Deep Learning Enabled Optimization and Mass Transfer Mechanism in Ultrasound-Assisted Enzymatic Extraction of Polyphenols from Tartary Buckwheat Hulls
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Tartary Buckwheat Hull
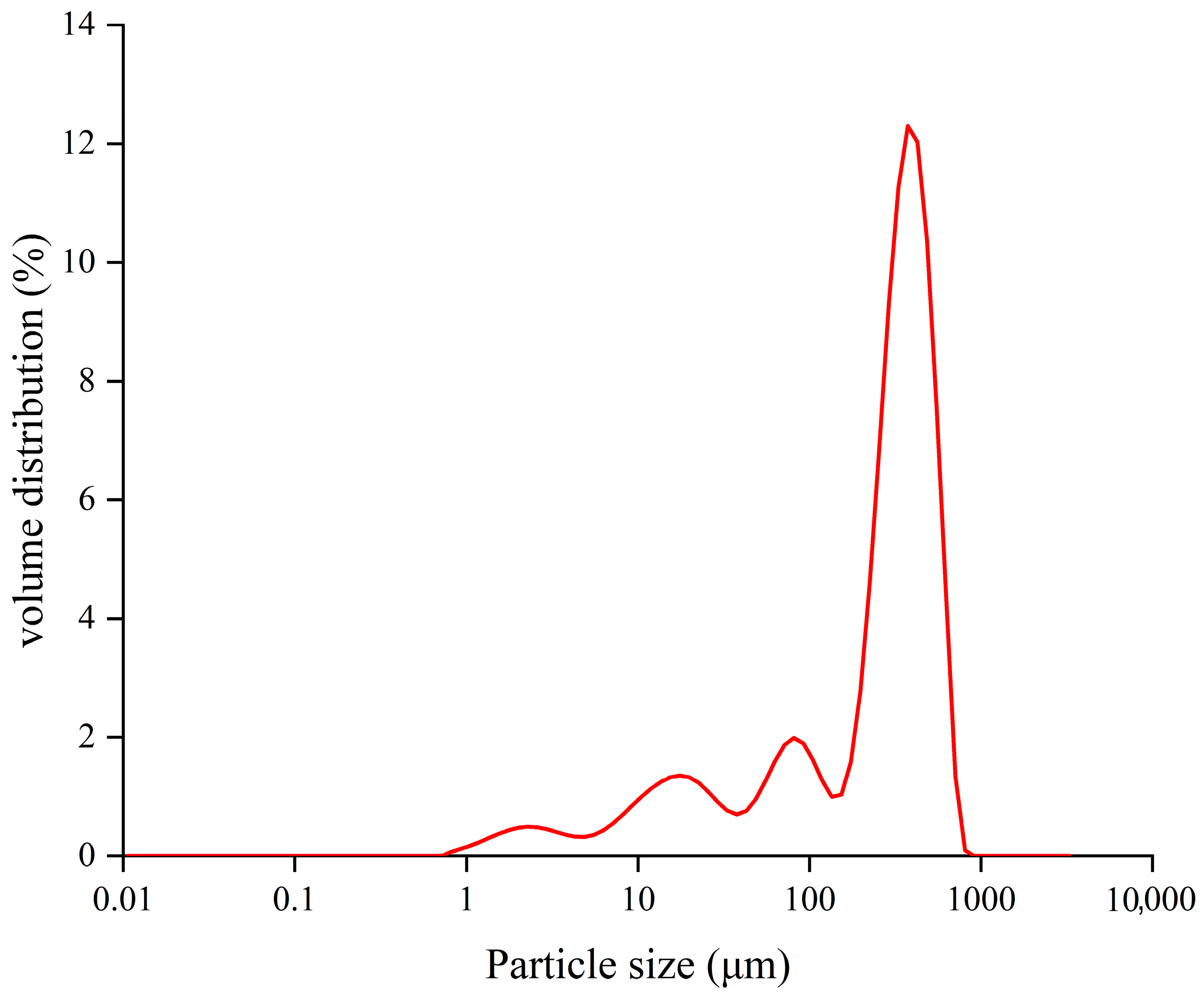
2.2. Particle Size Distribution of TBH
2.3. Extraction Protocol of Phenolics
2.4. Determination of Total Phenolic Content
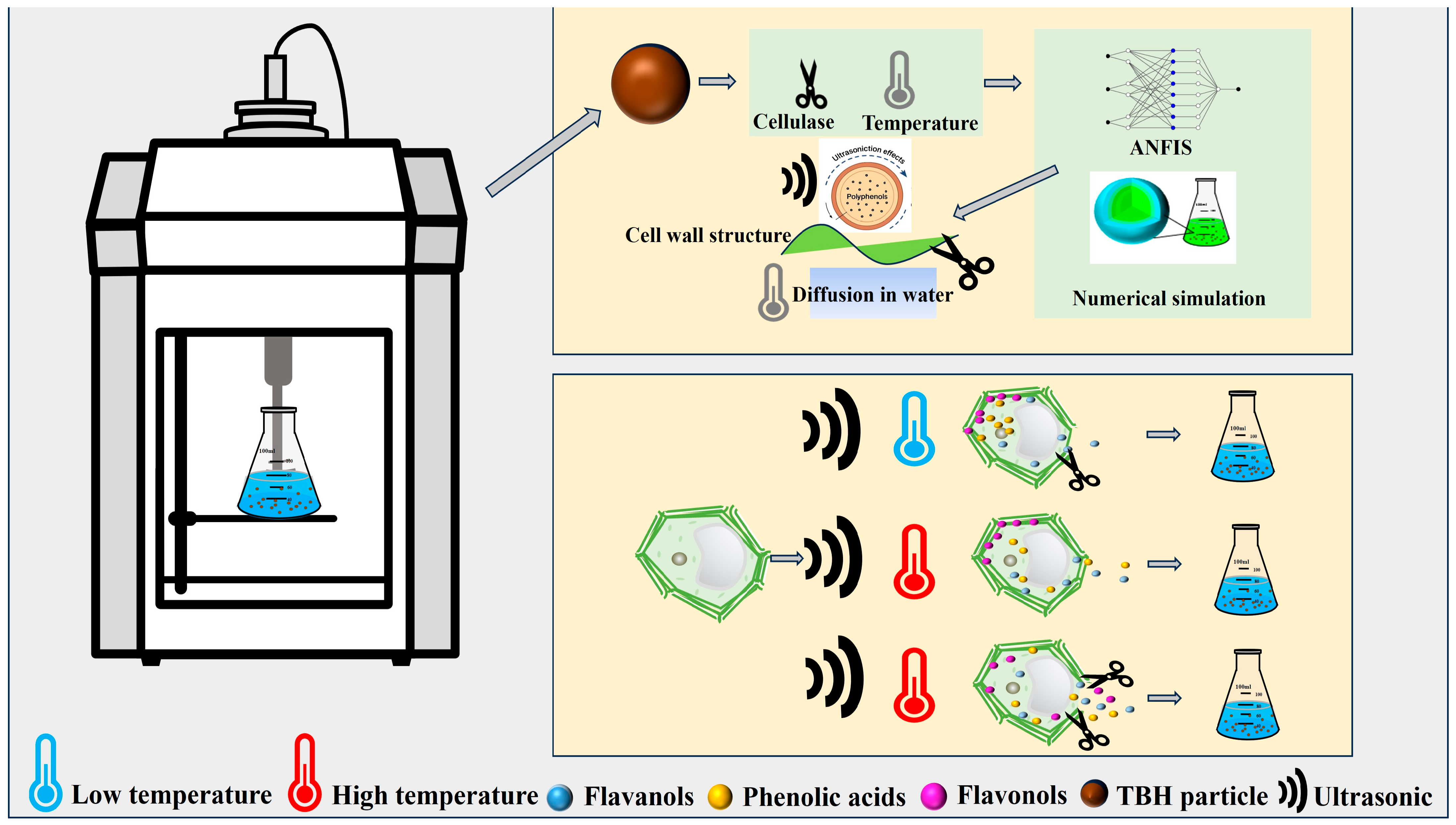
2.5. Modeling of Ultrasound-Assisted Extraction of Phenolics
- (a)
- TBH granules were modeled as spheres with an average diameter of 49.00 μm, containing uniformly distributed phenolic compounds.
