Engineering Edible Double Network Hydrogels with Abalone- and Squid-like Textures from Carrageenan and Konjac Glucomannan
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of κ-car-k+/KGM Double Networks and Thermally Processed Seafood Samples
2.3. Mechanical Properties Test
2.4. Texture Profile Test
2.5. Water Retention Ability and Swelling Characteristics Test
2.6. Cryogenic Scanning Electron Microscope (Cryo-SEM)
2.7. Microscopes-Fourier Transform Infrared Spectrometer (Micro-FTIR)
2.8. Sensory Assessment
2.9. Statistical Analysis
3. Results and Discussion
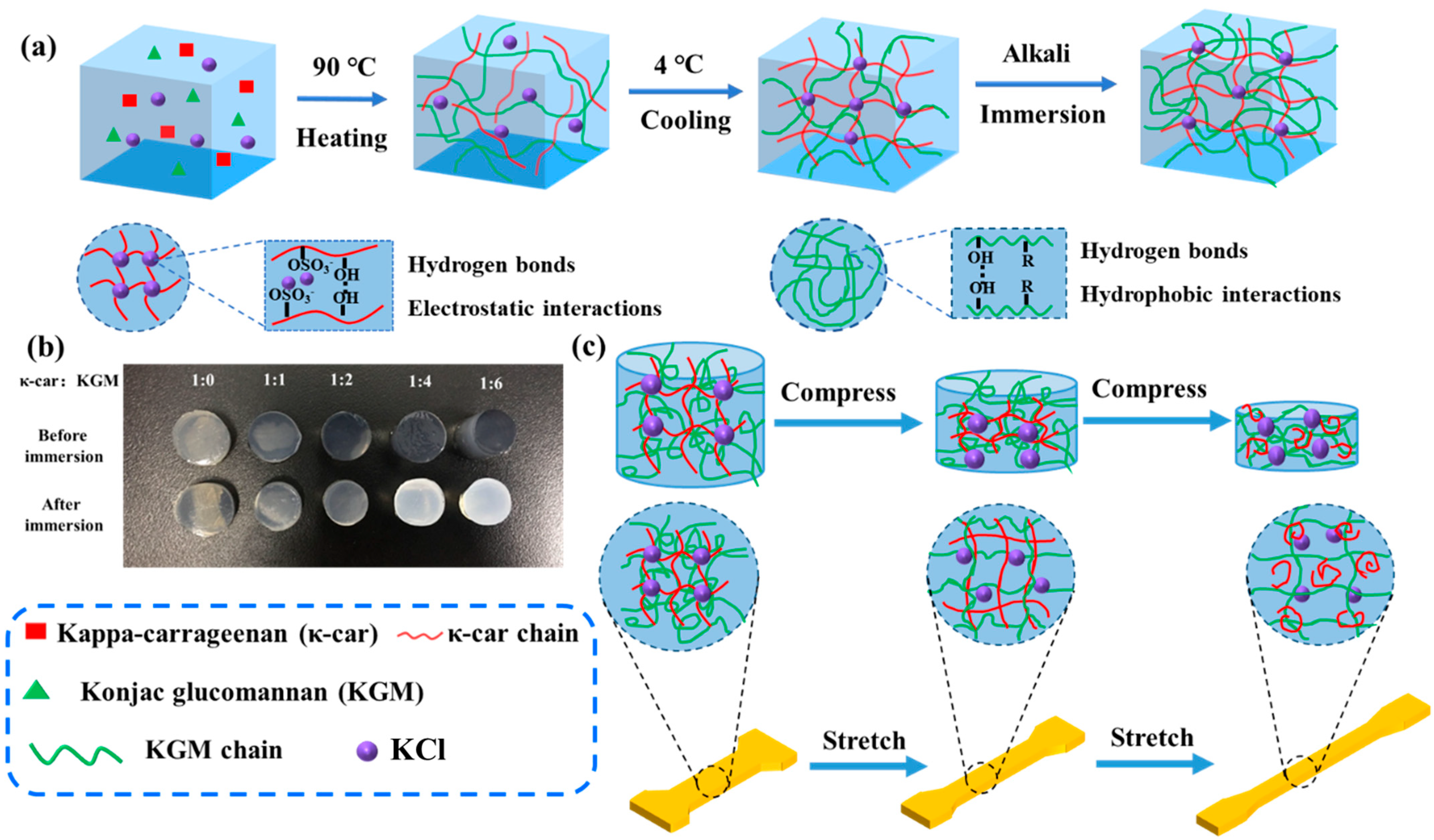
3.1. Fabrication and Analysis of κ-car-k+/KGM Double Networks Hydrogels
3.1.1. Fabrication of κ-car-k+/Konjac Glucomannan Double Networks
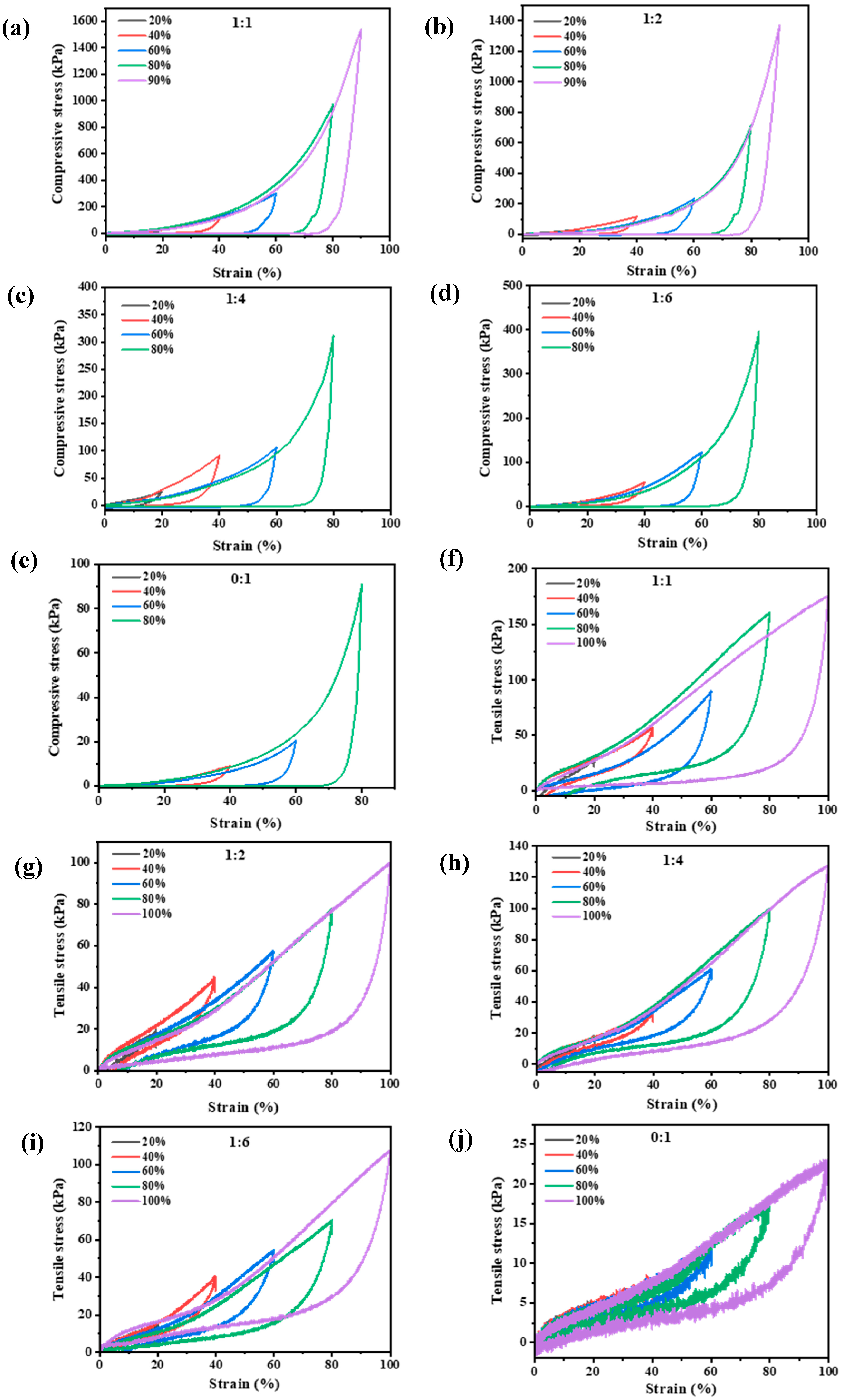
3.1.2. Mechanical Properties Analysis
3.1.3. Energy Dissipation and Self-Recovery
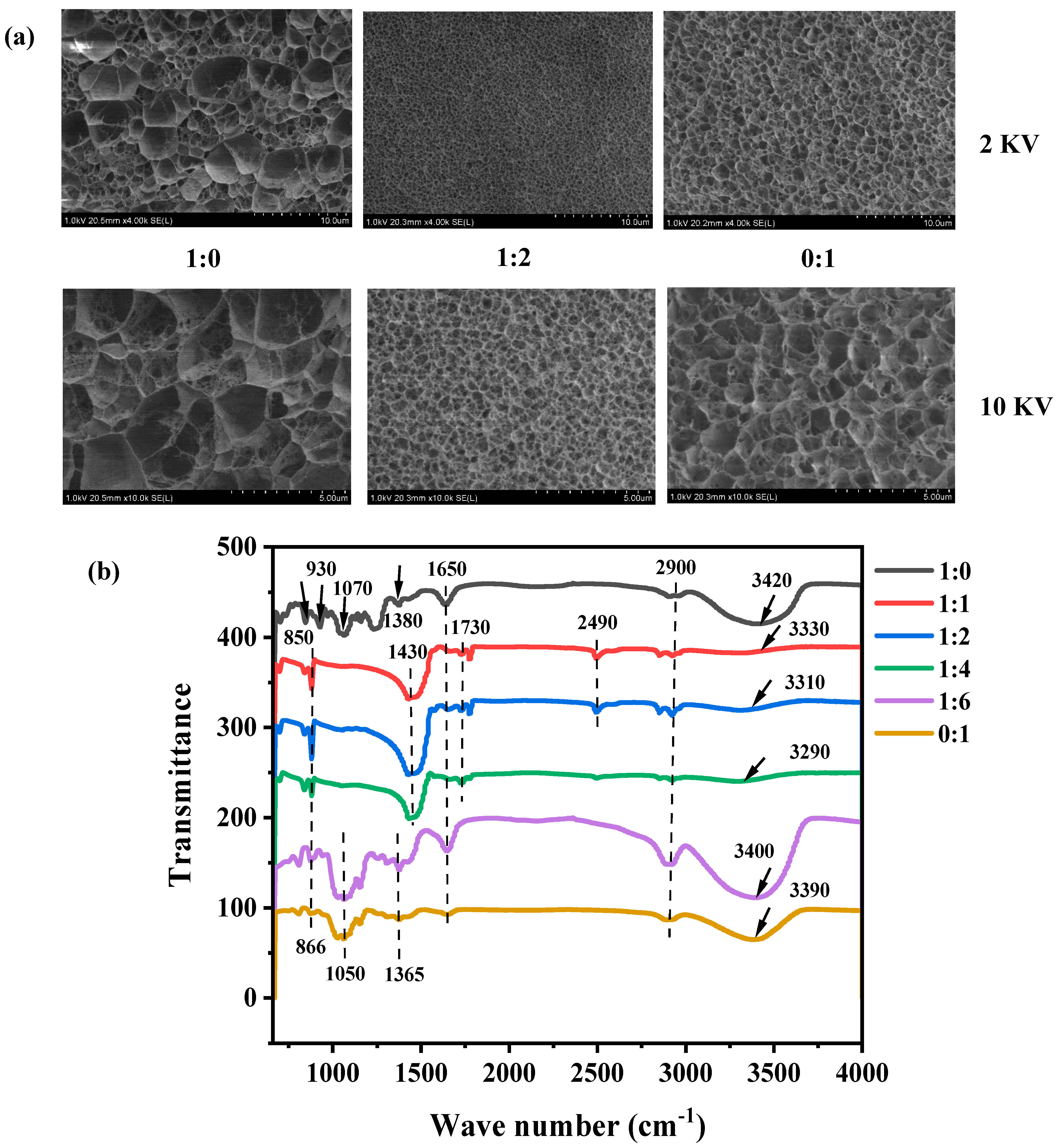
3.1.4. Structural and Molecular Characterization
3.2. Application as Biomimetic Seafood Analogues
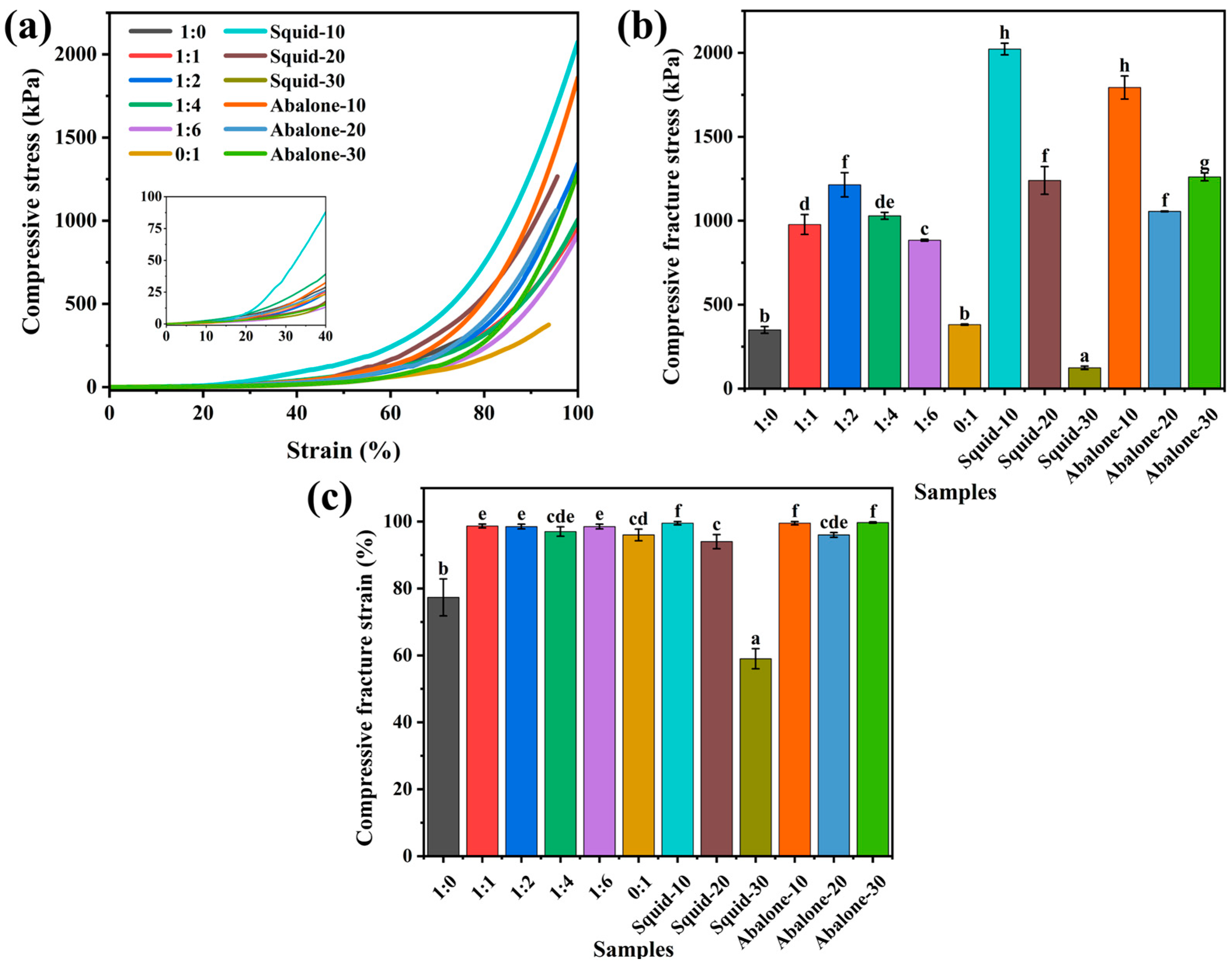
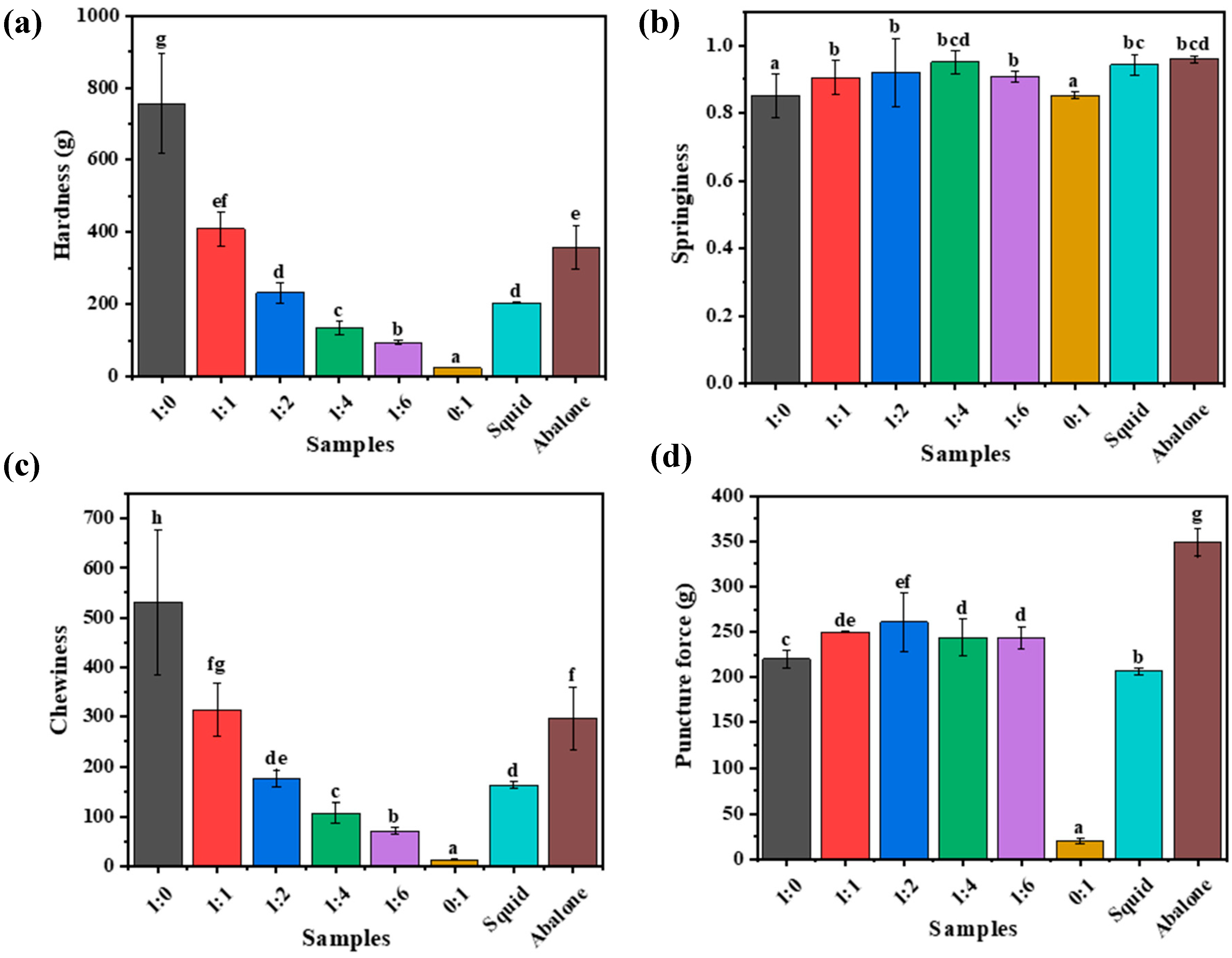
3.2.1. Mechanical and Textural Characteristics
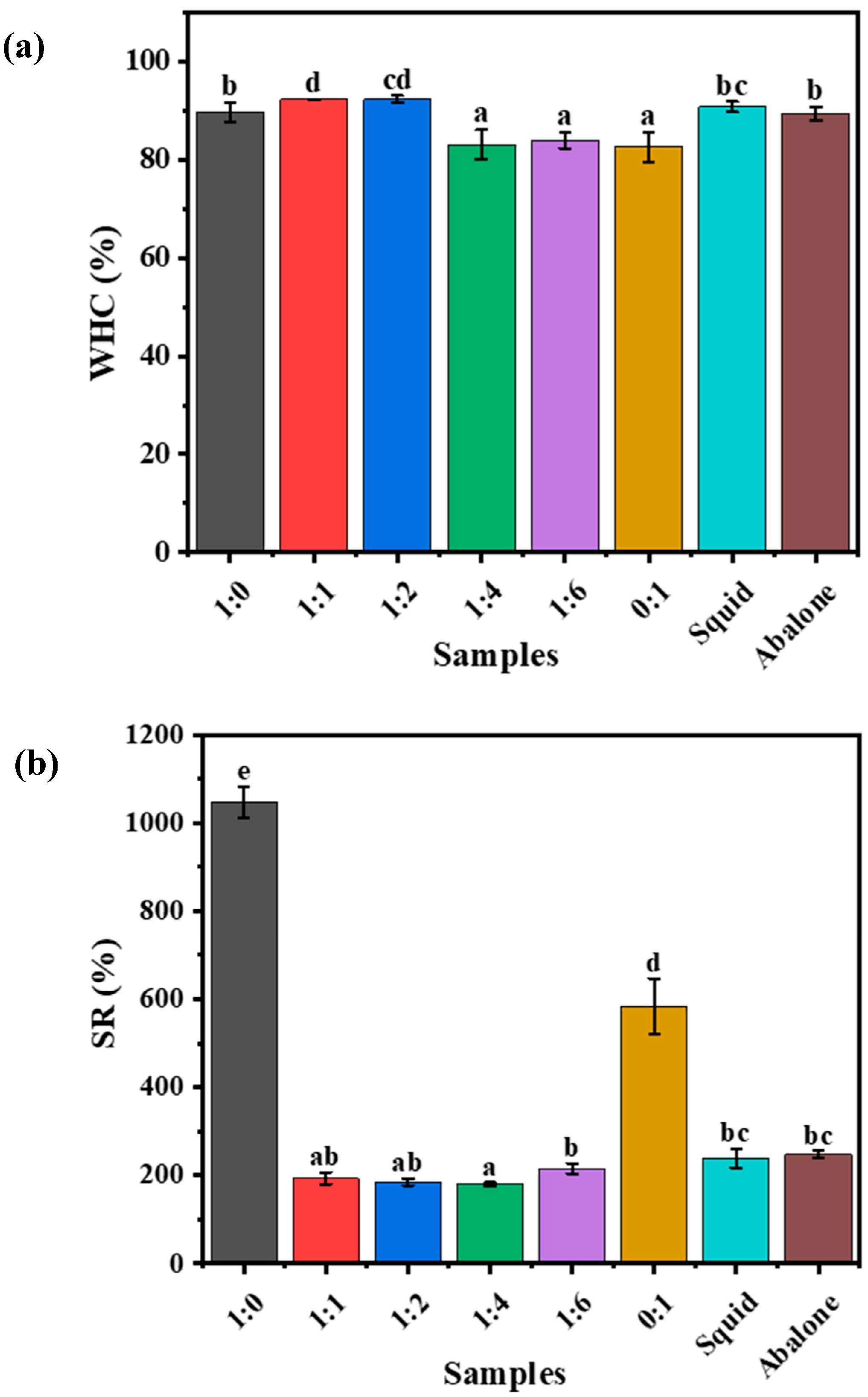
3.2.2. Water Retention Ability and Swelling Characteristics
3.2.3. Analogical Analysis of κ-car-k+/KGM Gels and Seafood in Statistics
Correlation Analysis
Hierarchical Cluster Associated with Heatmap (HCA Heatmap) Analysis
3.2.4. Sensory Assessment Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, S.; Zhang, J.; Zu, G.; Huang, J. Transparent, flexible, and multifunctional starch-based double-network hydrogels as high-performance wearable electronics. Carbohydr. Polym. 2021, 267, 118198. [Google Scholar] [CrossRef]
