Effects of Harvesting Periods and Cultivar on the Physicochemical and Sensory Properties of Two Coffee Bean Varieties
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Standards and Chemicals
2.3. Climatic Data
2.4. Characterization of Green Coffee Bean Physicochemical Properties
2.5. Bean Roasting, Grinding, and Brewing
2.6. Characterization of Roasted Coffee Bean Physicochemical Properties and Cupping Quality
2.7. Statistical Analysis
3. Results and Discussion
3.1. Green Coffee Bean Characteristics
3.1.1. Analysis of Defect Rate, Thousand-Grain Weight, pH, and TDS
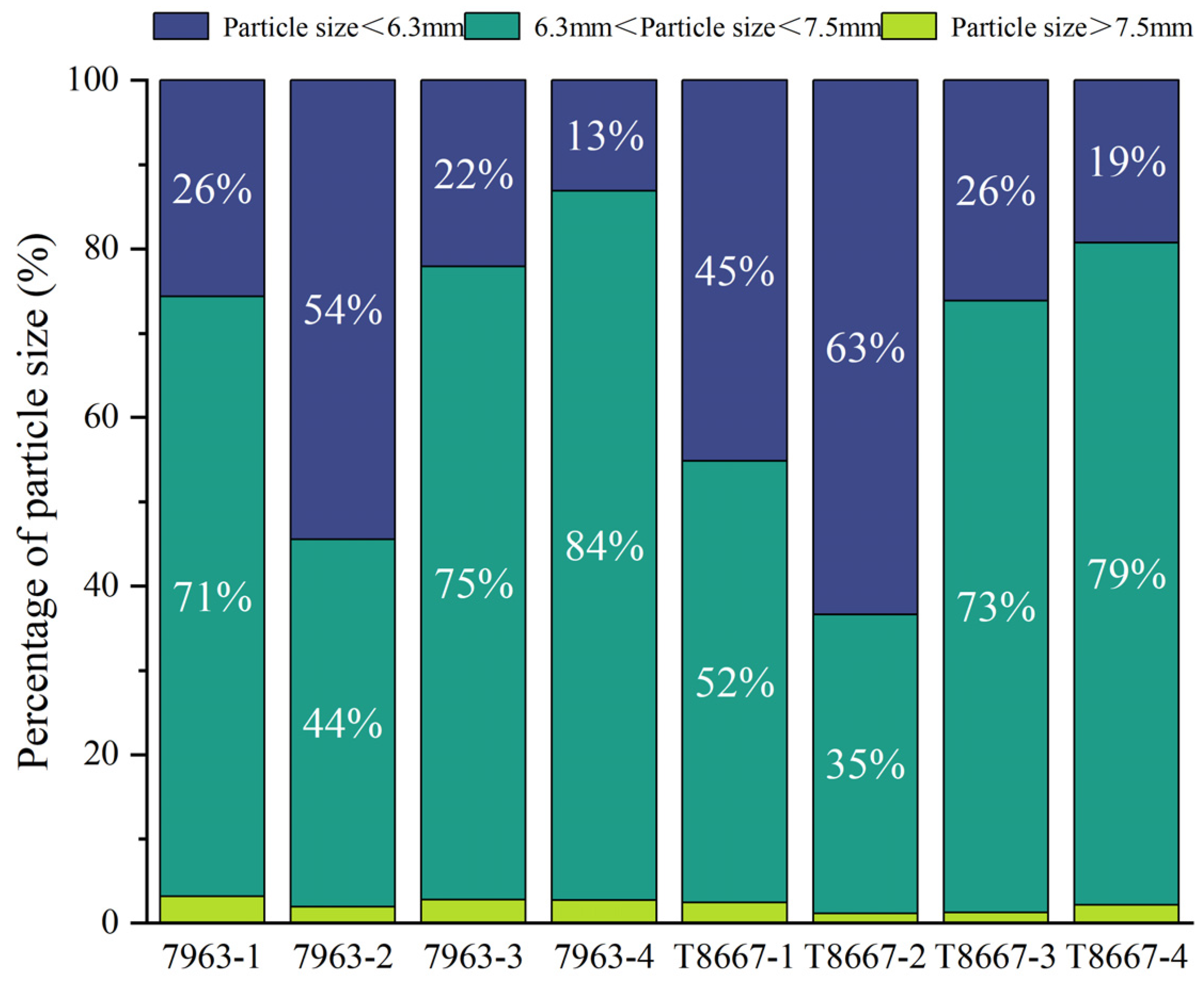
3.1.2. Analysis of Particle Size Distribution
3.1.3. Analysis of Lipid and Protein Contents
3.1.4. Analysis of Chlorogenic Acid, Caffeine and Trigonelline Content
