Improving the Surface Color and Delaying Softening of Peach by Minimizing the Harmful Effects of Ethylene in the Package
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Treatments
2.2. Determination of the Weight Loss and Firmness of Peach
2.3. Determination of the Respiratory Rate and Ethylene Production of Peach
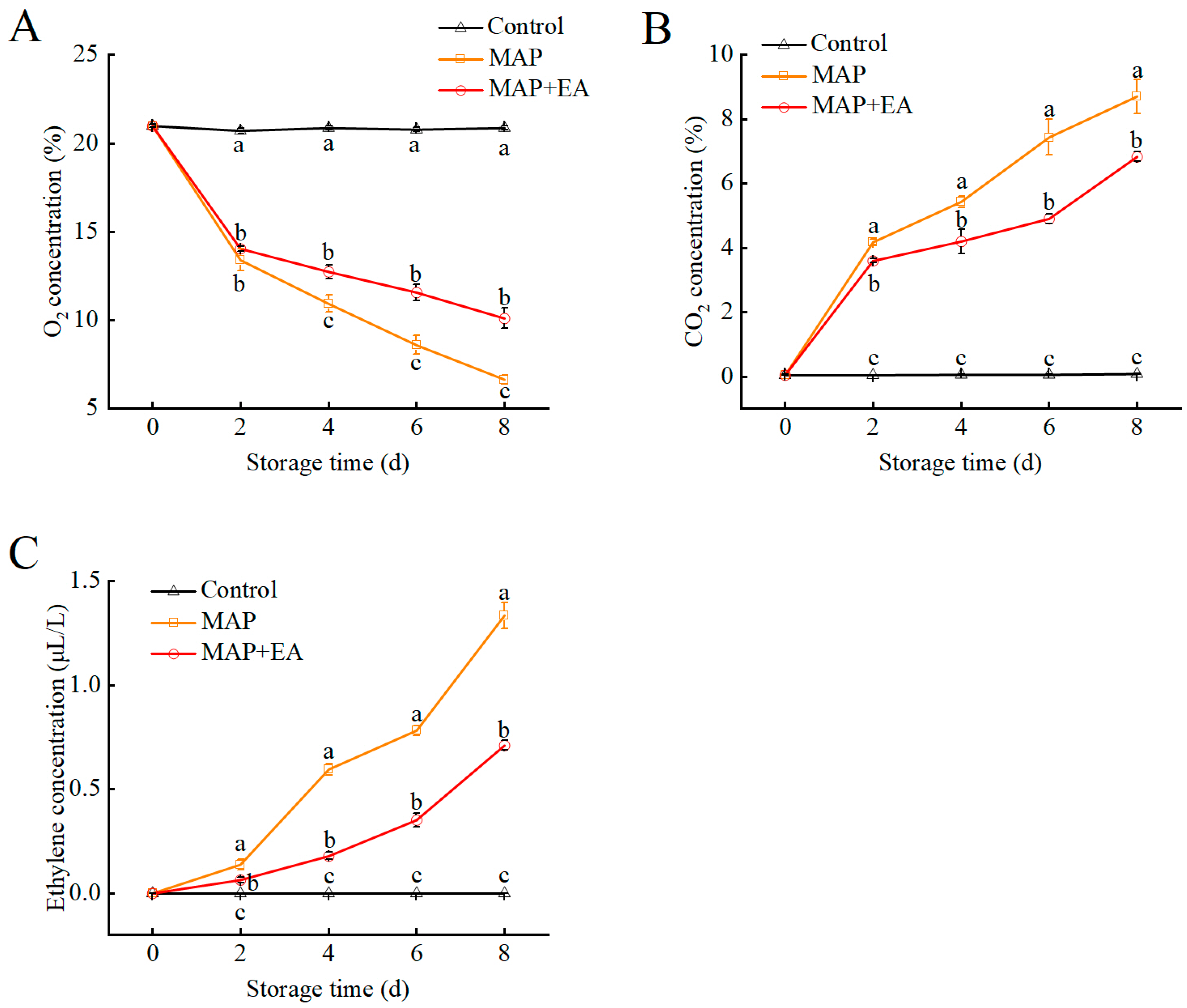
2.4. Determination of the Gaseous Composition in the Package
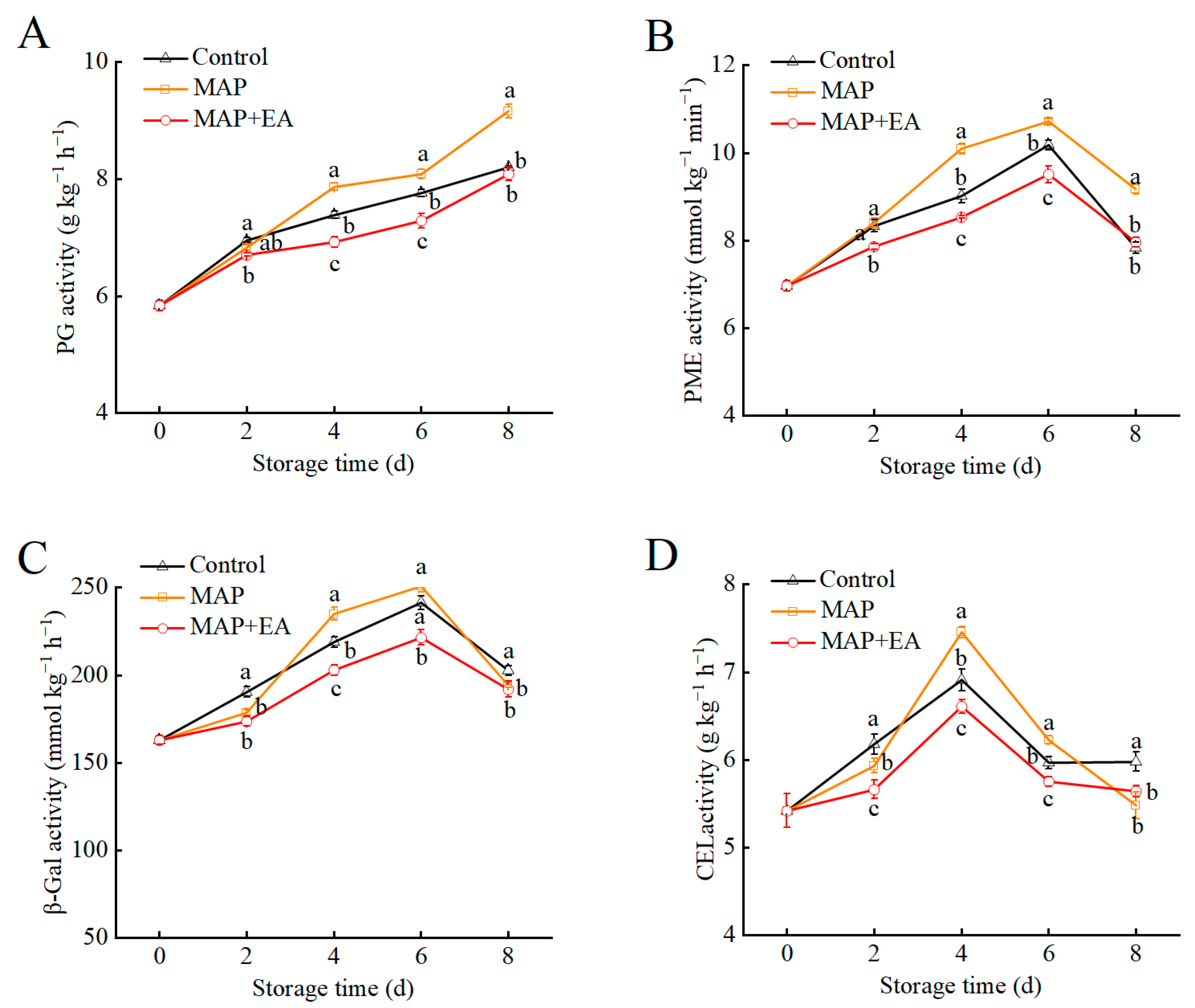
2.5. Measurement of Enzyme Activities of Cell-Wall-Modifying Enzymes
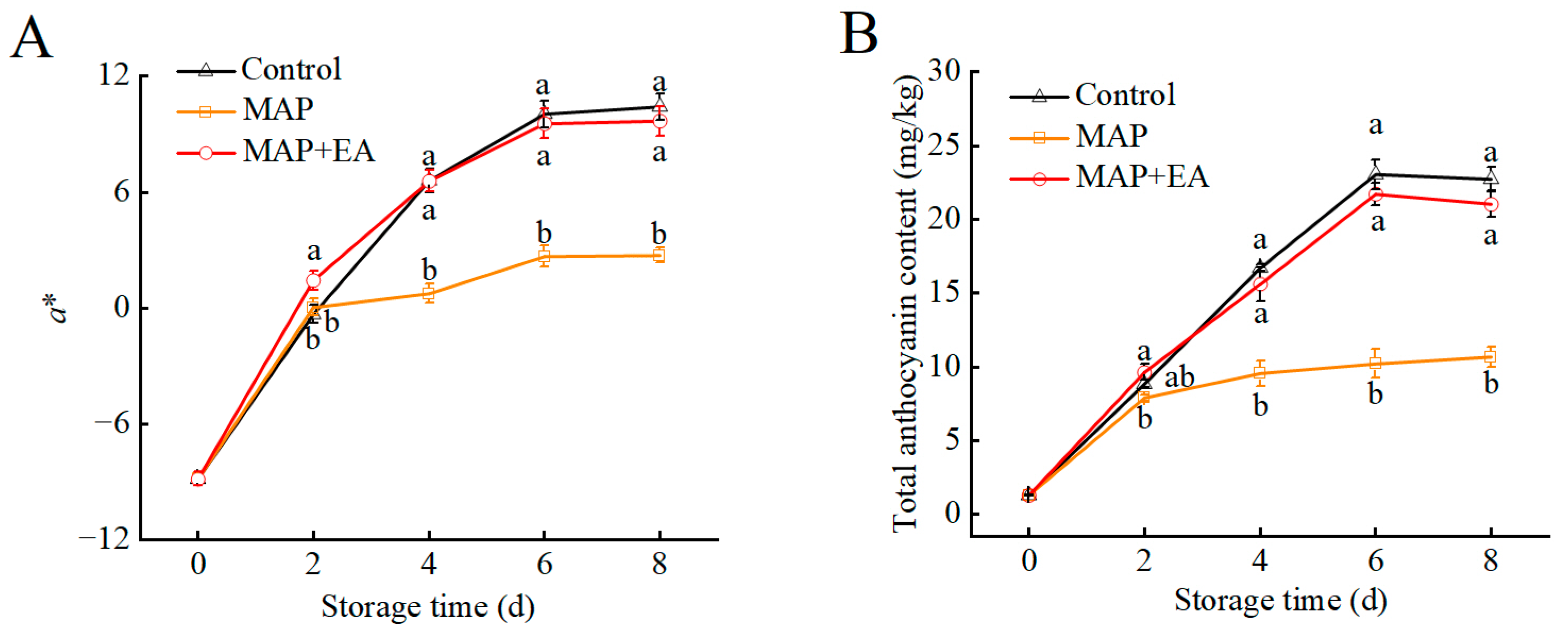
2.6. Determination of the Peel Color of Peach
2.7. Determination of the Contents of Anthocyanin
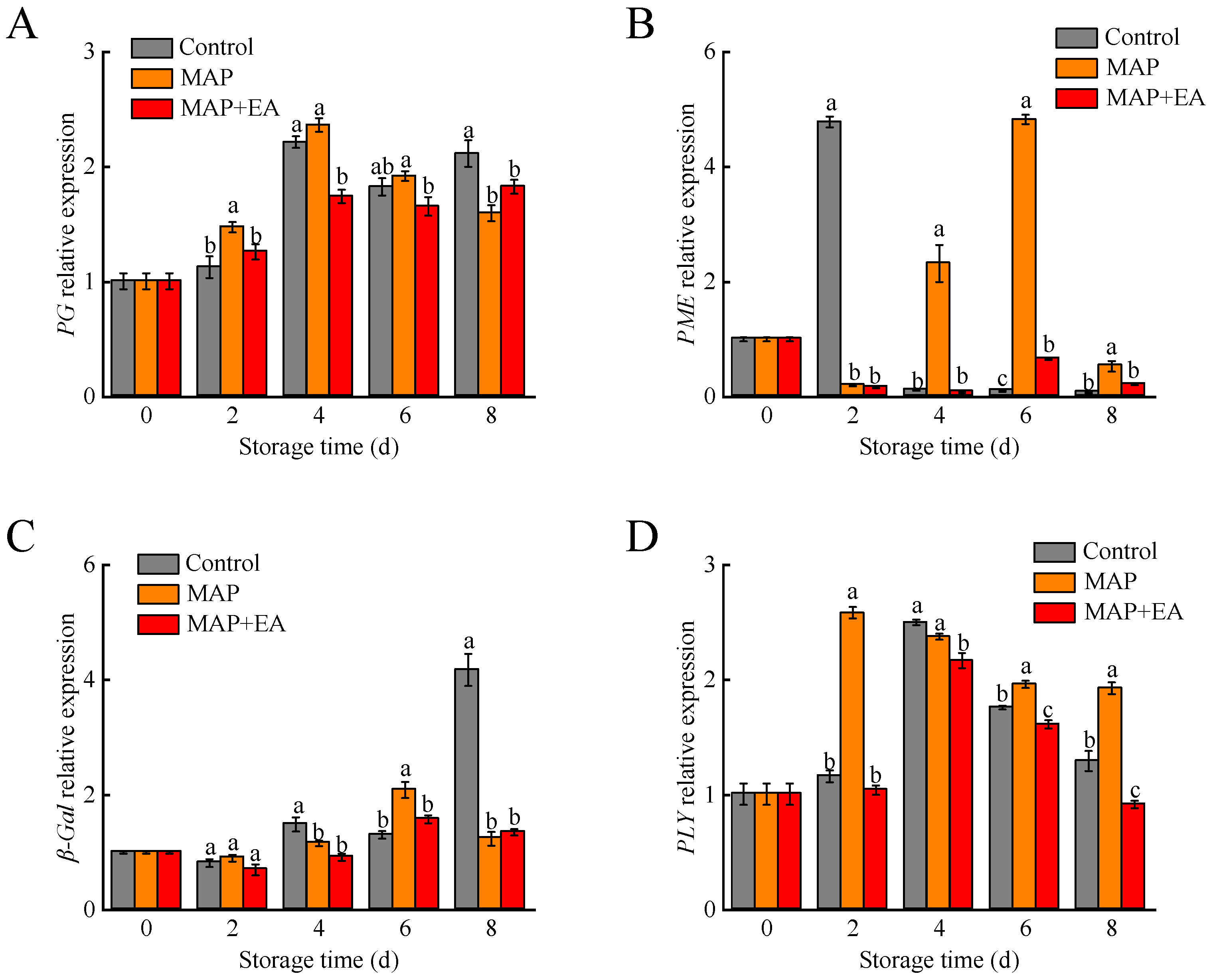
2.8. RNA Isolation and Quantitative Real-Time PCR Analysis
2.9. Statistical Analysis
3. Results
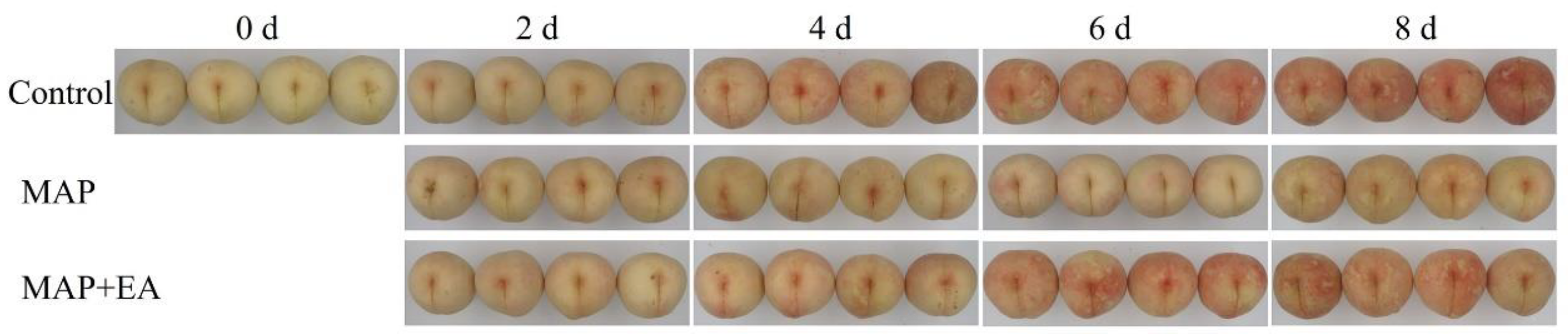
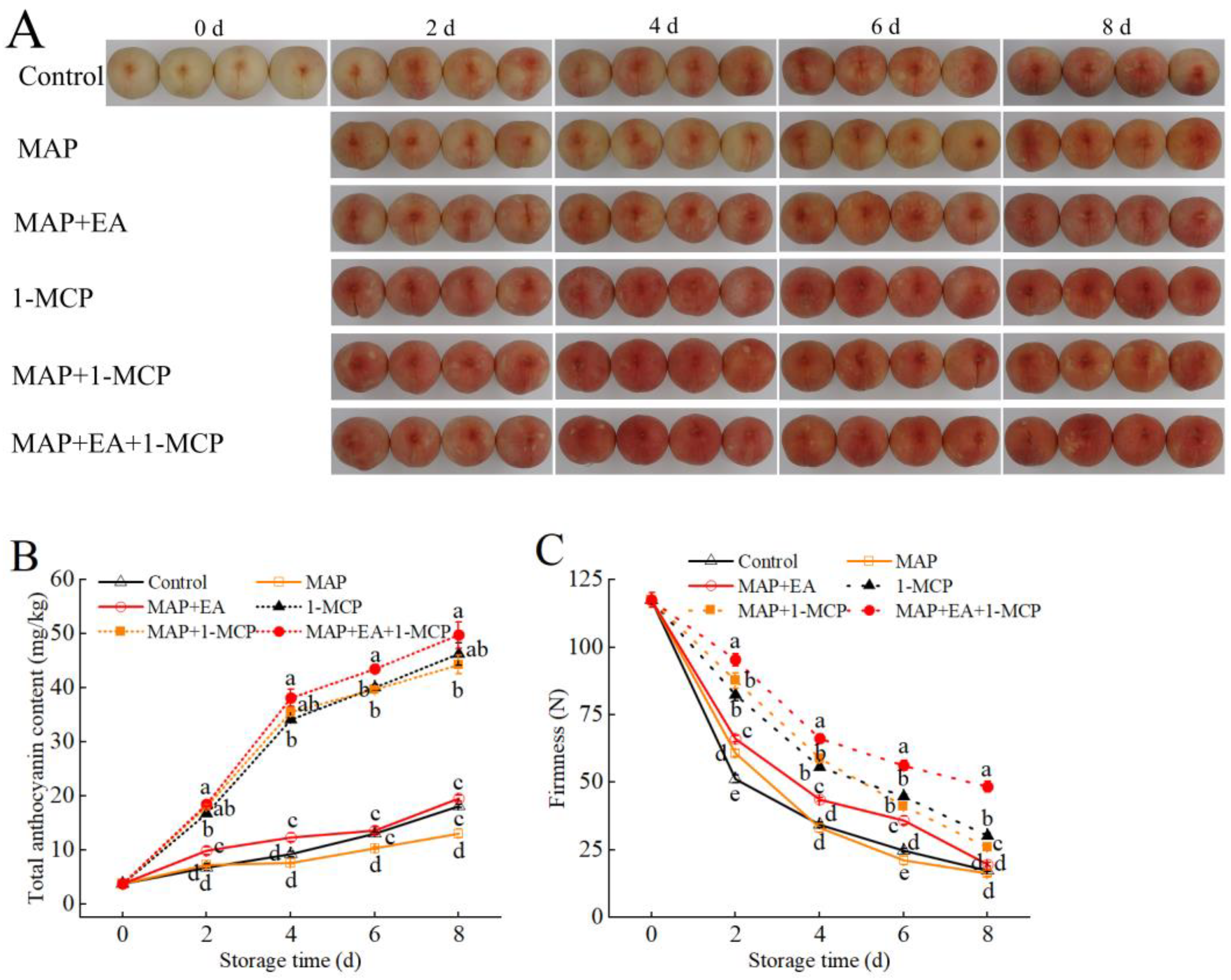
3.1. Visual Appearance Changes of the Peaches
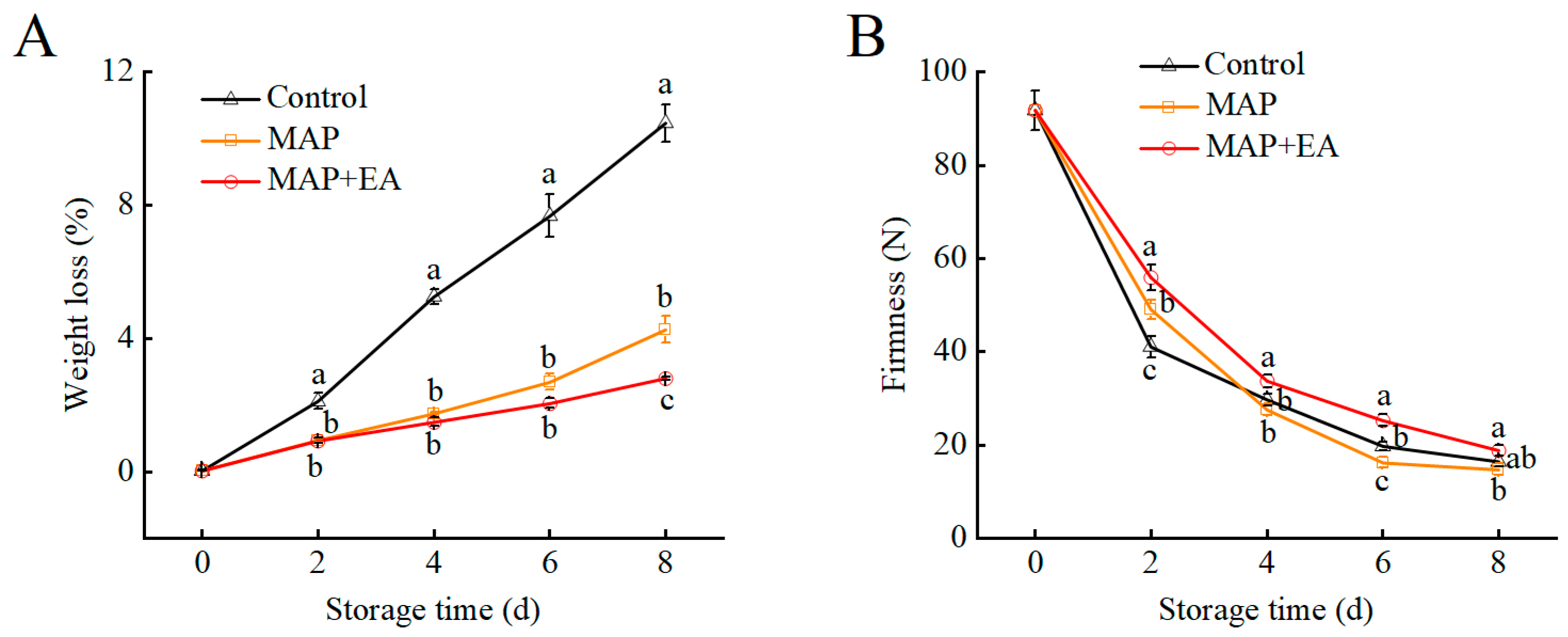
3.2. Changes in Weight Loss and Firmness of Peach During Storage
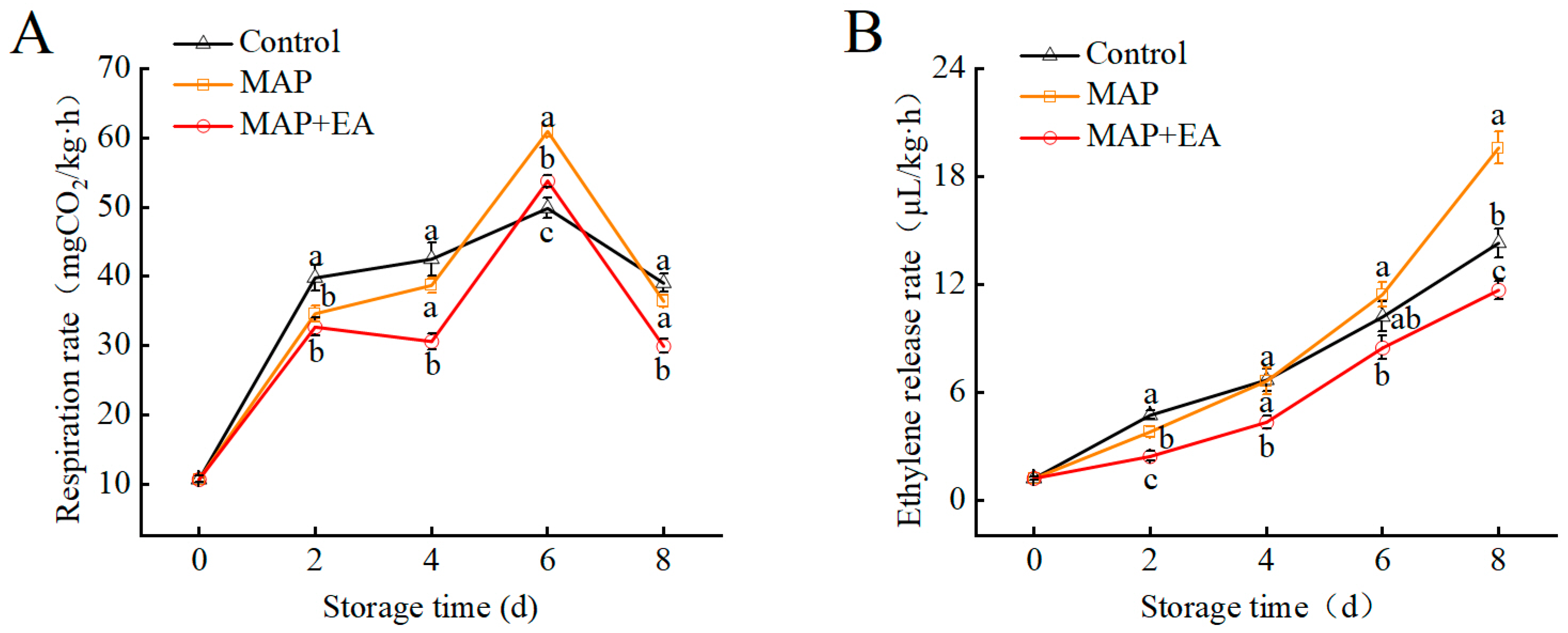
3.3. Respiration Rate and Ethylene Production Rate of Peach During Storage
3.4. Gas Composition Inside the Packages
3.5. Changes in the Activity of Cell-Wall-Modifying Enzymes of Peaches During Storage
3.6. Changes in the Expression of Genes Associated with Flesh Softening
3.7. Changes in the Color Difference Value and Total Anthocyanin Contents of Peach Peel
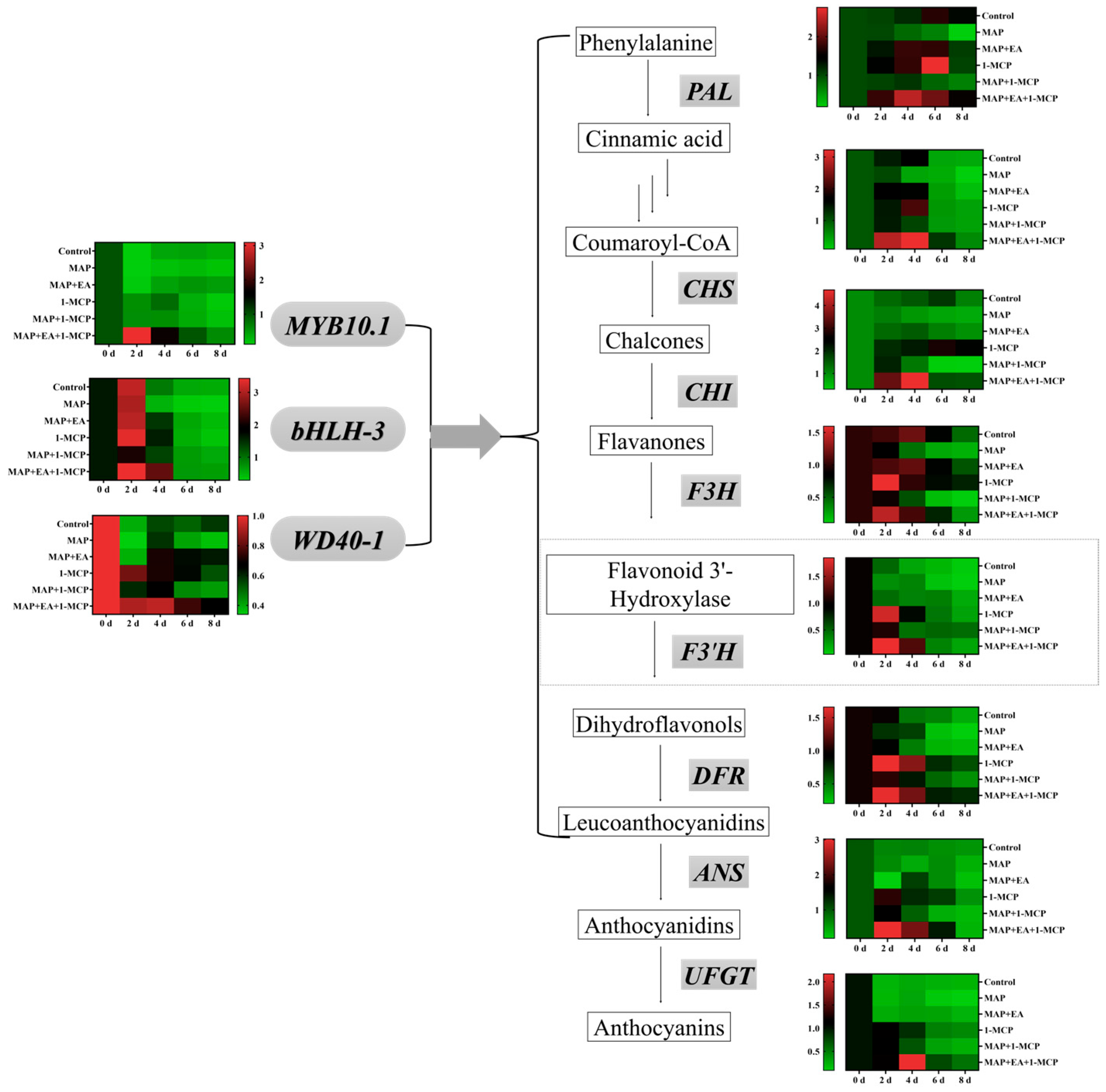
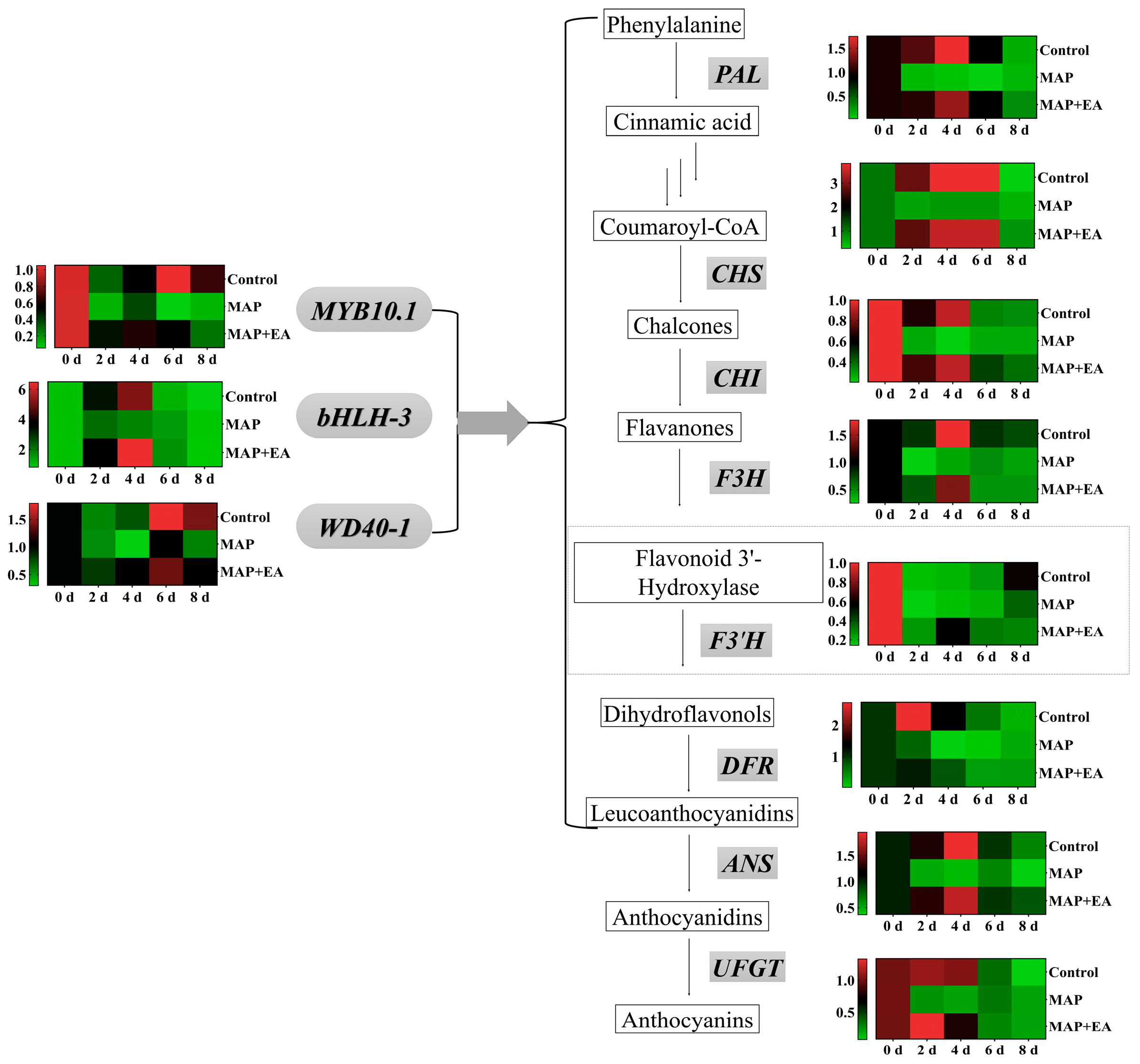
3.8. Changes in the Expression of Genes Associated with Anthocyanin Metabolism
3.9. Effects of 1-MCP Combined EA with Treatment on the Anthocyanin Content and Firmness of Peach
