Characterization of the Flavors and Organoleptic Attributes of Petit Manseng Noble Rot Wines from the Eastern Foothills of Helan Mountain in Ningxia, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Grape Material
2.2. Chemicals and Reagents
2.3. Instruments
2.4. Test Methods
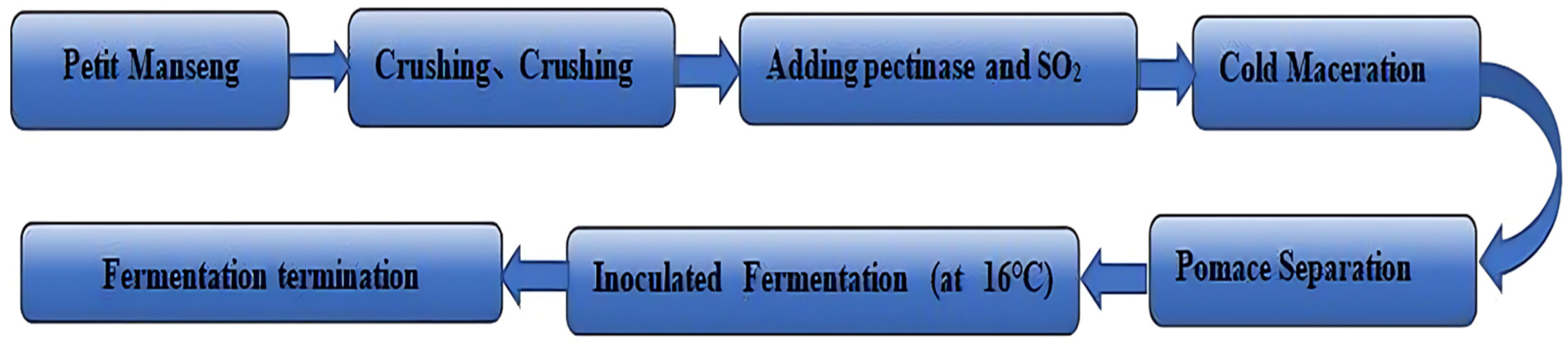
2.4.1. Petit Manseng Sweet Wines
2.4.2. Determining Physicochemical Parameters of Wines with Different Levels of Infestation
2.4.3. Determination of Phenolic Substances in Wine
2.4.4. Determining Volatile Compound Composition of Wines with Different Levels of Infestation
2.4.5. Wine Electronic Nose Measurement
2.4.6. Sensory Evaluation of Aromatic Characteristics
2.5. Data Processing
3. Results and Discussion
3.1. Physical and Chemical Analysis of Grapes and Wines
3.1.1. Physicochemical Analysis of Three Different Infestation Levels of Petit Manseng Grapes
3.1.2. Physicochemical Analysis of the Three Different Groups of Petit Manseng Wines
3.2. Analysis of Volatile Compounds in Three Wines with Different Levels of Infestation
3.2.1. Screening for Characteristic Flavor Substances
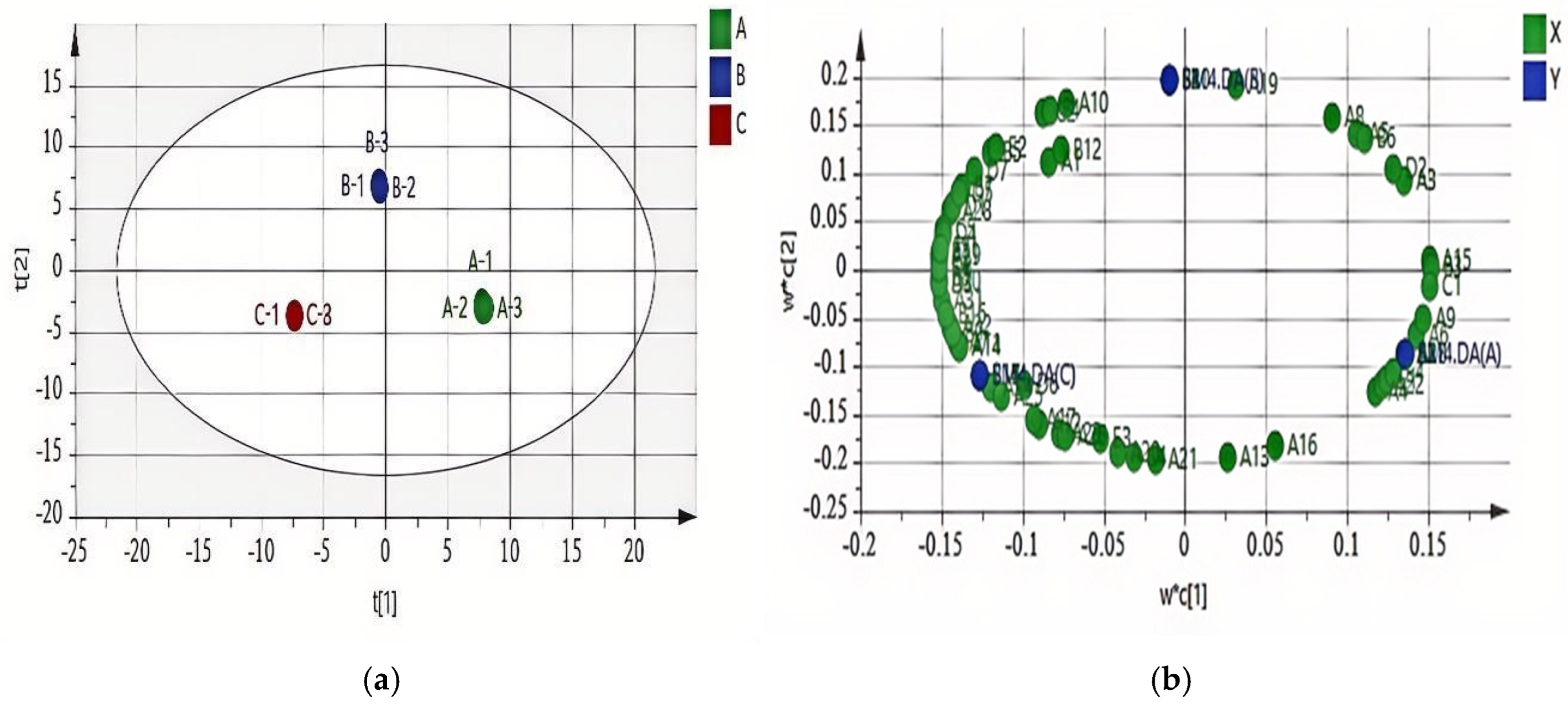
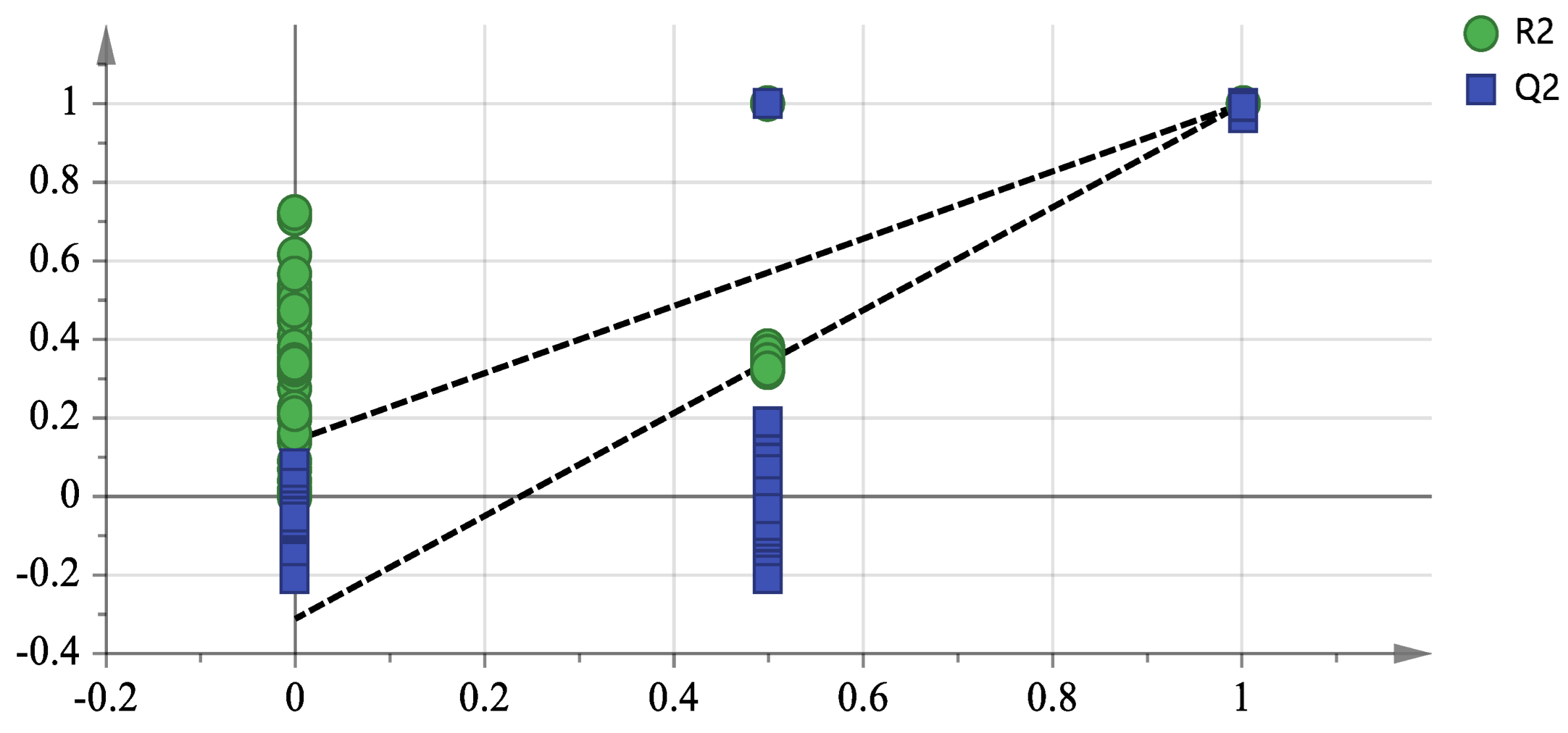
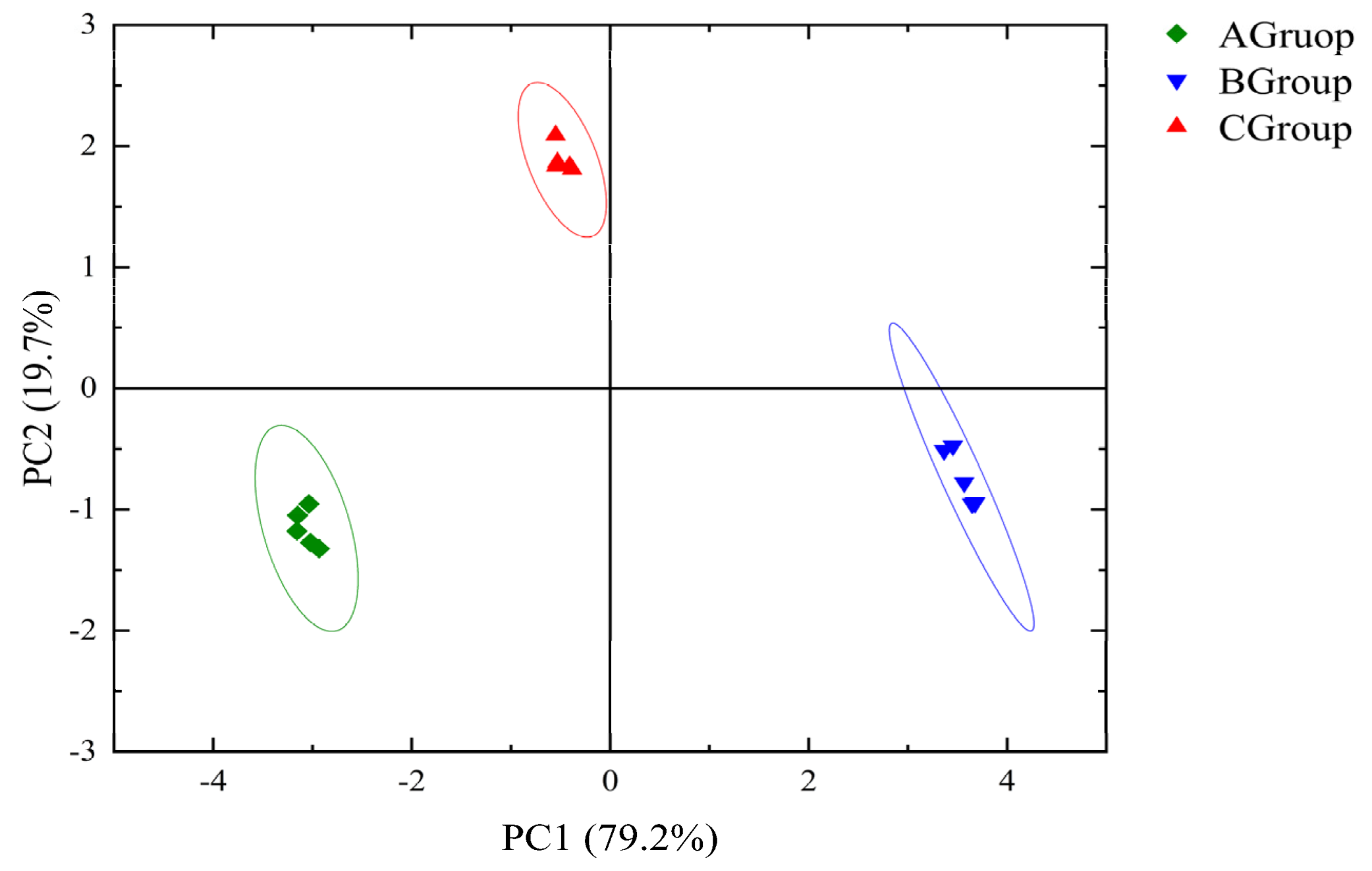
3.2.2. PLS-DA of GC-MS Data
3.2.3. OAV Analysis of GC-MS Data
3.3. Electronic Nose Analysis
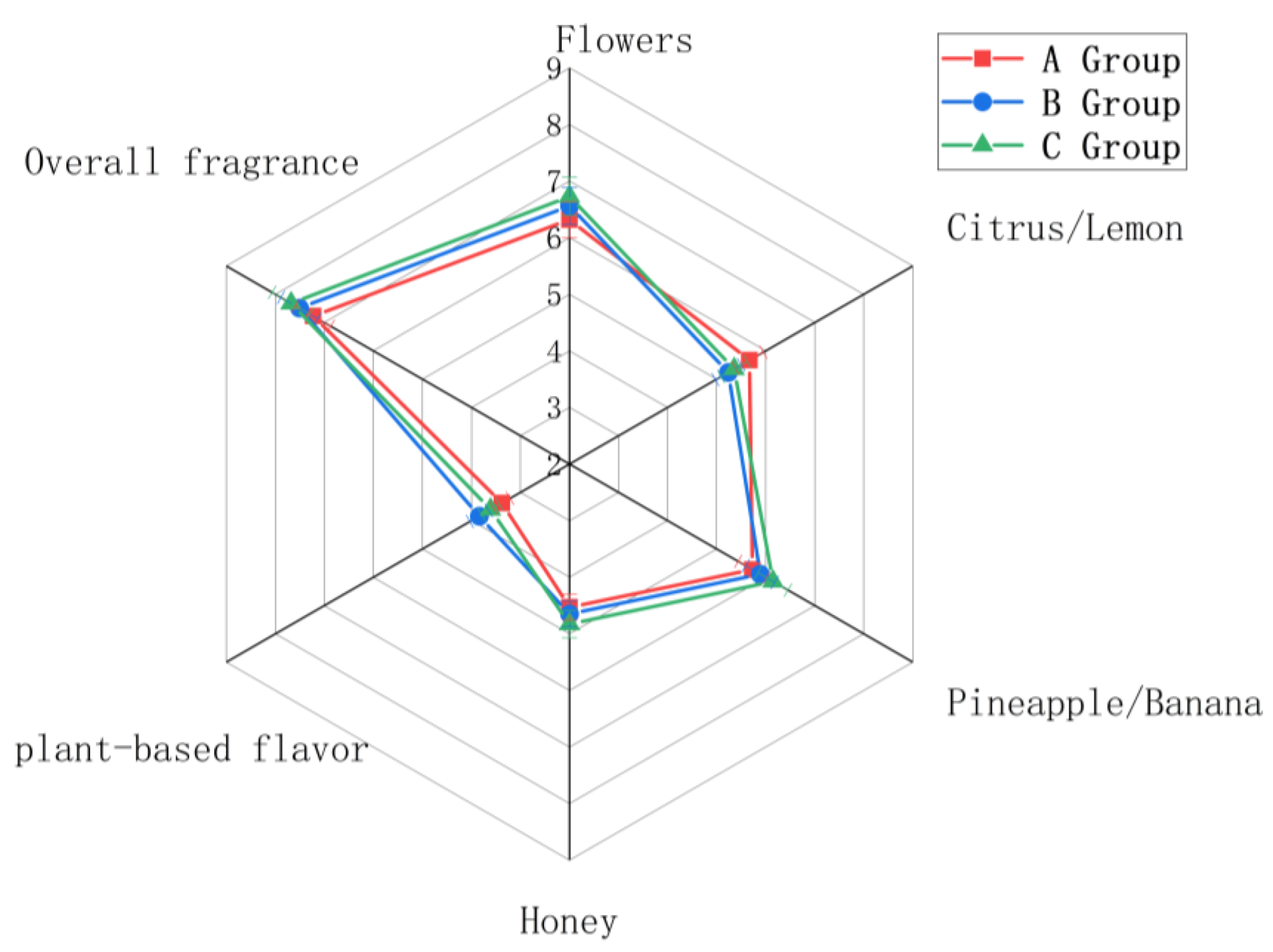
3.4. Analysis of Aromatic Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magyar, I. Botrytized Wines. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2011; Volume 63, pp. 147–206. ISBN 1043-4526. [Google Scholar]
- Sarrazin, E.; Dubourdieu, D.; Darriet, P. Characterization of key-aroma compounds of botrytized wines, influence of grape botrytization. Food Chem. 2007, 103, 536–545. [Google Scholar] [CrossRef]
- Bailly, S.; Jerkovic, V.; Marchand-Brynaert, J.; Collin, S. Aroma Extraction Dilution Analysis of Sauternes Wines. Key Role of Polyfunctional Thiols. J. Agric. Food Chem. 2006, 54, 7227–7234. [Google Scholar] [CrossRef]
- Campo, E.; Cacho, J.; Ferreira, V. The Chemical Characterization of the Aroma of Dessert and Sparkling White Wines (Pedro Ximénez, Fino, Sauternes, and Cava) by Gas Chromatography−Olfactometry and Chemical Quantitative Analysis. J. Agric. Food Chem. 2008, 56, 2477–2484. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Yang, H.; Sun, L.; Xia, H.; Sun, W.; Wang, Z.; Zhang, J. Bacterial Communities Related to Aroma Formation during Spontaneous Fermentation of ‘Cabernet Sauvignon’ Wine in Ningxia, China. Foods 2022, 11, 2775. [Google Scholar] [CrossRef]
