Maillard Reaction in Flour Product Processing: Mechanism, Impact on Quality, and Mitigation Strategies of Harmful Products
Abstract
1. Introduction
2. Substrate Sources of the Maillard Reaction in Flour Product Processing
2.1. Carbonyl Sources
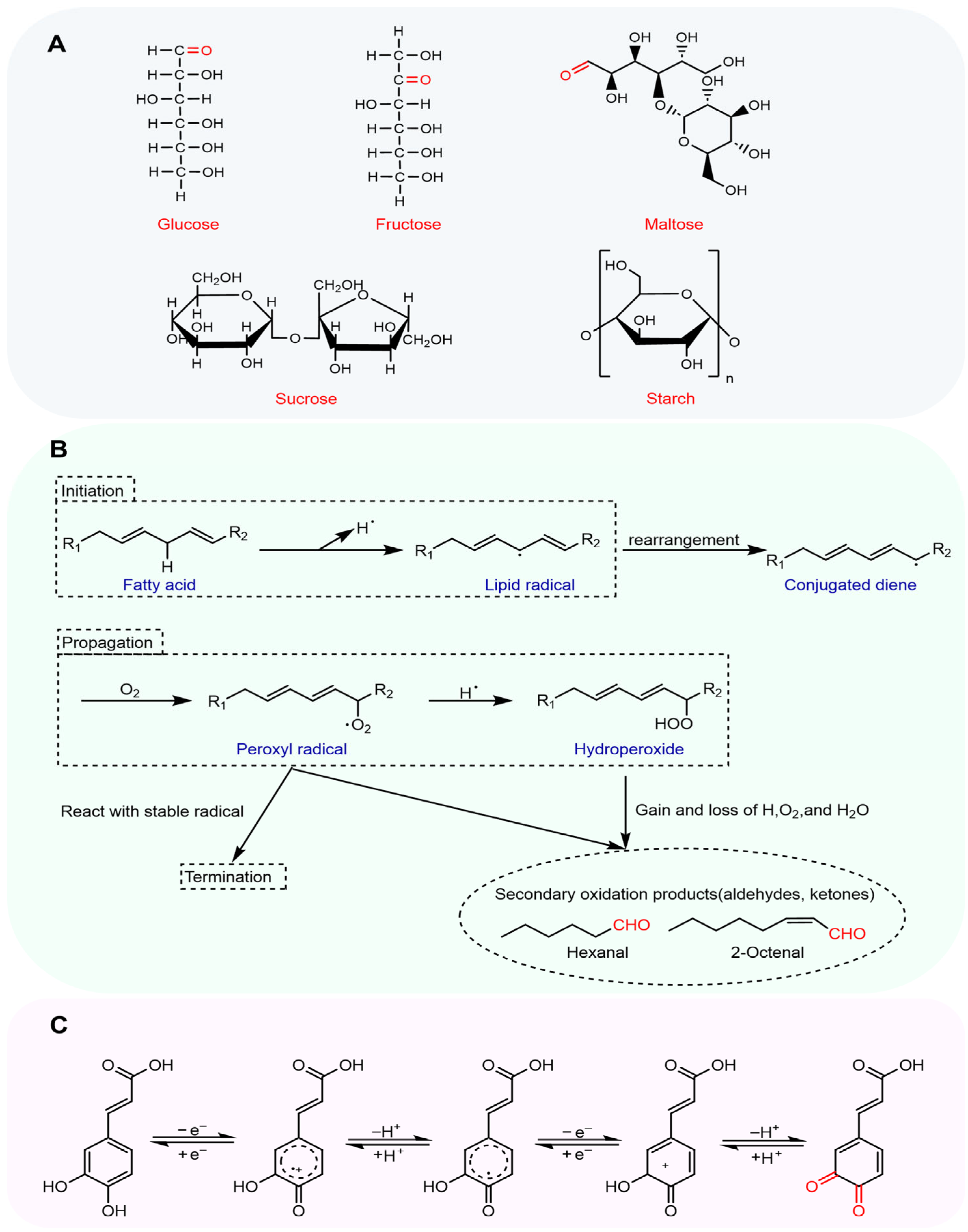
2.1.1. Reducing Sugars
Natural Reducing Sugars in Raw Materials
Reducing Sugars from the Milling Process
Reducing Sugars Derived from Polysaccharide Degradation During Dough Fermentation
2.1.2. Lipid Degradation Products
2.1.3. O-Quinones
2.1.4. The Carbonyl Group Provided by Exogenous Excipients
2.2. Amino Sources
2.2.1. Amino Acids
Natural Free Amino Acids in Raw Materials
Amino Acids from Protein Hydrolysis
2.2.2. Protein Amino Terminus
2.2.3. The Amino Groups Provided by Exogenous Excipients
3. Maillard Reaction Process and Influencing Factors in Flour Product Processing
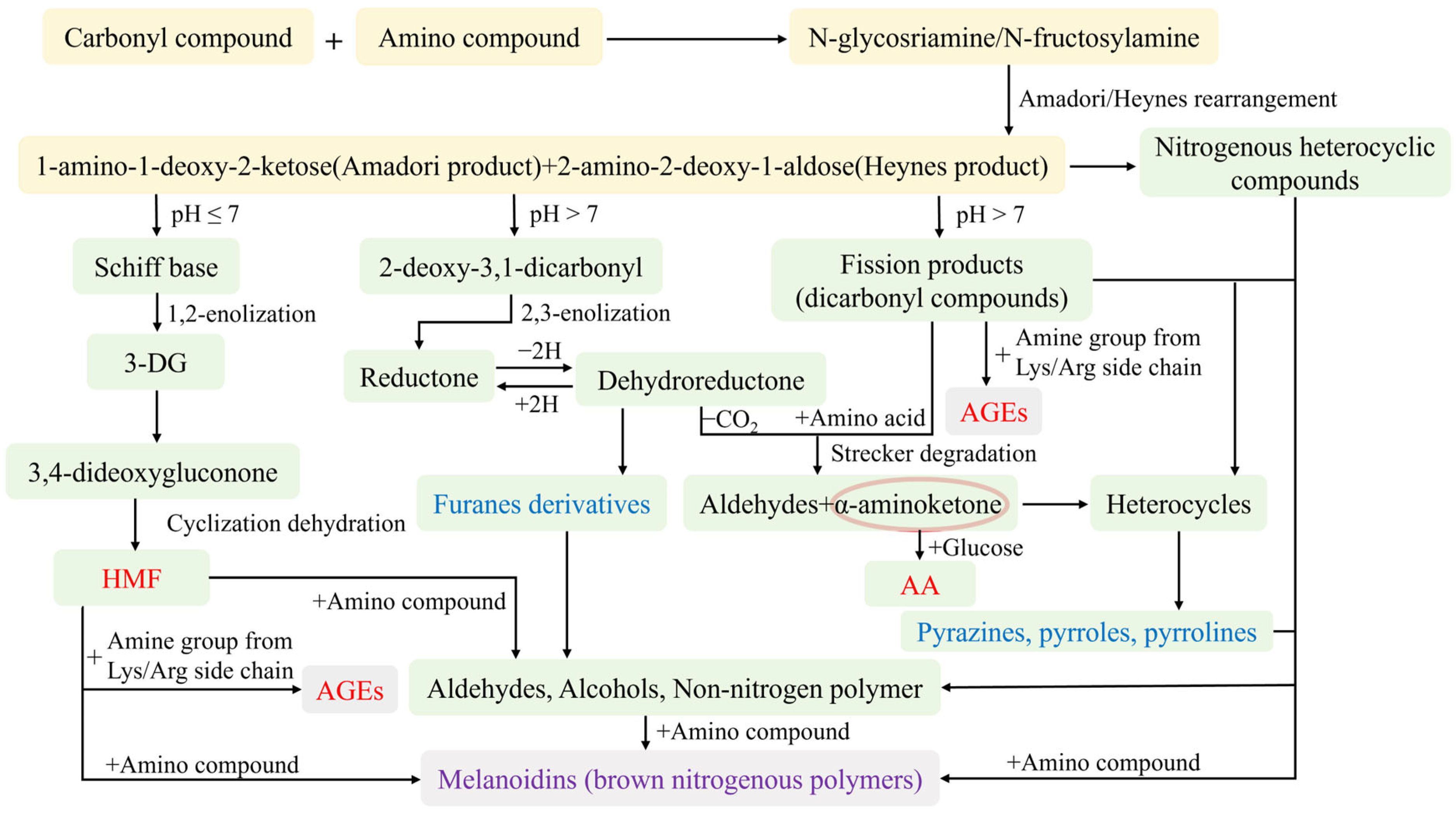
3.1. Reaction Process
3.1.1. Initial Stage
3.1.2. Intermediate Stage
3.1.3. Final Stage
3.2. Influencing Factors
3.2.1. Reaction Substrates
- (1)
- Type and content of carbonyl compounds
- (2)
- Type and content of amino compounds
3.2.2. Heat Input
3.2.3. Moisture
3.2.4. pH
4. Outcomes of the Maillard Reaction in Flour Food Processing
4.1. Color and Browning
4.2. Flavor Substances
| Category | Breads | Cookies | Fried Bread Sticks | Steamed Breads |
|---|---|---|---|---|
| Alcohols | Ethanol Nonanol 1-Propanol 1-Butanol 1-Octen-3-ol 2,3-Butanediol Phenethyl alcohol | n-Octanol 2,3-Butanediol 2-Nonyl alcohol Phenethyl alcohol | 1-Octen-3-ol Heptanol Octanol | 1-Heptanol 1-Hexanol 1-Pentanol Phenethyl alcohol 1-Octen-3-ol 2-Phenylethanol |
| Esters | Ethyl formate Ethyl acetate Ethyl valerate γ-Nonalactone | Ethyl nonanoate Ethyl caprylate Ethyl caprate | γ-Nonalactone | Ethyl Lactate Ethyl acetate Hexenyl butyrate γ-Nonalactone |
| Aldehydes | Hexanal Nonanal Heptanal Benzaldehyde (E)-2-Nonenal (E)-2-Heptenal (E)-2-Octenal 2-Methylbutanal 3-Methylbutanal | 2-Methylpropanal 2-Methylbutanal Hexanal Nonanal Benzaldehyde | Hexanal Heptanal Nonanal Decanal (E)-2-Hexenal (E)-2-Octenal (E)-2-Nonenal | Nonanal Hexanal Octanal Benzaldehyde (E,E)-2,4-Decadienal (E)-2-Nonenal |
| Ketones | 2-Pentanone 2-Heptanone 1-Octen-3-one 2,3-Pentanedione 3-Hydroxy-2-butanone 1-Hydroxy-2-propanone 2,3-Butanedione 3-Hydroxy-2-butanone | Methyl-heptenon 2-Heptanone | 3-Hydroxy-2-butanone | Octanone Acetophenone Geranylgeranylacetone 2,3-Pentanedione |
| Oxygen-containing heterocyclic compounds | 5-Methylfuranal 2-Furylcarbinol 2-Pentylfuran 2-Methyl furan Furfural | Furfural | 2-Pentylfuran 2,5-Dimethyl-4-hydroxy-3(2H)-furanone Furfural | Dihydro-5-pentyl-2(3H)-furanone 2-Pentylfuran |
| Nitrogen-containing heterocyclic compounds | 2-Methylpyrazine 2-Acetyl-1-pyrroline 2,3-Dimethylpyrazine 2,5-Dimethylpyrazine 2,6-Dimethylpyrazine 2,3,5-Trimethylpyrazine | 2-Methylpyrazine 2,3-Dimethylpyrazine 2,5-Dimethylpyrazine 2,6-Dimethylpyrazine 2-Acetylpyridine | 2-Ethyl-3,5-dimethylpyrazine 3-Ethyl-2,5-dimethylpyrazine 2,3-Dimethylpyrazine | |
| Sulfur-containing heterocyclic compounds | 1,3-Thiazole 2-Acetyl-2-thiazoline | 2-Acetylthiazole |
4.3. Nutritional Changes
4.4. Harmful Products
4.4.1. Acrylamide
4.4.2. 5-Hydroxymethylfurfural
4.4.3. Advanced Glycation End Products
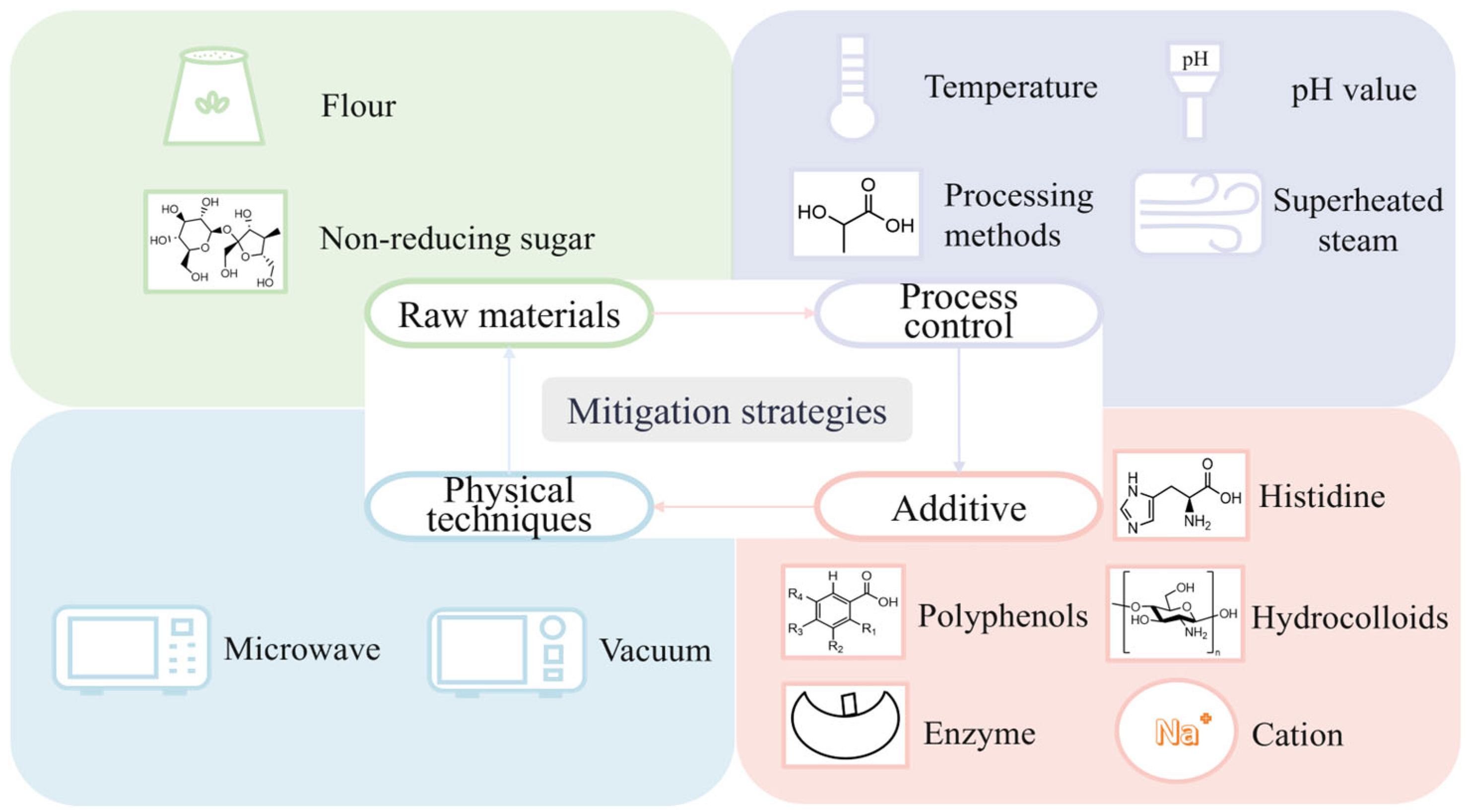
5. Mitigation Strategies for Maillard Reaction Harmful Products in Flour Product Processing
5.1. Raw Material Selection and Processing
5.1.1. Selection of Suitable Flour
5.1.2. Optimization of Reducing Sugar
5.2. Processing Control
5.2.1. Optimization of Processing Parameters
5.2.2. Improvement of Processing Methods
5.3. Additive Applications
5.3.1. Polyphenols
5.3.2. Hydrocolloids
5.3.3. Enzymes
5.3.4. Cations
5.3.5. Amino Acids
5.4. Physical Processing Techniques
5.4.1. Microwave Processing
5.4.2. Vacuum Treatment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Acrylamide |
| HMF | 5-Hydroxymethylfurfural |
| AGEs | Advanced glycation end products |
| SMF | Sulfoxymethylfurfural |
| CML | Nε-carboxymethyl-lysine |
| CEL | Nε-carboxyethyl-lysine |
| PPOs | Polyphenol oxidases |
| ARPs | Amadori rearrangement products |
| HRPs | Heyns rearrangement products |
| MGO | Methylglyoxal |
| 3-DG | 3-Deoxyglucosone |
| GO | Glyoxal |
References
- Liu, R.; Yang, Y.; Cui, X.; Mwabulili, F.; Xie, Y. Effects of Baking and Frying on the Protein Oxidation of Wheat Dough. Foods 2023, 12, 4479. [Google Scholar] [CrossRef]
- Chang, X.; Huang, X.; Tian, X.; Wang, C.; Aheto, J.H.; Ernest, B.; Yi, R. Dynamic characteristics of dough during the fermentation process of Chinese steamed bread. Food Chem. 2020, 312, 126050. [Google Scholar] [CrossRef]
- Tian, X.; Fang, Q.; Zhang, X.; Yu, S.; Dai, C.; Huang, X. Visualization of Moisture Content, Reducing Sugars, and Chewiness in Bread During Oral Processing Based on Hyperspectral Imaging Technology. Foods 2024, 13, 3589. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huang, Q.; Xia, Q.; Zha, B.; Sun, J.; Xu, B.; Shi, Y.-C. Intact endosperm cells in buckwheat flour limit starch gelatinization and digestibility in vitro. Food Chem. 2020, 330, 127318. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zeng, X.A.; Brennan, C.S.; Ma, H.; Aadil, R.M. Preparation and characterisation of novelty food preservatives by Maillard reaction between ε-polylysine and reducing sugars. Int. J. Food Sci. Technol. 2019, 54, 1824–1835. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, S.; Pei, J.; Jiang, P.; Jin, W.; Gao, R.; Rebollo-Hernanz, M. Antioxidative Activity and Volatile Profiles of Maillard Reaction Products between Giant Salamander (Andrias davidianus) Peptides and Glucose during the Heating Process. J. Food Qual. 2023, 2023, 8804009. [Google Scholar] [CrossRef]
- Chen, X.; Zou, Y.; Wang, D.; Xiong, G.; Xu, W. Effects of ultrasound pretreatment on the extent of Maillard reaction and the structure, taste and volatile compounds of chicken liver protein. Food Chem. 2020, 331, 127369. [Google Scholar] [CrossRef]
