Dry-Cured Bísaro Ham: Differences in Physicochemical Characteristics, Fatty Acid Profile and Volatile Compounds Between Muscles
Abstract
1. Introduction
2. Materials and Methods
2.1. Dry-Cured Bísaro Ham Production Process
2.2. Dry-Cured Bísaro Ham Muscle Sampling
2.3. Proximate Composition, Sodium Chloride, Collagen, Heme Pigment Contents and aw
2.4. Fatty Acid Composition Analysis
2.5. Volatile Compound Profile
2.6. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition, Sodium Chloride, Collagen and Heme Pigment Contents, aw and pH
3.2. Fatty Acid Composition
3.3. Volatile Compounds
3.4. Muscle Profiles from Principal Component and Discriminant Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leite, A.; Vasconcelos, L.; Ferreira, I.; Domínguez, R.; Pereira, E.; Rodrigues, S.; Lorenzo, J.M.; Teixeira, A. Effect of the inclusion of olive cake in the diet on the physicochemical characteristics of dry-cured loin and dry-cured “cachaço” of Bísaro pig. Appl. Sci. 2023, 13, 1439. [Google Scholar] [CrossRef]
- Despacho nº16840/2005. 2ª Série. Available online: https://files.diariodarepublica.pt/2s/2005/08/149000000/1112411129.pdf (accessed on 15 September 2024).
- Fernández, M.; Ordoñez, J.A.; Cambero, I.; Santos, C.; Pin, C.; Hoz, L. Fatty acids compositions of selected varieties of Spanish dry ham related to their nutritional implications. Food Chem. 2007, 101, 107–112. [Google Scholar] [CrossRef]
- Domínguez, R.; Purriños, L.; Pérez-Santaescolástica, C.; Pateiro, M.; Barba, J.F.; Tomasevic, I.; Bastianello-Campagnol, P.C.; Lorenzo, J.M. Caracterization of Volatile Compounds of dry-cured meat products using HS-SPME-GC/MS Techique. Food Anal. Methods 2019, 12, 1263–1284. [Google Scholar] [CrossRef]
- Ivanovic, S.; Nesic, K.; Pisinov, B.; Pavlovic, I. The impact of diet on the quality of fresh meat and smoked ham in goat. Small Rumin. Res. 2016, 138, 53–59. [Google Scholar] [CrossRef]
- Belloch, C.; Neef, A.; Salafia, C.; López-Diez, J.J.; Flores, M. Microbiota and volatilome of dry-cured pork loins manufactured with paprika and reduced concentration of nitrite and nitrate. Food Res. Int. 2021, 149, 110691. [Google Scholar] [CrossRef]
- Martín-Mateos, M.J.; Amaro-Blanco, G.; Manzano, R.; Andrés, A.I.; Ramírez, R. Efficacy of modified active packaging with oxygen scavengers for the preservation of sliced Iberian dry-cured shoulder. Food Sci. Technol. Int. 2022, 29, 318–330. [Google Scholar] [CrossRef]
- Flores, M.; Durá, M.A.; Marco, A.; Toldrá, F. Effect of Debaryomyces spp. on aroma formation and sensory quality of dry fermented sausages. Meat Sci. 2004, 68, 439–446. [Google Scholar] [CrossRef]
- Bermúdez, R.; Franco, D.; Carballo, J.; Lorenzo, J.M. Physicochemical changes during manufacture and final sensory characteristics of dry-cured Celta ham. Effect of muscle type. Food Control. 2014, 43, 263–269. [Google Scholar] [CrossRef]
- Martínez-Onandi, N.; Rivas-Cañedo, A.; Ávila, M.; Garde, S.; Nuñez, M.; Picon, A. Influence of physicochemical characteristics and high-pressure processing on the volatile fraction of Iberian dry cured ham. Meat Sci. 2017, 131, 40–47. [Google Scholar] [CrossRef]
- Paié-Ribeiro, J.; Pinheiro, V.; Guedes, C.; Gomes, M.J.; Teixeira, J.; Leite, A.; Vasconcelos, L.; Teixeira, A.; Outor-Monteiro, D. Exploring the Potential of Olive By-Products in Bísaro Pig Feed: Effects on the Chemical Compositions and Fatty Acid Profiles of Three Different Muscles. Foods 2025, 14, 836. [Google Scholar] [CrossRef]
