New Functional Food for the Treatment of Gastric Ulcer Based on Bioadhesive Microparticles Containing Sage Extract: Anti-Ulcerogenic, Anti-Helicobacter pylori, and H+/K+-ATPase-Inhibiting Activity Enhancement
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Plant Material
2.1.2. Chemical Reagents
2.2. Preparation and Characterization of the Extract
2.2.1. Soxhlet Extraction of Bioactive Molecules
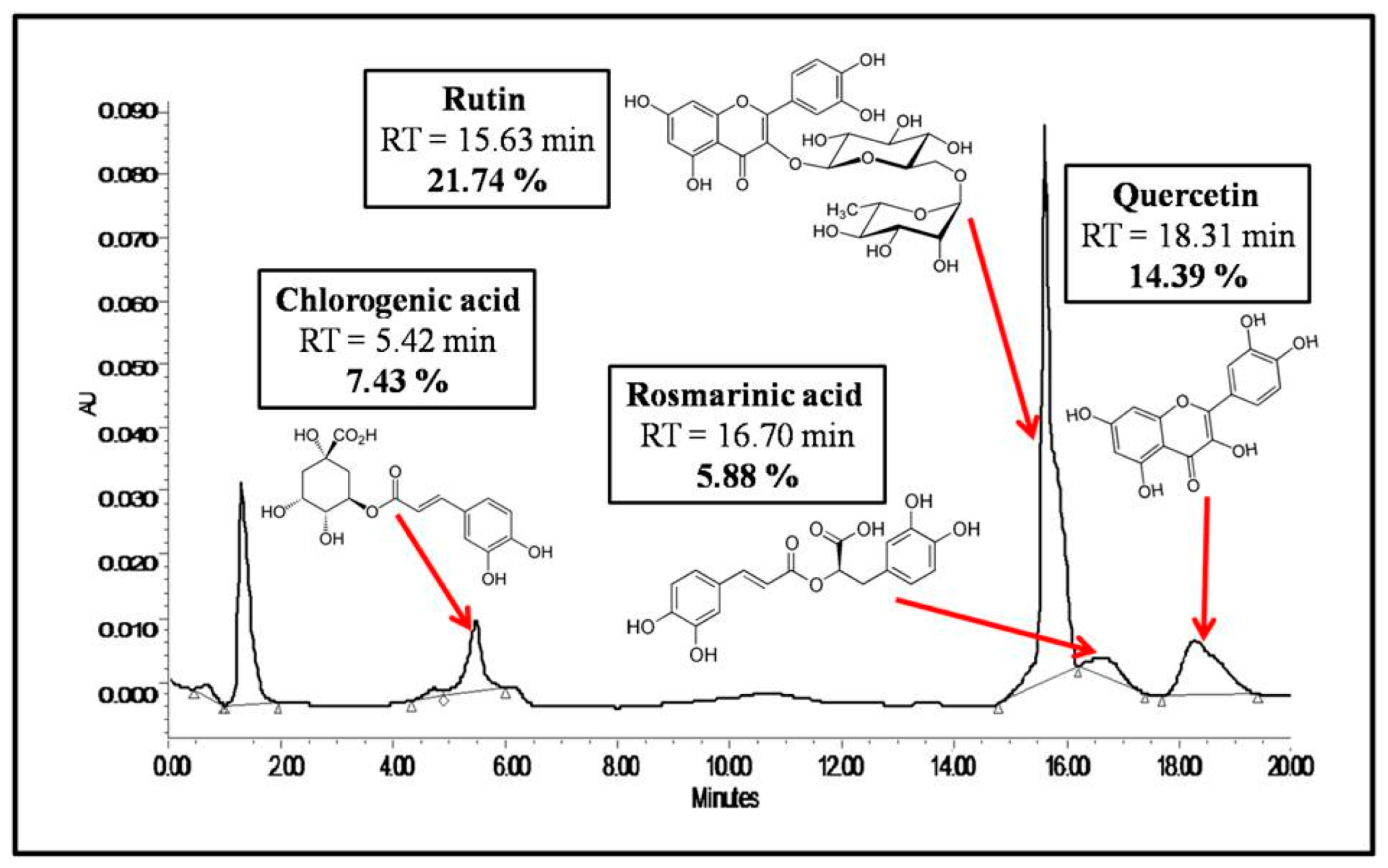
2.2.2. HPLC-UV/DAD Analysis of the Extract
2.3. Preparation and Characterization of Microcapsules
2.3.1. Preparation of Microcapsules
2.3.2. Encapsulation Rate
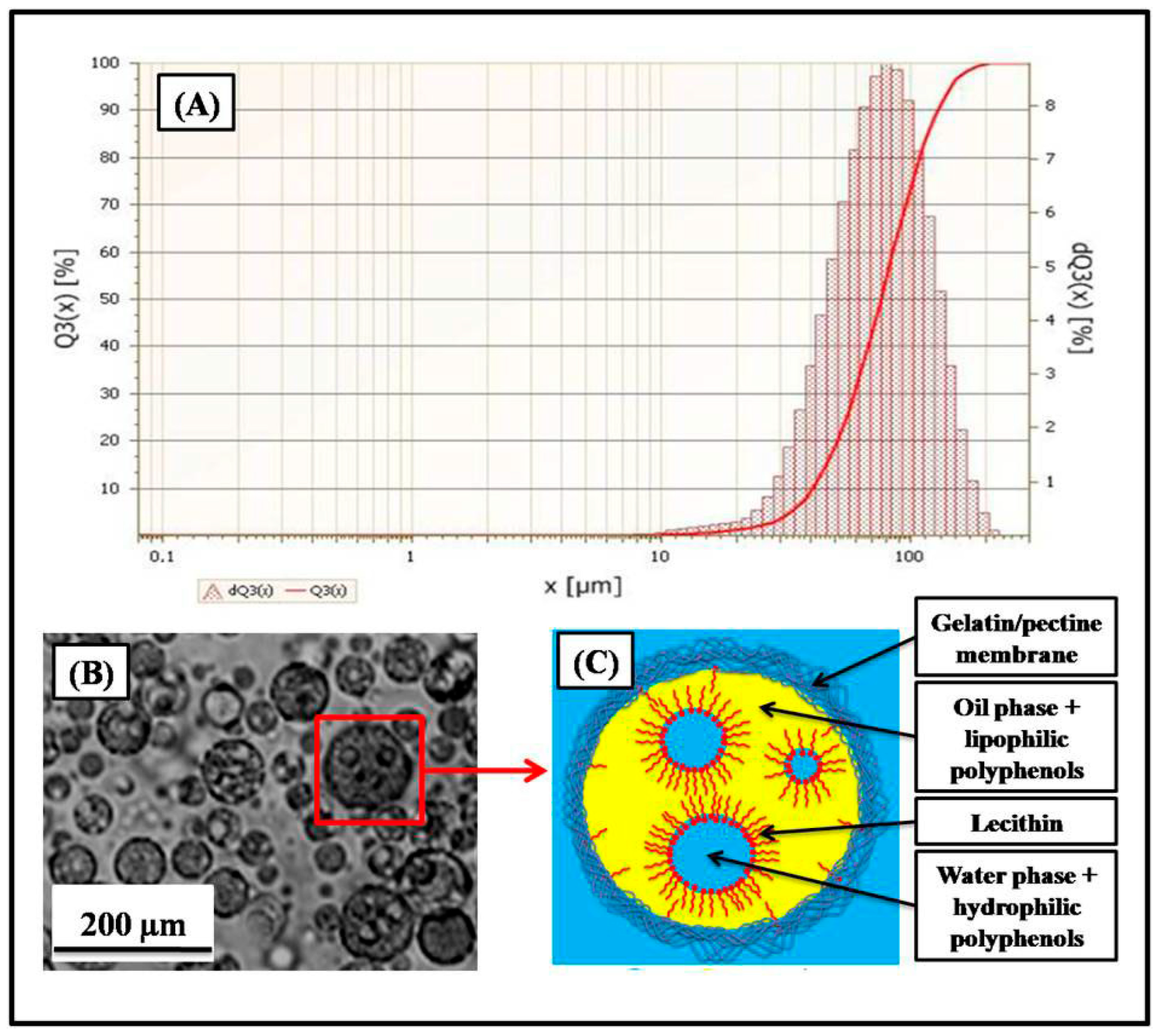
2.3.3. Particle Size Analysis
2.3.4. Microscopic Observation
2.3.5. In Vitro Dissolution Kinetics Study
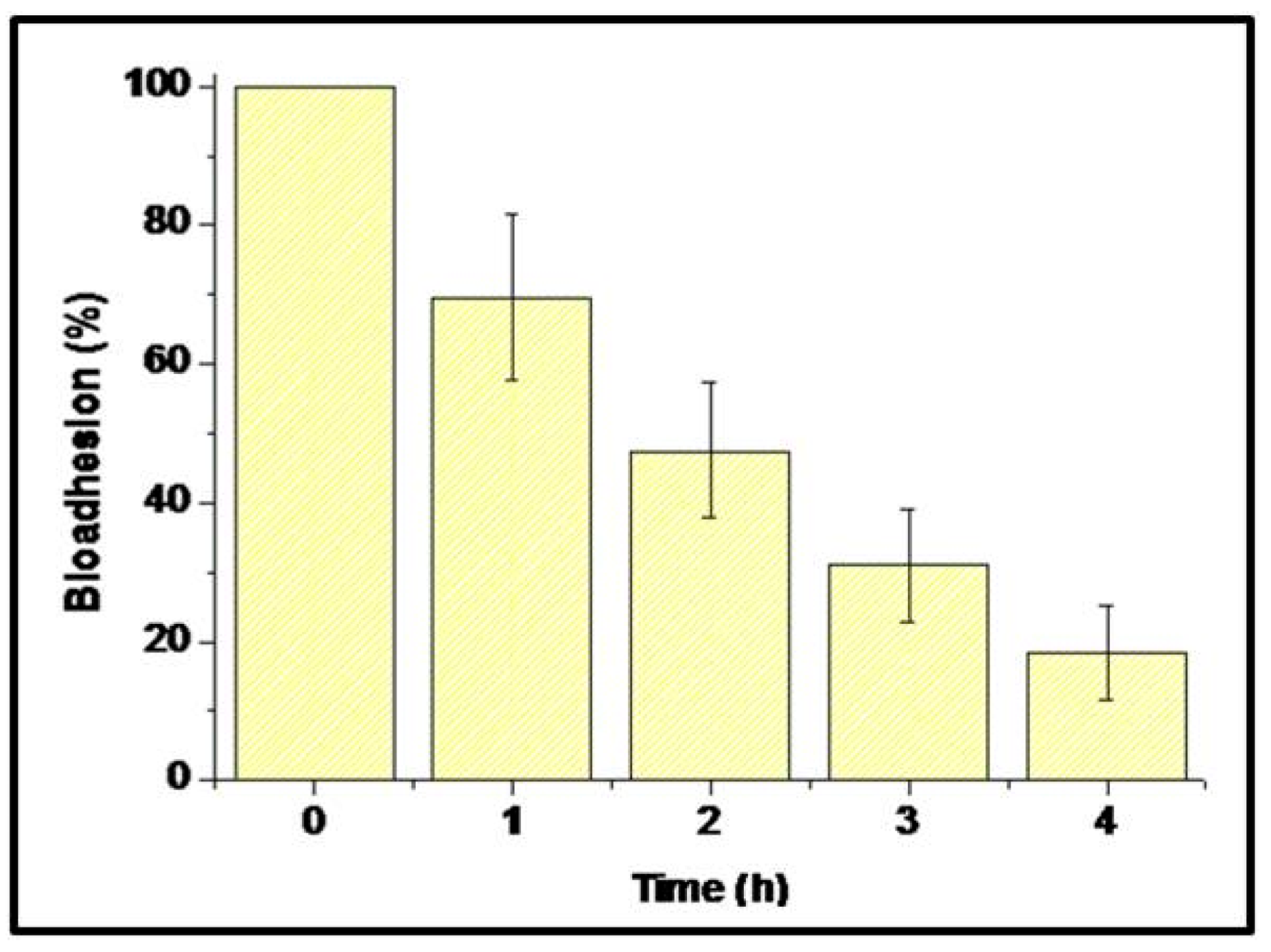