3.10. Effect of 1-MCP Combined with EA Treatment on the Expression of Genes Associated with Anthocyanin Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, C.; Liang, X.; Wei, W.; Zhang, N.; Yao, G.; Yan, R. Enhanced shelf life quality of peaches (Prunus persica L.) using ethylene manipulating active packaging in e-commerce logistics. Sci. Hortic. 2024, 326, 112701. [Google Scholar] [CrossRef]
- Zhang, Y.; Ling, J.; Zhou, H.; Tian, M.y.; Huang, W.; Luo, S.; Hu, H.; Li, P. 1-Methylcyclopropene counteracts ethylene inhibition of anthocyanin accumulation in peach skin after harvest. Postharvest Biol. Technol. 2022, 183, 111737. [Google Scholar] [CrossRef]
- Wang, X.; Fu, D.; Fruk, G.; Chen, E.; Zhang, X. Improving quality control and transparency in honey peach export chain by a multi-sensors-managed traceability system. Food Control 2018, 88, 169–180. [Google Scholar] [CrossRef]
- Leng, P.; Hu, H.; Cui, A.; Tang, H.; Liu, Y. HS-GC-IMS with PCA to analyze volatile flavor compounds of honey peach packaged with different preservation methods during storage. LWT-Food Sci. Technol. 2021, 149, 111963. [Google Scholar] [CrossRef]
- Hayama, H.; Tatsuki, M.; Ito, A.; Kashimura, Y. Ethylene and fruit softening in the stony hard mutation in peach. Postharvest Biol. Technol. 2006, 41, 16–21. [Google Scholar] [CrossRef]
- Qian, J.; Zhao, Y.; Shi, Y.; Chen, K. Transcriptome analysis of peach fruit under 1-MCP treatment provides insights into regulation network in melting peach softening. Food Qual. Saf. 2022, 6, fyac048. [Google Scholar] [CrossRef]
- Zheng, Y.; Jia, X.; Duan, L.; Li, X.; Zhao, Z. Synergistic effects of 1-MCP fumigation and ε-Poly-L-Lysine treatments on delaying softening and enhancing disease resistance of flat peach fruit. Foods 2023, 12, 3683. [Google Scholar] [CrossRef]
- Sortino, G.; Saletta, F.; Puccio, S.; Scuderi, D.; Allegra, A.; Inglese, P.; Farina, V. Extending the shelf life of white peach fruit with 1-methylcyclopropene and aloe arborescens edible coating. Agriculture 2020, 10, 151. [Google Scholar] [CrossRef]
