Silver Nanocluster–Based Label-Free Aptasensor for the Turn-On Fluorescent Detection of Ochratoxin A
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. AgNC Synthesis
2.4. Fluorescent Detection of OTA
2.5. Specificity of OTA Detection
2.5.1. Specificity Verification of Aptamer-AgNCs Interaction
2.5.2. Specificity Verification of OTA Recognition by Apt-OTA
2.6. Application
2.7. Statistical Analysis
3. Results and Discussion
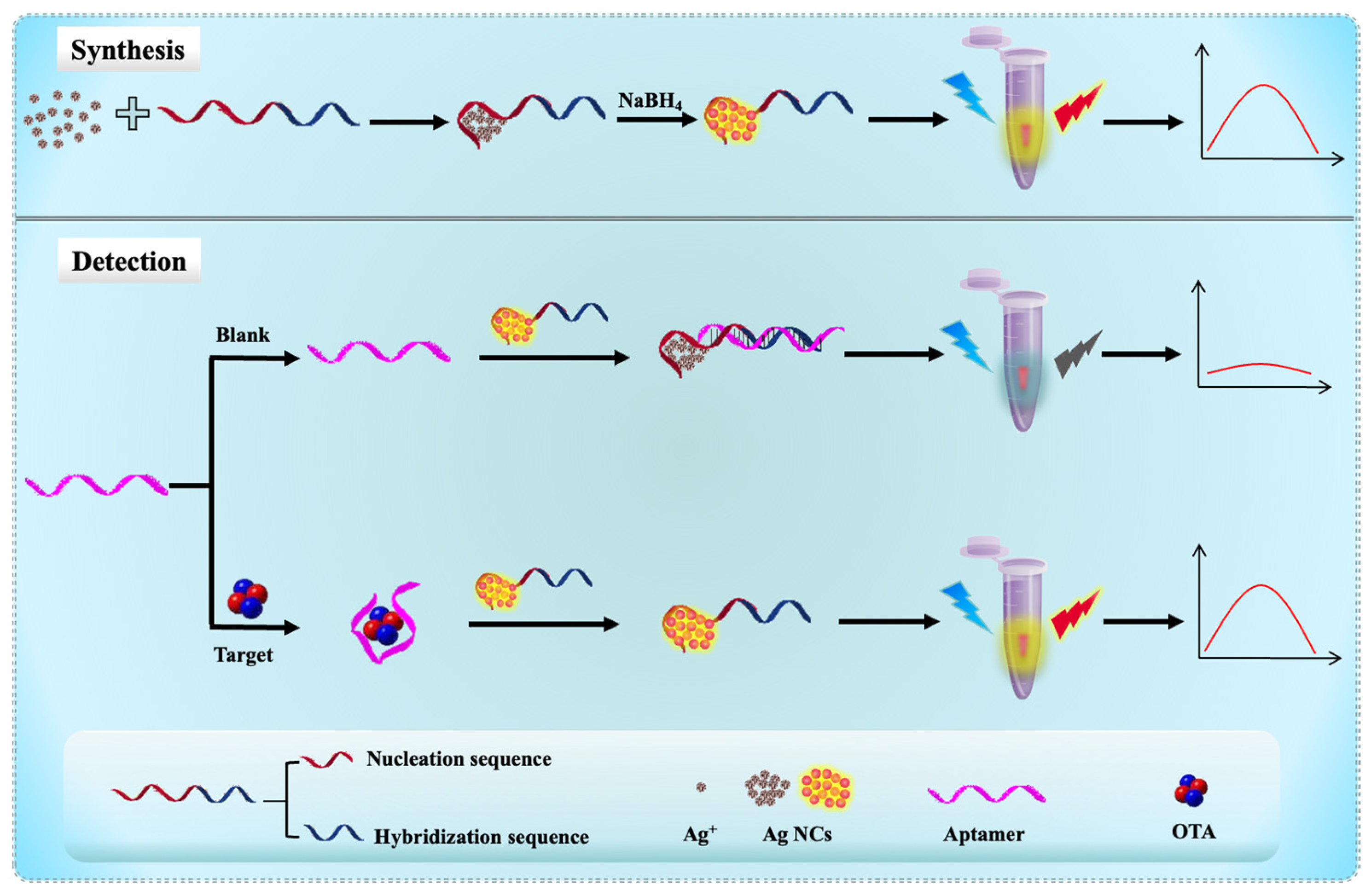
3.1. Design Strategy for OTA Detection
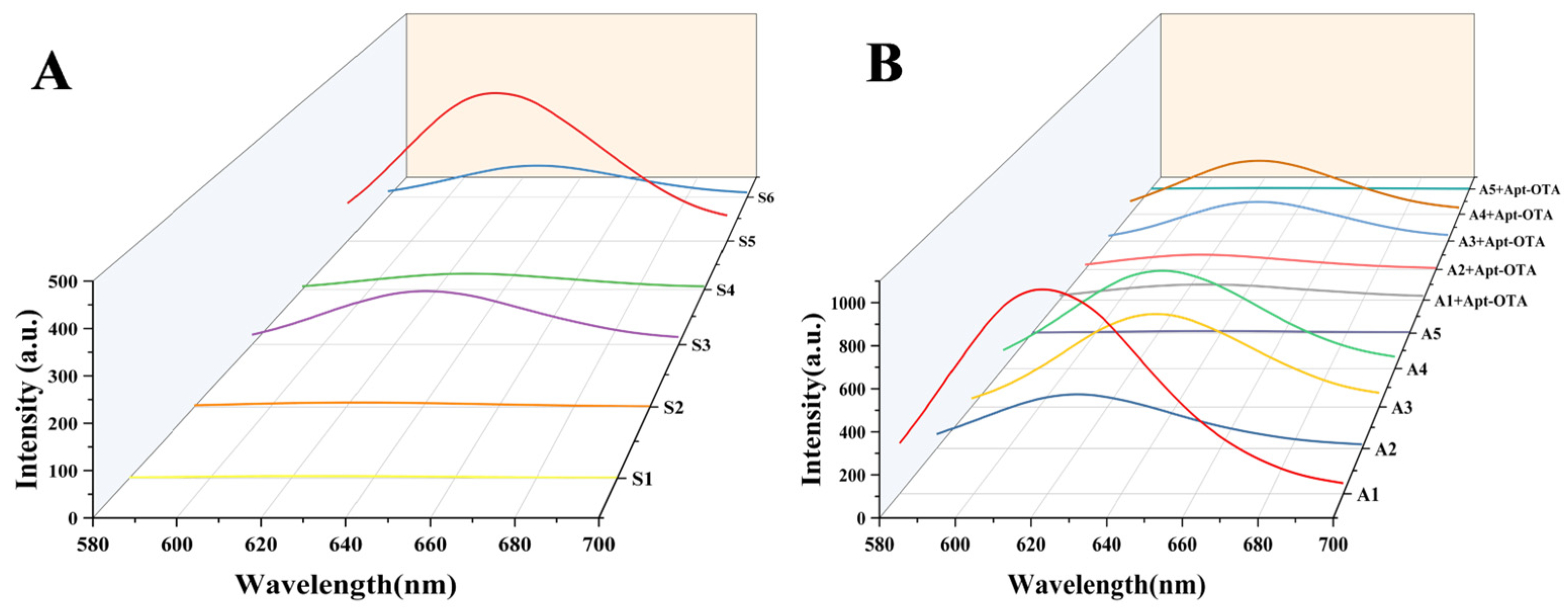
3.2. Selection of Template DNA for AgNCs
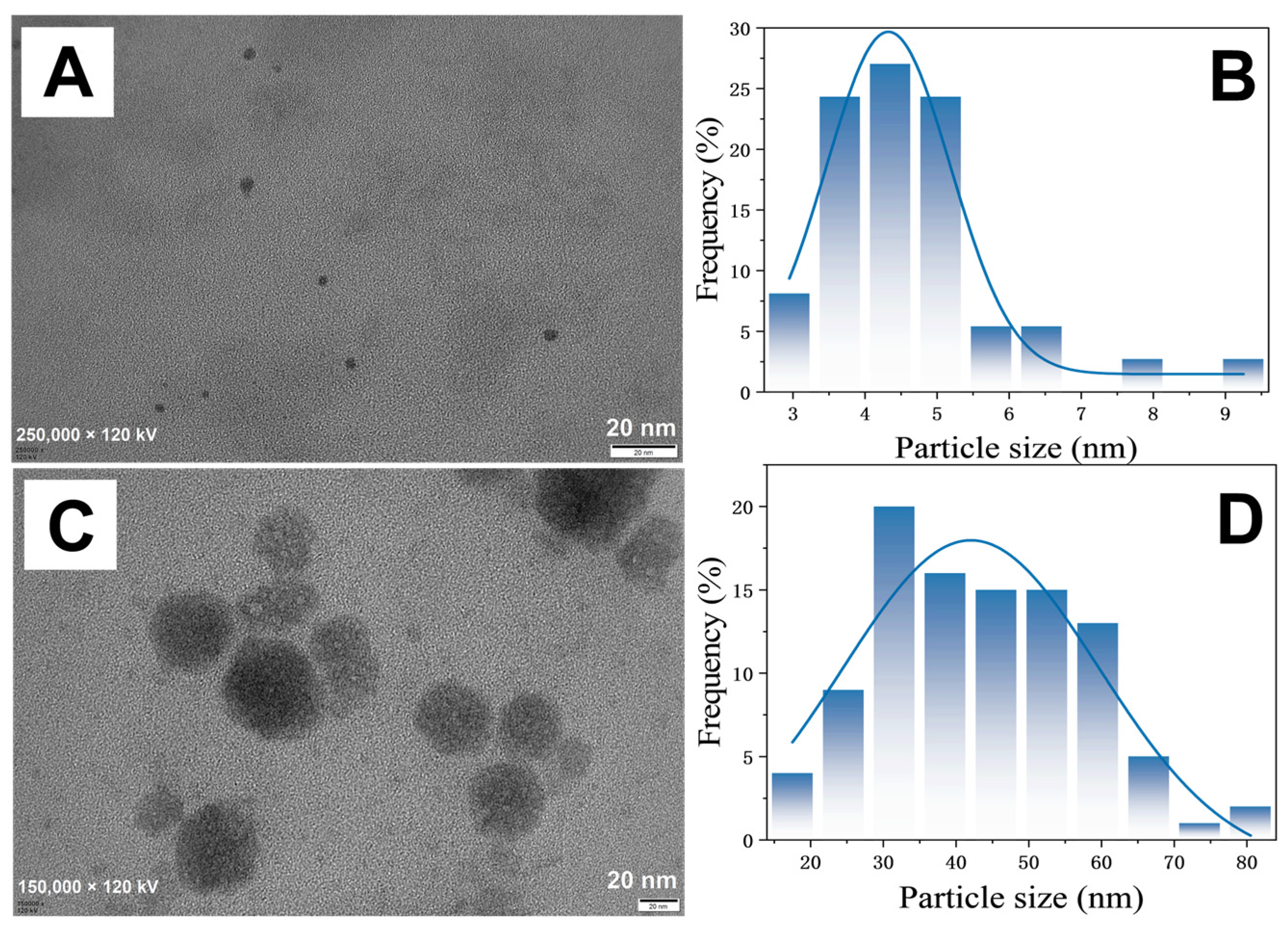
3.3. Optimisation of AgNC Preparation Parameters
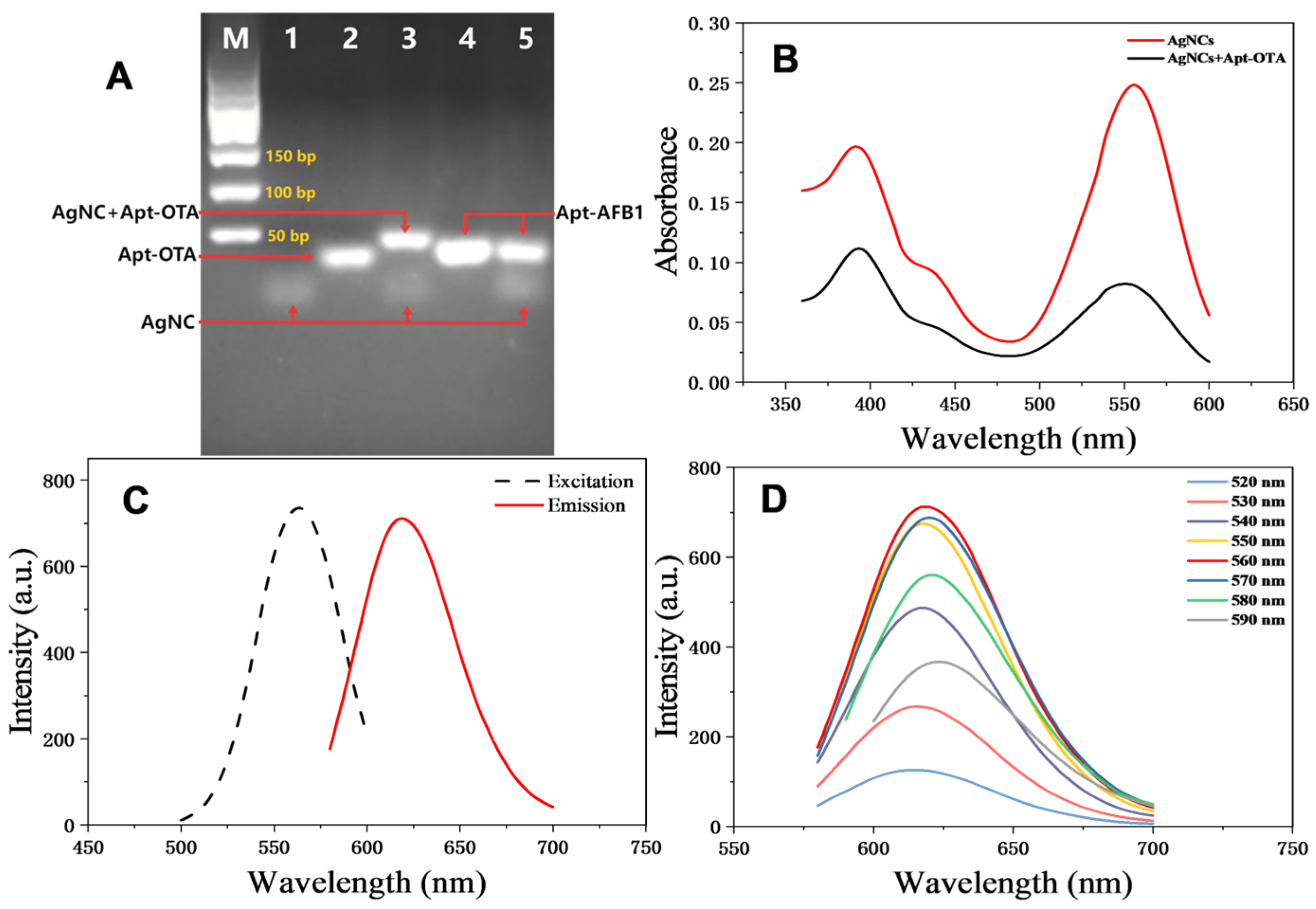
3.4. Characterisation of AgNCs and AgNCs + Apt-OTA Mixtures
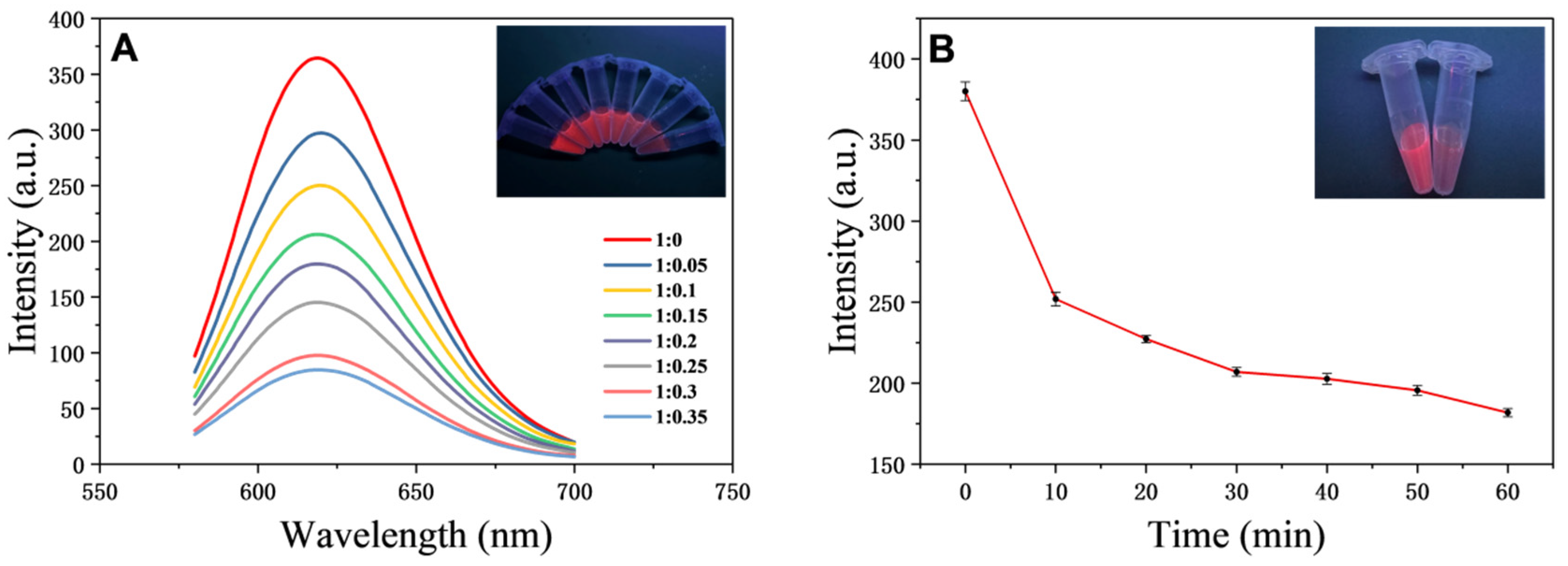
3.5. Apt-OTA Parameter Optimisation
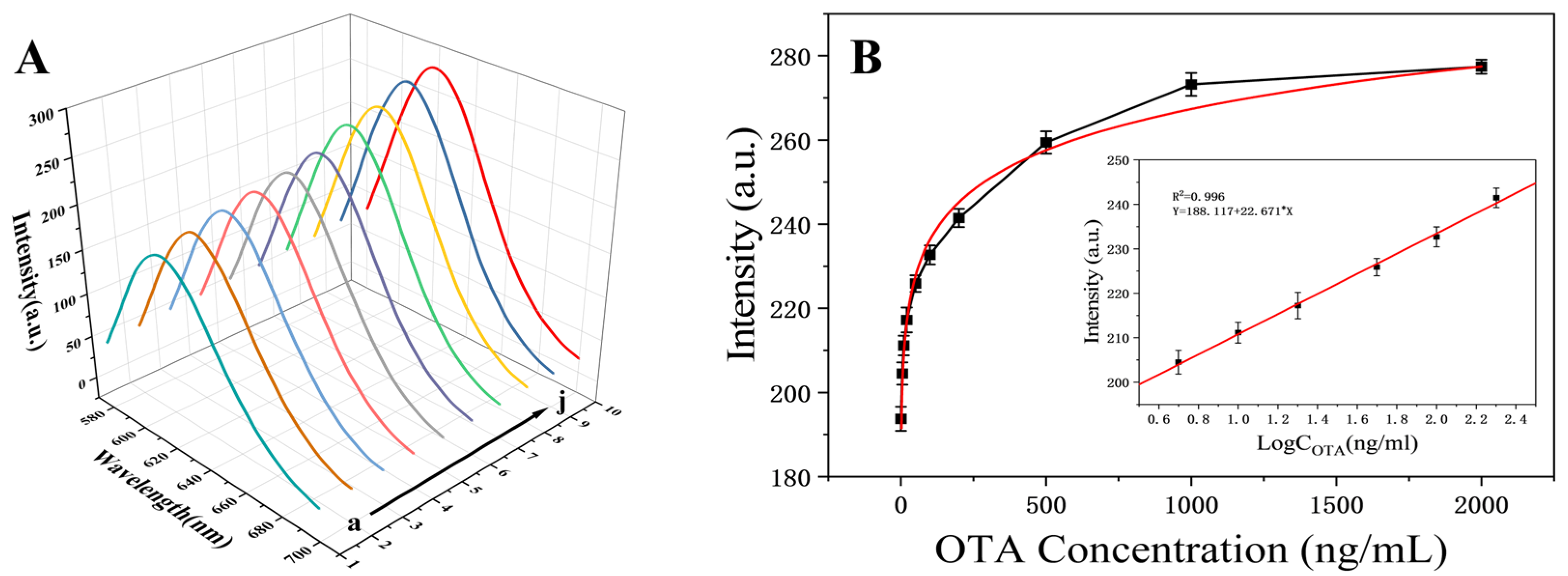
3.6. Sensitivity of OTA Detection
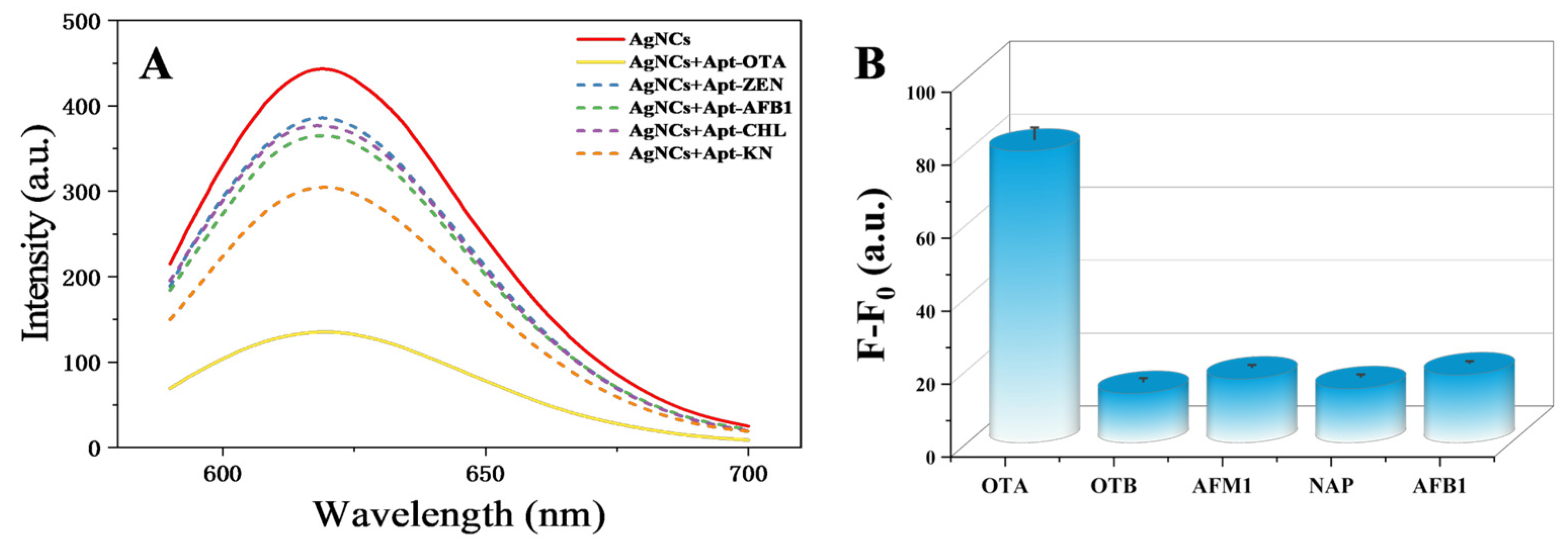
3.7. OTA Detection Specificity
3.8. Evaluation of OTA in Practical Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bryła, M.; Ksieniewicz-Woźniak, E.; Stępniewska, S.; Modrzewska, M.; Waśkiewicz, A.; Szymczyk, K.; Szafrańska, A. Transformation of ochratoxin A during bread-making processes. Food Control 2021, 125, 107950. [Google Scholar] [CrossRef]
- Gu, K.; Ryu, D.; Lee, H.J. Ochratoxin A and its reaction products affected by sugars during heat processing. Food Chem. 2021, 348, 129038. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hua, X.; Shi, J.; Jing, N.; Ji, T.; Lv, B.; Liu, L.; Chen, Y. Ochratoxin A: Occurrence and recent advances in detoxification. Toxicon 2022, 210, 11–18. [Google Scholar] [CrossRef]