3.2. Characteristics of Roasted Coffee Beans
3.2.1. Sensory Analysis
3.2.2. Analysis of pH and TDS
3.2.3. Analysis of CGA, Caffeine and Trigonelline Content
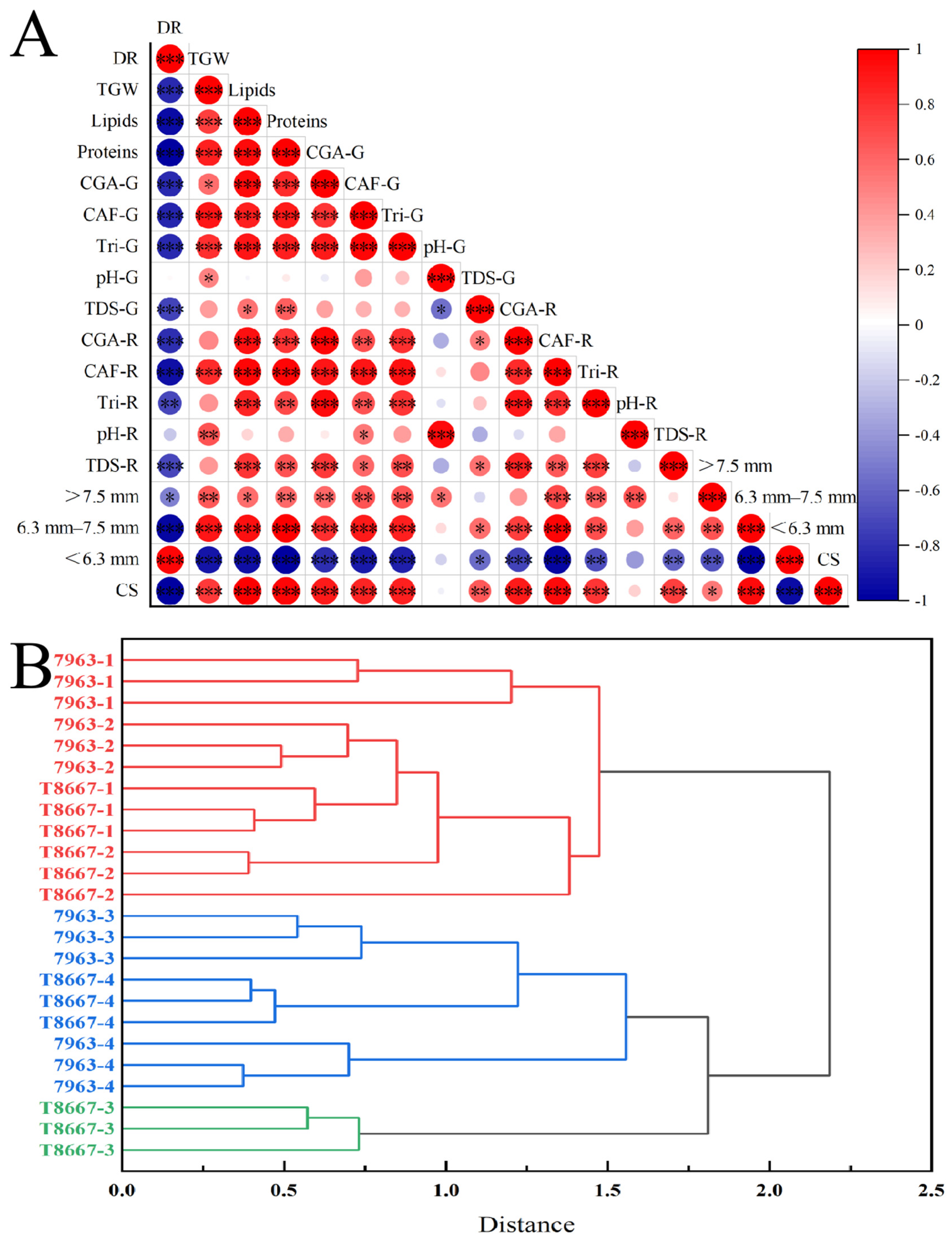
3.2.4. Correlation and Hierarchical Cluster Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Dam, R.M.; Hu, F.B.; Willett, W.C. Coffee, Caffeine, and Health. N. Engl. J. Med. 2020, 383, 369–378. [Google Scholar] [CrossRef]
- Grossman, O.; Rachamim, M. How can coffee shops draw customers back after COVID-19? The influence of psychological distance on coffee versus tea preference. J. Bus. Res. 2024, 172, 114431. [Google Scholar] [CrossRef]
- Coffee: World Markets and Trade. Available online: https://www.fas.usda.gov/sites/default/files/2024-12/coffee.pdf (accessed on 24 June 2025).