2.3.6. Measurement of Ex Vivo Bioadhesion:
2.4. Evaluation of Biological Activities
2.4.1. In Vivo Evaluation of Anti-Ulcerogenic Activity
Animal Material and Experimental Design
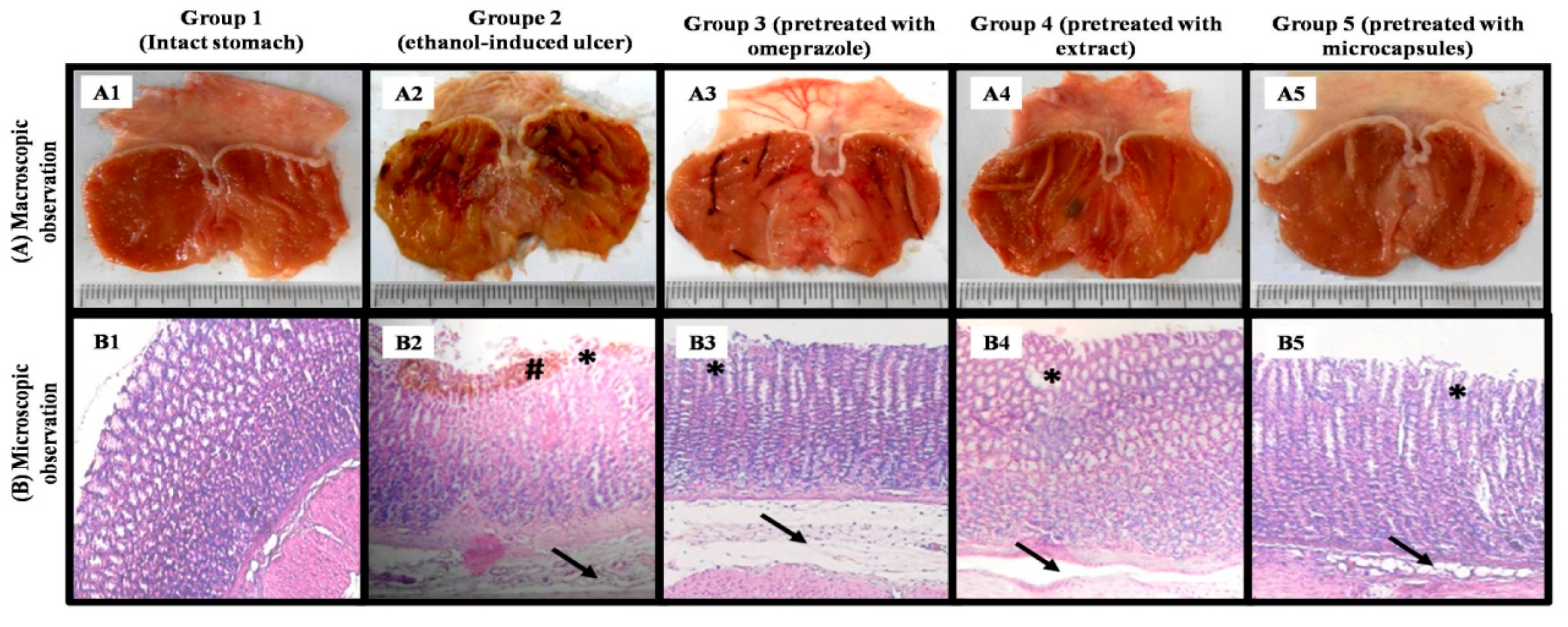
Determination of Ulcer and Inhibition Percentages
Histopathological Study
- Epithelial desquamations.
- Destruction of glands.
- Swelling of the sub-mucous membrane.
