Untargeted Metabolomics Uncovers Food Safety Risks: Polystyrene Nanoplastics Induce Metabolic Disorders in Chicken Liver
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. NPs Characterization
2.3. Experimental Animals
2.4. Measurement of the Serum Biochemical
2.5. Hematoxylin–Eosin (HE) Staining
2.6. Oil Red O (ORO) Staining
2.7. Metabolomics Methodology
2.7.1. Metabolite Extraction
2.7.2. HPLC-MS/MS Analysis
2.7.3. Data Processing and Metabolite Identification
2.7.4. Statistical and Bioinformatics Analysis
2.8. Statistical Analysis
3. Results
3.1. Characterization of Fluorescent NPs
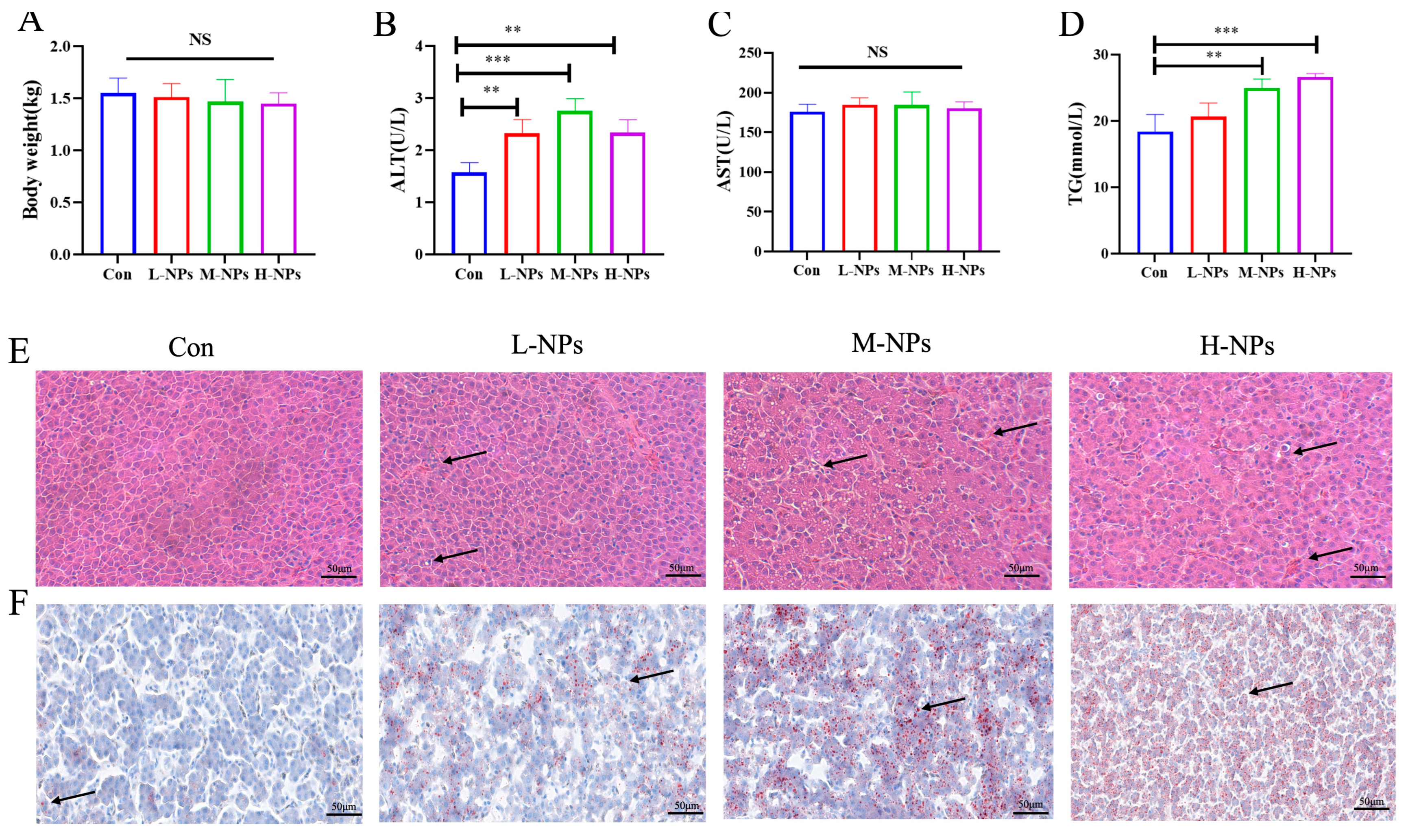
3.2. The Influence of Exposed NPs on the Organ Coefficients and Morphology of Chicken Liver
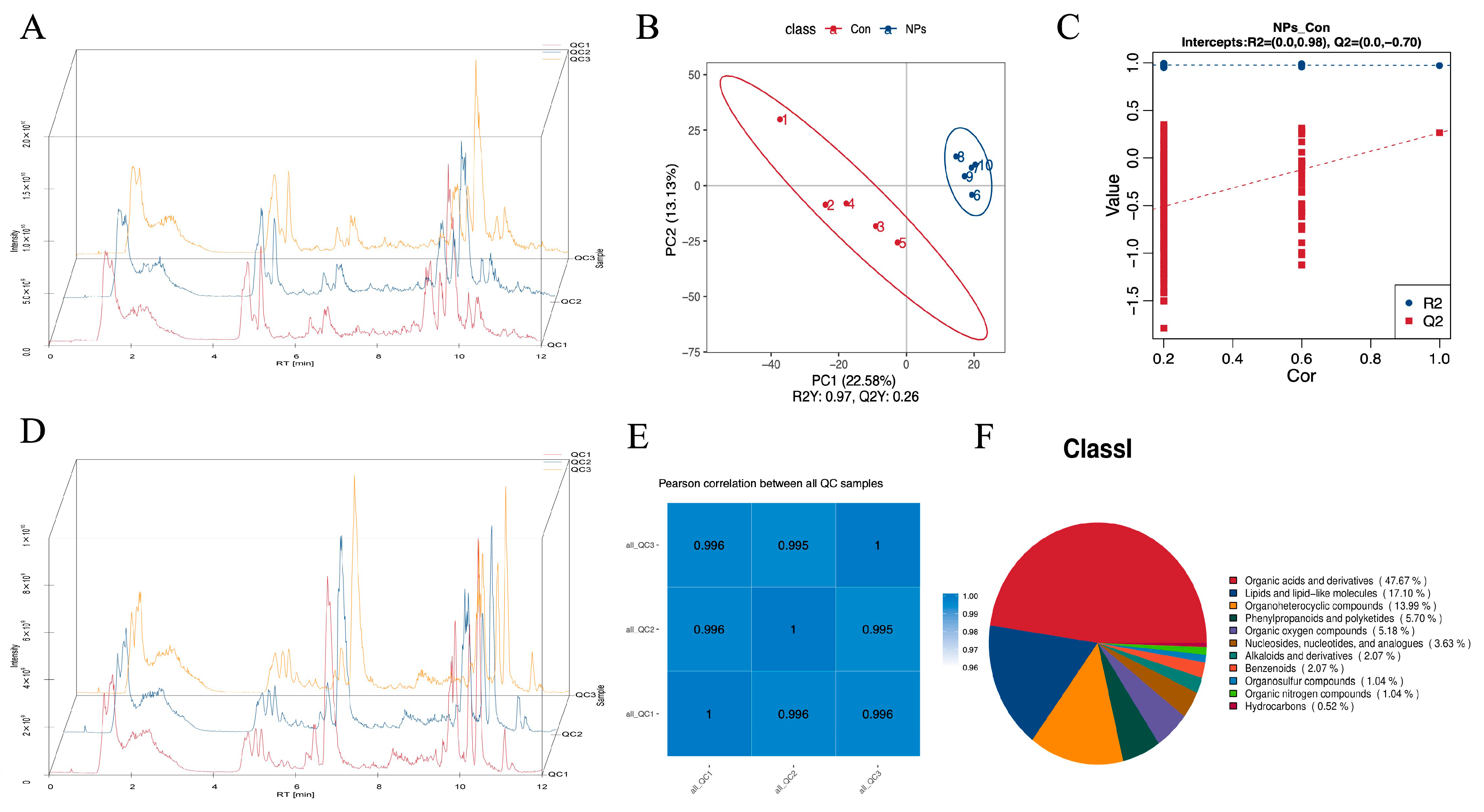
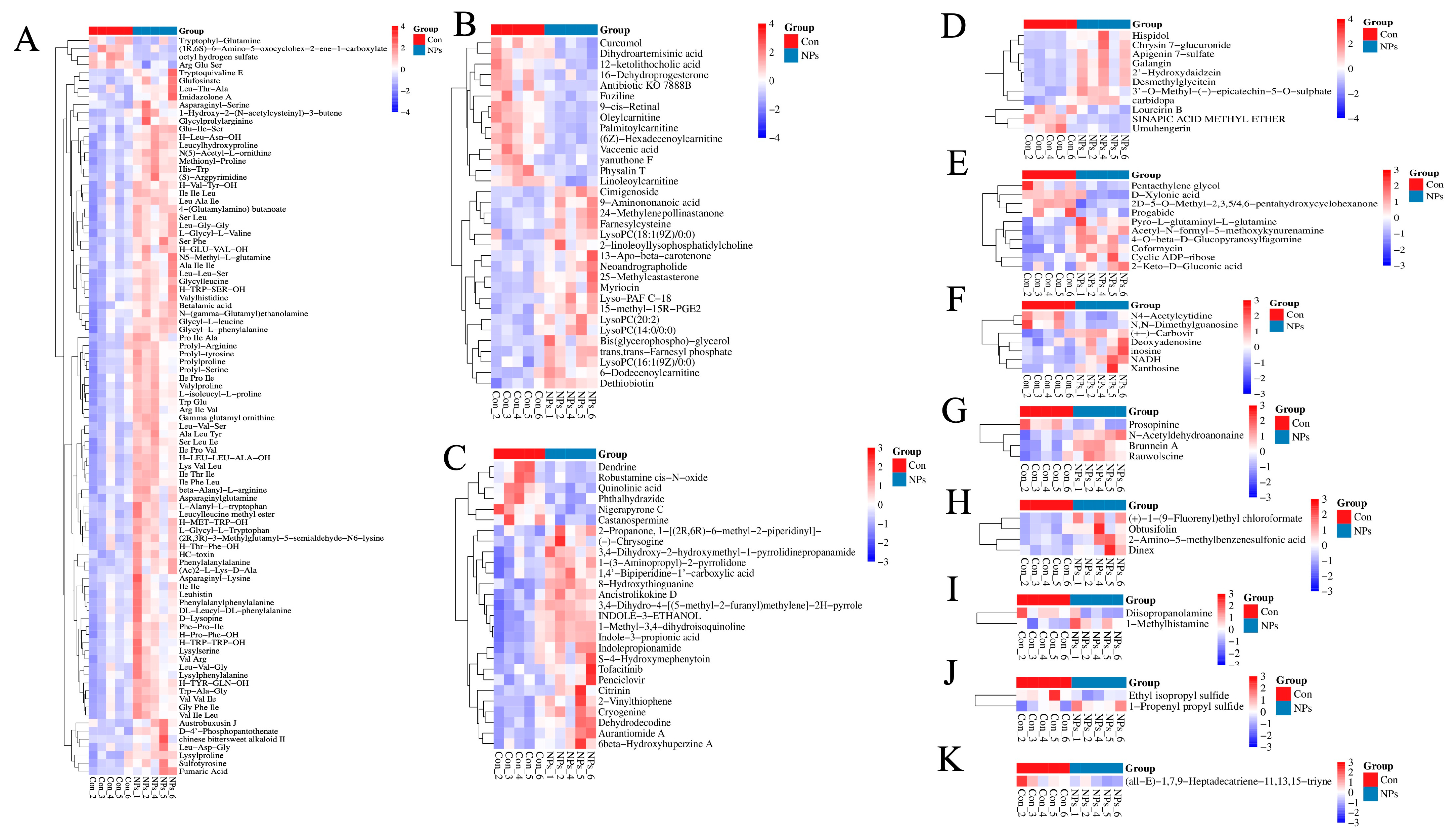
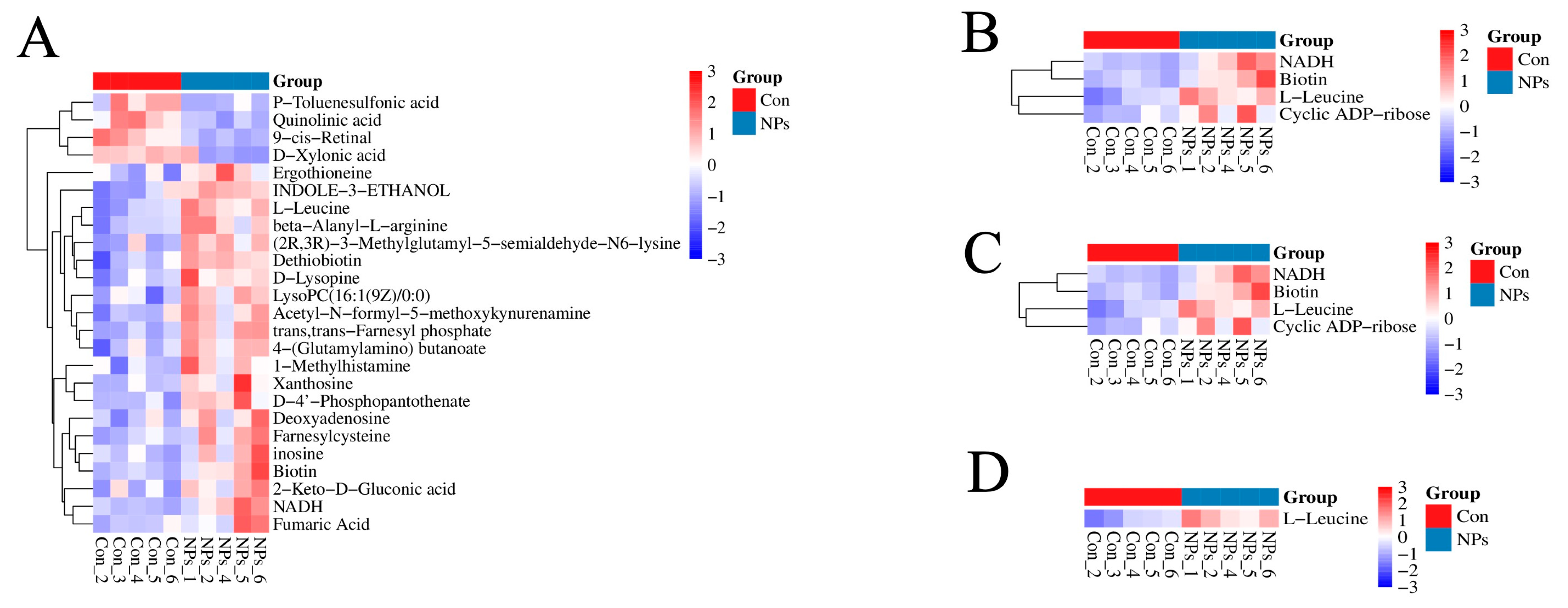
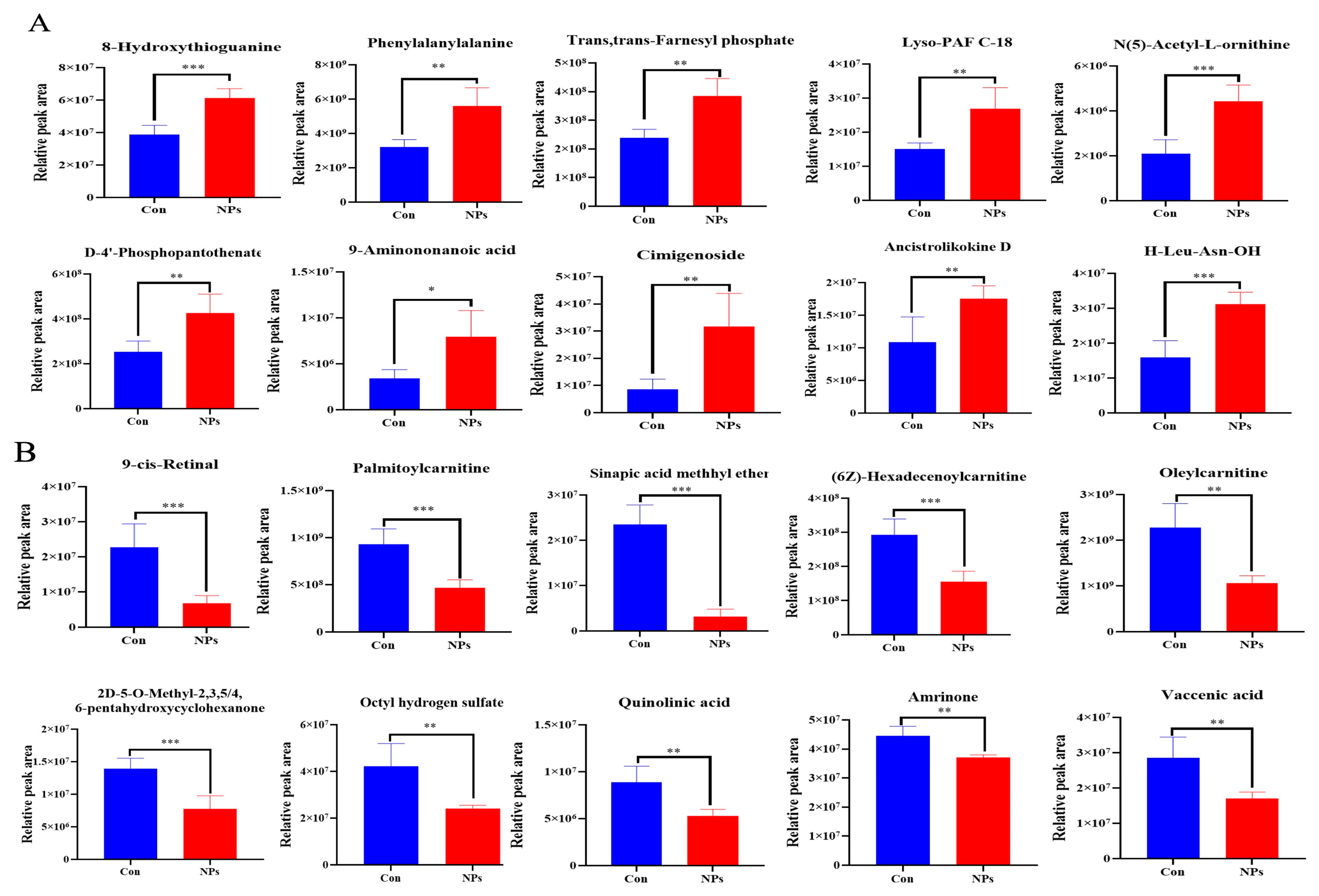
3.3. Metabolite Profiling with Data Analysis of Chicken Liver
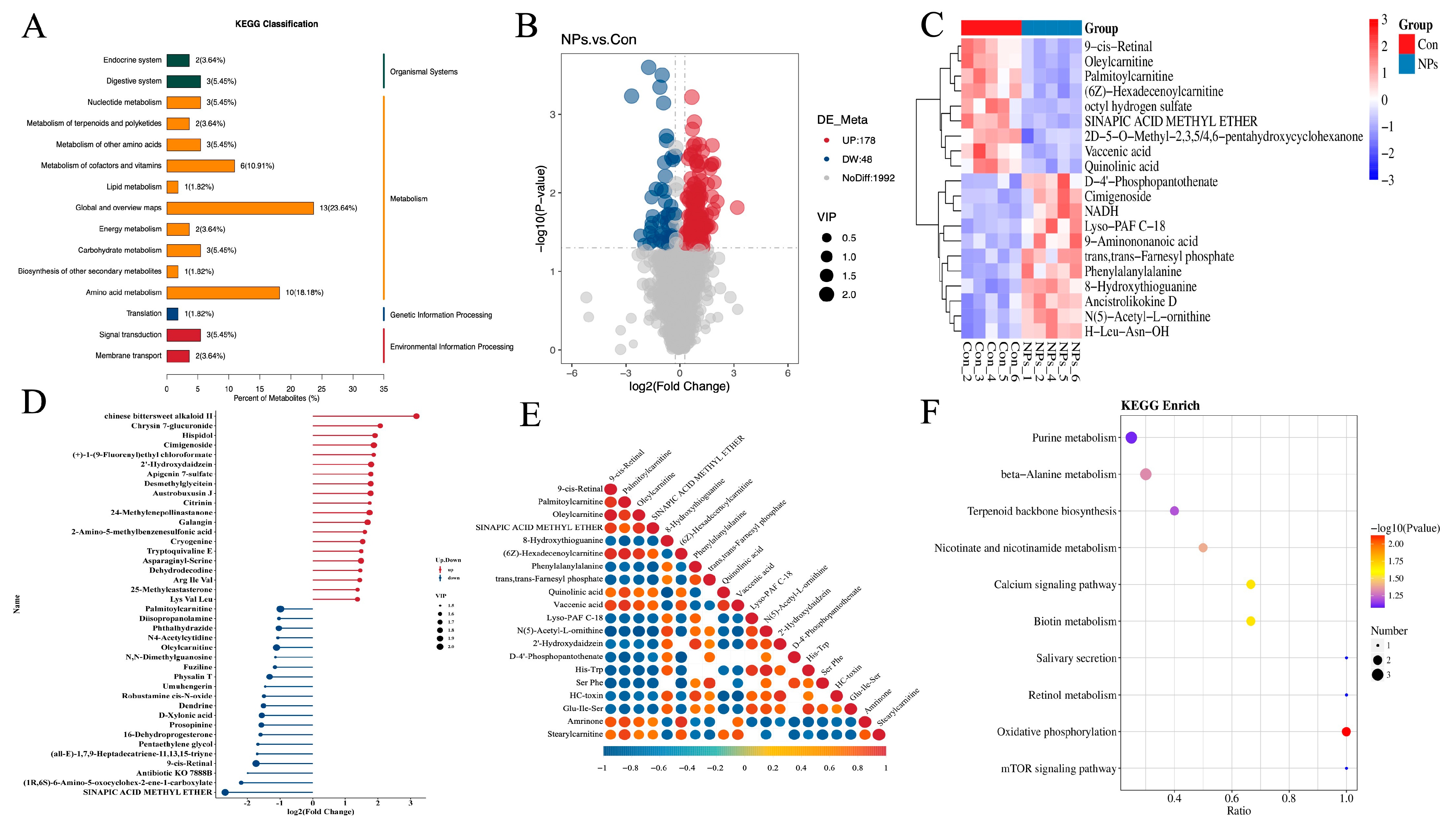
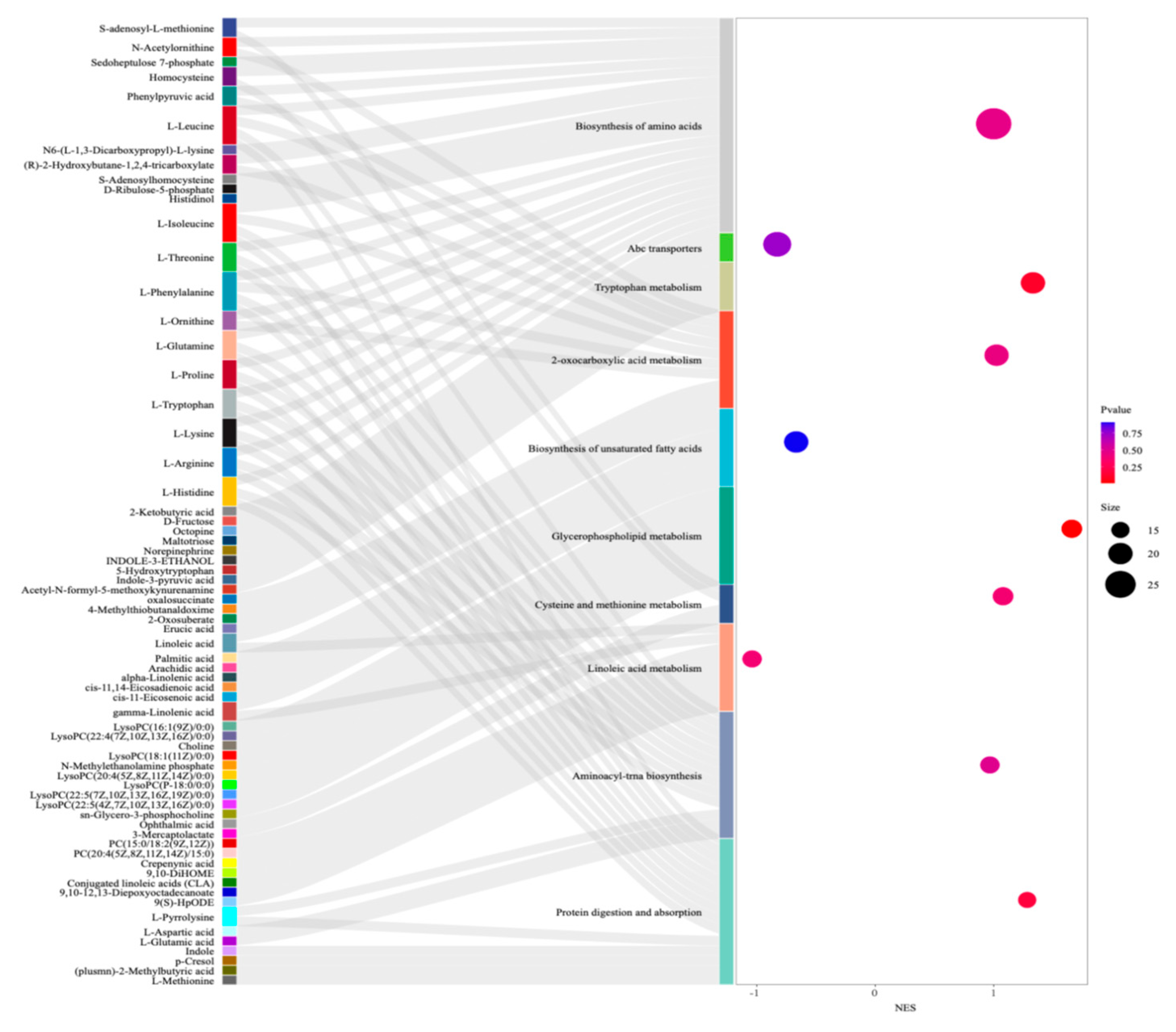
3.4. KEGG Classification and Differential Metabolite Analysis of Chicken Liver
3.5. GSEA Function Enrichment Analysis of Chicken Liver
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mattsson, K.; Hansson, L.-A.; Cedervall, T. Nano-Plastics in the Aquatic Environment. Environ. Sci. Process. Impacts 2015, 17, 1712–1721. [Google Scholar] [CrossRef]
- Sun, S.; Jin, Y.; Luo, P.; Shi, X. Polystyrene Microplastics Induced Male Reproductive Toxicity and Transgenerational Effects in Freshwater Prawn. Sci. Total Environ. 2022, 842, 156820. [Google Scholar] [CrossRef]
- Hantoro, I.; Löhr, A.J.; Van Belleghem, F.G.A.J.; Widianarko, B.; Ragas, A.M.J. Microplastics in Coastal Areas and Seafood: Implications for Food Safety. Food Addit. Contam. Part A 2019, 36, 674–711. [Google Scholar] [CrossRef]
