Microbial Fermentation Affects the Structure–Activity Relationship of Bioactive Compounds in Ginseng and Its Applications in Fermentation Products: A Review
Abstract
1. Introduction
2. Bibliometric Analysis of Ginseng Fermentation
3. Utilization of Different Microorganisms in Ginseng Fermentation
3.1. Lactic Acid Bacteria
3.2. Aspergillus spp.
3.3. Macrofungal
| Microorganisms | Substrates | Products | Outputs | References |
|---|---|---|---|---|
| Aspergillus tubingensis KCTC 14,166 | American ginseng extract | C-K | 8.06 g/L | [23] |
| Rhizopus oligosporus | Wild Ginseng | Total saponins | 2299 mg/kg | [91] |
| Total phenolic | 5.65 ± 0.72 mM GAE/g | |||
| L-Carnitine | 630 mg/kg | |||
| Lacticaseibacillus paracasei B16NY2107 and B04WI2501 | Panax ginseng | Rg3 | 92.981 ± 3.188 mg/L | [92] |
| Saccharomyces cerevisiae F6 | Ginsenoside extract | Rh4 | 2.65 mg/g | [93] |
| Rg5 | 2.56 mg/g | |||
| Cordyceps militaris KCCM 60304 | Red ginseng | Rb3 | 9.16% | [18] |
| Rd | 513.93% | |||
| Rg2 | 63.12% | |||
| Rg3 (20S) | 101.17% | |||
| Rg3 (20R) | 112.53% | |||
| cordycepin | 34.8 mg/kg | |||
| Bacillus subtilis CCTCC M 2,020,002 and Trichoderma reese CICC 2626 | Ginseng powder | Total saponins | 21.79 mg/g | [94] |
| Lactiplantibacillus plantarum B1 | Ginseng extract | C-K | 0.7706 mg/kg | [38] |
| Rk1 | 0.7348 mg/kg | |||
| Rh4 | 3.3924 mg/kg | |||
| Rg5 | 1.3648 mg/kg | |||
| Saccharomyces cerevisiae GIW-1 | Panax ginseng | Uronic acid | - | [36] |
| Acidic polysaccharide | - | |||
| Lactiplantibacillus plantarum KCCM 11613P | Panax ginseng Meyer | Rd | 55.74 ppm | [52] |
| Total phenolic | 37.67 ± 0.37 mg GAE/g | |||
| Aspergillus awamori | Black ginseng | Acidic polysaccharide | 74.2% | [77] |
| Rg3, Rg5, and Rk1 | 4.13 mg/g | |||
| Bacillus licheniformis IDCK 30 and Bacillus subtilis IDCK 40 | Mountain-cultivated ginseng | Rg3 | 166.90 μg/g | [95] |
| C-K | 231.33 μg/g | |||
| Aspergillus tubingensis KCTC 14166 | American ginseng extract | C-K | 17.1 mg/L/h | [76] |
| Leuconostoc mesenteroides KCCM 12010P | Hydroponic ginseng | Total phenolic | 107.19% | [96] |
| Total flavonoid | 645.59% | |||
| Lactiplantibacillus plantarum MB11 | ginsenoside extract | Rh2 | 62.37 mg/g | [97] |
| Cordyceps militaris KCCM 60304 | Korean red ginseng | Rd | 2.23 ± 0.28 mg/g | [89] |
| Rg3 | 3.50 ± 0.29 mg/g | |||
| Monascus pilosus KMU103 | Red ginseng | Rh1, Rh2, Rg3 | 838.7 mg/kg | [98] |
| Monacolin K | 3089 mg/kg | |||
| Chaetomium sp. F24-W and Aspergillus niger | Panax notoginseng | Rg3 | 108.95 mg/L | [99] |
4. Biotransformation and Structure–Activity Relationship of Bioactive Compounds in Fermented Ginseng
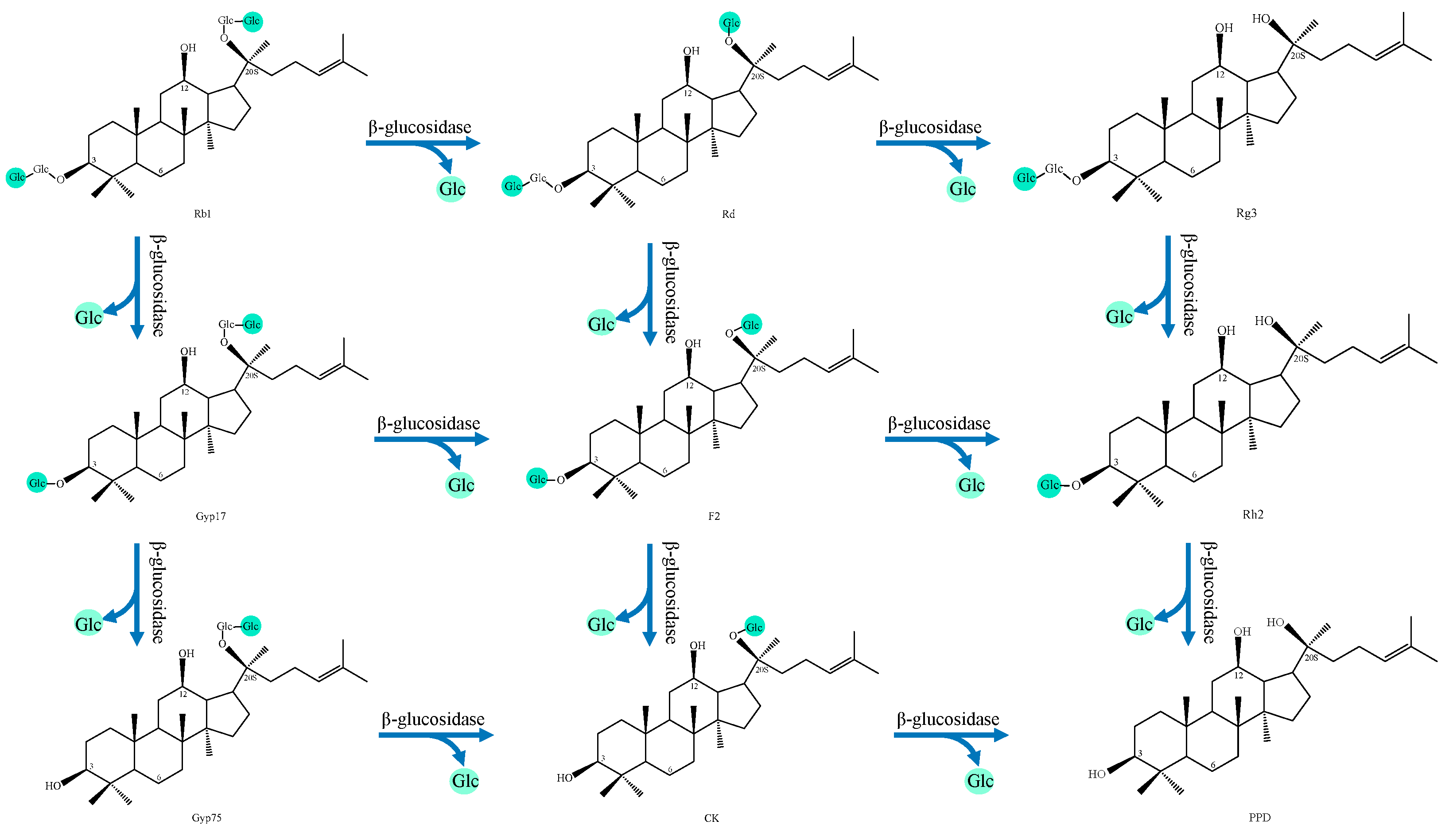
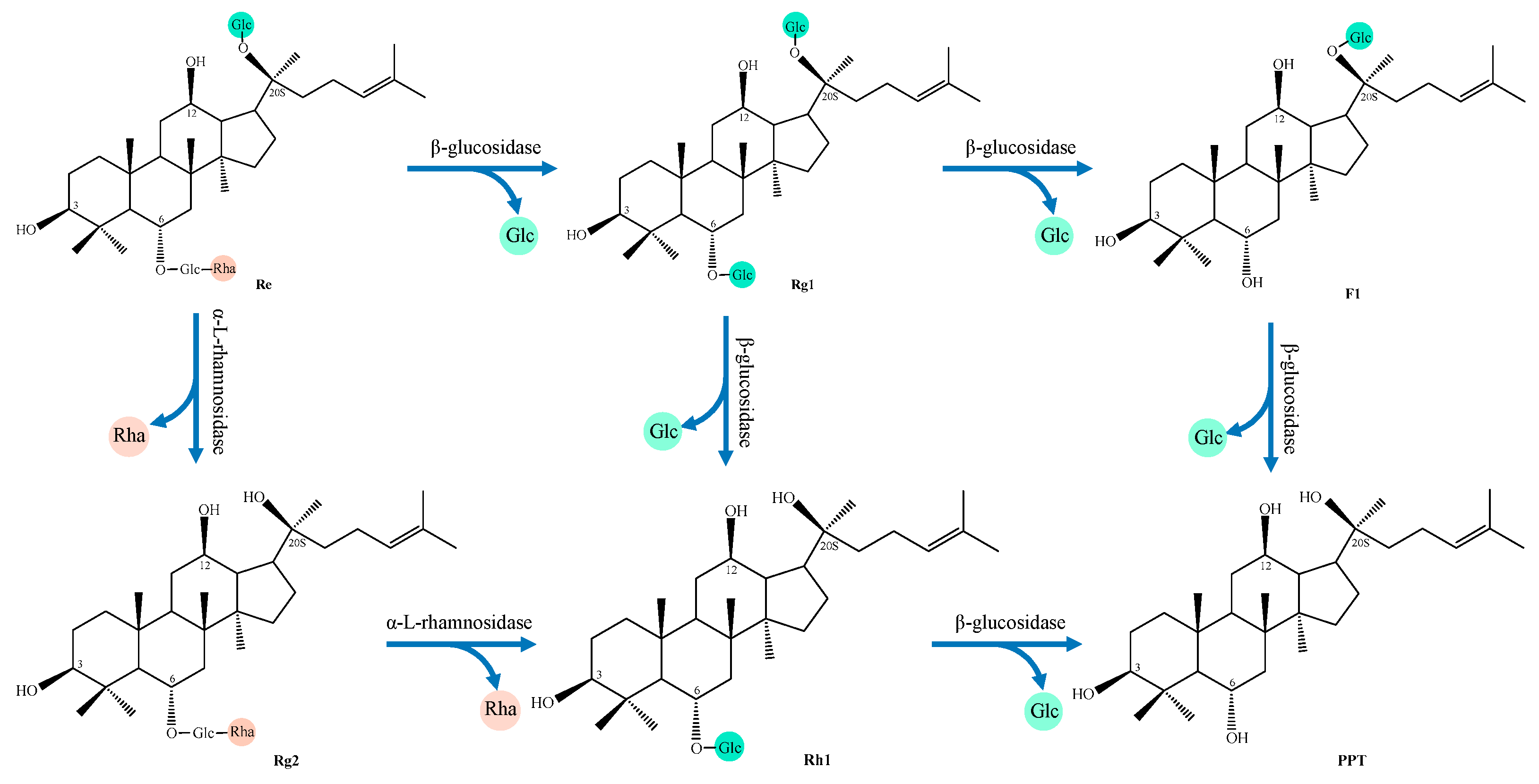
4.1. Ginsenoside
- (1)
- PPD type
- (2)
- PPT type
4.2. Ginseng Polysaccharide
4.3. Polyphenols
5. Ginseng Fermented Products
5.1. Ginseng Fermented Wine
5.2. Ginseng Fermented Milk
5.3. Ginseng Vinegar
| Product Names | Starter Cultures | Functions | References |
|---|---|---|---|
| Ginseng wine | Saccharomyces cerevisiae | Hepatoprotective effect | [162] |
| Ginseng alcoholic drink | Saccharomyces cerevisiae, Saccharomyces bayanus | - | [163] |
| Ginseng wine | Saccharomyces cerevisiae, Saccharomyces carlsbergensis | - | [164] |
| Red ginseng wine | Saccharomyces cerevisiae | - | [165] |
| Panax ginseng sprout wine | Saccharomyces cerevisiae | Antioxidant | [157] |
| Ginseng beer | Saccharomyces cerevisiae | - | [156] |
| Ginseng makgeolli | Saccharomyces cerevisiae | - | [166] |
| Ginseng rice wine | Kefir grain | - | [167] |
| Ginseng makgeolli | Saccharomyces cerevisiae | - | [168] |
| Ginseng-cactus wine | Saccharomyces cerevisiae | Anti-fatigue | [169] |
| Ginseng fermented milk | Lactobacillus acidophilus, Streptococcus thermophilus | Antioxidant | [158] |
| Ginseng fermented milk | Lactobacillus acidophilus, Bifidobacterium longum subsp. longum, Streptococcus thermophilus | Antioxidant | [66] |
| Ginseng fermented milk | Lacticaseibacillus rhamnosus GR-1, Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus delbrueckii, Streptococcus thermophilus | - | [159] |
| Ginseng fermented milk | Lactiplantibacillus plantarum NK181, Streptococcus thermophilus | Antioxidant | [170] |
| Ginseng fermented milk | Lactiplantibacillus plantarum SY46, Levilactobacillus brevis SY65 | Antioxidant | [171] |
| Ginseng fermented milk | Lactobacillus delbrueckii subsp. bulgaricus, Streptococcus thermophilus, Bifidobacterium bifidum | Antioxidant | [172] |
| Ginseng fermented milk | Bifidobacterium minimum KK-1, Bifidobacterium cholerium KK-2 | - | [173] |
| Ginseng fermented milk | Lactobacillus acidophilus KCTC3150, Ligilactobacillus salivarius ssp. CNU27 | - | [174] |
| Ginseng vinegar | Acetobacter aceti | - | [160] |
| Ginseng vinegar | Acetobacter aceti | Anti-obesity | [161] |
| Ginseng persimmon vinegar | Acetobacter aceti | Lipid-lowering effect | [175] |
| Ginseng vinegar | Mix microbial powder | - | [176] |
| Ginseng vinegar | Acetobacter pasteurianus JBA190503 | Anti-inflammatory effect | [42] |
| Ginseng vinegar | Acetobacter aceti | Antioxidant | [177] |
| Ginseng fruit vinegar | Acetobacter aceti | - | [178] |
| Ginseng-prunus mume fruit vinegar | Acetobacter aceti | Anti-fatigue effect | [179] |
| Effervescent tablets of lactobacilli | Lactobacillus acidophilus Lacticaseibacillus rhamnosus Lactiplantibacillus plantarum | - | [180] |
| Ginseng cheese | Lactobacillus acidophilus | - | [181] |
| Ginseng cheese | Flora Danica (Lactococcus lactis subsp. lactis, Lactococcus lactis subsp. cremoris, Lactococcus lactis subsp. lactis biovar diacetylactis, Leuconostoc mesenteroides subsp. cremoris) | - | [182] |
| Ginseng fermented milk | Ligilactobacillus salivarius, Lactobacillus delbrueckii subsp. bulgaricus, Streptococcus thermophilus | Antioxidant | [183] |
| Ginseng fermented milk | Lactobacillus acidophilus, Bifidobacterium longum subsp. longum, Streptococcus thermophilus | Antibacterial effect | [184] |
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.B.; Lu, X.Y.; Hu, Y.; Fan, X.H. Chemical constituents of Panax ginseng and Panax notoginseng explain why they differ in therapeutic efficacy. Pharmacol. Res. 2020, 161, 105263. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.S.; Sun, J.; Tang, N.Q.; Xu, M.; Cao, Y.; Fu, L.H.; Zhou, X.; Wu, X.H. Nondestructive detection for Panax notoginseng powder grades based on hyperspectral imaging technology combined with CARS-PCA and MPA-LSSVM. J. Food Process Eng. 2021, 44, e13718. [Google Scholar] [CrossRef]
- Zhang, J.J.; Liu, Y.; An, C.; Liu, C.; Ma, S.J.; Zhang, Q.W.; Ding, H.; Shao, J.J.; Xue, W.J. Protective Effect of Ginsenoside CK against Autoimmune Hepatitis Induced by Concanavalin A. Foods 2023, 12, 4379. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.W.; Li, T.; Wei, X.R.; Zheng, Y.F.; Zhang, Y.M.; Li, G.; Zhao, Y.Q. The Antioxidant and Anti-Fatigue Effects of Rare Ginsenosides and γ-Aminobutyric Acid in Fermented Ginseng and Germinated Brown Rice Puree. Int. J. Mol. Sci. 2024, 25, 10359. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, S.; Li, Y.; Lu, X.; Zhang, R.; Shen, X. Anti-aging Effect of Ginseng Radix et Rhizoma:A Review. Chin. J. Exp. Tradit. Med. Formulae 2024, 30, 196–207. [Google Scholar]
