Whey Protein–Quercetin–Gellan Gum Complexes Prepared Using pH-Shift Treatment: Structural and Functional Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experimental Methods
2.2.1. Preparation of Ternary Complexes
Preparation of Whey Protein–Quercetin Complexes
Preparation of Whey Protein–Quercetin–Gellan Gum Complexes
2.2.2. SDS-PAGE Analysis
2.2.3. Endogenous Fluorescence Spectroscopy Analysis
2.2.4. Ultraviolet-Visible (UV-Vis) Absorption Spectroscopy Analysis
2.2.5. Fourier Transform Infrared Spectra
2.2.6. Measurement of Particle Size and Potential
2.2.7. Determination of Polyphenol Binding Capacity
2.2.8. Determination of Turbidity of the Complexes
2.2.9. Determination of the Emulsifying Ability of the Complexes
Foaming
Emulsification
2.2.10. Thermal Analysis of Complexes
2.2.11. Scanning Electron MICROSCOPY (SEM) of Complexes
2.2.12. Determination of the Antioxidant Properties of Compounds
DPPH Radical Scavenging Rate
ABTS Radical Scavenging Rate
2.3. Statistical Analysis
3. Results and Discussion
3.1. SDS-PAGE
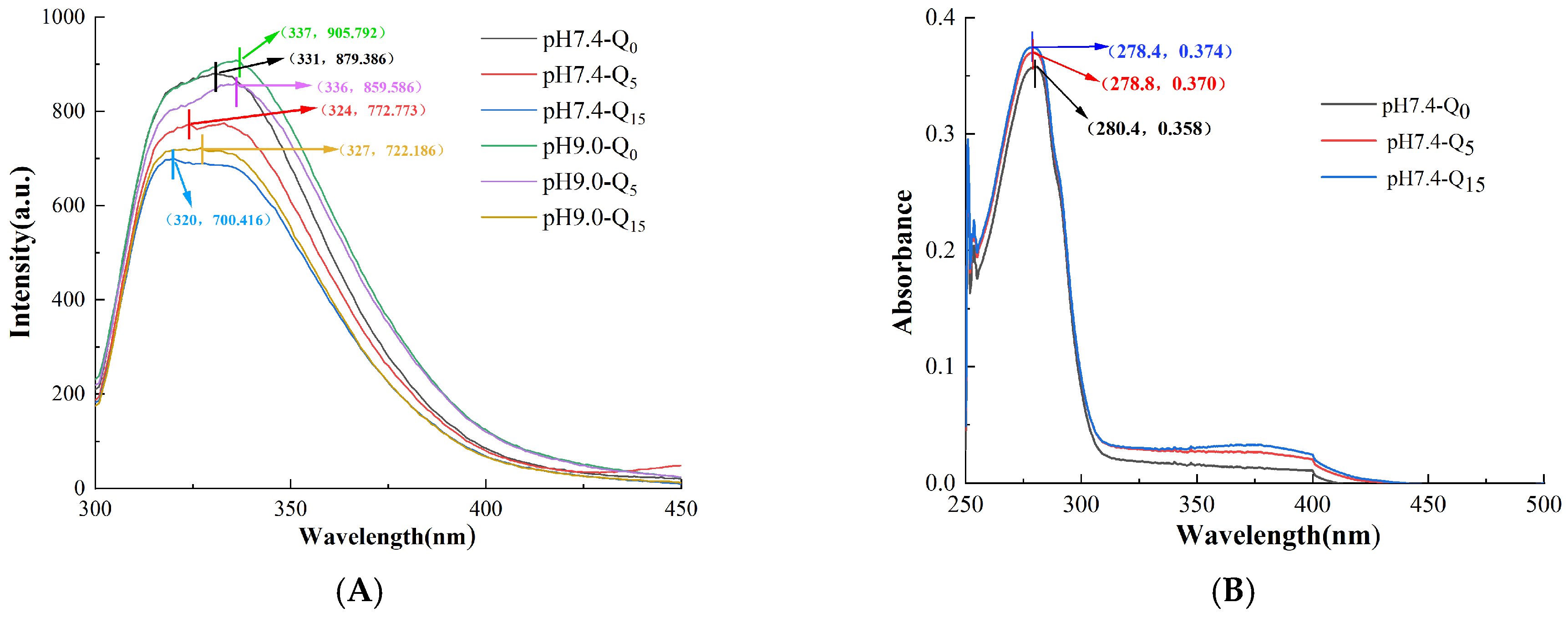
3.2. Analysis of Endogenous Fluorescence Spectra
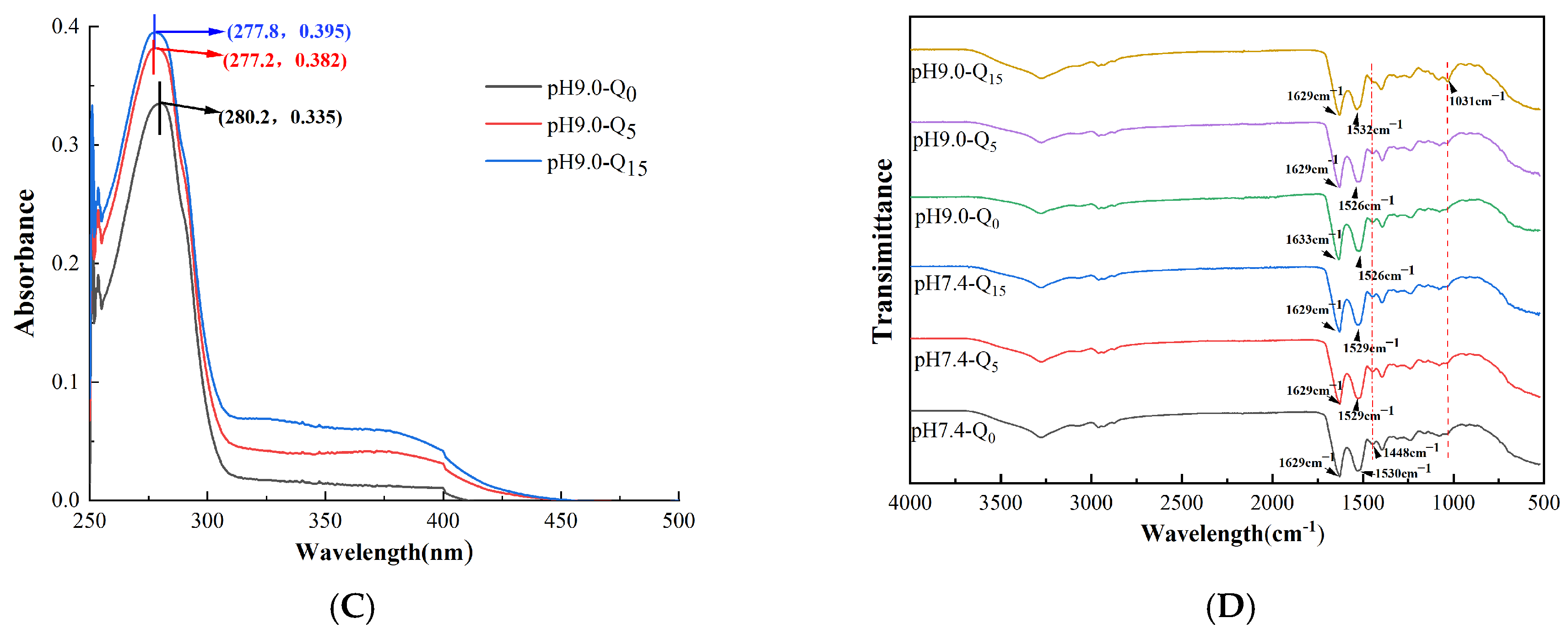
3.3. UV-Vis Absorption Spectra
3.4. Infrared Spectral Analysis
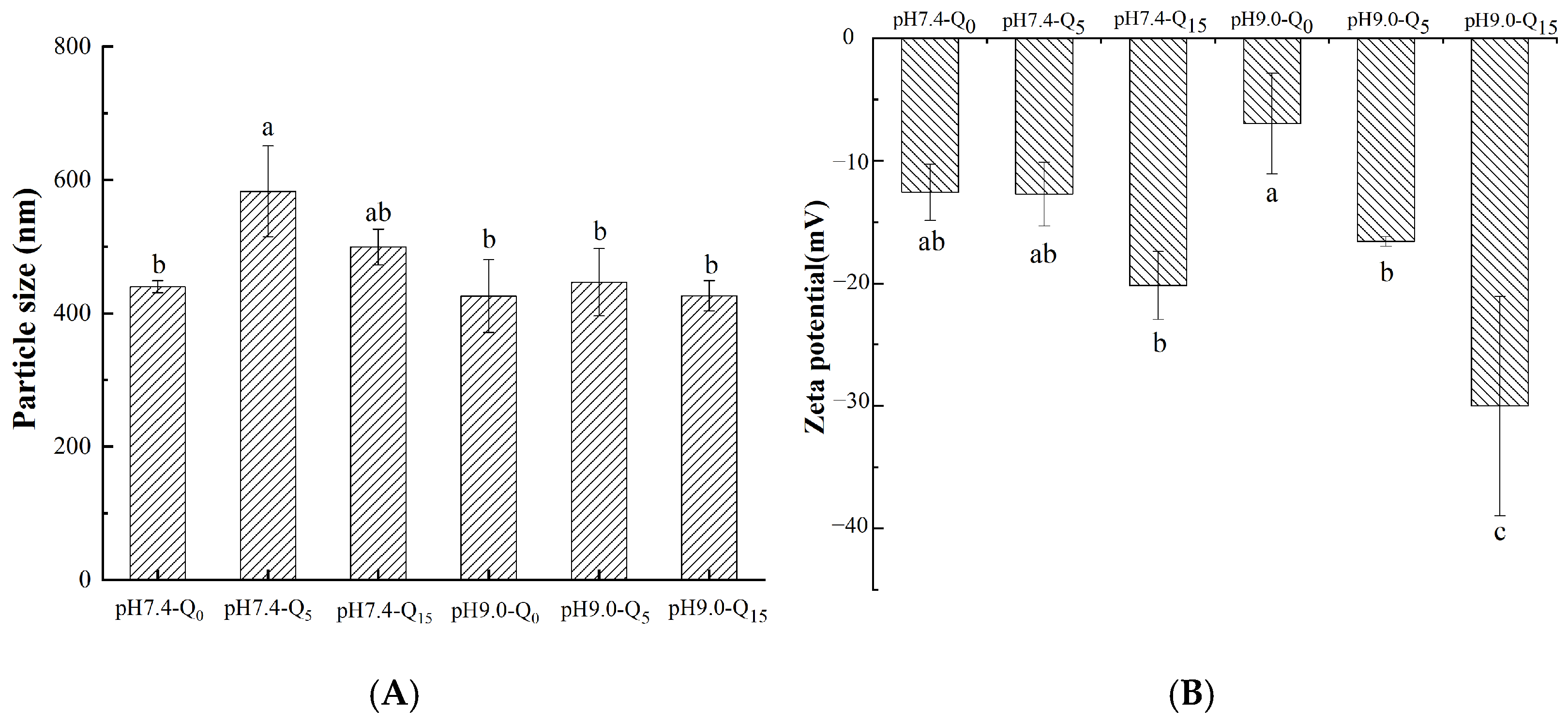
3.5. Particle Size and Zeta Potential