- Pugliese, C.; Sirtori, F.; Škrlep, M.; Piasentier, E.; Calamai, L.; Franci, O.; Čandek-Potokar, M. The effect of ripening time on the chemical, textural, volatile and sensorial traits of Bicep femoris and Semimembranosus muscles of the Slovenian drycured ham Kraški pršut. Meat Sci. 2015, 100, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Savić, B.; Škrlep, M.; Marušić Radovčić, N.; Petričević, S.; Čandek-Potokar, M. Quality of Slovenian dry-cured ham from Krškopolje and hybrid pigs: Influence of skin trimming methods. Meat Sci. 2025, 222, 109762. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-RamÍrez, J.; Arnau, J.; Serra, X.; Gou, P. Effect of pH24, NaCl content and proteolysis index on the rela-tionship between water content and texture parameters in Biceps femoris and semimembranosus muscles in dry-cured ham. Meat Sci. 2006, 72, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Marušić Radovčić, N.; Poljanec, I.; Petričević, S.; Mora, L.; Medić, H. Influence of Muscle Type on Physicochemical Parameters, Lipolysis, Proteolysis, and Volatile Compounds throughout the Processing of Smoked Dry-Cured Ham. Foods 2021, 10, 1228. [Google Scholar] [CrossRef]
- Poljanec, I.; Marušić Radovčić, N.; Petričević, S.; Karolyi, D.; Listes, E.; Medić, H. Proteolysis and protein oxidation throughout the smoked dry-cured ham process. Food Chem. 2021, 362, 130207. [Google Scholar] [CrossRef]
- Petričević, S.; Radovčić, N.M.; Lukić, K.; Listeš, E.; Medić, H. Differentiation of dry-cured hams from dif-ferent processing methods by means of volatile compounds, physico-chemical and sensory analysis. Meat Sci. 2018, 137, 217–227. [Google Scholar] [CrossRef]
- Council Regulation (EC). No 1099/2009 of 24 September 2009 on the protection of animals at the time of killing. Off. J. Eur. Communities 2009, 1–30. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:02009R1099-20180518 (accessed on 10 September 2024).
- Álvarez-Rodríguez, J.; Teixeira, A. Slaughter weight rather than sex affects carcass cuts and tissue composition of Bisaro pigs. Meat Sci. 2019, 154, 54–60. [Google Scholar] [CrossRef]
- Specifications for “Presunto de Vinhais or Presunto Bísaro de Vinhais—PGI”. Available online: https://tradicional.dgadr.gov.pt/pt/cat/salsicharia-fumados-presuntos-e-paletas/1043-presunto-de-vinhais-igp-ou-presunto-bisaro-de-vinhais-igpentonces (accessed on 20 September 2024).
- NP-ISO-3441/2008; Determinação do pH (Método de Referência); In Portuguese Norm–Meat and Meat Products. Portuguese Institute of Quality, Ministry of Economy and Innovation: Caparica, Portugal, 2008.
- NP-ISO-1614/2002; Determination of Moisture Content. Reference Method (ISO 1442:1197). In Portuguese Norm-Meat and Meat Products. Portuguese Institute of Quality, Ministry of Economy, and Innovation: Caparica, Portugal, 2002.
- NP-ISO-1615/2002; Determination of Total Ashes. Reference Method (ISO 3496:1994). In Portuguese Norm-Meat and Meat Products. Portuguese Institute of Quality, Ministry of Economy, and Innovation: Caparica, Portugal, 2002.
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- NP-ISO-1612/2002; Determination of Total Nitrogen Content. Determination of Total Nitrogen Content. Reference Method (ISO 937:1978). In Portuguese Norm-Meat and Meat Products. Portuguese Institute of Quality, Ministry of Economy, and Innovation: Caparica, Portugal, 2002.
- NP1845/1982; Meat and Meat Products—Determination of Chloride Content—Standard Method. Portuguese Institute of Quality, Ministry of Economy Innovation: Caparica, Portugal, 1982.
- NP-ISO-1987/2002; Determination of Hydroxyproline Content. Reference Method. Portuguese Institute of Quality, Ministry of Economy, and Innovation: Caparica, Portugal, 2002.