- Zhou, G.L.; Wang, C.Z.; Mohammadi, S.; Sawadogo, W.R.; Ma, Q.E.; Yuan, C.S. Pharmacological Effects of Ginseng: Multiple Constituents and Multiple Actions on Humans. Am. J. Chin. Med. 2023, 51, 1085–1104. [Google Scholar] [CrossRef]
- Geng, X.; Wang, J.; Liu, Y.; Liu, L.; Liu, X.; Zhao, Y.; Wang, C.; Liu, J. Research progress on chemical diversity of saponins in Panax ginseng. Chin. Herb. Med. 2024, 16, 529–547. [Google Scholar] [CrossRef]
- Li, B.; Zhang, C.; Song, K.; Lu, W. Research Progress in Biosynthesis of Rare Ginsenosides. Chin. Biotechnol. 2021, 41, 71–88. [Google Scholar]
- Wang, Q.L.; Wang, Y.P.; Xie, Y.J.; Adu-Frimpong, M.; Wei, C.M.; Yang, X.; Cao, X.; Deng, W.W.; Toreniyazov, E.; Ji, H.; et al. Nonionic surfactant vesicles as a novel drug delivery system for increasing the oral bioavailability of Ginsenoside Rb1. Food Biosci. 2021, 42, 101064. [Google Scholar] [CrossRef]
- Tuly, J.A.; Ma, H.L. Bioconversion of food industrial waste okara by microbial fermentation: Scope of omics study and possibility. Trends Food Sci. Technol. 2024, 146, 104391. [Google Scholar] [CrossRef]
- Su, Y.Y.; Bai, Q.; Tao, H.X.; Bin, X. Prospects for the application of traditional Chinese medicine network pharmacology in food science research. J. Sci. Food Agric. 2023, 103, 5183–5200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Guo, L.; Du, X. Research progress on probiotic fermentation of ginseng and its products. Food Ferment. Ind. 2022, 48, 311–319. [Google Scholar]
- Park, S.E.; Seo, S.H.; Lee, K.I.; Na, C.S.; Son, H.S. Metabolite profiling of fermented ginseng extracts by gas chromatography mass spectrometry. J. Ginseng Res. 2018, 42, 57–67. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Tang, Y.Q.; Liu, T.; Liu, H.C.; Yang, J.G.; Meng, L. Optimization of Rare Ginsenosides and Antioxidant Activity Quality of Ginseng Jiaosu Based on Probiotic Strains and Fermentation Technology. J. Food Qual. 2023, 2023, 5686929. [Google Scholar] [CrossRef]
- Jang, G.Y.; Joung, E.M.; Lee, S.H.; Jeong, J.H.; Hwang, B.Y.; Hong, J.T.; Lee, J.; Jeong, H.S. Isolation and identification of antiproliferative substances from ginseng fermented using Ganoderma lucidum mycelia. Food Sci. Biotechnol. 2015, 24, 567–574. [Google Scholar] [CrossRef]
- Hsu, B.Y.; Chen, C.H.; Lu, T.J.; Pan, M.H.; Ho, C.T.; Hwang, L.S.; Hung, W.L. Bioconversion of Ginsenosides in American Ginseng Extraction Residue by Fermentation with Ganoderma lucidum Improves Insulin-like Glucose Uptake in 3T3-L1 Adipocytes. Fermentation 2021, 7, 297. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Cui, F.J.; Sun, L.; Zan, X.Y.; Sun, W.J. Recent advances in Ganoderma lucidum polysaccharides: Structures/ bioactivities, biosynthesis and regulation. Food Biosci. 2023, 56, 103281. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lim, J.M.; Ku, B.H.; Cho, H.R.; Choi, J.S. Alteration in ginsenoside and cordycepin content by solid-state fermentation of red ginseng with Cordyceps militaris. Czech J. Food Sci. 2021, 39, 487–492. [Google Scholar] [CrossRef]
- Li, L.; Zuo, J.H.; Yi, F.; Yang, Y.L.; Dong, Y.M.; Li, Q.Y.; Li, M.H. Improved bioactivity and composition of Cordyceps militaris cultured with Panax ginseng. Food Sci. Technol. 2021, 41, 660–666. [Google Scholar] [CrossRef]
- Xu, L.; Wang, F.; Zhang, Z.C.; Terry, N. Optimization of Polysaccharide Production from Cordyceps militaris by Solid-State Fermentation on Rice and Its Antioxidant Activities. Foods 2019, 8, 590. [Google Scholar] [CrossRef]
- Siva, D.; Srivethi, G.; Vasan, P.T.; Rajesh, D.; Alfarhan, A.; Rajagopal, R. Enhanced cellulase enzyme production by Aspergillus niger using cellulase/iron oxide magnetic nano-composites. J. King Saud Univ. Sci. 2022, 34, 101695. [Google Scholar] [CrossRef]
- Odoch, M.; Buys, E.M.; Taylor, J.R.N. Solid-State Fermentation of Cassava Roots Using Cellulolytic-Type Alkaliphilic Bacillus spp. Cultures to Modify the Cell Walls and Assist Starch Release. Appl. Biochem. Biotechnol. 2020, 191, 1395–1410. [Google Scholar] [CrossRef]
- Lee, Y.I.; Song, W.S.; Oh, D.K. Enhanced production of ginsenoside compound K by synergistic conversion of fermentation with Aspergillus tubingensis and commercial cellulase. Front. Bioeng. Biotechnol. 2025, 12, 1538031. [Google Scholar] [CrossRef]
- Choi, H.J.; Kim, E.A.; Kim, D.H.; Shin, K.S. The Bioconversion of Red Ginseng Ethanol Extract into Compound K by Saccharomyces cerevisiae HJ-014. Mycobiology 2014, 42, 256–261. [Google Scholar] [CrossRef]
- You, X.; Li, Y.; Bu, Q.; Ren, G. Biotransformation of ginsenoside Rd by fermentation of ginseng rhizome by Bacillus subtilis LM 4-2. Food Ferment. Ind. 2024, 50, 38–46. [Google Scholar]
- Yao, M.J.; Yang, Y.; Fan, J.; Ma, C.M.; Liu, X.F.; Wang, Y.; Wang, B.; Sun, Z.H.; McClements, D.J.; Zhang, J.X.; et al. Production, purification, and functional properties of microbial fibrinolytic enzymes produced by microorganism obtained from soy-based fermented foods: Developments and challenges. Crit. Rev. Food Sci. Nutr. 2024, 64, 3725–3750. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ravikumar, Y.; Zhang, G.Y.; Yun, J.H.; Zhang, Y.F.; Zabed, H.M.; Qi, X.H. L-arabinose isomerase from Lactobacillus parabuchneri and its whole cell biocatalytic application in D-tagatose biosynthesis from D-galactose. Food Biosci. 2021, 41, 101034. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Q.; Zhao, T.; Zhang, Z.; Mao, G.H.; Feng, W.W.; Wu, X.Y.; Yang, L.Q. Biotransformation and metabolism of three mulberry anthocyanin monomers by rat gut microflora. Food Chem. 2017, 237, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lee, H.N.; Hong, S.J.; Kang, H.J.; Cho, J.Y.; Kim, D.; Ameer, K.; Kim, Y.M. Enhanced biotransformation of the minor ginsenosides in red ginseng extract by Penicillium decumbens β-glucosidase. Enzym. Microb. Technol. 2022, 153, 109941. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.H.; Lee, P.Y.; Bae, K.H.; Cho, S.; Park, B.C.; Shin, H.; Park, S.G. Ginsenoside Rb1 is Transformed into Rd and Rh2 by Microbacterium trichothecenolyticum. J. Microbiol. Biotechnol. 2013, 23, 1802–1805. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, P.; Liu, Y.; Fu, R.; Duan, Z.; Fan, D. Recent advances in biotransformation of ginsenosides. Chem. Ind. Eng. Prog. 2021, 40, 1238–1247. [Google Scholar]
- Li, W.N.; Fan, D.D. Biocatalytic strategies for the production of ginsenosides using glycosidase: Current state and perspectives. Appl. Microbiol. Biotechnol. 2020, 104, 3807–3823. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.X.; Zhu, L.; Xie, S.H.; Ren, Z.; Chen, X.; Liu, M.J.; Yin, G.S. Transcriptome Profiling, Cloning, and Characterization of An Glu04478, a Ginsenoside Hydrolyzing β-Glucosidase from Aspergillus niger NG1306. Curr. Microbiol. 2025, 82, 56. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, Q.; Zhang, D.W.; Gong, Y.Y.; Jiang, Q.Y.; Ma, C.; Si, L.B.; Zhang, T.H.; Zhang, J.; Ma, Z. Ginsenoside CK ameliorates tumor growth in lung cancer mice via inhibiting EGFR. J. Funct. Foods 2024, 121, 106446. [Google Scholar] [CrossRef]