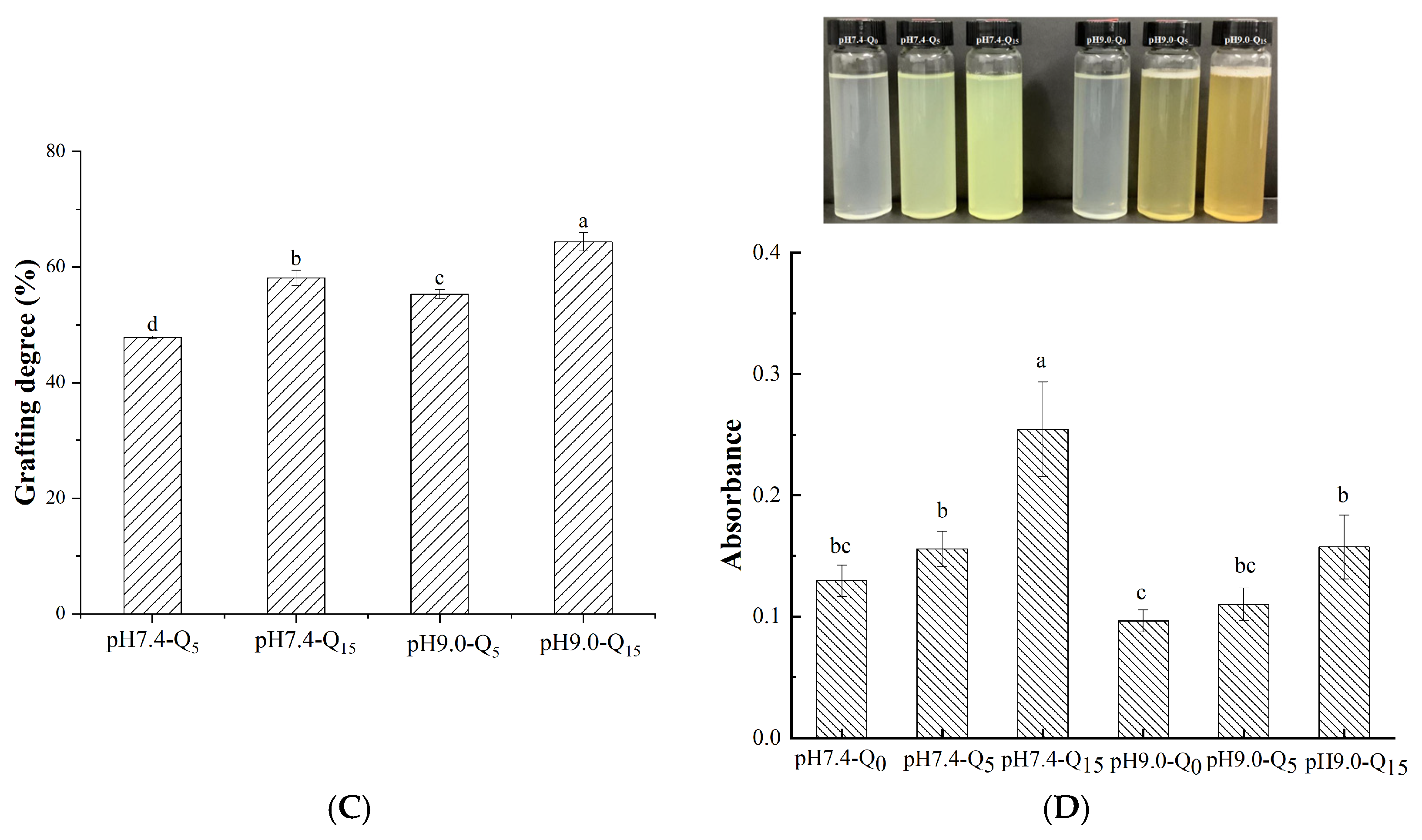
3.6. Polyphenol Binding Capacity
3.7. Turbidity
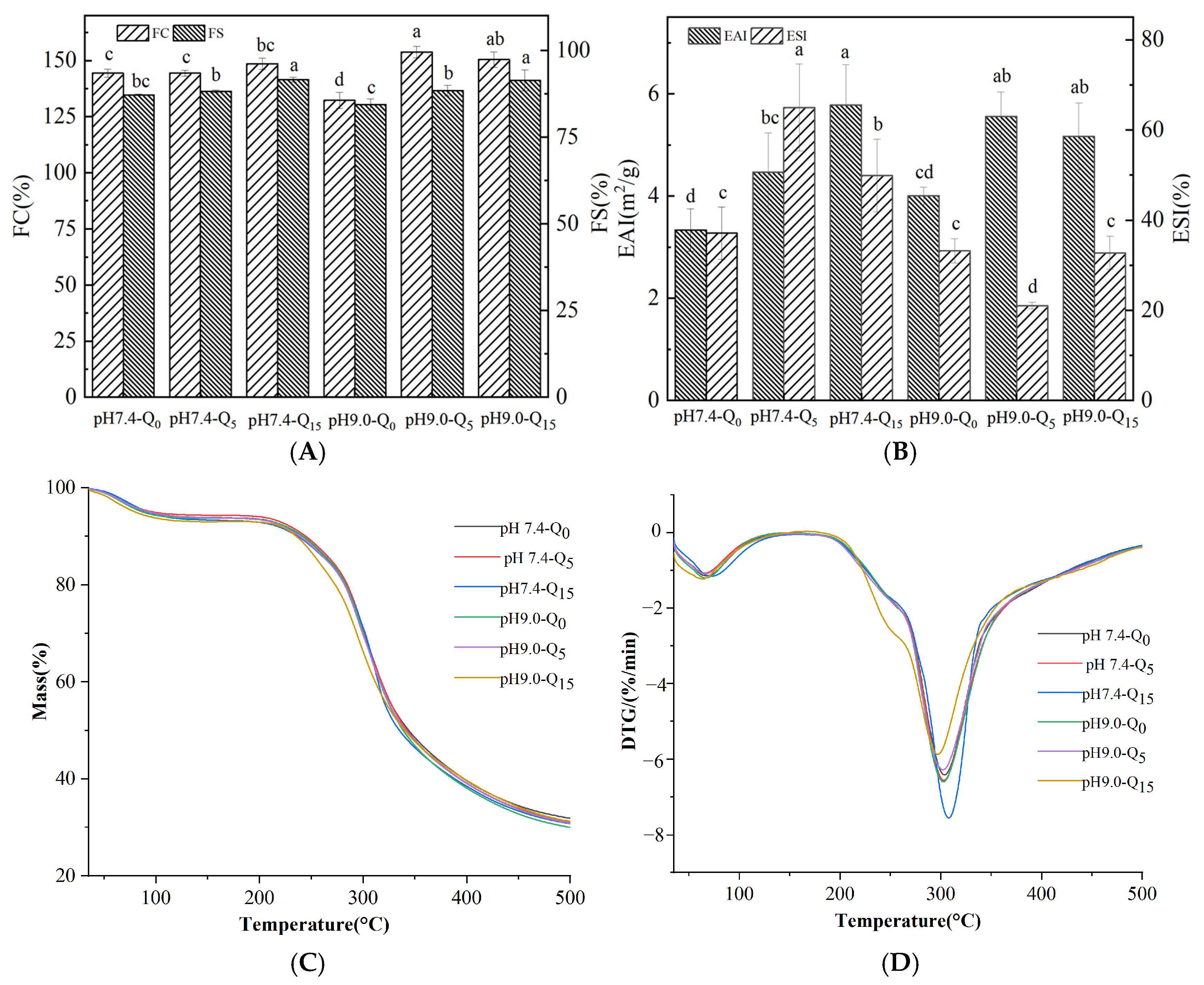
3.8. Emulsification Properties
3.9. Thermal Analysis
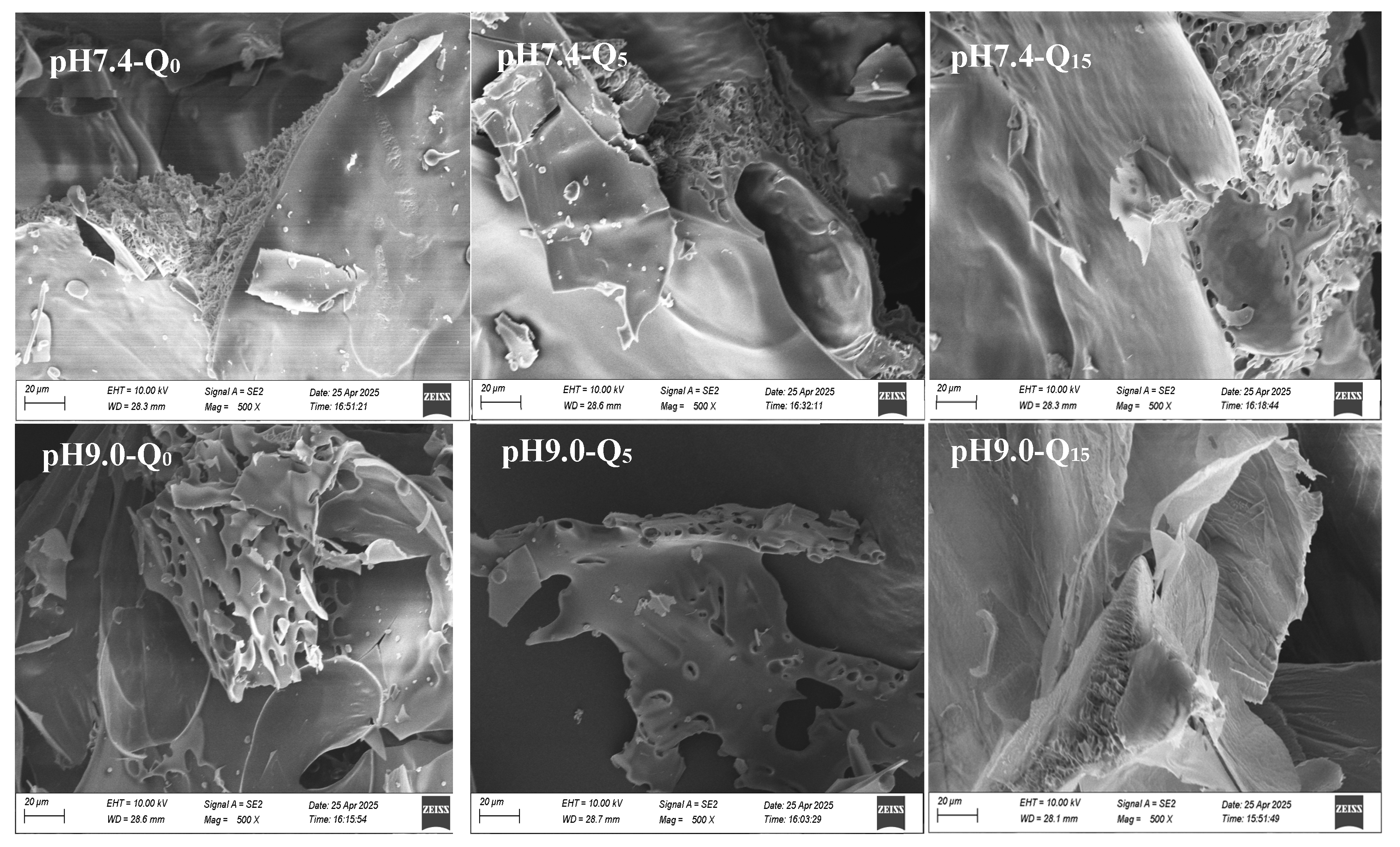
3.10. Scanning Electron Microscopy
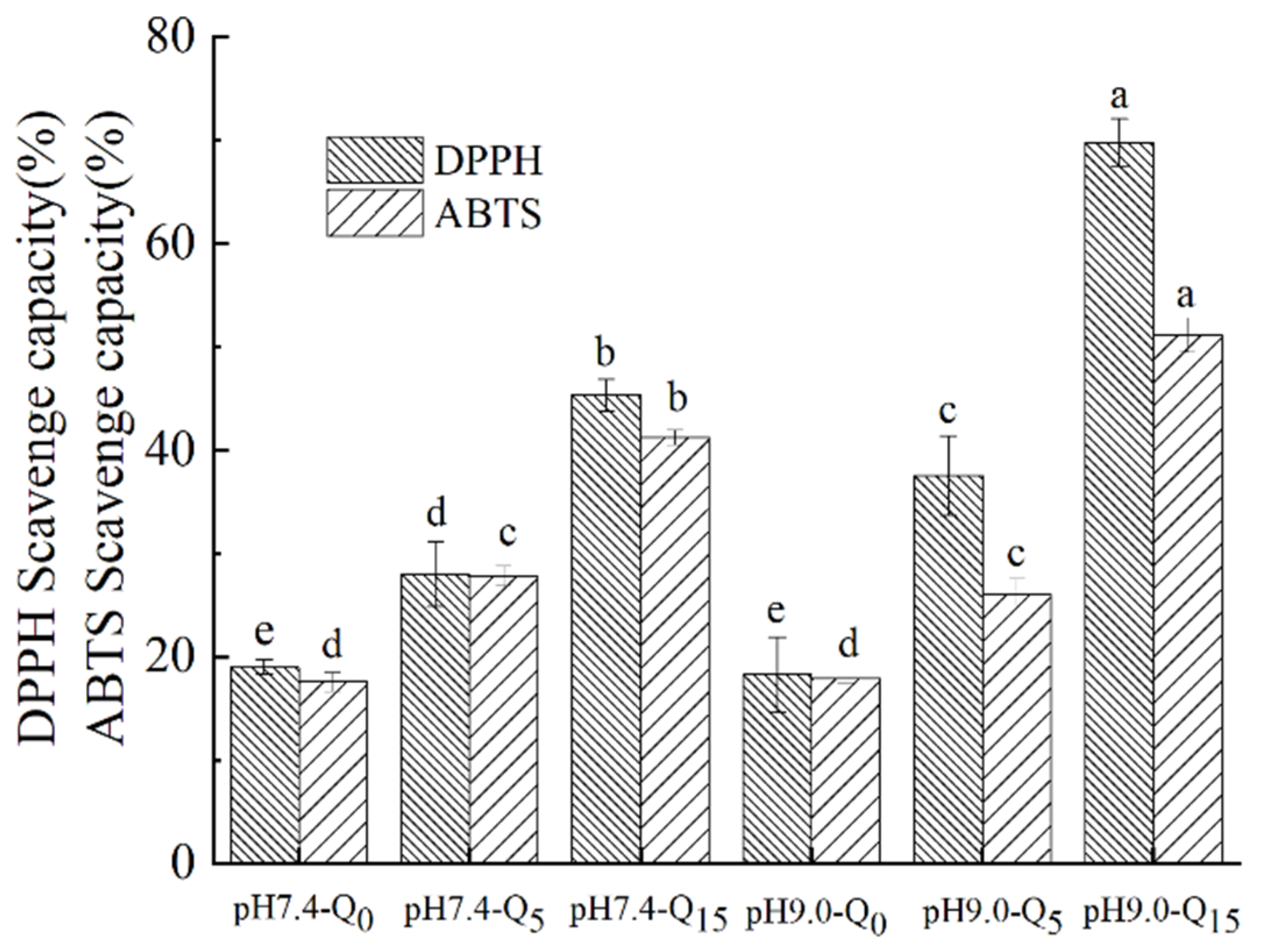
3.11. Antioxidant Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jian, X.; Shi, C.; Luo, W.; Zhou, L.; Jiang, L.; Liu, K. Therapeutic effects and molecular mechanisms of quercetin in gynecological disorders. Biomed. Pharmacother. 2024, 173, 116418. [Google Scholar] [CrossRef]
- Xu, S.-Q.; Liu, H.-X.; Yan, J.-N.; Wang, C.; Lai, B.; Wu, H.-T. Interaction mechanism and binding mode between different polyphenols and gellan gum. Food Hydrocoll. 2024, 153, 110014. [Google Scholar] [CrossRef]
- Li, H.; Wang, D.; Liu, C.; Zhu, J.; Fan, M.; Sun, X.; Wang, T.; Xu, Y.; Cao, Y. Fabrication of stable zein nanoparticles coated with soluble soybean polysaccharide for encapsulation of quercetin. Food Hydrocoll. 2019, 87, 342–351. [Google Scholar] [CrossRef]
- Gong, J.; Wang, H.; Xie, C.; Dai, Y.; Wang, Y.; Guo, W. A multifunctional injectable hydrogel for boosted diabetic wound healing assisted by Quercetin-ZIF system. Chem. Eng. J. 2024, 495, 153425. [Google Scholar] [CrossRef]
