Enhancement of Phytochemicals and Antioxidant Activity of Thai Fermented Soybean Using Box–Behnken Design Guided Microwave-Assisted Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Sample and Thai Fermented Soybean (TFS) Preparation
2.3. Crude TFS Extract Preparation by Conventional Extraction
2.4. Quantification of Phenolic and Isoflavone Derivative Contents Using HPLC Analysis
2.5. Cells and Cell Culture
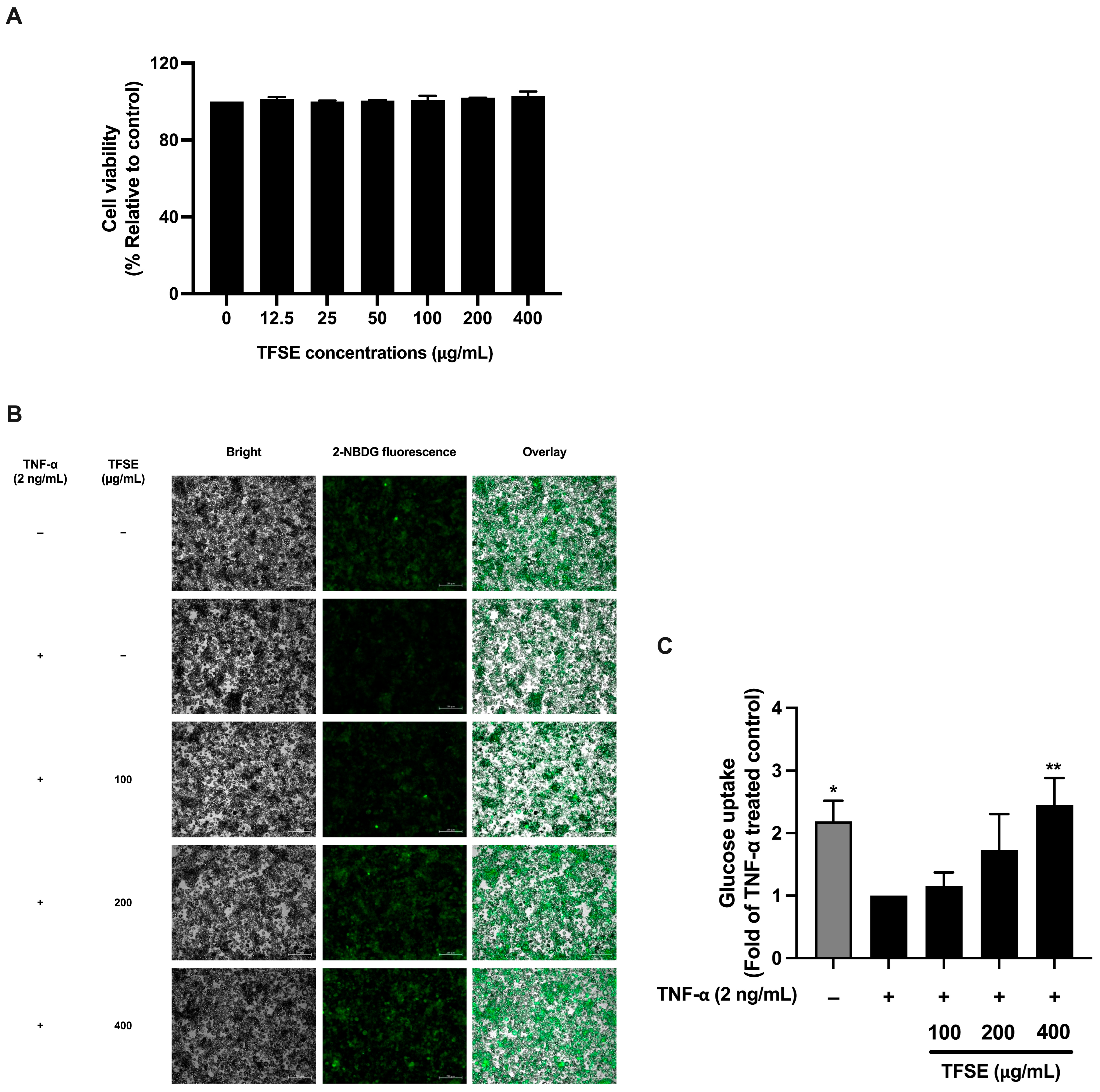
2.6. Cytotoxicity Testing
2.7. Determination of Anti-Insulin Resistance Activity Using Cellular Glucose Uptake Assay
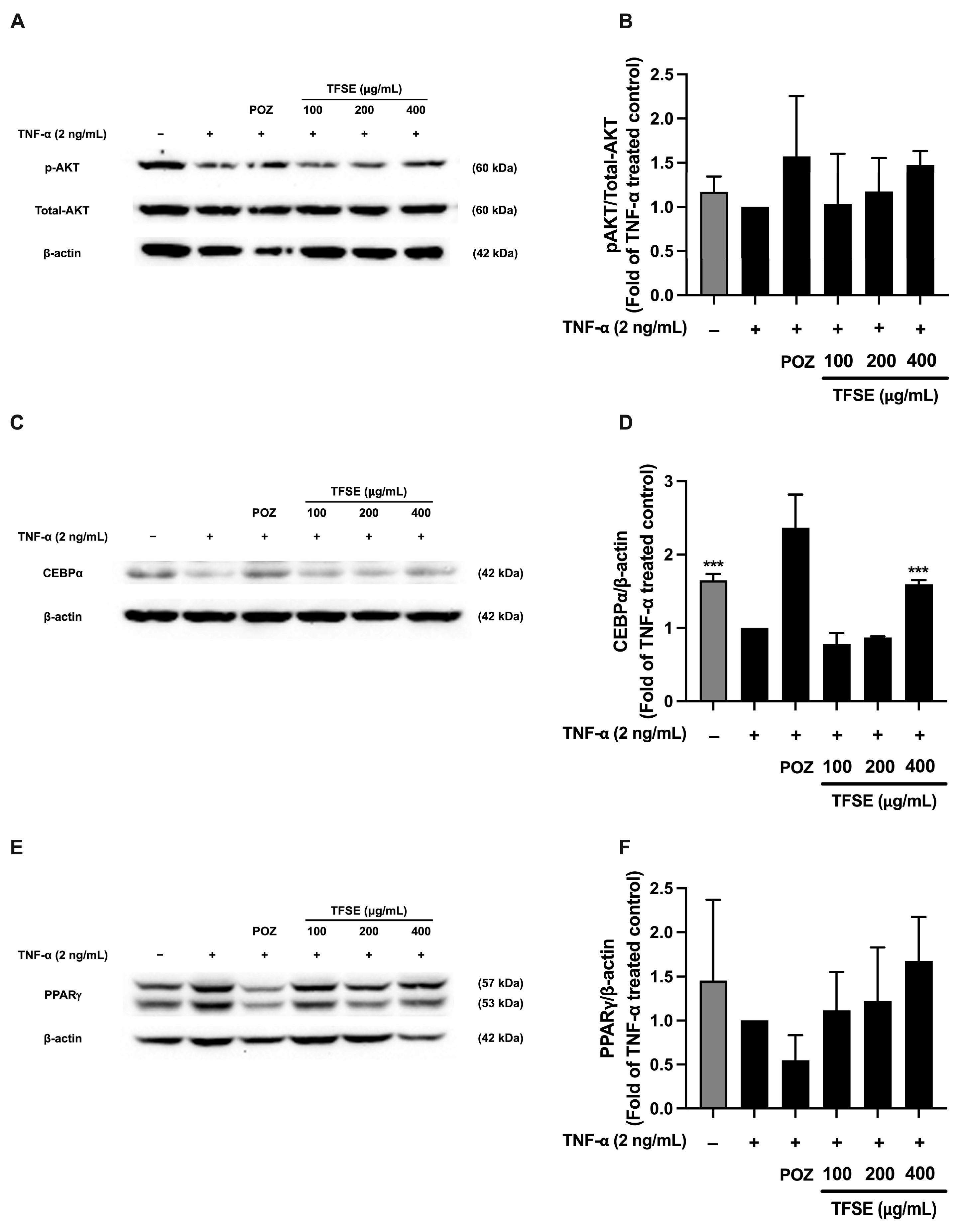
2.8. Measurement of Phosphorylation of AKT and Expression of C/EBPα and PPARγ via Western Blotting
2.9. Box–Behnken Design (BBD) and Response Surface Methodology (RSM) for the Optimization of Microwave-Assisted Extraction
2.10. Determination of Total Phenolic Content (TPC), Total Flavonoid Content (TFC), and Antioxidant Activities
2.11. Determination of Enzyme Inhibitory Activity
2.11.1. α-Glucosidase Inhibition Assay
2.11.2. α-Amylase Inhibition Assay
2.12. Metabolomic Profiling of Phytochemicals of TFSE and O-TFSE Using HPLC-qTOF-MS
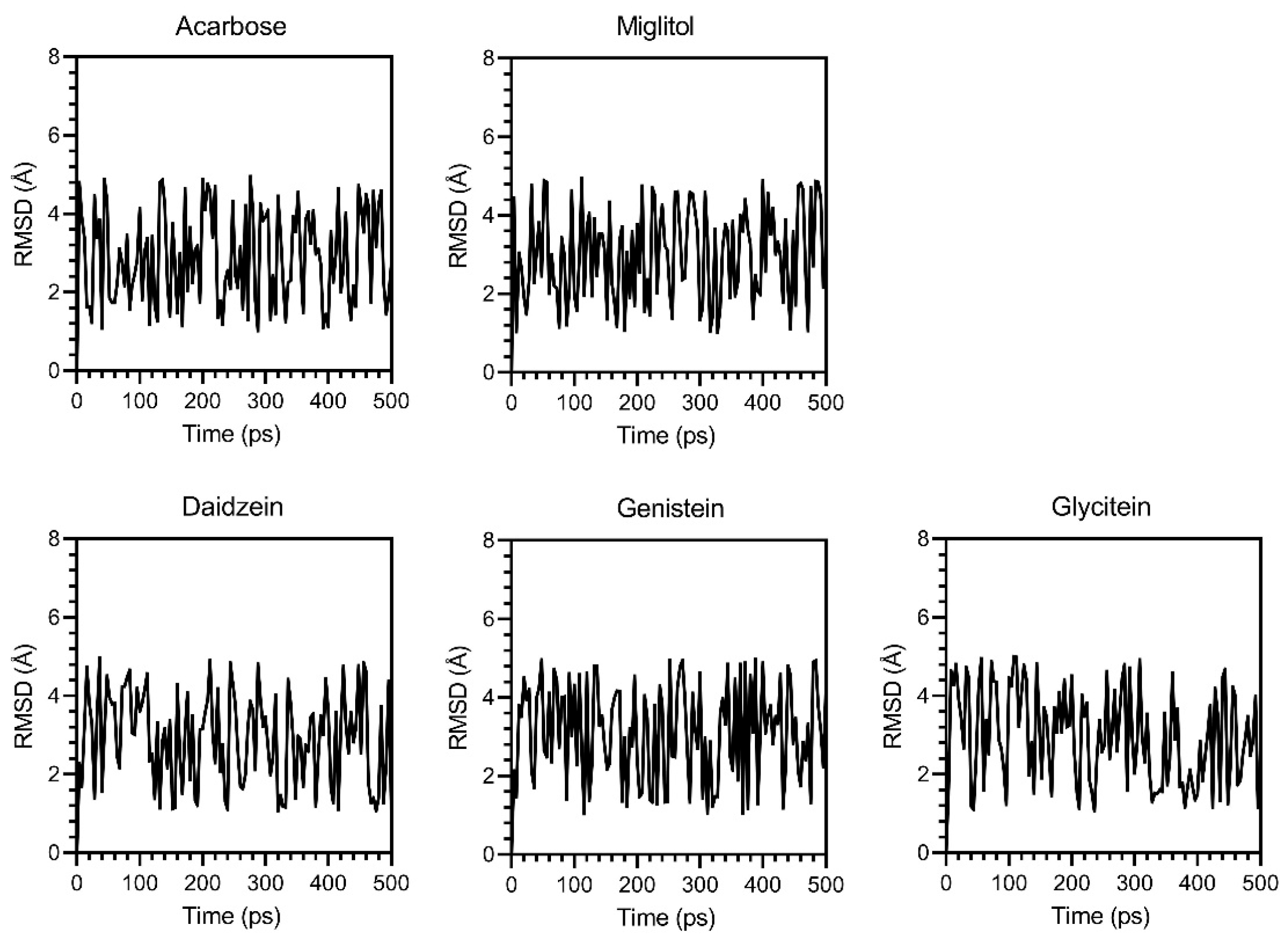
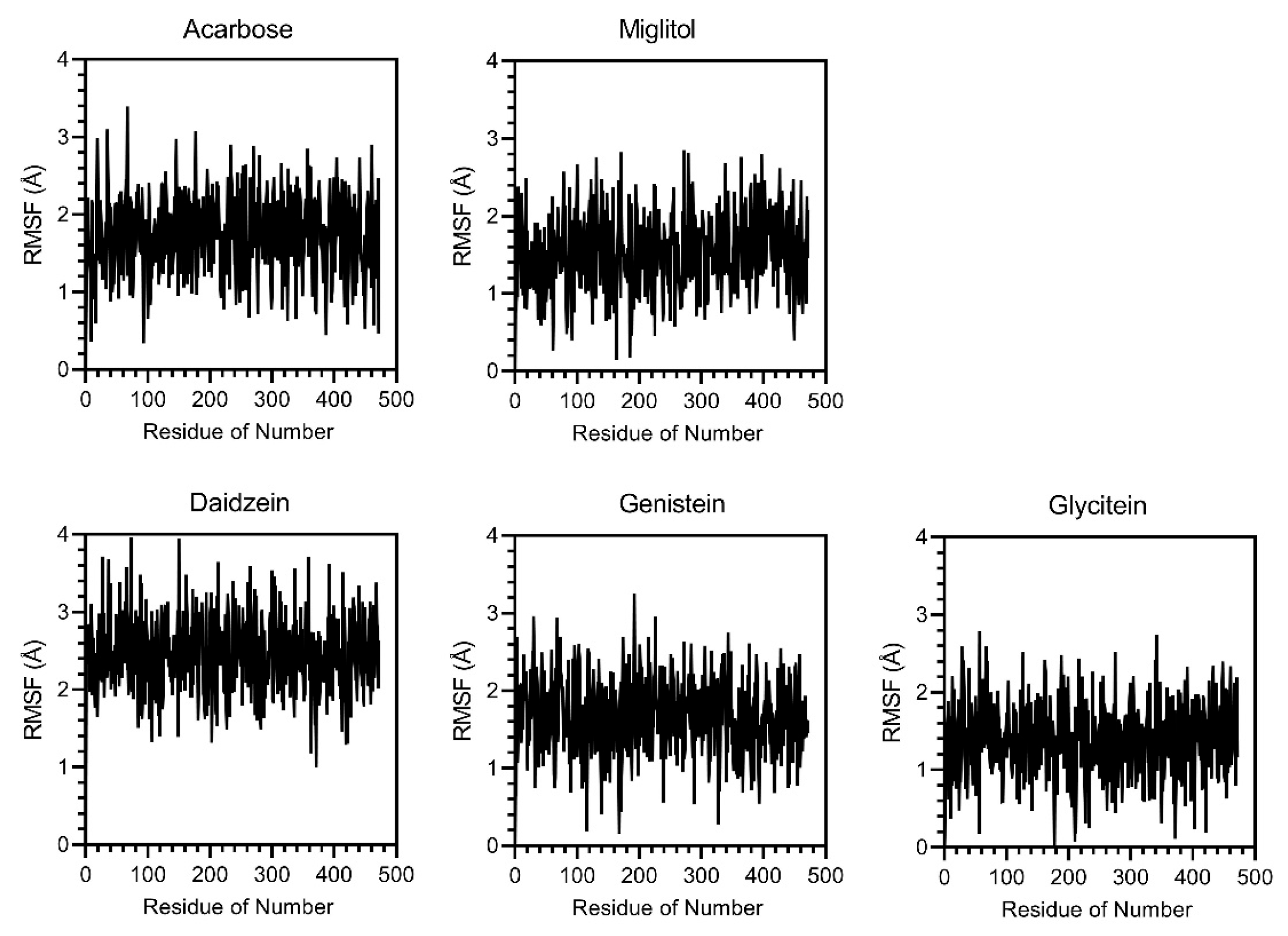
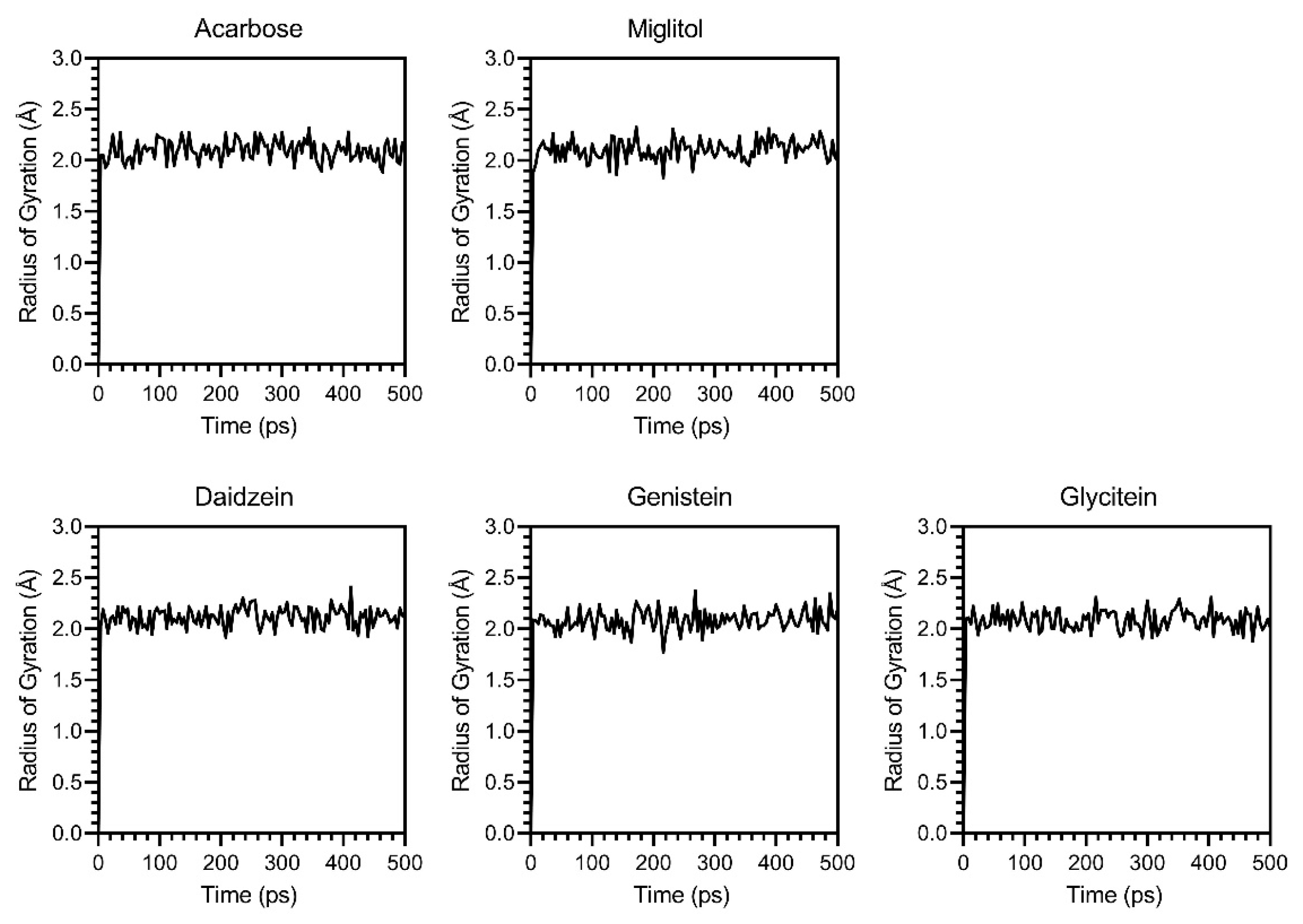
2.13. Molecular Docking and Molecular Dynamics (MD) Simulation
2.14. Statistical Analysis
3. Results
3.1. The Ethanolic Extract of Thai Fermented Soybean (TFSE) Suppresses Inflammation-Induced Insulin Resistance in TNF-α-Treated 3T3-L1 Adipocytes
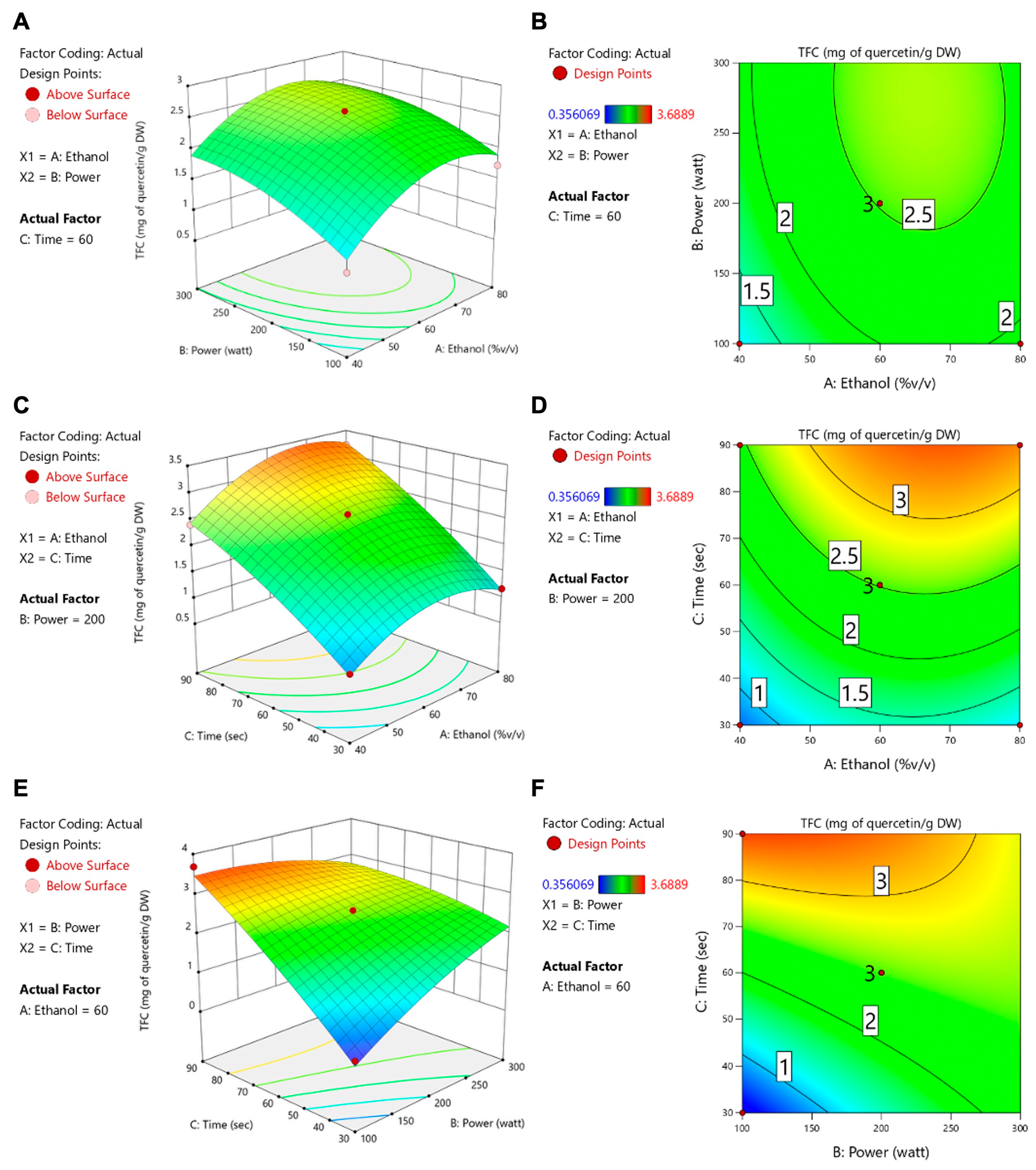
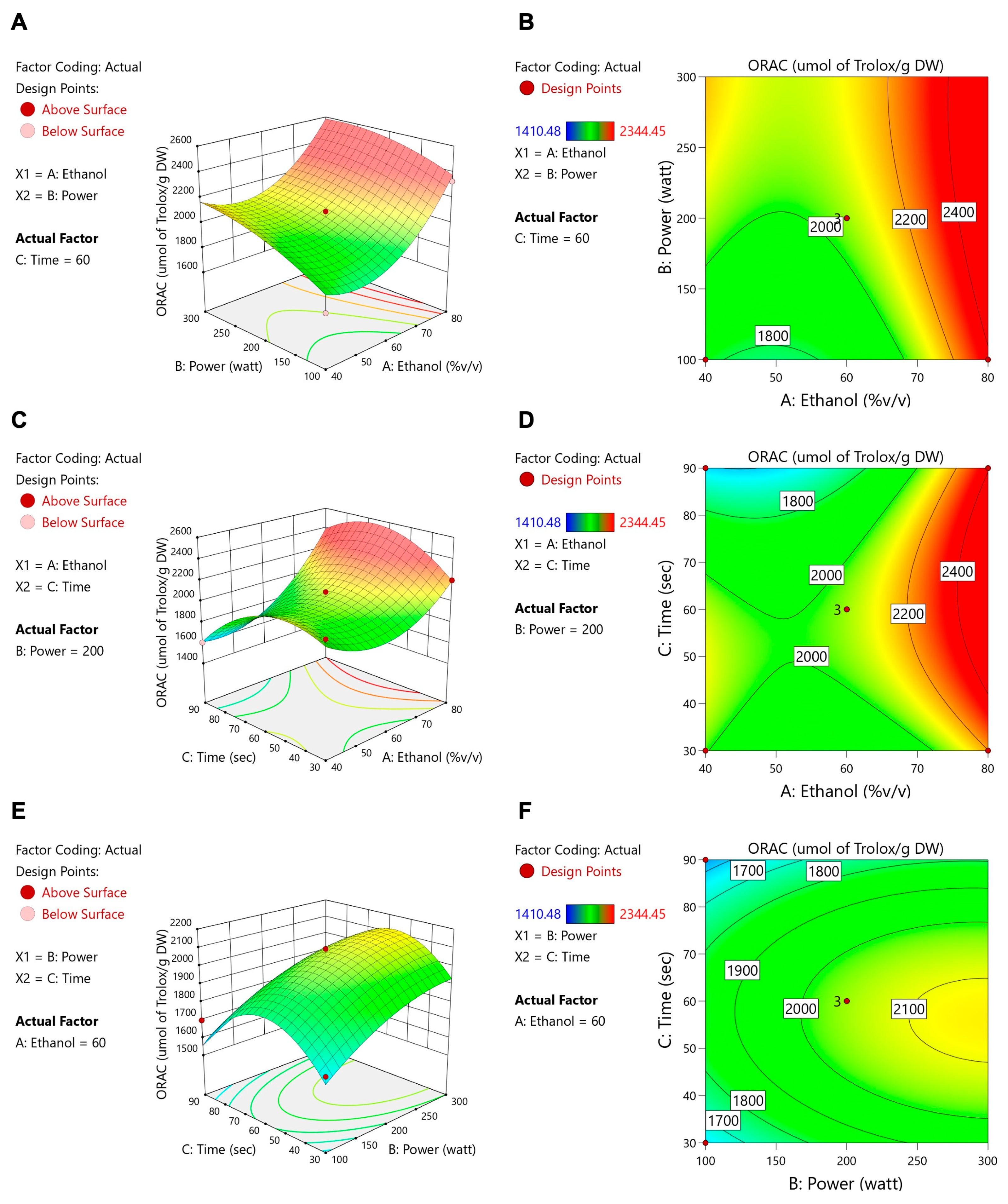
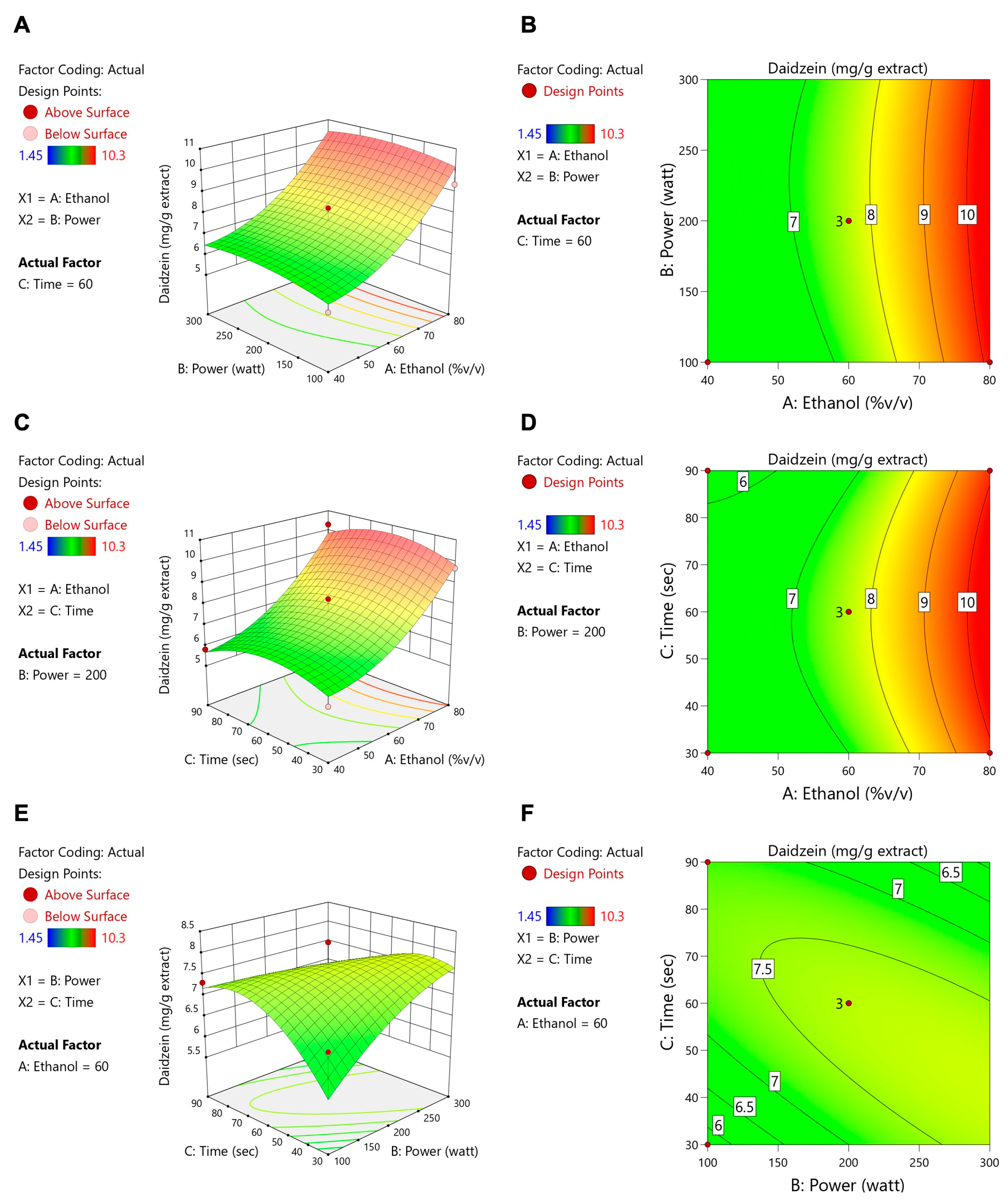
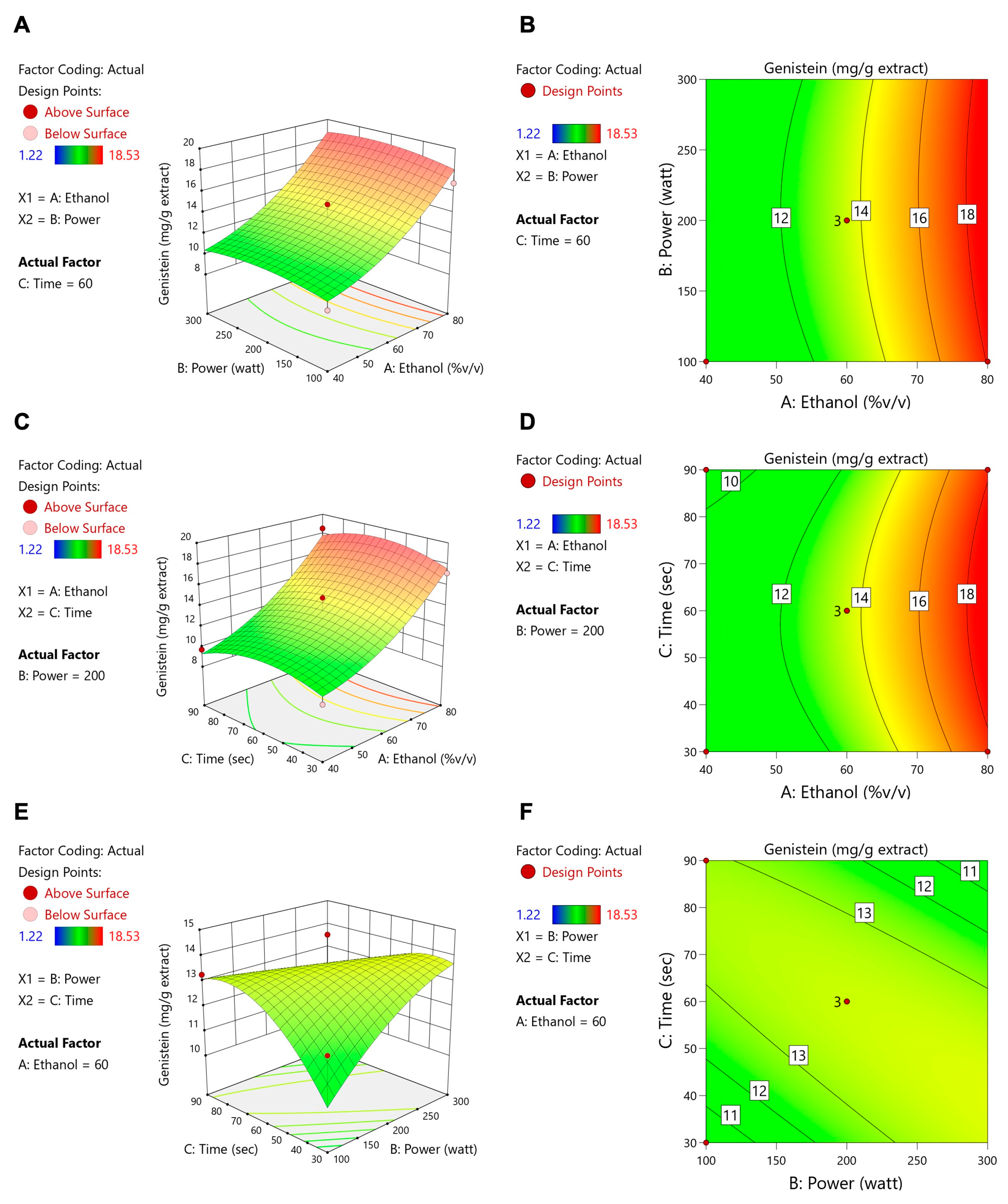
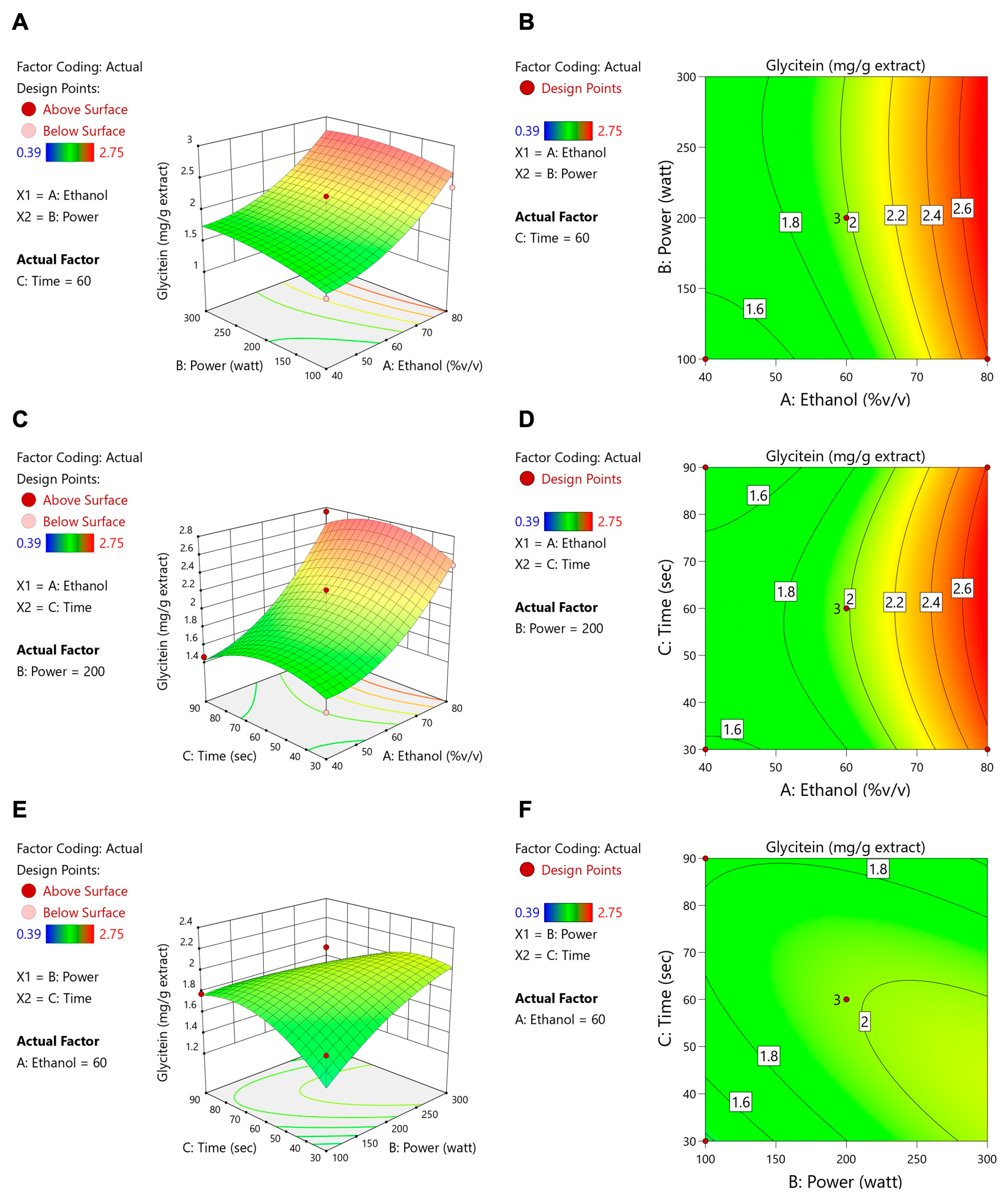
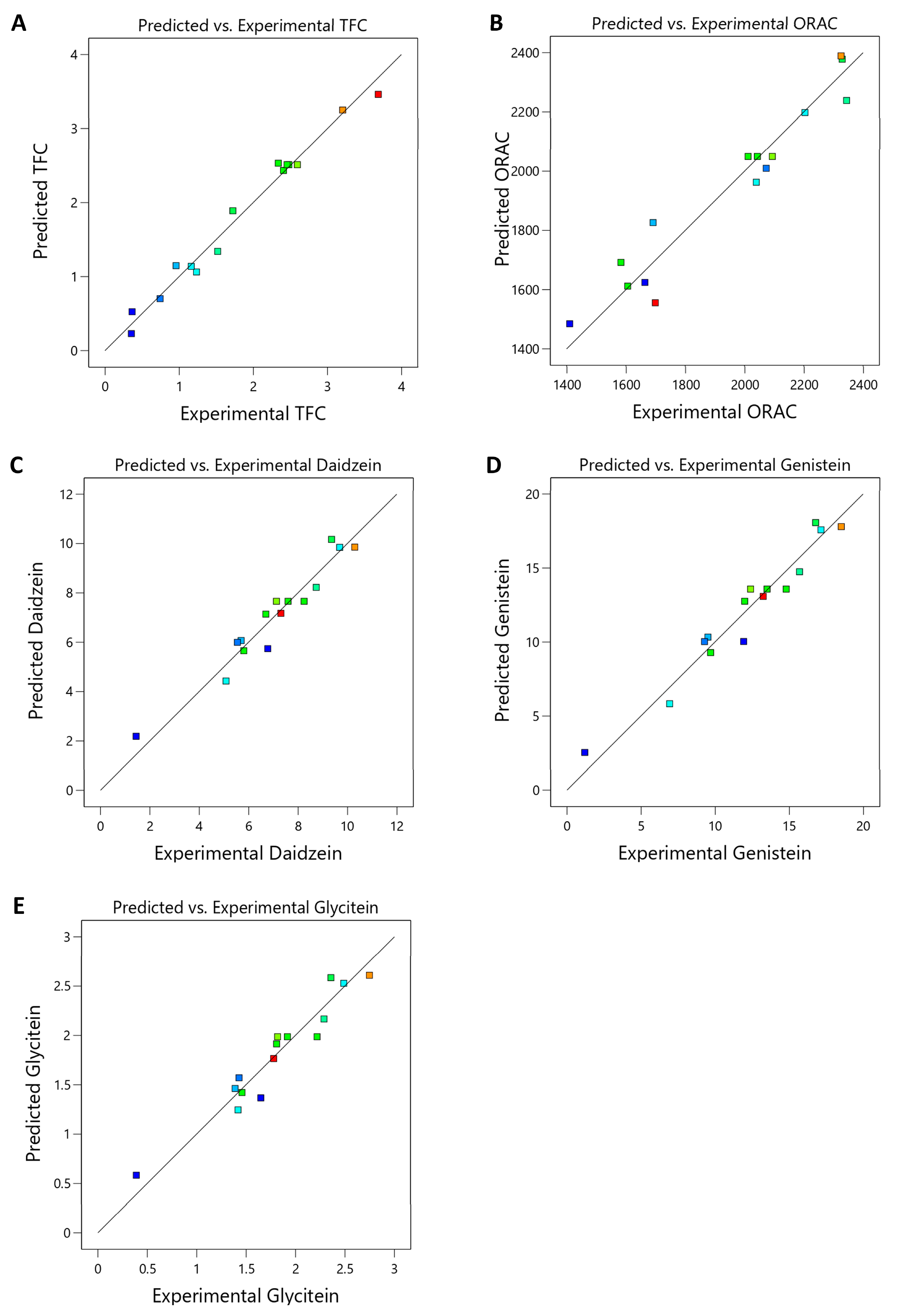
3.2. Microwave-Assisted Extraction Optimization of TFSE Using Box–Behnken Design (BBD) and Response Surface Methodology (RSM)
- Equation for Variable Optimization
3.3. The O-TFSE and TFSE Exhibited Distinctive Levels of Phytochemicals
3.4. Optimized TFSE (O-TFSE) Demonstrated Superior Antioxidant Activity Compared to TFSE Derived from Conventional Extraction
3.5. Optimized TFSE (O-TFSE) Exhibited Higher Inhibitory Effect on α-Glucosidase and α-Amylase Compared to TFSE Obtained from Conventional Extraction
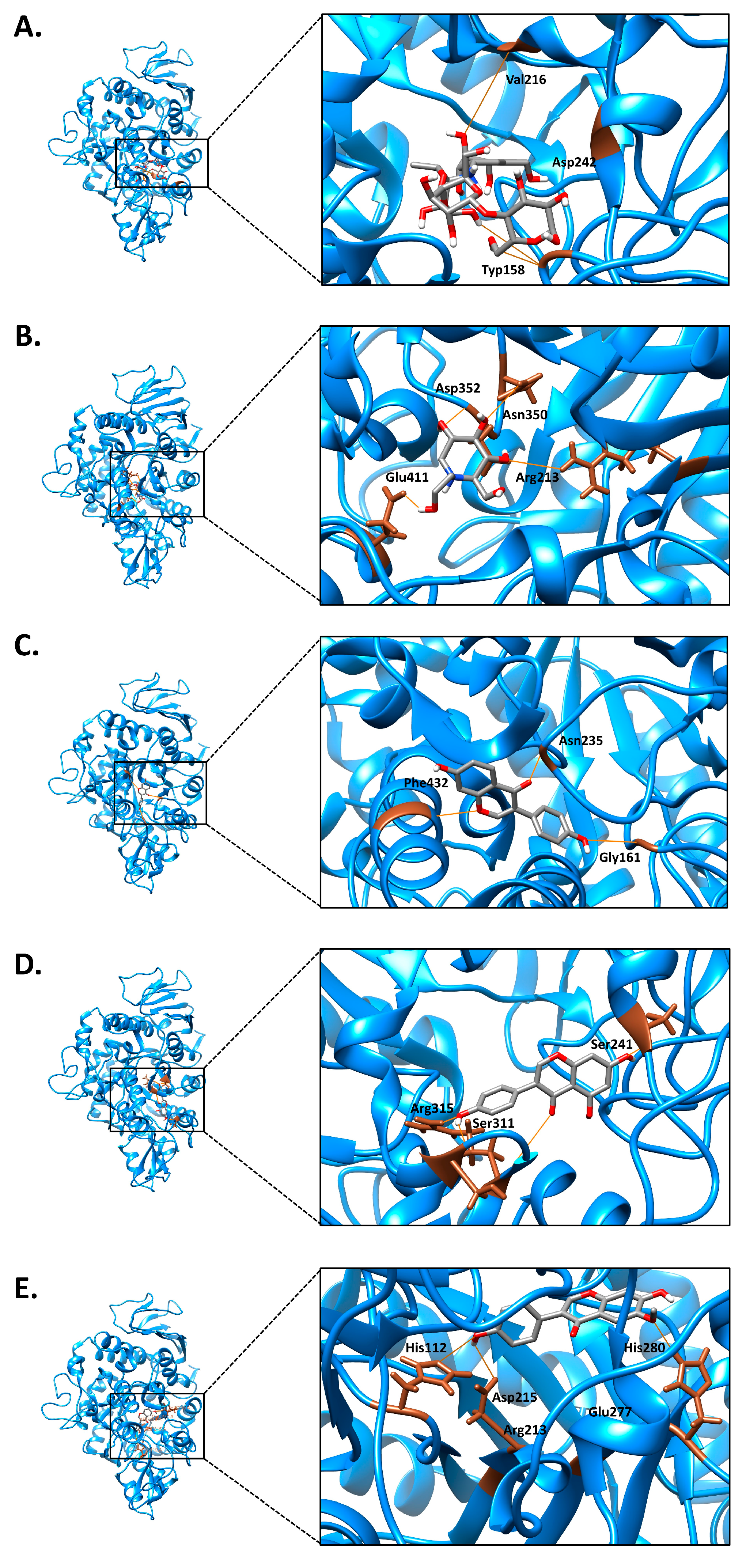
3.6. Molecular Docking and Molecular Dynamics (MDs) Simulations
3.7. Optimized TSFE and Its Active Isoflavones Suppress Inflammation-Induced Insulin Resistance in TNF-α-Treated 3T3-L1 Adipocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, P.; Weiskirchen, R. The Role of Obesity in Type 2 Diabetes Mellitus-An Overview. Int. J. Mol. Sci. 2024, 25, 1882. [Google Scholar] [CrossRef] [PubMed]
- Silveira, E.A.; Mendonca, C.R.; Delpino, F.M.; Elias Souza, G.V.; Pereira de Souza Rosa, L.; de Oliveira, C.; Noll, M. Sedentary behavior, physical inactivity, abdominal obesity and obesity in adults and older adults: A systematic review and meta-analysis. Clin. Nutr. ESPEN 2022, 50, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Bellary, S.; Kyrou, I.; Brown, J.E.; Bailey, C.J. Type 2 diabetes mellitus in older adults: Clinical considerations and management. Nat. Rev. Endocrinol. 2021, 17, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, N.; Curtis, A.J.; Heritier, S.; Gadowski, A.M.; Pavkov, M.E.; Kenealy, T.; Owens, D.R.; Thomas, R.L.; Song, S.; Wong, J.; et al. Impact of age at type 2 diabetes mellitus diagnosis on mortality and vascular complications: Systematic review and meta-analyses. Diabetologia 2021, 64, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Galiero, R.; Caturano, A.; Vetrano, E.; Beccia, D.; Brin, C.; Alfano, M.; Di Salvo, J.; Epifani, R.; Piacevole, A.; Tagliaferri, G.; et al. Peripheral Neuropathy in Diabetes Mellitus: Pathogenetic Mechanisms and Diagnostic Options. Int. J. Mol. Sci. 2023, 24, 3554. [Google Scholar] [CrossRef] [PubMed]