- Li, C.F.; Zhang, Q.P.; Cheng, J.; Xu, G.H.; Zhu, J.X.; Yi, L.T. Role of ginsenoside Rb1 in attenuating depression-like symptoms through astrocytic and microglial complement C3 pathway. Metab. Brain Dis. 2024, 39, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Ai, Z.Y.; You, Y.; Li, W.C.; Fan, J.J.; Wang, Y.H.; Huang, J.; Wang, Y.; Wang, Y.H.; Liu, J.S. Enhanced uronic acid content, antioxidant, and anti-inflammatory activities of polysaccharides from ginseng fermented by Saccharomyces cerevisiae GIW-1. J. Food Process. Preserv. 2020, 44, e14885. [Google Scholar] [CrossRef]
- Bai, S.W.; Zhang, G.Y.; Han, Y.Q.; Ma, J.W.; Bai, B.; Gao, J.J.; Zhang, Z.M. Ginsenosides and Polysaccharides from Ginseng Co-Fermented with Multi-Enzyme-Coupling Probiotics Improve In Vivo Immunomodulatory Effects. Nutrients 2023, 15, 2434. [Google Scholar] [CrossRef]
- Liu, S.; Liu, S.; Mi, Q.; Yin, P.; Xue, T.; Yu, X.; Meng, X.; Wang, L.; Bi, Y. Changes in Active Components and Antioxidant Properties of Ginseng Fermented by Lactobacillus plantarum. Food Sci. 2023, 44, 252–259. [Google Scholar]
- Shishir, M.R.I.; Saifullah, M.; Hashim, S.B.H.; Aalim, H.; Bilal, M.; Khan, S.; Marappan, G.; Tahir, H.E.; Zhihua, L.; Zhai, X.D.; et al. Micro and nano-encapsulated natural products in yogurt: An emerging trend to achieve multifunctional benefits in product quality and human health. Food Hydrocoll. 2024, 154, 110124. [Google Scholar] [CrossRef]
- Farag, M.A.; Jomaa, S.A.; Abd El-Wahed, A.; El-Seedi, H.R. The Many Faces of Kefir Fermented Dairy Products: Quality Characteristics, Flavour Chemistry, Nutritional Value, Health Benefits, and Safety. Nutrients 2020, 12, 346. [Google Scholar] [CrossRef]
- Jung, J.; Lee, N.K.; Paik, H.D. Bioconversion, health benefits, and application of ginseng and red ginseng in dairy products. Food Sci. Biotechnol. 2017, 26, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.E.; Oh, H.H.; Jeong, D.Y.; Soo, K.Y. Ginsenoside Conversion and Anti-Inflammatory Effect on RAW 264.7 Cells of Ginseng Extract Vinegar. J. Korean Soc. Food Sci. Nutr. 2021, 50, 226–235. [Google Scholar] [CrossRef]
- Kim, M.E.; Lee, J.S. The Potential of Korean Bioactive Substances and Functional Foods for Immune Enhancement. Int. J. Mol. Sci. 2024, 25, 1334. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Liu, M. Application of machine learning based genome sequence analysis in pathogen identification. Front. Microbiol. 2024, 15, 1474078. [Google Scholar] [CrossRef]
- Jamka, K.; Wróblewska-Luczka, P.; Adamczuk, P.; Zawadzki, P.; Bojar, H.; Raszewski, G. Methodology for preparing a cosmetic sample for the development of Microorganism Detection System (SDM) software and artificial intelligence learning to recognize specific microbial species. Ann. Agric. Environ. Med. 2021, 28, 681–685. [Google Scholar] [CrossRef]
- Zheng, Z.W.; Li, H.T.; Wang, X.; Yang, S.Y.; Li, X.K. Trends and Hotspots of Starch Fermentation Research: Bibliometric Analysis Based on CiteSpace. Starch-Starke 2023, 75, 2200216. [Google Scholar] [CrossRef]
- Ren, M.N.; Yu, X.J.; Mujumdar, A.S.; Yagoub, A.A.; Chen, L.; Zhou, C.S. Visualizing the knowledge domain of pulsed light technology in the food field: A scientometrics review. Sci. Emerg. Technol. 2021, 74, 102823. [Google Scholar] [CrossRef]
- Zeng, T.X.; Pei, J.; Miao, Y.J.; Zheng, Y.; Gu, S.J.; Zhao, L.; Huang, L.F. Current Status and Research Trends of Panax Between 1900-2019: A Bibliometric Analysis. Chin. J. Integr. Med. 2022, 28, 547–553. [Google Scholar] [CrossRef]
- Liu, S.J.; Zhao, L.J.; Li, M.Y.; Zhu, Y.D.; Liang, D.; Ma, Y.Y.; Sun, L.X.; Zhao, G.M.; Tu, Q.C. Probiotic Bacillus as fermentation agents: Status, potential insights, and future perspectives. Food Chem. X 2024, 22, 101465. [Google Scholar] [CrossRef]
- Lee, N.K.; Kim, W.S.; Paik, H.D. Bacillus strains as human probiotics: Characterization, safety, microbiome, and probiotic carrier. Food Sci. Biotechnol. 2019, 28, 1297–1305. [Google Scholar] [CrossRef]
- Li, F.; Wu, Z.X.; Sui, X. Biotransformation of ginsenoside Rb1 with wild Cordyceps sinensis and Ascomycota sp. and its antihyperlipidemic effects on the diet-induced cholesterol of zebrafish. J. Food Biochem. 2020, 44, e13192. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Jang, H.J.; Eom, S.J.; Choi, N.S.; Lee, N.K.; Paik, H.D. Fermentation of red ginseng extract by the probiotic Lactobacillus plantarum KCCM 11613P: Ginsenoside conversion and antioxidant effects. J. Ginseng Res. 2019, 43, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Deng, K.W.; Zhang, Y. Isolation and screening of high-quality lactic acid bacteria and yeast strains in kefir grains and preparation of kefir compound fermentation starter. J. Food Process. Preserv. 2022, 46, e17073. [Google Scholar] [CrossRef]
- Shi, C.; Chen, Y.Y.; Li, C.Z.; Al-Asmari, F.; Cui, H.Y.; Lin, L. Potential Application of Lactiplantibacillus plantarum in Food Bio-preservation—A Comprehensive Review with a Focus on the Antibacterial and Anti-Virulence Effects on Foodborne Pathogens. Food Rev. Int. 2024, 40, 2993–3019. [Google Scholar] [CrossRef]
- Virk, M.S.; Virk, M.A.; He, Y.F.; Tufail, T.; Gul, M.; Qayum, A.; Rehman, A.; Rashid, A.; Ekumah, J.N.; Han, X.; et al. The Anti-Inflammatory and Curative Exponent of Probiotics: A Comprehensive and Authentic Ingredient for the Sustained Functioning of Major Human Organs. Nutrients 2024, 16, 546. [Google Scholar] [CrossRef]
- Vasquez, R.; Song, J.H.; Park, Y.S.; Paik, H.D.; Kang, D.K. Application of probiotic bacteria in ginsenoside bioconversion and enhancing its health-promoting benefits: A review. Food Sci. Biotechnol. 2025, 34, 1631–1659. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Lee, J.H.; Shin, E.C.; Cho, D.Y.; Jung, J.G.; Kim, M.J.; Jeong, J.B.; Kang, D.; Kang, S.S.; Cho, K.M. Changes in Chemical Compositions and Antioxidant Activities from Fresh to Fermented Red Mountain-Cultivated Ginseng. Molecules 2022, 27, 4550. [Google Scholar] [CrossRef]
- Zhu, J.; Qian, Y.; Wu, Q.; Cui, J.; Lin, G.; Li, R.; Li, L.; Chen, S. Screening of ginseng fermented bacteria strains and research on transformation abilities of ginsenoside. Food Ferment. Ind. 2023, 49, 159–163. [Google Scholar]
- Ban, M.S.; Kim, Y.; Lee, S.; Han, B.; Yu, K.S.; Jang, I.J.; Chung, H.K.; Lee, S. Pharmacokinetics of Ginsenoside Compound K From a Compound K Fermentation Product, CK-30, and From Red Ginseng Extract in Healthy Korean Subjects. Clin. Pharmacol. Drug Dev. 2021, 10, 1358–1364. [Google Scholar] [CrossRef]
- Shin, M.B.; Kim, S.A.; Lee, S.; Shim, W.S.; Lee, K.T.; Lee, S.K.; Yim, S.V.; Kim, B.H. Pharmacokinetic Comparison of Ginsenosides between Fermented and Non-Fermented Red Ginseng in Healthy Volunteers. Pharmaceutics 2022, 14, 2807. [Google Scholar] [CrossRef]