- Modesti, M.; Alfieri, G.; Chieffo, C.; Mencarelli, F.; Vannini, A.; Catalani, A.; Chilosi, G.; Bellincontro, A. Destructive and non-destructive early detection of postharvest noble rot (Botrytis cinerea) in wine grapes aimed at producing high-quality wines. J. Sci. Food Agric. 2024, 104, 2314–2325. [Google Scholar] [CrossRef] [PubMed]
- Boido, E.; Alcalde-Eon, C.; Carrau, F.; Dellacassa, E.; Rivas-Gonzalo, J.C. Aging Effect on the Pigment Composition and Color of Vitis vinifera L. Cv. Tannat Wines. Contribution of the Main Pigment Families to Wine Color. J. Agric. Food Chem. 2006, 54, 6692–6704. [Google Scholar] [CrossRef]
- i Cortiella, M.G.; Úbeda, C.; Covarrubias, J.I.; Peña-Neira, Á. Chemical, physical, and sensory attributes of Sauvignon blanc wine fermented in different kinds of vessels. Innov. Food Sci. Emerg. Technol. 2020, 66, 102521. [Google Scholar] [CrossRef]
- Yue, F.; Zhang, J.; Xu, J.; Niu, T.; Lü, X.; Liu, M. Effects of monosaccharide composition on quantitative analysis of total sugar content by phenol-sulfuric acid method. Front. Nutr. 2022, 9, 963318. [Google Scholar] [CrossRef]
- Huang, M.D.; Becker-Ross, H.; Florek, S.; Heitmann, U.; Okruss, M.; Patz, C. Determination of sulfur forms in wine including free and total sulfur dioxide based on molecular absorption of carbon monosulfide in the air–acetylene flame. Anal. Bioanal. Chem. 2008, 390, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Míguez, M.J.; González-Miret, M.L.; Hernanz, D.; Fernández, M.Á.; Vicario, I.M.; Heredia, F.J. Effects of prefermentative skin contact conditions on colour and phenolic content of white wines. J. Food Eng. 2007, 78, 238–245. [Google Scholar] [CrossRef]
- Cliff, M.A.; King, M.C.; Schlosser, J. Anthocyanin, phenolic composition, colour measurement and sensory analysis of BC commercial red wines. Food Res. Int. 2007, 40, 92–100. [Google Scholar] [CrossRef]
- Figueiredo-González, M.; Cancho-Grande, B.; Simal-Gándara, J. Garnacha Tintorera-based sweet wines: Chromatic properties and global phenolic composition by means of UV–Vis spectrophotometry. Food Chem. 2013, 140, 217–224. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Z.; Zhao, Y.; Li, W.; Wang, L.; Shang, Q.; Du, J.; Jin, L. Effect of Protease Combined with Heat Treatment on the Volatile Composition and Aroma Quality in Liqueur Wine. Molecules 2023, 28, 5129. [Google Scholar] [CrossRef]