- Infiltration of eosinophils.
- Bleeding.
2.4.2. H+/K+-ATPase-Inhibiting Activity
Isolation of H+, K+ ATPase Enzyme
Determination of H+, K+ ATPase-Inhibiting Activity
2.4.3. Anti-Helicobacter pylori Activity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of the Extract
3.2. Preparation and Characterization of Microcapsules
3.2.1. Encapsulation Rate and Structure of Obtained Microcapsules
3.2.2. In Vitro Dissolution Kinetics
3.2.3. Evaluation of Ex Vivo Bioadhesion
3.3. Evaluation of Biological Activities
3.3.1. In Vivo Evaluation of Anti-Ulcerogenic Activity
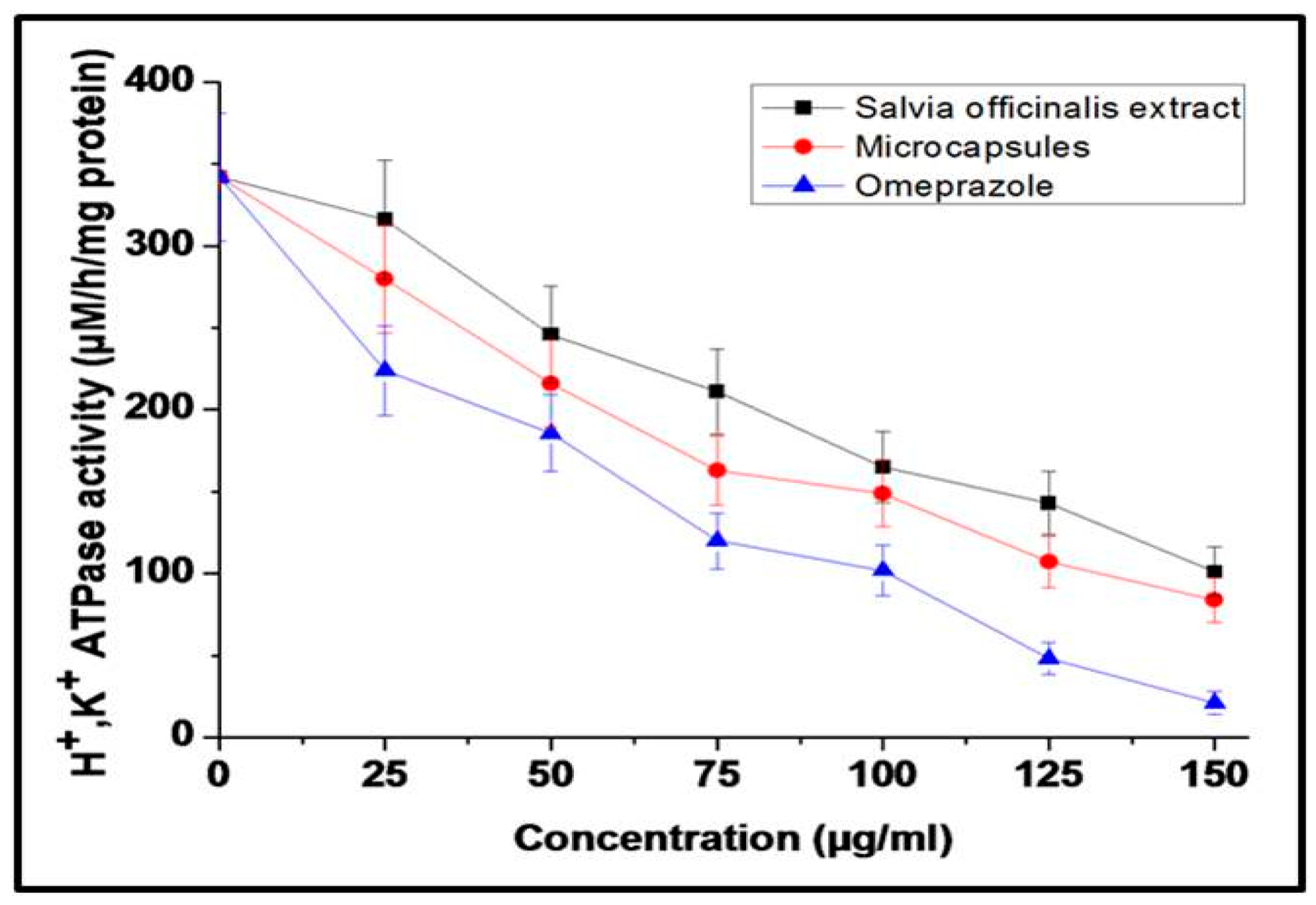
3.3.2. Evaluation of H+/K+ ATPase-Inhibiting Activity
3.3.3. Evaluation of Anti-Helicobacter pylori Activity
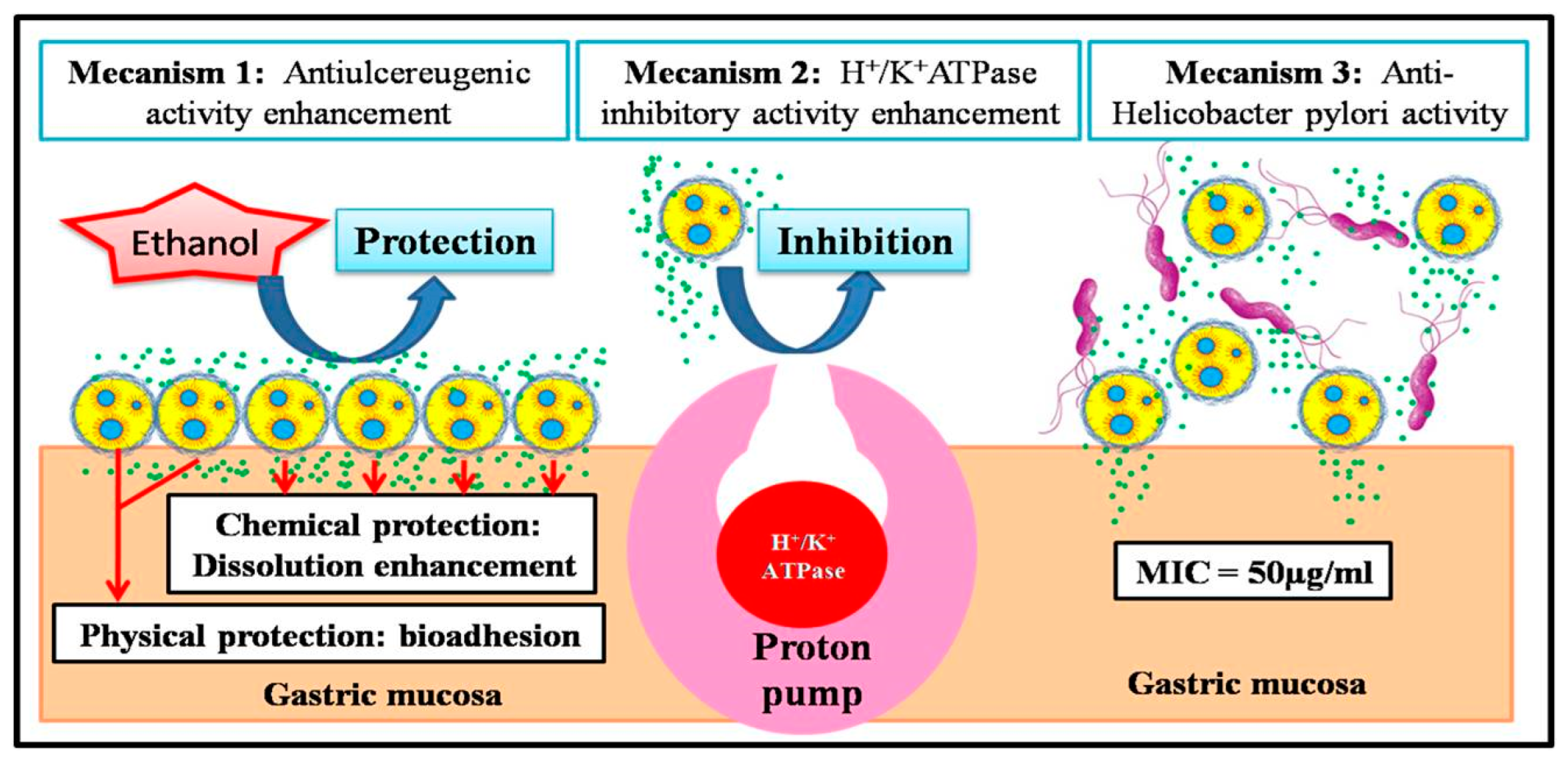
3.4. Discussion of Biological Activities and Biopharmaceutical Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Graham, D.Y. History of Helicobacter pylori, Duodenal Ulcer, Gastric Ulcer and Gastric Cancer. World J. Gastroenterol. 2014, 20, 5191–5204. [Google Scholar] [CrossRef] [PubMed]
- Shiotani, A.; Graham, D.Y. Pathogenesis and Therapy of Gastric and Duodenal Ulcer Disease. Med. Clin. N. Am. 2002, 86, 1447–1466. [Google Scholar] [CrossRef] [PubMed]
- Nabi, I.; Megateli, I.; Nait Bachir, Y.; Djellouli, S.; Hadj-Ziane-Zafour, A. Effect of Stevia and Pectin Supplementation on Physicochemical Properties, Preservation and In-vivo Hypoglycemic Potential of Orange Nectar. J. Food Process. Preserv. 2021, 45, e15124. [Google Scholar] [CrossRef]
- Labdi, A.; Amiali, M.; Nait Bachir, Y.; Merouane, A.; Dahman-Zouambi, A.; Koceir, E.A.; Bitam, A. Green Tea Extract Attenuates Non Alcoholic Fatty Liver Disease by Decreasing Hyperlipidemia and Enhancing Superoxide Dismutase Activity in Cholesterol-Fed Rats. Med. J. Nutr. Metab. 2018, 11, 295–306. [Google Scholar] [CrossRef]
- Nabi, I.; Nait Bachir, Y.; Djellouli, S.; Smain, M.; Hadj-Ziane-Zafour, A. In Vivo Antidiabetic Effect and Antioxidant Potential of Stevia Rebaudiana Mixed with Tragacanth Gum in Orange Nectar. Food Hydrocoll. Health 2023, 4, 100147. [Google Scholar] [CrossRef]
- Nait Bachir, Y. Natural Drugs for the Treatment of Severe Acute Respiratory Syndrome Coronaviruses Infections (SARS-CoV and COVID-19). In Coronavirus Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 73–153. [Google Scholar]
- Wu, Y.B.; Ni, Z.Y.; Shi, Q.W.; Dong, M.; Kiyota, H.; Gu, Y.C.; Cong, B. Constituents from Salvia Species and Their Biological Activities. Chem. Rev. 2012, 112, 5967–6026. [Google Scholar] [CrossRef]
- Hamidpour, M.; Hamidpour, R.; Hamidpour, S.; Shahlari, M. Chemistry, Pharmacology, and Medicinal Property of Sage (Salvia) to Prevent and Cure Illnesses Such as Obesity, Diabetes, Depression, Dementia, Lupus, Autism, Heart Disease, and Cancer. J. Tradit. Complement. Med. 2014, 4, 82–88. [Google Scholar] [CrossRef]