- (b)
- Micro-turbulence and cavitation bubbles ensured thorough mixing of the extraction suspension, rendering external mass transfer resistance negligible.
- (c)
- The effective diffusion coefficient () maintained constant values during extraction due to insignificant changes in particle size and external temperature.
- (d)
- In the extraction process, the swelling of TBH particles were not considered, and changes in particle size were neglected.
- (e)
- No degradation of phenolic compounds during sonication was considered.
- (f)
- The phenolic concentration at the particle-solvent interface equilibrated with that in the adjacent solvent phase.
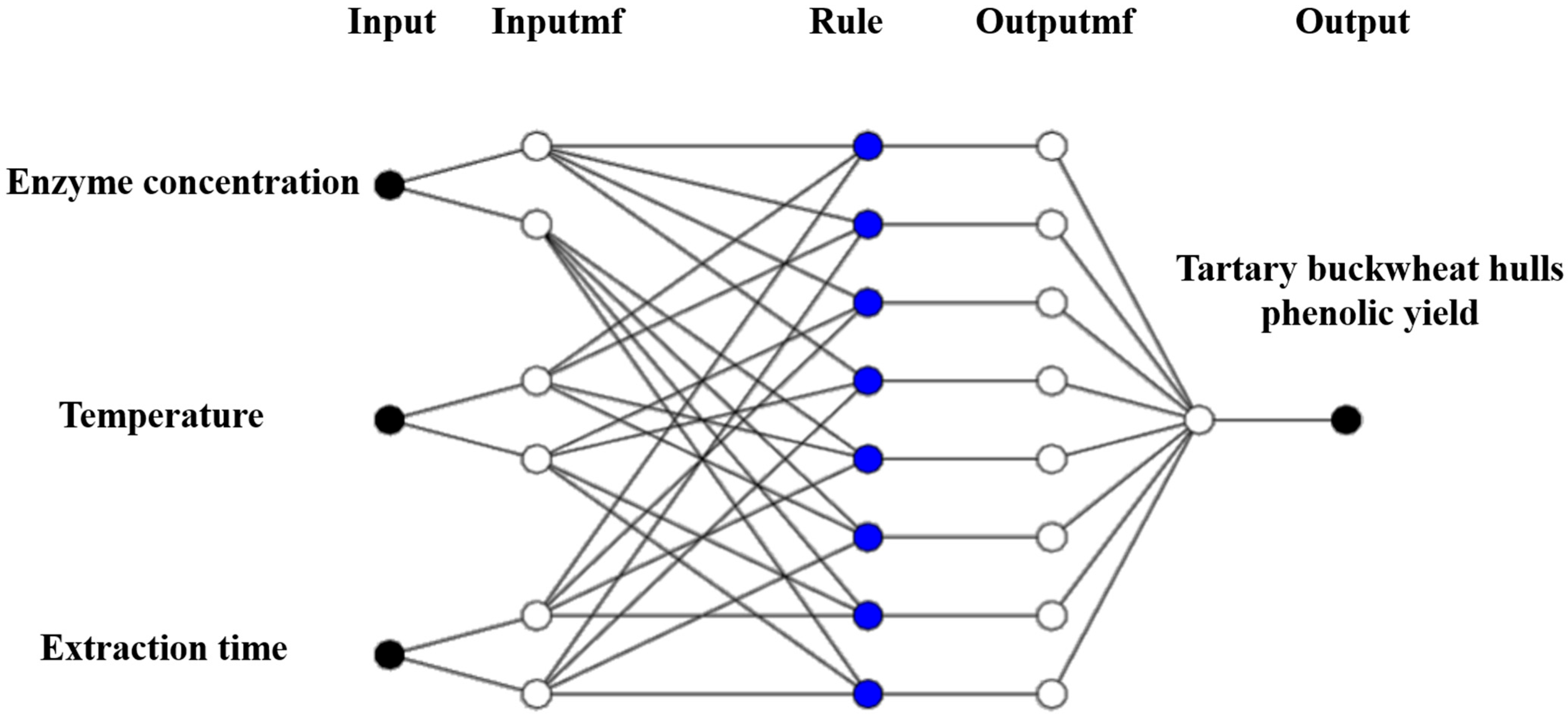
2.6. ANFIS Modeling
2.7. Quantification of Phenolic Compounds Through HPLC Analysis
2.8. Statistical Analysis
3. Results
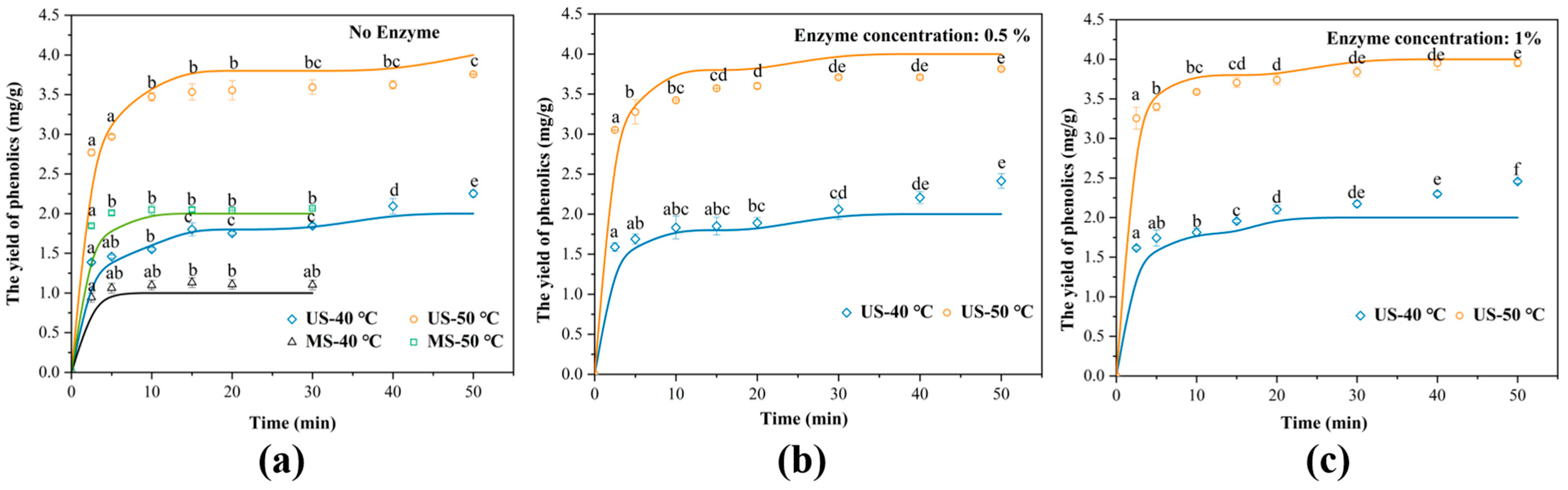
3.1. Comparative Analysis of the Kinetic Behavior of Phenolic Yields Under Different Extraction Conditions
3.2. Analysis of Mass Transfer Dynamic Parameters Based on Diffusion Model
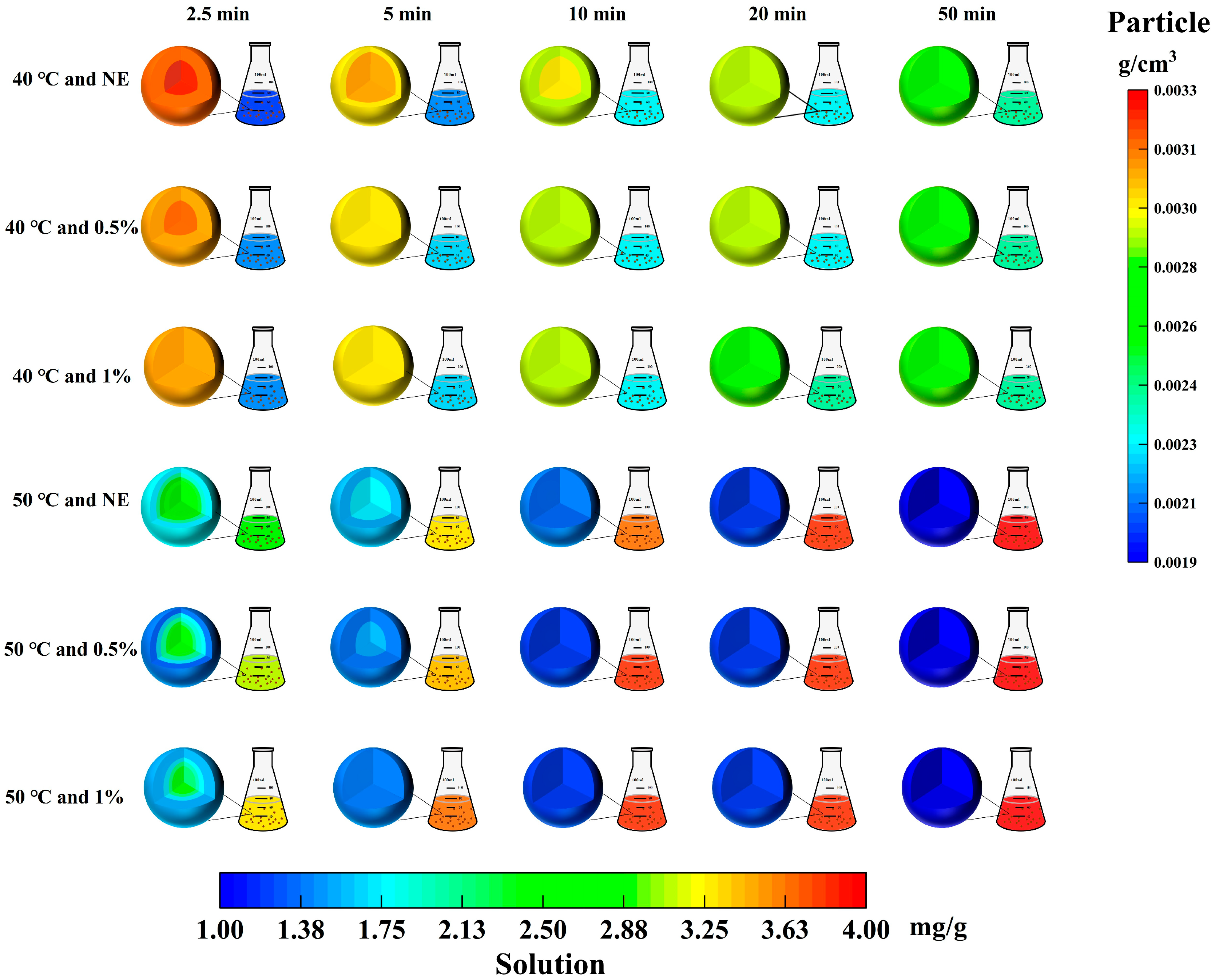
3.3. Phenolic Concentration Gradient Within TBH Particles Under Different Ultrasonic-Assisted Enzymatic Extraction Conditions
3.4. ANFIS Modeling Under Different Enzyme-Assisted Ultrasound Conditions
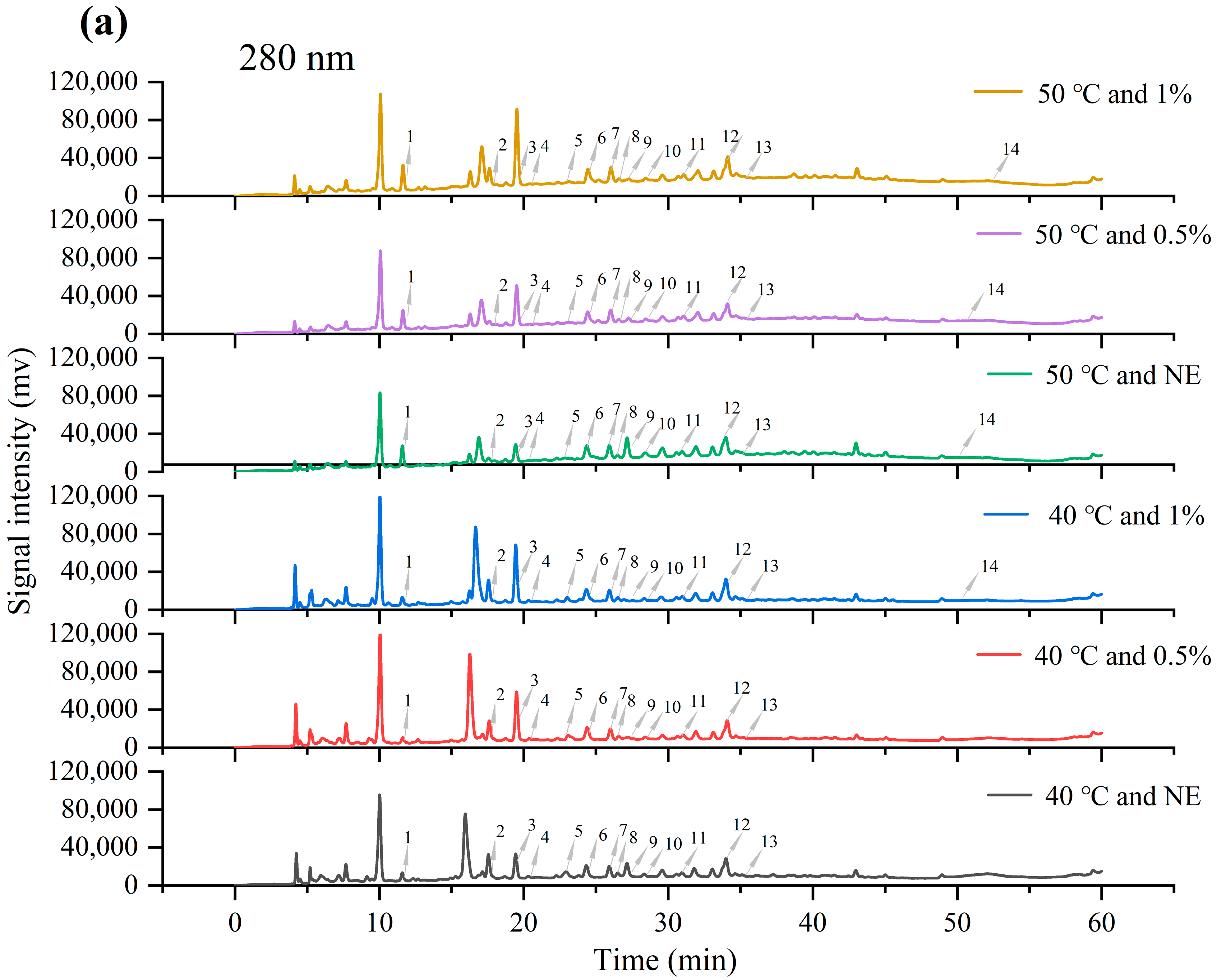
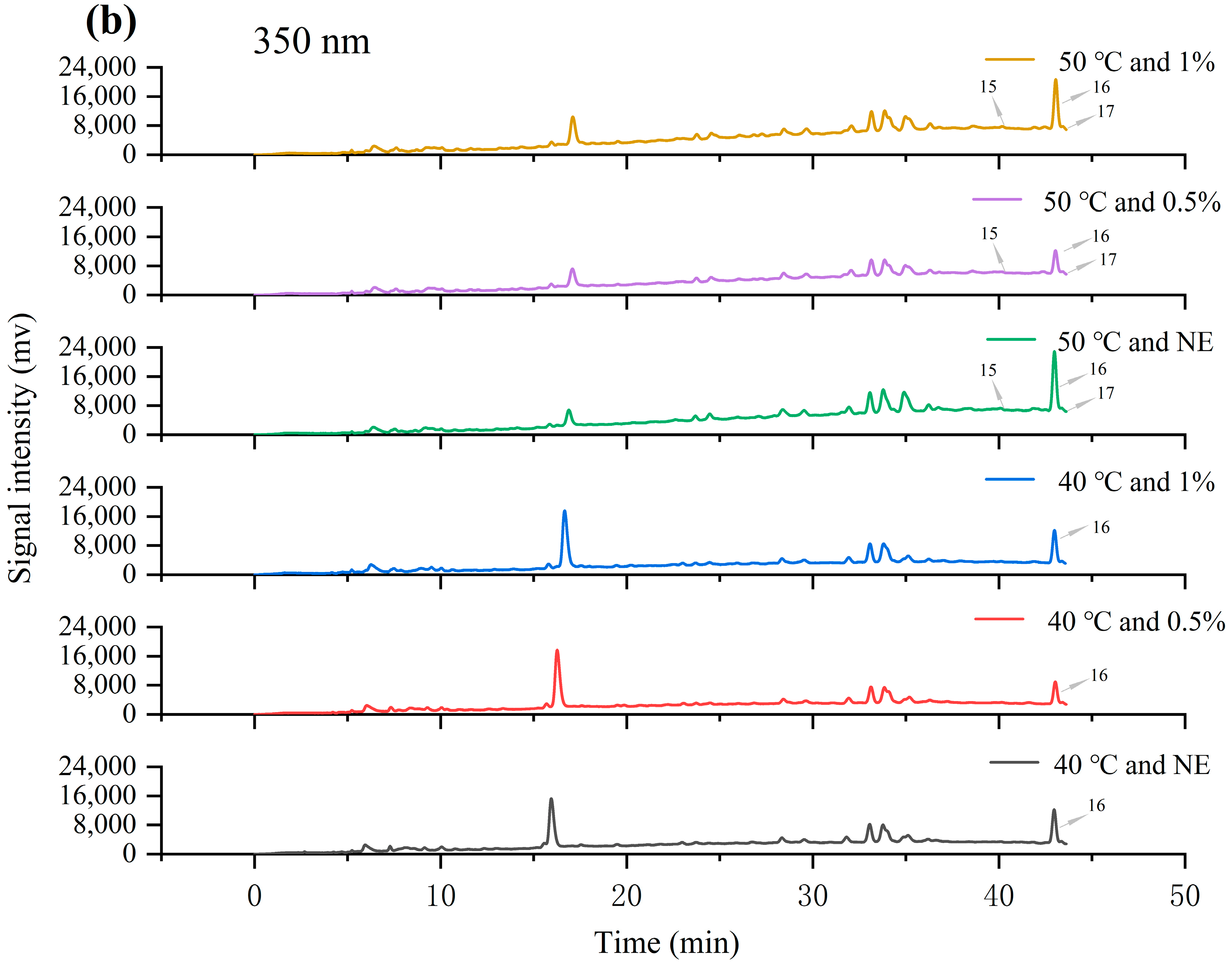
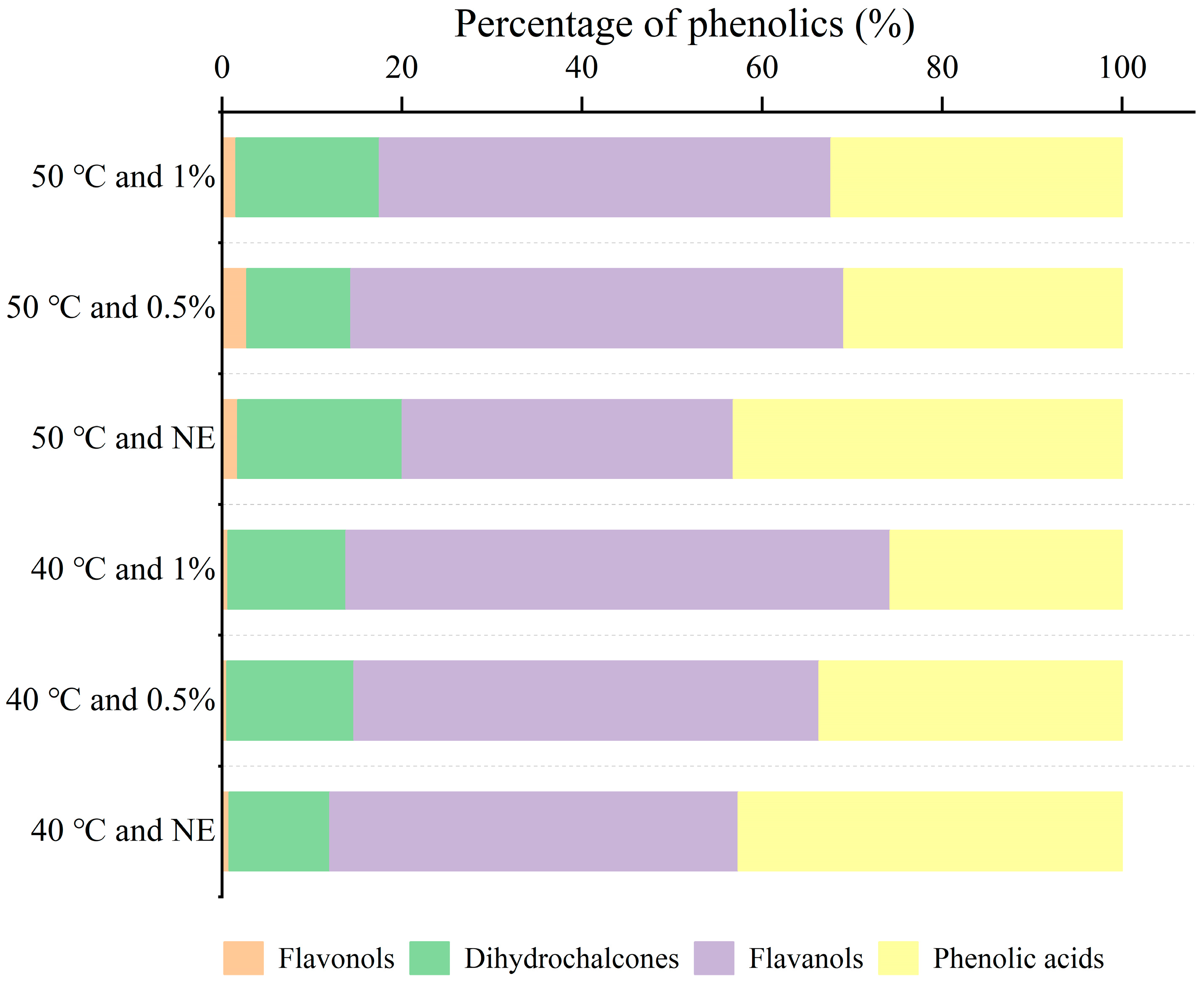
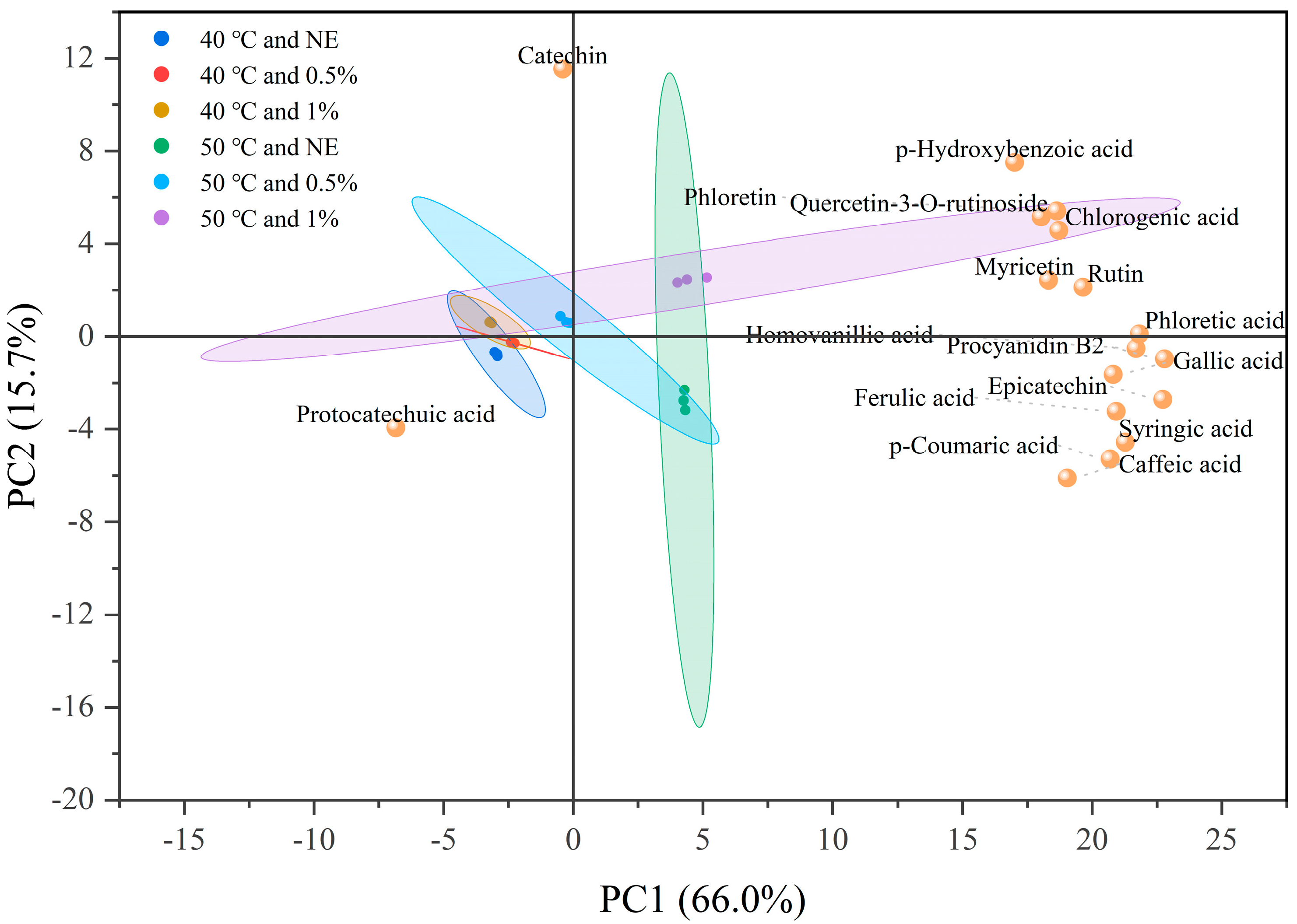
3.5. Identification and Content Analysis of Main Phenolic Components in Phenolic Mixtures
3.6. Construction of Mass Transfer Mechanism and Structural Response of Phenolics in TBH Particles
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| TBH | Tartary buckwheat hulls |
| NE | No Enzyme |
| ANFIS | adaptive neuro-fuzzy inference system |
| ANN | artificial neural network |
| RSM | surface methodology model |
| FIS | fuzzy inference system |
| RMSE | root mean square error (mg/g) |
| R2 | coefficient of determination |
| AAD | absolute average deviation (%) |
| MFs | membership functions |
| effective diffusion coefficient (m2/s) | |
| concentration of the TBH phenolic (g/cm3) | |
| extraction time (min) | |
| spherical particle radial coordinate (m) | |
| total phenolic content in liquid phase (g/mL) | |
| contacting area among the extracting solvent and particles (m2) | |
| the volume of suspension (mL) | |
| particle radius (m) | |
| total phenolic content from TBH granules predicted by diffusion model (g/mL) | |
| total phenolic content from TBH granules acquired through experiments (g/mL) | |
| experimentally determined mass of phenolic extracted from TBH granules (mg/g) | |
