.; Franco, G.; Lavie, C.J.; Harris, W.S. Omega-3 and Risk of atrial fibrillation: Vagally-mediated double-edged sword. Prog. Cardiovasc. Dis. 2024. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Y.; Zhang, N.; Che, H.; Wang, Z.; Han, J.; Wen, M. DHA and EPA alleviate depressive-like behaviors in chronic sleep-deprived mice: Involvement of iron metabolism, oligodendrocyte-lipids peroxidation and the LCN2-NLRP3 signaling axis. Free Radic. Biol. Med. 2024, 225, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, X.; Hu, M.; Huang, J.; Yu, S.; Zeng, H.; Mao, L. EPA and DHA differentially improve insulin resistance by reducing adipose tissue inflammation—Targeting GPR120/PPARγ pathway. J. Nutr. Biochem. 2024, 130, 109648. [Google Scholar] [CrossRef] [PubMed]
- Suryati, M.A.; Azrina, A.; Amin, I.; Nor-Khaizura, M.A.R. Retention of omega-3 fatty acids (DHA and EPA) and cooking yield in fish cooked using wet-heat methods: Steaming vs. bake-in-foil. J. Food Compos. Anal. 2025, 142, 107482. [Google Scholar] [CrossRef]
- Afrin, S.; Akter, S.; Begum, S.; Hossain, M.N. The Prospects of Lactobacillus oris as a Potential Probiotic With Cholesterol-Reducing Property From Mother’s Milk. Front. Nutr. 2021, 8, 619506. [Google Scholar] [CrossRef]
- Kathiriya, M.R.; Vekariya, Y.V.; Hati, S. Understanding the Probiotic Bacterial Responses Against Various Stresses in Food Matrix and Gastrointestinal Tract: A Review. Probiotics Antimicrob. Proteins 2023, 15, 1032–1048. [Google Scholar] [CrossRef]
- Ryabtseva, S.A.; Khramtsov, A.G.; Sazanova, S.N.; Budkevich, R.O.; Fedortsov, N.M.; Veziryan, A.A. The Probiotic Properties of Saccharomycetes (Review). Appl. Biochem. Microbiol. 2023, 59, 111–121. [Google Scholar] [CrossRef]
- Ravindhiran, R.; Subramanian, M.; Rajalingam, M.; Gunasekaran, M.; Sivarajan, K.; Chidambaram, K.; Dhandapani, K. Functional and biological characterization of Lactobacillus plantarum with isatin for use as probiotics for therapeutic options. J. Asian Nat. Prod. Res. 2025, 1–17. [Google Scholar] [CrossRef]
- Karimi, R.; Hosseinzadeh, D. Probiotics and Gastro-Intestinal Disorders: Augmentation, Enhancement, and Strengthening of Epithelial Lining. In Probiotics; CRC Press: Boca Raton, FL, USA, 2024; pp. 188–215. [Google Scholar]
- Lima, W.G.; Pessoa, R.M.; Vital, K.D.; Takenaka, I.; Cardoso, V.N.; Fernandes, S.O.A. Effect of probiotics on the maintenance of intestinal homeostasis after chemotherapy: Systematic review and meta-analysis of pre-clinical studies. Benef. Microbes 2020, 11, 305–318. [Google Scholar] [CrossRef]
- Yang, K.M.; Kim, J.-S.; Kim, H.-S.; Kim, Y.-Y.; Oh, J.-K.; Jung, H.-W.; Park, D.-S.; Bae, K.-H. Lactobacillus reuteri AN417 cell-free culture supernatant as a novel antibacterial agent targeting oral pathogenic bacteria. Sci. Rep. 2021, 11, 1631. [Google Scholar] [CrossRef]
- Yamamura, R.; Inoue, K.Y.; Nishino, K.; Yamasaki, S. Intestinal and fecal pH in human health. Front. Microbiomes 2023, 2, 1192316. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Chen, F.; Chen, Z.; Jiang, H. Research in advances in the bioactivity of plant polyphenols. Int. J. Food Sci. Technol. 2024, 59, 8037–8044. [Google Scholar] [CrossRef]
- Barik, S.K.; Sengupta, S.; Arya, R.; Kumar, S.; Kim, J.J.; Chaurasia, R. Dietary Polyphenols as Potential Therapeutic Agents in Type 2 Diabetes Management: Advances and Opportunities. Adv. Nutr. 2025, 16, 100346. [Google Scholar] [CrossRef] [PubMed]
- Nollet, L.M.; Otles, S. Handbook of Analysis and Extraction Methods of Anthocyanins; CRC Press: Boca Raton, FL, USA, 2025. [Google Scholar]
- Ding, W.; Liu, H.; Qin, Z.; Liu, M.; Zheng, M.; Cai, D.; Liu, J. Dietary Antioxidant Anthocyanins Mitigate Type II Diabetes through Improving the Disorder of Glycometabolism and Insulin Resistance. J. Agric. Food Chem. 2021, 69, 13350–13363. [Google Scholar] [CrossRef]
- Rolt, A.; Cox, L.S. Structural basis of the anti-ageing effects of polyphenolics: Mitigation of oxidative stress. BMC Chem. 2020, 14, 50. [Google Scholar] [CrossRef]
- Davinelli, S.; Scapagnini, G. Interactions between dietary polyphenols and aging gut microbiota: A review. BioFactors 2021, 48, 274–284. [Google Scholar] [CrossRef]
- Alsahli, M.A.; Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Anwar, S.; Almutary, A.G.; Alrumaihi, F.; Rahmani, A.H.; Roscetto, E. 6-Gingerol, a Major Ingredient of Ginger Attenuates Diethylnitrosamine-Induced Liver Injury in Rats through the Modulation of Oxidative Stress and Anti-Inflammatory Activity. Mediat. Inflamm. 2021, 2021, 6661937. [Google Scholar] [CrossRef]
- Amin, I.; Hussain, I.; Rehman, M.U.; Mir, B.A.; Ganaie, S.A.; Ahmad, S.B.; Mir, M.U.R.; Shanaz, S.; Muzamil, S.; Arafah, A.; et al. Zingerone prevents lead-induced toxicity in liver and kidney tissues by regulating the oxidative damage in Wistar rats. J. Food Biochem. 2020, 45, e13241. [Google Scholar] [CrossRef] [PubMed]
- Opšivač, D.; Musić, L.; Badovinac, A.; Šekelja, A.; Božić, D. Therapeutic Manuka Honey as an Adjunct to Non-Surgical Periodontal Therapy: A 12-Month Follow-Up, Split-Mouth Pilot Study. Materials 2023, 16, 1248. [Google Scholar] [CrossRef] [PubMed]
- Paunică-Panea, G.; Teodorescu, S.; Preda, A.; Gligor, L.E.; Silaghi, A.; Constantin, V.D. Chronic wound management; surgical therapy and complementary nursing with Manuka honey. J. Mind Med. Sci. 2023, 10, 139–147. [Google Scholar] [CrossRef]
- Onuoha, E.O.; Adekunle, A.A.; Ajike, S.O.; Gbotolorun, O.M.; Adeyemo, W.L. Effect of manuka honey socket dressing on postoperative sequelae and complications following third molar extraction: A randomized controlled study. J. Cranio-Maxillofac. Surg. 2023, 51, 252–260. [Google Scholar] [CrossRef]
- Basilicata, M.; Di Lauro, M.; Campolattano, V.; Marrone, G.; Celotto, R.; Mitterhofer, A.P.; Bollero, P.; Di Daniele, N.; Noce, A. Natural Bioactive Compounds in the Management of Oral Diseases in Nephropathic Patients. Int. J. Environ. Res. Public Health 2022, 19, 1665. [Google Scholar] [CrossRef]
- Ademosun, A.O.; Ajeigbe, O.F.; Ademosun, M.T.; Ogunruku, O.O.; Oboh, G. Improving gut microbiome through diet rich in dietary fibre and polyphenols: The case for orange peels. Hum. Nutr. Metab. 2025, 39, 200295. [Google Scholar] [CrossRef]
- Seo, S.-H.; Ham, D.-W.; Lee, J.-E.; Jong, S.-M.; Kim, S.-K.; Lee, S.-H.; Yu, H.-Y.; Shin, E.-H. Effects of red ginseng extract and red ginseng dietary fiber on the maintenance of intestinal immune and functional homeostasis in diet-induced obese mice. J. Ginseng Res. 2025, 49, 271–281. [Google Scholar] [CrossRef]
- Wei, R.; Su, Z.; Mackenzie, G.G. Chlorogenic acid combined with epigallocatechin-3-gallate mitigates d-galactose-induced gut aging in mice. Food Funct. 2023, 14, 2684–2697. [Google Scholar] [CrossRef]
- Choi, H.; Choi, J.; Jang, Y.; Lee, Y.-M.; Kang, M.-H.; Kwon, H.-S.; Kim, S.; Kwon, J. Anti-Obesity and Antidiabetic Effects of Fig (Ficus carica L.) Fermented Extract Using Lactobacillus plantarum BT-LP-01. Appl. Sci. 2024, 14, 6412. [Google Scholar] [CrossRef]
- Li, L.; Tan, L.; Zhang, Q.; Cheng, Y.; Liu, Y.; Li, R.; Hou, S. Nose-to-brain delivery of self-assembled curcumin-lactoferrin nanoparticles: Characterization, neuroprotective effect and in vivo pharmacokinetic study. Front. Bioeng. Biotechnol. 2023, 11, 1168408. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Yan, X.; Julian McClements, D.; Ma, C.; Liu, X.; Liu, F. Ultrasound-assisted preparation of lactoferrin-EGCG conjugates and their application in forming and stabilizing algae oil emulsions. Ultrason. Sonochem. 2022, 89, 106110. [Google Scholar] [CrossRef]