- Jassbi, A.R.; Zare, S.; Firuzi, O.; Xiao, J. Bioactive Phytochemicals from Shoots and Roots of Salvia Species. Phytochem. Rev. 2016, 15, 829–867. [Google Scholar] [CrossRef]
- Lu, Y.; Yeap Foo, L. Polyphenolics of Salvia-A Review. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef]
- Nait Bachir, Y.; Zafour, A.; Medjkane, M. Formulation of Stable Microcapsules Suspensions Content Salvia officinalis Extract for Its Antioxidant Activity Preservation. J. Food Process. Preserv. 2018, 42. [Google Scholar] [CrossRef]
- Fiorentin, T.R.; De Mello, M.B.; Aquino, A.M.K.; Rigo, B.A.; Loss, C.G.; Schwanz, M.; Junior, A.E.H.; Macedo, S.M.D. Antiulcerogenic Potential of Salvia officinalis L. Extract in Rats. J. Appl. Pharm. Sci. 2013, 3, 32–35. [Google Scholar] [CrossRef][Green Version]
- Roman Junior, W.A.; Picolli, A.L.; Morais, B.; Loeblein, M.; Schönell, A.P. Atividade Antiulcerogênica Do Extrato Aquoso de Salvia officinalis L. (Lamiaceae). Rev. Bras. De Plantas Med. 2015, 17, 774–781. [Google Scholar] [CrossRef]
- Mayer, B.; Baggio, C.H.; Freitas, C.S.; dos Santos, A.C.; Twardowschy, A.; Horst, H.; Pizzolatti, M.G.; Micke, G.A.; Heller, M.; Dos Santos, É.P.; et al. Gastroprotective Constituents of Salvia officinalis L. Fitoterapia 2009, 80, 421–426. Fitoterapia 2009, 80, 421–426. [Google Scholar] [CrossRef]
- Nostro, A.; Cellini, L.; Di Bartolomeo, S.; Di Campli, E.; Grande, R.; Cannatelli, M.A.; Marzio, L.; Alonzo, V. Antibacterial Effect of Plant Extracts against Helicobacter pylori. Phytother. Res. 2005, 19, 198–202. [Google Scholar] [CrossRef]
- O’Mahony, R.; Al-Khtheeri, H.; Weerasekera, D.; Fernando, N.; Vaira, D.; Holton, J.; Basset, C. Bactericidal and Anti-Adhesive Properties of Culinary and Medicinal Plants against Helicobacter pylori. World J. Gastroenterol. 2005, 11, 7499–7507. [Google Scholar] [CrossRef]
- Mahady, G.B.; Pendland, S.L.; Stoia, A.; Hamill, F.A.; Fabricant, D.; Dietz, B.M.; Chadwick, L.R. In Vitro Susceptibility of Helicobacter pylori to Botanical Extracts Used Traditionally for the Treatment of Gastrointestinal Disorders. Phytother. Res. 2005, 19, 988–991. [Google Scholar] [CrossRef]
- Cwikla, C.; Schmidt, K.; Matthias, A.; Bone, K.M.; Lehmann, R.; Tiralongo, E. Investigations into the Antibacterial Activities of Phytotherapeutics against Helicobacter pylori and Campylobacter jejuni. Phytother. Res. 2010, 24, 649–656. [Google Scholar] [CrossRef]
- Arshady, R. Microspheres and Microcapsules, a Survey of Manufacturing Techniques Part II: Coacervation. Polym. Eng. Sci. 1990, 30, 905–914. [Google Scholar] [CrossRef]
- Nait Bachir, Y.; Sahraoui, N.; Cheurfa, Z.; Medjkane, M.; Ziane, A.H. Formulation of a Natural Nanosystem Based on β-Cyclodextrin/Arginine/Xanthan to Increase Antifungal Activity of Salvia officinalis Essential Oil from Algeria (Bejaïa, Kalaa n’Ath Abas). J. Res. Pharm. 2022, 26, 581–597. [Google Scholar] [CrossRef]