- Yousri, N.A.; Suhre, K.; Yassin, E.; Al-Shakaki, A.; Robay, A.; Elshafei, M.; Chidiac, O.; Hunt, S.C.; Crystal, R.G.; Fakhro, K.A. Metabolic and Metabo-Clinical Signatures of Type 2 Diabetes, Obesity, Retinopathy, and Dyslipidemia. Diabetes 2022, 71, 184–205. [Google Scholar] [CrossRef] [PubMed]
- Murussi, M.; Coester, A.; Luiz Gross, J.; Pinho Silveiro, S. Diabetic nephropathy in type 2 diabetes mellitus: Risk factors and prevention. Arq. Bras. Endocrinol. Metabol. 2003, 47, 207–219. [Google Scholar] [CrossRef]
- Alam, S.; Hasan, M.K.; Neaz, S.; Hussain, N.; Hossain, M.F.; Rahman, T. Diabetes Mellitus: Insights from Epidemiology, Biochemistry, Risk Factors, Diagnosis, Complications and Comprehensive Management. Diabetology 2021, 2, 36–50. [Google Scholar] [CrossRef]
- Tarry-Adkins, J.L.; Grant, I.D.; Ozanne, S.E.; Reynolds, R.M.; Aiken, C.E. Efficacy and Side Effect Profile of Different Formulations of Metformin: A Systematic Review and Meta-Analysis. Diabetes Ther. 2021, 12, 1901–1914. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, Y.; Ley, S.H.; Rajpathak, S.; Hu, F.B. Sulfonylurea use and incident cardiovascular disease among patients with type 2 diabetes: Prospective cohort study among women. Diabetes Care 2014, 37, 3106–3113. [Google Scholar] [CrossRef] [PubMed]
- Aroda, V.R.; Henry, R.R. Thiazolidinediones: Potential link between insulin resistance and cardiovascular disease. Diabetes Spectr. 2003, 16, 120–125. [Google Scholar] [CrossRef]
- Shi, Q.; Nong, K.; Vandvik, P.O.; Guyatt, G.H.; Schnell, O.; Ryden, L.; Marx, N.; Brosius, F.C., 3rd; Mustafa, R.A.; Agarwal, A.; et al. Benefits and harms of drug treatment for type 2 diabetes: Systematic review and network meta-analysis of randomised controlled trials. BMJ 2023, 381, e074068. [Google Scholar] [CrossRef] [PubMed]
- Blahova, J.; Martiniakova, M.; Babikova, M.; Kovacova, V.; Mondockova, V.; Omelka, R. Pharmaceutical Drugs and Natural Therapeutic Products for the Treatment of Type 2 Diabetes Mellitus. Pharmaceuticals 2021, 14, 806. [Google Scholar] [CrossRef] [PubMed]
- Le, Y.; Wang, B.; Xue, M. Nutraceuticals use and type 2 diabetes mellitus. Curr. Opin. Pharmacol. 2022, 62, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; Oliveira, J.; Pinho, A.; Carvalho, E. The Role of Nutraceutical Containing Polyphenols in Diabetes Prevention. Metabolites 2022, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Mechanistic Insight into Oxidative Stress-Triggered Signaling Pathways and Type 2 Diabetes. Molecules 2022, 27, 950. [Google Scholar] [CrossRef] [PubMed]
- Čolak, E.; Pap, D. The role of oxidative stress in the development of obesity and obesity-related metabolic disorders. J. Med. Biochem. 2021, 40, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ayer, A.; Fazakerley, D.J.; James, D.E.; Stocker, R. The role of mitochondrial reactive oxygen species in insulin resistance. Free Radic. Biol. Med. 2022, 179, 339–362. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, N.; Vaziri, N.D.; Dafoe, D.C.; Ichii, H. The Role of Oxidative Stress in Pancreatic beta Cell Dysfunction in Diabetes. Int. J. Mol. Sci. 2021, 22, 1509. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.T.; Bhat, A.A.; Nisar, S.; Fakhro, K.A.; Al-Shabeeb Akil, A.S. The role of dietary antioxidants in type 2 diabetes and neurodegenerative disorders: An assessment of the benefit profile. Heliyon 2023, 9, e12698. [Google Scholar] [CrossRef] [PubMed]