- Kumar, P.; Mahato, D.K.; Sharma, B.; Borah, R.; Haque, S.; Mahmud, M.M.C.; Shah, A.K.; Rawal, D.; Bora, H.; Bui, S. Ochratoxins in food and feed: Occurrence and its impact on human health and management strategies. Toxicon 2020, 187, 151–162. [Google Scholar] [CrossRef]
- Tao, Y.; Xie, S.; Xu, F.; Liu, A.; Wang, Y.; Chen, D.; Pan, Y.; Huang, L.; Peng, D.; Wang, X.; et al. Ochratoxin A: Toxicity, oxidative stress and metabolism. Food Chem. Toxicol. 2018, 112, 320–331. [Google Scholar] [CrossRef]
- Klingelhöfer, D.; Braun, M.; Schöffel, N.; Oremek, G.M.; Brüggmann, D.; Groneberg, D.A. Ochratoxin–Characteristics, influences and challenges of global research. Food Control 2020, 114, 107230. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Wang, S.; Cai, R.; Yuan, Y.; Yue, T.; Wang, Z. Bio-control on the contamination of Ochratoxin A in food: Current research and future prospects. Curr. Res. Food Sci. 2022, 5, 1539–1549. [Google Scholar] [CrossRef]
- Yang, Q.; Dhanasekaran, S.; Ngea, G.L.N.; Tian, S.; Li, B.; Zhang, H. Unveiling ochratoxin a controlling and biodetoxification molecular mechanisms: Opportunities to secure foodstuffs from OTA contamination. Food Chem. Toxicol. 2022, 169, 113437. [Google Scholar] [CrossRef]
- Wei, W.; Liu, C.; Ke, P.; Chen, X.; Zhou, T.; Xu, J.; Zhou, Y. Toxicological and physiological effects of successive exposure to ochratoxin A at food regulatory limits. Food Chem. Toxicol. 2021, 151, 112128. [Google Scholar] [CrossRef]
- Campone, L.; Piccinelli, A.L.; Celano, R.; Pagano, I.; Russo, M.; Rastrelli, L. Rapid and automated on-line solid phase extraction HPLC–MS/MS with peak focusing for the determination of ochratoxin A in wine samples. Food Chem. 2018, 244, 128–135. [Google Scholar] [CrossRef]