- Sadeghi, S.; Madadlou, A.; Yarmand, M. Microemulsification–cold gelation of whey proteins for nanoencapsulation of date palm pit extract. Food Hydrocoll. 2014, 35, 590–596. [Google Scholar] [CrossRef]
- Falsafi, S.R.; Karaca, A.C.; Deng, L.; Wang, Y.; Li, H.; Askari, G.; Rostamabadi, H. Insights into whey protein-based carriers for targeted delivery and controlled release of bioactive components. Food Hydrocoll. 2022, 133, 108002. [Google Scholar] [CrossRef]
- Zang, Z.; Chou, S.; Geng, L.; Si, X.; Ding, Y.; Lang, Y.; Cui, H.; Gao, N.; Chen, Y.; Wang, M.; et al. Interactions of blueberry anthocyanins with whey protein isolate and bovine serum protein: Color stability, antioxidant activity, in vitro simulation, and protein functionality. LWT-Food Sci. Technol. 2021, 152, 112269. [Google Scholar] [CrossRef]
- Ji, W.; Yang, F.; Yang, M. Effect of change in pH, heat and ultrasound pre-treatments on binding interactions between quercetin and whey protein concentrate. Food Chem. 2022, 384, 132508. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, P.; Ban, Q. Green development strategy for efficient quercetin- loaded whey protein complex: Focus on quercetin loading characteristics, component interactions, stability, antioxidant, and in vitro digestive properties. Food Chem. 2025, 472, 142939. [Google Scholar] [CrossRef]
