Inactivation of Emerging Opportunistic Foodborne Pathogens Cronobacter spp. and Arcobacter spp. on Fresh Fruit and Vegetable Products: Effects of Emerging Chemical and Physical Methods in Model and Real Food Systems—A Review
Abstract
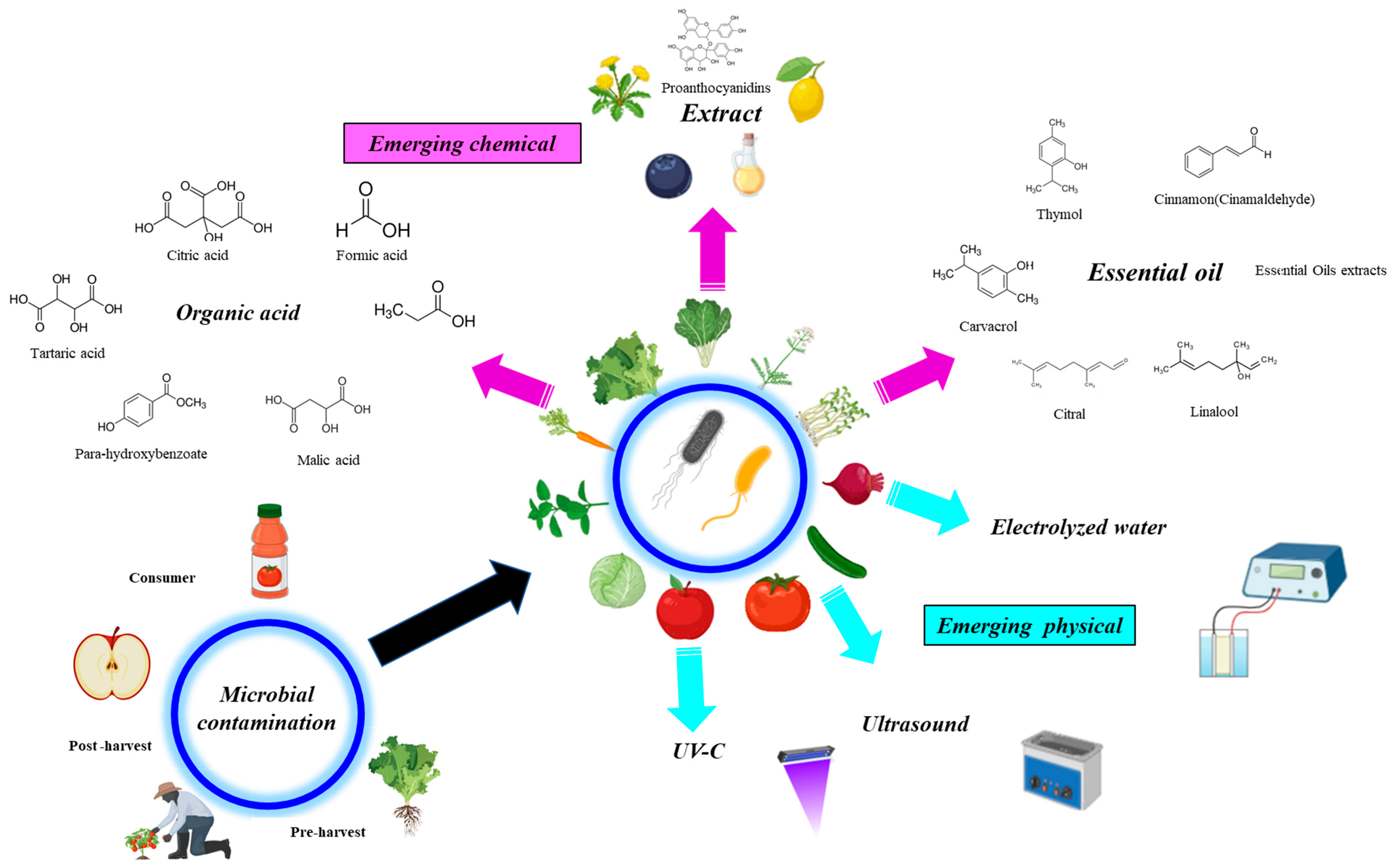
1. Introduction
2. Incidence of Arcobacter spp. and Cronobacter spp. in Fruit and Vegetable Products
3. Emerging Strategies Tested for Decontamination of Arcobacter spp. and Cronobacter spp. in Fruit and Vegetable Products
3.1. Emerging Chemical Methods
3.1.1. Organic Acid and Chlorine
3.1.2. Extracts
| Microorganism | Extract and Essential Oil | Matrix | Condition | Microbial Reduction | Potential Research Limitation | Reference |
|---|---|---|---|---|---|---|
| Arcobacter spp. | Extract virgin olive oils | In vitro test | Non-buffered (WEOO) and buffered (BEOO) extract virgin olive oils. | Reduction of 5 Log CFU/mL. | The effect of extract virgin olive oils on biofilm eradication has not been evaluated. | [26] |
| Arcobacter spp. | Orange oil fractions | In vitro test | Seven commercial orange oil fractions. | No inhibition of Arcobacter spp. was detected by 6 out of 7 orange fractions except CP terpeneless Valencia orange oil, which produced inhibition zones varying from 9.5 ± 0.7 to 29 ± 1.4 mm. | Composition of the tested orange-based fractions was not determined. | [28] |
| Cronobacter sakazakii | Blueberry proanthocyanidin (PAC) and commercial blueberry juice (BJ) | In vitro test | Blueberry PAC at 5 mg/mL, BJ (pH 2.8), neutralised BJ (pH 7), malic acid (pH 3.0), or PBS and incubated at 37 °C for 30 min, 1 h, 3 h, or 6 h. | Reductions of ~1 and 1.50 Log CFU/mL with BJ or blueberry PAC. Reduction of 8.25 ± 0.12 Log CFU/mL and 8.48 ± 0.03 Log CFU/mL, respectively, with BJ (pH 2.8) or blueberry PAC after 1 h, while malic acid (pH 3.0) showed 1.3 Log CFU/mL reduction for both strains. | Composition of blueberry polyphenolic fractions in commercial blueberry juice and blueberry proanthocyanidin (PAC) fraction has not been characterised. | [33] |
| Cronobacter sakazakii | Chrysanthemum bud crude extract (CBCE) | In vitro test | Different concentrations of CBCE (0, 0.3125, 0.625, 1.25, 2.5, 5, 10, and 20 mg/mL). | Diameter of inhibition zone (DIZ), minimum inhibitory concentration (MIC), and minimum bactericide concentration (MBC) of CBCE against C. sakazakii were 14.55 ± 0.44–14.84 ± 0.38 mm, 10 mg/mL, and 20 mg/mL, respectively. | The composition (principal bioactive compounds) of the chrysanthemum bud crude extract (CBCE) has not been determined. | [39] |
| Cronobacter spp. | Bacterial cellulose (BC) impregnated with plant extract and essential oil | In vitro test | Bacterial cellulose combined with extracts (Tulsi, Brahmi, lemon, blackberry, nettle root, and nettle leaf) and essential oils (cinnamon, sage, clove, mint, thyme, lemongrass, rosemary, lemon, anise, tea tree, lime, grapefruit, and tangerine) in agar diffusion methods. | The cellulose matrix with a 50% extract from Brahmi was found to effectively inhibit the growth of the selected Cronobacter strains. | Composition of the extract and essential oil fraction was not determined. | [63] |
| Cronobacter spp. | Biocellulose impregnated with oregano essential oil | In vitro test | Biocellulose impregned with oregano essential oil (100%) in agar diffusion methods. | Bacterial cellulose impregnated with oregano essential oil had strong and moderate antimicrobial activity against all presented strains of the genus Cronobacter. | Composition of the extract and essential oil fraction was not determined. | [64] |
| Cronobacter spp. | Thyme, cinnamon, clove, peppermint, marjoram, cumin, rosemary, fennel, basil, lime, bergamot orange, orange, lemon, grapefruit, mandarin, cardamom, anise, and ginger | In vitro test | Disc-diffusion method was used to screen the EOs. | Most effective EOs: thyme > cinnamon > marjoram. The clove, cumin, and fennel oils had moderately inhibiting effects on only some of the tested strains. | The effect of the essential oils on biofilm eradication was not evaluated. | [65] |
3.1.3. Essential Oils
3.2. Emerging Physical Methods
3.2.1. Combination of UV-C with Electrolysed Water