- Zhao, Q.; Jin, M.; Guo, L.; Pei, H.; Nan, Y.; Rao, J. Modified atmosphere packaging and 1-methylcyclopropene alleviate chilling injury of ‘Youhou’ sweet persimmon during cold storage. Food Packag. Shelf Life 2020, 24, 100479. [Google Scholar] [CrossRef]
- Özkaya, O.; Yildirim, D.; Dündar, Ö.; Tükel, S.S. Effects of 1-methylcyclopropene (1-MCP) and modified atmosphere packaging on postharvest storage quality of nectarine fruit. Sci. Hortic. 2016, 198, 454–461. [Google Scholar] [CrossRef]
- Akbudak, B.; Eris, A. Physical and chemical changes in peaches and nectarines during the modified atmosphere storage. Food Control 2004, 15, 307–313. [Google Scholar] [CrossRef]
- Zhou, H.; Ye, Z.; Su, M. Effects of MAP treatment on aroma compounds and enzyme activities in flat peach during storage and shelf life. HortScience 2018, 53, 511–523. [Google Scholar] [CrossRef]
- Sadeghi, K.; Lee, Y.; Seo, J. Ethylene Scavenging Systems in Packaging of Fresh Produce: A Review. Food Rev. Int. 2019, 37, 155–176. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Q.; Zhou, X.; Wei, B.; Ji, S. The effect of ethylene absorbent treatment on the softening of blueberry fruit. Food Chem. 2018, 246, 286–294. [Google Scholar] [CrossRef]
- Chen, H.; Lai, X.; Wang, L.; Li, X.; Chen, W.; Zhu, X.; Song, Z. Ethylene response factor MaERF012 modulates fruit ripening by regulating chlorophyll degradation and softening in banana. Foods 2022, 11, 3882. [Google Scholar] [CrossRef]
- Alonso-Salinas, R.; López-Miranda, S.; Pérez-López, A.J.; Acosta-Motos, J.R. Strategies to delay ethylene-mediated ripening in climacteric fruits: Implications for shelf life extension and postharvest quality. Horticulturae 2024, 10, 840. [Google Scholar] [CrossRef]