- Tang, Y.; Yu, M.; Liu, C.; Gao, X.; Song, H. Sensory-directed characterization of key odor-active compounds in fermented milk. J. Food Compos. Anal. 2024, 126, 105904. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, Z.; Weng, P. Comparison of bayberry fermented wine aroma from different cultivars by GC-MS combined with electronic nose analysis. Food Sci. Nutr. 2020, 8, 830–840. [Google Scholar] [CrossRef]
- Liu, R.; Liu, Y.; Zhu, Y.; Kortesniemi, M.; Zhu, B.; Li, H. Aromatic Characteristics of Passion Fruit Wines Measured by E-Nose, GC-Quadrupole MS, GC-Orbitrap-MS and Sensory Evaluation. Foods 2022, 11, 3789. [Google Scholar] [CrossRef] [PubMed]
- Rolle, L.; Giacosa, S.; Gerbi, V.; Bertolino, M.; Novello, V. Varietal Comparison of The Chemical, Physical, and Mechanical Properties of Five Colored Table Grapes. Int. J. Food Prop. 2013, 16, 598–612. [Google Scholar] [CrossRef]
- Niimi, J.; Danner, L.; Li, L.; Bossan, H.; Bastian, S.E.P. Wine consumers’ subjective responses to wine mouthfeel and understanding of wine body. Food Res. Int. 2017, 99, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Munir, M.T.; Yu, W.; Young, B. Wavelength Selection FOR Rapid Identification of Different Particle Size Fractions of Milk Powder Using Hyperspectral Imaging. Sensors 2020, 20, 4645. [Google Scholar] [CrossRef]
- Xiao, Z.; He, J.; Niu, Y.; Xiong, J.; Zhang, J. Characterization and comparison of aroma profiles of orange pulp and peel by GC–MS/O, OAV, aroma recombination and omission tests. Eur. Food Res. Technol. 2023, 249, 619–630. [Google Scholar] [CrossRef]
- Tosi, E.; Fedrizzi, B.; Azzolini, M.; Finato, F.; Simonato, B.; Zapparoli, G. Effects of noble rot on must composition and aroma profile of Amarone wine produced by the traditional grape withering protocol. Food Chem. 2012, 130, 370–375. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Varela, C.; Barker, A.; Tran, T.; Borneman, A.; Curtin, C. Sensory profile and volatile aroma composition of reduced alcohol Merlot wines fermented with Metschnikowia pulcherrima and Saccharomyces uvarum. Int. J. Food Microbiol. 2017, 252, 1–9. [Google Scholar] [CrossRef]
- Boban, A.; Vrhovsek, U.; Masuero, D.; Milanović, V.; Budić-Leto, I. Effect of Indigenous Non-Saccharomyces Yeasts on Lipid Compositions of Maraština Wine. Foods 2025, 14, 269. [Google Scholar] [CrossRef]
