Enhanced Antioxidant and Antiproliferative Activities of Apple and Korean Green Chili Pepper Extracts Cultivated with Mineral Supplementation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- •
- Standard Organic Cultivation (Control: Organic): Crops were grown using compost and certified organic soil amendments, with no additional mineral supplementation.
- •
- Mineral-Supplemented Organic Cultivation (Treatment: DSWM): In addition to organic inputs, crops were treated with deep sea water minerals (DSWM, iCOOP Natural Dream Cooperative, Gurye, Jeollanam-do, Republic of Korea) containing 27 ppm nitrogen (N), 10.8 ppm phosphorus (P), 11.59 ppm potassium (K), 57 ppm magnesium (Mg), 9.18 ppm sodium (Na), 0.86 ppm zinc (Zn), 0.02 ppm manganese (Mn), 1.17 ppm copper (Cu), 0.63 ppm molybdenum (Mo), and 0.02 ppm selenium (Se).
2.2. Preparation of Extracts
2.3. Determination of Total Phenolic and Flavonoid Contents
2.3.1. Total Phenolic Content (TPC)
2.3.2. Total Flavonoid Content (TFC)
2.4. Antioxidant Activity Assays
2.4.1. DPPH Radical Scavenging Assay
2.4.2. ABTS Radical Cation Scavenging Assay
2.5. Nrf2-Mediated Antioxidant Enzyme Assay Using HCT116-ARE Reporter Cells
2.5.1. Cell Culture and Treatment
2.5.2. Reporter Assay
2.6. ROS Scavenging Activity Assay
2.7. Protein Expression Analysis of Antioxidant Enzymes
2.8. Antiproliferative Activity Assay
2.8.1. Cell Culture and Treatment
2.8.2. CCK-8 Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic and Flavonoid Contents
3.1.1. Total Phenolic Contents
3.1.2. Total Flavonoid Contents
3.2. Antioxidant Activity
DPPH and ABTS Radical Scavenging Activity
3.3. Nrf2-Mediated Antioxidant Enzyme Activation
3.3.1. ARE Reporter Induction Activity
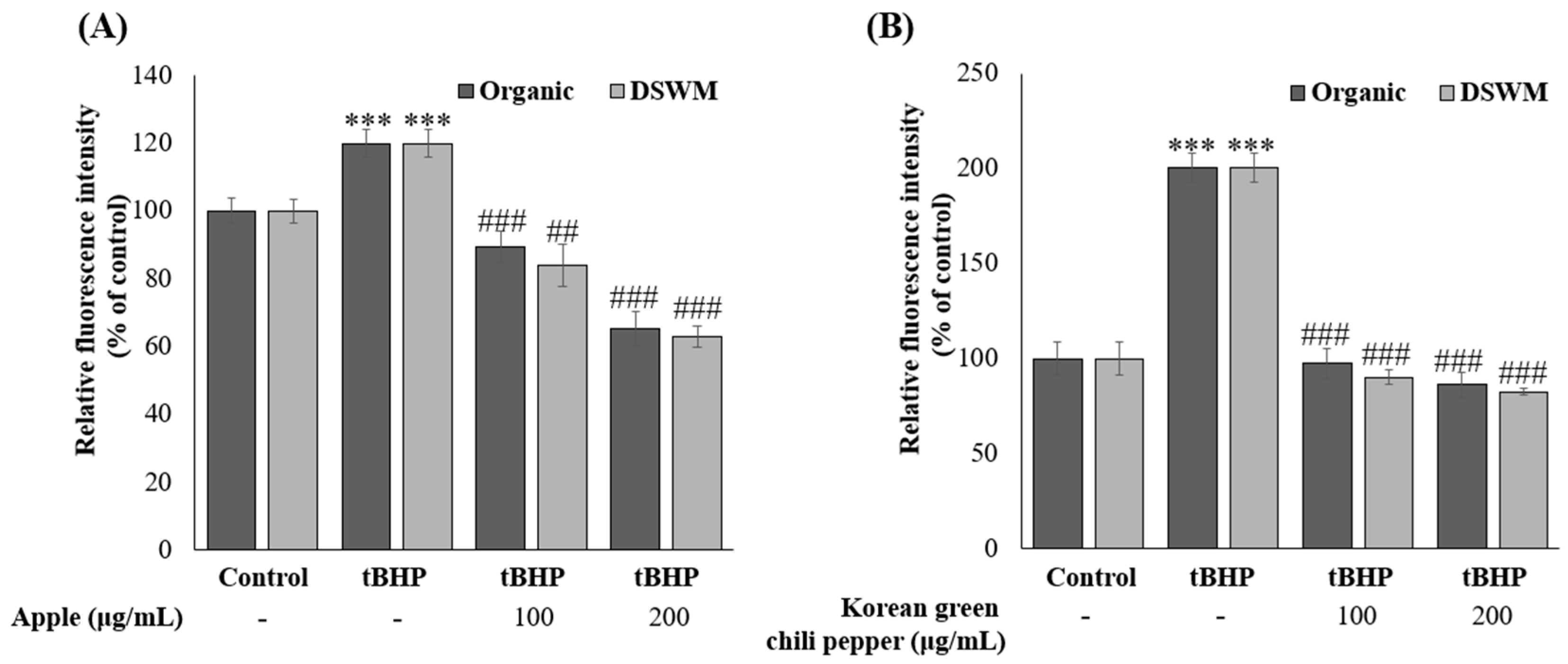
3.3.2. Intracellular ROS Measurement
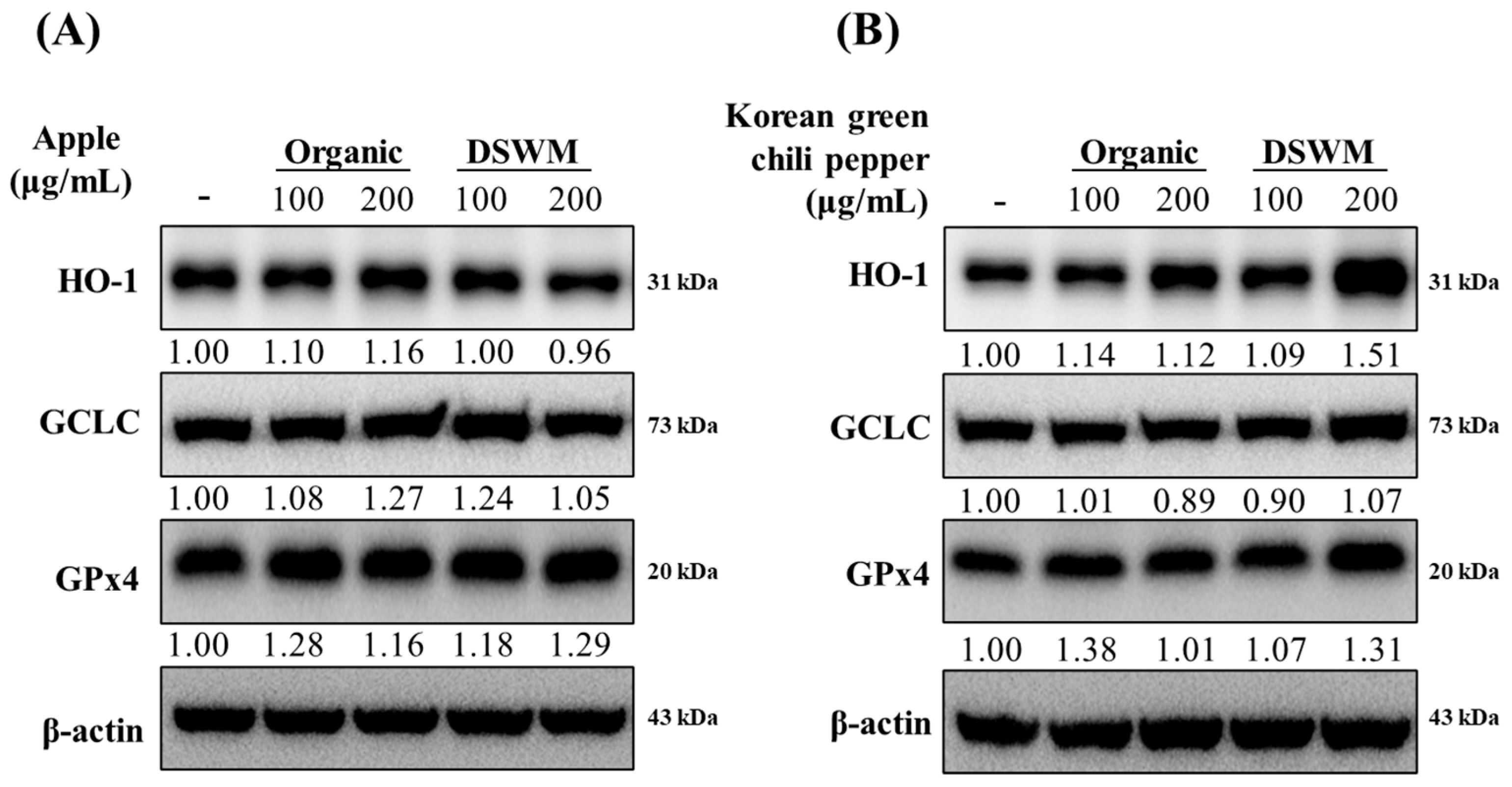
3.3.3. Expression of Antioxidant Enzyme Proteins
3.4. Antiproliferative Effects in Human Colorectal Cancer Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bourn, D.; Prescott, J. A comparison of the nutritional value, sensory qualities, and food safety of organically and conventionally produced foods. Crit. Rev. Food Sci. 2002, 42, 1–34. [Google Scholar] [CrossRef]
- Brandt, K.; Molgaard, J.P. Organic agriculture: Does it enhance or reduce the nutritional value of plant foods? J. Sci. Food Agr. 2001, 81, 924–931. [Google Scholar] [CrossRef]
- Soltoft, M.; Nielsen, J.; Holst Laursen, K.; Husted, S.; Halekoh, U.; Knuthsen, P. Effects of organic and conventional growth systems on the content of flavonoids in onions and phenolic acids in carrots and potatoes. J. Agric. Food Chem. 2010, 58, 10323–10329. [Google Scholar] [CrossRef]