3.2.2. Ultrasound
3.2.3. Methods Not Explored: Cold Plasma as an Emerging Technology for Decontamination of Fruit and Vegetable Products
4. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention—CDC: Outbreak of E. coli Infections Linked to Romaine Lettuce E. coli Infections November 2019 E. coli. (CDC). 2019. Available online: https://archive.cdc.gov/www_cdc_gov/ecoli/2019/o157h7-11-19/index.html (accessed on 22 December 2024).
- Kinsinger, N.M.; Mayton, H.M.; Luth, M.R.; Walker, S.L. Efficacy of post-harvest rinsing and bleach disinfection of E. coli O157:H7 on spinach leaf surfaces. Food Microbiol. 2017, 62, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Strysko, J.; Cope, J.R.; Martin, H.; Tarr, C.; Hise, K.; Collier, S.; Bowen, A. Food Safety and Invasive Cronobacter Infections during Early Infancy. Emerg. Infect. Dis. 2020, 26, 857–865. [Google Scholar] [CrossRef]
- González, A.; Ferrús, M.A. Study of Arcobacter spp. contamination in fresh lettuces detected by different cultural and molecular methods. Int. J. Food Microbiol. 2011, 145, 311–314. [Google Scholar] [CrossRef]
- Cechin, C.D.F.; Carvalho, G.G.; Bastos, C.P.; Kabuki, D.Y. Cronobacter spp. in foods of plant origin: Occurrence, contamination routes, and pathogenic potential. Crit. Rev. Food Sci. Nutr. 2023, 63, 12398–12412. [Google Scholar] [CrossRef]
- Mousavi, Z.E.; Hunt, K.; Koolman, L.; Butler, F.; Fanning, S. Cronobacter Species in the Built Food Production Environment: A Review on Persistence, Pathogenicity, Regulation and Detection Methods. Microorganisms 2023, 11, 1379. [Google Scholar] [CrossRef]
- Baltoiu, M.; Gradisteanu Pircalabioru, G.; Cristea, D.; Sorokin, M.; Dragomirescu, C.C.; Stoica, I. Genetic Diversity, Virulence, and Antibiotic Resistance Determinants of Campylobacter jejuni Isolates in Romania. Pathogens 2024, 13, 716. [Google Scholar] [CrossRef] [PubMed]
- Kietsiri, P.; Muangnapoh, C.; Lurchachaiwong, W.; Lertsethtakarn, P.; Bodhidatta, L.; Suthienkul, O.; Waters, N.C.; Demons, S.T.; Vesely, B.A. Characterization of Arcobacter spp. isolated from human diarrheal, non-diarrheal and food samples in Thailand. PLoS ONE 2021, 16, e0246598. [Google Scholar] [CrossRef] [PubMed]
- Dieguez, A.L.; Balboa, S.; Magnesen, T.; Romalde, J.L. Arcobacter lekithochrous sp. nov., isolated from a molluscan hatchery. Int. J. Syst. Evol. Microbiol. 2017, 67, 1327–1332. [Google Scholar] [CrossRef]
- Ferreira, S.; Queiroz, J.A.; Oleastro, M.; Domingues, F.C. Insights in the pathogenesis and resistance of Arcobacter: A review. Crit. Rev. Microbiol. 2016, 42, 364–383. [Google Scholar]
- Vandamme, P.; Falsen, E.; Rossau, R.; Hoste, B.; Segers, P.; Tytgat, R.; De Ley, J. Revision of Campylobacter, Helicobacter, and Wolinella taxonomy: Emendation of generic descriptions and proposal of Arcobacter gen. Int. J. Syst. Microbio 1991, 41, 88–103. [Google Scholar] [CrossRef]
- Vandamme, P.; Vancanneyt, M.; Pot, B.; Mels, L.; Hoste, B.; Dewettinck, D.; Vlaes, L.; van den Borre, C.; Higgins, R.; Hommez, J. Polyphasic taxonomic study of the emended genus Arcobacter with Arcobacter butzleri comb. nov. and Arcobacter skirrowii sp. nov., an aerotolerant bacterium isolated from veterinary specimens. Int. J. Syst. Microbio 1992, 42, 344–356. [Google Scholar] [CrossRef][Green Version]
- Collado, L.; Cleenwerck, I.; Van Trappen, S.; De Vos, P.; Figueras, M.J. Arcobacter mytili sp. nov., an indoxyl acetate-hydrolysis-negative bacterium isolated from mussels. Int. J. Syst. Microbio 2009, 59, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Tall, B.D.; Grim, C.J.; Franco, A.A.; Jarvis, K.G.; Hu, L.; Kothary, M.H.; Sathyamoorthy, V.; Gopinath, G.; Fanning, S. Chapter 16—Cronobacter Species (formerly Enterobacter sakazakii). In Food Science and Technology, Foodborne Infections and Intoxications, 4th ed.; Morris, J.G., Potter, M.E., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 251–258. [Google Scholar]
- Pelissari, E.M.R.; Covre, K.V.; do Rosario, D.K.A.; de São José, J.F.B. Application of chemometrics to assess the influence of ultrasound and chemical sanitizers on vegetables: Impact on natural microbiota, Salmonella Enteritidis and physicochemical nutritional quality. LWT 2021, 148, 111711. [Google Scholar] [CrossRef]