- Su, C.-Y.; Li, D.; Wang, L.-J. From micropores to mechanical strength: Fabrication and characterization of edible corn starch-sodium alginate double network hydrogels with Ca2+ cross-linking. Food Chem. 2025, 467, 142276. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ahmad, M.; Manzoor, K.; Purwar, R.; Ikram, S. A review on latest innovations in natural gums based hydrogels: Preparations & applications. Int. J. Biol. Macromol. 2019, 136, 870–890. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.P. Why are double network hydrogels so tough? Soft Matter 2010, 6, 2583. [Google Scholar] [CrossRef]
- Du, M.; Zhao, Y.; Zhang, Y.; Sun, S.; Fang, Y. Fabrication of agarose/fish gelatin double-network hydrogels with high strength and toughness for the development of artificial beef tendons. Food Funct. 2022, 13, 6975–6986. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chen, Q.; Zhu, L.; Tang, Z.; Li, Q.; Qin, G.; Yang, J.; Zhang, Y.; Ren, B.; Zheng, J. General Strategy To Fabricate Strong and Tough Low-Molecular-Weight Gelator-Based Supramolecular Hydrogels with Double Network Structure. Chem. Mater. 2018, 30, 1743–1754. [Google Scholar] [CrossRef]
- Tang, Z.; Lyu, X.; Xiao, A.; Shen, Z.; Fan, X. High-Performance Double-Network Ion Gels with Fast Thermal Healing Capability via Dynamic Covalent Bonds. Chem. Mater. 2018, 30, 7752–7759. [Google Scholar] [CrossRef]