- Fanasca, S.; Colla, G.; Maiani, G.; Venneria, E.; Rouphael, Y.; Azzini, E.; Saccardo, F. Changes in antioxidant content of tomato fruits in response to cultivar and nutrient solution composition. J. Agr. Food Chem. 2006, 54, 4319–4325. [Google Scholar] [CrossRef]
- Vanessa, B.G.; Alice, T.I.; William, D.A.; Adelaide, D.M.; Gisèle, L.E.; Condurache, N.N.; Milea, S.A.; Cotarlet, M.; Fabrice, D.D.; Inocent, G.; et al. Effect of fertilizers on yield, phytochemical, and antioxidant properties of fruits. Food Sci. Nutr. 2024, 12, 6742–6751. [Google Scholar] [CrossRef]
- Bloem, E.; Haneklaus, S.; Schnug, E. Influence of fertilizer practices on S-containing metabolites in garlic (Allium sativum L.) under field conditions. J. Agric. Food Chem. 2010, 58, 10690–10696. [Google Scholar] [CrossRef]
- Barrameda-Medina, Y.; Blasco, B.; Lentini, M.; Esposito, S.; Baenas, N.; Moreno, D.A.; Ruiz, J.M. Zinc biofortification improves phytochemicals and amino-acidic profile in cv. Bronco. Plant Sci. 2017, 258, 45–51. [Google Scholar] [CrossRef]
- Hassan, S.A.; Mijin, S.; Yusoff, U.K.; Ding, P.; Wahab, P.E.M. Nitrate, Ascorbic Acid, Mineral and Antioxidant Activities of in Response to Organic and Mineral-Based Fertilizer Rates. Molecules 2012, 17, 7843–7853. [Google Scholar] [CrossRef]
- Rahman, A.; Harker, T.; Lewis, W.; Islam, K.R. Nano and chelated iron fertilization influences marketable yield, phytochemical properties, and antioxidant capacity of tomatoes. PLoS ONE 2023, 18, e0294033. [Google Scholar] [CrossRef]
- Rahman, M.H.; Hasan, M.N.; Khan, M.Z.H. Study on different nano fertilizers influencing the growth, proximate composition and antioxidant properties of strawberry fruits. J. Agric. Food Res. 2021, 6, 100246. [Google Scholar] [CrossRef]
- Garza-Alonso, C.A.; Juárez-Maldonado, A.; González-Morales, S.; de la Fuente, M.C.; Cadenas-Pliego, G.; Morales-Díaz, A.B.; Trejo-Téllez, L.I.; Tortella, G.; Benavides-Mendoza, A. ZnO nanoparticles as potential fertilizer and biostimulant for lettuce. Heliyon 2023, 9, e12787. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Houldsworth, A. Role of oxidative stress in neurodegenerative disorders: A review of reactive oxygen species and prevention by antioxidants. Brain Commun. 2024, 6, fcad356. [Google Scholar] [CrossRef]
- Ranneh, Y.; Ali, F.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A. Crosstalk between reactive oxygen species and pro-inflammatory markers in developing various chronic diseases: A review. Appl. Biol. Chem. 2017, 60, 327–338. [Google Scholar] [CrossRef]