- Kim, H.K. Pharmacokinetics of ginsenoside Rb1 and its metabolite compound K after oral administration of Korean Red Ginseng extract. J. Ginseng Res. 2013, 37, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Boasiako, T.A.; Yinka, A.A.; Yuqing, X.; Boateng, I.D.; Ma, Y.K. Tri-cultured lactic-acetic acid co-fermentation improves stored jujube puree functionality, physicochemical, volatile compounds, and sensory characteristics. Food Biosci. 2024, 57, 103534. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.K.; Tchabo, W.; Apaliya, M.T.; Xiao, L.L.; Wu, M. Effect of lactic acid fermentation on the phytochemical, volatile profile and sensory attributes of mulberry juice. J. Food Nutr. Res. 2017, 56, 305–317. [Google Scholar]
- Shin, N.R.; Bose, S.; Choi, Y.; Kim, Y.M.; Chin, Y.W.; Song, E.J.; Nam, Y.D.; Kim, H. Anti-Obesity Effect of Fermented Panax notoginseng Is Mediated Via Modulation of Appetite and Gut Microbial Population. Front. Pharmacol. 2021, 12, 665881. [Google Scholar] [CrossRef]
- Kim, S.-H.; Woo, H.-G.; Choi, Y.-R.; Lee, C.-M.; Jeong, J.-H.; Lee, D.-H.; Lee, C.-Y.; Oh, I.-K.; Ha, H.-K.; Kim, J.-S. Quality characteristics of yakju added with lactic acid bacteria-fermented ginseng sprouts. Food Sci. Preserv. 2022, 29, 263–275. [Google Scholar]
- Lee, H.S.; Song, M.W.; Kim, K.T.; Hong, W.S.; Paik, H.D. Antioxidant Effect and Sensory Evaluation of Yogurt Supplemented with Hydroponic Ginseng Root Extract. Foods 2021, 10, 639. [Google Scholar] [CrossRef]
- Yoon, E.K.; Hong, J.H.; Lê, S.; Kim, K.O. Sensory Characteristics and Consumer Acceptability of Red Ginseng Extracts Produced with Different Processing Methods. J. Food Sci. 2011, 76, S270–S279. [Google Scholar] [CrossRef]
- Tu, T.T.; Ren, Y.; Gong, W.F.; Huang, J.Y.; Zhu, C.Y.; Salah, M.; Zhao, L.N.; Xia, X.S.; Wang, Y. Endoglucanase H from Aspergillus westerdijkiae Plays an Important Role in the Virulence on Pear Fruits. J. Agric. Food Chem. 2024, 72, 8415–8422. [Google Scholar] [CrossRef] [PubMed]
- Mamy, D.; Boateng, I.D.; Chen, X.M. Two-pot optimization of nutrient sources to enhance antioxidants in Citrus reticulata peel powder through solid-state fermentation with Aspergillus niger CGMCC 3.6189. Food Biosci. 2024, 59, 104145. [Google Scholar] [CrossRef]
- Gao, X.L.; Yin, Y.Y.; Zhou, C.S. Purification, characterisation and salt-tolerance molecular mechanisms of aspartyl aminopeptidase from Aspergillus oryzae 3.042. Food Chem. 2018, 240, 377–385. [Google Scholar] [CrossRef]
- Mamy, D.; Boateng, I.D.; Chen, X.M. Ultrasound-assisted solid-state fermentation by Aspergillus niger increased phenolics and antioxidants’ accumulation in Citrus reticulata peels. Food Biosci. 2025, 63, 105699. [Google Scholar] [CrossRef]
- Jang, J.H.; Bayaraa, U.; Lee, J.H.; Lee, O.R. Overexpression of the patatin-related phospholipase A gene, PgpPLAIIIf3, in ginseng adventitious roots reduces lignin and ginsenoside content while increasing fatty acid content. Plant Physiol. Biochem. 2025, 221, 109602. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhao, G.J.; Sun, R.; Wang, J.F.; Sun, T.X.; Xing, S.Y.; Lian, W.H.; Zhao, Y. Dynamic changes in cell wall-degrading enzymes and physio-biochemistry of ginseng in response to fusarium oxysporum infection. Eur. J. Plant Pathol. 2023, 165, 569–578. [Google Scholar] [CrossRef]
- Akpabli-Tsigbe, N.D.K.; Osabutey, J.; Mintah, B.K.; Tano-Debrah, K.; Ma, Y.K. Cleavage of macromolecule (protein/polysaccharide)-phenolic bond in soybean cell wall through Lactobacillus casei and Lactobacillus helviticus mixed culture solid-state fermentation for chlorogenic acid extraction. Food Biosci. 2023, 55, 102903. [Google Scholar] [CrossRef]
- Jeong, E.B.; Kim, S.A.; Shin, K.C.; Oh, D.K. Biotransformation of Protopanaxadiol-Type Ginsenosides in Korean Ginseng Extract into Food-Available Compound K by an Extracellular Enzyme from Aspergillus niger. J. Microbiol. Biotechnol. 2020, 30, 1560–1567. [Google Scholar] [CrossRef]
- Song, W.S.; Kim, M.J.; Shin, K.C.; Oh, D.K. Increased Production of Ginsenoside Compound K by Optimizing the Feeding of American Ginseng Extract during Fermentation by Aspergillus tubingensis. J. Microbiol. Biotechnol. 2022, 32, 902–910. [Google Scholar] [CrossRef]
- Shon, M.Y.; Kim, Y.-S. Analysis of Acidic Polysaccharide and Biological Activitiesof Fermented Stichopus japonicus and Black Ginseng. J. Chitin Chitosan 2021, 26, 53–62. [Google Scholar] [CrossRef]
- Ramadhania, Z.M.; Yang, D.U.; Moektiwardojo, M.; Han, Y.; Park, J.K.; Rupa, E.J.; Yang, D.C.; Lee, S.J.; Kang, S.C. Enhanced Anti-Skin Aging Effects of Fermented Black Ginseng (Panax ginseng CA Meyer) by Aspergillus nige KHNT-1. Appl. Sci. 2023, 13, 550. [Google Scholar] [CrossRef]
- Mamy, D.; Boateng, I.D.; Chen, X.M. Metabolomic changes in Citrus reticulata peel after conventional and ultrasound-assisted solid-state fermentation with Aspergillus niger: A focus on flavonoid metabolism. Food Chem. 2025, 467, 142224. [Google Scholar] [CrossRef]
- Pouris, J.; Kolyva, F.; Bratakou, S.; Vogiatzi, C.A.; Chaniotis, D.; Beloukas, A. The Role of Fungi in Food Production and Processing. Appl. Sci. 2024, 14, 5046. [Google Scholar] [CrossRef]
- Vásquez-Bonilla, J.N.; Barranco-Florido, J.E.; Ponce-Alquicira, E.; Rincón-Guevara, M.A.; Loera, O. Improvement of beauvericin production by Fusarium oxysporum AB2 under solid-state fermentation using an optimised liquid medium and co-cultures. Mycotoxin Res. 2022, 38, 175–183. [Google Scholar] [CrossRef]
- Xu, X.Q.; Yu, Y.F.; Shi, Y.J. Evaluation of inert and organic carriers for Verticillium lecanii spore production in solid-state fermentation. Biotechnol. Lett. 2011, 33, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, X.C.; Zhou, Y.Y.; Chen, H.Z. Porous inert material as promising carrier enhanced cellulase production from Trichoderma reesei in solid-state fermentation. Process Biochem. 2022, 122, 316–322. [Google Scholar] [CrossRef]
- Fu, X.; Zan, X.Y.; Sun, L.; Tan, M.; Cui, F.J.; Liang, Y.Y.; Meng, L.J.; Sun, W.J. Functional Characterization and Structural Basis of the β-1,3-Glucan Synthase CMGLS from Mushroom Cordyceps militaris. J. Agric. Food Chem. 2022, 70, 8725–8737. [Google Scholar] [CrossRef]
- Meng, X.Y.; Yang, Y.L.; Wu, Y.H.; Zhang, Y.; Zhang, H.Y.; Zhou, W.Q.; Guo, M.M.; Li, L. Inflammatory factor expression in HaCaT cells and melanin synthesis in melanocytes: Effects of Ganoderma lucidum fermentation broth containing Chinese medicine. Int. J. Food Prop. 2022, 25, 1604–1621. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, X.; Li, Z.; Yang, Y.; Guo, M.; Li, L.; Zhou, W. Inhibition of cyclooxygenase-2 and antioxidant activity of lyophilized powder of Ganoderma lucidum bidirectional fermentation broth. Food Ferment. Ind. 2023, 49, 47–52. [Google Scholar]
- Yang Hsu, B.; Hui Chen, C.; Jang Lu, T.; Sun Hwang, L. Bioconversion of ginsenosides in the american ginseng ( xi yang shen) extraction residue by fermentation with lingzhi ( ling zhi, ganoderma lucidum). J. Tradit. Complement. Med. 2013, 3, 95–101. [Google Scholar]
- Rae, S.H.; Lee, H.S.; Kim, M.R.; Kim, S.Y.; Kim, J.M.; Suh, H.J. Changes of Ginsenoside Content by Mushroom Mycelial Fermentation in Red Ginseng Extract. J. Ginseng Res. 2011, 35, 235–242. [Google Scholar]