- Hou, J.-J.; Guo, J.; Wang, J.-M.; He, X.-T.; Yuan, Y.; Yin, S.-W.; Yang, X.-Q. Edible double-network gels based on soy protein and sugar beet pectin with hierarchical microstructure. Food Hydrocoll. 2015, 50, 94–101. [Google Scholar] [CrossRef]
- Sun, J.; Ren, F.; Chang, Y.; Wang, P.; Li, Y.; Zhang, H.; Luo, J. Formation and structural properties of acid-induced casein–agar double networks: Role of gelation sequence. Food Hydrocoll. 2018, 85, 291–298. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, A.; Qiu, C.; Liu, Q.; Yang, Y.; Bian, S.; Zeng, F.; Jin, Z. A combined enzymatic and ionic cross-linking strategy for pea protein/sodium alginate double-network hydrogel with excellent mechanical properties and freeze-thaw stability. Food Hydrocoll. 2022, 131, 107737. [Google Scholar] [CrossRef]
- Yiu, C.C.Y.; Wang, Y.; Selomulya, C. Double Network as a Design Paradigm for Structuring Emulsion Gels in Food. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70201. [Google Scholar] [CrossRef]
- Chen, H.; Gan, J.; Ji, A.; Song, S.; Yin, L. Development of double network gels based on soy protein isolate and sugar beet pectin induced by thermal treatment and laccase catalysis. Food Chem. 2019, 292, 188–196. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, B.; Yadav, M.P.; Feng, L.; Yan, J.; Jia, X.; Yin, L. Corn fiber gum-soybean protein isolate double network hydrogel as oral delivery vehicles for thermosensitive bioactive compounds. Food Hydrocoll. 2020, 107, 105865. [Google Scholar] [CrossRef]
- Du, M.; Lu, W.; Zhang, Y.; Mata, A.; Fang, Y. Natural polymer-sourced interpenetrating network hydrogels: Fabrication, properties, mechanism and food applications. Trends Food Sci. Technol. 2021, 116, 342–356. [Google Scholar] [CrossRef]