- de Vries, H.E.; Witte, M.; Hondius, D.; Rozemuller, A.J.; Drukarch, B.; Hoozemans, J.; van Horssen, J. Nrf2-induced antioxidant protection: A promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic. Biol. Med. 2008, 45, 1375–1383. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V.; Kazantsev, A.G. The role of Nrf2 signaling in counteracting neurodegenerative diseases. FEBS J. 2018, 285, 3576–3590. [Google Scholar] [CrossRef]
- Tu, W.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The Anti-Inflammatory and Anti-Oxidant Mechanisms of the Keap1/Nrf2/ARE Signaling Pathway in Chronic Diseases. Aging Dis. 2019, 10, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L. Citation Classic-Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Cc/Agr. Biol. Environ. 1985, 48, 18. [Google Scholar]
- Zhang, Q.; Zhang, J.Z.; Shen, J.K.; Silva, A.; Dennis, D.A.; Barrow, C.J. A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Sari, K.R.P.; Ikawati, Z.; Danarti, R.; Hertiani, T. Micro-titer plate assay for measurement of total phenolic and total flavonoid contents in medicinal plant extracts. Arab. J. Chem. 2023, 16, 105003. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free-Radical Method to Evaluate Antioxidant Activity. Food Sci. Technol-Leb. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Bio. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.H.; Moon, N.; Oh, J.; Kim, J.S. Luteolin Shifts Oxaliplatin-Induced Cell Cycle Arrest at G/G to Apoptosis in HCT116 Human Colorectal Carcinoma Cells. Nutrients 2019, 11, 770. [Google Scholar] [CrossRef]
- Jang, C.H.; Moon, N.; Lee, J.; Kwon, M.J.; Oh, J.; Kim, J.S. Luteolin Synergistically Enhances Antitumor Activity of Oxaliplatin in Colorectal Carcinoma via AMPK Inhibition. Antioxidants 2022, 11, 626. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R.; Yang, R.; Xie, S.; Sockovie, E.; Khanizadeh, S. Which Polyphenolic Compounds Contribute to the Total Antioxidant Activities of Apple? J. Agric. Food chemistry 2005, 53, 7. [Google Scholar] [CrossRef]
- Shaha, R.K.; Rahman, S.; Asrul, A. Bioactive compounds in chilli peppers (Capsicum annuum L.) at various ripening (green, yellow and red) stages. Ann. Biol. Res. 2013, 4, 27–34. [Google Scholar]
- Khatun, M.N.; Kowser, J.; Wazed, M.A.; Asaduzzaman, M. Effect of different drying parameters on green chili nutritional quality. Eur. J. Agric. Food Sci. 2022, 4, 5. [Google Scholar] [CrossRef]