- Cooke, A.S.; Machekano, H.; Gwiriri, L.C.; Tinsley, J.H.I.; Silva, G.M.; Nyamukondiwa, C.; Safalaoh, A.; Morgan, E.R.; Lee, M.R.F. The Nutritional Feed Gap: Seasonal Variations in Ruminant Nutrition and Knowledge Gaps in Relation to Food Security in Southern Africa. Food Secur. 2024, 17, 73. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, G.; Peng, H.; Qi, L.; Zhang, D.; Nie, Q.; Zhang, X.; Luo, W. Microplastic Exposure Induces Muscle Growth but Reduces Meat Quality and Muscle Physiological Function in Chickens. Sci. Total Environ. 2023, 882, 163305. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.-T.; Cai, Y.-F.; Chen, Y.-X.; Yang, Y.-W.; Xing, S.-C.; Liao, X.-D. Occurrence of Microplastic in Livestock and Poultry Manure in South China. Environ. Pollut. 2021, 277, 116790. [Google Scholar] [CrossRef]
- Xu, S.; Li, M.; Zheng, Y.; Xu, M.; Zhou, J.; Wang, S.; Li, S.; Wang, M. Nanoplastics Disrupt Hepatic Lipid Metabolism via the Inhibition of PPARγ: A Study Based on Digestive System Exposure. Toxicology 2025, 516, 154194. [Google Scholar] [CrossRef]
- Zimmermann, L.; Geueke, B.; Parkinson, L.V.; Schür, C.; Wagner, M.; Muncke, J. Food Contact Articles as Source of Micro- and Nanoplastics: A Systematic Evidence Map. Npj Sci. Food 2025, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Collard, F.; Gilbert, B.; Compère, P.; Eppe, G.; Das, K.; Jauniaux, T.; Parmentier, E. Microplastics in Livers of European Anchovies (Engraulis encrasicolus, L.). Environ. Pollut. 2017, 229, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Dzierżyński, E.; Blicharz-Grabias, E.; Komaniecka, I.; Panek, R.; Forma, A.; Gawlik, P.J.; Puźniak, D.; Flieger, W.; Choma, A.; Suśniak, K.; et al. Post-Mortem Evidence of Microplastic Bioaccumulation in Human Organs: Insights from Advanced Imaging and Spectroscopic Analysis. Arch. Toxicol. 2025, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, G.; Wang, J.; Lu, L.; Li, X.; Zheng, Y.; Zhang, Z.; Ru, S. Microplastics Increase the Accumulation of Phenanthrene in the Ovaries of Marine Medaka (Oryzias melastigma) and Its Transgenerational Toxicity. J. Hazard. Mater. 2022, 424, 127754. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, L.; Weng, J.; Jin, Z.; Cao, Y.; Jiang, H.; Zhang, Z. Detection and Characterization of Microplastics in the Human Testis and Semen. Sci. Total Environ. 