Mulberry Leaf Protein: Extraction Technologies, Functional Attributes and Food Applications
Abstract
1. Introduction
2. Literature Search Strategy
3. Extraction Technologies for MLP
3.1. Alkali-Acid Precipitation Method and Salting-Out Method
3.2. Microbial Fermentation
3.3. Foam Separation Method
3.4. Cellulase-Assisted Extraction Method
3.5. Ultrasound/Microwave-Assisted Extraction Method
4. Properties of MLP
4.1. The Nutritional Value of MLP
4.2. Functional Properties of MLP
4.3. Biological Activity of MLP
4.3.1. Antioxidant Properties
4.3.2. Inhibiting ACE Activity
4.3.3. Other Bioactivities
5. Summary and Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MLP | mulberry leaf protein |
| FAO | Food and Agriculture Organization |
| WHO | World Health Organization |
| EAA | essential amino acids |
| TAA | total amino acids |
| NEAA | non-essential amino acids |
| RC | ratio coefficient |
| RAA | ratio of amino acid |
| SRC | score of the ratio coefficient |
| SRCAA | standard ratio coefficient of amino acid |
| ACE | angiotensin-converting enzyme |
| RAS | renin-angiotensin system |
| T2D | type 2 diabetes |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| TGase | transglutaminase |
| AAPH | 2,2′-Azobis(2-methylpropionamidine) dihydrochloride |
| GSH | glutathione |
| GSSH | oxidized glutathione |
| SOD | superoxide dismutase |
| CAT | catalase |
| GSH-Px | cellular glutathione peroxidase |
| MDA | malondialdehyde |
| LDH | lactate dehydrogenase |
| AST | aspartate aminotransferase |
| ALT | alanine aminotransferase |
| Res | resveratrol |
| Cla | chlorogenic acid |
| NO | nitric oxide |
| IL-6 | interleukin-6 |
| TNF-α | tumor necrosis factor alpha |
| ROS | reactive oxygen species |
| NaOH | sodium hydroxide |
| EDTA-2Na | ethylenediaminetetraacetic acid disodium salt |
| CNKI | China National Knowledge Infrastructure |
| OpenLCA | Open Life Cycle Assessment |
| GBIF | Global Biodiversity Information Facility |
| PBS | Phosphate-Buffered Saline |
| PrH | compound protease hydrolysate |
| AH | alkaline protease hydrolysate |
| NH | neutral protease hydrolysate |
| HMP | hydrolysate of mulberry leaf protein |
| SCFAs | short-chain fatty acids |
| BCFAs | branched-chain fatty acids |
| PI3K | Phosphatidylinositol 3-kinase |
| Akt | Protein kinase B |
| PPARalpha | Peroxisome proliferator-activated receptor alpha |
| CPT-1 | Carnitine palmitoyltransferase-1 |
| IC50 | Half maximal inhibitory concentration |
References
- Maqsood, M.; Anam Saeed, R.; Sahar, A.; Khan, M.I. Mulberry plant as a source of functional food with therapeutic and nutritional applications: A review. J. Food Biochem. 2022, 46, e14263. [Google Scholar] [CrossRef]
- Cao, T.; Hao, J.; Li, W. Optimization of Protein Extraction and Analysis of Hypoglycemic Activity in Vitro of 6 Different Enzymatic Hydrolysates from Protein Mulberry Leaves. Sci. Technol. Food Ind. 2023, 44, 232–241. [Google Scholar] [CrossRef]
- Liu, X.; Qin, Y.; Huang, Q. Determination of Protein and Selenium in Mulberry Leaves Collected from Yizhou City, Guangxi Province. J. Hechi Univ. 2009, 29, 61–65. [Google Scholar]
- Zhou, K. Research on the origins of silkworm cultivation. Agric. Archaeol. 1982, 133–138. Available online: https://kns.cnki.net/kcms2/article/abstract?v=qEs6_XgQVxwu0T2GJPFgIUJVU705kDkl1eeeURHXSWHnCx_PiGD3CaCh0W2l76Ut_DDxBQ4tUL2P-K4RhO5Gtvh6K9LptEYxl6rqI1oHCm5gwQYkENRjzLD-euo9u6Nsr8uAkfpOx4kydo2vaaQpDivdlKaNb3rjxahwRPECQTbjYTjUZpgOj1VmArAYR44J&uniplatform=NZKPT&language=CHS (accessed on 12 July 2025).
- Ouyang, Z.; Chen, J. Research Progress on the Chemical Constituents and Pharmacological Effects of Mulberry Leaves. J. Jiangsu Univ. (Nat. Sci. Ed.) 2003, 39–44. Available online: https://kns.cnki.net/kcms2/article/abstract?v=tQ02qvqxHS_iOPBCX8KfE7A2yZt9_cdvmLrHj7_1dpexNA66VMxg5ejLoYtutZFcTH2JRK4Xy0W2Szp4SEqeMxvQc-h_UanpxE5ISqmlSGNVKYbKI5hy0_z6kxItW5hZQFV9hnZykCl2hHsh6d-P-6vRdCA21Fj8s5MBvcN_1PAE8mo8uAzljWXObW8NEwDR9lcn1kZ1Z2A=&uniplatform=NZKPT&language=CHS (accessed on 12 July 2025).