- Donatti Leão Alvarenga, P.; Mileib Vasconcelos, C.; de São José, J.F.B. Application of Ultrasound Combined with Acetic Acid and Peracetic Acid: Microbiological and Physicochemical Quality of Strawberries. Molecules 2021, 26, 16. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Nou, X.; Millner, P.; Zhou, B.; Shen, C.; Yang, Y.; Wu, Y.; Wang, Q.; Feng, H.; Shelton, D. A pilot plant scale evaluation of a new process aid for enhancing chlorine efficacy against pathogen survival and cross-contamination during produce wash. Int. J. Food Microbiol. 2012, 158, 133–139. [Google Scholar] [CrossRef]
- Petri, E.; Rodríguez, M.; García, S. Evaluation of Combined Disinfection Methods for Reducing Escherichia coli O157:H7 Population on Fresh-Cut Vegetables. Int. J. Environ. Res. Public Health 2015, 12, 8678–8690. [Google Scholar] [CrossRef]
- Garg, R.; Abela, D.; Tiwari, B.; Valdramidis, V. Chapter 18—Potential industrial applications of decontamination technologies for fresh produce. In Parthena Kotzekidou, Food Hygiene and Toxicology in Ready-to-Eat Foods; Academic Press: Cambridge, MA, USA, 2016; pp. 313–336. [Google Scholar]
- Ahmed, S.; Zaman, S.; Ahmed, R.; Uddin, M.N.; Acedo, A.; Bari, M.L. Effectiveness of non-chlorine sanitizers in improving the safety and quality of fresh betel leaf. LWT 2017, 78, 77–81. [Google Scholar] [CrossRef]
- Parveen, N.; Chowdhury, S.; Goel, S. Environmental impacts of the widespread use of chlorine-based disinfectants during the COVID-19 pandemic. Environ. Sci. Pollut. Res. Int. 2022, 57, 85742–85760. [Google Scholar] [CrossRef]
- Kim, H.; Beuchat, L.R. Effectiveness of disinfectants in killing Enterobacter sakazakii in suspension, dried on the surface of stainless steel, and in a biofilm. Appl. Environ. Microbiol. 2007, 73, 1256–1265. [Google Scholar] [CrossRef]
- Šilha, D.; Šilhová, L.; Vytřasová, J.; Brožková, I.; Pejchalová, M. Survival of selected bacteria of Arcobacter genus in disinfectants and possibility of acquired secondary resistance to disinfectants. JMBFS 2016, 5, 326–329. [Google Scholar] [CrossRef][Green Version]
- Hausdorf, L.; Neumann, M.; Bergmann, I.; Sobiella, K.; Mundt, K.; Fröhling, A.; Klocke, M. Occurrence and genetic diversity of Arcobacter spp. in a spinach-processing plant and evaluation of two Arcobacter-specific quantitative PCR assays. Syst. Appl. Microbiol. 2013, 36, 235–243. [Google Scholar] [CrossRef]
- Kim, H.; Ryu, J.H.; Beuchat, L.R. Survival of Enterobacter sakazakii on fresh produce as affected by temperature, and effectiveness of sanitizers for its elimination. Int. J. Food Microbiol. 2006, 111, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Švarcová, K.; Hofmeisterová, L.; Švecová, B.; Šilha, D. In Vitro Activity of Water Extracts of Olive Oil against Planktonic Cells and Biofilm Formation of Arcobacter-like Species. Molecules 2022, 14, 4509. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Rowe, C.; Phillips, C.A. The survival of three strains of Arcobacter butzleri in the presence of lemon, orange and bergamot essential oils and their components in vitro and on food. Lett. Appl. Microbiol. 2007, 44, 495–499. [Google Scholar] [CrossRef]
- Nannapaneni, R.; Chalova, V.I.; Crandall, P.G.; Ricke, S.C.; Johnson, M.G.; O’Bryan, C.A. Campylobacter and Arcobacter species sensitivity to commercial orange oil fractions. Int. J. Food Microbiol. 2009, 129, 43–49. [Google Scholar] [CrossRef]
- Arroyo, C.; Gayán, E.; Pagán, R.; Condón, S. UV-C Inactivation of Cronobacter sakazakii. Foodborne Pathog. Dis. 2012, 9, 10. [Google Scholar] [CrossRef]
- Cervenka, L.; Zachova, I.; Minrikova, P.; Vytrasova, J. Effect of pH and water activity on the growth of Arcobacter sp. in culture. Czech J. Food Sci. 2003, 21, 203–209. [Google Scholar] [CrossRef]
- Gao, Z.; Ge, C.; Baker, R.C.; Tikekar, V.R.; Buchanan, R.L. Enhancement of Thermal Inactivation of Cronobacter sakazakii in Apple Juice at 58 °C by Inclusion of Butyl Para-Hydroxybenzoate and Malic Acid. J. Food Prot. 2022, 85, 1515–1521. [Google Scholar] [CrossRef]