- Gong, J.; Wang, L.; Wu, J.; Yuan, Y.; Mu, R.-J.; Du, Y.; Wu, C.; Pang, J. The rheological and physicochemical properties of a novel thermosensitive hydrogel based on konjac glucomannan/gum tragacanth. LWT 2019, 100, 271–277. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, X.; Zhang, Q.; Zhang, D.; Xie, X.; Zhou, H.; Wu, Z.; Liu, R.; Pang, J. Review of Konjac Glucomannan Structure, Properties, Gelation Mechanism, and Application in Medical Biology. Polymers 2023, 15, 1852. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Vadrale, A.P.; Singhania, R.R.; Michaud, P.; Pandey, A.; Chen, S.-J.; Chen, C.-W.; Dong, C.-D. Algal polysaccharides: Current status and future prospects. Phytochem. Rev. 2023, 22, 1167–1196. [Google Scholar] [CrossRef]
- Heidarian, P.; Kouzani, A.Z.; Kaynak, A.; Paulino, M.; Nasri-Nasrabadi, B.; Zolfagharian, A.; Varley, R. Dynamic plant-derived polysaccharide-based hydrogels. Carbohydr. Polym. 2020, 231, 115743. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, C.; Hu, S.; Zhao, G.; Zhou, Y. A fracture mechanics approach to investigating the crunchy texture of konjac glucomannan gels through imitative chewing tests. Food Hydrocoll. 2025, 164, 111212. [Google Scholar] [CrossRef]
- Lin, H.-T.V.; Wu, H.-X.; Sung, W.-C. Hardness and quality of abalone (Haliotis discus hannai diversicolor diversicolor) muscle as suitably softened for seniors. Int. J. Food Prop. 2021, 24, 579–591. [Google Scholar] [CrossRef]