- Yu, Y.; Li, H.; Zhang, B.; Wang, J.; Shi, X.; Huang, J.; Yang, J.; Zhang, Y.; Deng, Z. Nutritional and functional components of mulberry leaves from different varieties: Evaluation of their potential as food materials. Int. J. Food Prop. 2018, 21, 1495–1507. [Google Scholar] [CrossRef]
- Chon, S.; Kim, Y.; Park, Y.; Heo, B.; Park, Y.; Gorinstein, S. Antioxidant and antiproliferative effects of methanol extracts from raw and fermented parts of mulberry plant (Morus alba L.). Eur. Food Res. Technol. 2009, 230, 231–237. [Google Scholar] [CrossRef]
- Shan, Z.; Zhang, H.; He, C.; An, Y.; Huang, Y.; Fu, W.; Wang, M.; Du, Y.; Xie, J.; Yang, Y.; et al. High-Protein Mulberry Leaves Improve Glucose and Lipid Metabolism via Activation of the PI3K/Akt/PPARalpha/CPT-1 Pathway. Int. J. Mol. Sci. 2024, 25, 8726. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, X.; Feng, W.; Wang, M. Efficacy and Application Status of Mulberry Leaf Powder and Mulberry Leaf Extract. Storage Process 2024, 24, 91–96. [Google Scholar]
- Wang, F.; Li, J.R. Research Progress on Chemical Constituents, Physiological Function and Application of Mulberry Leaves. Food Sci. 2005, 111–117. Available online: https://kns.cnki.net/kcms2/article/abstract?v=tQ02qvqxHS9he71BJNgwyUjhXNFDEFnNsXvHMZCLs8N3hB6sk9I0wPLWwLtnIPjf-bh9NdmdVlRfulyeVUv41RsH5tzCrjtAWxYnflSw6ogyczF9yUMcA9RM7B6fGqnIVh2Aw8fj82Ldm9pRJ54tTFHF5FXm4csfLMa5liY-6oXbJwq1Ugpe05JxaxCy_AVsqbqa9mPIytQ=&uniplatform=NZKPT&language=CHS (accessed on 12 July 2025).
- Zhao, J.; Lin, Y.; Zhang, J.; Han, X.; Wang, C.; Gao, L.; Han, Q.; Liao, X. Sustainable Plant-based Protein Processing: Innovation and Challenges. J. Chin. Inst. Food Sci. Technol. 2025, 25, 67–76. [Google Scholar]
- Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Plant proteins as high-quality nutritional source for human diet. Trends Food Sci. Technol. 2020, 97, 170–184. [Google Scholar] [CrossRef]
- Sim, S.Y.; SRV, A.; Chiang, J.H.; Henry, C.J. Plant Proteins for Future Foods: A Roadmap. Foods 2021, 10, 1967. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Li, Q.; Yang, K.; Yu, K.; Li, J.; Yang, M.; Qian, X.; Qin, Y. Research progress development and utilization of mulberry leaf as unconventional feed resource. Feed Res. 2023, 46, 133–136. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, L. Research on Comprehensive Utilization of Mulberry Leaves. Guangdong Seric. 2025, 59, 3–5. [Google Scholar]
- Liu, Y.; Liu, J.; Zhang, C. Research Progress on the Function and Extraction of Mulberry Leaf Protein. J. Anhui Agric. Sci. 2017, 45, 89–91. [Google Scholar] [CrossRef]
- Li, X.; Liu, G.; Xiao, J.; Ye, T.; Yan, X.; Li, F.; Luo, A. Nutritional Value and Development and Utilization of Mulberry Leaf Protein. Sichuan Seric. 2019, 47, 15–17. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J. The Optimization for Extraction Technique of Leaf Protein Concentrate (LPC). from Morus alba L. Food Res. Dev. 2006, 79–81. Available online: https://kns.cnki.net/kcms2/article/abstract?v=79O6ZE_Rn2obBUa8JvhZdiK58Yrojn9NYKBS5aNQCS934fxX0sIlXoY4rSWbD7xwb4GJNazym8HVLVjlDB5-0BcWt6U5x6kKQASTljFuGaqZZ5CBKVT0by3gtI9sgd0pyXBQYGd840FxFl7u5xwXjjm7JYe7-zHtuvAYVTONjvDsfqdoahcg2FckeylSHniVnHp4q5JIb1M=&uniplatform=NZKPT&language=CHS (accessed on 12 July 2025).
- Kong, W.; Gao, L.; Zhou, Q.; Zhang, R. Advancements in protein extraction, separation, purification, and characterization. Food Nutr. China 2023, 1–7. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Chen, Y.; Ma, J.; Guo, X.; Fan, P.; Gao, D. Study on Extraction of Protein from Potato by Different Extraction Methods. China Condiment 2024, 49, 54–58. [Google Scholar]
- Yang, T.; Zhang, T.; Ji, H.; Zheng, W.; Pan, D. Mulberry Leaf Protein Extraction and EDTA-2Na Affect Its Extraction Process. Chin. Agric. Sci. Bull. 2014, 30, 57–61. [Google Scholar]
- D’Alvise, N.; Lesueur Lambert, C.; Fertin, B.; Dhulster, P.; Guillochon, D. Hydrolysis and large scale ultrafiltration study of alfalfa protein concentrate enzymatic hydrolysate. Enzym. Microb. Technol. 2000, 27, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Duo; You; Wu, Q.; Jia, J.; Gui, Z. Optimization of Technology for Preparation of Mulberry Leaf Protein by Fermentation and Determination of in Vitro Digestibility of the Prepared Product. Acta Sericologica Sin. 2012, 38, 885–892. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, W.; Sui, C.; Jing, Y.; Zhao, H.; Sun, P. Optimization of Foam Separation Process of Cladophora Protein and Analysis of Functional Characteristics. Food Res. Dev. 2025, 46, 119–128. [Google Scholar]
- Jiang, C.; Wu, Z.; Li, R.; Liu, Q. Technology of protein separation from whey wastewater by two-stage foam separation. Biochem. Eng. J. 2011, 55, 43–48. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, W.; Chen, Y.; Li, X.; Chen, L.; Gao, Z. Optimization of Foam Separation of Mulberry Leaf Protein by Response Surface Methodology. Food Sci. 2015, 36, 97–102. [Google Scholar]
- Zhang, K.; Hu, N.; Li, H.; Zhang, Z. Advances in foam fractionation technology of plant protein. Food Ferment. Ind. 2020, 46, 278–283. [Google Scholar] [CrossRef]