- Kathuria, D.; Hamid; Gautam, S.; Thakur, A. Maillard reaction in different food products: Effect on product quality, human health and mitigation strategies. Food Control 2023, 153, 109911. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Z.; Li, J.; Niu, Y.; Yu, L.L. Inhibition Mechanism of L-Cysteine on Maillard Reaction by Trapping 5-Hydroxymethylfurfural. Foods 2021, 10, 1391. [Google Scholar] [CrossRef] [PubMed]
- Murata, M. Browning and pigmentation in food through the Maillard reaction. Glycoconj. J. 2020, 38, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, T.; He, Y.; Chen, M.; Zhou, J.; Yin, L.; Ma, H.; Sun, Q. Preliminary Study on Ultrasonic Ageing Zhenjiang Vinegar Mechanism Based on Maillard Simulation System. J. Food Qual. 2020, 2020, 1087863. [Google Scholar] [CrossRef]
- Gan, Z.; Zhang, W.; Arslan, M.; Hu, X.; Zhang, X.; Li, Z.; Shi, J.; Zou, X. Ratiometric Fluorescent Metal–Organic Framework Biosensor for Ultrasensitive Detection of Acrylamide. J. Agric. Food Chem. 2022, 70, 10065–10074. [Google Scholar] [CrossRef]
- Jung, M.Y.; Baek, C.H.; Ma, Y.; Lee, H.W. Acrylamide formation in air-fryer roasted legumes as affected by legume species and roasting degree: The correlation of acrylamide with asparagine and free sugars. Food Sci. Biotechnol. 2024, 33, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wang, Y.; Li, Y.; Hou, J.; Liang, Y.; Zhang, Z. Investigation of the formation of furfural compounds in apple products treated with pasteurization and high pressure processing. Food Res. Int. 2024, 190, 114546. [Google Scholar] [CrossRef]
- Wei, Q.; Liu, T.; Sun, D.-W. Advanced glycation end-products (AGEs) in foods and their detecting techniques and methods: A review. Trends Food Sci. Technol. 2018, 82, 32–45. [Google Scholar] [CrossRef]
- Li, M.; Shen, M.; Lu, J.; Yang, J.; Huang, Y.; Liu, L.; Fan, H.; Xie, J.; Xie, M. Maillard reaction harmful products in dairy products: Formation, occurrence, analysis, and mitigation strategies. Food Res. Int. 2022, 151, 110839. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, F.; Xia, X.; Liu, Q. Excessive oil absorption and maillard reaction products in fried muscle foods: Formation mechanisms, potential health risks and mitigation strategies. Food Chem. 2025, 468, 142456. [Google Scholar] [CrossRef]
- Yıltırak, S.; Kocadağlı, T.; Çelik, E.E.; Özkaynak Kanmaz, E.; Gökmen, V. Effects of Sprouting and Fermentation on Free Asparagine and Reducing Sugars in Wheat, Einkorn, Oat, Rye, Barley, and Buckwheat and on Acrylamide and 5-Hydroxymethylfurfural Formation During Heating. J. Agric. Food Chem. 2021, 69, 9419–9433. [Google Scholar] [CrossRef]
- Žilić, S.; Aktağ, I.G.; Dodig, D.; Gökmen, V. Investigations on the formation of Maillard reaction products in sweet cookies made of different cereals. Food Res. Int. 2021, 144, 110352. [Google Scholar] [CrossRef]
- Li, Q.; Liu, S.; Obadi, M.; Jiang, Y.; Zhao, F.; Jiang, S.; Xu, B. The impact of starch degradation induced by pre-gelatinization treatment on the quality of noodles. Food Chem. 2020, 302, 125267. [Google Scholar] [CrossRef]
- Jost, T.; Henning, C.; Heymann, T.; Glomb, M.A. Comprehensive Analyses of Carbohydrates, 1,2-Dicarbonyl Compounds, and Advanced Glycation End Products in Industrial Bread Making. J. Agric. Food Chem. 2021, 69, 3720–3731. [Google Scholar] [CrossRef]
- Xiao, Z.; Hou, X.; Zhang, T.; Yuan, Y.; Xiao, J.; Song, W.; Yue, T. Starch-digesting product analysis based on the hydrophilic interaction liquid chromatography coupled mass spectrometry method to evaluate the inhibition of flavonoids on pancreatic α-amylase. Food Chem. 2022, 372, 131175. [Google Scholar] [CrossRef]
- Sun, J.; Sun, L.; Chen, X.; Raza, H.; Wu, G.; Liang, Q.; Ren, X.; Di Stefano, V. Characterization of Arrowhead-Derived Type 3 Resistant Starch Prepared by Ultrasound-Assisted α-Amylase Degradation. J. Food Qual. 2023, 2023, 2301485. [Google Scholar] [CrossRef]
- Wei, X.; Huang, W.; Han, Y.; Chen, L.; Wang, Y.; Yu, S.; Yang, F. Allosteric mechanism of synergistic effect in α- and β-amylase mixtures. Int. J. Biol. Macromol. 2024, 280, 135653. [Google Scholar] [CrossRef]
- Li, Z.; Dong, Y.; Xiao, X.; Zhou, X.H. Mechanism by which β-glucanase improves the quality of fermented barley flour-based food products. Food Chem. 2020, 311, 126026. [Google Scholar] [CrossRef]
- Timmermans, E.; Bautil, A.; Brijs, K.; Scheirlinck, I.; Van der Meulen, R.; Courtin, C.M. Sugar Levels Determine Fermentation Dynamics During Yeast Pastry Making and Its Impact on Dough and Product Characteristics. Foods 2022, 11, 1388. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, X.; Liu, L.; Zhao, Y.; Bai, F.; Wang, J.; Gao, R.; Xu, X. The influence mechanism of phospholipids structure and composition changes caused by oxidation on the formation of flavor substances in sturgeon caviar. Food Chem. 2024, 460, 140585. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, Y.; An, X.; Zhang, H.; Gong, W.; Liang, Y.; Wang, J. Changes in Lipid Metabolites and Enzyme Activities of Wheat Flour During Maturation. Foods 2024, 13, 2537. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Yu, Q.; Bao, Y.; Chen, L.; Luo, Y.; Tan, Y.; Hong, H. Myofibrillar protein lipoxidation in fish induced by linoleic acid and 4-hydroxy-2-nonenal: Insights from LC-MS/MS analysis. Food Res. Int. 2024, 187, 114357. [Google Scholar] [CrossRef] [PubMed]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Boateng, N.A.S.; Ma, H. Ultrasound-induced lipid peroxidation: Effects on phenol content and extraction kinetics and antioxidant activity of Tartary buckwheat (Fagopyrum tataricum) water extract. Food Biosci. 2020, 37, 100719. [Google Scholar] [CrossRef]
- Bassam, S.M.; Noleto-Dias, C.; Farag, M.A. Dissecting grilled red and white meat flavor: Its characteristics, production mechanisms, influencing factors and chemical hazards. Food Chem. 2022, 371, 131139. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, A.; Cao, H.; Xiao, J.; Simal-Gandara, J. Advantages of techniques to fortify food products with the benefits of fish oil. Food Res. Int. 2020, 137, 109353. [Google Scholar] [CrossRef]