- Cordente, A.G.; Espinase Nandorfy, D.; Solomon, M.; Schulkin, A.; Kolouchova, R.; Francis, I.L.; Schmidt, S.A. Aromatic Higher Alcohols in Wine: Implication on Aroma and Palate Attributes during Chardonnay Aging. Molecules 2021, 26, 4979. [Google Scholar] [CrossRef]
- Zheng, N.; Jiang, S.; He, Y.; Chen, Y.; Zhang, C.; Guo, X.; Ma, L.; Xiao, D. Production of low-alcohol Huangjiu with improved acidity and reduced levels of higher alcohols by fermentation with scarless ALD6 overexpression yeast. Food Chem. 2020, 321, 126691. [Google Scholar] [CrossRef]
- Kishimoto, K.; Matsui, K.; Ozawa, R.; Takabayashi, J. Direct fungicidal activities of C6-aldehydes are important constituents for defense responses in Arabidopsis against Botrytis cinerea. Phytochemistry 2008, 69, 2127–2132. [Google Scholar] [CrossRef]
- Chehab, E.W.; Kaspi, R.; Savchenko, T.; Rowe, H.; Negre-Zakharov, F.; Kliebenstein, D.; Dehesh, K. Distinct roles of jasmonates and aldehydes in plant-defense responses. PLoS ONE 2008, 3, e1904. [Google Scholar] [CrossRef]
- Genovese, A.; Gambuti, A.; Piombino, P.; Moio, L. Sensory properties and aroma compounds of sweet Fiano wine. Food Chem. 2007, 103, 1228–1236. [Google Scholar] [CrossRef]
- Šernaitė, L.; Rasiukevičiūtė, N.; Valiuškaitė, A. Application of Plant Extracts to Control Postharvest Gray Mold and Susceptibility of Apple Fruits to B. cinerea from Different Plant Hosts. Foods 2020, 9, 1430. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; VanderWeide, J.; Yan, Y.; Tindjau, R.; Pico, J.; Deluc, L.; Zandberg, W.F.; Castellarin, S.D. Impact of hormone applications on ripening-related metabolites in Gewürztraminer grapes (Vitis vinifera L.): The key role of jasmonates in terpene modulation. Food Chem. 2022, 388, 132948. [Google Scholar] [CrossRef] [PubMed]
- Mele, M.A.; Kang, H.; Lee, Y.; Islam, M.Z. Grape terpenoids: Flavor importance, genetic regulation, and future potential. Crit. Rev. Food. Sci. Nutr. 2021, 61, 1429–1447. [Google Scholar] [CrossRef]
- Siebert, T.E.; Barker, A.; Pearson, W.; Barter, S.R.; de Barros Lopes, M.A.; Darriet, P.; Herderich, M.J.; Francis, I.L. Volatile Compounds Related to ‘Stone Fruit’ Aroma Attributes in Viognier and Chardonnay Wines. J. Agric. Food Chem. 2018, 66, 2838–2850. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, L.; Du, H.; Lu, K.; Chen, C.; Xue, Q.; Hu, Y. Microbial community succession and their relationship with the flavor formation during the natural fermentation of Mouding sufu. Food Chem. X 2023, 18, 100686. [Google Scholar] [CrossRef]