- Kim, S.J.; Bae, Y.M.; Lee, S.Y. Stress response of acid-shocked Cronobacter sakazakii against subsequent acidic pH, mild heat, and organic acids. Food Sci. Biotechnol. 2012, 21, 205–210. [Google Scholar] [CrossRef]
- Joshi, S.S.; Howell, A.B.; D’Souza, D.H. Cronobacter sakazakii reduction by blueberry proanthocyanidins. Food Microbiol. 2014, 39, 127–131. [Google Scholar] [CrossRef]
- Park, S.Y.; Mizan, F.R.; Ha, S.D. Inactivation of Cronobacter sakazakii in head lettuce by using a combination of ultrasound and sodium hypochlorite. Food Control 2016, 60, 582–587. [Google Scholar] [CrossRef]
- Bang, H.-J.; Park, S.Y.; Kim, S.E.; Rahaman, F.M.M.; Ha, S.-D. Synergistic effects of combined ultrasound and peroxyacetic acid treatments against Cronobacter sakazakii biofilms on fresh cucumber. LWT 2017, 84, 91–98. [Google Scholar] [CrossRef]
- Webb, A.L.; Taboada, E.N.; Selinger, L.B.; Boras, V.F.; Inglis, G.D. Efficacy of wastewater treatment on Arcobacter butzleri density and strain diversity. Water Res. 2016, 105, 291–296. [Google Scholar] [CrossRef]
- Santo, D.; Graça, A.; Nunes, C.; Quintas, C. Escherichia coli and Cronobacter sakazakii in ‘Tommy Atkins’ minimally processed mangos: Survival, growth and effect of UV-C and electrolyzed water. Food Microbiol. 2018, 70, 49–54. [Google Scholar] [CrossRef]
- Berthold-Pluta, A.; Garbowska, M.; Stefańska, I.; Pluta, A. Microbiological quality of selected ready-to-eat leaf vegetables, sprouts and non-pasteurized fresh fruit-vegetable juices including the presence of Cronobacter spp. Food Microbiol. 2017, 65, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Xing, M.; Hu, X.; Feng, H.; Wang, Y.; Guo, B.; Sun, M.; Ma, L.; Fei, P. Antibacterial Activity of Chrysanthemum buds Crude Extract Against Cronobacter sakazakii and Its Application as a Natural Disinfectant. Front. Microbiol. 2021, 3, 632177. [Google Scholar] [CrossRef] [PubMed]
- Behling, R.G.; Eifert, J.; Erickson, M.C.; Gurtler, J.B.; Kornacki, J.L.; Line, E.; Radcliff, R.; Ryser, E.T.; Stawick, B.; Yan, Z. Selected Pathogens of Concern to Industrial Food Processors: Infectious, Toxigenic, Toxico-Infectious, Selected Emerging Pathogenic Bacteria. In Principles of Microbiological Troubleshooting in the Industrial Food Processing Environment; Food Microbiology and Food Safety; Kornacki, J., Ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Mottola, A.; Ciccarese, G.; Sinisi, C.; Savarino, A.E.; Marchetti, P.; Terio, V.; Tantillo, G.; Barrasso, R.; Di Pinto, A. Occurrence and characterization of Arcobacter spp. from ready-to-eat vegetables produced in Southern Italy. Ital. J. Food Saf. 2021, 10, 8585. [Google Scholar] [CrossRef]
- Ramees, T.P.; Rathore, R.S.; Kumar, A.; Arun, T.R.; Kumar, G.V.P.P.S.R.; Karthik, K.; Malik, Y.S.; Dhama, K.; Singh, R.K. Phylogenetic analysis of Arcobacter butzleri and Arcobacter skirrowii isolates and their detection from contaminated vegetables by multiplex PCR. J. Exp. Biol. Agric. 2018, 6, 307–314. [Google Scholar]
- Berthold-Pluta, A.; Stefańska, I.; Forsythe, S.; Aleksandrzak-Piekarczyk, T.; Stasiak-Różańska, L.; Garbowska, M. Genomic Analysis of Cronobacter condimenti s37: Identification of Resistance and Virulence Genes and Comparison with Other Cronobacter and Closely Related Species. Int. J. Mol. Sci. 2024, 25, 8622. [Google Scholar] [CrossRef]
- Hansson, I.S.; Marianne, H.; Lowman, R.I.; Engvall, E. Knowledge gaps in control of Campylobacter for prevention of campylobacteriosis. Transbound. Emerg. Dis. 2016, 65, 29663680. [Google Scholar]
- Echandi, M.L.A.; Soto, A.H.; Blanco, J.M.C.; Fernández, F.; Jaramillo, H.F. Occurrence of Aliarcobacter spp. in fresh and pre-cut vegetables of common use in San José, Costa Rica. Ital. J. Food Saf. 2023, 12, 10344. [Google Scholar]
- Mottola, A.; Bonerba, E.; Bozzo, G.; Marchetti, P.; Celano, G.V.; Colao, V.; Terio, V.; Tantillo, G.; Figueras, M.J.; Di Pinto, A. Occurrence of emerging food-borne pathogenic Arcobacter spp. isolated from pre-cut (ready-to-eat) vegetables. Int. J. Food Microbiol. 2016, 236, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Choi, C. Survival of Arcobacter butzleri in Apple and Pear Purees. J. Food Saf. 2013, 33, 333–339. [Google Scholar] [CrossRef]