- Soleimani, J.; Zarrinbal, M. Comparison of the storage effect of straw and some ethylene absorbents in apricot fruit packaging. J. Food Res. 2022, 32, 93–108. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Zhang, Y.; Cao, J.; Jiang, W. Synergistic effects of 1-MCP and hot air treatments on delaying softening and promoting anthocyanin biosynthesis in nectarines. Postharvest Biol. Technol. 2021, 180, 111598. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, K.; Wu, C.; Zhao, Y.; Yin, X.; Zhang, B.; Grierson, D.; Chen, K.; Xu, C. Effect of ethylene on cell wall and lipid metabolism during alleviation of postharvest chilling injury in peach. Cells 2019, 8, 1612. [Google Scholar] [CrossRef]
- Zheng, Y.; Duan, L.; Jiang, Y.; Yang, X.; Wang, H.; Li, W.; Pan, N.; Wang, X.; Liang, F.; Pan, Y.; et al. Ozone mitigates the flesh discoloration in response to 1-methylcyclopropene by promoting anthocyanin biosynthesis in postharvest nectarines. Sci. Hortic. 2023, 321, 112253. [Google Scholar] [CrossRef]
- Wei, H.; Seidi, F.; Zhang, T.; Jin, Y.; Xiao, H. Ethylene scavengers for the preservation of fruits and vegetables: A review. Food Chem. 2021, 337, 127750. [Google Scholar] [CrossRef] [PubMed]
- Tipu, M.M.H.; Sherif, S.M. Ethylene and its crosstalk with hormonal pathways in fruit ripening: Mechanisms, modulation, and commercial exploitation. Front. Plant Sci. 2024, 15, 1475496. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Chen, C.; Luo, H.; Xu, W.; Zhang, H.; Tian, J.; Ju, R.; Wang, L. The combined effect of ozone treatment and polyethylene packaging on postharvest quality and biodiversity of Toona sinensis (A.Juss.) M.Roem. Postharvest Biol. Technol. 2019, 154, 1–10. [Google Scholar] [CrossRef]
- Goswami, M.; Mondal, K.; Prasannavenkadesan, V.; Bodana, V.; Katiyar, V. Effect of guar gum-chitosan composites edible coating functionalized with essential oils on the postharvest shelf life of Khasi mandarin at ambient condition. Int. J. Biol. Macromol. 2024, 254, 127489. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Yang, L.; Shu, C.; Liu, J.; Zhu, Z.; Yang, Z.; Zhu, X.; Jiang, W. Near freezing temperature storage alleviates cell wall polysaccharide degradation and softening of apricot (Prunus armeniaca L.) fruit after simulated transport vibration. Sci. Hortic. 2021, 288, 110296. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, L.; Rahman, F.U.; Luo, J.; Liu, T.; Chen, W.; Li, X.; Zhu, X. 1-methylcyclopropene combined with ethylene absorbent delays the ripening of ‘Fenjiao’ banana (Musa ABB Pisang Awak). Sci. Hortic. 2024, 326, 112772. [Google Scholar] [CrossRef]
- Sang, X.; Yang, L.; Li, D.; Xu, W.; Fu, Y.; Shi, J. New passive modified atmosphere packaging to extend peaches shelf life at ambient temperature to reduce economic losses. Brit. Food J. 2022, 125, 1504–1515. [Google Scholar] [CrossRef]
- Deng, L.; Zhou, Y.; Zeng, K. Pre-harvest spray of oligochitosan induced the resistance of harvested navel oranges to anthracnose during ambient temperature storage. Crop Prot. 2015, 70, 70–76. [Google Scholar] [CrossRef]
- Fan, X.; Jiang, W.; Gong, H.; Yang, Y.; Zhang, A.; Liu, H.; Cao, J.; Guo, F.; Cui, K. Cell wall polysaccharides degradation and ultrastructure modification of apricot during storage at a near freezing temperature. Food Chem. 2019, 300, 125194. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.; Zhang, Y.; Xu, Y.; Chen, X.; Huang, W.; Li, P. The MYB transcriptional factor BrMYB108 regulates Auxin-mediated delayed leaf senescence in postharvest Pak Choi. Postharvest Biol. Technol. 2025, 219, 113181. [Google Scholar] [CrossRef]
- Hayat, U.; Li, W.; Bie, H.; Liu, S.; Guo, D.; Cao, K. An overview on post-harvest technological advances and ripening techniques for increasing peach fruit quality and shelf life. Horticulturae 2023, 10, 4. [Google Scholar] [CrossRef]