2023, 877, 162713. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Chao, X.; Williams, J.; Fulte, S.; Li, T.; Yang, L.; Ding, W.-X. Autophagy in Liver Diseases: A Review. Mol. Aspects Med. 2021, 82, 100973. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Chen, C.-Y.; Lu, T.-H.; Liao, C.-M. Toxicity-Based Toxicokinetic/Toxicodynamic Assessment for Bioaccumulation of Polystyrene Microplastics in Mice. J. Hazard. Mater. 2019, 366, 703–713. [Google Scholar] [CrossRef]
- Fan, X.; Wei, X.; Hu, H.; Zhang, B.; Yang, D.; Du, H.; Zhu, R.; Sun, X.; Oh, Y.; Gu, N. Effects of Oral Administration of Polystyrene Nanoplastics on Plasma Glucose Metabolism in Mice. Chemosphere 2022, 288, 132607. [Google Scholar] [CrossRef] [PubMed]
- Horvatits, T.; Tamminga, M.; Liu, B.; Sebode, M.; Carambia, A.; Fischer, L.; Püschel, K.; Huber, S.; Fischer, E.K. Microplastics Detected in Cirrhotic Liver Tissue. eBioMedicine 2022, 82, 104147. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, W.; Lin, T.; Liu, S.; Sun, Z.; Liu, F.; Yuan, Y.; Xiang, X.; Kuang, H.; Yang, B.; et al. Maternal Exposure to Polystyrene Nanoplastics during Gestation and Lactation Induces Hepatic and Testicular Toxicity in Male Mouse Offspring. Food Chem. Toxicol. 2022, 160, 112803. [Google Scholar] [CrossRef]
- Meng, X.; Ge, L.; Zhang, J.; Xue, J.; Gonzalez-Gil, G.; Vrouwenvelder, J.S.; Li, Z. Systemic Effects of Nanoplastics on Multi-Organ at the Environmentally Relevant Dose: The Insights in Physiological, Histological, and Oxidative Damages. Sci. Total Environ. 2023, 892, 164687. [Google Scholar] [CrossRef]
- Jiang, B.; Kauffman, A.E.; Li, L.; McFee, W.; Cai, B.; Weinstein, J.; Lead, J.R.; Chatterjee, S.; Scott, G.I.; Xiao, S. Health Impacts of Environmental Contamination of Micro- and Nanoplastics: A Review. Environ. Health Prev. Med. 2020, 25, 29. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Hu, R.; Liu, X.; Yang, W.; Wu, Y.; Zhang, L.; Zeng, X.; Chen, R.; Liu, C.; et al. Exposure Memory and Susceptibility to Ambient PM2.5: A Perspective from Hepatic Cholesterol and Bile Acid Metabolism. Ecotoxicol. Environ. Saf. 2024, 280, 116589. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-Y.; Lu, L.; Ren, H.-Y.; Hua, W.; Zheng, N.; Huang, F.-Y.; Wang, J.; Tian, M.; Huang, Q. The Size-Dependence and Reversibility of Polystyrene Nanoplastics-Induced Lipid Accumulation in Mice: Possible Roles of Lysosomes. Environ. Int. 2024, 185, 108532. [Google Scholar] [CrossRef]
- Hamed, M.; Soliman, H.A.M.; Osman, A.G.M.; Sayed, A.E.-D.H. Assessment the Effect of Exposure to Microplastics in Nile Tilapia (Oreochromis niloticus) Early Juvenile: I. Blood Biomarkers. Chemosphere 2019, 228, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Banaee, M.; Soltanian, S.; Sureda, A.; Gholamhosseini, A.; Haghi, B.N.; Akhlaghi, M.; Derikvandy, A. Evaluation of Single and Combined Effects of Cadmium and Micro-Plastic Particles on Biochemical and Immunological Parameters of Common Carp (Cyprinus carpio). Chemosphere 2019, 236, 124335. [Google Scholar] [CrossRef]
- Liu, M.; Mu, J.; Wang, M.; Hu, C.; Ji, J.; Wen, C.; Zhang, D. Impacts of Polypropylene Microplastics on Lipid Profiles of Mouse Liver Uncovered by Lipidomics Analysis and Raman Spectroscopy. J. Hazard. Mater. 2023, 458, 131918. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, L.; Wu, J.; Yuan, X.; Hong, L.; Pu, L.; Qin, S.; Li, L.; Yang, H.; Zhang, J. Multi-Omics Analyses Reveal Differences in Intestinal Flora Composition and Serum Metabolites in Cherry Valley Broiler Ducks of Different Body Weights. Poult. Sci. 2025, 104, 105275. [Google Scholar] [CrossRef]
- Tian, J.; Wang, Y.; Yan, S.; Sun, S.; Jia, J.; Hu, X. Advances in Metabolomics Technology and Its Applications in Agricultural and Livestock Research. Genetics 2020, 42, 452–465. [Google Scholar] [CrossRef]
- Dai, W.; Xie, D.; Lu, M.; Li, P.; Lv, H.; Yang, C.; Peng, Q.; Zhu, Y.; Guo, L.; Zhang, Y.; et al. Characterization of White Tea Metabolome: Comparison against Green and Black Tea by a Nontargeted Metabolomics Approach. Food Res. Int. 2017, 96, 40–45. [Google Scholar] [CrossRef]