- Zhu, T.; Yang, X.; Yang, W.; Chen, X. Study on Cellulase Assisted Extraction Process of Leaf Protein from Morus alba Leaves. J. Xihua Univ. (Nat. Sci. Ed.) 2016, 35, 77–81. [Google Scholar]
- Ju, X.; Li, J.; Yu, J.; Ding, Y.; Zou, R.; Zhang, J.; Xu, T. Advances in Enzyme-Assisted Extraction of Natural Active Substances from Plants. Food Res. Dev. 2024, 45, 202–211. [Google Scholar]
- Yang, W.; Li, K.; Wang, T.; Jia, J.; Liu, X. Optimization of Ultrasonic-assisted Extraction Process of Mulberry Leaf Protein and Analysis of Its Amino Acid Composition. Food Res. Dev. 2022, 43, 106–113. [Google Scholar]
- Wang, F.; Qiao, L.; Zhang, Q.; Gao, Y. Study on Ultrasonic Extraction of Mulberry Leaf Protein. J. Shanxi Agric. Sci. 2014, 42, 174–177. [Google Scholar]
- Zhou, Y.; Wei, X.; Li, Z.; Zhang, H.; Zhang, R. Optimization of New Ultrasound-Assisted Extraction Process for Mulberry Leaf Protein and Investigation of Its Antioxidant Activity. Chin. J. Anim. Nutr. 2025, 37, 2716–2727. [Google Scholar]
- Yin, P.; Sun, J.; Yang, Z.; Chen, C.; Huang, Q. Extraction and Characterization of Mulberry Leaf Protein from ‘Guosang Da’. Technol. Innov. Appl. 2018, 191–192. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, M.; Xue, Z.; Jiao, F.; Song, X.; Su, C. Technology for Mulberry Leaf Protein Extraction and Protein Powder Preparation. Acta Sericologica Sin. 2015, 41, 518–524. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, G.; Lei, J.; Wang, L. Optimization of Ultrasonic Extraction Combined with Ultrafiltration for Purification of Mulberry Leaf Protein and Its Nutritional Evaluation. Sci. Technol. Food Ind. 2023, 44, 236–245. [Google Scholar] [CrossRef]
- Fan, W.; Duan, H.; Ren, X.; Guo, X.; Zhang, Y.; Li, J.; Zhang, F.; Chen, J.; Yang, X. Optimization of ultrasound-assisted cellulase degradation method on the extraction of mulberry leaf protein and its effect on the functional characteristics. Ultrason. Sonochem. 2023, 99, 106561. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, Y. Study on Cellulase-ultrasonic Assisted Extraction of Protein from Mulberry Leaves. Agric. Prod. Process. 2022, 40–46. [Google Scholar] [CrossRef]
- Ren, X.; Duan, H.; Yang, X.; Ye, J.; Zhang, F.; Shang, Y. Ultrasonic Grading Extraction and Antioxidant Activity Analysis of Protein from Mulberry Leaf. Feed Ind. 2023, 44, 15–24. [Google Scholar] [CrossRef]
- Deng, X.; Li, X.; Tang, B.; Qin, Q.; Yang, C. Response Surface Optimization of Microwave-assisted Extraction of Mulberry Leaf Protein. Guangdong Agric. Sci. 2021, 48, 141–148. [Google Scholar] [CrossRef]
- Zhao, L.; Ouyang, D.; Cheng, X.; Zhou, X.; Lin, L.; Wang, J.; Wu, Q.; Jia, J. Multi-frequency ultrasound-assisted cellulase extraction of protein from mulberry leaf: Kinetic, thermodynamic, and structural properties. Ultrason. Sonochem. 2023, 99, 12. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, L.; Chen, F. Research progress of ultrasonic effect on protein extraction and modification. Food Mach. 2015, 31, 256–259. [Google Scholar] [CrossRef]
- Vilg, J.V.; Undeland, I. pH-driven solubilization and isoelectric precipitation of proteins from the brown seaweed Saccharina latissima—Effects of osmotic shock, water volume and temperature. J. Appl. Phycol. 2017, 29, 585–593. [Google Scholar] [CrossRef]
- Liu, X.; Wu, M.; Geng, Q.; Zhao, Z.; Liu, H.; Xu, F.; Shen, Y. Optimization of Protein Extraction Process from Feed Mulberry Leaves. Anim. Husb. Vet. Med. 2014, 46, 56–59. [Google Scholar]
- Zhang, Z.; Feng, J.; Zheng, Q.; Tu, C.; Li, J.; Zhang, C. Research on the Extraction Technology of Protein from Mulberry Leaves for Feed. Agric. Technol. 2016, 36, 3–4. [Google Scholar]
- Mian, X.; Mingwei, Z.; Yuanyuan, D.; Xiaojun, T.; Guang, L.; Pengfei, Z.; Zhihao, Z.; Jiarui, Z.; Jiajia, W.; Lihuang, Z.; et al. Structure, Extraction Methods, and Application of Mung Bean Protein. Modern Food Science and Technology 2025, 41, 97–108. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, J.; Su, W.; Jiao, F.; Su, C. Effect of Different Protein Precipitation and Drying Technics on Nutrition Evaluation of Protein Powder from Mulberry Leaves. North Seric. 2016, 37, 7–10. [Google Scholar] [CrossRef]
- Sun, C.; Wu, W.; Min, T.; Liu, Y.; Zhu, J.; Lai, F.; Wu, H. Functional Properties of Mulberry (Morus atropurpurea Roxb.) Leaf Proteins Extracted by Different Methods. Mod. Food Sci. Technol. 2015, 31, 242–249. [Google Scholar] [CrossRef]
- Liang, J.; Yu, Y.; Deng, Z.; Zhang, B. Effects of Different Drying Methods on the Physicochemical Properties and Nutritional Properties of Mulberry Leaf Proteins. Food Res. Dev. 2023, 44, 1–10. [Google Scholar]
- Liu, P.; Huang, H.; Luo, C.; Ge, D. Studies on Reaction Process and Mechanism of L eafProtein Flocculated by L actic Fermentation Acid. J. Hunan Agric. Univ. (Nat. Sci.) 1999, 41–46. Available online: https://kns.cnki.net/kcms2/article/abstract?v=79O6ZE_Rn2qD4w6aWXbSkkrcahbxuTo5G03k2AoExHefHu4Ooc_52JoQfv_l6epuSc9zkvAFOzA-8OzlUxizamrc8ccmBFhb0T64YEriqxgACqHEUk48SJMqZD5RVSSjSxBU0mdQoaFKgaO1YkocLUUOFDrtzWFCPRcykCnHq71XyJ_7P5IgRfCL9idox-Bn&uniplatform=NZKPT&language=CHS (accessed on 12 July 2025).