- Méndez, L.; Medina, I. Polyphenols and Fish Oils for Improving Metabolic Health: A Revision of the Recent Evidence for Their Combined Nutraceutical Effects. Molecules 2021, 26, 2438. [Google Scholar] [CrossRef] [PubMed]
- Fernando, W.; Clark, R.F.; Rupasinghe, H.P.V.; Hoskin, D.W.; Coombs, M.R.P. Phloridzin Docosahexaenoate Inhibits Spheroid Formation by Breast Cancer Stem Cells and Exhibits Cytotoxic Effects Against Paclitaxel-Resistant Triple Negative Breast Cancer Cells. Int. J. Mol. Sci. 2023, 24, 4577. [Google Scholar] [CrossRef] [PubMed]
- Delerue, T.; Fatima Barroso, M.; Dias-Teixeira, M.; Figueiredo-Gonzalez, M.; Delerue-Matos, C.; Grosso, C. Interactions between Ginkgo biloba L. and Scutellaria baicalensis Georgi in multicomponent mixtures towards cholinesterase inhibition and ROS scavenging. Food Res. Int. 2021, 140, 109857. [Google Scholar] [CrossRef] [PubMed]
- Kraithong, S.; Teerapattarakan, N.; Balasubramanian, B.; Issara, U. Bioactive compounds in tea: Effect of imbalanced intake on digestive enzymes activity, cytochrome inhibition and drug interaction. S. Afr. J. Bot. 2022, 150, 58–68. [Google Scholar] [CrossRef]
- Ibrahim Fouad, G.; Ahmed, K.A. Curcumin Ameliorates Doxorubicin-Induced Cardiotoxicity and Hepatotoxicity Via Suppressing Oxidative Stress and Modulating iNOS, NF-kappaB, and TNF-alpha in Rats. Cardiovasc. Toxicol. 2022, 22, 152–166. [Google Scholar] [CrossRef]
- Fan, W.; Huang, Y.; Zheng, H.; Li, S.; Li, Z.; Yuan, L.; Cheng, X.; He, C.; Sun, J. Ginsenosides for the treatment of metabolic syndrome and cardiovascular diseases: Pharmacology and mechanisms. Biomed. Pharmacother. 2020, 132, 110915. [Google Scholar] [CrossRef]
- Mehra, R.; Kumar, S.; Singh, R.; Kumar, N.; Rathore, D.; Nayik, G.A.; Alabdallah, N.M.; Monteiro, A.; Guiné, R.F.F.; Kumar, H. Biochemical, dielectric and surface characteristics of freeze-dried bovine colostrum whey powder. Food Chem. X 2022, 15, 100364. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, M.; Fan, H.; Wu, J. Emerging proteins as precursors of bioactive peptides/hydrolysates with health benefits. Curr. Opin. Food Sci. 2022, 48, 100914. [Google Scholar] [CrossRef]
- García-Encinas, J.P.; Ruiz-Cruz, S.; Juárez, J.; de Jesús Ornelas-Paz, J.; Del Toro-Sánchez, C.L.; Márquez-Ríos, E. Proteins from Microalgae: Nutritional, Functional and Bioactive Properties. Foods 2025, 14, 921. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, Y.; Zhu, F. An updated review of functional ingredients of Manuka honey and their value-added innovations. Food Chem. 2024, 440, 138060. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.R.; Sanjay, B.S.; Gupta, D.; Mehta, S.; Chauhan, O.P. Bioactive compounds of foods: Phytochemicals and peptides. Food Humanit. 2024, 3, 100354. [Google Scholar] [CrossRef]
- Galanakis, C.M. Nutraceutical and Functional Food Components: Effects of Innovative Processing Techniques; Academic Press: New York, NY, USA, 2021. [Google Scholar]
- Zou, X.; Dai, K.; Zhang, M.; Zhang, R.; Jia, X.; Dong, L.; Ma, Q.; Liang, S.; Wang, Z.; Deng, M.; et al. Dietary fiber from sweet potato residue with different processing methods: Physicochemical, functional properties, and bioactivity in vitro. LWT 2024, 206, 116581. [Google Scholar] [CrossRef]
- Sui, W.; Wang, S.; Chen, Y.; Li, X.; Zhuang, X.; Yan, X.; Song, Y. Insights into the Structural and Nutritional Variations in Soluble Dietary Fibers in Fruits and Vegetables Influenced by Food Processing Techniques. Foods 2025, 14, 1861. [Google Scholar] [CrossRef]
- Speroni, C.S.; Bender, A.B.B.; Stiebe, J.; Ballus, C.A.; Ávila, P.F.; Goldbeck, R.; Morisso, F.D.P.; da Silva, L.P.; Emanuelli, T. Granulometric fractionation and micronization: A process for increasing soluble dietary fiber content and improving technological and functional properties of olive pomace. LWT 2020, 130, 109526. [Google Scholar] [CrossRef]
- Polia, F.; Pastor-Belda, M.; Martínez-Blázquez, A.; Horcajada, M.-N.; Tomás-Barberán, F.A.; García-Villalba, R. Technological and Biotechnological Processes to Enhance the Bioavailability of Dietary (Poly)phenols in Humans. J. Agric. Food Chem. 2022, 70, 2092–2107. [Google Scholar] [CrossRef]
- Zadeike, D.; Vaitkeviciene, R.; Degutyte, R.; Bendoraitiene, J.; Rukuiziene, Z.; Cernauskas, D.; Svazas, M.; Juodeikiene, G. A comparative study on the structural and functional properties of water-soluble and alkali-soluble dietary fibres from rice bran after hot-water, ultrasound, hydrolysis by cellulase, and combined pre-treatments. Int. J. Food Sci. Technol. 2021, 57, 1137–1149. [Google Scholar] [CrossRef]
- Soleimanian, Y.; Sanou, I.; Turgeon, S.L.; Canizares, D.; Khalloufi, S. Natural plant fibers obtained from agricultural residue used as an ingredient in food matrixes or packaging materials: A review. Compr. Rev. Food Sci. Food Saf. 2021, 21, 371–415. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Puri, V.; Nagpal, M.; Singh, I.; Singh, M.; Dhingra, G.A.; Huanbutta, K.; Dheer, D.; Sharma, A.; Sangnim, T. A Comprehensive Review on Nutraceuticals: Therapy Support and Formulation Challenges. Nutrients 2022, 14, 4637. [Google Scholar] [CrossRef]
- Refaat, H.; Mady, F.M.; Sarhan, H.A.; Rateb, H.S.; Alaaeldin, E. Optimization and evaluation of propolis liposomes as a promising therapeutic approach for COVID-19. Int. J. Pharm. 2021, 592, 120028. [Google Scholar] [CrossRef] [PubMed]
- Ramli, N.A.; Ali, N.; Hamzah, S.; Yatim, N.I. Physicochemical characteristics of liposome encapsulation of stingless bees’ propolis. Heliyon 2021, 7, e06649. [Google Scholar] [CrossRef] [PubMed]
- Mendake, R.A.; Hatwar, P.R.; Bakal, R.L.; Kohale, N.B. Microencapsulation: A Review. Int. J. Multidiscip. Res. 2024, 8, 36–43. [Google Scholar] [CrossRef]
- Tressa, A.; Singh, A.; Pal, R.; Kumar, N. Novel Nanocarriers Microencapsulation: Current, Patents and Clinical Trials Comprehensive Review. J. Drug Deliv. Ther. 2025, 15, 188–208. [Google Scholar] [CrossRef]
- Emon, D.D.; Islam, M.D.S.; Mazumder, M.A.R.; Aziz, M.G.; Rahman, M.S. Recent applications of microencapsulation techniques for delivery of functional ingredient in food products: A comprehensive review. Food Chem. Adv. 2025, 6, 100923. [Google Scholar] [CrossRef]
- Dehnad, D.; Emadzadeh, B.; Ghorani, B.; Rajabzadeh, G.; Kharazmi, M.S.; Jafari, S.M. Nano-vesicular carriers for bioactive compounds and their applications in food formulations. Crit. Rev. Food Sci. Nutr. 2022, 64, 5583–5602. [Google Scholar] [CrossRef]
- Tirado-Gallegos, J.M.; Bustillos-Rodríguez, J.C.; Ordóñez-García, M.; Juárez-Enriquez, E.; Neder-Suárez, D.; Rodríguez-Roque, M.J.; De Dios-Avila, N. Polysaccharides as Wall Materials in Microencapsulation of Bioactive Compounds for Food Preservation. In Sustainable Agricultural and Food Systems Engineering; Apple Academic Press: New York, NY, USA, 2024; pp. 153–184. [Google Scholar]
- D’Amico, V.; Cavaliere, M.; Ivone, M.; Lacassia, C.; Celano, G.; Vacca, M.; la Forgia, F.M.; Fontana, S.; De Angelis, M.; Denora, N.; et al. Microencapsulation of Probiotics for Enhanced Stability and Health Benefits in Dairy Functional Foods: A Focus on Pasta Filata Cheese. Pharmaceutics 2025, 17, 185. [Google Scholar] [CrossRef]