- Wang, X.; Tao, Y.; Wu, Y.; An, R.; Yue, Z. Aroma compounds and characteristics of noble-rot wines of Chardonnay grapes artificially botrytized in the vineyard. Food Chem. 2017, 226, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Cai, Y.; Wu, X.; Gai, S.; Wang, B.; Liu, D. Characterization of selected commercially available grilled lamb shashliks based on flavor profiles using GC-MS, GC × GC-TOF-MS, GC-IMS, E-nose and E-tongue combined with chemometrics. Food Chem. 2023, 423, 136257. [Google Scholar] [CrossRef] [PubMed]


| Grape Samples | pH | Total Acid (g/L) | Total Sugars (g/L) | Glycerol (g/L) |
|---|---|---|---|---|
| A group | 3.37 ± 0.1 a | 7.48 ± 0.06 a | 300.42 ± 1.61 c | 0.44 ± 0.01 c |
| B group | 3.36 ± 0.02 a | 7.45 ± 0.03 a | 305.06 ± 1.31 b | 1.04 ± 0.03 b |
| C group | 3.36 ± 0.05 a | 7.37 ± 0.05 a | 309.87 ± 1.46 a | 5.97 ± 0.02 a |
| Grape Samples | Tartaric Acid (g/L) | Malic Acid (g/L) | Citric Acid (g/L) |
|---|---|---|---|
| A group | 11.54 ± 0.61 a | 2.52 ± 0.13 b | 0.55 ± 0.05 b |
| B group | 11.11 ± 0.69 a | 2.73 ± 0.1 b | 0.66 ± 0.04 a |
| C group | 10.65 ± 0.64 a | 4.21 ± 0.07 a | 0.66 ± 0.03 a |
| Physical and Chemical Indicators | Wine Samples | ||
|---|---|---|---|
| A Group | B Group | C Group | |
| Alcohol content/% | 12.03 ± 0.02 c | 12.12 ± 0.01 b | 12.27 ± 0.05 a |
| pH | 3.42 ± 0.04 c | 3.55 ± 0.03 b | 3.71 ± 0.03 a |
| Total acid/(g/L) | 7.29 ± 0.06 b | 7.34 ± 0.07 b | 7.61 ± 0.04 a |
| Volatile acids/(g/L) | 0.77 ± 0.02 b | 0.69 ± 0.04 c | 0.86 ± 0.01 a |
| Free sulfur/(mg/L) | 48 ± 1.00 c | 51 ± 1.18 b | 53 ± 0.11 a |
| Total sulfur/(mg/L) | 152 ± 1.00 c | 157 ± 0.37 b | 162.33 ± 0.58 a |
| glycerol/(g/L) | 8.28 ± 0.04 c | 9.89 ± 0.02 b | 15.69 ± 0.07 a |
| Color-Related Parameters | Wine Samples | ||
|---|---|---|---|
| A Group | B Group | C Group | |
| L* | 95.47 ± 0.46 a | 95.44 ± 0.46 a | 91.95 ± 0.33 b |
| A* | −8.28 ± 0.03 a | −7.50 ± 0.14 b | −7.19 ± 0.01 c |
| B* | 42.96 ± 1.46 b | 45.03 ± 1.39 b | 52.80 ± 0.27 a |
| Chromaticity | 0.58 ± 0.02 c | 0.62 ± 0.01 b | 0.86 ± 0.01 a |
| hue | 3.34 ± 0.04 a | 2.87 ± 0.17 b | 2.89 ± 0.09 b |
| Total phenols (mg/L) | 321.67 ± 1.15 c | 332.67 ± 2.52 b | 413.67 ± 0.58 a |
| Tartrate (mg/L) | 15.64 ± 0.31 c | 16.71 ± 0.17 b | 18.68 ± 0.56 a |