- World Coffee Research. Available online: https://worldcoffeeresearch.org (accessed on 24 August 2025).
- Freitas, V.V.; Borges, L.L.R.; Vidigal, M.C.T.R.; Dos Santos, M.H.; Stringheta, P.C. Coffee: A comprehensive overview of origin, market, and the quality process. Trends Food Sci. Technol. 2024, 146, 104411. [Google Scholar] [CrossRef]
- Abubakar, Y.; Siregar, M.M.; Juanda, J.; Nilda, C. Cherry and bean size distribution of Gayo arabica coffee harvested from different farm elevations. Iop Conf. Ser. Earth Environ. Sci. 2025, 1476, 12099. [Google Scholar] [CrossRef]
- Parada-Molina, P.C.; Cerdán-Cabrera, C.R.; Cervantes-Pérez, J.; Barradas, V.L.; Ortiz-Ceballos, G.C. Impact of climate on water status, growth, yield, and phenology of coffee (Coffea arabica) plants in the central region of the state of Veracruz, Mexico. PLoS ONE 2025, 20, e319670. [Google Scholar] [CrossRef]
- Getachew, M.; Tolassa, K.; De Frenne, P.; Verheyen, K.; Tack, A.J.M.; Hylander, K.; Ayalew, B.; Boeckx, P. The relationship between elevation, soil temperatures, soil chemical characteristics, and green coffee bean quality and biochemistry in southwest Ethiopia. Agron. Sustain. Dev. 2022, 42, 61. [Google Scholar] [CrossRef]
- Kim, J.; Pak, J.; Choi, J.; Park, S.; Bae, S.; Cho, H.; Kwak, S.; Son, H. Factors influencing metabolite profiles in global Arabica green coffee beans: Impact of continent, altitude, post-harvest processing, and variety. Food Res. Int. 2025, 208, 116187. [Google Scholar] [CrossRef]
- Angeloni, G.; Guerrini, L.; Masella, P.; Bellumori, M.; Daluiso, S.; Parenti, A.; Innocenti, M. What kind of coffee do you drink? An investigation on effects of eight different extraction methods. Food Res. Int. 2019, 116, 1327–1335. [Google Scholar] [CrossRef]
- Freitas, V.V.; Borges, L.L.R.; Castro, G.A.D.; Almeida, L.F.; Crepalde, L.T.; Kobi, H.D.B.; Vidigal, M.C.T.R.; Dos Santos, M.H.; Fernandes, S.A.; Maitan-Alfenas, G.P.; et al. Influence of roasting levels on chemical composition and sensory quality of Arabica and Robusta coffee: A comparative study. Food Biosci. 2024, 59, 104171. [Google Scholar] [CrossRef]
- Febrianto, N.A.; Zhu, F. Coffee bean processing: Emerging methods and their effects on chemical, biological and sensory properties. Food Chem. 2023, 412, 135489. [Google Scholar] [CrossRef]
- Arévalo, V.; Mejía, W.; Cevallos-Cevallos, J.M.; Ortiz-Ulloa, J. Effect of different drying airflows and harvest periods on the quality of specialty coffee (Coffea arabica L.). Bionatura 2023, 8, 17. [Google Scholar] [CrossRef]
- Tolessa, K.; D’Heer, J.; Duchateau, L.; Boeckx, P. Influence of growing altitude, shade and harvest period on quality and biochemical composition of Ethiopian specialty coffee. J. Sci. Food. Agric. 2017, 97, 2849–2857. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, F.; Topuz, A. Changes in dry matter, oil content and fatty acids composition of avocado during harvesting time and post-harvesting ripening period. Food Chem. 2004, 86, 79–83. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Bremer, V.; Ferguson, L.; Crisosto, G.M. Evaluating Quality Attributes of Four Fresh Fig (Ficus carica L.) Cultivars Harvested at Two Maturity Stages. Hortsci. Horts. 2010, 45, 707–710. [Google Scholar] [CrossRef]