- Ling, N.; Jiang, X.; Forsythe, S.; Zhang, D.; Shen, Y.; Ding, Y.; Wang, J.; Zhang, J.; Wu, Q.; Ye, Y. Food Safety Risks and Contributing Factors of Cronobacter spp. J. Eng. 2022, 12, 128–138. [Google Scholar] [CrossRef]
- Ling, N.; Chengsi, L.; Jumei, Z.; Qingping, W.; Haiyan, Z.; Wenjing, H.; Yingwang, Y.; Juan, W.; Yu, D.; Moutong, C.; et al. Prevalence and Molecular and Antimicrobial Characteristics of Cronobacter spp. Isolated from Raw Vegetables in China. Front. Microbiol. 2018, 9, 1149. [Google Scholar] [CrossRef]
- Moravkova, M.; Verbikova, V.; Huvarova, V.; Babak, V.; Cahlikova, H.; Karpiskova, R.; Kralik, P. Occurrence of Cronobacter spp. in Ready-to-Eat Vegetable Products, Frozen Vegetables, and Sprouts Examined Using Cultivation and Real-Time PCR Methods. J. Food Sci. 2018, 83, 3054–3058. [Google Scholar] [CrossRef]
- Turcovský, I.; Kuniková, K.; Drahovská, H.; Kaclíková, E. Biochemical and molecular characterization of Cronobacter spp. (formerly Enterobacter sakazakii) isolated from foods. Antonie Van Leeuwenhoek 2011, 99, 257–269. [Google Scholar] [CrossRef]
- Leclercq, A.; Wanegue, C.; Baylac, P. Comparison of fecal coliform agar and violet red bile lactose agar for fecal coliform enumeration in foods. Appl. Environ. Microbiol. 2002, 68, 1631–1638. [Google Scholar] [CrossRef]
- Gheller, L.S.; Ghizzi, L.G.; Marques, J.A.; Takiya, C.S.; Grigoletto, N.T.S.; Dias, M.S.S.; Silva, T.B.P.; Nunes, A.T.; da Silva, G.G.; Fernandes, L.G.X.; et al. Effects of organic acid-based products added to total mixed ration on performance and ruminal fermentation of dairy cows. AFST 2020, 261, 114406. [Google Scholar] [CrossRef]
- Abay, S.; Yaman, A.; Karakaya, E.; Aydin, F. Prevalence and antibacterial susceptibilities of Arcobacter spp. and Campylobacter spp. from fresh vegetables. World J. Microbiol. Biotechnol. 2022, 38, 132. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M. Eduardo Sobarzo-Sánchez, Seyed Fazel Nabavi, Seyed Mohammad Nabavi, Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2016, 196, 44–68. [Google Scholar] [CrossRef] [PubMed]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts Against Food Pathogens and Spoilage Microorganisms. Front. Microbiol. 2018, 24, 1639. [Google Scholar] [CrossRef]
- Huang, W.Y.; Zhang, H.C.; Liu, W.X.; Li, C.Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef]
- Meireles, A.; Giaouris, E.; Simões, M. Alternative disinfection methods to chlorine for use in the fresh-cut industry. Int. Food Res. 2016, 82, 71–85. [Google Scholar] [CrossRef]
- Roe, A.J.; O’Byrne, C.; McLaggan, D.; Booth, I.R. Inhibition of Escherichia coli growth by acetic acid: A problem with methionine biosynthesis and homocysteine toxicity. Microbiology 2002, 148, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Gurtler, J.B.; Kornacki, J.L.; Beuchat, L.R. Review Enterobacter sakazakii: A coliform of increased concern to infant health. Int. J. Food Microbiol. 2005, 104, 1–34. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, W. UV treatment improved the quality of postharvest fruit and vegetables by inducing resistance. Trends Food Sci. Technol. 2019, 92, 71–80. [Google Scholar] [CrossRef]
- Stasiak-Różańska, L.; Berthold-Pluta, A.; Aleksandrzak-Piekarczyk, T.; Koryszewska-Bagińska, A.; Garbowska, M. Antimicrobial Activity against Cronobacter of Plant Extracts and Essential Oils in a Matrix of Bacterial Cellulose. Polymers 2024, 16, 2316. [Google Scholar] [CrossRef]
- Nagmetova, G.; Berthold-Pluta, A.; Garbowska, M.; Kurmanbayev, A.; Stasiak-Różańska, L. Antibacterial Activity of Biocellulose with Oregano Essential Oil against Cronobacter Strains. Polymers 2020, 12, 1647. [Google Scholar] [CrossRef]
- Berthold-Pluta, A.; Stasiak-Różańska, L.; Pluta, A.; Garbowska, M. Antibacterial activities of plant-derived compounds and essential oils against Cronobacter strains. Eur. Food Res. Technol. 2019, 245, 1137–1147. [Google Scholar] [CrossRef]