- D’Abrosca, B.S.P.; Cefarelli, G.; Mastellone, C.; Fiorentino, A. ‘Limoncella’ apple, an Italian apple cultivar: Phenolic and flavonoid contents and antioxidant activity. Food Chem. 2007, 104, 1333–1337. [Google Scholar] [CrossRef]
- Youn, S.J.; Rhee, J.K.; Lee, H. Comparison of total phenolics, total flavonoids contents, and antioxidant capacities of an Apple Cultivar (Malus domestica cv. Fuji) peel powder prepared by different powdering methods. Food Eng. Prog. 2017, 21, 326–331. [Google Scholar] [CrossRef]
- Ab Rahman, M.H.; Jusoh, Y.M.M.; Hashim, Z. Total Phenolic, Total Flavonoid and Antioxidant Activity of Capsicum annuum Seed and Pericarp Extracts. J. Bioprocess. Biomass Technol. 2024, 3, 107–111. [Google Scholar] [CrossRef]
- Ribes-Moya, A.M.; Adalid, A.M.; Raigon, M.D.; Hellin, P.; Fita, A.; Rodriguez-Burruezo, A. Variation in flavonoids in a collection of peppers (Capsicum sp.) under organic and conventional cultivation: Effect of the genotype, ripening stage, and growing system. J. Sci. Food Agric. 2020, 100, 2208–2223. [Google Scholar] [CrossRef]
- Orsavova, J.; Jurikova, T.; Bednarikova, R.; Mlcek, J. Total Phenolic and Total Flavonoid Content, Individual Phenolic Compounds and Antioxidant Activity in Sweet Rowanberry Cultivars. Antioxidants 2023, 12, 913. [Google Scholar] [CrossRef]
- Chen, Q.R.; Wang, X.X.; Yuan, X.L.; Shi, J.; Zhang, C.S.; Yan, N.; Jing, C.L. Comparison of Phenolic and Flavonoid Compound Profiles and Antioxidant and α-Glucosidase Inhibition Properties of Cultivated Soybean and Wild Soybean. Plants 2021, 10, 813. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Eisner, P. How Does the Phenol Structure Influence the Results of the Folin-Ciocalteu Assay? Antioxidants 2021, 10, 811. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Kschonsek, J.; Wolfram, T.; Stockl, A.; Bohm, V. Polyphenolic Compounds Analysis of Old and New Apple Cultivars and Contribution of Polyphenolic Profile to the In Vitro Antioxidant Capacity. Antioxidants 2018, 7, 20. [Google Scholar] [CrossRef]
- Thuphairo, K.; Sornchan, P.; Suttisansanee, U. Bioactive Compounds, Antioxidant Activity and Inhibition of Key Enzymes Relevant to Alzheimer’s Disease from Sweet Pepper (Capsicum annuum) Extracts. Prev. Nutr. Food Sci. 2019, 24, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, R.; Aires, A.; Rodrigues, N.; Peres, A.M.; Pereira, J.A. Phenolics and Antioxidant Activity of Green and Red Sweet Peppers from Organic and Conventional Agriculture: A Comparative Study. Agriculture 2020, 10, 652. [Google Scholar] [CrossRef]