- Leh, H.E.; Lee, L.K. Lycopene: A Potent Antioxidant for the Amelioration of Type II Diabetes Mellitus. Molecules 2022, 27, 2335. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fan, Y.; Zhang, X.; Hou, W.; Tang, Z. Fruit and vegetable intake and risk of type 2 diabetes mellitus: Meta-analysis of prospective cohort studies. BMJ Open 2014, 4, e005497. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Zhuo, S.; Lu, W.; He, K.; Sui, Y.; Li, Y.; Chen, Y.; Wu, S.; Chen, P.; Fang, S. A diet rich in fruit and whole grains is associated with a low risk of type 2 diabetes mellitus: Findings from a case-control study in South China. Public Health Nutr. 2022, 25, 1492–1503. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, S.H.; Doroudi, A. Review of the antioxidant potential of flavonoids as a subgroup of polyphenols and partial substitute for synthetic antioxidants. Avicenna J. Phytomed. 2023, 13, 354–376. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Yu, J.S.; Lee, H.S.; Choi, C.I.; Kim, K.H. Antidiabetic Flavonoids from Fruits of Morus alba Promoting Insulin-Stimulated Glucose Uptake via Akt and AMP-Activated Protein Kinase Activation in 3T3-L1 Adipocytes. Pharmaceutics 2021, 13, 526. [Google Scholar] [CrossRef] [PubMed]
- Rowaiye, A.B.; Emenyonu, L.C.; Nwonu, E.J.; Okpalefe, O.A.; Ogugua, J.A.; Akinseye, V.O.; Ibeanu, G.C. Bioactive molecules from soybeans (Glycine max) with anti-type 2 diabetes activity: A systematic review. Academia Biology 2024, 2, 1–21. [Google Scholar] [CrossRef]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Food processing strategies to enhance phenolic compounds bioaccessibility and bioavailability in plant-based foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2531–2548. [Google Scholar] [CrossRef] [PubMed]
- Peres Fabbri, L.; Cavallero, A.; Vidotto, F.; Gabriele, M. Bioactive Peptides from Fermented Foods: Production Approaches, Sources, and Potential Health Benefits. Foods 2024, 13, 3369. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Singh, V.; Sharma, K.; Sliti, A.; Baunthiyal, M.; Shin, J.H. Significance of Soy-Based Fermented Food and Their Bioactive Compounds Against Obesity, Diabetes, and Cardiovascular Diseases. Plant Foods Hum. Nutr. 2024, 79, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chukeatirote, E. Thua nao: Thai fermented soybean. J. Ethn. Foods 2015, 2, 115–118. [Google Scholar] [CrossRef]
- Piao, Y.Z.; Eun, J.B. Physicochemical characteristics and isoflavones content during manufacture of short-time fermented soybean product (cheonggukjang). J. Food Sci. Technol. 2020, 57, 2190–2197. [Google Scholar] [CrossRef] [PubMed]
- Kulprachakarn, K.; Chaipoot, S.; Phongphisutthinant, R.; Paradee, N.; Prommaban, A.; Ounjaijean, S.; Rerkasem, K.; Parklak, W.; Prakit, K.; Saengsitthisak, B.; et al. Antioxidant Potential and Cytotoxic Effect of Isoflavones Extract from Thai Fermented Soybean (Thua-Nao). Molecules 2021, 26, 7432. [Google Scholar] [CrossRef] [PubMed]