- Choi, S.Y.; Park, J.S.; Shon, C.H.; Lee, C.Y.; Ryu, J.M.; Son, D.J.; Hwang, B.Y.; Yoo, H.S.; Cho, Y.C.; Lee, J.; et al. Fermented Korean Red Ginseng Extract Enriched in Rd and Rg3 Protects against Non-Alcoholic Fatty Liver Disease through Regulation of mTORC1. Nutrients 2019, 11, 2963. [Google Scholar] [CrossRef]
- Yan, M.C.; Chen, Y.; Feng, Y.; Saeed, M.; Fang, Z.; Zhen, W.; Ni, Z.; Chen, H.Y. Perspective on Agricultural Industrialization: Modification Strategies for Enhancing the Catalytic Capacity of Keratinase. J. Agric. Food Chem. 2024, 72, 12915–12929. [Google Scholar] [CrossRef]
- Lee, G.; Nguyen, T.T.H.; Lim, T.Y.; Lim, J.; Park, B.; Lee, S.; Mok, I.K.; Pal, K.; Lim, S.; Kim, D. Fermented Wild Ginseng by Rhizopus oligosporus Improved l-Carnitine and Ginsenoside Contents. Molecules 2020, 25, 2111. [Google Scholar] [CrossRef]
- Yan, J.; Lu, L.; Fu, S.; Fang, L.; Wang, Y.; Zhang, X. Processing technology of fermentation transformation of Ginsenoside Rg3 by lactic acid bacteria. Food Ferment. Ind. 2023, 49, 222–230. [Google Scholar]
- Liu, S.N.; Wang, H.Y.; Liu, S.W.; Yin, P.; Song, S.X.; Xiong, B.Y.; Wang, L.N.; Bi, Y.F.; Yu, L. Fermented Ginsenosides Alleviate Acute Liver Injury Induced by CCl4 in Mice by Regulating the AKT/mTOR Signaling Pathway. J. Med. Food 2024, 27, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Guo, B.Q.; Li, X.M.; Liu, S.; Liu, H.X.; Wang, Y.Z. Enhancement of biotransformation of ginsenosides in white ginseng roots by aerobic co-cultivation of Bacillus subtilis and Trichoderma reesei. Appl. Microbiol. Biotechnol. 2021, 105, 8265–8276. [Google Scholar] [CrossRef]
- Seong, J.; Lee, H.Y.; Jeong, J.B.; Cho, D.Y.; Kim, D.H.; Lee, J.H.; Lee, G.Y.; Jang, M.Y.; Lee, J.H.; Cho, K.M. Comparison in Bioactive Compounds and Antioxidant Activity of Cheonggukjang Containing Mountain-Cultivated Ginseng Using Two Bacillus Genus. Foods 2024, 13, 3155. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.E.; Kim, K.T.; Paik, H.D. Improved Antioxidant, Anti-inflammatory, and Anti-adipogenic Properties of Hydroponic Ginseng Fermented by Leuconostoc mesenteroides KCCM 12010P. Molecules 2019, 24, 3359. [Google Scholar] [CrossRef]
- Shen, Y.J.; Gao, Y.S.; Yang, G.; Zhao, Z.J.; Zhao, Y.J.; Gao, L.; Zhao, L.; Li, S.Y. Transformation of Ginsenosides by Lactiplantibacillus plantarum MB11 Fermentation: Minor Ginsenosides Conversion and Enhancement of Anti-Colorectal Cancer Activity. Molecules 2024, 29, 27. [Google Scholar] [CrossRef]
- Hong, S.Y.; Oh, J.H.; Lee, I. Simultaneous Enrichment of Deglycosylated Ginsenosides and Monacolin K in Red Ginseng by Fermentation with Monascus pilosus. Biosci. Biotechnol. Biochem. 2011, 75, 1490–1495. [Google Scholar] [CrossRef]
- Qi, G.Y.; Ji, B.Y.; Zhang, Y.A.; Huang, L.Q.; Wang, J.; Gao, W.Y. Microbiome-based screening and co-fermentation of rhizospheric microorganisms for highly ginsenoside Rg3 production. Microbiol. Res. 2022, 261, 127054. [Google Scholar] [CrossRef]
- Ji, T.L.; Liaqat, F.; Khazi, M.I.; Liaqat, N.; Nawaz, M.Z.; Zhu, D.C. Lignin biotransformation: Advances in enzymatic valorization and bioproduction strategies. Ind. Crops Prod. 2024, 216, 118759. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Shi, T.; Bao, Y.L.; Tan, Y.Q.; Luo, Y.K.; Hong, H. Exploring Release, Isomerization, and Absorption of Cypermethrin in Pacific Oysters (Crassostrea gigas) with Different Processing Methods during In Vivo Digestion: Insights from a Gastrointestinal Tract Quantitative Tracing Method. J. Agric. Food Chem. 2024, 72, 14364–14374. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, H.; Zhang, W.J.; Ding, Y.Y.; Zhao, T.; Zhang, M.; Mao, G.H.; Feng, W.W.; Wu, X.Y.; Yang, L.Q. Bioaccessibility and biotransformation of anthocyanin monomers following in vitro simulated gastric-intestinal digestion and in vivo metabolism in rats. Food Funct. 2019, 10, 6052–6061. [Google Scholar] [CrossRef]
- Tanko, A.I.; Hosawi, S.; Moglad, E.; Afzal, M.; Ghaboura, N.; Alzareaa, S.I.; Osman, A.; Nadeem, M.S.; Kazmi, I. Ginsenoside Rg3 in Cancer Research: Current Trends and Future Prospects—A Review. Curr. Med. Chem. 2025, 32, 1–24. [Google Scholar] [CrossRef]
- Cao, J.; Cao, Y.; Zhang, J.; Zhou, Y.; Guo, Z.; Shao, J. Research progress on large-scale preparation biological activity of ginsenoside, C.K. Food Ferment. Ind. 2025, 51, 371–379. [Google Scholar]
- Piao, X.M.; Huo, Y.; Kang, J.P.; Mathiyalagan, R.; Zhang, H.; Yang, D.U.; Kim, M.; Yang, D.C.; Kang, S.C.; Wang, Y.P. Diversity of Ginsenoside Profiles Produced by Various Processing Technologies. Molecules 2020, 25, 4390. [Google Scholar] [CrossRef]
- Xu, L.F.; Li, J.; Hou, N.N.; Han, F.; Sun, X.D.; Li, Q.Y. 20(S)-Ginsenoside Rh2 inhibits hepatocellular carcinoma by suppressing angiogenesis and the GPC3-mediated Wnt/β-catenin signaling pathway. Acta Biochim. Et Biophys. Sin. 2024, 56, 688–696. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, W.Q.; Yang, L.C.; Yan, F.; Cui, D.J. Ginsenoside Rh2 suppresses ferroptosis in ulcerative colitis by targeting specific protein 1 by upregulating microRNA-125a-5p. Eur. J. Med. Res. 2024, 29, 450. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.L.; Huy, N.Q.; Tung, N.H. Microorganisms for Ginsenosides Biosynthesis: Recent Progress, Challenges, and Perspectives. Molecules 2023, 28, 1437. [Google Scholar] [CrossRef]
- Yang, W.; Gu, Q.; Yu, X. Protopanaxadiol-type ginsenoside hydrolases and their application in the preparation of ginsenoside Compound K: A review. Chin. J. Biotechnol. 2023, 39, 978–992. [Google Scholar]
- Jiang, Y.Y.; Li, W.N.; Fan, D.D. Biotransformation of Ginsenoside Rb1 to Ginsenoside CK by Strain XD101: A Safe Bioconversion Strategy. Appl. Biochem. Biotechnol. 2021, 193, 2110–2127. [Google Scholar] [CrossRef]
- Renchinkhand, G.; Magsar, U.; Bae, H.C.; Choi, S.H.; Nam, M.S. Identification of β-Glucosidase Activity of Lentilactobacillus buchneri URN103L and Its Potential to Convert Ginsenoside Rb1 from Panax ginseng. Foods 2022, 11, 529. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.C.; Oh, D.K. Classification of glycosidases that hydrolyze the specific positions and types of sugar moieties in ginsenosides. Crit. Rev. Biotechnol. 2016, 36, 1036–1049. [Google Scholar] [CrossRef]
- Lee, J.-S.; Choi, S.-W. Changes in effective components of the fermented ginseng berry extract using lactic acid bacteria. Curr. Top. Lact. Acid Bact. Probiotics 2023, 9, 86–93. [Google Scholar] [CrossRef]
- Quan, L.H.; Piao, J.Y.; Min, J.W.; Yang, D.U.; Lee, H.N.; Yang, D.C. Bioconversion of ginsenoside Rb1 into compound K by Leuconostoc citreum LH1 isolated from kimchi. Braz. J. Microbiol. 2011, 42, 1227–1237. [Google Scholar] [CrossRef]
- Yi, E.-J.; Lee, J.-M.; Yi, T.-H.; Cho, S.-C.; Park, Y.-J.; Kook, M.-C. Biotransformation of Ginsenoside by Lactobacillus brevis THK-D57 Isolated from Kimchi. Korean J. Food Nutr. 2012, 25, 629–636. [Google Scholar] [CrossRef]