- Feng, Y.; Cao, H.; Song, H.; Huang, K.; Zhang, Y.; Zhang, Y.; Li, S.; Li, Y.; Lu, J.; Guan, X. The formation mechanism, analysis strategies and regulation measures of cereal aroma: A review. Trends Food Sci. Technol. 2024, 147, 104452. [Google Scholar] [CrossRef]
- Yin, W.-t.; Yang, C.-j.; Yang, H.-j.; Hu, B.-b.; Zhang, F.; Wang, X.-d.; Liu, H.-m.; Miao, H.-m. Sesame lignans modulate aroma formation in sesame oil through the Maillard reaction and lipid oxidation in model systems. Food Chem. 2024, 457, 140079. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. Coordinate Contribution of Lipid Oxidation and Maillard Reaction to the Nonenzymatic Food Browning. Crit. Rev. Food Sci. Nutr. 2005, 45, 49–59. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Serwah Boateng, N.A.; Ma, H. Latest developments in polyphenol recovery and purification from plant by-products: A review. Trends Food Sci. Technol. 2020, 99, 375–388. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, Y.; Hassane Hamadou, A.; Shen, Q.; Xu, B. Tempering–preservation treatment inactivated lipase in wheat bran and retained phenolic compounds. Int. J. Food Sci. Technol. 2021, 57, 2104–2112. [Google Scholar] [CrossRef]
- Obadi, M.; Guo, Q.; Sun, J.; Xu, B. Recent developments in the application of physical processing techniques for controlling browning in fresh wet noodles: A review. J. Cereal Sci. 2024, 118, 103951. [Google Scholar] [CrossRef]
- Boateng, I.D.; Yang, X.M. Effect of different drying methods on product quality, bioactive and toxic components of Ginkgo biloba L. seed. J. Sci. Food Agric. 2021, 101, 3290–3297. [Google Scholar] [CrossRef]
- Su, J.; Geng, Y.; Yao, J.; Huang, Y.; Ji, J.; Chen, F.; Hu, X.; Ma, L. Quinone-mediated non-enzymatic browning in model systems during long-term storage. Food Chem. X 2022, 16, 100512. [Google Scholar] [CrossRef] [PubMed]
- Carcea, M.; Narducci, V.; Turfani, V.; Giannini, V. Polyphenols in Raw and Cooked Cereals/Pseudocereals/Legume Pasta and Couscous. Foods 2017, 6, 80. [Google Scholar] [CrossRef]
- Udeh, H.O.; Duodu, K.G.; Jideani, A.I.O. Malting Period Effect on the Phenolic Composition and Antioxidant Activity of Finger Millet (Eleusine coracana L. Gaertn) Flour. Molecules 2018, 23, 2091. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, D.; Li, W.; Mou, Y.; Geng, Y.; Chen, F.; Hu, X.; Ji, J.; Ma, L. Quinone reactivity: Investigation of their contribution to nonenzymatic browning. Food Front. 2023, 4, 945–954. [Google Scholar] [CrossRef]
- Kang, J.; Yeo, J. Critical overview of mass spectrometry-based lipidomics approach for evaluating lipid oxidation in foods. Food Sci. Biotechnol. 2024, 34, 837–849. [Google Scholar] [CrossRef]
- Süli, J.; Hamarová, I.; Sobeková, A. Possible consequences of the sucrose replacement by a fructose-glucose syrup. Potravin. Slovak J. Food Sci. 2017, 11, 425–430. [Google Scholar] [CrossRef]
- Mustafa, A.; Aman, P.; Andersson, R.; Kamaleldin, A. Analysis of free amino acids in cereal products. Food Chem. 2007, 105, 317–324. [Google Scholar] [CrossRef]
- Fu, B.; Xu, X.; Zhang, X.; Cheng, S.; El-Seedi, H.R.; Du, M. Identification and characterisation of taste-enhancing peptides from oysters (Crassostrea gigas) via the Maillard reaction. Food Chem. 2023, 424, 136412. [Google Scholar] [CrossRef] [PubMed]
- Gänzle, M.G. Enzymatic and bacterial conversions during sourdough fermentation. Food Microbiol. 2014, 37, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ames, J.M.; Smith, R.D.; Baynes, J.W.; Metz, T.O. A Perspective on the Maillard Reaction and the Analysis of Protein Glycation by Mass Spectrometry: Probing the Pathogenesis of Chronic Disease. J. Proteome Res. 2009, 8, 754–769. [Google Scholar] [CrossRef]
- Mei, X.; Qi, Y.; Ahmed, Z.; Chen, Z.; Bai, J.; Xiao, W.; Liu, S.; Yao, Y.; Xu, B. Salt Reduction in Dough Production: A Comprehensive Review of Quality Changes and Regulation Strategies. J. Texture Stud. 2025, 56, e70032. [Google Scholar] [CrossRef] [PubMed]
- Hannß, M.; Hubbe, N.; Henle, T. Acid-Induced Gelation of Caseins Glycated with Lactose: Impact of Maillard Reaction-Based Glycoconjugation and Protein Cross-Linking. J. Agric. Food Chem. 2018, 66, 11477–11485. [Google Scholar] [CrossRef]
- Boateng, I.D.; Yang, X.-M. Thermal and non-thermal processing affect Maillard reaction products, flavor, and phytochemical profiles of Ginkgo biloba seed. Food Biosci. 2021, 41, 101044. [Google Scholar] [CrossRef]
- Cuomo, F.; Quiquero, M.; Trivisonno, M.C.; Angelicola, M.; Messia, M.C.; Marconi, E. Mitigation of Maillard reaction in spaghetti by optimization of the drying conditions. LWT 2023, 184, 114990. [Google Scholar] [CrossRef]
- Gao, Y.; Miao, J.; Lai, K. Study on Maillard reaction mechanism by quantum chemistry calculation. J. Mol. Model. 2023, 29, 81. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Dai, X.; Shilong, F.; Zhu, M.; Shen, X.; Zhang, K.; Li, S. Antimicrobial and antioxidant capacity of glucosamine-zinc(II) complex via non-enzymatic browning reaction. Food Sci. Biotechnol. 2018, 27, 1–7. [Google Scholar] [CrossRef]
- Geng, Y. Mechanism and Examples of Maillard Reaction. Int. J. Food Sci. Agric. 2024, 8, 54–58. [Google Scholar] [CrossRef]
- Yıltırak, S.; Kocadağlı, T.; Evrim Çelik, E.; Özkaynak Kanmaz, E.; Gökmen, V. Effects of sprouting and fermentation on the formation of Maillard reaction products in different cereals heated as wholemeal. Food Chem. 2022, 389, 133075. [Google Scholar] [CrossRef]
- Shengbu, M.; Ai, L.; Shi, Q.; Zhao, Q.; Liu, X.; Lai, X. Research Progress of Maillard Reaction and its Application in Processing of Traditional Chinese Medicine. Nat. Prod. Commun. 2024, 19, 1934578X241290620. [Google Scholar] [CrossRef]
- Liu, S.; Sun, H.; Ma, G.; Zhang, T.; Wang, L.; Pei, H.; Li, X.; Gao, L. Insights into flavor and key influencing factors of Maillard reaction products: A recent update. Front. Nutr. 2022, 9, 973677. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, E.; Zhang, J.; Yang, M.; Chen, S.; Liu, Z.; Ma, H.; Hu, F. Effects of sonication during moromi fermentation on antioxidant activities of compounds in raw soy sauce. LWT 2019, 116, 108605. [Google Scholar] [CrossRef]