- Hernández-Carranza, P.; Ávila-Sosa, R.; Guerrero-Beltrán, J.A.; Navarro-Cruz, A.R.; Corona-Jiménez, E.; Ochoa-Velasco, C.E. Optimization of antioxidant compounds extraction from fruit by-products: Apple pomace, orange and banana peel. J. Food Process. Preserv. 2016, 13, 103–115. [Google Scholar] [CrossRef]
- Ghasemnezhad, M.; Sherafati, M.; Payvast, G.A. Variation in phenolic compounds, ascorbic acid and antioxidant activity of five coloured bell pepper (Capsicum annum) fruits at two different harvest times. J. Funct. Foods 2011, 3, 44–49. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environ. Sci. Pollut. Res. 2022, 29, 81112–81129. [Google Scholar] [CrossRef]
- Oli, P.; Rawat, P.; Punetha, S.; Shukla, S. Antioxidant activities and biochemical analysis of Capsicum annuum L. varieties at different ripening stages. Curr. Sci. 2025, 128, 999. [Google Scholar] [CrossRef]
- Atia, A.; Abdullah, A. Review of Nrf2-Regulated Genes Induced in Response to Antioxidants. Int. J. Med. Res. Health 2014, 3, 428–435. [Google Scholar] [CrossRef]
- Zhang, M.; An, C.; Gao, Y.; Leak, R.K.; Chen, J.; Zhang, F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 2013, 100, 30–47. [Google Scholar] [CrossRef]
- Jaiswal, A.K. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol. Med. 2004, 36, 1199–1207. [Google Scholar] [CrossRef]
- Pouremamali, F.; Pouremamali, A.; Dadashpour, M.; Soozangar, N.; Jeddi, F. An update of Nrf2 activators and inhibitors in cancer prevention/promotion. Cell Commun. Signal 2022, 20, 100. [Google Scholar] [CrossRef]
- He, W.J.; Lv, C.H.; Chen, Z.; Shi, M.; Zeng, C.X.; Hou, D.X.; Qin, S. The Regulatory Effect of Phytochemicals on Chronic Diseases by Targeting Nrf2-ARE Signaling Pathway. Antioxidants 2023, 12, 236. [Google Scholar] [CrossRef]
- Luo, M.; Zhou, L.; Huang, Z.; Li, B.; Nice, E.C.; Xu, J.; Huang, C. Antioxidant Therapy in Cancer: Rationale and Progress. Antioxidants 2022, 11, 1128. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Huang, H.; Zhao, H.; Liu, R.; Sun, Z.; Liu, Y.; Chen, N.; Zhang, Z. Edaravone dexborneol protects against cerebral ischemia/reperfusion-induced blood-brain barrier damage by inhibiting ferroptosis via activation of nrf-2/HO-1/GPX4 signaling. Free Radic. Biol. Med. 2024, 217, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Hao, L.; Li, S.; Deng, J.; Yu, F.; Zhang, J.; Nie, A.; Hu, X. The NRF-2/HO-1 Signaling Pathway: A Promising Therapeutic Target for Metabolic Dysfunction-Associated Steatotic Liver Disease. J. Inflamm. Res. 2024, 17, 8061–8083. [Google Scholar] [CrossRef]