- Deiana, P.; Santona, M.; Dettori, S.; Culeddu, N.; Dore, A.; Molinu, M.G. Multivariate approach to assess the chemical composition of Italian virgin olive oils as a function of variety and harvest period. Food Chem. 2019, 300, 125243. [Google Scholar] [CrossRef]
- Kath, J.; Mittahalli Byrareddy, V.; Mushtaq, S.; Craparo, A.; Porcel, M. Temperature and rainfall impacts on robusta coffee bean characteristics. Clim. Risk Manag. 2021, 32, 100281. [Google Scholar] [CrossRef]
- Brighenti, C.; Cirillo, M.A. Analysis of defects in coffee beans compared to biplots for simultaneous tables. Rev. Cienc. Agron. 2018, 49, 62–69. [Google Scholar] [CrossRef]
- Latimer, G.W., Jr. Official Methods of Analysis of AOAC INTERNATIONAL; AOAC International: Oxford, UK, 2023; ISBN 9780197610138. [Google Scholar]
- Murthy, P.S.; Manonmani, H.K. Physico-chemical, antioxidant and antimicrobial properties of Indian monsooned coffee. Eur. Food Res. Technol. 2009, 229, 645–650. [Google Scholar] [CrossRef]
- Yüksel, A.N.; Özkara Barut, K.T.; Bayram, M. The effects of roasting, milling, brewing and storage processes on the physicochemical properties of Turkish coffee. LWT 2020, 131, 109711. [Google Scholar] [CrossRef]
- Worku, M.; Astatkie, T.; Boeckx, P. Effect of growing conditions and postharvest processing on arabica coffee bean physical quality features and defects. Heliyon 2022, 8, e9201. [Google Scholar] [CrossRef] [PubMed]
- Casas, M.I.; Vaughan, M.J.; Bonello, P.; McSpadden Gardener, B.; Grotewold, E.; Alonso, A.P. Identification of biochemical features of defective Coffea arabica L. beans. Food Res. Int. 2017, 95, 59–67. [Google Scholar] [CrossRef]
- Fujioka, K.; Shibamoto, T. Chlorogenic acid and caffeine contents in various commercial brewed coffees. Food Chem. 2008, 106, 217–221. [Google Scholar] [CrossRef]
- Camargo, M.B.P.D. The impact of climatic variability and climate change on arabic coffee crop in Brazil. Bragantia 2010, 69, 239–247. [Google Scholar] [CrossRef]
- Zhang, X.; Peck, L.D.; Flood, J.; Ryan, M.J.; Barraclough, T.G. Temperature contributes to host specialization of coffee wilt disease (Fusarium xylarioides) on arabica and robusta coffee crops. Sci. Rep. 2023, 13, 9327. [Google Scholar] [CrossRef] [PubMed]
- Mouen Bedimo, J.A.; Cilas, C.; Nottéghem, J.L.; Bieysse, D. Effect of temperatures and rainfall variations on the development of coffee berry disease caused by Colletotrichum kahawae. Crop Prot. 2012, 31, 125–131. [Google Scholar] [CrossRef]
- Gebisa, M.D.R.L. Impact of Climate Change on Coffee Quality and Yield. Cross Curr. Int. J. Agric. Vet. Sci. 2024, 6, 118–125. [Google Scholar] [CrossRef]