- Yu, M.-M.; Li, D.-Y.; Liu, Z.-Q.; Liu, Y.-X.; Zhou, J.-Z.; Zhang, M.; Zhou, D.-Y.; Zhu, B.-W. Effects of heat treatments on texture of abalone muscles and its mechanism. Food Biosci. 2021, 44, 101402. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Z.; Lu, W.; Zhou, X.; Liu, S.; Zhu, S.; Ding, Y. Improving the texture attributes of squid meat (Sthenoteuthis oualaniensis) with slight oxidative and phosphate curing treatments. Food Res. Int. 2024, 176, 113829. [Google Scholar] [CrossRef]
- Chen, F.; Tang, Z.; Lu, S.; Zhu, L.; Wang, Q.; Gang, Q.; Yang, J.; Chen, Q. Fabrication and mechanical behaviors of novel supramolecular/polymer hybrid double network hydrogels. Polymer 2019, 168, 159–167. [Google Scholar] [CrossRef]
- Fatehi, F.; Krizsan, S.J.; Gidlund, H.; Huhtanen, P. A comparison of ruminal or reticular digesta sampling as an alternative to sampling from the omasum of lactating dairy cows. J. Dairy Sci. 2015, 98, 3274–3283. [Google Scholar] [CrossRef] [PubMed]
- Sow, L.C.; Tan, S.J.; Yang, H. Rheological properties and structure modification in liquid and gel of tilapia skin gelatin by the addition of low acyl gellan. Food Hydrocoll. 2019, 90, 9–18. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, J.; Hemar, Y.; Cui, B. Improvement of the rheological and textural properties of calcium sulfate-induced soy protein isolate gels by the incorporation of different polysaccharides. Food Chem. 2020, 310, 125983. [Google Scholar] [CrossRef]
- Xu, C.; Zhan, W.; Tang, X.; Mo, F.; Fu, L.; Lin, B. Self-healing chitosan/vanillin hydrogels based on Schiff-base bond/hydrogen bond hybrid linkages. Polym. Test. 2018, 66, 155–163. [Google Scholar] [CrossRef]