- Hornsey, H.C. The colour of cooked cured pork. I-Estimation of the nitric oxide-haem pigments. J. Sci. Food Agric. 1956, 7, 534–540. [Google Scholar] [CrossRef]
- AOAC International; Cunniff, P. AOAC Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Rockville, MD, USA, 1995; ISBN 9780935584547. [Google Scholar]
- Domínguez, R.; Borrajo, P.; Lorenzo, J.M. The effect of cooking methods on nutritional value of foal meat. J. Food Compos. Anal. 2015, 43, 61–67. [Google Scholar] [CrossRef]
- Teixeira, A.; Fernandes, A.; Pereira, E. Fat content reduction and lipid profile improvement in Portuguese fermented sausages alheira. Heliyon 2020, 6, e05306. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, R.; Franco, D.; Carballo, J.; Lorenzo, J.M. Influence of type of muscle on volatile compounds throughout the manufacture of Celta dry-cured ham. Food Sci. Technol. Int. 2015, 21, 581–592. [Google Scholar]
- de Bianchi, T.L.C.F.P. Comparação de Processos Proteolíticos e Lipolíticos em Músculos de Presuntos Curados de uma População Suína Seleccionada de Acordo com Critérios Tecnológicos. Mestrado de Inovação e Qualidade na Produção Alimentar. Master’s Thesis, Instituto Politécnico de Castelo Branco, Castelo Branco, Portugal, 2013. [Google Scholar]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Dominguez, V.R. Influencia de la Alimentación Sobre los Ácidos Grasos, el Colesterol y el Retinol en Distintos Depósitos Grasos del Cerdo de Raza Celta. Ph.D. Thesis, Universidad de Vigo, Vigo, Spain, 2012. [Google Scholar]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Department of Health and Social Care. Nutritional aspects of cardiovascular disease. Report of the Cardiovascular Review Group Committee on Medical Aspects of Food Policy. Rep. Health Soc. Subj. 1994, 46, 1–186. [Google Scholar]
- Ramirez, R.; Cava, R. Volatile profiles of dry-cured meat products from three different Iberian x Duroc genotypes. J. Agric. Chem. 2007, 55, 1923–1931. [Google Scholar] [CrossRef]