| Flavanols (mg/L) | 36.82 ± 0.27 c | 35.51 ± 0.32 b | 38.24 ± 0.85 a |
| Serial Number | Aromatic Substances | Threshold (μg/L) | Mass Concentration (μg/L) | Odor Description | ||
|---|---|---|---|---|---|---|
| A Group | B Group | C Group | ||||
| Esters | ||||||
| A1 | Ethyl acetate | 7500 | 9934 ± 1683 | 12,857 ± 901 | 11,890 ± 602 | Fruity, sweet, nail polish |
| A2 | Isobutyl acetate | \ | 4301 ± 5.5 | nd | nd | Aroma of ripe fruits |
| A3 | Ethyl butyrate | 20 | 1053 ± 17 | 970 ± 17 | 341 ± 1.66 | Strawberries, apples, bananas |
| A4 | Isoamyl acetate | 30 | 46,334 ± 970 | 2260 ± 273 | 12,537 ± 155 | Intense banana flavor, fruity, sweet |
| A5 | Ethyl valerate | \ | 81 ± 1.43 | 104 ± 4.9 | nd | \ |
| A6 | Hexyl acetate | 5 | 14,505 ± 653 | 4583 ± 312 | 3208 ± 28 | Fruity, pear, cherry |
| A7 | Ethyl hexanoate | 80 | 6188 ± 150 | 8343 ± 154 | 16,547 ± 227 | \ |
| A8 | Ethyl caprate | 100 | 71,988 ± 1647 | 90,942 ± 953 | 35,825 ± 1234 | Coconut fruity aroma |
| A9 | Ethyl 3-hexenoate | \ | 26 ± 0.67 | 11 ± 0.82 | 7.0 ± 0.04 | \ |
| A10 | Ethyl heptanoate | 300 | 191 ± 1.46 | 253 ± 4.1 | 217 ± 1.01 | Fresh and fruity |
| A11 | Ethyl 9-hexadecenoate | \ | 11 ± 0.55 | 57 ± 0.65 | 261 ± 0.87 | \ |
| A12 | Heptyl acetate | 670 | 117 ± 0.75 | nd | 330 ± 1.69 | Cherry and pear flavors |
| A13 | Octyl acetate | \ | 137 ± 9.4 | nd | 120 ± 1.11 | \ |
| A14 | Methyl caprylate | 200 | 200 ± 2.6 | 245 ± 3.2 | 460 ± 0.86 | Intense citrus flavor |
| A15 | Ethyl caprylate | 580 | 145,402 ± 11,456 | 90,798 ± 564 | 38,020 ± 668 | Pineapple, pear, floral, fruity, brandy |
| A16 | Propyl caprylate | \ | 82 ± 1.99 | 47 ± 1.13 | 70 ± 0.91 | \ |
| A17 | Isoamyl caproate | \ | 180.31 ± 4.29 | 108 ± 0.80 | 330 ± 1.78 | Apples, pineapples |
| A18 | N-butyl caprylate | \ | 142 ± 2.7 | nd | nd | |
| A19 | Phenylethyl acetate | 250 | 7758 ± 64 | 8629 ± 93 | 7422 ± 25. | Floral, fruity, woody |
| A20 | Ethyl laurate | 83 | 16,846 ± 1008 | 5153 ± 100 | 22,846 ± 432 | Sweet, floral, fruity, creamy |
| A21 | Caprylate 3-methylbutyl ester | 125 | 848 ± 12 | 466 ± 5.5 | 938 ± 7.1 | Pear, wax, soap |
| A22 | Diethyl succinate | 200,000 | 191 ± 5.7 | nd | 367 ± 0.95 | Grape, melon and fruity aromas |
| A23 | N-propyl caprate | \ | 82 ± 2.2 | 31 ± 1.12 | 146 ± 1.25 | \ |
| A24 | Ethyl undecanoate | \ | 46 ± 2.15 | 41 ± 1.46 | 97 ± 0.65 | Coconut, nutty aroma |