- Fan, J.; Hao, X.; Wu, X.; Li, H.; Zhang, H.; Feng, Z. Effects of Optimized Fermentation on the Protein Contents in Mulberry Leaves by Response Surface Methodology. Tianjin Agric. Sci. 2022, 28, 1–7. [Google Scholar]
- Burghoff, B. Foam fractionation applications. J. Biotechnol. 2012, 161, 126–137. [Google Scholar] [CrossRef]
- Hamid, S.B.A.; Islam, M.M.; Das, R. Cellulase biocatalysis: Key influencing factors and mode of action. Cellulose 2015, 22, 2157–2182. [Google Scholar] [CrossRef]
- Ding, X.; Yu, C.; Yu, J.; Xiong, Y.; Luo, Z.; Wu, P. Optimization of Enzyme-Assisted Extraction of Curcumin by Response Surface Methodology. Food Ind. 2025, 46, 6–10. [Google Scholar]
- Zhao, R.; Wang, M.; Zhang, K.; Li, Y.; Wang, Z.; Wang, K.; He, J. Optimization of Extraction Method of Total Flavonoids from Eucommia ulmoides Leaves by Ultrasonic Combine Ethanol. Feed Ind. 2025, 46, 151–158. [Google Scholar] [CrossRef]
- Liu, Z.; Lv, S.; Zhao, L.; Zhang, Y.; Li, Q.; Tan, W. Optimization of Microwave Assisted Extraction of Anthocyanins from Grape Skin Residue and Their Antioxidant Properties Study. Food Ind. 2024, 45, 73–77. [Google Scholar]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef]
- Block, R.J. The Amino Acid Composition of Food Proteins. Adv. Protein Chem. 1945, 2, 119–134. [Google Scholar] [CrossRef]
- Shi, J.; Feng, R.; Gao, W.; He, L.; Wang, X. Determination of amino acids by pre-column derivatization-high performance liquid chromatography and nutritional evaluation of Morindae officinalis Radix. Food Ferment. Ind. 2024, 50, 308–318. [Google Scholar] [CrossRef]
- Wang, F.; Qiao, L.; Zhang, Q.; Shen, B. Amino Acid Composition and Nutritional Evaluation of Mulberry Leaves. Food Sci. 2015, 36, 225–228. [Google Scholar]
- Wu, X.; Yang, J.; Mumby, W.; Zhang, Y.; Zhang, Y.; Wang, C.; Chen, X.; Suo, H.; Song, J. Silkworm pupa protein and its peptides: Preparation, biological activity, applications in foods, and advantages. Trends Food Sci. Technol. 2023, 139, 11. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Dong, M. Functional Properties of Proteins from Mulberry Leaves. Food Sci. 2010, 31, 81–86. [Google Scholar]
- Xie, Y.; Li, H.; Deng, Z.; Peng, H.; Yu, Y.; Zhang, B. Preparation and characterization of a new food-grade Pickering emulsion stabilized by mulberry-leaf protein nanoparticles. J. Sci. Food Agric. 2025, 105, 1080–1090. [Google Scholar] [CrossRef]
- Zhi, M.; Xie, Y.; Li, H.; Deng, Z.; Peng, H.; Yu, Y.; Zhang, B. TGase-induced crosslinking of mulberry leaf protein particles as stabilizer of high-internal-phase Pickering emulsions: Characterization and stability. J. Sci. Food Agric. 2025, 105, 4388–4399. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Qiu, M.; Meng, C.; Lu, X.; Han, S.; Ning, J.; Li, F.; Jiao, F. Preparation of Enzymatic Hydrolysate of Mulberry Leaf and Evaluation of Its Antioxidant Activity. Acta Sericologica Sin. 2020, 46, 485–492. [Google Scholar] [CrossRef]
- Zhao, R.; Sun, X.; Wang, X.; Wang, Z. Optimization of the Extraction Process and Antioxidant Activity of Mulberry Leaf Protein in vitro. Food Res. Dev. 2022, 43, 138–144. [Google Scholar]
- Sun, C.; Shan, Y.; Tang, X.; Han, D.; Wu, X.; Wu, H.; Hosseininezhad, M. Effects of enzymatic hydrolysis on physicochemical property and antioxidant activity of mulberry (Morus atropurpurea Roxb.) leaf protein. Food Sci. Nutr. 2021, 9, 5379–5390. [Google Scholar] [CrossRef]
- Sun, C.; Tang, X.; Sun, C.; Han, D.; Lai, F.; Shao, X.; Yang, S.; Wu, X.; Wu, H. Enzymatic Preparation of Antioxidant Peptides of Mulberry (Morus atropurpurea Roxb.) Leaf Protein. Mod. Food Sci. Technol. 2020, 36, 192–201. [Google Scholar] [CrossRef]
- Sun, C.; Wu, W.; Yin, Z.; Fan, L.; Ma, Y.; Lai, F.; Wu, H. Effects of simulated gastrointestinal digestion on the physicochemical properties, erythrocyte haemolysis inhibitory ability and chemical antioxidant activity of mulberry leaf protein and its hydrolysates. Int. J. Food Sci. Technol. 2018, 53, 282–295. [Google Scholar] [CrossRef]
- Sun, C.; Li, H.; Hui, X.; Ma, Y.; Yin, Z.; Chen, Q.; Chen, C.; Wu, H.; Wu, X. Protective Effects of Mulberry (Morus atropurpurea Roxb.) Leaf Protein Hydrolysates and Their In Vitro Gastrointestinal Digests on AAPH-Induced Oxidative Stress in Human Erythrocytes. Foods 2023, 12, 3468. [Google Scholar] [CrossRef]
- Sun, C.; Tang, X.; Ren, Y.; Wang, E.; Shi, L.; Wu, X.; Wu, H. Novel Antioxidant Peptides Purified from Mulberry (Morus atropurpurea Roxb.) Leaf Protein Hydrolysates with Hemolysis Inhibition Ability and Cellular Antioxidant Activity. J. Agric. Food Chem. 2019, 67, 7650–7659. [Google Scholar] [CrossRef] [PubMed]
- Ren, X. Preparation of Antioxidant Peptides from Mulberry Leaves and Their Protective Effect Against Oxidative Damage in BRL-3A Liver Cells. Dissertation, Chengde Medical College, 2023. Available online: https://link.cnki.net/doi/10.27691/d.cnki.gcdyx.2023.000136 (accessed on 12 July 2025).