- Jiang, S.; Xia, D.; Wang, X.; Zhu, Y.; Chen, G.; Liu, Y. Analysis of aroma-active compounds in four Chinese dry-cured hams based on GC-O combined with AEDA and frequency detection methods. Food Sci. Technol. 2022, 153, 112497. [Google Scholar] [CrossRef]
- Sánchez-Peña, C.M.; Luna, G.; García-González, D.L.; Aparicio, R. Characterization of French and Spanish dry-cured hams: Influence of the volatiles from the muscles and the subcutaneous fat quantified by SPME-GC. Meat Sci. 2005, 69, 635–645. [Google Scholar] [CrossRef]
- Sirtori, F.; Dimauro, C.; Bozzi, R.; Aquilani, C.; Franci, O.; Pezzati, A.; Pugliese, C. Evolution of volatile compounds and physical, chemical and sensory characteristics of Toscano PDO ham from fresh to dry-cured product. Eur. Food Res. Technol. 2020, 246, 409–424. [Google Scholar] [CrossRef]
- Segura-Borrego, M.P.; Callejón, R.M.; Morales, M.L. Iberian dry-cured ham sliced: Influence of vacuum packaging on volatile profile during chill-storage. Food Packag. Shelf Life 2023, 39, 101155. [Google Scholar] [CrossRef]
- Górska, E.; Nowicka, K.; Jaworska, D.; Prybylski, W.; Tambor, K. Relationship between sensory attributes and volatile compounds of polish dry-cured loin. Asian-Australas. J. Anim. Sci. 2017, 30, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Carballo, J.; Franco, D. Effect of the inclusion of chestnut in the finishing diet on volatile compounds of dry-cured ham from celta pig breed. J. Integr. Agric. 2013, 12, 2002–2012. [Google Scholar] [CrossRef]
- Nárváez-Rivas, M.; Vicario, I.M.; Alcade, M.J.; León-Camacho, M. Volatile hydrocarbon profile of Iberian dry-cured hams. A possible tool for authentication of hams according to the fattening diet. Talanta 2010, 81, 1224–1228. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, Y.; Wang, G.; Zhu, R.; Ge, C.; Liao, G. Changes in the physicochemical properties and volatile flavor compounds of dry-cured Chinese Laowo ham during processing. J. Food Process Preserv. 2020, 44, e14593. [Google Scholar] [CrossRef]