- An, J.; Zhang, M.; Zhan, Z. Effect of packaging film on the quality of ‘Chaoyang’ honey peach fruit in modified atmosphere packages. Packag. Technol. Sci. 2006, 20, 71–76. [Google Scholar] [CrossRef]
- Mahajan, B.V.C.; Dhillon, W.S.; Kumar, M.; Singh, B. Effect of different packaging films on shelf life and quality of peach under super and ordinary market conditions. J. Food Sci. Technol. 2015, 52, 3756–3762. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, H.; Zhang, L.; Luo, S.; Hu, H.; Li, P. Screening of packaging materials suitable for preservation of peach fruits during shelf-life. Jiangsu Agric. Sci. 2018, 46, 215–219. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, D.; Wang, B.; Khan, I.; Ni, Y. Ethylene control technologies in extending postharvest ahelf life of climacteric fruit. J. Agric. Food Chem. 2017, 65, 7308–7319. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Zabihzadeh Khajavi, M.; Ahmadi, S.; Mortazavian, A.M.; Abdolshahi, A.; Rafiee, S.; Farhoodi, M. Novel strategies to control ethylene in fruit and vegetables for extending their shelf life: A review. Int. J. Environ. Sci. Technol. 2021, 19, 4599–4610. [Google Scholar] [CrossRef]
- Jacxsens, L.; Devliegherre, F.; Van Der Steen, C.; Siro, I.; Debevere, J. Application of ethylene adsorbers in combination with high oxygen atmospheres for the storage of strawberries and raspberries. Acta Hortic. 2003, 600, 311–318. [Google Scholar] [CrossRef]
- Böhmer-Maas, B.W.; Fonseca, L.M.; Otero, D.M.; da Rosa Zavareze, E.; Zambiazi, R.C. Photocatalytic zein-TiO2 nanofibers as ethylene absorbers for storage of cherry tomatoes. Food Packag. Shelf Life 2020, 24, 100508. [Google Scholar] [CrossRef]
- Li, S.; Hu, X.; Chen, S.; Wang, X.; Shang, H.; Zhou, Y.; Dai, J.; Xiao, L.; Qin, W.; Liu, Y. Synthesis of γ-cyclodextrin metal-organic framework as ethylene absorber for improving postharvest quality of kiwi fruit. Food Hydrocoll. 2023, 136, 108294. [Google Scholar] [CrossRef]