- Meng, Y.; Chen, W.; Zhou, J.; Li, X. Interaction between main phenols and proteins in mulberry leaves and analysis of complex properties. Food Ferment. Ind. 2023, 49, 161–168. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Wang, J.; Yang, L.; Zhu, X.; Li, J.; Song, L.; Wang, X. Research Progress of ACE Inhibitory Peptides Derived from Almond Protein. J. Hebei North Univ. (Nat. Sci. Ed.) 2024, 40, 13–21. [Google Scholar]
- Liao, M.; Cheng, L.; Liao, Y. Review of renin-angiotensin system. J. Clin. Cardiol. 2012, 28, 83–87. [Google Scholar] [CrossRef]
- Xiang, L.; Qiu, Z.; Zhao, R.; Zheng, Z.; Qiao, X. Advancement and prospects of production, transport, functional activity and structure-activity relationship of food-derived angiotensin converting enzyme (ACE) inhibitory peptides. Crit. Rev. Food Sci. 2023, 63, 1437–1463. [Google Scholar] [CrossRef]
- Liu, X.; Liu, F.; Zhao, J.; Song, W.; Han, Y.; Jia, M. Research progress on enzymatic hydrolysis preparation of blood pressure lowering peptide from mulberry source products. North Seric. 2025, 46, 7–11. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Qi, Q.; Liang, F.; Wang, N.; Chen, Q.; Li, X.; Sun, S.; Wang, X.; Bai, K.; et al. Preparation and activity evaluation of angiotensin-I converting enzyme inhibitory peptides from protein hydrolysate of mulberry leaf. Front. Nutr. 2023, 9, 19. [Google Scholar] [CrossRef]
- Jia, M.; Yang, X.; Wang, B.; Li, N.; Li, J.; Fan, W. Enzymatic Hydrolysis Preparation of Mulberry Leaf Protein Active Peptides. Food Res. Dev. 2023, 44, 112–118. [Google Scholar]
- Fang, P.; Shi, M.; Zhu, Y.; Bo, P.; Zhang, Z. Type 2 diabetes mellitus as a disorder of galanin resistance. Exp. Gerontol. 2016, 73, 72–77. [Google Scholar] [CrossRef]
- Feng, X.; Wang, X.; Wang, D.; Ma, X.; Liu, Y.; Liu, B.; Yang, W. Functional Evaluation of Mulberry Leaf Peptides in Maintaining Animal Blood Sugar Health. Acta Chin. Med. Pharmacol. 2022, 50, 32–35. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Bester, M.J.; Neitz, A.W.H.; Gaspar, A.R.M. Structural properties of bioactive peptides with α-glucosidase inhibitory activity. Chem. Biol. Drug Des. 2018, 91, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhao, H.; Pan, X.; Orfila, C.; Lu, W.; Ma, Y. Preparation of bioactive peptides with antidiabetic, antihypertensive, and antioxidant activities and identification of α-glucosidase inhibitory peptides from soy protein. Food Sci. Nutr. 2019, 7, 1848–1856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Zhang, X.; Man, Q.; Yang, F.; Li, Y.; Zhu, M.; Fan, B.; Tang, G.; Xiang, X. Lipid-lowering effect of sodium alginate in patients with hypercholesterolemia. J. Hyg. Res. 2025, 54, 495–499. [Google Scholar] [CrossRef]
- Duan, H.; Guo, M.; Tan, J.; Dong, Y.; Zhao, J.; Yang, X. Optimization of extraction process of cholesterol-lowering peptides from mulberry leaves based on Plackett-Burman and Box-Behnken methods. Cereals Oils 2024, 37, 103–108. [Google Scholar]
- Marques, M.R.; Freitas, R.A.M.S.; Carlos, A.C.C.; Siguemoto, E.S.; Fontanari, G.G.; Areas, J.A.G. Peptides from cowpea present antioxidant activity, inhibit cholesterol synthesis and its solubilisation into micelles. Food Chem. 2015, 168, 288–293. [Google Scholar] [CrossRef]
- Gasparrini, M.; Mazzoni, L. Special Issue “Dietary Bioactive Components in Inflammatory Bowel Disease”. Int. J. Mol. Sci. 2024, 25, 3569. [Google Scholar] [CrossRef]
- Sun, C.; Tang, X.; Shao, X.; Han, D.; Zhang, H.; Shan, Y.; Gooneratne, R.; Shi, L.; Wu, X.; Hosseininezhad, M. Mulberry (Morus atropurpurea Roxb.) leaf protein hydrolysates ameliorate dextran sodium sulfate-induced colitis via integrated modulation of gut microbiota and immunity. J. Funct. Foods 2021, 84, 15. [Google Scholar] [CrossRef]
- Sun, C. Enzymatic Preparation of Antioxidant Peptides from Mulberry Leaves, Structural Identification, and Immunological Activity Analysis. Dissertation, South China University of Technology, 2017. Available online: https://link.cnki.net/doi/10.27151/d.cnki.ghnlu.2017.000006 (accessed on 12 July 2025).