- Chen, H.; Shi, P.; Fan, F.; Chen, H.; Wu, C.; Xu, X.; Wang, Z.; Du, M. Hofmeister effect-assisted one step fabrication of fish gelatin hydrogels. LWT 2020, 121, 108973. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, M.; Sun, D.; Hou, Y.; Li, Y.; Dong, T.; Wang, X.; Zhang, L.; Yang, W. Dual Physically Cross-Linked κ-Carrageenan-Based Double Network Hydrogels with Superior Self-Healing Performance for Biomedical Application. ACS Appl. Mater. Interfaces 2018, 10, 37544–37554. [Google Scholar] [CrossRef]
- Kanniyappan, H.; Thangavel, P.; Chakraborty, S.; Arige, V.; Muthuvijayan, V. Design and evaluation of Konjac glucomannan-based bioactive interpenetrating network (IPN) scaffolds for engineering vascularized bone tissues. Int. J. Biol. Macromol. 2020, 143, 30–40. [Google Scholar] [CrossRef]
- Hua, J.; Liu, C.; Ng, P.F.; Fei, B. Bacterial cellulose reinforced double-network hydrogels for shape memory strand. Carbohydr. Polym. 2021, 259, 117737. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Xue, Y.; Zhang, T.; Li, Z.; Xue, C. The effects of microwave processing on the structure and various quality parameters of Alaska pollock surimi protein-polysaccharide gels. Food Hydrocoll. 2017, 63, 77–84. [Google Scholar] [CrossRef]
- Weiss, J.; Salminen, H.; Moll, P.; Schmitt, C. Use of molecular interactions and mesoscopic scale transitions to modulate protein-polysaccharide structures. Adv. Colloid Interface Sci. 2019, 271, 101987. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Wu, S.; Ma, Y.; Zhao, Y.; Chen, Z.; Frenkel, I.; Strzalka, J.; Zhou, H.; Zhu, X.; He, X. Strong tough hydrogels via the synergy of freeze-casting and salting out. Nature 2021, 590, 594–599. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Y.; Ding, Y.; Xiang, X.; Yang, Q.; Wei, Z.; Song, H.; Liu, S.; Zhou, X. Improved texture properties and toughening mechanisms of surimi gels by double network strategies. Food Hydrocoll. 2024, 152, 109900. [Google Scholar] [CrossRef]
- Wang, J.J.; Wang, Y.; Wang, Q.; Yang, J.; Hu, S.-Q.; Chen, L. Mechanically Strong and Highly Tough Prolamin Protein Hydrogels Designed from Double-Cross-Linked Assembled Networks. ACS Appl. Polym. Mater. 2019, 1, 1272–1279. [Google Scholar] [CrossRef]
- Liu, S.; Li, L. Recoverable and Self-Healing Double Network Hydrogel Based on κ-Carrageenan. ACS Appl. Mater. Interfaces 2016, 8, 29749–29758. [Google Scholar] [CrossRef]
- Shang, X.; Wang, Q.; Li, J.; Zhang, G.; Zhang, J.; Liu, P.; Wang, L. Double-network hydrogels with superior self-healing properties using starch reinforcing strategy. Carbohydr. Polym. 2021, 257, 117626. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Zhang, L.; Chen, X.; Dong, Y.; Zou, L.; Liu, W. Engineering soy protein/konjac glucomannan double network hydrogels with low voltage-electric field: Anisotropic structure and properties. Food Res. Int. 2025, 213, 116559. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, H.; Liu, X.; Li, Z.; Liu, B.; Wu, J.; Shi, M.; Norde, W.; Li, Y. TEMPO-oxidized Konjac glucomannan as appliance for the preparation of hard capsules. Carbohydr. Polym. 2016, 143, 262–269. [Google Scholar] [CrossRef]