- Williams, S.D.; Barkla, B.J.; Rose, T.J.; Liu, L. Does Coffee Have Terroir and How Should It Be Assessed? Foods 2022, 11, 1907. [Google Scholar] [CrossRef]
- Barbosa, M.D.S.G.; Scholz, M.B.D.S.; Kitzberger, C.S.G.; Benassi, M.D.T. Correlation between the composition of green Arabica coffee beans and the sensory quality of coffee brews. Food Chem. 2019, 292, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, J.C.; Pais, I.P.; Leitao, A.E.; Guerra, M.; Reboredo, F.H.; Máguas, C.M.; Carvalho, M.L.; Scotti-Campos, P.; Ribeiro-Barros, A.I.; Lidon, F.; et al. Can Elevated Air [CO2] Conditions Mitigate the Predicted Warming Impact on the Quality of Coffee Bean? Front. Plant Sci. 2018, 9, 287. [Google Scholar] [CrossRef] [PubMed]
- Perini Da Silva, M.M.; Tarone, A.G.; Giomo, G.S.; Ferrarezzo, E.M.; Guerreiro Filho, O.; Teramoto, J.R.S. Predicting best planting location and coffee cup quality from chemical parameters: An evaluation of raw Arabica coffee beans from São Paulo over two harvests. Food Res. Int. 2024, 195, 114911. [Google Scholar] [CrossRef]
- Cheng, B.; Furtado, A.; Smyth, H.E.; Henry, R.J. Influence of genotype and environment on coffee quality. Trends Food Sci. Technol. 2016, 57, 20–30. [Google Scholar] [CrossRef]
- Zhu, M.; Long, Y.; Ma, Y.; Chen, Y.; Yu, Q.; Xie, J.; Li, B.; Tian, J. Comparison of chemical and fatty acid composition of green coffee bean (Coffea arabica L.) from different geographical origins. LWT 2021, 140, 110802. [Google Scholar] [CrossRef]
- Casal, S.; Mendes, E.; Oliveira, M.B.P.P.; Ferreira, M.A. Roast effects on coffee amino acid enantiomers. Food Chem. 2005, 89, 333–340. [Google Scholar] [CrossRef]
- Speer, K.; Koelling-Speer, I. The lipid fraction of the coffee bean. Braz. J. Plant Physiol. 2006, 18, 201–216. [Google Scholar] [CrossRef]
- Arruda, N.P.; Hovell, A.M.C.; Rezende, C.M.; Freitas, S.P.; Couri, S.; Bizzo, H.R. Correlação entre precursores e voláteis em café arábica brasileiro processado pelas vias seca, semiúmida e úmida e discriminação através da análise por componentes principais. Química Nova. 2012, 35, 2044–2051. [Google Scholar] [CrossRef][Green Version]
- Silva, E.A.D.; Mazzafera, P.; Brunini, O.; Sakai, E.; Arruda, F.B.; Mattoso, L.H.C.; Carvalho, C.R.L.; Pires, R.C.M. The influence of water management and environmental conditions on the chemical composition and beverage quality of coffee beans. Braz. J. Plant Physiol. 2005, 17, 229–238. [Google Scholar] [CrossRef]
- Hall, R.D.; Trevisan, F.; de Vos, R.C.H. Coffee berry and green bean chemistry—Opportunities for improving cup quality and crop circularity. Food Res. Int. 2022, 151, 110825. [Google Scholar] [CrossRef]
- Mehari, B.; Redi-Abshiro, M.; Chandravanshi, B.S.; Atlabachew, M.; Combrinck, S.; McCrindle, R. Simultaneous Determination of Alkaloids in Green Coffee Beans from Ethiopia: Chemometric Evaluation of Geographical Origin. Food Anal. Methods 2016, 9, 1627–1637. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, M.K.; Lee, K. Effect of reversed coffee grinding and roasting process on physicochemical properties including volatile compound profiles. Innov. Food Sci. Emerg. Technol. 2017, 44, 97–102. [Google Scholar] [CrossRef]