- Baba, W.N.; Abdelrahman, R.; Maqsood, S. Conjoint application of ultrasonication and redox pair mediated free radical method enhances the functional and bioactive properties of camel whey-quercetin conjugates. Ultrason. Sonochemistry 2021, 79, 105784. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Xu, J.; Zhang, X.; Xie, F.; Zhang, S.; Jiang, L.; Qi, B.; Li, Y. Effect of pH-shifting treatment on the structural and functional properties of soybean protein isolate and its interactions with (–)-epigallocatechin-3-gallate. Process Biochem. 2021, 101, 190–198. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Zhang, J.; Zhang, L. Interaction between whey protein isolate and rose anthocyanin extracts at different pHs: Structure, emulsification and digestibility of complexes. Food Biosci. 2022, 49, 101888. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, L.; Niu, X.; Zhang, Y.; Yang, X.; Li, L. Soy protein-gellan gum noncovalent complexes stabilized emulsion: Effect of heating and pH on emulsion stability. Int. J. Biol. Macromol. 2025, 301, 140067. [Google Scholar] [CrossRef]
- Miwa, N.; Yokoyama, K.; Nio, N.; Sonomoto, K. Effect of Enzymatic Deamidation on the Heat-induced Conformational Changes in Whey Protein Isolate and Its Relation to Gel Properties. J. Agric. Food Chem. 2013, 61, 2205–2212. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, J.; Zou, Y.; Mao, X. The enhanced affinity of moderately hydrolyzed whey protein to EGCG promotes the isoelectric separation and unlocks the protective effects on polyphenols. Food Chem. 2024, 450, 138833. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, N.; Zhang, F.; Zhuo, K.; Zhu, G. Improving stability and bioavailability of ACNs based on Gellan gum-whey protein isolate nanocomplexes. Food Chem. X 2024, 24, 102050. [Google Scholar] [CrossRef]
- Yin, L.; Cao, Y.; Wang, M.; Kong, B.; Liu, Q.; Wang, H.; Wang, H. Zein-quercetin covalent nanoparticles encapsulating oregano essential oil: Improved stability, antioxidant, and antibacterial properties. Process. Biochem. 2025, 149, 248–259. [Google Scholar] [CrossRef]
- Baba, W.; Khan, H.; Nazir, A.; Maqsood, S. Innovative use of camel whey proteins, quercetin, and starch as ternary complexes for emulsion stabilization at the Micro and Nano scale. Food Chem. 2025, 473, 142880. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Dudu, O.E.; Zhang, J.; Wang, Y.; Meng, L.; Wei, W.; Li, X.; Yan, T. Impact of binding interaction modes between whey protein concentrate and quercetin on protein structural and functional characteristics. Food Hydrocoll. 2023, 142, 108787. [Google Scholar] [CrossRef]