- Taya, S.; Dissook, S.; Ruangsuriya, J.; Yodkeeree, S.; Boonyapranai, K.; Chewonarin, T.; Wongpoomchai, R. Thai Fermented Soybean (Thua-Nao) Prevents Early Stages of Colorectal Carcinogenesis Induced by Diethylnitrosamine and 1,2-Dimethylhydrazine Through Modulations of Cell Proliferation and Gut Microbiota in Rats. Nutrients 2024, 16, 3506. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, R.; Wulandari, P. Methods of Extraction: Maceration, Percolation and Decoction. Eureka Herba Indones. 2021, 2, 68–74. [Google Scholar] [CrossRef]
- Pereira, A.G.; Cruz, L.; Cassani, L.; Chamorro, F.; Lourenço-Lopes, C.; Freitas, V.; Otero, P.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J.; et al. Comparative Study of Microwave-Assisted Extraction and Ultrasound-Assisted Extraction Techniques (MAE vs. UAE) for the Optimized Production of Enriched Extracts in Phenolic Compounds of Camellia japonica var Eugenia de Montijo. Eng. Proc. 2023, 37, 124. [Google Scholar] [CrossRef]
- Karami, Z.; Emam-Djomeh, Z.; Mirzaee, H.A.; Khomeiri, M.; Mahoonak, A.S.; Aydani, E. Optimization of microwave assisted extraction (MAE) and soxhlet extraction of phenolic compound from licorice root. J. Food Sci. Technol. 2015, 52, 3242–3253. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, B.; Christen, P. Recent extraction techniques for natural products: Microwave-assisted extraction and pressurised solvent extraction. Phytochem. Anal. 2002, 13, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Destandau, E.; Michel, T.; Elfakir, C. Microwave-assisted Extraction. In Natural Product Extraction: Principles and Applications; Rostagno, M.A., Prado, J.M., Eds.; Green Chemistry Series; The Royal Society of Chemistry: Cambridge, UK, 2013; p. 516. [Google Scholar]
- Wang, N.; Zhu, H.; Wang, M.; Zhao, S.; Sun, G.; Li, Z. Recent Advancements in Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioprocess Technol. 2024, 18, 2083–2100. [Google Scholar] [CrossRef]
- Yu, I.-T. Factor screening with modified one-factor-at-a-time experiments. Commun. Stat. Simul. Comput. 2022, 53, 4449–4463. [Google Scholar] [CrossRef]
- Weremfo, A.; Abassah-Oppong, S.; Adulley, F.; Dabie, K.; Seidu-Larry, S. Response surface methodology as a tool to optimize the extraction of bioactive compounds from plant sources. J. Sci. Food Agric. 2023, 103, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Szpisják-Gulyás, N.; Al-Tayawi, A.N.; Horváth, Z.H.; László, Z.; Kertész, S.; Hodúr, C. Methods for experimental design, central composite design and the Box–Behnken design, to optimise operational parameters: A review. Acta Aliment. 2023, 52, 521–537. [Google Scholar] [CrossRef]
- Kongsung, S.; Inthachat, W.; Chantong, B.; Suttisansanee, U.; On-Nom, N.; Chupeerach, C.; Thangsiri, S.; Pitchakarn, P.; Temviriyanukul, P. Box-Behnken Design-Based Optimization of Phytochemical Extraction from Diplazium esculentum (Retz.) Sw. Associated with Its Antioxidant and Anti-Alzheimer’s Properties. Molecules 2024, 29, 2204. [Google Scholar] [CrossRef] [PubMed]
- Inthachat, W.; Temviriyanukul, P.; On-Nom, N.; Kanoongon, P.; Thangsiri, S.; Chupeerach, C.; Suttisansanee, U. Optimization of Phytochemical-Rich Citrus maxima Albedo Extract Using Response Surface Methodology. Molecules 2023, 28, 4121. [Google Scholar] [CrossRef] [PubMed]
- On-Nom, N.; Thangsiri, S.; Inthachat, W.; Temviriyanukul, P.; Sahasakul, Y.; Aursalung, A.; Chupeerach, C.; Suttisansanee, U. Optimized Conditions for the Extraction of Phenolic Compounds from Aeginetia indica L. and Its Potential Biological Applications. Molecules 2024, 29, 1050. [Google Scholar] [CrossRef] [PubMed]