- Vásquez-Reyes, S.; Velázquez-Villegas, L.A.; Vargas-Castillo, A.; Noriega, L.G.; Torres, N.; Tovar, A.R. Dietary Bioactive Compounds as Modulators of Mitochondrial Function. J. Nutr. Biochem. 2021, 96, 108768. [Google Scholar] [CrossRef]
- Wang, X.; Sang, W.; Xie, Y.; Xu, J.; Sun, T.; Cuthbertson, A.G.S.; Wu, J.; Ali, S. Comparative Proteomic Analysis Reveals Insights into the Response of Cryptolaemus montrouzieri to Bottom-up Transfer of Cadmium and Lead across a Multi-Trophic Food Chain. Ecotoxicol. Environ. Saf. 2022, 241, 113852. [Google Scholar] [CrossRef]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.-F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1α-Responsive Genes Involved in Oxidative Phosphorylation Are Coordinately Downregulated in Human Diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Grabon, A.; Bankaitis, V.A.; McDermott, M.I. The Interface between Phosphatidylinositol Transfer Protein Function and Phosphoinositide Signaling in Higher Eukaryotes. J. Lipid Res. 2019, 60, 242–268. [Google Scholar] [CrossRef] [PubMed]
- Sofou, K.; Shahim, P.; Tulinius, M.; Blennow, K.; Zetterberg, H.; Mattsson, N.; Darin, N. Cerebrospinal Fluid Neurofilament Light Is Associated with Survival in Mitochondrial Disease Patients. Mitochondrion 2019, 46, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, F.M.; von Stockum, S.; Giacomello, M.; Goliand, I.; Kakimoto, P.; Marchesan, E.; De Stefani, D.; Kowaltowski, A.J.; Ziviani, E.; Shirihai, O.S. A New Target for an Old DUB: UCH-L1 Regulates Mitofusin-2 Levels, Altering Mitochondrial Morphology, Function and Calcium Uptake. Redox Biol. 2020, 37, 101676. [Google Scholar] [CrossRef]
- Cantó, C.; Menzies, K.J.; Auwerx, J. NAD+ Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015, 22, 31–53. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Kadioglu, O.; Fu, Y.; Wiench, B.; Zu, Y.; Efferth, T. Synthetic Cajanin Stilbene Acid Derivatives Inhibit C-MYC in Breast Cancer Cells. Arch. Toxicol. 2016, 90, 575–588. [Google Scholar] [CrossRef]