- Piao, J.-Y.; Kim, Y.-J.; Quan, L.-H.; Yang, D.-U.; Min, J.-W.; Son, S.-H.; Kim, S.-M.; Yang, D.-C. Bioconversion of Ginsenoside Rb1 to Compound K using Leuconostoc lactis DC201. Korean J. Plant Reources 2011, 24, 712–718. [Google Scholar] [CrossRef]
- Quan, L.H.; Kim, Y.J.; Li, G.H.; Choi, K.T.; Yang, D.C. Microbial transformation of ginsenoside Rb1 to compound K by Lactobacillus paralimentarius. World J. Microbiol. Biotechnol. 2013, 29, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.L.; Zhang, Y.Y.; Zhou, Y.K.; Xu, M.H.; Yu, S.S. Production of Gypenoside XVII from Ginsenoside Rb1 by Enzymatic Transformation and Their Anti-Inflammatory Activity In Vitro and In Vivo. Molecules 2023, 28, 7001. [Google Scholar] [CrossRef]
- Ku, S.; You, H.J.; Park, M.S.; Ji, G.E. Whole-Cell Biocatalysis for Producing Ginsenoside Rd from Rb1 Using Lactobacillus rhamnosus GG. J. Microbiol. Biotechnol. 2016, 26, 1206–1215. [Google Scholar] [CrossRef]
- Hu, Y.B.; Li, Y.M.; Cao, Y.; Shen, Y.Z.; Zou, X.J.; Liu, J.X.; Zhao, J. Advancements in enzymatic biotransformation and bioactivities of rare ginsenosides: A review. J. Biotechnol. 2024, 392, 78–89. [Google Scholar] [CrossRef]
- Li, F.; Huang, Q.; Sui, X.; Xie, Y. Activities of beta-Glucosidase and alpha-L-Arabinofuranosidase from Cordyceps militaris and Their Applications in the Transformation of Ginsenoside Rg1 and Rc. Food Sci. 2023, 44, 152–161. [Google Scholar]
- Sui, X.; Liu, J.S.; Xin, Y.; Qu, M.; Qiu, Y.; He, T.Z.; Luo, H.M.; Wang, W.N.; Qiu, Z.D. Highly regioselective biotransformation of ginsenoside Rg1 to 25-OH derivatives of 20(S/R)-Rh1 by Cordyceps Sinensis. Bioorganic Med. Chem. Lett. 2020, 30, 127504. [Google Scholar] [CrossRef] [PubMed]
- Shim, K.-S.; Park, G.-G.; Park, Y.-S. Bioconversion of Puffed Red Ginseng Extract Using β-Glucosidase-producing Lactic Acid Bacteria. Food Eng. Prog. 2014, 18, 332–340. [Google Scholar] [CrossRef]
- Palaniyandi, S.A.; Le, B.; Kim, J.-M.; Yang, S.H. Fermentative transformation of ginsenosides by a combination of probiotic Lactobacillus helveticus and Pediococcus pentosaceus. Korean J. Microbiol. 2018, 54, 436–441. [Google Scholar]
- Jiang, M.C.; Chi, J.X.; Qiao, Y.F.; Wang, J.P.; Zhang, Z.X.; Liu, J.; Sheng, X.H.; Yuan, L.J. Ginsenosides Rg1, Rb1 and rare ginsenosides: Promising candidate agents for Parkinson’s disease and Alzheimer’s disease and network pharmacology analysis. Pharmacol. Res. 2025, 212, 107578. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Tan, H.Y.; Chai, J.Y.; Han, L.L.; Zhai, C.Z.; Lee, J.J.; Li, X.M.; Zhao, Y.Q. Ginseng fruit rare saponins (GFRS) improved inflammatory response: In vitro and in vivo assessment. Fitoterapia 2024, 179, 106244. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Y.Y.; Gu, M.M.; Liu, Z.Z.; Zhang, J.Y.; Zeng, Q.; Zhu, D.H. Biotransformation of ginsenoside Rc to Rd by endophytic bacterium Bacillus sp. G9y isolated from Panax quinquefolius. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2021, 114, 437–444. [Google Scholar] [CrossRef]
- Li, Y.; Liang, Y.; Cui, X.; Shao, L.; Lou, D.; Yang, X. Screening of a strain for ginsenoside Rb1 transformation and analysis of its saponin products. Microbiol. China 2023, 50, 2127–2136. [Google Scholar]
- Wang, P.H.; Gao, Y.S.; Yang, G.; Zhao, Y.J.; Zhao, Z.J.; Gao, G.; Zhao, L.; Li, S.Y. Enhancing the inhibition of cell proliferation and induction of apoptosis in H22 hepatoma cells through biotransformation of notoginsenoside R1 by Lactiplantibacillus plantarum S165 into 20(S/R)-notoginsenoside R2. Rsc Adv. 2023, 13, 29773–29783. [Google Scholar] [CrossRef]
- Renchinkhand, G.; Cho, S.H.; Park, Y.W.; Song, G.Y.; Nam, M.S. Biotransformation of Major Ginsenoside Rb1 to Rd by Dekkera anomala YAE-1 from Mongolian Fermented Milk (Airag). J. Microbiol. Biotechnol. 2020, 30, 1536–1542. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, Y.F.; Dai, Z.P.; Liang, Y.; Zhu, C.Y.; Su, C.; Song, L.S.; Wang, K.P.; Li, J.; Wei, X.Y. Gypenoside biotransformation into ginsenoside F2 by endophytic Aspergillus niger from Gynostemma pentaphyllum. Nat. Prod. Res. 2024, 38, 3086–3092. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y. Biotransformation of ginsenoside Rb1 to Gyp-XVII and minor ginsenoside Rg3 by endophytic bacterium Flavobacterium sp. GE 32 isolated from Panax ginseng. Lett. Appl. Microbiol. 2019, 68, 134–141. [Google Scholar] [CrossRef]
- Fu, Y.; Yin, Z.H.; Wu, L.P.; Yin, C.R. Biotransformation of ginsenoside Rb1 to ginsenoside C-K by endophytic fungus Arthrinium sp GE 17-18 isolated from Panax ginseng. Lett. Appl. Microbiol. 2016, 63, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Ten, L.N.; Chae, S.M.; Yoo, S.A. Biotransformation of Ginsenosides Re and Rg1 by the Bacterium Microbacterium sp GT35. Chem. Nat. Compd. 2015, 51, 81–86. [Google Scholar] [CrossRef]
- Liu, X.; Qiao, L.R.; Xie, D.; Zhang, Y.; Zou, J.H.; Chen, X.G.; Dai, J.G. Microbial transformation of ginsenoside-Rg1 by Absidia coerulea and the reversal activity of the metabolites towards multi-drug resistant tumor cells. Fitoterapia 2011, 82, 1313–1317. [Google Scholar] [CrossRef]
- Hu, Y.B.; Wang, N.; Yan, X.C.; Yuan, Y.; Luo, F.; Jiang, Z.Y.; Zhou, Y.F. Ginsenoside Re impacts on biotransformation products of ginsenoside Rb1 by Cellulosimicrobium cellulans sp. 21 and its mechanisms. Process Biochem. 2019, 77, 57–62. [Google Scholar] [CrossRef]
- Guo, M.K.; Shao, S.; Wang, D.D.; Zhao, D.Q.; Wang, M.X. Recent progress in polysaccharides from Panax ginseng C. A. Meyer. Food Funct. 2021, 12, 494–518. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Bryant, D.L.; Farone, A.L. Panax quinquefolius (North American Ginseng) Polysaccharides as Immunomodulators: Current Research Status and Future Directions. Molecules 2020, 25, 5854. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Yin, F. Physicochemical Properties and Biological Effects of Ginseng Polysaccharide and Its Application in Animal Production. Chin. J. Anim. Nutr. 2024, 36, 7626–7634. [Google Scholar]
- Lee, S.J.; In, G.; Han, S.T.; Lee, M.H.; Lee, J.W.; Shin, K.S. Structural characteristics of a red ginseng acidic polysaccharide rhamnogalacturonan I with immunostimulating activity from red ginseng. J. Ginseng Res. 2020, 44, 570–579. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G.L. Antioxidant activity of polysaccharides from different sources of ginseng. Int. J. Biol. Macromol. 2019, 125, 906–908. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.S.; Sun, L.; Ji, L.; Zhu, J.J.; Fan, Y.Y.; Tai, G.H.; Zhou, Y.F. Further analysis of the structure and immunological activity of an RG-I type pectin from Panax ginseng. Carbohydr. Polym. 2012, 89, 519–525. [Google Scholar] [CrossRef]
- Kim, H.; Suh, H.J.; Kwon, K.H.; Hwang, J.H.; Yu, K.W. Immunostimulation activity of a polysaccharide from fermented ginseng with Hericium erinaceum mycelia in solid-state culture. Food Sci. Biotechnol. 2016, 25, 311–318. [Google Scholar] [CrossRef] [PubMed]