| predicted mass of phenolic extracted from TBH granules (mg/g) | |
| the average value of the mass of phenolic extracted from TBH granules across all the experimental data (mg/g) |
Appendix A
Appendix B
References
- Li, J.; Gong, Y.; Li, J.; Fan, L. In vitro inhibitory effects of polyphenols from Tartary buckwheat on xanthine oxidase: Identification, inhibitory activity, and action mechanism. Food Chem. 2022, 379, 132100. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.H.; Wang, H. Nutrient components and bioactive compounds in tartary buckwheat bran and flour as affected by thermal processing. Int. J. Food Prop. 2020, 23, 127–137. [Google Scholar] [CrossRef]
- Park, B.I.; Kim, J.; Lee, K.; Lim, T.; Hwang, K.T. Flavonoids in common and tartary buckwheat hull extracts and antioxidant activity of the extracts against lipids in mayonnaise. J. Food Sci. Technol. 2019, 56, 2712–2720. [Google Scholar] [CrossRef]
- Noore, S.; Joshi, A.; Kumari, B.; Zhao, M.; O’Donnell, C.; Tiwari, B.K. Effects of Novel Extraction Strategies on the Recovery of Phenolic Compounds and Associated Antioxidant Properties from Buckwheat Hull (Fagopyrum esculentum). Processes 2022, 10, 365. [Google Scholar] [CrossRef]
- Dziadek, K.; Kopeć, A.; Pastucha, E.; Piątkowska, E.; Leszczyńska, T.; Pisulewska, E.; Witkowicz, R.; Francik, R. Basic chemical composition and bioactive compounds content in selected cultivars of buckwheat whole seeds, dehulled seeds and hulls. J. Cereal Sci. 2016, 69, 1–8. [Google Scholar] [CrossRef]
- Dzah, C.S.; Yuqing, D.; Haihui, Z.; Kwaku, G.M.; Ma, H. Enhanced screening of key ultrasonication parameters: Total phenol content and antioxidant activity assessment of Tartary buckwheat (Fagopyrum tataricum) water extract. Sep. Sci. Technol. 2020, 55, 3242–3251. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Boateng, N.A.S.; Ma, H. Ultrasound-induced lipid peroxidation: Effects on phenol content and extraction kinetics and antioxidant activity of Tartary buckwheat (Fagopyrum tataricum) water extract. Food Biosci. 2020, 37, 100719. [Google Scholar] [CrossRef]
- Sinkovič, L.; Kokalj Sinkovič, D.; Meglič, V. Milling fractions composition of common (Fagopyrum esculentum Moench) and Tartary (Fagopyrum tataricum L. Gaertn.) buckwheat. Food Chem. 2021, 365, 130459. [Google Scholar] [CrossRef]
- Guo, X.-D.; Wu, C.-S.; Ma, Y.-J.; Parry, J.; Xu, Y.-Y.; Liu, H.; Wang, M. Comparison of milling fractions of tartary buckwheat for their phenolics and antioxidant properties. Food Res. Int. 2012, 49, 53–59. [Google Scholar] [CrossRef]