- Oussalah, M.; Caillet, S.; Saucier, L.; Lacroix, M. Antimicrobial effects of selected plant essential oils on the growth of a Pseudomonas putida strain isolated from meat. Meat Sci. 2006, 73, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Johny, K.A.; Darre, M.J.; Donoghue, M.A.; Donoghue, J.D.; Venkitanarayanan, K. Antibacterial effect of trans-cinnamaldehyde, eugenol, carvacrol, and thymol on Salmonella Enteritidis and Campylobacter jejuni in chicken cecal contents in vitro1 1Mention of a trade name, proprietary product, or specific equipment does not constitute a guarantee or warranty by the USDA and does not imply its approval to the exclusion of other products that are suitable. J. Appl. Poult. Res. 2005, 19, 237–244. [Google Scholar]
- Dutra da Silva, B.; Bernardes, P.C.; Pinheiro, P.F.; Fantuzzi, E.; Roberto, C.D. Chemical composition, extraction sources and action mechanisms of essential oils: Natural preservative and limitations of use in meat products. Meat Sci. 2021, 176, 108463. [Google Scholar] [CrossRef]
- Graça, A.; Salazar, M.; Quintas, C.; Nunes, C. Low dose UV-C illumination as an eco-innovative disinfection system on minimally processed apples. Postharvest Biol. Technol. 2013, 85, 1–7. [Google Scholar] [CrossRef]
- Rahman, S.M.E.; Khan, I.; Oh, D.-H. Electrolyzed Water as a Novel Sanitizer in the Food Industry: Current Trends and Future Perspectives. Compr. Rev. Food Sci. Food Saf. 2016, 15, 471–490. [Google Scholar] [CrossRef]
- Chen, B.K.; Wang, C.K. Electrolyzed Water and Its Pharmacological Activities: A Mini-Review. Molecules 2022, 11, 1222. [Google Scholar] [CrossRef]
- Beirao-da-Costa, S.; Moura-Guedes, M.C.; Ferreira-Pinto, M.M.; Empis, J.; Moldao-Martins, M. Alternative sanitizing methods to ensure safety and quality of fresh-cut kiwifruit. J. Food Process. Preserv. 2014, 38, 1–10. [Google Scholar] [CrossRef]
- Yun, J.; Yan, R.; Fan, X.; Gurtler, J.; Phillips, J. Fate of E. coli O157:H7, Salmonella spp. and potential surrogate bacteria on apricot fruit, following exposure to UV-C light. Int. J. Food Microbiol. 2013, 16, 356–363. [Google Scholar] [CrossRef]
- Manzocco, L.; Pieve, S.D.; Maifreni, M. Impact of UV-C light on safety and quality of fresh-cut melon. Innov. Food Sci. Emerg. Technol. 2011, 12, 13–17. [Google Scholar] [CrossRef]
- George, D.S.; Razali, Z.; Santhirasegaram, V.; Somasundram, C. Effects of Ultraviolet Light (UV-C) and heat treatment on the quality of fresh-cut Chokanan mango and Josephine pineapple. J. Food Sci. 2015, 80, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Artés-Hernández, F.; Robles, A.P.; Gómez, A.P.; Tomás-Callejas, A.; Artés, F. Low UV-C illumination for keeping overall quality of fresh-cut watermelon. Postharvest Biol. Technol. 2010, 55, 114–120. [Google Scholar] [CrossRef]
- Dai, J.; Bai, M.; Li, C.; Cui, H.; Lin, L. Advances in the mechanism of different antibacterial strategies based on ultrasound technique for controlling bacterial contamination in food industry. Trends Food Sci. Technol. 2020, 105, 211–222. [Google Scholar] [CrossRef]
- Kim, S.G.; Ha, J.H. Formation and speciation of hazardous trihalomethanes and haloacetic acids during chlorinated washing of brined kimchi cabbage in the presence of bromide. J. Hazard Mater 2020, 455, 131557. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.; Dar, A.H.; Dash, K.K.; Srivastava, S.; Pandey, V.K.; Ayoub, W.S.; Pandiselvam, R.; Manzoor, S.; Kaur, M. Cold plasma treatment advancements in food processing and impact on the physiochemical characteristics of food products. Food Sci. Biotechnol. 2023, 32, 621–638. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Yang, Y.; Mulati, A.; Hong, J.; Wang, J. The Effects of Cold-Plasma Technology on the Quality Properties of Fresh-Cut Produce: A Review. Foods 2025, 14, 149. [Google Scholar] [CrossRef]
- Molina-Hernandez, J.B.; Landi, L.; De Flaviis, R.; Laika, J.; Romanazzi, G.; Chaves-Lopez, C. Understanding the mechanisms of action of atmospheric cold plasma towards the mitigation of the stress induced in molds: The case of Aspergillus chevalieri. Innov. Food Sci. Emerg. Technol. 2023, 90, 1466–8564. [Google Scholar] [CrossRef]
- Boehm, D.; Heslin, C.; Cullen, P.J.; Bourke, P. Cytotoxic and mutagenic potential of solutions exposed to cold atmospheric plasma. Sci. Rep. 2016, 6, 21464. [Google Scholar] [CrossRef]
- Phan, N.L.B.; Nguyen, T.; Pedley, J.; Flint, S. Inactivation of Cronobacter sakazakii biofilms using high voltage atmospheric cold plasma on various food-contact surfaces—A preliminary study. Lett. Appl. Microbiol. 2023, 76, ovac046. [Google Scholar] [CrossRef]