- Orefice, L.L.; Zimmerman, A.L.; Chirila, A.M.; Sleboda, S.J.; Head, J.P.; Ginty, D.D. Peripheral Mechanosensory Neuron Dysfunction Underlies Tactile and Behavioral Deficits in Mouse Models of ASDs. Cell 2016, 166, 299–313. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Yan, J.; Xie, J. Coculture of Acinetobacter Johnsonii and Shewanella Putrefaciens Contributes to the ABC Transporter That Impacts Cold Adaption in the Aquatic Food Storage Environment. J. Agric. Food Chem. 2024, 72, 10605–10615. [Google Scholar] [CrossRef]
- Bai, Y.; Nan, Y.; Wu, T.; Zhu, A.; Xie, X.; Sun, Y.; Deng, Y.; Dou, Z.; Hu, X.; Zhou, R.; et al. Lipid Nanoparticle-Mediated Delivery of CRISPR-Cas9 Against Rubicon Ameliorates NAFLD by Modulating CD36 Along with Glycerophospholipid Metabolism. Adv. Sci. 2024, 11, 2400493. [Google Scholar] [CrossRef] [PubMed]

| Map Sum Spectrum | ||
|---|---|---|
| Element | Wt% | Atomic% |
| C | 89.96 | 91.64 |
| N | 6.3 | 5.51 |
| O | 3.73 | 2.86 |
| Total | 100 | 100 |
| KO Pathway Level 1 | KO Pathway Level 2 | Metabolites | FC | log2FC | p Value | VIP | Trend |
|---|---|---|---|---|---|---|---|
| Metabolism | Amino acid metabolism | Ergothioneine | 1.26 | 0.33 | 0.032 | 1.798 | up |
| 4-(Glutamylamino) butanoate | 1.53 | 0.62 | 0.028 | 1.652 | up | ||
| D-Lysopine | 1.98 | 0.98 | 0.026 | 1.649 | up | ||
| Fumaric Acid | 2.46 | 1.3 | 0.044 | 1.468 | up | ||
| Quinolinic acid | 0.59 | −0.8 | 0.002 | 2.037 | down | ||
| L-Leucine | 1.38 | 0.47 | 0.004 | 1.884 | up | ||
| (2R,3R)-3-Methylglutamyl- 5-semialdehyde-N6-lysine | 1.67 | 0.74 | 0.01 | 1.793 | up | ||
| Acetyl-N-formyl-5- methoxykynurenamine | 1.54 | 0.62 | 0.021 | 1.644 | up | ||
| 1-Methylhistamine | 1.7 | 0.77 | 0.043 | 1.539 | up | ||
| INDOLE-3-ETHANOL | 2.18 | 1.12 | 0.025 | 1.737 | up | ||
| Biosynthesis of other secondary metabolites | Xanthosine | 1.56 | 0.64 | 0.029 | 1.583 | up | |