- Gaspardo, B.; Procida, G.; Toso, B.; Stefanon, B. Determination of volatile compounds in San Daniele ham using headspace GC-MS. Meat Sci. 2008, 80, 204–209. [Google Scholar] [CrossRef]
- Sidira, M.; Kandylis, P.; Kanellaki, M.; Kourkoutas, Y. Effect of immobilized Lactobacillus casei on volatile compounds of heat treated probiotic dry-fermented sausages. Food Chem. 2015, 178, 201–207. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Liu, Y.; Zhu, J.; Zhou, G. Changes in the volatile flavour components of Jinhua ham during the traditional ageing process. Int. J. Food Sci. Techol. 2006, 41, 1033–1039. [Google Scholar] [CrossRef]
- López-Salas, L.; Cea, I.; Borrás-Linares, I.; Emanuelli, T.; Robert, P.; Segura-Carretero, A.; Lozano-Sánchez, J. Preliminary investigation of different drying systems to preserve hydroxytyrosol and its derivatives in olive oil filter cake pressurized liquid extracts. Foods 2021, 10, 1407. [Google Scholar] [CrossRef]
- Bosse, R.; Wirth, M.; Becker, T.; Weiss, J.; Gibis, M. Determination of volatile marker compounds in raw ham using headspace-trap gas chromatography. Food Chem. 2017, 219, 249–259. [Google Scholar] [CrossRef]
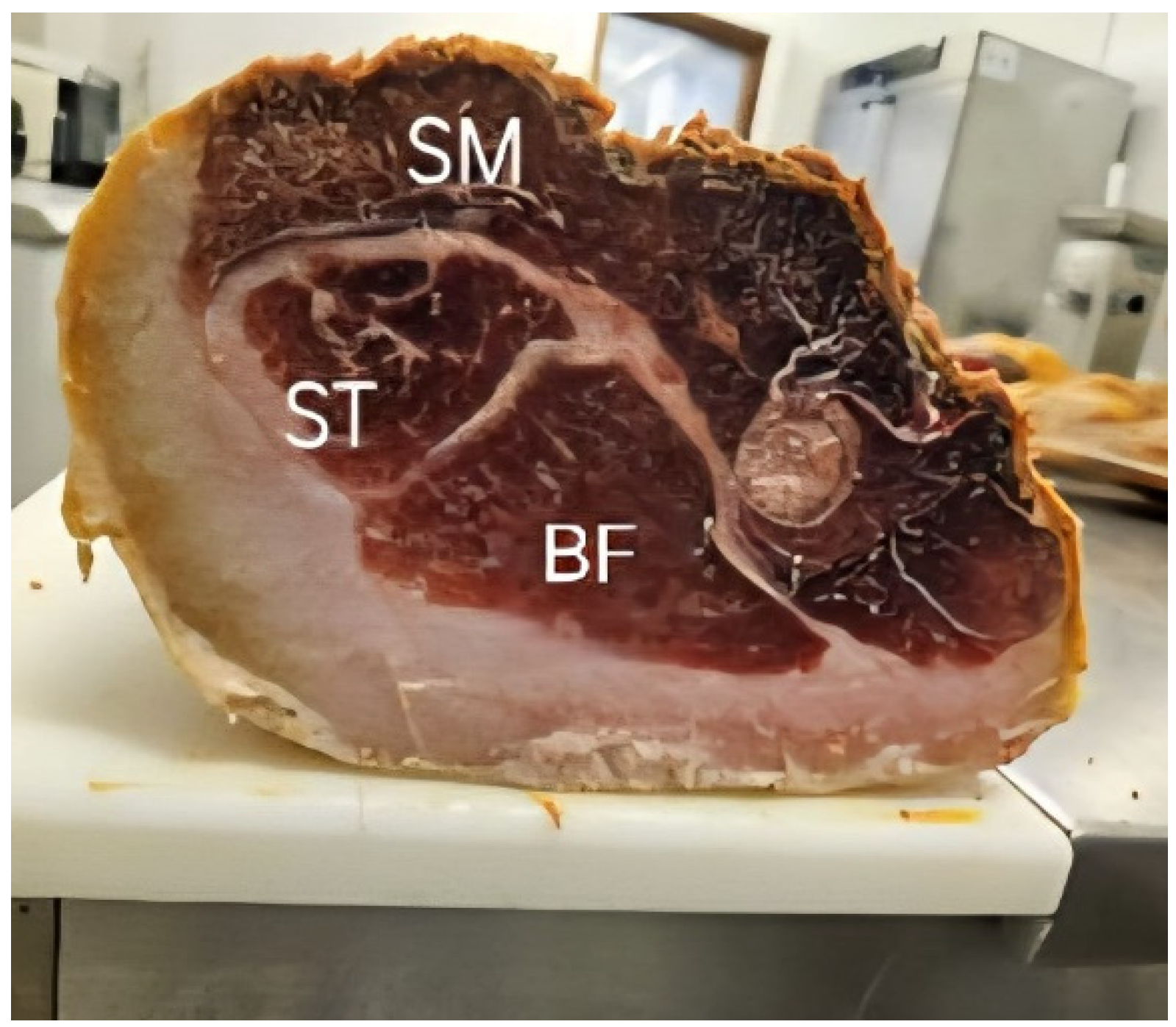
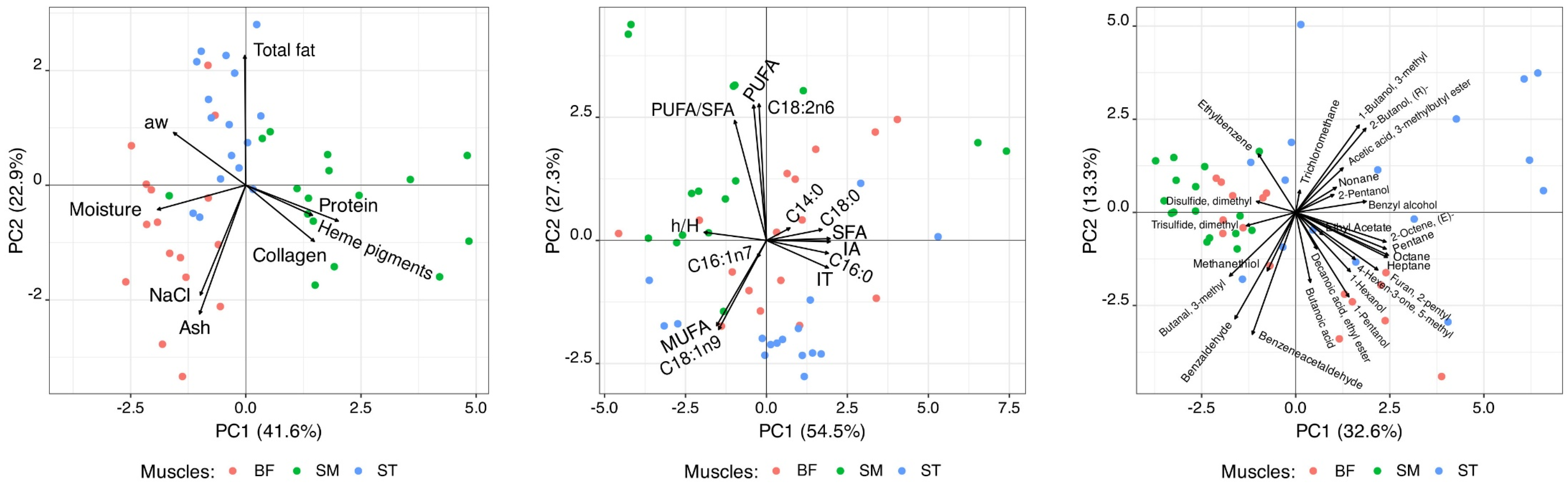
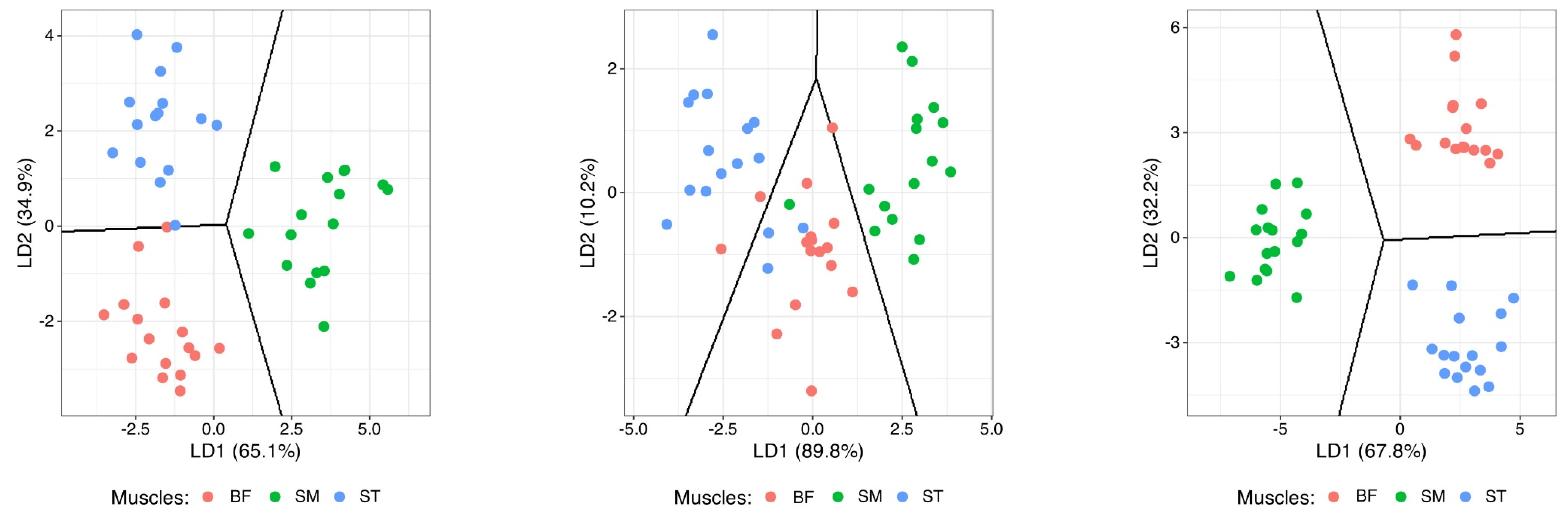
| Parameter | Muscle | SEM | SIG | ||
|---|---|---|---|---|---|
| SM | BF | ST | |||
| aw | 0.84 b | 0.86 a | 0.85 ab | 0.004 | ** |
| Moisture (%) | 42.09 b | 49.30 a | 45.25 b | 0.953 | *** |
| Total Fat (%) | 7.09 b | 6.89 b | 12.78 a | 0.563 | *** |
| Protein (%) | 41.00 a | 29.65 b | 31.06 b | 0.774 | *** |
| Ash (%) | 6.53 b | 7.43 a | 6.82 b | 0.164 | ** |
| NaCl (%) | 5.86 b | 7.10 a | 6.17 b | 0.220 | ** |
| Heme Pigment (mg/g) | 2.60 a | 1.57 b | 1.92 b | 0.142 | *** |
| Collagen (%) | 1.79 a | 1.31 b | 1.10 c | 0.089 | *** |