| A25 | 3-methylbutyl caprate | \ | 465 ± 11 | 299 ± 4.9 | 1359 ± 13 | Fruity |
| A26 | Ethyl myristate | 2000 | 560 ± 2.4 | 265 ± 3.0 | 910 ± 4.9 | Coconut, beeswax scent |
| A27 | α, β-glucocaprylic acid γ-lactone | \ | 27 ± 3.2 | nd | nd | \ |
| A28 | Butyl caproate | \ | nd | 32 ± 1.26 | 38 ± 0.23 | \ |
| A29 | Methyl caprate | \ | nd | 136 ± 1.50 | 219 ± 1.02 | Fruity aromas |
| A30 | Isobutyl caprate | \ | nd | 72 ± 0.57 | 144 ± 1.62 | \ |
| A31 | Methyl 10-methylundecylate | \ | nd | 20 ± 0.60 | 47 ± 0.33 | \ |
| A32 alcohols | Ethyl palmitate | 1500 | nd | 458 ± 4.5 | 1596 ± 8.4 | Floral, Banana, Pear scents |
| B1 | Isobutanol | 40,000 | 927 ± 2.7 | nd | nd | Solvent flavor, Raw green flavor, mushroom aroma |
| B2 | N-butanol | 15,000 | nd | 25 ± 1.45 | nd | Medicinal smell, resin smell |
| B3 | 3-Methyl-1-butanol | \ | 20,439 ± 762 | 14,834 ± 109 | 10,176 ± 107 | \ |
| B4 | 1-pentanol | 64,000 | nd | 20 ± 0.80 | nd | Mellow and astringent |
| B5 | N-hexanol | 8000 | 25 ± 1.42 | 2174 ± 60 | 1719 ± 26 | Floral, fruity, grassy |
| B6 | 2-Hexanol | \ | 55 ± 3.3 | 67 ± 0.82 | nd | Fruity, milky |
| B7 | 3-Methyl-1-pentanol | \ | nd | 58 ± 0.95 | 60 ± 1.06 | Fruity flavor |
| B8 | 2,6,8-Trimethyl-4-nonanol | \ | nd | 107 ± 1.36 | 204 ± 1.29 | Flowers |
| B9 | (2R,3R)-(-)-2,3-butanediol | \ | nd | 307 ± 2.3 | 1207 ± 8.5 | \ |
| B10 | Benzyl alcohol | \ | nd | 71 ± 1.25 | nd | Jasmine |
| B11 | Heptylene ethylene glycol | \ | nd | 2.5 ± 0.04 | 4.01 ± 0.13 | \ |
| B12 | Phenylethanol | 7230 | 14,590 ± 1032 | 16,287 ± 336 | 15,567 ± 78 | Rose aroma, peach flavor |
| B13 | 2,3-Butanediol | 12,000 | nd | nd | 457 ± 5.6 | Creamy, fruity aroma |
| B14 | Heptanol | 1000 | nd | nd | 342 ± 2.9 | Raw green and sweet aroma |
| B15 | 3-Methylthiopropanol | 300 | nd | nd | 156 ± 1.04 | Fatty, cooked vegetables |
| B16 | 2-Nonanol | \ | nd | 51 ± 1.11 | 146 ± 0.89 | Strong fruity scent |
| B17Acid | 2-Heptanol | 200 | nd | nd | 17 ± 0.64 | Fresh and citrus aroma |
| C1 | Acetic acid | 300,000 | 4254 ± 132 | 2670 ± 21 | 1715 ± 19 | Acetic sourness |
| C2 | Hexanoic acid | 420 | 1166 ± 20 | 2468 ± 71 | 1846 ± 21 | Cheese, leaves, wood |
| C3 | 2-Methylhexanoic acid | \ | nd | 143 ± 1.03 | 241 ± 1.94 | \ |
| C4 | Capric acid | 1000 | 3077 ± 88 | 1339 ± 19.384 | 3740 ± 50 | Caramel, milk, fat |
| C5 Aldehydes and ketones | Octanoic acid | 500 | 2841 ± 27 | 7062 ± 25 | 8537 ± 81 | Buttery, almond |