- Gavrila, A.I.; Damian, E.J.; Rosca, A.; Calinescu, I.; Hodosan, C.; Popa, I. Optimization of Microwave-Assisted Extraction of Polyphenols from Crataegus monogyna L. Antioxidants 2025, 14, 357. Antioxidants 2025, 14, 357. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, G.; Marina, M.L.; Plaza, M. Enzyme-assisted extraction of bioactive non-extractable polyphenols from sweet cherry (Prunus avium L.) pomace. Food Chem. 2021, 339, 128086. [Google Scholar] [CrossRef]
- Gao, Y.; Shi, Y.; Miao, N.; Xing, W.; Yun, C.; Wang, S.; Wang, W.; Wang, H. A green ultrasound-assisted enzymatic extraction method for efficient extraction of total polyphenols from Empetrum nigrum and determination of its bioactivities. J. Ind. Eng. Chem. 2022, 109, 559–567. [Google Scholar] [CrossRef]
- Peiró, S.; Luengo, E.; Segovia, F.; Raso, J.; Almajano, M.P. Improving Polyphenol Extraction from Lemon Residues by Pulsed Electric Fields. Waste Biomass Valorization 2019, 10, 889–897. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Zhang, Y.; Ma, H.; Liang, Q.; Qu, W.; He, R.; Zhou, C.; Mahunu, G.K. Effects of ultrasound and ultrasound assisted alkaline pretreatments on the enzymolysis and structural characteristics of rice protein. Ultrason. Sonochemistry 2016, 31, 20–28. [Google Scholar] [CrossRef]
- Ma, Y.-r.; Xu, Y.-q.; Guo, W.; Shi, Y.-l.; Wu, Y.; Chen, Z.-g. Combined ANFIS and numerical methods to reveal the mass transfer mechanism of ultrasound-enhanced extraction of proteins from millet. Ultrason. Sonochemistry 2024, 111, 107153. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Fei, Q.; Manickam, S.; Li, D.; Xiao, H.; Han, Y.; Show, P.L.; Zhang, G.; Tao, Y. Mechanistic study of the solid-liquid extraction of phenolics from walnut pellicle fibers enhanced by ultrasound, microwave and mechanical agitation forces. Chemosphere 2022, 309, 136451. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Ma, X.; Zhang, K.; Li, S.; Wang, X.; Liu, X.; Liu, J.; Fan, W.; Li, Y.; et al. Study on the kinetic model, thermodynamic and physicochemical properties of Glycyrrhiza polysaccharide by ultrasonic assisted extraction. Ultrason. Sonochemistry 2019, 51, 249–257. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, Y.; Pan, M.; Zhong, S.; Wu, Y.; Yang, R.; Han, Y.; Zhou, J. Combined ANFIS and numerical methods to simulate ultrasound-assisted extraction of phenolics from chokeberry cultivated in China and analysis of phenolic composition. Sep. Purif. Technol. 2017, 178, 178–188. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, S.; Yang, X.; Guo, H.; Li, Y.; Xu, W.; Chen, J.; Wang, Z.; Guo, M.; Liu, D. Novel Accelerated Penetration Extraction for Polyphenol Extraction from Pomegranate Skins: Utilization of Fick’s Law. ACS Sustain. Chem. Eng. 2021, 9, 3702–3709. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, Z.; Sun, D.-W. Kinetic modeling of ultrasound-assisted extraction of phenolic compounds from grape marc: Influence of acoustic energy density and temperature. Ultrason. Sonochemistry 2014, 21, 1461–1469. [Google Scholar] [CrossRef]