| FA (%) | Muscle | SEM | SIG | ||
|---|---|---|---|---|---|
| SM | BF | ST | |||
| C16:0 | 24.20 | 24.76 | 24.91 | 0.293 | ns |
| C16:1n-7 | 3.15 | 3.03 | 2.97 | 0.069 | ns |
| C18:0 | 9.94 | 10.68 | 10.45 | 0.342 | ns |
| C18:1n-9 | 50.08 | 50.30 | 51.81 | 0.620 | ns |
| C18:2n-6 | 7.80 a | 6.84 b | 5.67 c | 0.205 | *** |
| SFAs | 35.68 | 36.90 | 35.26 | 1.057 | ns |
| MUFAs | 54.10 | 54.27 | 57.37 | 1.106 | ns |
| PUFAs | 10.21 a | 8.83 b | 7.37 c | 0.283 | *** |
| PUFA/SFA ratio | 0.28 a | 0.24 b | 0.21 c | 0.266 | *** |
| IA | 0.44 | 0.46 | 0.44 | 0.018 | ns |
| IT | 0.99 | 1.07 | 1.04 | 0.041 | ns |
| h/H | 2.36 | 2.26 | 2.25 | 1.897 | ns |
| VOCs | Muscle | SEM | SIG | |||
|---|---|---|---|---|---|---|
| M/Z | BF | SM | ST | |||
| 2-Butanol, (R)- | 45 | 0.87 b | 0.77 b | 2.37 a | 0.332 | 0.002 |
| 1-Butanol | 56 | 1.12 | 0.82 | 1.33 | 0.187 | 0.175 |
| 2-Pentanol | 45 | 14.21 a | 8.73 b | 12.85 ab | 1.541 | 0.041 |
| 1-Pentanol | 70 | 7.58 ab | 5.78 b | 9.03 a | 0.872 | 0.039 |
| 1-Hexanol | 69 | 16.96 ab | 12.54 b | 23.69 a | 2.543 | 0.012 |
| 1-Butanol, 3-methyl- | 70 | 57.52 b | 81.80 b | 144.25 a | 14.320 | <0.001 |
| Benzyl alcohol (aromatic) | 79 | 6.20 b | 4.33 c | 8.53 a | 0.540 | <0.001 |
| TOTAL ALCOHOL CONTENT | 104.45 b | 114.77 b | 202.05 a | 15.605 | <0.001 | |
| Butanal, 3-methyl- | 58 | 51.20 a | 65.05 a | 19.26 b | 6.107 | <0.001 |
| Hexanal | 56 | 27.48 | 20.59 | 12.68 | 4.383 | 0.068 |
| Benzaldehyde (aromatic) | 106 | 30.67 a | 31.09 a | 17.02 b | 2.473 | <0.001 |
| Benzeneacetaldehyde (aromatic) | 91 | 32.62 a | 32.60 a | 19.27 b | 2.912 | 0.002 |
| Nonanal | 57 | 6.82 | 6.29 | 5.20 | 0.873 | 0.416 |
| TOTAL ALDEHYDE CONTENT | 148.79 a | 155.61 a | 73.43 b | 13.195 | <0.001 | |
| Pentane | 72 | 2.06 a | 0.44 b | 2.79 a | 0.453 | 0.002 |
| Heptane | 71 | 37.03 a | 8.96 b | 46.01 a | 6.667 | 0.001 |
| Octane | 85 | 121.25 a | 38.51 b | 156.27 a | 19.56 | <0.001 |
| 2-Octene, (E)- | 112 | 0.93 a | 0.36 b | 1.15 a | 0.150 | 0.002 |