| Carbohydrate metabolism | 2-Keto-D-Gluconic acid | 1.52 | 0.6 | 0.037 | 1.571 | up | |
| D-Xylonic acid | 0.34 | −1.6 | 0.024 | 1.803 | down | ||
| Fumaric Acid | 2.46 | 1.3 | 0.044 | 1.468 | up | ||
| Energy metabolism | Fumaric Acid | 2.46 | 1.3 | 0.044 | 1.468 | up | |
| NADH | 2.27 | 1.19 | 0.007 | 1.982 | up | ||
| Global and overview maps | 4-(Glutamylamino) butanoate | 1.53 | 0.62 | 0.028 | 1.652 | up | |
| P-Toluenesulfonic acid | 0.71 | −0.5 | 0.015 | 1.854 | down | ||
| Biotin | 1.42 | 0.51 | 0.013 | 1.829 | up | ||
| Fumaric Acid | 2.46 | 1.3 | 0.044 | 1.468 | up | ||
| Xanthosine | 1.56 | 0.64 | 0.029 | 1.583 | up | ||
| Quinolinic acid | 0.59 | −0.8 | 0.002 | 2.037 | down | ||
| Deoxyadenosine | 1.7 | 0.76 | 0.036 | 1.544 | up | ||
| L-Leucine | 1.38 | 0.47 | 0.004 | 1.884 | up | ||
| 2-Keto-D-Gluconic acid | 1.52 | 0.6 | 0.037 | 1.571 | up | ||
| NADH | 2.27 | 1.19 | 0.007 | 1.982 | up | ||
| Dethiobiotin | 1.86 | 0.89 | 0.042 | 1.639 | up | ||
| D-4′-Phosphopantothenate | 1.68 | 0.75 | 0.003 | 1.988 | up | ||
| inosine | 2.01 | 1.01 | 0.036 | 1.584 | up | ||
| Lipid metabolism | LysoPC(16:1(9Z)/0:0) | 1.72 | 0.79 | 0.038 | 1.569 | up | |
| Metabolism of cofactors and vitamins | Biotin | 1.42 | 0.51 | 0.013 | 1.829 | up | |
| 9-cis-Retinal | 0.3 | −1.7 | 0.0003 | 2.065 | down | ||
| D-4′-Phosphopantothenate | 1.68 | 0.75 | 0.003 | 1.988 | up | ||
| Dethiobiotin | 1.86 | 0.89 | 0.042 | 1.639 | up | ||
| Fumaric Acid | 2.46 | 1.3 | 0.044 | 1.468 | up | ||
| Quinolinic acid | 0.59 | −0.8 | 0.002 | 2.037 | down | ||
| Metabolism of other amino acids | beta-Alanyl-L-arginine | 1.92 | 0.94 | 0.019 | 1.65 | up | |
| D-4′-Phosphopantothenate | 1.68 | 0.75 | 0.003 | 1.988 | up | ||
| Quinolinic acid | 0.59 | −0.8 | 0.002 | 2.037 | down | ||
| Metabolism of terpenoids and polyketides | Farnesylcysteine | 2.39 | 1.26 | 0.028 | 1.549 | up | |
| trans,trans-Farnesyl phosphate | 1.61 | 0.69 | 0.002 | 1.959 | up | ||
| Nucleotide metabolism | inosine | 2.01 | 1.01 | 0.036 | 1.584 | up | |
| Xanthosine | 1.56 | 0.64 | 0.029 | 1.583 | up | ||