- Suttisansanee, U.; Thiyajai, P.; Chalermchaiwat, P.; Wongwathanarat, K.; Pruesapan, K.; Charoenkiatkul, S.; Temviriyanukul, P. Phytochemicals and In Vitro Bioactivities of Aqueous Ethanolic Extracts from Common Vegetables in Thai Food. Plants 2021, 10, 1563. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Luu, L.K.; Thangsiri, S.; Sahasakul, Y.; Aursalung, A.; Inthachat, W.; Temviriyanukul, P.; On-Nom, N.; Chupeerach, C.; Suttisansanee, U. Nutrients, Phytochemicals and In Vitro Disease Prevention of Nephelium hypoleucum Kurz Fruit. Nutrients 2023, 15, 950. [Google Scholar] [CrossRef] [PubMed]
- Rohn, S.; Rawel, H.M.; Kroll, J. Inhibitory effects of plant phenols on the activity of selected enzymes. J. Agric. Food Chem. 2002, 50, 3566–3571. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common Trends and Differences in Antioxidant Activity Analysis of Phenolic Substances Using Single Electron Transfer Based Assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wei, Y.; Shi, M.; Li, Z.; Zhang, S.H. Statistical optimization of a cellulase from Aspergillus glaucus CCHA for hydrolyzing corn and rice straw by RSM to enhance yield of reducing sugar. Biotechnol. Lett. 2020, 42, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Mali, P.S.; Kumar, P. Simulation and experimentation on parameters influencing microwave-assisted extraction of bioactive compounds from Punica granatum waste and its preliminary analysis. Food Chem. Adv. 2023, 3, 100344. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Wang, S.; Gao, X.; Zhang, X. Research Progress on Extraction and Detection Technologies of Flavonoid Compounds in Foods. Foods 2024, 13, 628. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H.; Ali, H.A.; Zain, N.M. Microwave-assisted extraction of phenolic compounds from Carica papaya leaves: An optimization study and LC-QTOF-MS analysis. Future Foods 2021, 3, 100035. [Google Scholar] [CrossRef]
- Georgiopoulou, I.; Tzima, S.; Louli, V.; Magoulas, K. Process Optimization of Microwave-Assisted Extraction of Chlorophyll, Carotenoid and Phenolic Compounds from Chlorella vulgaris and Comparison with Conventional and Supercritical Fluid Extraction. Appl. Sci. 2023, 13, 2740. [Google Scholar] [CrossRef]
- Cheng, M.; He, J.; Wang, H.; Li, C.; Wu, G.; Zhu, K.; Chen, X.; Zhang, Y.; Tan, L. Comparison of microwave, ultrasound and ultrasound-microwave assisted solvent extraction methods on phenolic profile and antioxidant activity of extracts from jackfruit (Artocarpus heterophyllus Lam.) pulp. LWT 2023, 173, 114395. [Google Scholar] [CrossRef]
- Kanitkar, A.; Sabliov, C.M.; Balasubramanian, S.; Lima, M.; Boldor, D. Microwave-assisted extraction of soybean and rice bran oil: Yield and extraction kinetics. Trans. ASABE 2011, 54, 1387–1394. [Google Scholar] [CrossRef]
- Khalfi, A.; Garrigos, M.C.; Ramos, M.; Jimenez, A. Optimization of the Microwave-Assisted Extraction Conditions for Phenolic Compounds from Date Seeds. Foods 2024, 13, 3771. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Isaac, A.T.; Akinyemi, A.J.; Ajani, R.A. Inhibition of key enzymes linked to type 2 diabetes and sodium nitroprusside induced lipid peroxidation in rats’ pancreas by phenolic extracts of avocado pear leaves and fruit. Int. J. Biomed. Sci. 2014, 10, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Dirir, A.M.; Daou, M.; Yousef, A.F.; Yousef, L.F. A review of alpha-glucosidase inhibitors from plants as potential candidates for the treatment of type-2 diabetes. Phytochem. Rev. 2022, 21, 1049–1079. [Google Scholar] [CrossRef] [PubMed]