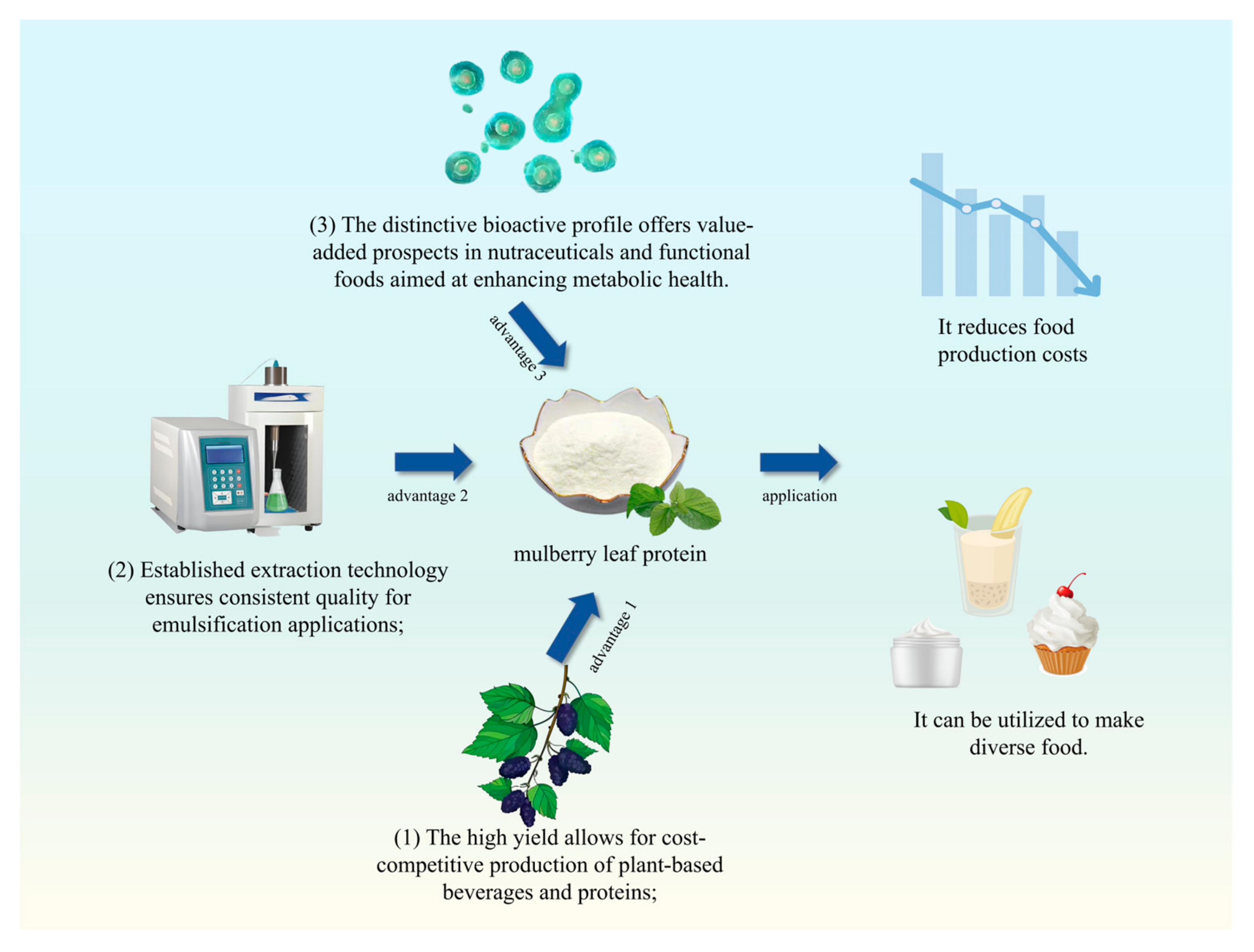
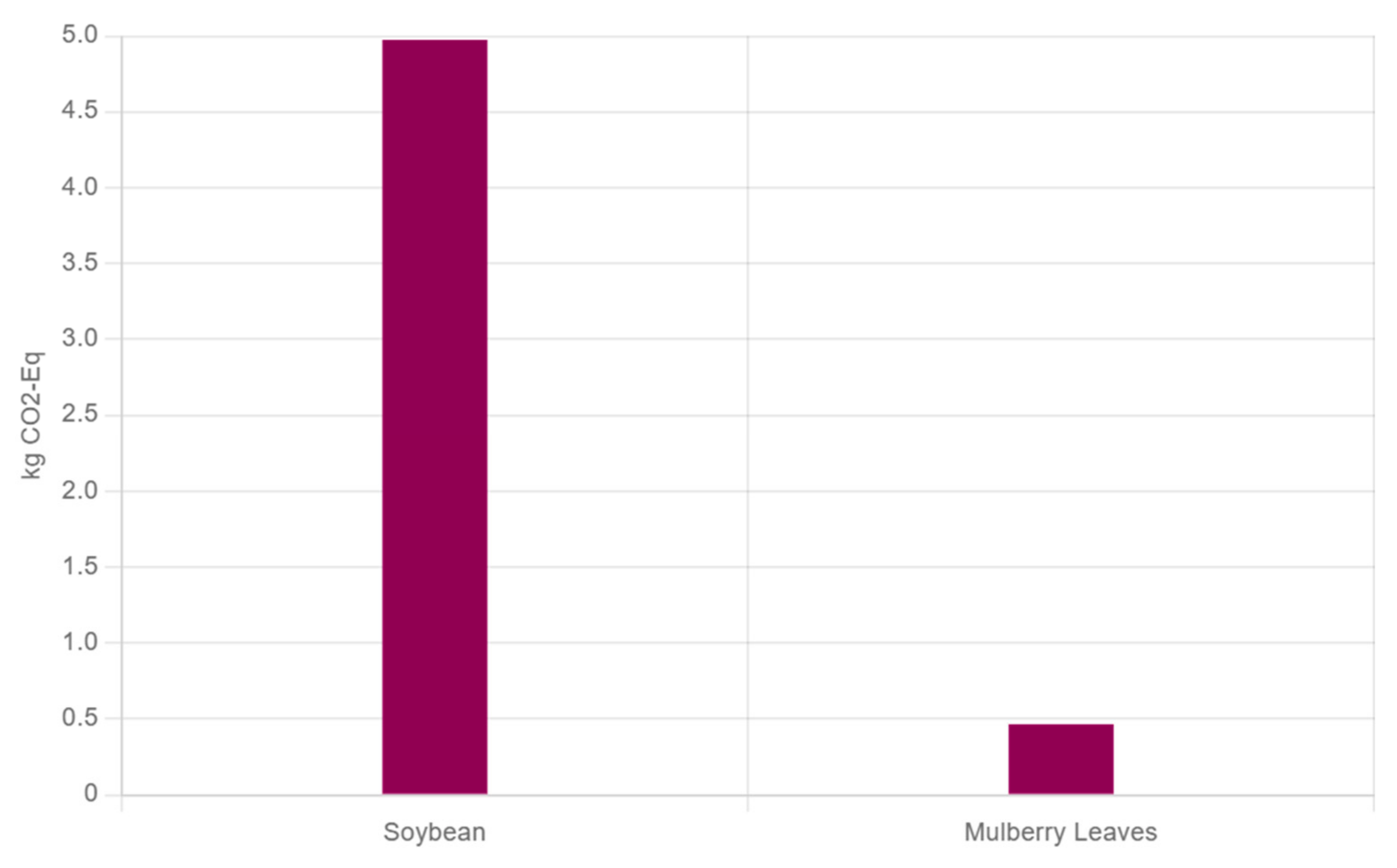
| Method | MLP Yield (From Single Studies) | Key Advantages | Limitations | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| Yield (%) | Dry/Wet Weight Basis | Protein Content Determination Method | Number of Repetitions | |||||
| Alkali–acid precipitation method | 5.17 | wet | Precipitation method | 5 | This method is simple and cost-effective. | The MLP obtained is prone to denaturation, resulting in a relatively low yield and purity. | [18,19,20] | |
| Salting-out method | 1.38 | wet | Precipitation method | 3 | This method does not compromise protein bioactivity and better preserves the nutritional value of MLP. | The yield is relatively low. | [21] | |
| Microbial fermentation | 16.29 | dry | Precipitation method | 3 | This method does not require heating and generates no waste or pollution. | The fermentation process is time-consuming and may result in nutrient loss; the production costs are higher. | [22,23] | |
| Foam separation method | 15.50 | dry | Coomassie Brilliant Blue colorimetric method | 5 | Simple equipment, straightforward operation, low energy consumption, environmental friendliness, and high efficiency. | When tasked with separating high-concentration solutions, the efficiency of this technology appears to be relatively low. | [24,25,26,27] | |