- Njumbe Ediage, E.; Van Poucke, C.; De Saeger, S. A multi-analyte LC–MS/MS method for the analysis of 23 mycotoxins in different sorghum varieties: The forgotten sample matrix. Food Chem. 2015, 177, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-S.; Zhao, J.; Ma, T.-T.; Li, Z.-Y.; Wang, L.-L.; Ji, S.-L.; Sun, M.-Y.; Liu, Y.-S.; Hu, Z.-H.; Liu, Q.-W.; et al. Magnetic covalent organic framework for effective solid-phase extraction and HPLC determination of ochratoxin A in food. Lwt 2023, 179, 114639. [Google Scholar] [CrossRef]
- Ouyang, M.; Liu, T.; Yuan, X.; Xie, C.; Luo, K.; Zhou, L. Nanomaterials-based aptasensors for rapid detection and early warning of key food contaminants: A review. Food Chem. 2025, 462, 140990. [Google Scholar] [CrossRef] [PubMed]
- Xavier, M.; Parente, I.A.; Rodrigues, P.M.; Cerqueira, M.A.; Pastrana, L.; Gonçalves, C. Safety and fate of nanomaterials in food: The role of in vitro tests. Trends Food Sci. Technol. 2021, 109, 593–607. [Google Scholar] [CrossRef]
- Eguílaz, M.; Gutierrez, F.; González-Domínguez, J.M.; Martínez, M.T.; Rivas, G. Single-walled carbon nanotubes covalently functionalized with polytyrosine: A new material for the development of NADH-based biosensors. Biosens. Bioelectron. 2016, 86, 308–314. [Google Scholar] [CrossRef]
- Guo, Z.; Tian, J.; Cui, C.; Wang, Y.; Yang, H.; Yuan, M.; Yu, H. A label-free aptasensor for turn-on fluorescent detection of ochratoxin a based on SYBR gold and single walled carbon nanohorns. Food Control 2021, 123, 107741. [Google Scholar] [CrossRef]
- Wu, G.; Qiu, H.; Liu, X.; Luo, P.; Wu, Y.; Shen, Y. Nanomaterials-based fluorescent assays for pathogenic bacteria in food-related matrices. Trends Food Sci. Technol. 2023, 142, 104214. [Google Scholar] [CrossRef]