- Cordoba, N.; Fernandez-Alduenda, M.; Moreno, F.L.; Ruiz, Y. Coffee extraction: A review of parameters and their influence on the physicochemical characteristics and flavour of coffee brews. Trends Food Sci. Technol. 2020, 96, 45–60. [Google Scholar] [CrossRef]
- Pereira, L.L.; Guarçoni, R.C.; Pinheiro, P.F.; Osório, V.M.; Pinheiro, C.A.; Moreira, T.R.; Ten Caten, C.S. New propositions about coffee wet processing: Chemical and sensory perspectives. Food Chem. 2020, 310, 125943. [Google Scholar] [CrossRef]
- Muzaifa, M.; Hasni, D.; Febriani; Patria, A.; Abubakar, A. Chemical composition of green and roasted coffee bean of Gayo arabica civet coffee (Kopi luwak). IOP Conf. Ser. Earth Environ. Sci. 2020, 425, 12001. [Google Scholar] [CrossRef]
- Bote, A.D.; Vos, J. Tree management and environmental conditions affect coffee (Coffea arabica L.) bean quality. NJAS-Wagening. J. Life Sci. 2017, 83, 39–46. [Google Scholar] [CrossRef]
- Marie, L.; Abdallah, C.; Campa, C.; Courtel, P.; Bordeaux, M.; Navarini, L.; Lonzarich, V.; Bosselmann, A.S.; Turreira-García, N.; Alpizar, E.; et al. G × E interactions on yield and quality in Coffea arabica: New F1 hybrids outperform American cultivars. Euphytica 2020, 216, 78. [Google Scholar] [CrossRef]
- Gamboa-Becerra, R.; Hernández-Hernández, M.C.; González-Ríos, Ó.; Suárez-Quiroz, M.L.; Gálvez-Ponce, E.; Ordaz-Ortiz, J.J.; Winkler, R. Metabolomic Markers for the Early Selection of Coffea canephora Plants with Desirable Cup Quality Traits. Metabolites 2019, 9, 214. [Google Scholar] [CrossRef]
- Franca, A.S.; Mendonça, J.C.F.; Oliveira, S.D. Composition of green and roasted coffees of different cup qualities. LWT-Food Sci. Technol. 2005, 38, 709–715. [Google Scholar] [CrossRef]
- Farah, A.; Monteiro, M.C.; Calado, V.; Franca, A.S.; Trugo, L.C. Correlation between cup quality and chemical attributes of Brazilian coffee. Food Chem. 2006, 98, 373–380. [Google Scholar] [CrossRef]
- Munyendo, L.M.; Njoroge, D.M.; Owaga, E.E.; Mugendi, B. Coffee phytochemicals and post-harvest handling—A complex and delicate balance. J. Food Compos. Anal. 2021, 102, 103995. [Google Scholar] [CrossRef]
- Sittipod, S.; Schwartz, E.; Paravisini, L.; Peterson, D.G. Identification of flavor modulating compounds that positively impact coffee quality. Food Chem. 2019, 301, 125250. [Google Scholar] [CrossRef] [PubMed]
- Fassio, L.O.; Malta, M.R.; Carvalho, G.R.; Liska, G.R.; Lima, P.M.; Nadaleti, D.H.S.; Fonseca, A.J.; Pimenta, C.J. Fatty Acids Profile of Coffea arabica L. Resistant to Leaf Rust Grown in Two Environments of Minas Gerais, Brazil. J. Agric. Sci. 2017, 9, 88. [Google Scholar] [CrossRef]

| Harvest Periods | Average Temperature (°C) | Precipitation (mm) | Average Temperature Range (°C) |
|---|---|---|---|