| Cellulase-assisted extraction method | 0.98 | dry | Coomassie Brilliant Blue colorimetric method | 3 | This extraction technique exhibits high selectivity and mild reaction conditions. | Relatively slow extraction rates and the tendency to introduce enzyme protein impurities during operation, which affects purity. | [28,29] | |
| Ultrasound/microwave-assisted extraction method | Ultrasound-assisted salting-out extraction | 9.19 | dry | Coomassie Brilliant Blue colorimetric method | 3 | Reduced extraction time and mild operating conditions. | Protein denaturation risks, equipment costs, energy consumption, and scalability for industrial production. | [30,31,32,33,34,35,36,37,38,39,40,41] |
| Ultrasound-assisted alkali dissolution and acid precipitation | 5.68 | dry | Coomassie Brilliant Blue colorimetric method | 3 | ||||
| Ultrasonic extraction combined with ultrafiltration | 5.56 | dry | Coomassie Brilliant Blue colorimetric method | 3 | ||||
| Ultrasound-assisted cellulase degradation | 16.06 | dry | Precipitation method | 3 | ||||
| Microwave-assisted extraction | 7.23 | dry | Coomassie Brilliant Blue colorimetric method | 3 | ||||
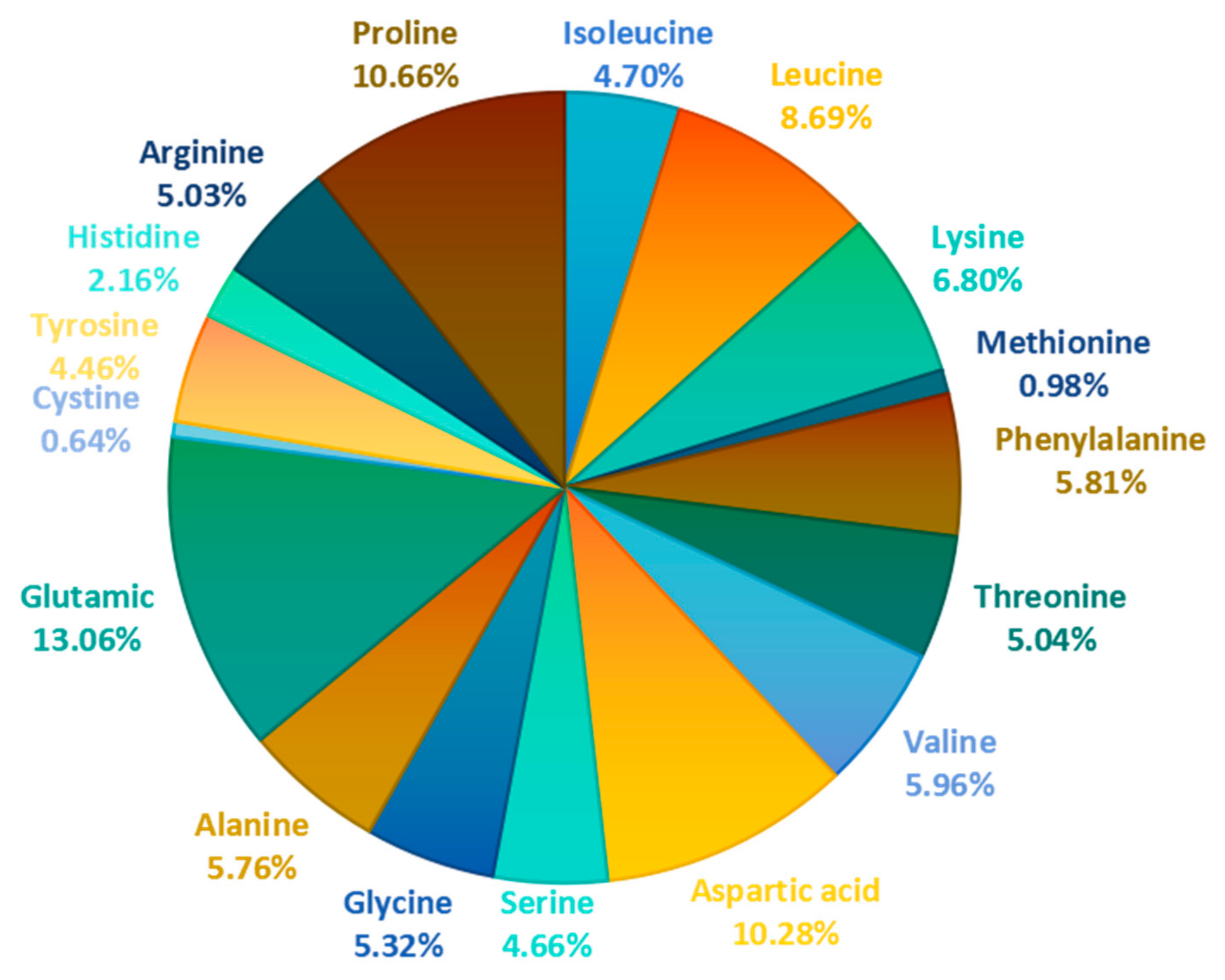
| Amino Acids | Content(mg/g) | Proportion | Amino Acids | Content (mg/g) | Proportion |
|---|---|---|---|---|---|
| Isoleucine | 19.3 | 4.70% | Glycine | 21.84 | 5.32% |
| Leucine | 35.68 | 8.69% | Alanine | 23.65 | 5.76% |
| Lysine | 27.92 | 6.80% | Glutamic | 53.65 | 13.06% |
| Methionine | 4.01 | 0.98% | Cystine | 2.64 | 0.64% |
| Phenylalanine | 23.85 | 5.81% | Tyrosine | 18.34 | 4.46% |
| Threonine | 20.72 | 5.04% | Histidine | 8.86 | 2.16% |