- Schrooyen, P.M.M.; Van der Meer, R.; Kruif, C.G. De Microencapsulation: Its Application in Nutrition. Proc. Nutr. Soc. 2001, 60, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Champagne, C.P.; Fustier, P. Microencapsulation for the Improved Delivery of Bioactive Compounds into Foods. Curr. Opin. Biotechnol. 2007, 18, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.L.; Gambari, R. Advanced Progress of Microencapsulation Technologies: In Vivo and in Vitro Models for Studying Oral and Transdermal Drug Deliveries. J. Control Release 2014, 178, 25–45. [Google Scholar] [CrossRef] [PubMed]
- Gîrd, C.E.; Nencu, I.; Costea, T.; Duţu, L.E.; Popescu, M.L.; Ciupitu, N. Quantitative Analysis of Phenolic Compounds from Salvia officinalis L. Leaves. Farmacia 2014, 62, 649–657. [Google Scholar]
- Rocha-Selmi, G.A.; Theodoro, A.C.; Thomazini, M.; Bolini, H.M.A.; Favaro-Trindade, C.S. Double Emulsion Stage Prior to Complex Coacervation Process for Microencapsulation of Sweetener Sucralose. J. Food Eng. 2013, 119, 28–32. [Google Scholar] [CrossRef]
- McMullen, J.N.; Newton, D.W.; Becker, C.H. Pectin-Gelatin Complex Coacervates I: Determinants of Microglobule Size, Morphology, and Recovery as Water-Dispersible Powders. J. Pharm. Sci. 1982, 71, 628–633. [Google Scholar] [CrossRef]
- McMullen, J.N.; Newton, D.W.; Becker, C.H. Pectin–Gelatin Complex Coacervates II: Effect of Microencapsulated Sulfamerazine on Size, Morphology, Recovery, and Extraction of Water-dispersible Microglobules. J. Pharm. Sci. 1984, 73, 1799–1803. [Google Scholar] [CrossRef]
- Saravanan, M.; Rao, K.P. Pectin-Gelatin and Alginate-Gelatin Complex Coacervation for Controlled Drug Delivery: Influence of Anionic Polysaccharides and Drugs Being Encapsulated on Physicochemical Properties of Microcapsules. Carbohydr. Polym. 2010, 80, 808–816. [Google Scholar] [CrossRef]
- Nait Bachir, Y.; Bensaibi, I.; Nantenainjanahary, I.; Medjkane, M.; Zafour, A. Optimisation Des Paramètres d’Extraction Des Molécules Bioactives de Salvia officinalis En Utilisant Les Plans d’Expériences. PhytoChem BioSub J. 2017, 11, 2017. [Google Scholar]
- Folin, O.; Ciocalteau, V. Tyrosine and Tryptophane in Proteins. J. Biol. Chem. 1927, 73, 627–648. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin-Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Corrêa Dias, P.; Foglio, M.A.; Possenti, A.; De Carvalho, J.E. Antiulcerogenic Activity of Crude Hydroalcoholic Extract of Rosmarinus officinalis L. J. Ethnopharmacol. 2000, 69, 57–62. [Google Scholar] [CrossRef]
- Jahovic, N.; Erkanli, G.; Işeri, S.; Arbak, S.; Alican, I. Gastric Protection by α-Melanocyte-Stimulating Hormone against Ethanol in Rats: Involvement of Somatostatin. Life Sci. 2007, 80, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.; Uehara, A.; Kubota, T.; Nozu, T.; Moriya, M.; Watanabe, Y.; Shoji, E.; Santos, S.; Harada, K.; Kohgo, Y. Effects of Ranitidine on Gastric Vesicles Containing H. Scand. J. Gastroenterol. 1995, 30, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Siddaraju, M.N.; Dharmesh, S.M. Inhibition of Gastric H+,K+-ATPase and Helicobacter pylori Growth by Phenolic Antioxidants of Zingiber officinale. Mol. Nutr. Food Res. 2007, 51, 324–332. [Google Scholar] [CrossRef]