- Kashtoh, H.; Baek, K.H. New Insights into the Latest Advancement in alpha-Amylase Inhibitors of Plant Origin with Anti-Diabetic Effects. Plants 2023, 12, 2944. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Sharma, M.; Singh, S.; Goyal, A. Recent Advances of alpha-Glucosidase Inhibitors: A Comprehensive Review. Curr. Top. Med. Chem. 2022, 22, 2069–2086. [Google Scholar] [CrossRef] [PubMed]
- Ae Park, S.; Choi, M.S.; Cho, S.Y.; Seo, J.S.; Jung, U.J.; Kim, M.J.; Sung, M.K.; Park, Y.B.; Lee, M.K. Genistein and daidzein modulate hepatic glucose and lipid regulating enzyme activities in C57BL/KsJ-db/db mice. Life Sci. 2006, 79, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Daily, J.W., 3rd; Kim, H.J.; Park, S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr. Res. 2010, 30, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Hamaguchi, M.; Fukui, M. Fermented soybean foods and diabetes. J. Diabetes Investig. 2023, 14, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Chen, C.L.; Shieh, C.C.; Chang, S.H.; Tsai, G.J. Evaluation of Hypoglycemic and Antioxidant Activities of Soybean Meal Products Fermented by Lactobacillus plantarum FPS 2520 and Bacillus subtilis N1 in Rats Fed with High-Fat Diet. Metabolites 2022, 12, 442. [Google Scholar] [CrossRef] [PubMed]
- Messina, M. Soy and Health Update: Evaluation of the Clinical and Epidemiologic Literature. Nutrients 2016, 8, 754. [Google Scholar] [CrossRef] [PubMed]
- Thormar, H.; Isaacs, C.E.; Brown, H.R.; Barshatzky, M.R.; Pessolano, T. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 1987, 31, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Bergsson, G.; Steingrimsson, O.; Thormar, H. Bactericidal effects of fatty acids and monoglycerides on Helicobacter pylori. Int. J. Antimicrob. Agents 2002, 20, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Kabara, J.J. (Ed.) The Pharmacological Effect of Lipids; The American Oil Chemists’ Society: Champaign, IL, USA, 1978; p. 77. [Google Scholar]
- Miao, R.; Fang, X.; Wei, J.; Wu, H.; Wang, X.; Tian, J. Akt: A Potential Drug Target for Metabolic Syndrome. Front. Physiol. 2022, 13, 822333. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.S.; Siersbaek, R.; Boergesen, M.; Nielsen, R.; Mandrup, S. Peroxisome proliferator-activated receptor gamma and C/EBPalpha synergistically activate key metabolic adipocyte genes by assisted loading. Mol. Cell Biol. 2014, 34, 939–954. [Google Scholar] [CrossRef] [PubMed]
- Odegaard, A.O.; Pereira, M.A. Trans fatty acids, insulin resistance, and type 2 diabetes. Nutr. Rev. 2006, 64, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Mandal, V.; Mohan, Y.; Hemalatha, S. Microwave Assisted Extraction-An Innovative and Promising Extraction Tool for Medicinal Plant Research. Phcog. Rev. 2007, 1, 7–18. [Google Scholar]
- Yamashita, Y.; Sakakibara, H.; Toda, T.; Ashida, H. Insights into the potential benefits of black soybean (Glycine max L.) polyphenols in lifestyle diseases. Food Funct. 2020, 11, 7321–7339. [Google Scholar] [CrossRef] [PubMed]

| Factors | Unit | Actual Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| (X1) Ethanol concentration | % v/v | 40 | 60 | 80 |
| (X2) Microwave power | W | 100 | 200 | 500 |
| (X3) Extraction time | sec | 30 | 60 | 90 |
| Run | X1: Ethanol Concentration (% v/v) | X2: Extraction Power (W) | X3: Extraction Time (sec) |
|---|---|---|---|
| 1 | 60 | 100 | 30 |
| 2 | 80 | 100 | 60 |
| 3 | 40 | 100 | 60 |
| 4 | 60 | 100 | 90 |
| 5 | 40 | 200 | 90 |
| 6 | 60 | 200 | 60 |
| 7 | 60 | 200 | 60 |
| 8 | 80 | 200 | 30 |
| 9 | 40 | 200 | 30 |
| 10 | 60 | 200 | 60 |