| Valine | 24.47 | 5.96% | Arginine | 20.65 | 5.03% |
| Aspartic acid | 42.22 | 10.28% | Proline | 43.79 | 10.66% |
| Serine | 19.16 | 4.66% | TAA 1 | 410.77 | 100.00% |
| EAA 2/TAA | 0.38 | EAA/NEAA 3 | 0.612 | ||
| Characteristic Value | FAO/WHO Essential Amino Acid Composition | SRC | ||||||
|---|---|---|---|---|---|---|---|---|
| Leucine | Lysine | Phenylalanine + Tyrosine | Threonine | Valine | Isoleucine | Methionine + Cystine | ||
| RAA | 0.52 | 0.52 | 0.76 | 0.52 | 0.50 | 0.50 | 0.22 | 69.29 |
| RCAA | 1.03 | 1.02 | 1.49 | 1.03 | 1.00 | 0.98 | 0.44 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, R.; Chen, L.; Sun, C.; Muhammad, A.; Shao, Y. Mulberry Leaf Protein: Extraction Technologies, Functional Attributes and Food Applications. Foods 2025, 14, 2602. https://doi.org/10.3390/foods14152602
Xue R, Chen L, Sun C, Muhammad A, Shao Y. Mulberry Leaf Protein: Extraction Technologies, Functional Attributes and Food Applications. Foods. 2025; 14(15):2602. https://doi.org/10.3390/foods14152602
Chicago/Turabian StyleXue, Rongxiang, Lichao Chen, Chao Sun, Abrar Muhammad, and Yongqi Shao. 2025. "Mulberry Leaf Protein: Extraction Technologies, Functional Attributes and Food Applications" Foods 14, no. 15: 2602. https://doi.org/10.3390/foods14152602
APA StyleXue, R., Chen, L., Sun, C., Muhammad, A., & Shao, Y. (2025). Mulberry Leaf Protein: Extraction Technologies, Functional Attributes and Food Applications. Foods, 14(15), 2602. https://doi.org/10.3390/foods14152602