- Chen, T.; Wei, C.-K.; Li, T.; Zhang, H.-L.; Ni, Z.-J.; Khan, M.R.; Wei, Z.-J. Effects of Reducing Sugars on the Structural and Flavor Properties of the Maillard Reaction Products of Lycium barbarum Seed Meal. Foods 2023, 12, 4346. [Google Scholar] [CrossRef]
- Chen, Z.; Mense, A.L.; Brewer, L.R.; Shi, Y.C. Wheat bran arabinoxylans: Chemical structure, extraction, properties, health benefits, and uses in foods. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13366. [Google Scholar] [CrossRef]
- Khalid, K.H.; Ohm, J.-B.; Simsek, S. Whole wheat bread: Effect of bran fractions on dough and end-product quality. J. Cereal Sci. 2017, 78, 48–56. [Google Scholar] [CrossRef]
- Xiang, J.; Liu, F.; Wang, B.; Chen, L.; Liu, W.; Tan, S. A Literature Review on Maillard Reaction Based on Milk Proteins and Carbohydrates in Food and Pharmaceutical Products: Advantages, Disadvantages, and Avoidance Strategies. Foods 2021, 10, 1998. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, F.H.; Zhang, W.C.; Zhang, Z.J.; Yu, S.J. Effects of moisture content on the enolization products formation in glucose–proline Maillard reaction models. J. Sci. Food Agric. 2022, 102, 7249–7258. [Google Scholar] [CrossRef]
- Shi, B.; Guo, X.; Liu, H.; Jiang, K.; Liu, L.; Yan, N.; Farag, M.A.; Liu, L. Dissecting Maillard reaction production in fried foods: Formation mechanisms, sensory characteristic attribution, control strategy, and gut homeostasis regulation. Food Chem. 2024, 438, 137994. [Google Scholar] [CrossRef] [PubMed]
- El Hosry, L.; Elias, V.; Chamoun, V.; Halawi, M.; Cayot, P.; Nehme, A.; Bou-Maroun, E. Maillard Reaction: Mechanism, Influencing Parameters, Advantages, Disadvantages, and Food Industrial Applications: A Review. Foods 2025, 14, 1881. [Google Scholar] [CrossRef]
- Buera, M.d.P.; Chirife, J.; Resnik, S.L.; Lozano, R.D. Nonenzymatic Browning in Liquid Model Systems of High Water Activity: Kinetics of Color Changes due to Caramelization of Various Single Sugars. J. Food Sci. 1987, 52, 1059–1062. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, X.; Karangwa, E.; Xia, S. Correlating enzymatic browning inhibition and antioxidant ability of Maillard reaction products derived from different amino acids. J. Sci. Food Agric. 2017, 97, 4210–4218. [Google Scholar] [CrossRef]
- Giovanelli, G.; Cappa, C. 5-Hydroxymethylfurfural Formation in Bread as a Function of Heat Treatment Intensity: Correlations with Browning Indices. Foods 2021, 10, 417. [Google Scholar] [CrossRef]
- Ameur, L.A.; Mathieu, O.; Lalanne, V.; Trystram, G.; Birlouez-Aragon, I. Comparison of the effects of sucrose and hexose on furfural formation and browning in cookies baked at different temperatures. Food Chem. 2006, 101, 1407–1416. [Google Scholar] [CrossRef]
- Ashoor, S.H.; Zent, J.B. Maillard Browning of Common Amino Acids and Sugars. J. Food Sci. 2006, 49, 1206–1207. [Google Scholar] [CrossRef]
- Purlis, E. Browning development in bakery products—A review. J. Food Eng. 2010, 99, 239–249. [Google Scholar] [CrossRef]
- Boateng, I.D.; Yang, X.-M. Do non-thermal pretreatments followed by intermediate-wave infrared drying affect toxicity, allergenicity, bioactives, functional groups, and flavor components of Ginkgo biloba seed? A case study. Ind. Crops Prod. 2021, 165, 113421. [Google Scholar] [CrossRef]
- Siddiquy, M.; JiaoJiao, Y.; Rahman, M.H.; Iqbal, M.W.; Al-Maqtari, Q.A.; Easdani, M.; Yiasmin, M.N.; Ashraf, W.; Hussain, A.; Zhang, L. Advances of Protein Functionalities Through Conjugation of Protein and Polysaccharide. Food Bioprocess Technol. 2024, 17, 2077–2097. [Google Scholar] [CrossRef]
- Hellwig, M.; Henle, T. Baking, Ageing, Diabetes: A Short History of the Maillard Reaction. Angew. Chem. Int. Ed. 2014, 53, 10316–10329. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Huang, Y.; Wang, W.; Xie, J.; Xie, M.; Shen, M. Quantitative assessment of furosine, furfurals, and advanced glycation end products in different types of commercially available cheeses. Food Control 2022, 136, 108866. [Google Scholar] [CrossRef]
- Huang, J.; Qi, Y.; Faisal Manzoor, M.; Guo, Q.; Xu, B. Effect of superheated steam treated wheat flour on quality characteristics and storage stability of fresh noodles. Food Control 2022, 133, 108666. [Google Scholar] [CrossRef]
- Starowicz, M.; Zieliński, H. How Maillard Reaction Influences Sensorial Properties (Color, Flavor and Texture) of Food Products? Food Rev. Int. 2019, 35, 707–725. [Google Scholar] [CrossRef]
- Jin, W.; Zhao, S.; Sun, H.; Pei, J.; Gao, R.; Jiang, P. Characterization and discrimination of flavor volatiles of different colored wheat grains after cooking based on GC-IMS and chemometrics. Curr. Res. Food Sci. 2023, 7, 100583. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, J.; Regenstein, J.M.; Yin, Y.; Zhou, C. Characterization of taste and aroma compounds in Tianyou, a traditional fermented wheat flour condiment. Food Res. Int. 2018, 106, 156–163. [Google Scholar] [CrossRef]
- Li, Y.; Leng, W.; Xue, J.; Yuan, L.; Liu, H.; Gao, R. A multi-omics-based investigation into the flavor formation mechanisms during the fermentation of traditional Chinese shrimp paste. Food Res. Int. 2023, 166, 112585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, W.; Jin, Y.; Xu, J.; Zhou, W.; Shen, T.; Yang, A.; Wu, Z.; Chen, H. Effect of Boiling and Roasting Treatments on the Nutrients, Lipid Quality, and Flavor of Peanuts. Food Sci. Nutr. 2024, 12, 9314–9324. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, X.; Wang, R. Research progress on the formation mechanism and detection technology of bread flavor. J. Food Sci. 2022, 87, 3724–3736. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Hossain, A. Role of Lipids in Food Flavor Generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhong, Y.; Zhang, X.; Wang, Y.; Sun, Y.; Li, X.; Liu, Z.; Liang, J. Flavor Characteristics, Antioxidant Activity and In Vitro Digestion Properties of Bread with Large-Leaf Yellow Tea Powder. Foods 2024, 13, 715. [Google Scholar] [CrossRef]