- Pinelo, M.; Zornoza, B.; Meyer, A.S. Selective release of phenols from apple skin: Mass transfer kinetics during solvent and enzyme-assisted extraction. Sep. Purif. Technol. 2008, 63, 620–627. [Google Scholar] [CrossRef]
- Pusty, K.; Kumar Dash, K.; Giri, S.; Raj, G.V.S.B.; Tiwari, A.; Shaikh, A.M.; Béla, K. Ultrasound assisted phytochemical extraction of red cabbage by using deep eutectic solvent: Modelling using ANFIS and optimization by genetic algorithms. Ultrason. Sonochemistry 2024, 102, 106762. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, J.; Li, J.; Li, P.; Zhao, M.; Xia, G. Optimization of ultrasonic-assisted extraction of polyphenol from Areca nut (Areca catechu L.) seeds using response surface methodology and its effects on osteogenic activity. Ultrason. Sonochemistry 2023, 98, 106511. [Google Scholar] [CrossRef]
- Liao, J.; Xue, H.; Li, J. Extraction of phenolics and anthocyanins from purple eggplant peels by multi-frequency ultrasound: Effects of different extraction factors and optimization using uniform design. Ultrason. Sonochemistry 2022, 90, 106174. [Google Scholar] [CrossRef]
- Shao, J.; Xiao, X.; Li, Y.; Chen, B.; Xing, L.; Zhu, Z.; Qi, C. Ultrasound-assisted enzymatic extraction of dark tea total polyphenols. Front. Nutr. 2025, 12, 1548103. [Google Scholar] [CrossRef]
- Bhinder, S.; Singh, B.; Kaur, A.; Singh, N.; Kaur, M.; Kumari, S.; Yadav, M.P. Effect of infrared roasting on antioxidant activity, phenolic composition and Maillard reaction products of Tartary buckwheat varieties. Food Chem. 2019, 285, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Han, F.; He, J.; Duan, C. HPLC-DAD-ESI-MS/MS Analysis and Antioxidant Activities of Nonanthocyanin Phenolics in Mulberry (Morus alba L.). J. Food Sci. 2008, 73, C512–C518. [Google Scholar] [CrossRef] [PubMed]
- Şahin, S.; Samlı, R. Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology. Ultrason. Sonochemistry 2012, 20, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Pinelo, M.; Arnous, A.; Meyer, A.S. Upgrading of grape skins: Significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci. Technol. 2006, 17, 579–590. [Google Scholar] [CrossRef]