- Kheirkhahnia, S.; Sadeghi, S. Copper metallic nanoclusters encapsulated within zinc-based metal organic framework for optical sensing of ciprofloxacin in aqueous solutions. J. Photochem. Photobiol. A: Chem. 2025, 462, 116259. [Google Scholar] [CrossRef]
- Xu, J.; Bi, Y.; Zhao, H.; Shi, L.; Zhang, N.; Xin, X. In situ synthesis of well-dispersed silver nanoparticles from silver nanoclusters hydrogel for catalytic reduction of 4-Nitrophenol. Appl. Surf. Sci. 2025, 683, 161759. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, X.; Zhou, X.; Khusbu, F.Y.; Ma, C. Recent advances in the bioanalytical and biomedical applications of DNA-templated silver nanoclusters. TrAC Trends Anal. Chem. 2020, 124, 115786. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, L.; Yang, W.; Xu, W. Nucleic acid-templated silver nanoclusters: A review of structures, properties, and biosensing applications. Coord. Chem. Rev. 2023, 491, 215247. [Google Scholar] [CrossRef]
- Zhou, B.; Khan, I.M.; Ding, X.; Niazi, S.; Zhang, Y.; Wang, Z. Fluorescent DNA-Silver nanoclusters in food safety detection: From synthesis to application. Talanta 2024, 273, 125834. [Google Scholar] [CrossRef]
- Petty, J.T.; Zheng, J.; Hud, N.V.; Dickson, R.M. DNA-Templated Ag Nanocluster Formation. J. Am. Chem. Soc. 2004, 126, 5207–5212. [Google Scholar] [CrossRef]
- Sharma, J.; Rocha, R.C.; Phipps, M.L.; Yeh, H.-C.; Balatsky, K.A.; Vu, D.M.; Shreve, A.P.; Werner, J.H.; Martinez, J.S. A DNA-templated fluorescent silver nanocluster with enhanced stability. Nanoscale 2012, 4, 4107–4110. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Pan, X.; Zhang, W.; Hu, Z.; Wong, K.-W.; He, Z.; Li, H.-W. Label-free probes using DNA-templated silver nanoclusters as versatile reporters. Biosens. Bioelectron. 2020, 150, 111926. [Google Scholar] [CrossRef]