| Ethylbenzene (aromatic) | 91 | 1.12 b | 3.09 a | 2.51 a | 0.338 | 0.001 |
| Nonane | 57 | 1.29 b | 1.06 b | 2.71 a | 0.295 | 0.001 |
| Nonane, 5-methylene- | 56 | 0.71 | 0.83 | 0.96 | 0.087 | 0.155 |
| 2,2,4,4- Tetramethyloctane | 57 | 48.50 | 54.51 | 54.05 | 5.094 | 0.653 |
| Undecane | 57 | 2.13 | 2.75 | 2.36 | 0.253 | 0.225 |
| Tridecane | 57 | 0.32 | 0.33 | 0.33 | 0.054 | 0.991 |
| Toluene (aromatic) | 91 | 84.83 | 106.67 | 130.27 | 26.520 | 0.485 |
| TOTAL HYDROCARBON CONTENT | 300.17 ab | 217.51 b | 399.40 a | 30.242 | 0.001 | |
| 2-Butanone | 72 | 5.29 | 5.50 | 4.37 | 0.412 | 0.129 |
| 2-Pentanone | 86 | 5.95 | 8.79 | 10.94 | 2.588 | 0.400 |
| Acetoin | 45 | 11.33 | 8.36 | 6.14 | 3.203 | 0.522 |
| 2-Hexanone | 58 | 2.55 | 3.92 | 3.46 | 1.010 | 0.626 |
| 2-Heptanone | 58 | 25.35 | 30.30 | 31.50 | 7.138 | 0.812 |
| 4-Hexen-3-one, 5-methyl | 112 | 2.95 a | 1.05 b | 2.78 a | 0.284 | 0.001 |
| 2-Nonanone | 58 | 4.21 | 4.96 | 6.45 | 1.395 | 0.517 |
| TOTAL KETONE CONTENT | 57.63 | 62.88 | 65.64 | 12.760 | ns | |
| Ethyl acetate | 61 | 5.09 | 1.37 | 5.73 | 1.281 | 0.050 |
| Propanoic acid, ethyl ester | 57 | 1.45 | 0.84 | 1.54 | 0.396 | 0.397 |
| Acetic acid, propyl ester | 61 | 0.25 | 0.05 | 0.39 | 0.101 | 0.071 |
| Propanoic acid, 2-methyl-, ethyl ester | 116 | 1.41 | 0.68 | 1.31 | 0.331 | 0.251 |
| Butanoic acid, ethyl ester | 88 | 13.80 | 6.71 | 11.59 | 2.975 | 0.236 |
| Butanoic acid, 2-methyl-, ethyl ester | 102 | 7.45 | 3.92 | 9.11 | 2.358 | 0.293 |
| Butanoic acid, 3-methyl-, ethyl ester | 88 | 13.09 | 6.81 | 15.50 | 4.197 | 0.328 |
| Acetic acid, 3-methylbutyl ester | 70 | 0.77 b | 0.60 b | 2.68 a | 0.504 | 0.009 |
| Acetic acid, 2-methylbutyl ester | 70 | 0.17 | 0.07 | 1.18 | 0.347 | 0.052 |
| Hexanoic acid, ethyl ester | 88 | 35.28 | 16.38 | 41.32 | 10.74 | 0.241 |
| Octanoic acid, ethyl ester | 88 | 11.24 | 3.37 | 14.36 | 3.381 | 0.071 |
| Decanoic acid, ethyl ester | 88 | 2.08 ab | 0.58 b | 3.41 a | 0.766 | 0.041 |