- Öztürk, M.; Ayhan, Z. Combined effects of ethylene scavenging-active packaging system and modified atmosphere to reduce postharvest losses of ethylene sensitive produce: Banana and kiwifruit. Packag. Technol. Sci. 2023, 36, 951–967. [Google Scholar] [CrossRef]
- Awalgaonkar, G.; Beaudry, R.; Almenar, E. Ethylene-removing packaging: Basis for development and latest advances. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3980–4007. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, S.; Zhang, M.; Nie, J.; Tang, A.; Sun, N.; Zeng, S.; Liu, X.; Ding, Y.; Yin, X.; et al. Integrated transcriptomic and metabolomic analyses revealed the effect of melatonin on delaying persimmon fruit softening. Postharvest Biol. Technol. 2024, 214, 113008. [Google Scholar] [CrossRef]
- Shi, W.; Yang, H.; Jiao, J.; Wang, F.; Lu, Y.; Deng, J. Effects of graft copolymer of chitosan and salicylic acid on reducing rot of postharvest fruit and retarding cell walldegradation in grapefruit during storage. Food Chem. 2019, 283, 92–100. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Zhang, Z.; Fang, Y.; Li, D.; Chen, X.; Feng, S. Ethylene precisely regulates anthocyanin synthesis in apple via a module comprising MdEIL1, MdMYB1, and MdMYB17. Hortic. Res. 2022, 9, uhac034. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Gu, H.; Wang, L.; Shao, Y.; Li, R.; Li, W. Exogenous ethylene promotes peel color transformation by regulating the degradation of chlorophyll and synthesis of anthocyanin in postharvest mango fruit. Front. Nutr. 2022, 9, 911542. [Google Scholar] [CrossRef]
- Xu, Y.; Li, S.e.; Huan, C.; Jiang, T.; Zheng, X.; Brecht, J.K. Effects of 1-methylcyclopropene treatment on quality and anthocyanin biosynthesis in plum (Prunus salicina cv. Taoxingli) fruit during storage at a non-chilling temperature. Postharvest Biol. Technol. 2020, 169, 111291. [Google Scholar] [CrossRef]