- Yang, S.W.; Vosch, T. Rapid Detection of MicroRNA by a Silver Nanocluster DNA Probe. Anal. Chem. 2011, 83, 6935–6939. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guan, B.; Xu, Z.; Wu, Y.; Wang, Y.; Ning, G. A fluorescent assay for sensitive detection of kanamycin by split aptamers and DNA-based copper/silver nanoclusters. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2023, 286, 121953. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Shan, L.; Guo, B.; Wang, J.; Huang, Q.; Wang, S.; Li, F.; Wu, S.; Wang, W.; Chen, J. A fluorescent aptasensor for kanamycin detection in milk, seafood and water samples using DNA-AgNCs and exonuclease I-assisted recycling amplification strategy. Food Chem. 2025, 478, 143291. [Google Scholar] [CrossRef]
- Fan, P.; Li, Q.; Zhang, Z.; Ni, S.; Jiang, P.; Sun, S.; Li, L. A novel and universal dual-channel signal amplification aptasensing platform for ultrasensitive and rapid detection of cardiac biomarkers based on the mutual regulation of bimetallic organic framework and silver nanoclusters. Talanta 2025, 288, 127745. [Google Scholar] [CrossRef]
- Yin, Y.; Han, L.; Liu, J.; Xu, Y.; Tian, Y.; Mou, Y.; Chen, D.; Sun, X.; Guo, Y.; Li, F.; et al. Dual-color AgNCs-enabled fluorescent aptasensor for the simultaneous detection of KAN and AFB1. Food Chem. 2025, 493, 145595. [Google Scholar] [CrossRef]
- Wu, Y.; Feng, Y.; Mei, X.; Zhu, Y.; Wu, K.; Deng, A.; Li, J. A visualizable-quantitative SERS-LFIA platform for ultrasensitive detection of ochratoxin a using size-tunable Au@Ag core-shell nanocubes as the substrate. Microchem. J. 2025, 216, 114763. [Google Scholar] [CrossRef]