- Peruzzolo, M.; Ceni, G.C.; Junges, A.; Zeni, J.; Cansian, R.L.; Backes, G.T. Probiotics: Health benefits, microencapsulation, and viability, combination with natural compounds, and applications in foods. Food Biosci. 2025, 66, 106253. [Google Scholar] [CrossRef]
- Qazi, H.J.; Ye, A.; Acevedo-Fani, A.; Singh, H. Delivery of encapsulated bioactive compounds within food matrices to the digestive tract: Recent trends and future perspectives. Crit. Rev. Food Sci. Nutr. 2025, 65, 2921–2942. [Google Scholar] [CrossRef]
- Ajeeshkumar, K.K.; Aneesh, P.A.; Raju, N.; Suseela, M.; Ravishankar, C.N.; Benjakul, S. Advancements in liposome technology: Preparation techniques and applications in food, functional foods, and bioactive delivery: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1280–1306. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, J.; Chandrapala, J.; Majzoobi, M.; Brennan, C.; Zeng, X.A.; Sun, B. Recent progress of food-derived bioactive peptides: Extraction, purification, function, and encapsulation. Food Front. 2024, 5, 1240–1264. [Google Scholar] [CrossRef]
- Shakeri, M.; Ghobadi, R.; Sohrabvandi, S.; Khanniri, E.; Mollakhalili-Meybodi, N. Co-encapsulation of omega-3 and vitamin D3 in beeswax solid lipid nanoparticles to evaluate physicochemical and in vitro release properties. Front. Nutr. 2024, 11, 1323067. [Google Scholar] [CrossRef]
- Nnamani, P.O.; Onokala, O.B.; Owodeha-Ashaka, K.; Cardoso-Daodu, I.M.; Ilomuanya, M.; Mbah, C.C.; Attama, A.A. Liposomes and Nanoliposomes as Potential Nano-Enabled Platforms for Enhanced Bioavailability of Phytoconstituents. In Advances in Novel Phytopharmaceuticals; CRC Press: Boca Raton, FL, USA, 2025; pp. 99–133. [Google Scholar]
- Kouame, K.J.E.; Falade, E.O.; Zhu, Y.; Zheng, Y.; Ye, X. Advances in innovative extraction techniques for polysaccharides, peptides, and polyphenols from distillery by-products: Common extraction techniques, emerging technologies, and AI-driven optimization. Food Chem. 2025, 476, 143326. [Google Scholar] [CrossRef]
- Yang, H.; Jiao, W.; Zouyi, L.; Diao, H.; Xia, S. Artificial intelligence in the food industry: Innovations and applications. Discov. Artif. Intell. 2025, 5, 60. [Google Scholar] [CrossRef]
- Elisha, C.; Bhagwat, P.; Pillai, S. Emerging production techniques and potential health promoting properties of plant and animal protein-derived bioactive peptides. Crit. Rev. Food Sci. Nutr. 2025, 65, 4729–4758. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.; Falch, E.; Slizyte, R.; Kumari, A.; Khushboo; Hjellnes, V.; Sharma, A.; Rajauria, G. Valorization of fish processing by-products for protein hydrolysate recovery: Opportunities, challenges and regulatory issues. Food Chem. 2024, 459, 140244. [Google Scholar] [CrossRef] [PubMed]
- Umego, E.C.; He, R.; Ren, W.; Xu, H.; Ma, H. Ultrasonic-assisted enzymolysis: Principle and applications. Process Biochem. 2021, 100, 59–68. [Google Scholar] [CrossRef]
- Rezvankhah, A.; Yarmand, M.S.; Ghanbarzadeh, B.; Mirzaee, H. Characterization of bioactive peptides produced from green lentil (Lens culinaris) seed protein concentrate using Alcalase and Flavourzyme in single and sequential hydrolysis. J. Food Process. Preserv. 2021, 45, e15932. [Google Scholar] [CrossRef]
- Ozon, B.; Cotabarren, J.; Valicenti, T.; Graciela Parisi, M.; David Obregon, W. Chia expeller: A promising source of antioxidant, antihypertensive and antithrombotic peptides produced by enzymatic hydrolysis with Alcalase and Flavourzyme. Food Chem. 2022, 380, 132185. [Google Scholar] [CrossRef]
- D’Opazo, V.; Calpe, J.; Escrivá, L.; Melo Nazareth, T.; Meca, G.; Luz, C. Identification and evaluation of antioxidant activity of bioactive casein-derived peptides during in vitro digestion and transepithelial transport in Caco-2 cells. Food Biosci. 2023, 53, 102763. [Google Scholar] [CrossRef]
- Chen, J.; Yu, X.; Chen, Q.; Wu, Q.; He, Q. Screening and mechanisms of novel angiotensin-I-converting enzyme inhibitory peptides from rabbit meat proteins: A combined in silico and in vitro study. Food Chem. 2022, 370, 131070. [Google Scholar] [CrossRef] [PubMed]
- Habinshuti, I.; Mu, T.H.; Zhang, M. Ultrasound microwave-assisted enzymatic production and characterisation of antioxidant peptides from sweet potato protein. Ultrason. Sonochem. 2020, 69, 105262. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, M.; Zeng, Y.; Liu, G. Application and Perspectives of Supercritical Fluid Technology in the Nutraceutical Industry. Adv. Sustain. Syst. 2022, 6, 2200055. [Google Scholar] [CrossRef]
- Waliat, S.; Arshad, M.S.; Hanif, H.; Ejaz, A.; Khalid, W.; Kauser, S.; Al-Farga, A. A review on bioactive compounds in sprouts: Extraction techniques, food application and health functionality. Int. J. Food Prop. 2023, 26, 647–665. [Google Scholar] [CrossRef]
- Khalid, W.; Benmebarek, I.E.; Zargarchi, S.; Kumar, P.; Javed, M.; Moreno, A.; Sharma, A.; Nayik, G.A.; Esatbeyoglu, T. Optimization of the effect of cold plasma treatment on UAE-NADES green extraction of chickpea roots (Cicer arietinum) bioactive compounds. Ultrason. Sonochem. 2025, 114, 107276. [Google Scholar] [CrossRef]
- Galanakis, C.M. Functionality of Food Components and Emerging Technologies. Foods 2021, 10, 128. [Google Scholar] [CrossRef]
- Saifullah, M.; Akanbi, T.O.; McCullum, R.; Vuong, Q.V. Optimization of Commercial Microwave Assisted-Extraction Conditions for Recovery of Phenolics from Lemon-Scented Tee Tree (Leptospermum petersonii) and Comparison with Other Extraction Techniques. Foods 2021, 11, 50. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, H.; Xu, J.; Chang, Y.; Liu, C.; Zhang, Y.; Yang, H.; Duan, C.; Huang, J.; Fu, Y. A sustainable and integrated natural surfactant mediated microwave-assisted extraction technique enhances the extraction of phytochemicals from plants. Ind. Crops Prod. 2022, 184, 115043. [Google Scholar] [CrossRef]
- Cameselle, C.; Maietta, I.; Torres, M.D.; Simón-Vázquez, R.; Domínguez, H. Optimization of ultrasound-assisted extraction of bioactive compounds and biopolymers from Ulva spp. using response surface methodology. J. Appl. Phycol. 2025, 37, 2031–2050. [Google Scholar] [CrossRef]
- Martínez-Zamora, L.; Bueso, M.C.; Kessler, M.; Zapata, R.; Gómez, P.A.; Artés-Hernández, F. Optimization of Extraction Parameters for Phenolics Recovery from Avocado Peels Using Ultrasound and Microwave Technologies. Foods 2025, 14, 2431. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ali, H.A.; Zain, N.M. Microwave-assisted extraction of phenolic compounds from Carica papaya leaves: An optimization study and LC-QTOF-MS analysis. Future Foods 2021, 3, 100035. [Google Scholar] [CrossRef]
- Verma, D.K.; Mahanti, N.K.; Thakur, M.; Chakraborty, S.K.; Srivastav, P.P. Microwave heating: Alternative thermal process technology for food application. In Emerging Thermal and Nonthermal Technologies in Food Processing; Apple Academic Press: New York, NY, USA, 2020; pp. 25–27. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef] [PubMed]
- Marić, B.; Pavlić, B.; Čolović, D.; Abramović, B.; Zeković, Z.; Bodroža-Solarov, M.; Ilić, N.; Teslić, N. Recovery of high-content ω–3 fatty acid oil from raspberry (Rubus idaeus L.) seeds: Chemical composition and functional quality. LWT 2020, 130, 109627. [Google Scholar] [CrossRef]