- Biswas, A.; Dey, S.; Xiao, A.; Deng, Y.; Birhanie, Z.M.; Roy, R.; Akhter, D.; Liu, L.; Li, D. Ultrasound-assisted extraction (UAE) of antioxidant phenolics from Corchorus olitorius leaves: A response surface optimization. Chem. Biol. Technol. Agric. 2023, 10, 64. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Pinela, J.; Pereira, C.; Calhelha, R.C.; Oliveira, I.; Heleno, S.; Oliveira, M.B.P.P.; Barros, L. Optimization and comparison of heat- and ultrasound-assisted extraction methods for anthocyanin recovery from Sicana odorifera fruit epicarp. Biomass Convers. Biorefinery 2025, 15, 1027–1040. [Google Scholar] [CrossRef]
- Oroian, M.; Ursachi, F.; Dranca, F. Influence of ultrasonic amplitude, temperature, time and solvent concentration on bioactive compounds extraction from propolis. Ultrason. Sonochemistry 2020, 64, 105021. [Google Scholar] [CrossRef]
- Khemakhem, I.; Ahmad-Qasem, M.H.; Catalán, E.B.; Micol, V.; García-Pérez, J.V.; Ayadi, M.A.; Bouaziz, M. Kinetic improvement of olive leaves’ bioactive compounds extraction by using power ultrasound in a wide temperature range. Ultrason. Sonochemistry 2016, 34, 466–473. [Google Scholar] [CrossRef]
- Bautista-Ortín, A.B.; Ben Abdallah, R.; Castro-López, L.D.; Jiménez-Martínez, M.D.; Gómez-Plaza, E. Technological Implications of Modifying the Extent of Cell Wall-Proanthocyanidin Interactions Using Enzymes. Int. J. Mol. Sci. 2016, 17, 123. [Google Scholar] [CrossRef]
- Hojnik, M.; Škerget, M.; Knez, Ž. Extraction of lutein from Marigold flower petals—Experimental kinetics and modelling. LWT—Food Sci. Technol. 2008, 41, 2008–2016. [Google Scholar] [CrossRef]
- Renard, C.M.G.C.; Watrelot, A.A.; Le Bourvellec, C. Interactions between polyphenols and polysaccharides: Mechanisms and consequences in food processing and digestion. Trends Food Sci. Technol. 2016, 60, 43–51. [Google Scholar] [CrossRef]
- Mosca, F.; Hidalgo, G.I.; Villasante, J.; Almajano, M.P. Continuous or Batch Solid-Liquid Extraction of Antioxidant Compounds from Seeds of Sterculia apetala Plant and Kinetic Release Study. Molecules 2018, 23, 1759. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Sun, D.-W. Enhancement of food processes by ultrasound: A review. Crit. Rev. Food Sci. Nutr. 2014, 55, 570–594. [Google Scholar] [CrossRef]
- Das, S.; Nadar, S.S.; Rathod, V.K. Integrated strategies for enzyme assisted extraction of bioactive molecules: A review. Int. J. Biol. Macromol. 2021, 191, 899–917. [Google Scholar] [CrossRef] [PubMed]
- Ganje, M.; Gharibi, S.; Nejatpour, F.; Deilamipour, M.; Goshadehrou, K.; Saberyan, S.; Abdi, G. The ANFIS-RSM based multi-objective optimization and modelling of ultrasound-assisted extraction of polyphenols from jamun fruit (Syzygium cumini). Ultrason. Sonochemistry 2025, 113, 107227. [Google Scholar] [CrossRef]
- Jirdehi, M.A.; Rezaei, A. Parameters estimation of squirrel-cage induction motors using ANN and ANFIS. Alex. Eng. J. 2016, 55, 357–368. [Google Scholar] [CrossRef]