- Sun, H.; Hu, K.; Wei, S.; Yao, G.; Zhang, H. Ethylene Response Factors 4.1/4.2 with an EAR motif repress anthocyanin biosynthesis in red-skinned pears. Plant Physiol. 2023, 192, 1892–1912. [Google Scholar] [CrossRef] [PubMed]
- Jayarajan, S.; Sharma, R.R. Influence of in-package use of ethylene absorbents on shelf life and quality of nectarine during supermarket conditions. Fruits 2019, 74, 180–186. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, Y.; Jiang, S.; Wang, X.; Xu, F.; Wang, H.; Shao, X. Flavor development in peach fruit treated with 1-methylcyclopropene during shelf storage. Food Res. Int. 2020, 137, 109653. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Ma, S.; Zhao, J.; Gao, Y.; Huang, W.; Zhang, Y.; Ling, J.; Zhou, Q.; Li, P. Improving the Surface Color and Delaying Softening of Peach by Minimizing the Harmful Effects of Ethylene in the Package. Foods 2025, 14, 2472. https://doi.org/10.3390/foods14142472
Zhou H, Ma S, Zhao J, Gao Y, Huang W, Zhang Y, Ling J, Zhou Q, Li P. Improving the Surface Color and Delaying Softening of Peach by Minimizing the Harmful Effects of Ethylene in the Package. Foods. 2025; 14(14):2472. https://doi.org/10.3390/foods14142472
Chicago/Turabian StyleZhou, Hongsheng, Siyu Ma, Jing Zhao, Ying Gao, Wen Huang, Yingtong Zhang, Jun Ling, Qian Zhou, and Pengxia Li. 2025. "Improving the Surface Color and Delaying Softening of Peach by Minimizing the Harmful Effects of Ethylene in the Package" Foods 14, no. 14: 2472. https://doi.org/10.3390/foods14142472
APA StyleZhou, H., Ma, S., Zhao, J., Gao, Y., Huang, W., Zhang, Y., Ling, J., Zhou, Q., & Li, P. (2025). Improving the Surface Color and Delaying Softening of Peach by Minimizing the Harmful Effects of Ethylene in the Package. Foods, 14(14), 2472. https://doi.org/10.3390/foods14142472