- You, S.Q.; Shi, X.Q.; Yu, D.; Zhao, D.; An, Q.; Wang, D.D.; Zhang, J.C.; Li, M.; Wang, C.T. Fermentation of Panax notoginseng root extract polysaccharides attenuates oxidative stress and promotes type I procollagen synthesis in human dermal fibroblast cells. Bmc Complement. Med. Ther. 2021, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Lv, C.N.; Lu, J.C. Natural occurring polysaccharides from Panax ginseng C. A. Meyer: A review of isolation, structures, and bioactivities. Int. J. Biol. Macromol. 2019, 133, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.R.; Ren, J.; Jiang, Z.Y.; Zhou, S.; Wang, E.P.; Li, H.; Wu, W.; Zhang, X.Y.; Wang, J.; Jiao, L.L. Structural characterization and immunostimulant activities of polysaccharides fractionated by gradient ethanol precipitation method from Panax ginseng C. A. Meyer. Front. Pharmacol. 2024, 15, 1388206. [Google Scholar] [CrossRef]
- Kang, J.; Zhao, J.; He, L.F.; Li, L.X.; Zhu, Z.K.; Tian, M.L. Extraction, characterization and anti-oxidant activity of polysaccharide from red Panax ginseng and Ophiopogon japonicus waste. Front. Nutr. 2023, 10, 1183096. [Google Scholar] [CrossRef]
- Zhao, Q.; Bai, L.; Zhu, D.W.; Li, T.Y.; Xu, J.; Xu, Y.; Zhou, X.M. Clinical efficacy and potential mechanism of ginseng polysaccharides in the treatment of non-small cell lung cancer based on meta-analysis associated with network pharmacology. Heliyon 2024, 10, e27152. [Google Scholar] [CrossRef]
- Ai, J.; Bao, B.; Battino, M.; Giampieri, F.; Chen, C.; You, L.J.; Cespedes-Acuña, C.L.; Ognyanov, M.; Tian, L.M.; Bai, W.B. Recent advances on bioactive polysaccharides from mulberry. Food Funct. 2021, 12, 5219–5235. [Google Scholar] [CrossRef]
- Chung, Y.; Park, J.Y.; Lee, J.E.; Kim, K.T.; Paik, H.D. Antioxidant Activity and Inhibitory Effect on Nitric Oxide Production of Hydroponic Ginseng Fermented with Lactococcus lactis KC24. Antioxidants 2021, 10, 1614. [Google Scholar] [CrossRef]
- Zhang, D.; Du, M.Z.; Wei, Y.; Wang, C.T.; Shen, L.Q. A review on the structure-activity relationship of dietary flavonoids for protecting vascular endothelial function: Current understanding and future issues. J. Food Biochem. 2018, 42, e12557. [Google Scholar] [CrossRef]
- Elmeligy, S.; Hathout, R.M.; Khalifa, S.A.M.; El-Seedi, H.R.; Farag, M.A. Pharmaceutical manipulation of citrus flavonoids towards improvement of its bioavailability and stability. A mini review and a meta-analysis study. Food Biosci. 2021, 44, 101428. [Google Scholar] [CrossRef]
- Rauf, A.; Ahmad, Z.; Formanowicz, D.; Ribaudo, G.; Alomar, T.S. Editorial: Antioxidant potential of polyphenolic and flavonoid compounds. Front. Chem. 2024, 12, 1463755. [Google Scholar] [CrossRef] [PubMed]
- Song, M.W.; Park, J.Y.; Kim, W.J.; Kim, K.T.; Paik, H.D. Fermentative effects by probiotic Lactobacillus brevis B7 on antioxidant and anti-inflammatory properties of hydroponic ginseng. Food Sci. Biotechnol. 2023, 32, 169–180. [Google Scholar] [CrossRef]
- Yoo, J.M.; Lee, J.Y.; Lee, Y.G.; Baek, S.; Kim, M.R. Enhanced production of compound K in fermented ginseng extracts by Lactobacillus brevis. Food Sci. Biotechnol. 2019, 28, 823–829. [Google Scholar] [CrossRef]
- Park, J.-W.; Kim, J.H.; Kwon, Y.-A.; Kim, W.J. Fermentation properties of beer produced from Korean two-row barley or malt (Gwangmaek) supplemented with Korean red ginseng extracts and Bokbunja (Rubus coreanus Miquel) juice. Korean J. Food Sci. Technol. 2019, 51, 596–603. [Google Scholar]
- Pyo, M.-J.; Cho, A.-R.; Kang, M.-J.; Kim, G.-W.; Shin, J.-H. Physicochemical characteristics and ginsenoside content of Korean traditional wine produced by fermentation of Panax ginseng sprouts. Food Sci. Preserv. 2018, 25, 659–667. [Google Scholar]
- Jung, J.; Paik, H.D.; Yoon, H.J.; Jang, H.J.; Jeewanthi, R.K.C.; Jee, H.S.; Li, X.; Lee, N.K.; Lee, S.K. Physicochemical Characteristics and Antioxidant Capacity in Yogurt Fortified with Red Ginseng Extract. Korean J. Food Sci. Anim. Resour. 2016, 36, 412–420. [Google Scholar] [CrossRef]
- Cimo, A.; Soltani, M.; Lui, E.; Hekmat, S. Fortification of probiotic yogurt with ginseng (Panax quinquefolius) extract. Food Nut Disord. 2013, 2, 1–5. [Google Scholar]
- Kim, D.-K.; Baik, M.-Y.; Kim, H.-K.; Hahm, Y.-T.; Kim, B.-Y. Manufacture of the Red Ginseng Vinegar Fermented with Red Ginseng Concentrate and Rice Wine, and its Quality Evaluation. Korean J. Food Sci. Technol. 2012, 44, 179–184. [Google Scholar] [CrossRef]
- Oh, Y.-J.; Kwon, S.-H.; Choi, K.B.; Kim, T.-S.; Yeo, I.-H. Effect of Vinegar Made with Hydroponic-cultured Panax ginseng C. A. Meyer on Body Weight and Lipid Metabolism in High-Fat Diet-Fed Mice. Korean J. Food Sci. Technol. 2014, 46, 743–749. [Google Scholar] [CrossRef]
- Li, K.; Wang, L.; Zhou, R.; Fan, H.; Sui, J. Amelioration of alcohol-induced liver injury in mice by ginsenosides in ginseng wine. J. Funct. Foods 2019, 54, 281–288. [Google Scholar] [CrossRef]
- Heon-Sang, J.; Tae-Su, K.; Koan-Sik, W.; Kee-Yeoup, P.; Kee-Won, Y.; Seung-Joon, Y. Effects of cultured wild ginseng roots on the alcoholic fermentation. Food Sci. Preserv. 2005, 12, 402–410. [Google Scholar]
- Jang, M.; Min, J.-W.; Yang, D.-U.; Jung, S.-K.; Kim, S.-Y.; Yang, D.-C. Ethanolic fermentation from red ginseng extract using Saccharomyces cerevisiae and Saccharomyces carlsbergensis. Food Sci. Biotechnol. 2011, 20, 131–135. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kang, B.-H.; Noh, S.-G.; Kim, J.-G.; Lee, S.-H.; Lee, J.-M. Monitoring on Alcohol Fermentation Properties of Red Ginseng Extracts. J. Life Sci. 2008, 18, 550–555. [Google Scholar] [CrossRef]
- Choi, K.H.; Sohn, E.-H.; Kim, S.J.; Lee, J.-H.; Jang, K.-H. Physicochemical characteristics and ginsenosides compositions of Makgeolli added with mountain ginsengs. J. East Asian Soc. Diet. Life 2013, 23, 437–443. [Google Scholar]
- Chu, Q.; Piao, C.; Zhou, Y.; Wang, Y.; Yu, H.; Liu, J.; Li, P. Optimization of fermentation technique of ginseng rice wine by kefir grain. Chin. J. Chem. Eng. 2017, 39, 343–348. [Google Scholar]
- Sung, J.-H.; Han, M.-J. Quality characteristics of Jeungpyun manufactured by ginseng Makgeolli. Korean J. Food Cook. Sci. 2008, 24, 837–848. [Google Scholar]
- Zheng, F.; Zhang, Y.; Han, M.; Qiao, M.; Dai, Y.; Yue, H.; Liu, S. Study on the Change of Ginsenosides in Fermenting Fresh Ginseng and Prickly Pear by RRLC-Q-TOF MS. J. Chin. Mass Spectrom. Soc. 2018, 39, 532–539. [Google Scholar] [CrossRef]
- Jang, H.J.; Jung, J.; Yu, H.S.; Lee, N.K.; Paik, H.D. Evaluation of the Quality of Yogurt Using Ginseng Extract Powder and Probiotic Lactobacillus plantarum NK181. Korean J. Food Sci. Anim. Resour. 2018, 38, 1160–1167. [Google Scholar] [CrossRef]
- Park, S.-Y.; Hwang, U.-S.; You, C.-B.; Lee, E.-S.; Park, H. Quality Characteristics of Red Ginseng Yogurt Produced with Probiotic Lactic Acid Bacteria Isolated from Kimchi. Curr. Top. Lact. Acid Bact. Probiotics 2021, 7, 67–76. [Google Scholar] [CrossRef]
- Lee, S.B.; Ganesan, P.; Kwak, H.S. Comparison of Nanopowdered and Powdered Ginseng-added Yogurt on Its Physicochemical and Sensory Properties during Storage. Korean J. Food Sci. Anim. Resour. 2013, 33, 24–30. [Google Scholar] [CrossRef]
- Kim, N.-Y.; Han, M.-J. Development of ginseng yogurt fermented by Bifidobacterium spp. Korean J. Food Cook. Sci. 2005, 21, 575–584. [Google Scholar]