- Chen, D.; Peng, P.; Zhou, N.; Cheng, Y.; Min, M.; Ma, Y.; Mao, Q.; Chen, P.; Chen, C.; Ruan, R. Evaluation of Cronobacter sakazakii inactivation and physicochemical property changes of non-fat dry milk powder by cold atmospheric plasma. Food Chem. 2019, 290, 270–276. [Google Scholar] [CrossRef]
- Oh, Y.J.; Lee, H.; Kim, J.E.; Lee, S.H.; Cho, H.Y.; Min, S.C. Cold Plasma Treatment Application to Improve Microbiological Safety of Infant Milk Powder and Onion Powder. Korean Journal of Food Science and Technology. Korean Soc. Food Sci. Technol. 2015, 47, 486–491. [Google Scholar] [CrossRef]
- Ziuzina, D.; Misra, N.N.; Han, L.; Cullen, P.J.; Moiseev, T.; Mosnier, J.P.; Keener, K.; Gaston, E.; Vilaro, I.; Bourke, P. Investigation of a large gap cold plasma reactor for continuous in-package decontamination of fresh strawberries and spinach. Innov. Food Sci. Emerg. Technol. 2020, 59, 102229. [Google Scholar] [CrossRef]
- Critzer, J.F.; Kelly-Wintenberg, K.; South, L.S.; Golden, A.D. Atmospheric Plasma Inactivation of Foodborne Pathogens on Fresh Produce Surfaces. J. Food Prot. 2007, 70, 2290–2296. [Google Scholar] [CrossRef]
- Donaghy, J.A.; Jagadeesan, B.; Goodburn, K.; Grunwald, L.; Jensen, O.N.; Jespers, A.D.; Kanagachandran, K.; Lafforgue, H.; Seefelder, W.; Quentin, M.C. Relationship of sanitizers, disinfectants, and cleaning agents with antimicrobial resistance. J. Food Prot. 2019, 82, 889–902. [Google Scholar] [CrossRef]
| Food Matrix | Genus | Occurrence | Reference |
|---|---|---|---|
| Lettuce | Arcobacter spp. | - | [4] |
| Alfalfa broccoli | Cronobacter sakazakii | 21/60 | [38] |
| Small radish | Cronobacter muytjensii | ||
| Lentil | Cronobacter turicensis | ||
| Sunflower | Cronobacter malonaticus | ||
| Leek and sprout mix | Cronobacter condiment | ||
| Rucola | Cronobacter sakazakii | ||
| Lamb’s lettuce | Cronobacter muytjensii | ||
| Endive escarole | Cronobacter turicensis | ||
| Leaf vegetables mix | Cronobacter malonaticus | ||
| Lettuce | Arcobacter butzleri | 16/110 | [41] |
| Rocket | Arcobacter butzleri | ||
| Arcobacter cryaerophilus | |||
| Carrot | Arcobacter skirrowii | ||
| Arcobacter butzleri | |||
| Beet root | Arcobacter skirrowii | 28/204 | [42] |
| Arcobacter butzleri | |||
| Cabbage | Arcobacter butzleri | ||
| Lettuce | Arcobacter spp. | 21/90 | [44] |
| Spinach | |||
| Arugula | |||
| Lettuce | Arcobacter butzleri | 44/160 | [46] |
| Spinach | Arcobacter cryaerophilus | ||
| Rocket | Arcobacter butzleri | ||
| Valerian | Arcobacter butzleri | ||
| Apple | Arcobacter spp. | 10/50 | [47] |
| Lettuce | Cronobacter spp. | 122/403 | [49] |
| Coriander | |||
| Tomato | |||
| Cucumber | |||
| Vegetables | Cronobacter turicensis, | 71/602 | [51] |
| Cronobacter muytjensii | |||
| Vegetable | Cronobacter sakazaki | - | [52] |
| Spinach | Arcobacter butzleri | 119/175 | [54] |
| Lettuce | Arcobacter cryaerophilus | ||
| Rocket | Arcobacter butzleri | ||
| Valerian | Arcobacter butzleri |
| Microorganism | Organic Acid and Chlorine | Matrix | Condition | Microbial Reduction | Potential Research Limitation | Reference |
|---|---|---|---|---|---|---|
| C. sakazakii | Chlorine, chlorine dioxide, and a peroxyacetic acid-based sanitiser | Surface of apples, cantaloupes, strawberries, lettuce, and tomatoes | Chlorine dioxide, and a peroxyacetic acid-based sanitiser (Tsunami 200) treatments (1 and 5 min). | Chlorine and chlorine dioxide 3.38 and 3.77 Log10 CFU/apple. Tsunami 200: 4 Log10 CFU/apple. Reductions of > or = 3.70 Log CFU/tomato with 10 ugml chlorine or chlorine dioxide or 40 microg/mL Tsunami 200 for 5 min. Treatment of lettuce with Tsunami 200 (40 and 80 microg/mL) reduction of 5.31 Log10 CFU/sample. | Only two treatment times (1 and 5 min) were evaluated. | [25] |
| Arcobacter butzleri and Arcobacter cryaerophilus | Propionic acid, lactic acid, malic acid, ascorbic acid, formic acid, and tartaric acid. | In vitro test | Propionic, lactic, malic, and ascorbic acids (pH 5.5–5.0), formic, citric, and tartaric acids in the pH range of 6.0–5.5 | Arcobacter butzleri grows at pH 5.5. No viable cells were detected in BHI broth acidified to pH 5.0. with ascorbic, malic, lactic, or propionic acid; no growth observed at pH < 5.5. Arcobacter cryaerophilus: more susceptible to the decrease in pH values, regardless of the acidulant used. | The effect of the pH of brain heart infusion (BHI) broth as a positive control. | [26] |