- Di Giacomo, C.; Malfa, G.A.; Tomasello, B.; Bianchi, S.; Acquaviva, R. Natural Compounds and Glutathione: Beyond Mere Antioxidants. Antioxidants 2023, 12, 1445. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D. Thirty years of NRF2: Advances and therapeutic challenges. Nat. Rev. Drug Discov. 2025, 24, 421–444. [Google Scholar] [CrossRef] [PubMed]


| Crop | Cultivar | Cultivation Method | Total Phenolics (μg GAE/g Dry wt) † |
|---|---|---|---|
| Apple | Gamhong | Organic (control) | 4554 ± 407 |
| Mineral supplemented | 5779 ± 453 * | ||
| Korean green chili pepper | Sunhangilsang | Organic (control) | 2509 ± 283 |
| Mineral supplemented | 4569 ± 283 * |
| Crop | Cultivar | Cultivation Method | Total Flavonoids (μg QE/g Dry wt) † |
|---|---|---|---|
| Apple | Gamhong | Organic (control) | 6847 ± 643 |
| Mineral supplemented | 11,780 ± 2498 * | ||
| Korean green chili pepper | Sunhangilsang | Organic (control) | 2430 ± 135 |
| Mineral supplemented | 4252 ± 33 * |
| Crop | Cultivation Method | DPPH Radical Scavenging Activity IC50 (mg/mL) | ABTS Radical Scavenging Activity IC50 (mg/mL) |
|---|---|---|---|
| Apple | Organic (control) | 42.0 ± 3.3 | 12.9 ± 2.0 |
| Mineral supplemented | 38.4 ± 4.9 * | 11.4 ± 1.6 | |
| Korean green chili pepper | Organic (control) | 125.7 ± 16.1 | 3.7 ± 0.7 |
| Mineral supplemented | 91.4 ± 14.6 * | 3.7 ± 2.7 | |
| Quercetin | 0.092 ± 0.004 | 0.084 ± 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.-S.; Yu, M.-H.; Choi, D.K.; Kim, H.W.; Park, S.-H.; Sin, S.-I.; Kim, J.-S. Enhanced Antioxidant and Antiproliferative Activities of Apple and Korean Green Chili Pepper Extracts Cultivated with Mineral Supplementation. Foods 2025, 14, 2685. https://doi.org/10.3390/foods14152685
Lim J-S, Yu M-H, Choi DK, Kim HW, Park S-H, Sin S-I, Kim J-S. Enhanced Antioxidant and Antiproliferative Activities of Apple and Korean Green Chili Pepper Extracts Cultivated with Mineral Supplementation. Foods. 2025; 14(15):2685. https://doi.org/10.3390/foods14152685
Chicago/Turabian StyleLim, Ji-Sun, Mi-Hee Yu, Dong Kyu Choi, Hae Won Kim, Seung-Hwan Park, Sin-Il Sin, and Jong-Sang Kim. 2025. "Enhanced Antioxidant and Antiproliferative Activities of Apple and Korean Green Chili Pepper Extracts Cultivated with Mineral Supplementation" Foods 14, no. 15: 2685. https://doi.org/10.3390/foods14152685
APA StyleLim, J.-S., Yu, M.-H., Choi, D. K., Kim, H. W., Park, S.-H., Sin, S.-I., & Kim, J.-S. (2025). Enhanced Antioxidant and Antiproliferative Activities of Apple and Korean Green Chili Pepper Extracts Cultivated with Mineral Supplementation. Foods, 14(15), 2685. https://doi.org/10.3390/foods14152685