| Harvest Period 1 | 19.4 | 15 | 11.7–19.8 |
| Harvest Period 2 | 14.1 | 0 | 6.8–14 |
| Harvest Period 3 | 15.4 | 30 | 9.4–21.1 |
| Harvest Period 4 | 20.8 | 2.9 | 12.7–26.9 |
| 7963 | T8667 | |
|---|---|---|
| Defective rate (%) | ||
| Harvest period 1 | 11.08 ± 1.99 bA | 12.7 ± 1.59 bA |
| Harvest period 2 | 14 ± 0.74 cA | 14.95 ± 0.48 cA |
| Harvest period 3 | 5.6 ± 0.35 aA | 4.72 ± 0.75 aA |
| Harvest period 4 | 4.19 ± 0.59 aA | 6.78 ± 0.85 bB |
| mean | 8.72 ± 4.28 A | 9.79 ± 4.45 A |
| Thousand-grain weight (g) | ||
| Harvest period 1 | 128.84 ± 2.47 bB | 119.75 ± 3.44 bA |
| Harvest period 2 | 120.89 ± 1.16 aB | 111.88 ± 0.99 aA |
| Harvest period 3 | 133.15 ± 1.1 cB | 128.14 ± 1.38 cA |
| Harvest period 4 | 139.07 ± 2.08 dA | 157.63 ± 0.63 dB |
| mean | 130.49 ± 7.09 A | 129.35 ± 18.15 A |
| pH | ||
| Harvest period 1 | 6.09 ± 0.03 aA | 6.13 ± 0.04 bA |
| Harvest period 2 | 6.1 ± 0.01 aA | 6.09 ± 0.03 bA |
| Harvest period 3 | 6.16 ± 0.01 bB | 5.96 ± 0.03 aA |
| Harvest period 4 | 6.11 ± 0.02 aA | 6.22 ± 0.01 cB |
| mean | 6.11 ± 0.03 A | 6.1 ± 0.1 A |
| TDS (%) | ||
| Harvest period 1 | 1.73 ± 0.12 aA | 1.7 ± 0.1 aA |
| Harvest period 2 | 1.77 ± 0.06 aA | 1.67 ± 0.06 aA |
| Harvest period 3 | 2.37 ± 0.06 bA | 3.5 ± 0.1 cB |
| Harvest period 4 | 1.79 ± 0.06 aA | 2.1 ± 0.1 bB |
| mean | 1.91 ± 0.28 A | 2.24 ± 0.78 A |
| Variables | Variety | Harvest Period | Interaction | |
|---|---|---|---|---|
| Green beans | Defect rate | * | ** | ns |
| Thousand-grain weight | ns | ** | ** | |
| Chlorogenic acid | ** | ** | ** | |
| Caffeine | ns | * | ns | |
| Trigonelline | ns | * | ns | |
| Lipids | ** | ** | ** | |
| Proteins | ** | ** | ** | |
| pH | ns | ** | ** | |
| TDS | ** | ** | ** | |
| Roasted beans | Chlorogenic acid | ** | * | ** |
| Caffeine | ** | ** | ** | |
| Trigonelline | ** | ** | ** | |
| pH | ** | ** | ** | |
| TDS | ns | ns | ns | |
| Cupping scores | ** | ** | ** | |
| 7963 | T8667 | |
|---|---|---|
| Lipid (% d.b.) | ||
| Harvest period 1 | 13.46 ± 0.08 aB | 13.29 ± 0.07 aA |
| Harvest period 2 | 13.34 ± 0.08 aA | 13.44 ± 0.07 bA |
| Harvest period 3 | 13.78 ± 0.09 bA | 13.62 ± 0.05 cA |
| Harvest period 4 | 14.16 ± 0.07 cB | 13.6 ± 0.05 cA |
| mean | 13.69 ± 0.34 A | 13.49 ± 0.15 A |
| Protein (% d.b.) | ||
| Harvest period 1 | 12.53 ± 0.05 aB | 11.36 ± 0.05 aA |
| Harvest period 2 | 12.60 ± 0.07 aB | 11.21 ± 0.1 aA |
| Harvest period 3 | 14.26 ± 0.06 cB | 13.33 ± 0.05 bA |
| Harvest period 4 | 13.96 ± 0.08 bB | 13.43 ± 0.2 bA |
| mean | 13.34 ± 0.82 B | 12.33 ± 1.1 A |
| CGA (% d.b.) | ||
| Harvest period 1 | 3.78 ± 0.27 aA | 3.39 ± 0.26 aA |
| Harvest period 2 | 3.76 ± 0.37 aA | 3.51 ± 0.25 aA |
| Harvest period 3 | 4.28 ± 0.26 bA | 3.74 ± 0.31 aA |
| Harvest period 4 | 4.99± 0.03 cB | 3.48 ± 0.32 aA |
| mean | 4.2 ± 0.57 B | 3.53 ± 0.28 A |
| Caffeine (% d.b.) | ||
| Harvest period 1 | 0.89 ± 0.09 aA | 0.86 ± 0.07 aA |
| Harvest period 2 | 0.94 ± 0.1 aA | 0.84 ± 0.1 aA |
| Harvest period 3 | 1.04 ± 0.12 abA | 0.92 ± 0.13 aA |