- Polykretis, C.; Chalkias, C.; Ferentinou, M. Adaptive neuro-fuzzy inference system (ANFIS) modeling for landslide susceptibility assessment in a Mediterranean hilly area. Bull. Eng. Geol. Environ. 2017, 78, 1173–1187. [Google Scholar] [CrossRef]
- Salleh, M.N.M.; Talpur, N.; Hussain, K. Adaptive Neuro-Fuzzy Inference System: Overview, Strengths, Limitations, and Solutions. In Data Mining and Big Data; Springer: Cham, Switzerland, 2017; pp. 527–535. [Google Scholar]
- Baruah, K.N.; Singha, S.; Mukherjee, P.; Uppaluri, R.V.S. Optimization of the enzymatic extraction of catechins from Assam tea leaves. Biomass Convers. Biorefinery 2023, 14, 24407–24425. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Flanagan, B.M.; D’Arcy, B.R.; Gidley, M.J. Binding selectivity of dietary polyphenols to different plant cell wall components: Quantification and mechanism. Food Chem. 2017, 233, 216–227. [Google Scholar] [CrossRef] [PubMed]
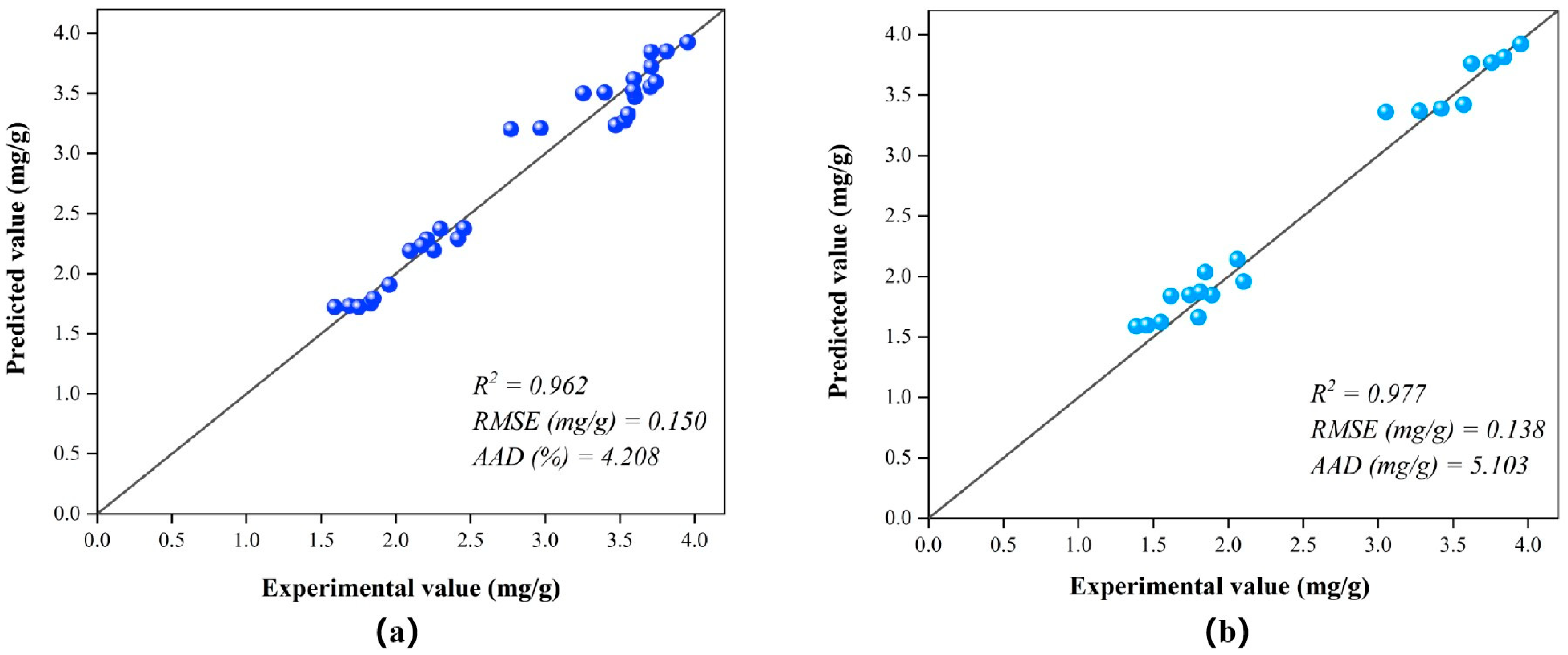
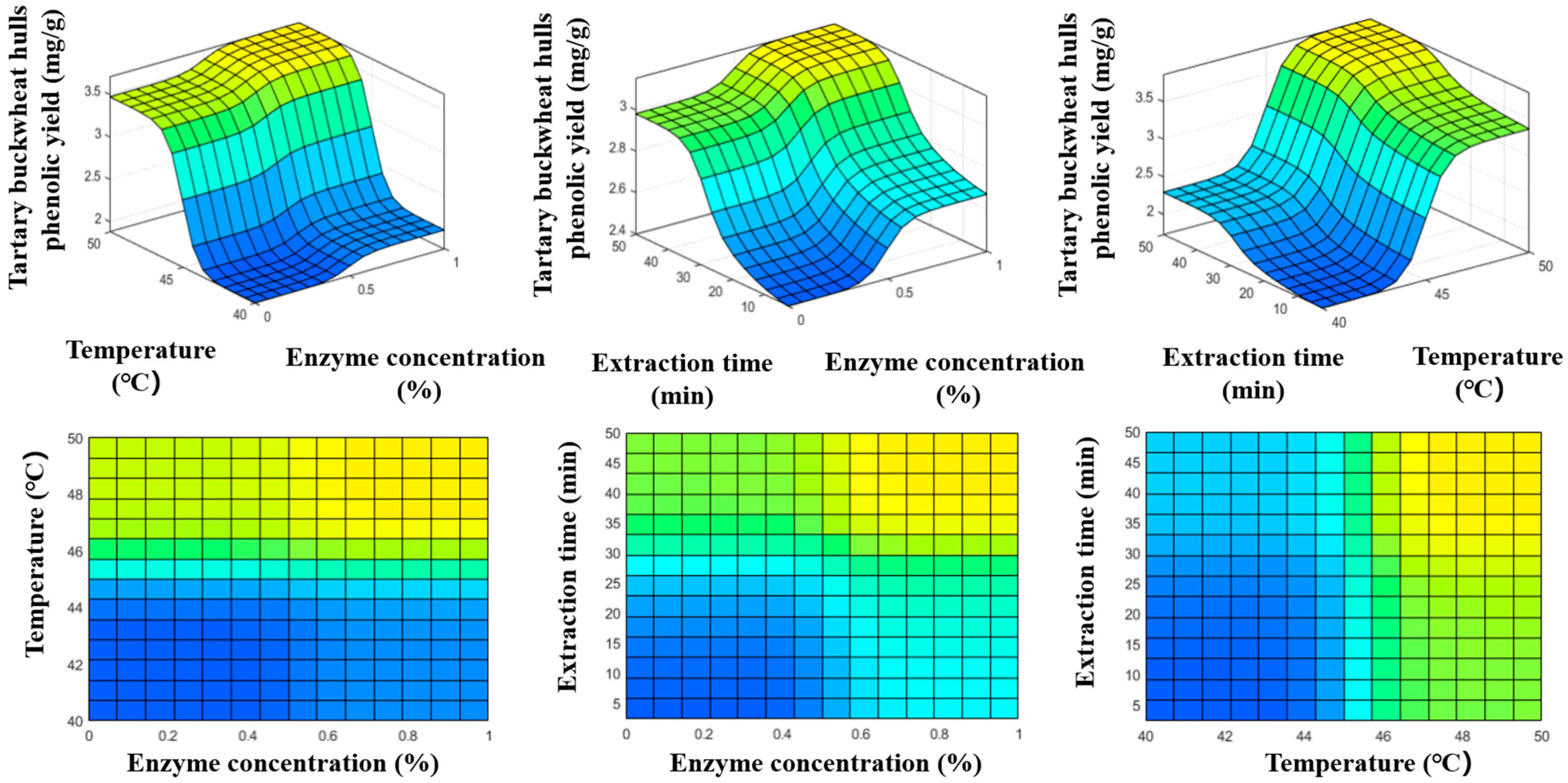
| Temperature (°C) | Extraction Method | Enzyme Concentration (%) | (m2/s) | R2 | RMSE (mg/g) | AAD (%) |
|---|---|---|---|---|---|---|
| 40 | US | NE | 9.15 × 10−7 | 0.963 | 0.115 | 5.229 |
| 0.5 | 1.86 × 10−6 | 0.917 | 0.175 | 7.025 | ||
| 1 | 2.00 × 10−6 | 0.880 | 0.219 | 9.346 | ||
| MS | 0 | 5.70 × 10−7 | 0.918 | 0.102 | 10.075 | |
| 50 | US | NE | 4.30 × 10−7 | 0.972 | 0.201 | 6.151 |
| 0.5 | 5.01 × 10−7 | 0.966 | 0.225 | 6.134 | ||
| 1 | 6.09 × 10−7 | 0.990 | 0.121 | 3.031 | ||
| MS | 0 | 3.01 × 10−7 | 0.964 | 0.129 | 5.640 |
| Category | Compound | Retention Time (min) | 40 °C and NE (μg/g) | 40 °C and 0.5% (μg/g) | 40 °C and 1% (μg/g) | 50 °C and NE (μg/g) | 50 °C and 0.5% (μg/g) | 50 °C and 1% (μg/g) |
|---|---|---|---|---|---|---|---|---|
| Phenolic acids | Homovanillic acid | 26.76 | 100.42 ± 0.26 cd | 139.05 ± 4.43 b | 91.74 ±0.88 e | 201.14 ± 5.34 a | 106.30 ± 0.49 c | 213.36 ± 0.26 a |
| p-Coumaric acid | 34.70 | 16.22 ± 1.87 c | 27.75 ± 1.04 c | 15.31 ± 0.27 c | 104.67 ± 13.35 a | 33.41 ± 9.17 c | 60.00 ± 0.33 b | |
| Chlorogenic acid | 23.01 | 142.92 ± 0.96 c | 108.15 ± 9.85 e | 99.36 ± 3.13 e | 158.11 ± 3.73 b | 125.42 ± 7.44 d | 240.74 ± 1.20 a | |
| p-Hydroxybenzoic acid | 24.81 | 23.74 ± 3.05 c | 50.50 ± 2.37 b | 24.13 ± 3.12 c | 47.90 ± 7.90 b | 53.34 ± 3.07 b | 140.97 ± 5.16 a | |
| Syringic acid | 28.05 | 34.90 ± 1.42 e | 56.84 ± 0.89 c | 32.39 ± 0.83 e | 148.27 ± 3.74 a | 47.00 ± 3.30 d | 101.14 ± 2.44 b | |
| Ferulic acid | 35.72 | 13.75 ± 2.08 c | 28.10 ± 1.34 b | 12.88 ± 0.65 c | 50.62 ± 2.36 a | 19.43 ± 3.02 bc | 40.57 ± 8.57 a | |