- Ma, S.; Youssef, M.; Albahi, A.; Li, J.; Zhou, P.; Li, B. Calcium alginate-cross-linked deacetylated konjac glucomannan-based double network hydrogels: Construction, characterizations and gelation kinetics. Int. J. Biol. Macromol. 2025, 309, 142634. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Yang, L.; Li, J.; Lin, L.; Zheng, G. Effect of Smilax china L. starch on the gel properties and interactions of calcium sulfate-induced soy protein isolate gel. Int. J. Biol. Macromol. 2019, 135, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Babaei, J.; Mohammadian, M.; Madadlou, A. Gelatin as texture modifier and porogen in egg white hydrogel. Food Chem. 2019, 270, 189–195. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Du, M.; Zhao, Y.; Fang, Y. Engineering Edible Double Network Hydrogels with Abalone- and Squid-like Textures from Carrageenan and Konjac Glucomannan. Foods 2025, 14, 3140. https://doi.org/10.3390/foods14173140
Zhao J, Du M, Zhao Y, Fang Y. Engineering Edible Double Network Hydrogels with Abalone- and Squid-like Textures from Carrageenan and Konjac Glucomannan. Foods. 2025; 14(17):3140. https://doi.org/10.3390/foods14173140
Chicago/Turabian StyleZhao, Jingwen, Mengjia Du, Yiguo Zhao, and Yapeng Fang. 2025. "Engineering Edible Double Network Hydrogels with Abalone- and Squid-like Textures from Carrageenan and Konjac Glucomannan" Foods 14, no. 17: 3140. https://doi.org/10.3390/foods14173140
APA StyleZhao, J., Du, M., Zhao, Y., & Fang, Y. (2025). Engineering Edible Double Network Hydrogels with Abalone- and Squid-like Textures from Carrageenan and Konjac Glucomannan. Foods, 14(17), 3140. https://doi.org/10.3390/foods14173140