| Harvest period 4 | 1.15 ± 0.08 bA | 1.14 ± 0.06 bA |
| mean | 1.01 ± 0.13 A | 0.94 ± 0.15 A |
| Trigonelline (% d.b.) | ||
| Harvest period 1 | 0.8 ± 0.08 aA | 0.81 ± 0.08 aA |
| Harvest period 2 | 0.82 ± 0.09 aA | 0.78 ± 0.09 aA |
| Harvest period 3 | 0.91 ± 0.11 abA | 0.82 ± 0.12 aA |
| Harvest period 4 | 1.07 ± 0.07 bA | 0.94 ± 0.05 aA |
| mean | 0.9 ± 0.14 A | 0.84 ± 0.1 A |
| 7963 | T8667 | |
|---|---|---|
| pH | ||
| Harvest period 1 | 5.23 ± 0.01 aA | 5.22 ± 0.01 bA |
| Harvest period 2 | 5.22 ± 0.01 aA | 5.21 ± 0.02 bA |
| Harvest period 3 | 5.32 ± 0.02 bB | 5.14 ± 0.01 aA |
| Harvest period 4 | 5.22 ± 0.02 aA | 5.31 ± 0.01 cB |
| mean | 5.25 ± 0.05 A | 5.22 ± 0.06 A |
| TDS (%) | ||
| Harvest period 1 | 1.27 ± 0.13 aA | 1.37 ± 0.05 aA |
| Harvest period 2 | 1.4 ± 0.12 abA | 1.35 ± 0.08 aA |
| Harvest period 3 | 1.37 ± 0.06 abA | 1.45 ± 0.09 aA |
| Harvest period 4 | 1.58 ± 0.16 cA | 1.4 ± 0.05 aA |
| mean | 1.41 ± 0.16 A | 1.39 ± 0.07 A |
| CGA (% d.b.) | ||
| Harvest period 1 | 1.21 ± 0.08 aA | 1.1 ± 0.06 aA |
| Harvest period 2 | 1.22 ± 0.07 aA | 1.21 ± 0.06 aA |
| Harvest period 3 | 1.36 ± 0.07 bA | 1.35 ± 0.09 bA |
| Harvest period 4 | 1.67± 0.05 cB | 1.09 ± 0.08 aA |
| mean | 1.36 ± 0.2 A | 1.19 ± 0.13 A |
| Caffeine (% d.b.) | ||
| Harvest period 1 | 0.82 ± 0.06 bB | 0.75 ± 0.01 aA |
| Harvest period 2 | 0.78 ± 0.002 aB | 0.76 ± 0.001 aA |
| Harvest period 3 | 0.83 ± 0.01 bB | 0.79 ± 0.001 bA |
| Harvest period 4 | 0.85 ± 0.01 cB | 0.81 ± 0.005 cA |
| mean | 0.82 ± 0.03 B | 0.78 ± 0.25 A |
| Trigonelline (% d.b.) | ||
| Harvest period 1 | 0.37 ± 0.015 aA | 0.39 ± 0.001 cA |
| Harvest period 2 | 0.38 ± 0.001 aB | 0.35 ± 0.001 aA |
| Harvest period 3 | 0.39 ± 0.06 bB | 0.37 ± 0.005 bA |
| Harvest period 4 | 0.42 ± 0.004 cB | 0.35 ± 0.002 aA |
| mean | 0.39 ± 0.02 A | 0.37 ± 0.02 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, G.; Liu, S.; Chen, G.-L.; Zhao, Y.; Huang, Q.; Cen, Q.; Ren, E.-F. Effects of Harvesting Periods and Cultivar on the Physicochemical and Sensory Properties of Two Coffee Bean Varieties. Foods 2025, 14, 3135. https://doi.org/10.3390/foods14173135
Huang G, Liu S, Chen G-L, Zhao Y, Huang Q, Cen Q, Ren E-F. Effects of Harvesting Periods and Cultivar on the Physicochemical and Sensory Properties of Two Coffee Bean Varieties. Foods. 2025; 14(17):3135. https://doi.org/10.3390/foods14173135
Chicago/Turabian StyleHuang, Guanru, Shuaimin Liu, Gan-Lin Chen, Yuan Zhao, Qiulan Huang, Qingjing Cen, and Er-Fang Ren. 2025. "Effects of Harvesting Periods and Cultivar on the Physicochemical and Sensory Properties of Two Coffee Bean Varieties" Foods 14, no. 17: 3135. https://doi.org/10.3390/foods14173135
APA StyleHuang, G., Liu, S., Chen, G.-L., Zhao, Y., Huang, Q., Cen, Q., & Ren, E.-F. (2025). Effects of Harvesting Periods and Cultivar on the Physicochemical and Sensory Properties of Two Coffee Bean Varieties. Foods, 14(17), 3135. https://doi.org/10.3390/foods14173135