- Cruz-Aguado, J.A.; Penner, G. Determination of Ochratoxin A with a DNA Aptamer. J. Agric. Food Chem. 2008, 56, 10456–10461. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhu, J.; Fan, D.; Teng, Y.; Zhu, X.; Dong, S. A Multicolor Chameleon DNA-templated Silver Nanocluster and Its Application for Ratiometric Fluorescence Target Detection with Exponential Signal Response. Adv. Funct. Mater. 2017, 27, 1704092. [Google Scholar] [CrossRef]
- Kondo, J.; Tada, Y.; Dairaku, T.; Saneyoshi, H.; Okamoto, I.; Tanaka, Y.; Ono, A. High-Resolution Crystal Structure of a Silver(I)–RNA Hybrid Duplex Containing Watson-Crick-like C-Silver(I)-C Metallo-Base Pairs. Angew. Chem. Int. Ed. 2015, 54, 13323–13326. [Google Scholar] [CrossRef]
- New, S.Y.; Lee, S.T.; Su, X.D. DNA-templated silver nanoclusters: Structural correlation and fluorescence modulation. Nanoscale 2016, 8, 17729–17746. [Google Scholar] [CrossRef]
- Li, J.; Wang, W.; Sun, D.; Chen, J.; Zhang, P.-H.; Zhang, J.-R.; Min, Q.; Zhu, J.-J. Aptamer-functionalized silver nanoclusters-mediated cell type-specific siRNA delivery and tracking. Chem. Sci. 2013, 4, 3514–3521. [Google Scholar] [CrossRef]
- Li, J.; Zhong, X.; Cheng, F.; Zhang, J.-R.; Jiang, L.-P.; Zhu, J.-J. One-Pot Synthesis of Aptamer-Functionalized Silver Nanoclusters for Cell-Type-Specific Imaging. Anal. Chem. 2012, 84, 4140–4146. [Google Scholar] [CrossRef]
- Li, R.; Zhu, L.; Yang, M.; Liu, A.; Xu, W.; He, P. Silver nanocluster-based aptasensor for the label-free and enzyme-free detection of ochratoxin A. Food Chem. 2024, 431, 137126. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Tseng, W.-L. Highly sensitive and selective detection of silver ions and silver nanoparticles in aqueous solution using an oligonucleotide-based fluorogenic probe. Chem. Commun. 2009, 43, 6619–6621. [Google Scholar] [CrossRef]