- Aili, Q.; Cui, D.; Li, Y.; Zhige, W.; Yongping, W.; Minfen, Y.; Dongbin, L.; Xiao, R.; Qiang, W. Composing functional food from agro-forest wastes: Selectively extracting bioactive compounds using supercritical fluid extraction. Food Chem. 2024, 455, 139848. [Google Scholar] [CrossRef] [PubMed]
- Argun, M.E.; Argun, M.Ş. Recovery of valuable compounds from apricot concentrate production waste using supercritical carbon dioxide extraction as a green separation method. J. Food Meas. Charact. 2025, 19, 2395–2408. [Google Scholar] [CrossRef]
- Osorio-Tobon, J.F. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef]
- Lin, D.; Ma, Q.; Zhang, Y.; Peng, Z. Phenolic compounds with antioxidant activity from strawberry leaves: A study on microwave-assisted extraction optimization. Prep. Biochem. Biotechnol. 2020, 50, 874–882. [Google Scholar] [CrossRef]
- Wang, M.; Li, D.; Zang, Z.; Sun, X.; Tan, H.; Si, X.; Tian, J.; Teng, W.; Wang, J.; Liang, Q.; et al. 3D food printing: Applications of plant-based materials in extrusion-based food printing. Crit. Rev. Food Sci. Nutr. 2021, 62, 7184–7198. [Google Scholar] [CrossRef]
- Navaf, M.; Sunooj, K.V.; Aaliya, B.; Akhila, P.P.; Sudheesh, C.; Mir, S.A.; George, J. 4D printing: A new approach for food printing; effect of various stimuli on 4D printed food properties. A comprehensive review. Appl. Food Res. 2022, 2, 100150. [Google Scholar] [CrossRef]
- Pereira, T.; Barroso, S.; Gil, M.M. Food Texture Design by 3D Printing: A Review. Foods 2021, 10, 320. [Google Scholar] [CrossRef]
- Kokane, S.B.; Arora, V.K.; Thangalakshmi, S. Potential of Bioactive Ingredients for Healthy 4D Food Printing: Applications and Challenges. Food Rev. Int. 2024, 40, 3792–3815. [Google Scholar] [CrossRef]
- Niu, D.; Zhang, M.; Mujumdar, A.S.; Li, J. Investigation of 3D printing of toddler foods with special shape and function based on fenugreek gum and flaxseed protein. Int. J. Biol. Macromol. 2023, 253, 127203. [Google Scholar] [CrossRef] [PubMed]
- Nian, B.; Xu, Y.-J.; Liu, Y. Molecular dynamics simulation for mechanism revelation of the safety and nutrition of lipids and derivatives in food: State of the art. Food Res. Int. 2021, 145, 110399. [Google Scholar] [CrossRef] [PubMed]
- Pallante, L.; Cannariato, M.; Vezzulli, F.; Lambri, M.; Deriu, M.A. Artificial intelligence-driven prediction of taste perception, molecular mechanisms, and food molecule trajectory. Sci. Talks 2024, 10, 100353. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, Y.; Wu, Y.; Ren, F.; Liu, H. Exploring the application of molecular simulation technology in food sector: Focusing on food component interactions and food quality. Food Biosci. 2024, 62, 105480. [Google Scholar] [CrossRef]
- Prabhakar, P.; Mukherjee, S.; Kumar, A.; Kumar, S.; Verma, D.K.; Dhara, S.; Maiti, M.K.; Banerjee, M. Optimization of MAE for Carica papaya phytochemicals, and its in silico, in vitro, and ex vivo evaluation: For functional food and drug applications. Food Biosci. 2023, 54, 102861. [Google Scholar] [CrossRef]
- Lin, P.Y.; Huang, S.C.; Chen, K.L.; Huang, Y.C.; Liao, C.Y.; Lin, G.J.; Lee, H.; Chen, P.Y. Analysing protein complexes in plant science: Insights and limitation with AlphaFold 3. Bot. Stud. 2025, 66, 14. [Google Scholar] [CrossRef]
- Ellaway, J.I.J.; Anyango, S.; Nair, S.; Zaki, H.A.; Nadzirin, N.; Powell, H.R.; Gutmanas, A.; Varadi, M.; Velankar, S. Identifying protein conformational states in the Protein Data Bank: Toward unlocking the potential of integrative dynamics studies. Struct. Dyn. 2024, 11, 034701. [Google Scholar] [CrossRef]
- Bagnulo, E.; Strocchi, G.; Bicchi, C.; Liberto, E. Industrial food quality and consumer choice: Artificial intelligence-based tools in the chemistry of sensory notes in comfort foods (coffee, cocoa and tea). Trends Food Sci. Technol. 2024, 147, 104415. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Mujumdar, A.S.; Chen, Y. From farm to market: Research progress and application prospects of artificial intelligence in the frozen fruits and vegetables supply chain. Trends Food Sci. Technol. 2024, 153, 104730. [Google Scholar] [CrossRef]
- Alasi, S.O.; Sanusi, M.S.; Sunmonu, M.O.; Odewole, M.M.; Adepoju, A.L. Exploring recent developments in novel technologies and AI integration for plant-based protein functionality: A review. J. Agric. Food Res. 2024, 15, 101036. [Google Scholar] [CrossRef]
- Doherty, A.; Wall, A.; Khaldi, N.; Kussmann, M. Artificial Intelligence in Functional Food Ingredient Discovery and Characterisation: A Focus on Bioactive Plant and Food Peptides. Front. Genet. 2021, 12, 768979. [Google Scholar] [CrossRef] [PubMed]
- Brankovic, A.; Hendrie, G.A. Perspectives, challenges and future of artificial intelligence in personalised nutrition research. Proc. Nutr. Soc. 2025, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Torrico, D.D.; Mehta, A.; Borssato, A.B. New methods to assess sensory responses: A brief review of innovative techniques in sensory evaluation. Curr. Opin. Food Sci. 2023, 49, 100978. [Google Scholar] [CrossRef]
- van Erp, M.; Reynolds, C.; Maynard, D.; Starke, A.; Ibáñez Martín, R.; Andres, F.; Leite, M.C.A.; de Toledo, D.A.; Schmidt Rivera, X.; Trattner, C.; et al. Using Natural Language Processing and Artificial Intelligence to Explore the Nutrition and Sustainability of Recipes and Food. Front. Artif. Intell. 2021, 3, 621577. [Google Scholar] [CrossRef]
- Iheanacho, C.A.; Vincent, O.R. Classification and recommendation of food intake in West Africa for healthy diet using Deep Learning. In Proceedings of the 2022 5th Information Technology for Education and Development (ITED), Abuja, Nigeria, 1–3 November 2022; pp. 1–6. [Google Scholar]
- Limketkai, B.N.; Mauldin, K.; Manitius, N.; Jalilian, L.; Salonen, B.R. The Age of Artificial Intelligence: Use of Digital Technology in Clinical Nutrition. Curr. Surg. Rep. 2021, 9, 20. [Google Scholar] [CrossRef]
- Cabitza, F.; Campagner, A. The need to separate the wheat from the chaff in medical informatics: Introducing a comprehensive checklist for the (self)-assessment of medical AI studies. Int. J. Med. Inf. 2021, 153, 104510. [Google Scholar] [CrossRef]
- Zulkarnain, A.H.B.; Gere, A. Virtual reality sensory analysis approaches for sustainable food production. Appl. Food Res. 2025, 5, 100780. [Google Scholar] [CrossRef]
- Galmarini, M.V. The role of sensory science in the evaluation of food pairing. Curr. Opin. Food Sci. 2020, 33, 149–155. [Google Scholar] [CrossRef]
- Baker, M.T.; Lu, P.; Parrella, J.A.; Leggette, H.R. Consumer Acceptance toward Functional Foods: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 1217. [Google Scholar] [CrossRef]
- Mahmoud, A.B.; VanDyke, M.S.; Lee, N.M.; Abitbol, A.; Rush, S.W. How message appeals and prior product use influence information processing, risk perceptions, trust, attitudes, and genetic test purchase intentions. PLoS ONE 2023, 18, e0283102. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Z.; Kuang, J. Artificial Intelligence-Driven Recommendations and Functional Food Purchases: Understanding Consumer Decision-Making. Foods 2025, 14, 976. [Google Scholar] [CrossRef]
- Liew, Y.W.; Vafaei-Zadeh, A.; Teoh, A.P.; Ramayah, T. Predicting Public Willingness to Use Autonomous Shuttles: Evidence from an Emerging Economy. Transp. Res. Rec. J. Transp. Res. Board 2023, 2678, 736–757. [Google Scholar] [CrossRef]