| Gallic acid | 11.84 | 27.86 ± 0.24 b | 12.21 ± 0.07 c | 28.23 ± 4.01 b | 61.96 ± 0.91 a | 38.00 ± 4.52 b | 52.86 ± 7.81 a | |
| Protocatechuic acid | 17.95 | 148.61 ± 3.36 b | 174.34 ± 6.91 a | 34.64 ± 1.54 e | 82.64 ± 2.15 c | 43.37 ± 3.22 e | 70.91 ± 3.39 d | |
| Caffeic acid | 27.37 | 78.96 ± 2.40 c | 31.49 ± 1.38 d | 19.48 ± 0.79 e | 171.52 ± 1.82 a | 74.47 ± 9.47 c | 98.12 ± 3.07 b | |
| Dihydrochalcones | Phloretic acid | 30.95 | 154.32 ± 1.94 e | 263.92 ± 6.46 c | 161.05 ± 3.66 e | 404.97 ± 3.78 b | 194.31 ± 13.44 d | 438.13 ± 1.34 a |
| Phloretin | 50.68 | - | - | 21.49 ± 1.51 c | 30.26 ± 5.35 b | 8.50 ± 2.02 d | 64.34 ± 1.79 a | |
| Flavanols | Procyanidin B2 | 20.23 | 136.16 ± 1.86 c | 183.73 ± 8.42 b | 131.19 ± 3.34 c | 328.96 ± 23.37 a | 227.33 ± 27.33 b | 311.20 ± 18.50 a |
| Catechin | 20.03 | 309.10 ± 5.72 d | 578.69 ± 14.18 b | 539.73 ± 2.50 b | 44.08 ± 3.45 e | 475.80 ± 45.72 c | 855.66 ± 10.93 a | |
| Epicatechin | 25.89 | 179.66 ± 1.27 d | 202.32 ± 33.64 d | 169.94 ± 1.44 d | 500.73 ± 4.35 a | 255.04 ± 12.89 c | 413.93 ± 8.69 b | |
| Flavonols | Rutin | 40.27 | - | - | - | 16.88 ± 1.34 a | 19.05 ± 3.05 a | 20.05 ± 3.88 a |
| Quercetin-3-O-rutinoside | 43.89 | - | - | - | 1.46 ± 0.96 ab | 2.02 ± 0.97 a | 3.06 ± 1.06 a | |
| Myricetin | 43.55 | 11.19 ± 1.34 b | 10.53 ± 0.13 b | 9.67 ± 0.57 b | 23.74 ± 4.23 a | 27.44 ± 3.26 a | 26.63 ± 6.63 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Ma, Y.; Li, R.; Zhang, R.; Song, Z.; Lu, Y.; Chen, Z.; Wang, Y.; Wu, Y. Deep Learning Enabled Optimization and Mass Transfer Mechanism in Ultrasound-Assisted Enzymatic Extraction of Polyphenols from Tartary Buckwheat Hulls. Foods 2025, 14, 2915. https://doi.org/10.3390/foods14162915
Shi Y, Ma Y, Li R, Zhang R, Song Z, Lu Y, Chen Z, Wang Y, Wu Y. Deep Learning Enabled Optimization and Mass Transfer Mechanism in Ultrasound-Assisted Enzymatic Extraction of Polyphenols from Tartary Buckwheat Hulls. Foods. 2025; 14(16):2915. https://doi.org/10.3390/foods14162915
Chicago/Turabian StyleShi, Yilin, Yanrong Ma, Rong Li, Ruiyu Zhang, Zizhen Song, Yao Lu, Zhigang Chen, Yufu Wang, and Yue Wu. 2025. "Deep Learning Enabled Optimization and Mass Transfer Mechanism in Ultrasound-Assisted Enzymatic Extraction of Polyphenols from Tartary Buckwheat Hulls" Foods 14, no. 16: 2915. https://doi.org/10.3390/foods14162915
APA StyleShi, Y., Ma, Y., Li, R., Zhang, R., Song, Z., Lu, Y., Chen, Z., Wang, Y., & Wu, Y. (2025). Deep Learning Enabled Optimization and Mass Transfer Mechanism in Ultrasound-Assisted Enzymatic Extraction of Polyphenols from Tartary Buckwheat Hulls. Foods, 14(16), 2915. https://doi.org/10.3390/foods14162915