- Ono, A.; Cao, S.; Togashi, H.; Tashiro, M.; Fujimoto, T.; Machinami, T.; Oda, S.; Miyake, Y.; Okamoto, I.; Tanaka, Y. Specific interactions between silver(i) ions and cytosine–cytosine pairs in DNA duplexes. Chem. Commun. 2008, 39, 4825–4827. [Google Scholar] [CrossRef]
- Xu, L.; Qu, W.; Zhu, Z.; Hao, X.; Yang, Q.; Liu, L.; Gong, Z.; Li, P. Tetrahedral DNA scaffold-based label-free electrochemical aptasensor for the detection of ochratoxin A. Lwt 2025, 218, 117475. [Google Scholar] [CrossRef]
- Briones, N.; Gómez, H.; Henríquez, R.; Rojas, V.; Villarroel, M. Photoelectrochemical aptasensor for detection of Ochratoxin A in synthetic red wine samples. J. Photochem. Photobiol. A: Chem. 2025, 463, 116311. [Google Scholar] [CrossRef]
- Xu, M.; Li, Y.; Hu, X.; Zhang, S.; Liu, Y. Smartphone-assisted antifouling colorimetric aptasensor for ultrasensitive ochratoxin A detection in coffee. Microchem. J. 2025, 218, 115167. [Google Scholar] [CrossRef]
- Li, Y.-L.; Chen, Y.-Y.; Xie, F.-T.; Li, Q.-X.; Yang, T.; Yang, Y.-H.; Hu, R. Smartphone-based dual-mode aptasensor with bifunctional metal-organic frameworks as signal probes for ochratoxin A detection. Food Chem. 2025, 464, 141540. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Yu, R. Nucleic acid aptamer based thermally oxidized porous silicon/zinc oxide microarray chip for detection of ochratoxin A in cereals. Food Chem. 2024, 442, 138384. [Google Scholar] [CrossRef]