- Yuwei, H.; Jialan, Z.; Shaojin, W.; Yingbao, L.; Li, L.; Mengxiang, G. Lactic acid bacteria synergistic fermentation affects the flavor and texture of bread. J. Food Sci. 2022, 87, 1823–1836. [Google Scholar] [CrossRef]
- Du, W.; Zhao, M.; Zhen, D.; Tan, J.; Wang, T.; Xie, J. Key aroma compounds in Chinese fried food of youtiao. Flavour Fragr. J. 2019, 35, 88–98. [Google Scholar] [CrossRef]
- Wei, G.; Zhang, Z.; Zhao, F.; Sang, Y.; Regenstein, J.M.; Zhou, P. Characteristic aroma compounds during the fermentation of Chinese steamed bread fermented with different starters. Food Chem. 2024, 457, 140151. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Yang, X.; Ruan, S.; Zhou, A.; Huang, S.; Ma, H.; Cai, S. The Preparation and Identification of Characteristic Flavour Compounds of Maillard Reaction Products of Protein Hydrolysate from Grass Carp (Ctenopharyngodon idella) Bone. J. Food Qual. 2021, 2021, 8394152. [Google Scholar] [CrossRef]
- Shakoor, A.; Zhang, C.; Xie, J.; Yang, X. Maillard reaction chemistry in formation of critical intermediates and flavour compounds and their antioxidant properties. Food Chem. 2022, 393, 133416. [Google Scholar] [CrossRef] [PubMed]
- Cerny, C.; Davidek, T. α-Mercaptoketone Formation during the Maillard Reaction of Cysteine and [1-13C]Ribose. J. Agric. Food Chem. 2004, 52, 958–961. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Ma, Y.-J.; Guo, Y.; Luo, X.-L.; Du, M.; Dong, L.; Yu, P.; Xu, X.-B. Reinvestigation of 2-acetylthiazole formation pathways in the Maillard reaction. Food Chem. 2021, 345, 128761. [Google Scholar] [CrossRef]
- Hwang, H.-I.; Hartman, T.G.; Rosen, R.T.; Lech, J.; Ho, C.-T. Formation of Pyrazines from the Maillard Reaction of Glucose and Lysine-.alpha.-amine-15N. J. Agric. Food Chem. 1994, 42, 1000–1004. [Google Scholar] [CrossRef]
- Wang, K.; Tang, N.; Bian, X.; Geng, D.; Chen, H.; Cheng, Y. Structural characteristics, chemical compositions and antioxidant activity of melanoidins during the traditional brewing of Monascus vinegar. LWT 2024, 209, 116760. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, G.; Li, Y. Bread characteristics and antioxidant activities of Maillard reaction products of white pan bread containing various sugars. LWT 2018, 95, 308–315. [Google Scholar] [CrossRef]
- Fayek, N.M.; Xiao, J.; Farag, M.A. A multifunctional study of naturally occurring pyrazines in biological systems; formation mechanisms, metabolism, food applications and functional properties. Crit. Rev. Food Sci. Nutr. 2021, 63, 5322–5338. [Google Scholar] [CrossRef]
- Morales, F.J.; Açar, Ö.Ç.; Serpen, A.; Arribas-Lorenzo, G.; Gökmen, V. Degradation of Free Tryptophan in a Cookie Model System and Its Application in Commercial Samples. J. Agric. Food Chem. 2007, 55, 6793–6797. [Google Scholar] [CrossRef] [PubMed]
- Tsen, C.C.; Reddy, P.R.K.; El-Samahy, S.K.; Gehrke, C.W. Effect of the Maillard Browning Reaction on the Nutritive Value of Breads and Pizza Crusts. In The Maillard Reaction in Foods and Nutrition, Proceedings of the ACS Symposium Series, Washington, DC, UAS, 29 April 1983; Waller, G.R., Feather, M.S., Eds.; American Chemical Society: Washington, DC, USA, 1983; pp. 379–394. [Google Scholar]
- Cheng, W.; Wang, X.; Zhang, Z.; Ma, L.; Liu, G.; Wang, Q.; Chen, F.; Cheng, K.W. Development of an Isotope Dilution UHPLC-QqQ-MS/MS-Based Method for Simultaneous Determination of Typical Advanced Glycation End Products and Acrylamide in Baked and Fried Foods. J. Agric. Food Chem. 2021, 69, 2611–2618. [Google Scholar] [CrossRef]
- Shyu, Y.-S.; Hwang, J.-Y.; Shen, S.-T.; Sung, W.-C. The Effect of Different Frying Methods and the Addition of Potassium Aluminum Sulfate on Sensory Properties, Acrylamide, and Oil Content of Fried Bread (Youtiao). Appl. Sci. 2021, 11, 549. [Google Scholar] [CrossRef]
- Khorshidian, N.; Yousefi, M.; Shadnoush, M.; Siadat, S.D.; Mohammadi, M.; Mortazavian, A.M. Using probiotics for mitigation of acrylamide in food products: A mini review. Curr. Opin. Food Sci. 2020, 32, 67–75. [Google Scholar] [CrossRef]
- Qi, Y.; Cheng, J.; Ding, W.; Wang, L.; Qian, H.; Qi, X.; Wu, G.; Zhu, L.; Yang, T.; Xu, B.; et al. Epicatechin-Promoted Formation of Acrylamide from 3-Aminopropionamide Via Postoxidative Reaction of B-Ring. J. Agric. Food Chem. 2024, 72, 15301–15310. [Google Scholar] [CrossRef]
- Li, J.; Shi, J.; Huang, X.; Wang, T.; Zou, X.; Li, Z.; Zhang, D.; Zhang, W.; Xu, Y. Effects of pulsed electric field pretreatment on frying quality of fresh-cut lotus root slices. LWT 2020, 132, 109873. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, C.; Cao, C. Production and Inhibition of Acrylamide during Coffee Processing: A Literature Review. Molecules 2023, 28, 3476. [Google Scholar] [CrossRef]
- Maan, A.A.; Anjum, M.A.; Khan, M.K.I.; Nazir, A.; Saeed, F.; Afzaal, M.; Aadil, R.M. Acrylamide Formation and Different Mitigation Strategies During Food Processing—A Review. Food Rev. Int. 2020, 38, 70–87. [Google Scholar] [CrossRef]
- Rong, Y.; Ali, S.; Ouyang, Q.; Wang, L.; Wang, B.; Chen, Q. A turn-on upconversion fluorescence sensor for acrylamide in potato chips based on fluorescence resonance energy transfer and thiol-ene Michael addition. Food Chem. 2021, 351, 129215. [Google Scholar] [CrossRef]
- Choudhary, A.; Kumar, V.; Kumar, S.; Majid, I.; Aggarwal, P.; Suri, S. 5-Hydroxymethylfurfural (HMF) formation, occurrence and potential health concerns: Recent developments. Toxin Rev. 2020, 40, 545–561. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, Y.; Mehmood, A.; Lu, T.; Chen, X. Unraveling the temporal changes of Maillard reaction products and aroma profile in coffee leaves during hot-air drying. J. Food Compos. Anal. 2024, 128, 106055. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, M.; Gong, Z.; Jiao, W.; Li, L.; Dong, M.; Xiang, T.; Feng, N.; Wu, Q. Inhibition of polyphenols on Maillard reaction products and their induction of related diseases: A comprehensive review. Phytomedicine 2024, 128, 155589. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, H.; Dong, L.; Liu, Y.; Liu, L.; Cao, H.; Wang, W.; Liu, L. Research advance on AGEs generation, detection, influencing factors and inhibition mechanism in bakery products processing. Food Biosci. 2024, 57, 103404. [Google Scholar] [CrossRef]