| C. sakazakii | Para-hydroxybenzoate (BPB) Malic acid | Apple Juice | pH values of 3.2 to 9.0, supplemented with selected concentrations of BPB ≤ 125 ppm and thermally treated (58 °C). | 6-Log10 reduction at 600 s, 125 ppm of BPB resulted in a 6-Log10 reduction in 30 s. | The effect on food quality has not been evaluated. | [31] |
| C. sakazakii | Acetic acid Propionic acid Malic acid | In vitro test | Acidic acetic acid, propionic acid, malic acid, and heat stress (55 °C). | The order of resistance of the acid-shocked C. sakazakii to the organic acids was acetic acid > propionic acid > malic acid. | The effect of pH has not been evaluated. | [32] |
| Microorganism | Emerging Physical | Matrix | Condition | Microbial Reduction | Potential Research Limitation | Reference |
|---|---|---|---|---|---|---|
| Cronobacter sakazakii | Combined ultrasound with organic acids | In situ test | Single treatment of ultrasound (frequency of 37 kHz and a power up to 380 W) treated with ultrasound alone for 5, 20, 40, 60, 80, and 100 min) or NaOCl 12% diluted with tap water to a final volume 80 mL and final concentrations of 50, 100, 150, and 200 ppm for 5 min. Combined ultrasound and NaOCl treatment, 24 combination treatments were compared with ultrasound (5, 20, 40,60, 80, and 100 min) and NaOCl (50, 100, 150, and 200 ppm for 5 min). | Despite the significant reduction in C. sakazakii with NaOCl treatment (200 ppm), the combination of 100 min ultrasound and 200 ppm NaOCl resulted in an additional 1.67 log-reduction in C. sakazakii (4.44 Log10 reduction = 2.77 + 1.67 Log10). | The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were not determined. | [34] |
| Cronobacter sakazakii | Ultraviolet-C (UV-C) radiation, acidic electrolysed (AEW), and neutral electrolysed (NEW) waters | In situ test | The fruits were contaminated by dip inoculation, contaminated mangoes were disinfected using UV-C (2.5, 5, 7.5, and 10 kJ/m2), AEW, NEW, and sodium hypochlorite (SH), and the microorganisms were monitored. | C. sakazakii grew after and UV-C was more effective in reducing C. sakazakii (2.6 Log CFU/g) when compared with AEW, NEW, and SH (1.8 Log CFU/g). | The combined effect of AEW, NEW, and UV-C was not evaluated. | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina-Hernandez, J.B.; Cellini, B.; Shanbeh Zadeh, F.; Vannini, L.; Rocculi, P.; Tappi, S. Inactivation of Emerging Opportunistic Foodborne Pathogens Cronobacter spp. and Arcobacter spp. on Fresh Fruit and Vegetable Products: Effects of Emerging Chemical and Physical Methods in Model and Real Food Systems—A Review. Foods 2025, 14, 2463. https://doi.org/10.3390/foods14142463
Molina-Hernandez JB, Cellini B, Shanbeh Zadeh F, Vannini L, Rocculi P, Tappi S. Inactivation of Emerging Opportunistic Foodborne Pathogens Cronobacter spp. and Arcobacter spp. on Fresh Fruit and Vegetable Products: Effects of Emerging Chemical and Physical Methods in Model and Real Food Systems—A Review. Foods. 2025; 14(14):2463. https://doi.org/10.3390/foods14142463
Chicago/Turabian StyleMolina-Hernandez, Junior Bernardo, Beatrice Cellini, Fatemeh Shanbeh Zadeh, Lucia Vannini, Pietro Rocculi, and Silvia Tappi. 2025. "Inactivation of Emerging Opportunistic Foodborne Pathogens Cronobacter spp. and Arcobacter spp. on Fresh Fruit and Vegetable Products: Effects of Emerging Chemical and Physical Methods in Model and Real Food Systems—A Review" Foods 14, no. 14: 2463. https://doi.org/10.3390/foods14142463
APA StyleMolina-Hernandez, J. B., Cellini, B., Shanbeh Zadeh, F., Vannini, L., Rocculi, P., & Tappi, S. (2025). Inactivation of Emerging Opportunistic Foodborne Pathogens Cronobacter spp. and Arcobacter spp. on Fresh Fruit and Vegetable Products: Effects of Emerging Chemical and Physical Methods in Model and Real Food Systems—A Review. Foods, 14(14), 2463. https://doi.org/10.3390/foods14142463