- Xue, F.; Li, C.; Adhikari, B. Physicochemical properties of soy protein isolates-cyanidin-3-galactoside conjugates produced using free radicals induced by ultrasound. Ultrason. Sonochemistry 2020, 64, 104990. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sun, Y.; Cheng, J.; Zhang, X.; Guo, M. Changes in conformation and functionality of whey proteins induced by the interactions with soy isoflavones. LWT-Food Sci. Technol. 2022, 163, 113555. [Google Scholar] [CrossRef]
- Sun, Z.; Li, D.; Lin, P.; Zhao, Y.; Zhang, J.; Sergeeva, I.; Li, Y.; Zheng, H. Preparation, characterization, and binding mechanism of quercetin-loaded composite nanoparticles based on zein-soybean protein isolate. Food Chem. 2025, 463, 141359. [Google Scholar] [CrossRef]
- Gao, J.; Liang, H.; Zhao, J.; Dai, Y.; Wan, C.; Zhou, B. Interactions between zein and polyphenols and characterization of their complexes. Food Sci. 2022, 43, 8–17. [Google Scholar] [CrossRef]
- Jia, Z.; Zheng, M.; Tao, F.; Chen, W.; Huang, G.; Jiang, J. Effect of covalent modification by (−)-epigallocatechin-3-gallate on physicochemical and functional properties of whey protein isolate. LWT-Food Sci. Technol. 2016, 66, 305–310. [Google Scholar] [CrossRef]