- Liu, D.; He, Y.; Xiao, J.; Zhou, Q.; Wang, M. The occurrence and stability of Maillard reaction products in various traditional Chinese sauces. Food Chem. 2021, 342, 128319. [Google Scholar] [CrossRef] [PubMed]
- Mencin, M.; Abramovič, H.; Vidrih, R.; Schreiner, M. Acrylamide levels in food products on the Slovenian market. Food Control 2020, 114, 107267. [Google Scholar] [CrossRef]
- Zilic, S.; Aktag, I.G.; Dodig, D.; Filipovic, M.; Gokmen, V. Acrylamide formation in biscuits made of different wholegrain flours depending on their free asparagine content and baking conditions. Food Res. Int. 2020, 132, 109109. [Google Scholar] [CrossRef] [PubMed]
- Mildner-Szkudlarz, S.; Barbara Rozanska, M.; Siger, A.; Zembrzuska, J.; Szwengiel, A. Formation of Maillard reaction products in a model bread system of different gluten-free flours. Food Chem. 2023, 429, 136994. [Google Scholar] [CrossRef]
- Qi, Y.; Cheng, J.; Chen, Y.; Xu, B. Effect of sodium carbonate on the properties of seventy percent of Tartary buckwheat composite flour-based doughs and noodles and the underlying mechanism. J. Texture Stud. 2023, 54, 947–957. [Google Scholar] [CrossRef]
- Aarabi, F.; Seyedain Ardebili, M. The effect of sugar type and baking condition on formation of acrylamide in industrial rotary moulded biscuit. J. Food Meas. Charact. 2020, 14, 2230–2239. [Google Scholar] [CrossRef]
- Cheng, L.; Jin, C.; Zhang, Y. Investigation of Variations in the Acrylamide and Nε-(Carboxymethyl) Lysine Contents in Cookies during Baking. J. Food Sci. 2014, 79, T1030–T1038. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, H.; Xi, J.; Jin, Y.; Chen, Y.; Guo, L.; Jin, Z.; Xu, X. Improving bread aroma using low-temperature sourdough fermentation. Food Biosci. 2020, 37, 100704. [Google Scholar] [CrossRef]
- Sarion, C.; Codina, G.G.; Dabija, A. Acrylamide in Bakery Products: A Review on Health Risks, Legal Regulations and Strategies to Reduce Its Formation. Int. J. Env. Res Public Health 2021, 18, 4332. [Google Scholar] [CrossRef]
- Iyota, H.; Sakai, H.; Mamiya, Y. Color Measurement Methods for Optimization of Oven Operation (Baking of Sliced Bread with Superheated Steam and Hot Air). Food Sci. Technol. Res. 2013, 19, 939–947. [Google Scholar] [CrossRef]
- Onacik-Gür, S.; Szafrańska, A.; Roszko, M.; Stępniewska, S. Interaction of dough preparation method, green tea extract and baking temperature on the quality of rye bread and acrylamide content. LWT 2022, 154, 112759. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, L.; Yuan, S.; Guo, Y.; Yao, W.; Zhou, W.; Yu, H. Formation of advanced glycation end-products and α-dicarbonyl compounds through Maillard reaction: Solutions from natural polyphenols. J. Food Compos. Anal. 2023, 120, 105350. [Google Scholar] [CrossRef]
- Chamorro, F.; Carpena, M.; Fraga-Corral, M.; Echave, J.; Riaz Rajoka, M.S.; Barba, F.J.; Cao, H.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Valorization of kiwi agricultural waste and industry by-products by recovering bioactive compounds and applications as food additives: A circular economy model. Food Chem. 2022, 370, 131315. [Google Scholar] [CrossRef]
- Zhou, Q.; Cheng, K.-W.; Xiao, J.; Wang, M. The multifunctional roles of flavonoids against the formation of advanced glycation end products (AGEs) and AGEs-induced harmful effects. Trends Food Sci. Technol. 2020, 103, 333–347. [Google Scholar] [CrossRef]
- Sun, Y.; Xie, W.; Huang, Y.; Chen, X. Coffee leaf extract inhibits advanced glycation end products and their precursors: A mechanistic study. J. Food Sci. 2024, 89, 3455–3468. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.S.; Reboredo-Rodríguez, P.; Süntar, I.; Sureda, A.; Belwal, T.; Loizzo, M.R.; Tundis, R.; Sobarzo-Sanchez, E.; Rastrelli, L.; Forbes-Hernandez, T.Y.; et al. Evaluation of the status quo of polyphenols analysis: Part I—Phytochemistry, bioactivity, interactions, and industrial uses. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3191–3218. [Google Scholar] [CrossRef]
- Mildner-Szkudlarz, S.; Siger, A.; Szwengiel, A.; Przygoński, K.; Wojtowicz, E.; Zawirska-Wojtasiak, R. Phenolic compounds reduce formation of Nε-(carboxymethyl)lysine and pyrazines formed by Maillard reactions in a model bread system. Food Chem. 2017, 231, 175–184. [Google Scholar] [CrossRef]
- Syeunda, C.; Awika, J.M. Mechanisms of flavonoid inhibition of Maillard reaction product formation in relation to whole grains processing. Food Chem. 2024, 449, 139237. [Google Scholar] [CrossRef]
- Zhang, Y.; An, X. Inhibitory mechanism of quercetin against the formation of 5-(hydroxymethyl)-2-furaldehyde in buckwheat flour bread by ultra-performance liquid chromatography coupled with high-resolution tandem mass spectrometry. Food Res. Int. 2017, 95, 68–81. [Google Scholar] [CrossRef]
- Liang, H.-Y.; Gao, H.-X.; Jing, Z.; He, Q.; Zeng, W.-C. Regulation of tea polyphenols in gluten-glucose Maillard reaction: Evaluation and analysis. LWT 2024, 205, 116508. [Google Scholar] [CrossRef]
- Li, W.; Sun, X.; Mariga, A.M.; Yang, Q.; Fang, Y.; Hu, Q.; Pei, F. Effects of ferulic acid on the polymerization behavior of gluten protein and its components. Food Hydrocoll. 2024, 147, 109388. [Google Scholar] [CrossRef]
- Zheng, J.; Guo, H.; Ou, J.; Liu, P.; Huang, C.; Wang, M.; Simal-Gandara, J.; Battino, M.; Jafari, S.M.; Zou, L.; et al. Benefits, deleterious effects and mitigation of methylglyoxal in foods: A critical review. Trends Food Sci. Technol. 2021, 107, 201–212. [Google Scholar] [CrossRef]
- Zhang, N.; Zhou, Q.; Fan, D.; Xiao, J.; Zhao, Y.; Cheng, K.-W.; Wang, M. Novel roles of hydrocolloids in foods: Inhibition of toxic maillard reaction products formation and attenuation of their harmful effects. Trends Food Sci. Technol. 2021, 111, 706–715. [Google Scholar] [CrossRef]
- Mousa, R.M.A. Simultaneous mitigation of 4(5)-methylimidazole, acrylamide, and 5-hydroxymethylfurfural in ammonia biscuits by supplementing with food hydrocolloids. Food Sci. Nutr. 2019, 7, 3912–3921. [Google Scholar] [CrossRef]