- Carrete, L.; Arroyo, P.; Arroyo, A. Should Green Products Be Advertised as Green? Exploring the Factors That Affect Ad Credibility. J. Promot. Manag. 2023, 29, 427–460. [Google Scholar] [CrossRef]
- Begho, T.; Liu, S. Does social proof and herd behaviour drive food choices of consumers? Br. Food J. 2023, 126, 1050–1064. [Google Scholar] [CrossRef]
- Drake, M.A.; Watson, M.E.; Liu, Y. Sensory Analysis and Consumer Preference: Best Practices. Annu. Rev. Food Sci. Technol. 2023, 14, 427–448. [Google Scholar] [CrossRef]
- Gere, A.; Zulkarnain, A.H.B.; Szakál, D.; Fehér, O.; Kókai, Z. Virtual reality applications in food science. Current knowledge and prospects. Prog. Agric. Eng. Sci. 2021, 17, 3–14. [Google Scholar] [CrossRef]
- Szakos, D.; Ózsvári, L.; Kasza, G. Health-related nutritional preferences of older adults: A segmentation study for functional food development. J. Funct. Foods 2022, 92, 105065. [Google Scholar] [CrossRef]
- Sproesser, G.; Ruby, M.B.; Arbit, N.; Akotia, C.S.; dos Santos Alvarenga, M.; Bhangaokar, R.; Furumitsu, I.; Hu, X.; Imada, S.; Kaptan, G.; et al. Similar or different? Comparing food cultures with regard to traditional and modern eating across ten countries. Food Res. Int. 2022, 157, 111106. [Google Scholar] [CrossRef]
- Kroger, T.; Dupont, J.; Busing, L.; Fiebelkorn, F. Acceptance of Insect-Based Food Products in Western Societies: A Systematic Review. Front. Nutr. 2021, 8, 759885. [Google Scholar] [CrossRef]
- Omuse, E.R.; Tonnang, H.E.Z.; Yusuf, A.A.; Machekano, H.; Egonyu, J.P.; Kimathi, E.; Mohamed, S.F.; Kassie, M.; Subramanian, S.; Onditi, J.; et al. The global atlas of edible insects: Analysis of diversity and commonality contributing to food systems and sustainability. Sci. Rep. 2024, 14, 5045. [Google Scholar] [CrossRef] [PubMed]
- Michaelsen, M.M.; Esch, T. Understanding health behavior change by motivation and reward mechanisms: A review of the literature. Front. Behav. Neurosci. 2023, 17, 1151918. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, F.P.; Adamashvili, N.; Contò, F. Factors affecting consumer purchasing behavior of functional food: A comparative analysis for consumer management. Br. Food J. 2021, 124, 1519–1536. [Google Scholar] [CrossRef]
- Ares, G.; Song, J.; Brown, M.K.; Tan, M.; MacGregor, G.A.; Webster, J.; Campbell, N.R.C.; Trieu, K.; Ni Mhurchu, C.; Cobb, L.K.; et al. Impact of color-coded and warning nutrition labelling schemes: A systematic review and network meta-analysis. PLoS Med. 2021, 18, e1003765. [Google Scholar] [CrossRef]
- Nagy, L.B.; Lakner, Z.; Temesi, A. Is it really organic? Credibility factors of organic food-A systematic review and bibliometric analysis. PLoS ONE 2022, 17, e0266855. [Google Scholar] [CrossRef]
- Nystrand, B.T.; Olsen, S.O. Consumers’ attitudes and intentions toward consuming functional foods in Norway. Food Qual. Prefer. 2020, 80, 103827. [Google Scholar] [CrossRef]
- Abdullah; Liu, L.; Javed, H.U.; Xiao, J. Engineering Emulsion Gels as Functional Colloids Emphasizing Food Applications: A Review. Front. Nutr. 2022, 9, 890188. [Google Scholar] [CrossRef]
- Daher, D.; Deracinois, B.; Courcoux, P.; Baniel, A.; Chollet, S.; Froidevaux, R.; Flahaut, C. Sensopeptidomic Kinetic Approach Combined with Decision Trees and Random Forests to Study the Bitterness During Enzymatic Hydrolysis Kinetics of Micellar Caseins. Foods 2021, 10, 1312. [Google Scholar] [CrossRef]
- Delgado-Pando, G.; Ekonomou, S.I.; Stratakos, A.C.; Pintado, T. Clean Label Alternatives in Meat Products. Foods 2021, 10, 1615. [Google Scholar] [CrossRef]
- Balivo, A.; d’Errico, G.; Genovese, A. Sensory properties of foods functionalised with milk proteins. Food Hydrocoll. 2024, 147, 109301. [Google Scholar] [CrossRef]
- Tosif, M.M.; Bains, A.; Goksen, G.; Kaushik, R.; Chawla, P. Application of mucilage-based functional and clean-label food ingredients as vegan fat replacers in different food products. Curr. Opin. Food Sci. 2025, 62, 101275. [Google Scholar] [CrossRef]
- Zulhendri, F.; Perera, C.O.; Chandrasekaran, K.; Ghosh, A.; Tandean, S.; Abdulah, R.; Herman, H.; Lesmana, R. Propolis of stingless bees for the development of novel functional food and nutraceutical ingredients: A systematic scoping review of the experimental evidence. J. Funct. Foods 2022, 88, 104902. [Google Scholar] [CrossRef]
- Gupta, A.; Sanwal, N.; Bareen, M.A.; Barua, S.; Sharma, N.; Joshua Olatunji, O.; Prakash Nirmal, N.; Sahu, J.K. Trends in functional beverages: Functional ingredients, processing technologies, stability, health benefits, and consumer perspective. Food Res. Int. 2023, 170, 113046. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Huan, S.; Zhu, Y.; Chu, G.; McClements, D.J.; Rojas, O.J. Recent Advances in Food Emulsions and Engineering Foodstuffs Using Plant-Based Nanocelluloses. Annu. Rev. Food Sci. Technol. 2021, 12, 383–406. [Google Scholar] [CrossRef]
- Rane, B.R.; Keservani, R.K.; Shelar, M.Y.; Shukla, U.D.; Jain, A.S. Efficacy, Safety, and Toxicity of Nutraceutical Fruits. In Nutraceutical Fruits; Apple Academic Press: New York, NY, USA, 2025; pp. 285–317. [Google Scholar]
- Yuan, L.; Li, G.; Yan, N.; Wu, J.; Due, J. Optimization of fermentation conditions for fermented green jujube wine and its quality analysis during winemaking. J. Food Sci. Technol. 2021, 59, 288–299. [Google Scholar] [CrossRef]
- Das, A.K.; Nanda, P.K.; Madane, P.; Biswas, S.; Das, A.; Zhang, W.; Lorenzo, J.M. A comprehensive review on antioxidant dietary fibre enriched meat-based functional foods. Trends Food Sci. Technol. 2020, 99, 323–336. [Google Scholar] [CrossRef]
- Inguglia, E.S.; Song, Z.; Kerry, J.P.; O’Sullivan, M.G.; Hamill, R.M. Addressing Clean Label Trends in Commercial Meat Processing: Strategies, Challenges and Insights from Consumer Perspectives. Foods 2023, 12, 2062. [Google Scholar] [CrossRef]
- Functional Food Ingredients Market by Type (Probiotics, Proteins & Amino Acids, Phytochemicals & Plant Extracts, Prebiotics, Omega-3 Fatty Acids, Carotenoids, Vitamins), Application, Source, Form, Health Benefits, and Region—Global Forecast to 2029. Available online: https://www.marketsandmarkets.com/Market-Reports/functional-food-ingredients-market-9242020.html (accessed on 30 July 2025).
- Khasanov, A.R.; Matveeva, N.A.; Gruzd, A.A. The development of a functional adaptogenic beverage, using plant extracts of Centella asiatica and Hoodia gordonii. IOP Conf. Ser. Earth Environ. Sci. 2021, 640, 022092. [Google Scholar] [CrossRef]
- Arafa, A.; Kokubo, Y.; Kashima, R.; Teramoto, M.; Sakai, Y.; Nosaka, S.; Nakao, Y.M.; Watanabe, E. The Lifelong Health Support 10: A Japanese prescription for a long and healthy life. Environ. Health Prev. Med. 2022, 27, 23. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Q.; Suo, H.; Xu, F.; Huang, W.; Wang, D.O. Nattokinase as a functional food ingredient: Therapeutic applications and mechanisms in age-related diseases. Food Sci. Hum. Wellness 2024, 13, 2401–2409. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, F.; Wang, J.; Pan, M.; Gao, Q.; Long, J.; Li, X. Nattokinase Supplementation and Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Rev. Cardiovasc. Med. 2023, 24, 234. [Google Scholar] [CrossRef]
- FDA. Dietary Supplements. Available online: https://www.fda.gov/food/dietary-supplements (accessed on 1 August 2025).