- Fiske, C.H.; Subbarow, Y. The colorimetric determination of phosphorus. J. Biol. Chem. 1925, 66, 375–400. [Google Scholar] [CrossRef]
- Bouaziz, M.; Yangui, T.; Sayadi, S.; Dhouib, A. Disinfectant Properties of Essential Oils from Salvia officinalis L. cultivated in Tunisia. Food Chem. Toxicol. 2009, 47, 2755–2760. [Google Scholar] [CrossRef]
- Jedidi, S.; Aloui, F.; Rtibi, K.; Sammari, H.; Selmi, H.; Rejeb, A.; Toumi, L.; Sebai, H. Individual and Synergistic Protective Properties of Salvia officinalis Decoction Extract and Sulfasalazine against Ethanol-Induced Gastric and Small Bowel Injuries. RSC Adv. 2020, 10, 35998–36013. [Google Scholar] [CrossRef] [PubMed]
- Fathi, F.; Ebrahimi, S.N.; Pereira, D.M.; Estevinho, B.N.; Rocha, F. Preliminary Studies of Microencapsulation and Anticancer Activity of Polyphenols Extract from Punica granatum Peels. Can. J. Chem. Eng. 2022, 100, 3240–3252. [Google Scholar] [CrossRef]
- Huang, K.; Yuan, Y.; Baojun, X. A Critical Review on the Microencapsulation of Bioactive Compounds and Their Application. Food Rev. Int. 2023, 39, 2594–2634. [Google Scholar] [CrossRef]
- Khanlari, S.; Dubé, M.A. Bioadhesives: A Review. Macromol. React. Eng. 2013, 7, 573–587. [Google Scholar] [CrossRef]
- Markov, P.A.; Krachkovsky, N.S.; Durnev, E.A.; Martinson, E.A.; Litvinets, S.G.; Popov, S.V. Mechanical Properties, Structure, Bioadhesion, and Biocompatibility of Pectin Hydrogels. J. Biomed. Mater. Res. A 2017, 105, 2572–2581. [Google Scholar] [CrossRef] [PubMed]
- Obidike, I.C.; Emeje, M.O. Microencapsulation Enhances the Anti-Ulcerogenic Properties of Entada africana Leaf Extract. J. Ethnopharmacol. 2011, 137, 553–561. [Google Scholar] [CrossRef]
- Wang, W.; Kang, Q.; Liu, N.; Zhang, Q.; Zhang, Y.; Li, H.; Zhao, B.; Chen, Y.; Lan, Y.; Ma, Q.; et al. Enhanced Dissolution Rate and Oral Bioavailability of Ginkgo Biloba Extract by Preparing Solid Dispersion via Hot-Melt Extrusion. Fitoterapia 2015, 102, 189–197. [Google Scholar] [CrossRef]
- Nait Bachir, Y.; Nait Bachir, R.; Hadj-Ziane-Zafour, A. Nanodispersions Stabilized by β-Cyclodextrin Nanosponges: Application for Simultaneous Enhancement of Bioactivity and Stability of Sage Essential Oil. Drug Dev. Ind. Pharm. 2019, 45, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Nait Bachir, Y.; Ouennoughi, N.; Daoud, K. Formulation, Characterization and In-vitro Dissolution Study of Glimepiride, Β-cyclodextrins Inclusion Complexes and Water Soluble Polymers Ternary Systems. Int. J. Pharma Bio Sci. 2013, 4, 272–287. [Google Scholar]