- Passos, C.P.; Ferreira, S.S.; Serôdio, A.; Basil, E.; Marková, L.; Kukurová, K.; Ciesarová, Z.; Coimbra, M.A. Pectic polysaccharides as an acrylamide mitigation strategy—Competition between reducing sugars and sugar acids. Food Hydrocoll. 2018, 81, 113–119. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, X.; Zheng, L.; Yang, Y.; Xiao, D.; Zhang, H.; Ai, B.; Sheng, Z. κ-Carrageenan inhibits the formation of advanced glycation end products in cakes: Inhibition mechanism, cake characteristics, and sensory evaluation. Food Chem. 2023, 429, 136583. [Google Scholar] [CrossRef]
- Heng, X.; Chen, H.; Lu, C.; Feng, T.; Li, K.; Gao, E. Study on synergistic fermentation of bean dregs and soybean meal by multiple strains and proteases. LWT 2022, 154, 112626. [Google Scholar] [CrossRef]
- Sun, Y.; Cao, Q.; Huang, Y.; Lu, T.; Ma, H.; Chen, X. Mechanistic study on the inhibition of α-amylase and α-glucosidase using the extract of ultrasound-treated coffee leaves. J. Sci. Food Agric. 2023, 104, 63–74. [Google Scholar] [CrossRef]
- El-Sayed, A.A.; Abdelhady, M.M.; Jaafari, S.A.; Alanazi, T.M.; Mohammed, A.S. Impact of Some Enzymatic Treatments on Acrylamide Content in Biscuits. Processes 2023, 11, 1041. [Google Scholar] [CrossRef]
- Jana, A.; Biswas, S.; Ghosh, R.; Modak, R. Recent advances in L-Asparaginase enzyme production and formulation development for acrylamide reduction during food processing. Food Chem. X 2025, 25, 102055. [Google Scholar] [CrossRef] [PubMed]
- Kocadağlı, T.; Gökmen, V. Effects of Sodium Chloride, Potassium Chloride, and Calcium Chloride on the Formation of α-Dicarbonyl Compounds and Furfurals and the Development of Browning in Cookies during Baking. J. Agric. Food Chem. 2016, 64, 7838–7848. [Google Scholar] [CrossRef]
- Göncüoğlu Taş, N.; Kocadağlı, T.; Balagiannis, D.P.; Gökmen, V.; Parker, J.K. Effect of salts on the formation of acrylamide, 5-hydroxymethylfurfural and flavour compounds in a crust-like glucose/wheat flour dough system during heating. Food Chem. 2023, 410, 135358. [Google Scholar] [CrossRef]
- Van Der Fels-Klerx, H.J.; Capuano, E.; Nguyen, H.T.; Ataç Mogol, B.; Kocadağlı, T.; Göncüoğlu Taş, N.; Hamzalıoğlu, A.; Van Boekel, M.A.J.S.; Gökmen, V. Acrylamide and 5-hydroxymethylfurfural formation during baking of biscuits: NaCl and temperature–time profile effects and kinetics. Food Res. Int. 2014, 57, 210–217. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, Y.; Zhou, T.; Zhang, F.; Xia, X.; Yu, J.; Song, S.; Hayat, K.; Zhang, X.; Ho, C.-T. Light-Colored Maillard Peptides: Formation from Reduced Fluorescent Precursors of Browning and Enhancement of Saltiness Perception. J. Agric. Food Chem. 2023, 71, 20251–20259. [Google Scholar] [CrossRef]
- Zhu, Y.; Luo, Y.; Sun, G.; Wang, P.; Hu, X.; Chen, F. The simultaneous inhibition of histidine on 5-hydroxymethylfurfural and acrylamide in model systems and cookies. Food Chem. 2022, 370, 131271. [Google Scholar] [CrossRef]
- Sun, J.; Mu, Y.; Mohammed, O.; Dong, S.; Xu, B. Effects of single-mode microwave heating and dextran conjugation on the structure and functionality of ovalbumin–dextran conjugates. Food Res. Int. 2020, 137, 109468. [Google Scholar] [CrossRef]
- Li, M.; Zhou, C.; Wang, B.; Zeng, S.; Mu, R.; Li, G.; Li, B.; Lv, W. Research progress and application of ultrasonic- and microwave-assisted food processing technology. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3707–3731. [Google Scholar] [CrossRef] [PubMed]
- Michalak, J.; Czarnowska-Kujawska, M.; Klepacka, J.; Gujska, E. Effect of Microwave Heating on the Acrylamide Formation in Foods. Molecules 2020, 25, 4140. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Qiu, C.; Wei, F.; Yu, Z.; Zhang, Y.; Wang, S. The Effect of Microwave Baking Conditions on the Quality of Biscuits and the Control of Thermal Processing Hazards in the Maillard Reaction. Front. Nutr. 2022, 9, 825365. [Google Scholar] [CrossRef]
- Palazoğlu, T.K.; Coşkun, Y.; Tuta, S.; Mogol, B.A.; Gökmen, V. Effect of vacuum-combined baking of cookies on acrylamide content, texture and color. Eur. Food Res. Technol. 2015, 240, 243–249. [Google Scholar] [CrossRef]
- Yıldız, H.G.; Palazoğlu, T.K.; Miran, W.; Kocadağlı, T.; Gökmen, V. Evolution of surface temperature and its relationship with acrylamide formation during conventional and vacuum-combined baking of cookies. J. Food Eng. 2017, 197, 17–23. [Google Scholar] [CrossRef]
- Song, X.; Ni, M.; Zhang, Y.; Zhang, G.; Pan, J.; Gong, D. Comparing the inhibitory abilities of epigallocatechin-3-gallate and gallocatechin gallate against tyrosinase and their combined effects with kojic acid. Food Chem. 2021, 349, 129172. [Google Scholar] [CrossRef]
- Hu, H.; Wang, Y.; Huang, Y.; Yu, Y.; Shen, M.; Li, C.; Nie, S.; Xie, M. Natural Antioxidants and Hydrocolloids as a Mitigation Strategy to Inhibit Advanced Glycation End Products (AGEs) and 5-Hydroxymethylfurfural (HMF) in Butter Cookies. Foods 2022, 11, 657. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.; Li, Y.; Yu, W.; Zhao, Y.; Hu, X.; Tao, N.P.; Wang, M. Naringenin, a common flavanone, inhibits the formation of AGEs in bread and attenuates AGEs-induced oxidative stress and inflammation in RAW264.7 cells. Food Chem. 2018, 269, 35–42. [Google Scholar] [CrossRef]
- Fang, M.; Ting, Y.-S.; Sung, W.-C. Effects of Sodium Alginate, Pectin and Chitosan Addition on the Physicochemical Properties, Acrylamide Formation and Hydroxymethylfurfural Generation of Air Fried Biscuits. Polymers 2022, 14, 3961. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, L.; Zheng, X.; Yang, Y.; Xiao, D.; Zhang, H.; Ai, B.; Sheng, Z. Chitosan inhibits advanced glycation end products formation in chemical models and bakery food. Food Hydrocoll. 2022, 128, 107600. [Google Scholar] [CrossRef]
- Levine, R.A.; Ryan, S.M. Determining the Effect of Calcium Cations on Acrylamide Formation in Cooked Wheat Products Using a Model System. J. Agric. Food Chem. 2009, 57, 6823–6829. [Google Scholar] [CrossRef]
| Type | Barley | Rye | Buckwheat | Oat | Wheat | References |