| Deoxyadenosine | 1.7 | 0.76 | 0.036 | 1.544 | up | ||
| Environmental Information Processing | Membrane transport | Biotin | 1.42 | 0.51 | 0.013 | 1.829 | up |
| L-Leucine | 1.38 | 0.47 | 0.004 | 1.884 | up | ||
| Signal transduction | Cyclic ADP-ribose | 1.94 | 0.96 | 0.019 | 1.625 | up | |
| L-Leucine | 1.38 | 0.47 | 0.004 | 1.884 | up | ||
| NADH | 2.27 | 1.19 | 0.007 | 1.982 | up | ||
| Organismal Systems | Endocrine system | Cyclic ADP-ribose | 1.94 | 0.96 | 0.019 | 1.625 | up |
| NADH | 2.27 | 1.19 | 0.007 | 1.982 | up | ||
| Digestive system | Cyclic ADP-ribose | 1.94 | 0.96 | 0.019 | 1.625 | up | |
| Biotin | 1.42 | 0.51 | 0.013 | 1.829 | up | ||
| L-Leucine | 1.38 | 0.47 | 0.004 | 1.884 | up | ||
| Genetic Information Processing | Translation | L-Leucine | 1.38 | 0.47 | 0.004 | 1.884 | up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Liu, Y.; Ma, Y.; Zhang, J.; Ma, L.; Chen, W.; Tang, X.; Lu, J.; Chen, L.; Cai, G.; et al. Untargeted Metabolomics Uncovers Food Safety Risks: Polystyrene Nanoplastics Induce Metabolic Disorders in Chicken Liver. Foods 2025, 14, 2781. https://doi.org/10.3390/foods14162781
Hu X, Liu Y, Ma Y, Zhang J, Ma L, Chen W, Tang X, Lu J, Chen L, Cai G, et al. Untargeted Metabolomics Uncovers Food Safety Risks: Polystyrene Nanoplastics Induce Metabolic Disorders in Chicken Liver. Foods. 2025; 14(16):2781. https://doi.org/10.3390/foods14162781
Chicago/Turabian StyleHu, Xuan, Yinyin Liu, Yinpeng Ma, Jing Zhang, Lina Ma, Wanqiang Chen, Xiujun Tang, Junxian Lu, Lingzhi Chen, Guodong Cai, and et al. 2025. "Untargeted Metabolomics Uncovers Food Safety Risks: Polystyrene Nanoplastics Induce Metabolic Disorders in Chicken Liver" Foods 14, no. 16: 2781. https://doi.org/10.3390/foods14162781
APA StyleHu, X., Liu, Y., Ma, Y., Zhang, J., Ma, L., Chen, W., Tang, X., Lu, J., Chen, L., Cai, G., Bian, J., & Gao, Y. (2025). Untargeted Metabolomics Uncovers Food Safety Risks: Polystyrene Nanoplastics Induce Metabolic Disorders in Chicken Liver. Foods, 14(16), 2781. https://doi.org/10.3390/foods14162781