| D1 | Furfural | 14,100 | nd | 287 ± 1.45 | 329 ± 1.33 | Roasted almonds |
| D2 | Trans-2-hexenal | \ | 16.78 ± 1.18 | 16 ± 0.61 | nd | \ |
| D3 | Benzaldehyde | 2000 | 125 ± 0.67 | 323 ± 3. 5 | 532 ± 3. 5 | Aromatic vegetative, bitter almond |
| D4 | Decanal | 10 | 252 ± 2. 6 | 311 ± 2.1 | 334 ± 1.75 | Orange peel |
| D5 | Methylheptenone | \ | nd | 47 ± 0.40 | 50 ± 0.79 | Citrus flavor |
| D6 | 2,6,8-Trimethyl-4-nonanone | \ | 633 ± 169 | 573 ± 1.95 | 919 ± 0.98 | \ |
| D7 | 3-Hydroxy-2-butanone | 800 | nd | 150 ± 1.15 | 136 ± 0.85 | Creamy, fatty taste |
| D8 Terpenes | 2-Nonanone | \ | nd | 90.67 ± 1.27 | 160.08 ± 1.54 | Fruity, floral, fatty |
| E1 | Damascenone | 50 | 270 ± 4.4 | 182 ± 1.74 | 197 ± 1.19 | Floral, fruity, lilac |
| E2 | Citronellol | 40 | nd | 170 ± 0.84 | 129 ± 1.46 | Grass, lilac flowers, roses |
| E3 | Linalool | 1 | 11 ± 0.72 | 9.5 ± 0.12 | 12 ± 0.38 | Fruity, citrus, rose |
| E4 Miscellaneous | Styrene | 100 | 412 ± 3.6 | 77 ± 0.22 | 116 ± 1.69 | Flowers |
| F1 | Trimethylbenzene | \ | 929 ± 2.8 | nd | nd | \ |
| F2 | Naphthalene | \ | nd | 38 ± 0.74 | 59 ± 0.51 | It has the smell of camphor wood |
| F3 | Dodecane | \ | nd | 39 ± 0.15 | nd | \ |
| F4 | 2,4-Di-tert-butylphenol | 200 | 127 ± 1.25 | 164 ± 1.17 | 145 ± 1.55 | Acrid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Yang, F.; Ji, Q.; Huo, L.; Qiao, C.; Pan, L. Characterization of the Flavors and Organoleptic Attributes of Petit Manseng Noble Rot Wines from the Eastern Foothills of Helan Mountain in Ningxia, China. Foods 2025, 14, 2723. https://doi.org/10.3390/foods14152723
Li F, Yang F, Ji Q, Huo L, Qiao C, Pan L. Characterization of the Flavors and Organoleptic Attributes of Petit Manseng Noble Rot Wines from the Eastern Foothills of Helan Mountain in Ningxia, China. Foods. 2025; 14(15):2723. https://doi.org/10.3390/foods14152723
Chicago/Turabian StyleLi, Fuqi, Fan Yang, Quan Ji, Longxuan Huo, Chen Qiao, and Lin Pan. 2025. "Characterization of the Flavors and Organoleptic Attributes of Petit Manseng Noble Rot Wines from the Eastern Foothills of Helan Mountain in Ningxia, China" Foods 14, no. 15: 2723. https://doi.org/10.3390/foods14152723
APA StyleLi, F., Yang, F., Ji, Q., Huo, L., Qiao, C., & Pan, L. (2025). Characterization of the Flavors and Organoleptic Attributes of Petit Manseng Noble Rot Wines from the Eastern Foothills of Helan Mountain in Ningxia, China. Foods, 14(15), 2723. https://doi.org/10.3390/foods14152723