|---|---|---|---|---|---|---|
| Maltose (g/kg) | 10.5 ± 1.7 | 10.6 ± 0.1 | 11.6 ± 0.9 | 4.8 ± 0.3 | 8.5 ± 0.1 | [18] |
| Glucose (g/kg) | 4.9 ± 1.2 | 4.7 ± 0.1 | 2.2 ± 0.2 | 1.7 ± 0.1 | 1.5 ± 0.1 | [18] |
| Fructose (g/kg) | 3.7 ± 0.3 | 2.7 ± 0.1 | 0.7 ± 0.1 | 4.5 ± 0.1 | 1.3 ± 0.1 | [18] |
| Total free amino acid (mg/kg) | 1704 ± 130 | 2314 ± 59 | 1960 ± 57 | 1994 ± 69 | 1299 ± 33 | [18] |
| Asparagine (mg/kg) | 309 ± 16 | 829 ± 24 | 113 ± 6 | 672 ± 22 | 292 ± 5 | [18] |
| Lysine (mg/kg) | 28.6 ± 1.7 | 16.3 ± 2.7 | — | 26.5 ± 2.9 | 18.3 ± 2.5 | [19] |
| Type | AA (μg/kg) | HMF (mg/kg) | CML (mg/kg) | CEL (mg/kg) |
|---|---|---|---|---|
| Breads | 6.66–134.8 [100,113] | 0.66–18.34 [108] | 4.5–617.86 [111] | 2.1–71.49 [111] |
| Cookies | 26.75–384.5 [100,113] | 1.65–82.78 [108] | 0.86–117.53 [111] | 3.59–50.79 [111] |
| Instant noodles | 12.91 [100] | — | 4.61 [100] | 3.39 [100] |
| Fried bread sticks | 21.34–2095.2 [100,101] | — | 4.48 [100] | 1.99 [100] |
| Mitigation Strategies | Types of Flour Products | Mitigation Mechanism | Mitigation Results | References |
|---|---|---|---|---|
| Selection of raw material | ||||
| Using flours with a low content of asparagine | Baking products | Reducing the content of precursor substances of AA | The AA contents of baked products made from refined wheat flour and red corn flour with low asparagine content are lower than those of baked products processed from hulled oat and rye flour. | [114] |
| Replacing reducing sugar with non-reducing sugar | Cookies | Reducing the content of carbonyl groups | Replacing part of the reducing sugar (invert syrup) with a non-reducing sugar (sucrose) reduces the production of AA. | [117] |
| Processing control | ||||
| Optimization of heat input | Cookies | When the temperature drops, the reaction rate decreases | Reducing the temperature within a short baking time inhibits the generation of CML. | [118] |
| Adjustment of pH | Cookies | A decrease in pH inhibits the formation of AA | Adding citric acid to the dough of semi-sweet cookies reduced the AA content by 20–30%. | [120] |
| Optimization of the drying process | Pasta | Reducing the degree of the Maillard reaction | High-temperature rapid treatment reduced the formation of HMF. | [153] |
| Additive applications | ||||
| Adding catechin | Butter biscuit | Trapping α-dicarbonyl compounds, scavenging free radicals, and shielding the amino group in proteins | The contents of free CML and CEL in butter biscuits with 0.3–5% catechins decreased by 31.89–84.19%, and the content of protein-bound CEL decreased by 15.32–30.64%. | [154] |
| Adding naringenin | Bread | Scavenging free radicals | The contents of CML and total fluorescent AGEs in the bread with 0.25–1% naringenin decreased by 9.67–54.27% and 11.79–35.19%, respectively. | [155] |
| Adding caffeic acid | Bread | Shielding the amino structure in proteins | The contents of CML in the bread crust and bread crumb with 0.1% caffeic acid decreased by 80% and 50%, respectively. | [128] |
| Adding quercetin | Bread | Forming adducts with HMF and its precursors | The HMF content in bread with 0.19% quercetin decreased by 86.0%. | [130] |
| Adding sodium alginate | Cookies | Mitigating the formation of intermediate products for the generation of AA | The AA content in biscuits with 1% sodium alginate decreased by 28%. | [156] |
| Adding chitosan | Cake | Inhibiting protein oxidation and capturing GO and MGO | The content of AGEs in the cake with 0.5% chitosan decreased by 30.31–61.22%. | [157] |
| Adding pectin | Cookies | Lowering the pH value | The AA content in biscuits with 5% pectin decreased by 67%. | [136] |
| Adding gum Arabic | Cookies | Forming a tight adhesive layer to inhibit water evaporation | In the biscuits with 0.28% gum Arabic, AA and HMF decreased by 58.6% and 74%, respectively. | [135] |
| Adding L-asparaginase | Bread Cookies | Removing asparagine | The AA contents in sweet bread and biscuits with L-asparaginase decreased by 81% and 84%, respectively. | [141] |
| Adding Na+ | Cookies | Inhibiting the formation of the Schiff base between reducing sugar and asparagine | The AA contents in biscuits with 0.65% NaCl at 180 °C and 190 °C decreased by 24% and 16.5%, respectively. | [144] |
| Adding Ca2+ | Wheat product | Lowering the pH value | The AA content in wheat products with 0.44% CaCl2 decreased by 36%. | [158] |
| Adding histidine | Cookies | Competing with asparagine for glucose | The inhibition rates of AA and HMF in biscuits with 2% histidine were 65% and 90%, respectively. | [146] |
| Physical techniques | ||||
| Microwave treatment | Fried product | Increasing temperature quickly to prevent excessive reaction | The AA content in microwave frying products were 37–83% lower than those in traditional frying ones. | [149] |
| Vacuum treatment | Cookies | Inhibiting oxidation reaction and accelerating water evaporation | The AA content in the biscuits prepared by vacuum combined baking was 30% lower than that in the samples baked by traditional baking. | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Wang, W.; Yang, T.; Ding, W.; Xu, B. Maillard Reaction in Flour Product Processing: Mechanism, Impact on Quality, and Mitigation Strategies of Harmful Products. Foods 2025, 14, 2721. https://doi.org/10.3390/foods14152721
Qi Y, Wang W, Yang T, Ding W, Xu B. Maillard Reaction in Flour Product Processing: Mechanism, Impact on Quality, and Mitigation Strategies of Harmful Products. Foods. 2025; 14(15):2721. https://doi.org/10.3390/foods14152721
Chicago/Turabian StyleQi, Yajing, Wenjun Wang, Tianxiang Yang, Wangmin Ding, and Bin Xu. 2025. "Maillard Reaction in Flour Product Processing: Mechanism, Impact on Quality, and Mitigation Strategies of Harmful Products" Foods 14, no. 15: 2721. https://doi.org/10.3390/foods14152721
APA StyleQi, Y., Wang, W., Yang, T., Ding, W., & Xu, B. (2025). Maillard Reaction in Flour Product Processing: Mechanism, Impact on Quality, and Mitigation Strategies of Harmful Products. Foods, 14(15), 2721. https://doi.org/10.3390/foods14152721