- Yang, J.; Roozalipour, S.P.L.; Berton-Carabin, C.C.; Nikiforidis, C.V.; van der Linden, E.; Sagis, L.M. Air-water interfacial and foaming properties of whey protein-sinapic acid mixtures. Food Hydrocoll. 2021, 112, 106467. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, J.; Zhu, R.; Wen, Y.; Xiong, T.; Peng, F. Non-covalent interaction of Millettia specisoa protein and quercetin: Mechanism and physicochemical property. Food Biosci. 2025, 63, 105711. [Google Scholar] [CrossRef]
- Wu, X.; Lu, Y.; Xu, H.; Lin, D.; He, Z.; Wu, H.; Liu, L.; Wang, Z. Reducing the allergenic capacity of β-lactoglobulin by covalent conjugation with dietary polyphenols. Food Chem. 2018, 256, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, X.; Yong, H.; Kan, J.; Zhang, N.; Jin, C. Preparation, characterization, digestibility and antioxidant activity of quercetin grafted Cynanchum auriculatum starch. Int. J. Biol. Macromol. 2018, 114, 130–136. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Z. A pH-sensitive microemulsion-filled gellan gum hydrogel encapsulated apigenin: Characterization and in vitro release kinetics. Colloids Surfaces B 2019, 178, 245–252. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Y.; Yang, X.-Q. A novel enzyme cross-linked gelation method for preparing food globular protein-based transparent hydrogel. Food Hydrocoll. 2012, 26, 277–285. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, J.; Chen, M.; Feng, L.; Zhu, G. Stabilization effect of ternary covalent complexes with different grafting sequences on blueberry anthocyanins. Food Sci. 2025, 46, 73–79. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, N.; Zhou, X.; Zhou, G.; Zhang, Y.; Yu, G.; Liu, Y.; Li, B.; Zhang, F.; Zhu, G. Whey Protein–Quercetin–Gellan Gum Complexes Prepared Using pH-Shift Treatment: Structural and Functional Properties. Foods 2025, 14, 2720. https://doi.org/10.3390/foods14152720
Guo N, Zhou X, Zhou G, Zhang Y, Yu G, Liu Y, Li B, Zhang F, Zhu G. Whey Protein–Quercetin–Gellan Gum Complexes Prepared Using pH-Shift Treatment: Structural and Functional Properties. Foods. 2025; 14(15):2720. https://doi.org/10.3390/foods14152720
Chicago/Turabian StyleGuo, Na, Xin Zhou, Ganghua Zhou, Yimeng Zhang, Guoqing Yu, Yangliu Liu, Beibei Li, Fangyan Zhang, and Guilan Zhu. 2025. "Whey Protein–Quercetin–Gellan Gum Complexes Prepared Using pH-Shift Treatment: Structural and Functional Properties" Foods 14, no. 15: 2720. https://doi.org/10.3390/foods14152720
APA StyleGuo, N., Zhou, X., Zhou, G., Zhang, Y., Yu, G., Liu, Y., Li, B., Zhang, F., & Zhu, G. (2025). Whey Protein–Quercetin–Gellan Gum Complexes Prepared Using pH-Shift Treatment: Structural and Functional Properties. Foods, 14(15), 2720. https://doi.org/10.3390/foods14152720