- Domínguez Díaz, L.; Fernández-Ruiz, V.; Cámara, M. An international regulatory review of food health-related claims in functional food products labeling. J. Funct. Foods 2020, 68, 103896. [Google Scholar] [CrossRef]
- European Food Safety Authority. Food Supplements. Available online: https://www.efsa.europa.eu/en/topics/topic/food-supplements (accessed on 2 August 2025).
- Sato, K.; Kodama, K.; Sengoku, S. The Co-Evolution of Markets and Regulation in the Japanese Functional Food Industry: Balancing Risk and Benefit. Foods 2025, 14, 1581. [Google Scholar] [CrossRef] [PubMed]
- Intrasook, J.; Tsusaka, T.W.; Anal, A.K. Trends and current food safety regulations and policies for functional foods and beverages containing botanicals. J. Food Drug Anal. 2024, 32, 112–139. [Google Scholar] [CrossRef]
- Pilolli, R.; Nitride, C.; Gillard, N.; Huet, A.-C.; van Poucke, C.; de Loose, M.; Tranquet, O.; Larré, C.; Adel-Patient, K.; Bernard, H.; et al. Critical review on proteotypic peptide marker tracing for six allergenic ingredients in incurred foods by mass spectrometry. Food Res. Int. 2020, 128, 108747. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
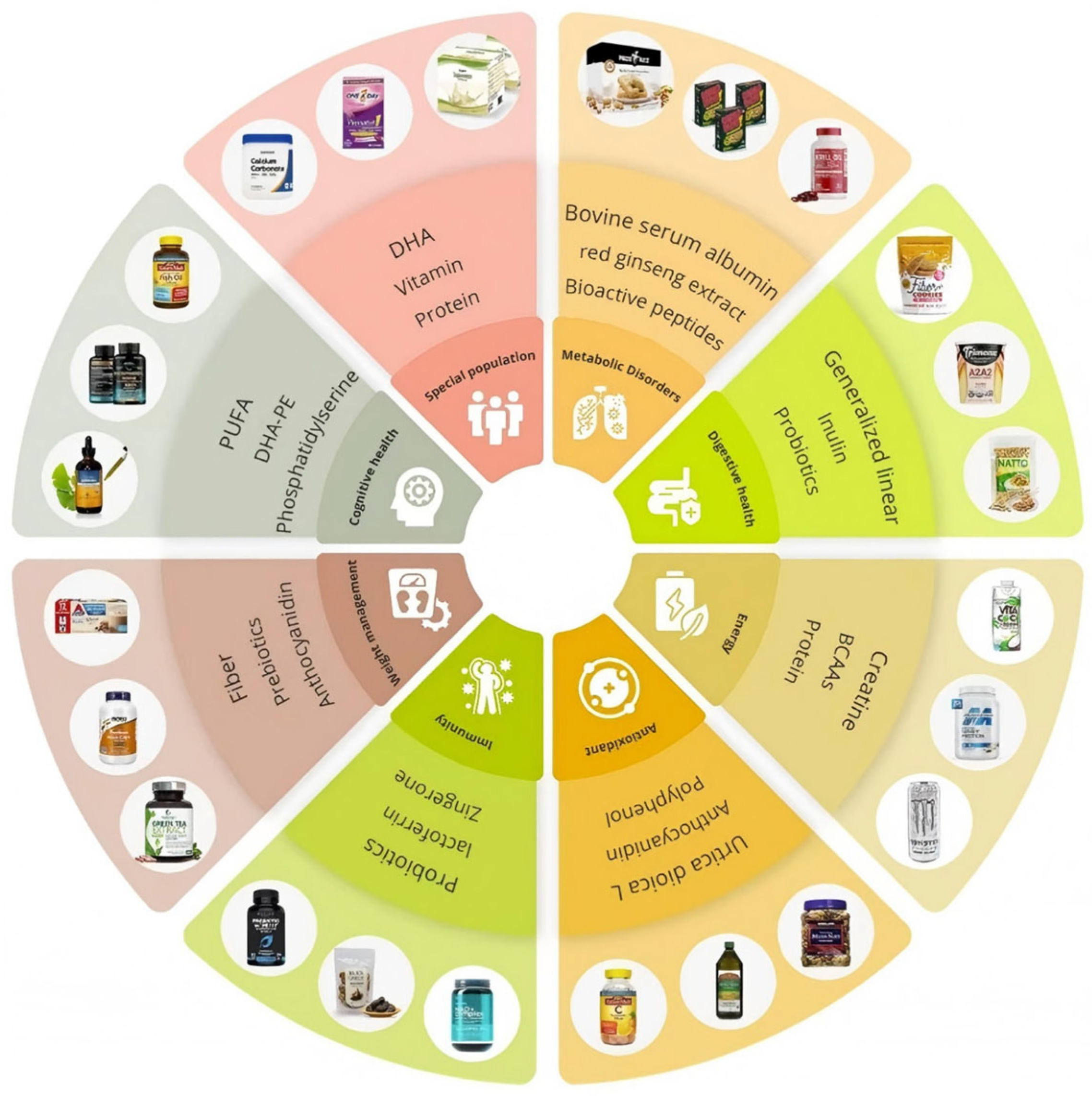

| Type | Type | Functional Ingredient | Source | Principal Biological Activities | Key Evidence/Outcomes | Reference |
|---|---|---|---|---|---|---|
| Carbohydrate-based Functional Ingredients | Dietary fiber | Psyllium husk (Plantago ovata) | Seed coat of P. ovata | Modulates bowel function and alleviates IBS pain | A 6-week double-blind RCT in 88 children showed psyllium reduced pain-episode frequency in boys versus placebo, with no effect in girls | [28,29] |
| Non-starch polysaccharides (cellulose, hemicellulose, pectin) | Multiple plant tissues | Improves glycemic and lipid profiles, modulates gut microbiota, lowers CVD and T2DM risk | Short-chain fatty acid–mediated gene regulation and anti-inflammatory effects observed | [16,18] | ||
| Curry-leaf polysaccharides (Murraya koenigii) | Leaves | Restores gut barrier function, regulates SCFA profile, exhibits anti-obesity effects | Alkaloid fraction from M. koenigii alleviated HFD-induced obesity and insulin resistance in mice | [23] | ||
| Prebiotic | Designed dietary-fiber prebiotic | Synthetic soluble fiber blend | Enhances SCFA-producing gut bacteria, improves barrier function, reduces inflammation | 10-day clinical study (20 g/day) in Parkinson’s patients decreased zonulin and plasma NfL levels | [32] | |
| Enzymatically modified pea-peel fiber | Pea by-product | High-solubility DF with prebiotic potential; increased SCFA in vitro | Boost cell biomass and relative growth rate of probiotic bacteria | [31] | ||
| Protein-based Functional Ingredients | Proteins and Peptides | Bovine serum albumin (BSA) | Whey | Bovine colostrum whey powder, Amino acids | Whey-derived products offer bioactive peptides, improved functionalities, and diverse food and health applications through optimized processing techniques | [83] |
| Food-derived bioactive peptides | Insects, seaweed, and oilseed by-products | ACE inhibition, antihypertensive, antioxidant, immunomodulatory | Emerging protein-derived hydrolysates show promise for managing hypertension, diabetes, obesity, and neurodegeneration | [84] | ||
| Alternative proteins (Tenebrio molitor, algal protein) | microalgae | Antioxidant and antimicrobial effects | Offer high-protein, amino acid–rich biomass; functional peptides; and sustainable extraction methods for food and pharma applications | [85] | ||
| Fat-based Functional Ingredients | ω-3 PUFA | EPA + DHA (fish oil) | Marine lipids | Lowers triglycerides, reduces inflammation, improves mitochondrial function | Improves lipid profiles, insulin sensitivity, blood pressure, liver function, inflammation, oxidative stress, and mitochondrial performance, and may reduce sudden cardiovascular event risk in diet-related disorders | [45] |
| EPA vs. DHA mechanistic differences | Steaming and baking in foil on the retention of EPA and DHA in three fish species | EPA superior in anti-depressant effect; DHA stronger for insulin resistance via GPR120/PPARγ | Steaming fish at 100 °C preserves significantly more EPA and DHA, with retention up to about 60% in Indian mackerel and over 500 mg per 100 g total EPA + DHA in steamed Indian scad, compared to lower retention when baking in foil at 160 °C | [49] | ||
| Other Functional Ingredients | Probiotic | Lactobacillus plantarum formulation with isatin | L. plantarum culture + Couroupita guianensis fruit isatin | Antioxidant, antimicrobial, and cholesterol-lowering | In vitro and oral-fluid model showed 67% LDL-C reduction and 98.8% probiotic survivability | [53] |
| Common probiotic genera (Lactobacillus, Bifidobacterium, S. boulardii) | Fermented foods (yogurt, kimchi, kefir) | Strengthens mucosal barrier, increases IgA, and reduces intestinal inflammation | Gut health by enhancing barrier function, immunity and reducing inflammation; Bifidobacterium raises IgA levels and cuts harmful bacteria in the gut | [54] | ||
| Phenolic compound | 6-Gingerol | Ginger rhizome | Provides hepatoprotective and anti-inflammatory effects | In DEN-induced liver-injury rats, 50 mg/kg reduced ALT, AST, and ALP, and mitigated tissue damage | [65] | |
| Zingerone | Ginger rhizome | Antioxidant, anti-inflammatory, anti-apoptotic | Zingerone pretreatment in lead-exposed rats improved antioxidant enzymes and protected organs | [66] | ||
| Quercetin, catechins | Tea, fruit, veg. | Geroprotective; interact with ageing pathways | Polyphenol-rich diets of Blue Zone centenarians may promote healthy ageing and longevity by targeting conserved biological mechanisms to reduce age-related disease risk | [64] | ||
| Manuka honey polyphenols | Manuka honey | Antimicrobial, antioxidative, wound healing | Demonstrated antioxidant, anti-inflammatory, immunomodulatory, antimicrobial, and anticancer effects | [86] | ||
| Combination therapy | Red-ginseng extract (RGEP) + red-ginseng dietary fiber (RGDF) | Panax ginseng root and fiber | Gut-barrier protection; anti-inflammatory | RGEP and RGDF supplementation in DIO mice (4–8 weeks) reduced markers of inflammation and intestinal permeability, including α-1-antitrypsin, CRP, iNOS, NF-κB, MPO, calprotectin, urinary indican, and β-glucuronidase | [72] | |