| 11 | 80 | 200 | 90 |
| 12 | 60 | 500 | 30 |
| 13 | 80 | 500 | 60 |
| 14 | 40 | 500 | 60 |
| 15 | 60 | 500 | 90 |
| Run | Factor 1 | Factor 2 | Factor 3 | Response 1 | Response 2 | Response 3 | Response 4 | Response 5 | Response 6 | Response 7 |
|---|---|---|---|---|---|---|---|---|---|---|
| A: Ethanol (%v/v) | B: Power (Watt) | C: Time (s) | TPCs (mg of GAE/g DW) | TFCs (mg of QE/g DW) | ORAC (µmol of Trolox/g DW) | Protocatechuic Acid (mg/g Extract) | Daidzein (mg/g Extract) | Genistein (mg/g Extract) | Glycitein (mg/g Extract) | |
| 1 | 60 | 100 | 30 | 61.57 | 0.36 | 1663.95 | 3.97 | 6.78 | 11.94 | 1.65 |
| 2 | 80 | 100 | 60 | 56.07 | 1.73 | 2329.63 | 4.64 | 9.36 | 16.8 | 2.36 |
| 3 | 40 | 100 | 60 | 52.82 | 0.96 | 1691.65 | 3.88 | 5.70 | 9.53 | 1.39 |
| 4 | 60 | 100 | 90 | 52.86 | 3.69 | 1699.22 | 4.18 | 7.31 | 13.26 | 1.78 |
| 5 | 40 | 200 | 90 | 54.53 | 2.41 | 1606.04 | 3.82 | 5.81 | 9.71 | 1.46 |
| 6 | 60 | 200 | 60 | 60.85 | 2.48 | 2012.04 | 4.49 | 7.6 | 13.52 | 1.92 |
| 7 | 60 | 200 | 60 | 59.13 | 2.60 | 2093.82 | 4.18 | 7.13 | 12.41 | 1.82 |
| 8 | 80 | 200 | 30 | 58.60 | 1.16 | 2203.88 | 4.55 | 9.69 | 17.16 | 2.49 |
| 9 | 40 | 200 | 30 | 52.38 | 0.74 | 2073.23 | 3.82 | 5.55 | 9.3 | 1.43 |
| 10 | 60 | 200 | 60 | 58.01 | 2.46 | 2043.39 | 4.67 | 8.25 | 14.81 | 2.22 |
| 11 | 80 | 200 | 90 | 61.76 | 3.21 | 2325.70 | 5.48 | 10.3 | 18.53 | 2.75 |
| 12 | 60 | 500 | 30 | 56.56 | 2.34 | 1583.18 | 3.98 | 6.7 | 12.01 | 1.81 |
| 13 | 80 | 500 | 60 | 64.85 | 1.52 | 2344.45 | 4.69 | 8.74 | 15.71 | 2.29 |
| 14 | 40 | 500 | 60 | 61.28 | 1.24 | 2039.74 | 3.97 | 5.09 | 6.95 | 1.42 |
| 15 | 60 | 500 | 90 | 52.57 | 0.37 | 1410.48 | 3.07 | 1.45 | 1.22 | 0.39 |
| Source | TFCs | ORAC | ||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | F-Value | p Value | Significant | Coefficient | F-Value | p Value | Significant | |
| Model | −2.51 | 29.21 | 0.0008 | *** | 2049.75 | 7.69 | 0.0185 | * |
| A-Ethanol | 0.3129 | 13.02 | 0.0154 | * | 241.36 | 23.64 | 0.0046 | ** |
| B-Power | 0.3165 | 8.93 | 0.0305 | * | 136.44 | 5.06 | 0.0742 | |
| C-Time | 0.9610 | 122.78 | 0.0001 | *** | −51.68 | 1.08 | 0.3455 | |
| AB | −0.0580 | 1.12 | 0.3388 | −34.46 | 1.20 | 0.3224 | ||
| AC | 0.0950 | 0.6671 | 0.4512 | 147.25 | 4.89 | 0.0780 | ||
| BC | −0.6548 | 142.51 | <0.0001 | **** | −17.33 | 0.3047 | 0.6047 | |
| A2 | −0.4790 | 15.64 | 0.0108 | * | 257.31 | 13.78 | 0.0138 | * |
| B2 | −0.1979 | 21.53 | 0.0056 | ** | −68.43 | 7.86 | 0.0378 | * |
| C2 | −0.1519 | 1.57 | 0.2653 | −254.85 | 13.51 | 0.0144 | * | |
| Residual | ||||||||
| Lack of Fit | 15.55 | 0.0610 | NS | 16.71 | 0.0570 | NS | ||
| R2 | 0.9813 | 0.9326 | ||||||
| Adjusted R2 | 0.9477 | 0.8112 | ||||||
| Source | Daidzein | Genistein | Glycitein | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | F-Value | p Value | Significant | Coefficient | F-Value | p Value | Significant | Coefficient | F-Value | p Value | Significant | |
| Model | 7.66 | 8.08 | 0.0166 | * | 13.58 | 9.94 | 0.0105 | * | 1.99 | 6.71 | 0.0248 | * |
| A-Ethanol | 2.01 | 33.04 | 0.0022 | ** | 4.01 | 39.45 | 0.0015 | ** | 0.5364 | 28.84 | 0.0030 | ** |
| B-Power | 0.1581 | 0.1369 | 0.7265 | 0.1708 | 0.0479 | 0.8355 | 0.1166 | 0.9134 | 0.3831 | |||
| C-Time | −0.0825 | 0.0556 | 0.8230 | −0.1322 | 0.0428 | 0.8443 | −0.0169 | 0.0288 | 0.8719 | |||
| AB | −0.0378 | 0.0291 | 0.8711 | 0.1475 | 0.1331 | 0.7302 | −0.0253 | 0.1601 | 0.7056 | |||
| AC | 0.0875 | 0.0347 | 0.8595 | 0.2400 | 0.0783 | 0.7908 | 0.0575 | 0.1841 | 0.6857 | |||