- Hyoung-Churl, B.; Myoung-Soo, N. Properties of the Mixed Fermentation Milk Added with Red Ginseng Extract. Food Sci. Anim. Resour. 2006, 26, 127–135. [Google Scholar]
- Seo, H.; Jeon, B.-D.; Ryu, S. Persimmon vinegar ripening with the mountain-cultivated ginseng ingestion reduces blood lipids and lowers inflammatory cytokines in obese adolescents. J. Exerc. Nutr. Biochem. 2015, 19, 1–10. [Google Scholar] [CrossRef]
- Supeno, D.; Kwon, S.; Sung-Won, C.; Goo, K.S.; JongMin, P.; Kim, J.; Choi, W. A Study on the Vinegar Fermentation Processes of Fresh Korean Ginseng Extract Using Mix Microbial Yinkin. J. Korean Soc. Ind. Converg. 2017, 20, 345–350. [Google Scholar]
- Ahn, C.H.; Yang, B.W.; Sung-Kwon, K. Functional Ingredients and Antioxidant Activity of Korean Traditional Wild Simulated Ginseng Vinegar and Red Ginseng Vinegar. Food Eng. Prog. 2021, 25, 51–60. [Google Scholar] [CrossRef]
- Nie, Y.S.; Sun, G.R.; Zhang, X.L. Design of Green Processing Chain for Processing of Ginseng Fruit Vinegar. Adv. Mater. Res. 2014, 933, 988–993. [Google Scholar] [CrossRef]
- Park, W.-L.; Kim, J.-H.; Seo, K.-I. Anti-fatigue effect of a beverage mixture containing red ginseng and Prunus mume fruit vinegar on high-intensity exercised rats. Food Sci. Preserv. 2023, 30, 514–525. [Google Scholar]
- Zhao, F.; Li, M.; Meng, L.L.; Yu, J.H.; Zhang, T.H. Characteristics of Effervescent Tablets of Lactobacilli Supplemented with Chinese Ginseng (Panax ginseng CA Meyer) and Polygonatum sibiricum. Appl. Sci. 2020, 10, 3194. [Google Scholar] [CrossRef]
- Park, J.-H.; Moon, H.-J.; Oh, J.-H.; Lee, J.-H.; Jung, H.-K.; Choi, K.-M.; Cha, J.-D.; Lim, J.-Y.; Han, S.-B.; Lee, T.-B. Changes in the functional components of Lactobacillus acidophilus-fermented red ginseng extract and its application to fresh cheese production. J. Dairy Sci. Biotechnol. 2014, 32, 47–53. [Google Scholar]
- Choi, K.-H.; Min, J.-Y.; Ganesan, P.; Bae, I.-H.; Kwak, H.-S. Physicochemical and sensory properties of red ginseng extracts or red ginseng hydrolyzates-added asiago cheese during ripening. Asian-Australas. J. Anim. Sci. 2015, 28, 120. [Google Scholar] [CrossRef] [PubMed]
- You, W. The Physicochemical Properties, Antioxidant Activity, and Sensory Evaluation of Red Ginseng Added to Greek Yogurt Made With Cow’s Milk. Master’s Thesis, 2024. [Google Scholar]
- Eom, S.J.; Hwang, J.E.; Kim, K.T.; Paik, H.D. Antibacterial Effects against Various Foodborne Pathogens and Sensory Properties of Yogurt Supplemented with Panax ginseng Marc Extract. Korean J. Food Sci. Anim. Resour. 2017, 37, 787–791. [Google Scholar] [CrossRef]
- Jiang, S.; Li, H.; Zhang, L.; Mu, W.; Zhang, Y.; Chen, T.; Wu, J.; Tang, H.; Zheng, S.; Liu, Y.; et al. Generic Diagramming Platform (GDP): A comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. 2025, 53, D1670–D1676. [Google Scholar] [CrossRef] [PubMed]
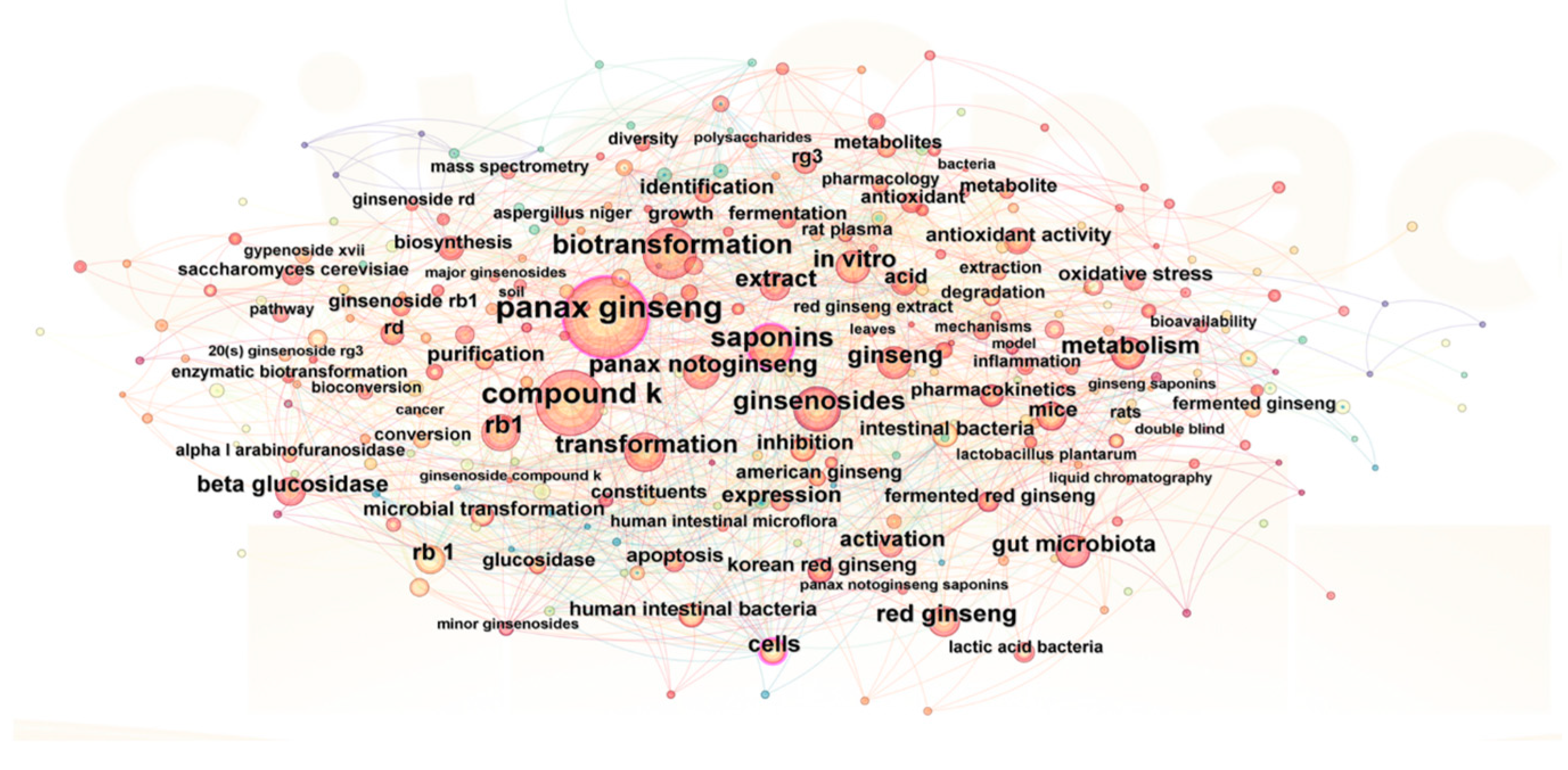

| Classification | Keyword | Frequency |
|---|---|---|
| Ginseng | Panax ginseng | 270 |
| Red ginseng | 62 | |
| Korean red ginseng | 28 | |
| Fermented red ginseng | 27 | |
| Panax notoginseng | 64 | |
| Notoginseng | 6 | |
| American ginseng | 23 | |
| Panax quinquefolius | 8 | |
| Black ginseng | 5 | |
| Fermented black ginseng | 5 | |
| Microorganism | Lactic acid bacteria | 20 |
| Lactobacillus | 4 | |
| Lactiplantibacillus plantarum | 13 | |
| Limosilactobacillus fermentum | 3 | |
| Aspergillus niger | 16 | |
| Aspergillus tubingensis | 5 | |
| Ganoderma lucidum | 10 | |
| Saccharomyces cerevisiae | 7 | |
| Bacillus amyloliquefaciens | 3 | |
| Bacillus subtilis | 3 | |
| Active ingredient | Compound k | 181 |
| Ginsenoside compound k | 11 | |
| C K | 7 | |
| Ginsenosides | 105 | |
| Rb1 | 134 | |
| Ginsenoside Rb1 | 26 | |
| Rd | 31 | |
| Ginsenoside Rd | 17 | |
| Rg3 | 30 | |
| Ginsenoside Rg3 | 2 | |
| Rh2 | 9 | |
| 20(s) ginsenoside Rh2 | 2 | |
| F2 | 11 | |
| Ginsenoside Rb2 | 2 | |
| Rc | 5 | |
| Panax notoginseng saponins | 11 | |
| Polysaccharides | 19 | |
| Phenolic compounds | 12 |
| NO. | Transformation Pathway |
|---|---|
| 1 | Rb1→Gyp17→Gyp75→C-K→PPD |
| 2 | Rb1→Gyp17→F2→C-K→PPD |
| 3 | Rb1→Gyp17→F2→Rh2→PPD |
| 4 | Rb1→Rd→F2→C-K→PPD |
| 5 | Rb1→Rd→F2→Rh2→PPD |
| 6 | Rb1→Rd→Rg3→Rh2→PPD |
| NO. | Transformation Pathway |
|---|---|
| 1 | Re→Rg1→Rh1→PPT |
| 2 | Re→Rg1→F1→PPT |
| 3 | Re→Rg2→Rh1→PPT |
| Microorganisms | Substrates | Conversion Rates | Transformation Pathways | References |
|---|---|---|---|---|
| Aspergillus niger XD101 | Rb1 | 94.4% | Rb1→Rd→F2→C-K | [110] |
| Endophytic bacterium G9y | Rc | 98% | Rc→Rd | [127] |
| Pestalotiopsis biciliata | Rb1 | - | Rb1→Rd→F2→C-K | [128] |
| Cordyceps militaris C03 | Rg1 | 54.9% | Rg1→Rh1 | [121] |
| Rg1→F1 | ||||
| Rc | 83.44% | Rc→Rd→Rg3→CK | ||
| Rc→CMc | ||||
| Lentilactobacillus buchneri URN103L | Rb1 | - | Rb1→Rd→Rg3 | [111] |
| Lactiplantibacillus plantarum S165 | R1 | 82.85% | R1→20(S/R)-R2 | [129] |
| Dekkera anomala YAE-1 | Rb1 | - | Rb1→Rd | [130] |
| Aspergillus niger JGL8 | Gypenoside | - | Gyp-V→Rd→F2 | [131] |
| Gyp-XVII→F2 | ||||
| Penicillium decumbens | Rb1 | - | Rb1→Gyp17→F2→C-K | [29] |
| Rb1→Rd→F2→C-K | ||||
| Rb1→Rd→Rg3→Rh2 | ||||
| Flavobacterium sp. GE 32 | Rb1 | - | Rb1→Gyp-XVII | [132] |
| Rb1→Rd→Rg3 | ||||
| Microbacterium trichothecenolyticum KCTC 19343 | Rb1 | - | Rb1→Rd→Rh2 | [30] |
| Endophytic fungi GE 17-18 | Rb1 | - | Rb1→Rd→F2→C-K | [133] |
| Microbacterium sp. GT35 | Re | 72% | Re→Rg2 | [134] |
| Rg1 | - | Rg1→Rh1 | ||
| Absidia coerulea AS 3.2462 | Rg1 | - | Rg1→F1 | [135] |
| Cordyceps Sinensis CICC14017 | Rg1 | 82.5% | Rg1→20(S/R)-Rh1→25-OH-20(S/R)-Rh1 | [122] |
| Cellulosimicrobium cellulans sp. 21 | Rb1 | - | Rb1→Rd→Rg3→Rh2→PPD | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, J.; Zhu, Z.; Luo, W.; Jang, M.; Pan, B.; Zhu, Y.; Zhang, J.; Zhao, Y.; Xiao, X. Microbial Fermentation Affects the Structure–Activity Relationship of Bioactive Compounds in Ginseng and Its Applications in Fermentation Products: A Review. Foods 2025, 14, 2473. https://doi.org/10.3390/foods14142473
Bai J, Zhu Z, Luo W, Jang M, Pan B, Zhu Y, Zhang J, Zhao Y, Xiao X. Microbial Fermentation Affects the Structure–Activity Relationship of Bioactive Compounds in Ginseng and Its Applications in Fermentation Products: A Review. Foods. 2025; 14(14):2473. https://doi.org/10.3390/foods14142473
Chicago/Turabian StyleBai, Juan, Zixian Zhu, Wei Luo, Miran Jang, Beibei Pan, Ying Zhu, Jiayan Zhang, Yansheng Zhao, and Xiang Xiao. 2025. "Microbial Fermentation Affects the Structure–Activity Relationship of Bioactive Compounds in Ginseng and Its Applications in Fermentation Products: A Review" Foods 14, no. 14: 2473. https://doi.org/10.3390/foods14142473
APA StyleBai, J., Zhu, Z., Luo, W., Jang, M., Pan, B., Zhu, Y., Zhang, J., Zhao, Y., & Xiao, X. (2025). Microbial Fermentation Affects the Structure–Activity Relationship of Bioactive Compounds in Ginseng and Its Applications in Fermentation Products: A Review. Foods, 14(14), 2473. https://doi.org/10.3390/foods14142473