| Cranberry polyphenols + EPA/DHA | Cranberry juice (200 mL) + fish-oil capsule (180 mg EPA + 120 mg DHA, BID) | Glycemic control; cardiometabolic and periodontal benefit | An 8-week study in diabetic individuals with periodontitis showed decreased HbA1c, increased HDL-C, and better periodontal indices | [77] |
| Theme/Tool | Main Purpose | Core Mechanism and Key Insight | Representative Bioactives Involved | Typical Food or R&D Application | Key Reference |
|---|---|---|---|---|---|
| Bioavailability fundamentals | Disease prevention and therapeutic support | Modulate inflammation, oxidative stress, lipid metabolism, gene expression; exert antimicrobial and anticancer effects | Polyphenols, carotenoids, glucosinolates, terpenes, alkaloids, ω-3 PUFA, CLA, chitosan, probiotics, marine bioactives | Functional foods, dietary supplements, drug development | [87] |
| Fermentation of dietary fiber | Enhances the release of bound phenolics and increases their solubility | Microbial enzymes cleave cell wall matrices, creating shorter, more fermentable fragments | Sweet-potato, cereal, or legume DF | Fermented DF powders for gut-health beverages | [90] |
| Micronization of DF | The increased surface area promotes greater release and absorption of polyphenols and carboxyl groups | Jet-milling reduces particle size (<50 µm), exposes functional moieties | Fruit and veg by-product fibers | High-fiber smoothies, bakery powders | [13] |
| Excipient foods | Act as co-ingested carriers that enhance uptake of lipophilic or labile actives | Add digestible lipids, binding proteins, or permeability enhancers to promote micellization and protect against oxidation | Curcumin, quercetin, carotenoids, CoQ10 | “Bioavailability-boost” snack bars, shots | [96] |
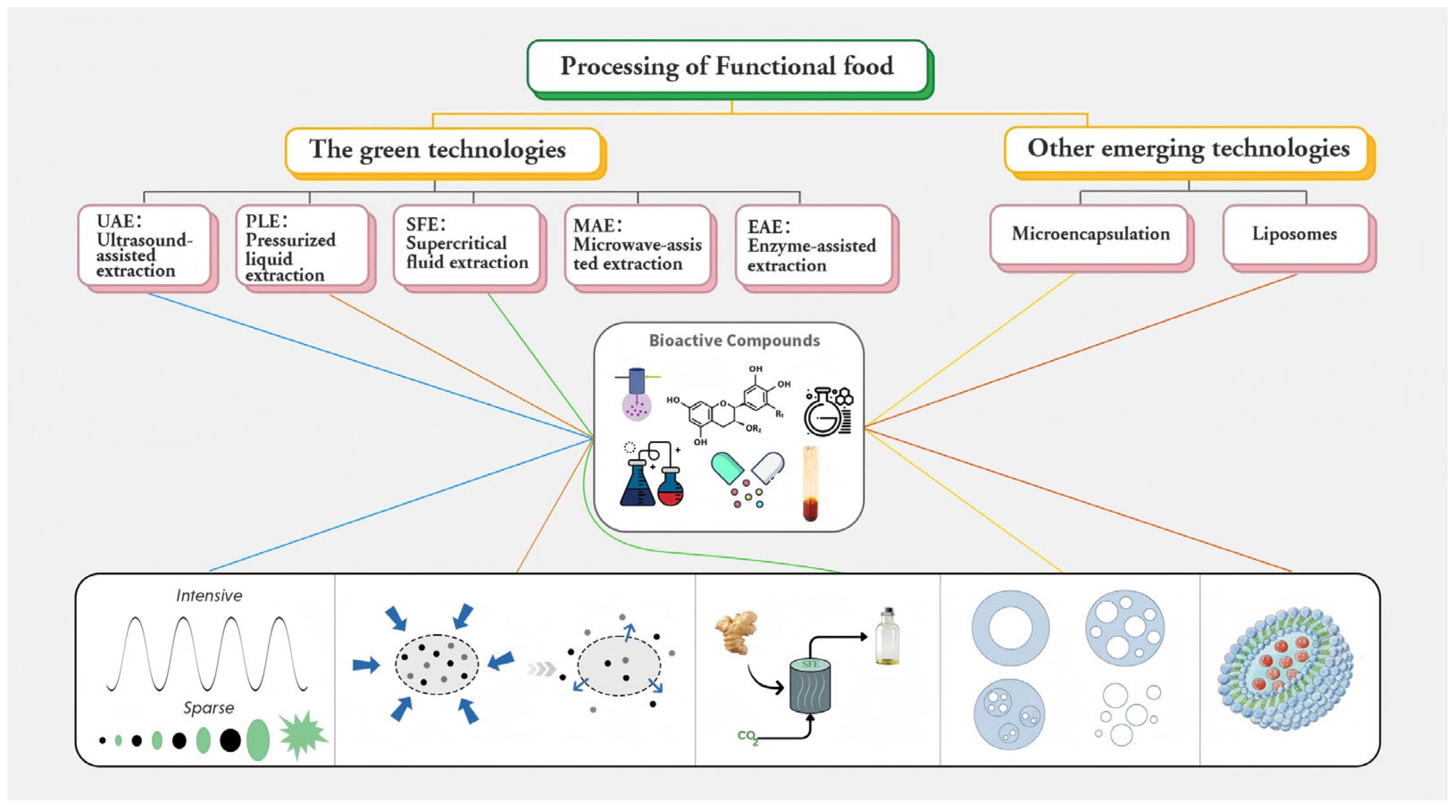
| Green extraction (UAE, MAE, EAE, PLE, SFE) | Obtains high-purity actives while lowering solvent, time and thermal damage | Ultrasound/microwave cavitation, enzyme hydrolysis, pressurized liquids, supercritical CO2 | Polyphenols, carotenoids, essential oils, peptides | Clean-label extracts for functional beverages, gummies, capsules | [123] |
| Supercritical CO2 (SFE) | Green extraction of raspberry seed oil e | SFE achieved comparable yield to Soxhlet, but higher ω–3 FA content; optimized via Box–Behnken design; better oil quality and full material exhaustion | ω–3 fatty acids, unsaturated fatty acids, tocopherols | Clean-label lipid ingredients for functional foods, nutraceutical oils | [132] |
| Microencapsulation | Enhances probiotic stability and viability during processing and digestion | Provides a protective matrix that shields probiotics from heat, oxygen, and acidity; enables targeted release in the GI tract | Lactobacillus, Bifidobacterium strains | Functional dairy foods (e.g., pasta filata cheese), dietary supplements | [104] |
| Enzymatic hydrolysis | Production of functional food ingredients | Catalyzing the hydrolysis of peptide bonds and releasing functional food components such as bioactive peptides | Peptide | Dairy, meat, plant, seafood | [113] |
| Nano-vesicular carriers (NVCs) | Improve stability, bioavailability, and functionality of bioactives in foods | NVCs (e.g., liposomes, niosomes, phytosomes, and transfersomes) enhances antioxidant capacity, control release at various pH/storage conditions, reduce cytotoxicity, improve digestibility, mask taste/smell, inhibit biofilm gene expression, and maintains sensory properties in fortified foods | Anthocyanins, d-limonene, tannic acid, ω-3 fatty acids, iron | Omega-3 fortified juices, powdered drink mixes; Functional foods with antioxidant, anti-inflammatory, or sensory-sensitive bioactives | [102] |
| 3D/4D food printing | Personalized nutrition; time-dependent shape or flavor change | Extrusion of stimuli-responsive biopolymers; programmable geometry | Fenugreek-gum + flax protein toddler snacks; protein-rich insect pastes | Tailor-made textures for dysphagia, space-saving packaging | [141] |
| Molecular simulation (MS) | In silico prediction of binding, stability, ADME–Tox | Atomistic docking and MD reveal enzyme inhibition, receptor targeting | C. papaya polyphenols vs. α-amylase/α-glucosidase | Accelerates lead-ingredient screening before wet lab | [144] |
| Artificial intelligence (AI/ML) | Accelerate ingredient development for improved sensory quality | Use of AI models to classify compounds by taste category and uncover structural determinants of taste; supports predictive organoleptic profiling | Mediterranean bioactives, phytochemicals, synthetic flavorants | AI-enabled R&D for functional food, nutraceuticals, and reformulated products | [143] |
| AI-driven fraud detection | Identify food adulteration and preserve consumer trust | Combines chemical fingerprints with machine learning to detect anomalies or counterfeit patterns; addresses issues driven by financial motives and geopolitical instability in supply chains | Authentication markers (e.g., isotopic, molecular) | Anti-fraud testing for raw/processed coffee, cocoa, and tea | [148] |
| AI and digital tools | Enable intelligent ingredient discovery and process control | AI predicts protein–function relationships, identifies health-promoting molecules, supports real-time quality monitoring through sensor-integrated digital platforms | Predictive models for peptides, flavor precursors | Smart formulation tools, health-enhancing plant-based foods | [150] |
| Focus Area | Core Insight | Practical Implication for Product Developers | Reference |
|---|---|---|---|
| Advanced sensory evaluation tool-box | Traditional discrimination, descriptive, and hedonic tests are now complemented by biometrics (facial EMG, HR, GSR, eye-tracking), virtual/augmented reality (VR/AR) and electronic-nose/e-tongue systems. | Combines objective physiological data with self-report, allows testing in immersive, context-rich environments and shortens time-to-insight. | [153] |
| VR-enabled panels | VR can synchronously manipulate sight, sound, odor, haptics, and taste. Immersive scenes reshape liking and purchase intent. | Prototype “farm-to-table” or “carbon-neutral” contexts to quantify eco-premium consumers are willing to pay. | [158] |
| Bitterness of bioactive peptides | 5–6 human T2R receptors respond strongly to food-derived peptides; bitterness limits acceptance. | Spray-dry encapsulation with maltodextrin/cyclodextrin, exopeptidase trimming, or QSAR-guided sequence selection masks bitterness while preserving bioactivity. | [7] |