| BC | −0.7975 | 12.99 | 0.0155 | * | −1.66 | 16.82 | 0.0093 | ** | −0.2161 | 11.70 | 0.0188 | * |
| A2 | 0.9200 | 3.55 | 0.1184 | 1.37 | 2.35 | 0.1861 | 0.2517 | 3.26 | 0.1310 | |||
| B2 | −0.3031 | 3.11 | 0.1383 | −0.5742 | 3.34 | 0.1272 | −0.0980 | 3.98 | 0.1025 | |||
| C2 | −0.7425 | 2.31 | 0.1891 | −1.27 | 2.03 | 0.2133 | −0.2058 | 2.18 | 0.2001 | |||
| Residual | ||||||||||||
| Lack of Fit | 3.98 | 0.2074 | NS | 2.73 | 0.2793 | NS | 2.10 | 0.3391 | NS | |||
| R2 | 0.9357 | 0.9470 | 0.9235 | |||||||||
| Adjusted R2 | 0.8199 | 0.8517 | 0.7859 | |||||||||
| Assay | O-TFSE | TFSE |
|---|---|---|
| TPCs (mg of GAE/g DW) | 45.36 ± 0.62 **** | 2.06 ± 0.02 |
| TFCs (mg of QE/g DW) | 3.16 ± 0.01 **** | 0.11 ± 0.001 |
| Antioxidant | ||
| ORAC (µmol of TE/g DW) | 2613.75 ± 26.60 **** | 110.78 ± 1.43 |
| FRAP (µmol of TE/g DW) | 44.92 ± 2.19 **** | 1.99 ± 0.10 |
| DPPH (µmol of TE/g DW) | 18.12 ± 1.21 **** | 1.09 ± 0.04 |
| Isoflavone compounds | ||
| Daidzein (mg/g extract) | 12.8 ± 0.03 **** | 72.8 ± 0.04 |
| Genistein (mg/g extract) | 23.4 ± 0.19 **** | 107.4 ± 0.24 |
| Glycitein (mg/g extract) | 3.2 ± 0.01 **** | 6.8 ± 0.01 |
| Sample | % Inhibition | |
|---|---|---|
| Alpha-Glucosidase | Alpha-Amylase | |
| O-TFSE | 72.91 ± 1.19 *** | 64.31 ± 1.44 **** |
| TFSE | 59.77 ± 1.37 | 37.09 ± 0.90 |
| Miglitol | 50.03 ± 0.20 | ND |
| Acarbose | 51.76 ± 1.60 | 48.34 ± 0.28 |
| Chemical Compounds | Vina Score (kcal/mol) | Grid Box (Å) | Size Box | Interactions with Enzyme |
|---|---|---|---|---|
| Acarbose | −8.94 | 22, −5, 23 | 40, 40, 40 | TYP158 VAL216 ASP242 |
| Miglitol | −5.80 | 22, −5, 23 | 40, 40, 40 | ARG213 ASN350 ASP352 GLU411 |
| Daidzein | −8.55 | 22, −5, 23 | 40, 40, 40 | GLY161 ASN235 PHE432 |
| Genistein | −8.36 | 22, −5, 23 | 40, 40, 40 | SER241 SER311 ARG315 |
| Glycitein | −8.41 | 22, −5, 23 | 40, 40, 40 | HIS112 ARG213 ASP215 GLU277 HIS280 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Temviriyanukul, P.; Inthachat, W.; Jaiaree, A.; Karinchai, J.; Buacheen, P.; Yodkeeree, S.; Laowanitwattana, T.; Chewonarin, T.; Suttisansanee, U.; Imsumran, A.; et al. Enhancement of Phytochemicals and Antioxidant Activity of Thai Fermented Soybean Using Box–Behnken Design Guided Microwave-Assisted Extraction. Foods 2025, 14, 2603. https://doi.org/10.3390/foods14152603
Temviriyanukul P, Inthachat W, Jaiaree A, Karinchai J, Buacheen P, Yodkeeree S, Laowanitwattana T, Chewonarin T, Suttisansanee U, Imsumran A, et al. Enhancement of Phytochemicals and Antioxidant Activity of Thai Fermented Soybean Using Box–Behnken Design Guided Microwave-Assisted Extraction. Foods. 2025; 14(15):2603. https://doi.org/10.3390/foods14152603
Chicago/Turabian StyleTemviriyanukul, Piya, Woorawee Inthachat, Ararat Jaiaree, Jirarat Karinchai, Pensiri Buacheen, Supachai Yodkeeree, Tanongsak Laowanitwattana, Teera Chewonarin, Uthaiwan Suttisansanee, Arisa Imsumran, and et al. 2025. "Enhancement of Phytochemicals and Antioxidant Activity of Thai Fermented Soybean Using Box–Behnken Design Guided Microwave-Assisted Extraction" Foods 14, no. 15: 2603. https://doi.org/10.3390/foods14152603
APA StyleTemviriyanukul, P., Inthachat, W., Jaiaree, A., Karinchai, J., Buacheen, P., Yodkeeree, S., Laowanitwattana, T., Chewonarin, T., Suttisansanee, U., Imsumran, A., Wongnoppavich, A., & Pitchakarn, P. (2025). Enhancement of Phytochemicals and Antioxidant Activity of Thai Fermented Soybean Using Box–Behnken Design Guided Microwave-Assisted Extraction. Foods, 14(15), 2603. https://doi.org/10.3390/foods14152603








_Bonness.jpeg)