- Nait Bachir, Y.; Medjkane, M.; Benaoudj, F.; Sahraoui, N.; Hadj-ziane, A. Formulation of β-Cyclodextrin Nanosponges by Polycondensation Method: Application for Natural Drugs Delivery and Preservation. J. Mater. Process. Environ. 2017, 5, 80–85. [Google Scholar]
- Slamani, M.; Zaouadi, N.; Gharbi, D.; Nait Bachir, Y.; Hadj Ziane-Zafour, A. Formulation of an oral emulsion for the delivery of active substances contained in Atriplex halimus L. Leaves. J. Fundam. Appl. Sci. 2020, 12, 35–48. [Google Scholar]

| Groups | Nom | Number of Rats | Gavage at t0 (Treatment) | Gavage at t0 + 30 min (Ethanol) |
|---|---|---|---|---|
| Group 1 | physiological | 6 | no | no |
| Group 2 | negative control | 6 | no | yes |
| Group 3 | positive control | 6 | omeprazole at 50 mg/kg | yes |
| Group 4 | Treatment 1 | 6 | Salvia officinalis extract (100 mg/kg) | yes |
| Group 5 | Treatment 2 | 6 | Microcapsules (equivalent to 100 mg/kg of Salvia officinalis extract) | yes |
| Groups | Macroscopic | Microscopic | |||||
|---|---|---|---|---|---|---|---|
| Percentages of Ulcer (%) | Percentages of Inhibitions (%) | Epithelial Desquamations | Glandular Destruction | Edema of the Sub-mucosa | Infiltration of Eosinophils | Hemorrhages | |
| Group 1 | - | - | - | - | - | ||
| Group 2 | 10.25 ± 0.17 | +++ | +++ | +++ | ++ | ++ | |
| Group 3 | 4.73 ± 0.18 a | 53.85 ± 1.61 | + | + | ++ | + | - |
| Group 4 | 2.9 ± 0.26 a,b | 71.71 ± 2.43 | ++ | + | +++ | + | - |
| Group 5 | 1.05 ± 0.25 a,b,c | 89.76 ± 2.54 | + | - | + | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bachir, Y.N.; Nait Bachir, R.; Medjkane, M.; Boudjema, N.; Foligni, R. New Functional Food for the Treatment of Gastric Ulcer Based on Bioadhesive Microparticles Containing Sage Extract: Anti-Ulcerogenic, Anti-Helicobacter pylori, and H+/K+-ATPase-Inhibiting Activity Enhancement. Foods 2025, 14, 2757. https://doi.org/10.3390/foods14152757
Bachir YN, Nait Bachir R, Medjkane M, Boudjema N, Foligni R. New Functional Food for the Treatment of Gastric Ulcer Based on Bioadhesive Microparticles Containing Sage Extract: Anti-Ulcerogenic, Anti-Helicobacter pylori, and H+/K+-ATPase-Inhibiting Activity Enhancement. Foods. 2025; 14(15):2757. https://doi.org/10.3390/foods14152757
Chicago/Turabian StyleBachir, Yacine Nait, Ryma Nait Bachir, Meriem Medjkane, Nouara Boudjema, and Roberta Foligni. 2025. "New Functional Food for the Treatment of Gastric Ulcer Based on Bioadhesive Microparticles Containing Sage Extract: Anti-Ulcerogenic, Anti-Helicobacter pylori, and H+/K+-ATPase-Inhibiting Activity Enhancement" Foods 14, no. 15: 2757. https://doi.org/10.3390/foods14152757
APA StyleBachir, Y. N., Nait Bachir, R., Medjkane, M., Boudjema, N., & Foligni, R. (2025). New Functional Food for the Treatment of Gastric Ulcer Based on Bioadhesive Microparticles Containing Sage Extract: Anti-Ulcerogenic, Anti-Helicobacter pylori, and H+/K+-ATPase-Inhibiting Activity Enhancement. Foods, 14(15), 2757. https://doi.org/10.3390/foods14152757