| Flavor pairing science | Most foods/beverages are co-consumed; sensory synergy or suppression drives overall liking. | Optimize functional beverages with compatible carriers (e.g., plant sterol juice + citrus notes) to avoid off-flavors. | [159] |
| Sociodemographic drivers | Age, gender, education, household type, nationality, and marital status modulate acceptance. Nordic and Finnish consumers show higher readiness than US/Danish counterparts; Chinese > German for health-claim products. | Segmentation is essential—tailor message/format to demographic clusters. | [160] |
| Message framing in DTC genetic testing | Message features (e.g., sidedness, hedging) and prior experience with genetic tests significantly affect trust, information processing, risk perception, and attitudes. | To boost purchase intention, companies should enhance trust in both message and brand by using two-way refutational messaging and hedging, especially for experienced users. | [161] |
| Health motivation and self-efficacy | Preventive-health orientation, self-efficacy and self-esteem are top internal motivators. | Emphasize tangible health outcomes (e.g., cholesterol reduced by X%) and empower consumers with usage guidance. | [162] |
| Adoption of autonomous shuttles | Perceived usefulness and enjoyment drive perceived value, which predicts adoption intention; perceived risk has no significant effect. | Developers should prioritize enhancing perceived usefulness and enjoyment of autonomous shuttles to increase public adoption, especially in emerging markets. | [163] |
| Green advertising credibility | Eco-brand familiarity boosts ad credibility when the product is inherently green; green purchasing orientation can lower ad credibility, especially for low-cost items. | To strengthen ad effectiveness, brands should build familiarity and launch truly green products; avoid overemphasis on claims for low-cost items that may trigger skepticism. | [164] |
| Social proof and herd behavior in food choice | Social proof (e.g., reviews/ratings) significantly influences food choices, while herd behavior and influential sources have limited effect; health preference can override social influence. | Use consumer reviews/ratings as persuasive tools to promote healthy products; focus less on influencer marketing and more on visible peer-generated feedback. | [165] |
| Theme | Core Focus | Key Technical/Nutritional Insight | Market Relevance | Reference |
|---|---|---|---|---|
| Functional food categories | Functional meat, beverages, dairy, fruit products and snacks (biscuits, yoghurts, cereals) | Demonstrates the breadth of matrices that can deliver bioactives without compromising sensory quality | Signals continued diversification of “every-day” products carrying health claims | [177] |
| Bioactive peptides | Peptides from diverse raw materials | Several peptides already commercialized; incorporated into bars, drinks, dairy | Peptide-fortified foods add scientifically validated benefits beyond basic nutrition | [178] |
| Antioxidant dietary fiber (ADF) in meat | Plant by-products rich in DF + polyphenols | ADF improves fiber content and retards oxidative spoilage in meat | Meets demand for “clean-label” meat products with added health halo | [179] |
| Milk-protein functionalization | Whey/casein combined with polyphenols or volatiles | Protein–polyphenol nanoparticles and whey-based emulsions enhance delivery, mask bitterness | Customizable protein systems widen application in high-protein functional foods | [180] |
| Mucilage as vegan fat replacer | Plant-derived hydrophilic polymer (chia, basil, okra, etc.) | 7.5% chia mucilage yogurt reduced syneresis, preserved texture, and offered dietary fiber-related benefits on cholesterol and glycemic control | Clean-label, sustainable, allergen-free alternative to dairy or animal fat | [181] |
| Propolis in confectionery | Stingless-bee propolis chewing gum | Clinical studies in children demonstrated a reduction in Streptococcus mutans biofilm formation, an increase in salivary calcium and phosphate concentrations, and a decrease in dental plaque accumulation | Demonstrates delivery platform for antimicrobial phytochemicals; opens oral-health snacking niche | [182] |
| Plant-based functional beverages | Coconut water, maple sap, minimally processed drinks | Consumers prefer high bioavailability of native phytochemicals | Growth of “natural and barely processed” beverage segment in US/CA/EU | [183] |
| Engineered food colloids | Nano-/micro-structured emulsions, nanocellulose | Enables healthier, tastier, safer, more sustainable foods | Colloidal design tackles reformulation challenges (fat, salt, sugar reduction) | [184] |
| Berries as nutraceuticals | Polyphenol-rich berries | High convenience and palatability | Rising demand for ready-to-eat antioxidant sources | [185] |
| Clean-label plant beverages | Coconut water, maple sap, low-processed juices | “Raw” nutrient profile, natural antioxidants retained | Younger consumers value authenticity, low processing, and combined energy-drink formats | [186] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Ju, W.-M.; Wang, L.-L.; Ye, Y.-B.; Liu, Z.-Y.; Cavender, G.; Sun, Y.-J.; Sun, S.-Q. Functional Ingredients: From Molecule to Market—AI-Enabled Design, Bioavailability, Consumer Impact, and Clinical Evidence. Foods 2025, 14, 3141. https://doi.org/10.3390/foods14173141
Zhao L, Ju W-M, Wang L-L, Ye Y-B, Liu Z-Y, Cavender G, Sun Y-J, Sun S-Q. Functional Ingredients: From Molecule to Market—AI-Enabled Design, Bioavailability, Consumer Impact, and Clinical Evidence. Foods. 2025; 14(17):3141. https://doi.org/10.3390/foods14173141
Chicago/Turabian StyleZhao, Lei, Wen-Ming Ju, Lin-Lin Wang, Yu-Bin Ye, Zheng-Yang Liu, George Cavender, Yong-Jun Sun, and Sheng-Qian Sun. 2025. "Functional Ingredients: From Molecule to Market—AI-Enabled Design, Bioavailability, Consumer Impact, and Clinical Evidence" Foods 14, no. 17: 3141. https://doi.org/10.3390/foods14173141
APA StyleZhao, L., Ju, W.-M., Wang, L.-L., Ye, Y.-B., Liu, Z.-Y., Cavender, G., Sun, Y.-J., & Sun, S.-Q. (2025). Functional Ingredients: From Molecule to Market—AI-Enabled Design, Bioavailability, Consumer Impact, and Clinical Evidence. Foods, 14(17), 3141. https://doi.org/10.3390/foods14173141