| TOTAL ESTER CONTENT | 92.09 | 41.35 | 108.12 | 24.707 | ns | |
| Methanethiol | 48 | 0.12 ab | 0.15 a | 0.07 b | 0.018 | 0.015 |
| Trichloromethane | 83 | 23.55 b | 16.08 b | 65.26 a | 11.990 | 0.012 |
| Disulfide, dimethyl | 79 | 10.84 b | 19.02 a | 8.98 b | 2.091 | 0.003 |
| Butanoic acid | 60 | 17.71 a | 8.56 b | 12.44 b | 1.357 | <0.001 |
| Furan, 2-pentyl- | 81 | 12.31 | 13.96 | 14.71 | 1.919 | 0.667 |
| Benzoic acid, m-hydroxyphenyl ester | 105 | 1.11 | 0.64 | 0.76 | 0.220 | 0.305 |
| Trisulfide, dimethyl | 126 | 6.37 b | 10.80 a | 5.00 b | 1.250 | 0.005 |
| TOTAL CONTENT OF OTHER COMPOUNDS | 72.01 | 69.20 | 107.23 | 13.388 | ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasconcelos, L.; Dias, L.G.; Leite, A.; Lorenzo, J.M.; Teixeira, A.; Rodrigues, S.S.Q.; Mateo, J. Dry-Cured Bísaro Ham: Differences in Physicochemical Characteristics, Fatty Acid Profile and Volatile Compounds Between Muscles. Foods 2025, 14, 2474. https://doi.org/10.3390/foods14142474
Vasconcelos L, Dias LG, Leite A, Lorenzo JM, Teixeira A, Rodrigues SSQ, Mateo J. Dry-Cured Bísaro Ham: Differences in Physicochemical Characteristics, Fatty Acid Profile and Volatile Compounds Between Muscles. Foods. 2025; 14(14):2474. https://doi.org/10.3390/foods14142474
Chicago/Turabian StyleVasconcelos, Lia, Luís G. Dias, Ana Leite, José M. Lorenzo, Alfredo Teixeira, Sandra S. Q. Rodrigues, and Javier Mateo. 2025. "Dry-Cured Bísaro Ham: Differences in Physicochemical Characteristics, Fatty Acid Profile and Volatile Compounds Between Muscles" Foods 14, no. 14: 2474. https://doi.org/10.3390/foods14142474
APA StyleVasconcelos, L., Dias, L. G., Leite, A., Lorenzo, J. M., Teixeira, A., Rodrigues, S. S. Q., & Mateo, J. (2025). Dry-Cured Bísaro Ham: Differences in Physicochemical Characteristics, Fatty Acid Profile and Volatile Compounds Between Muscles. Foods, 14(14), 2474. https://doi.org/10.3390/foods14142474