- Zhao, Y.; Niu, M.; Wei, D.; Wang, B.; Guan, T.; Lv, L.; Guo, Z. Exonuclease III-mediated label-free fluorometric aptasensor for ochratoxin A detection in food samples based on aggregation-induced emission probe. J. Food Compos. Anal. 2025, 148, 108272. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, C.; Zhao, F.; Suo, Z.; Liu, Y.; Wei, M.; Jin, B. Ratiometric fluorescent aptasensor based on DNA-gated Fe3O4@Uio-66-NH2 and Exo I-assisted signal amplification. Analytica Chimica Acta 2025, 1340, 343665. [Google Scholar] [CrossRef] [PubMed]
| Sample Number | Added (ng/mL) | Detected (ng/mL) | Recovery (%) | Relative Standard Deviation (%) | |||
|---|---|---|---|---|---|---|---|
| Present Method | HPLC | Present Method | HPLC | Present Method | HPLC | ||
| 1 | 20 | 20.8 | 19.5 | 104 | 97.5 | 4.17 | 4.27 |
| 2 | 60 | 59.7 | 61.4 | 99.5 | 102.3 | 3.47 | 3.78 |
| 3 | 100 | 97.2 | 102.1 | 97.2 | 102.1 | 5.54 | 5.06 |
| 4 | 140 | 141.2 | 139.6 | 100.9 | 99.7 | 4.48 | 4.47 |
| 5 | 180 | 183.9 | 181.2 | 102.2 | 100.7 | 5.27 | 5.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nan, J.; Cui, C.; Guo, Z. Silver Nanocluster–Based Label-Free Aptasensor for the Turn-On Fluorescent Detection of Ochratoxin A. Foods 2025, 14, 3271. https://doi.org/10.3390/foods14183271
Nan J, Cui C, Guo Z. Silver Nanocluster–Based Label-Free Aptasensor for the Turn-On Fluorescent Detection of Ochratoxin A. Foods. 2025; 14(18):3271. https://doi.org/10.3390/foods14183271
Chicago/Turabian StyleNan, Jinyan, Chengbi Cui, and Zhijun Guo. 2025. "Silver Nanocluster–Based Label-Free Aptasensor for the Turn-On Fluorescent Detection of Ochratoxin A" Foods 14, no. 18: 3271. https://doi.org/10.3390/foods14183271
APA StyleNan, J., Cui, C., & Guo, Z. (2025). Silver Nanocluster–Based Label-Free Aptasensor for the Turn-On Fluorescent Detection of Ochratoxin A. Foods, 14(18), 3271. https://doi.org/10.3390/foods14183271





