Effects of Umami Taste on Visual Food Cue Processing: An ERP Study with Source Localization
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
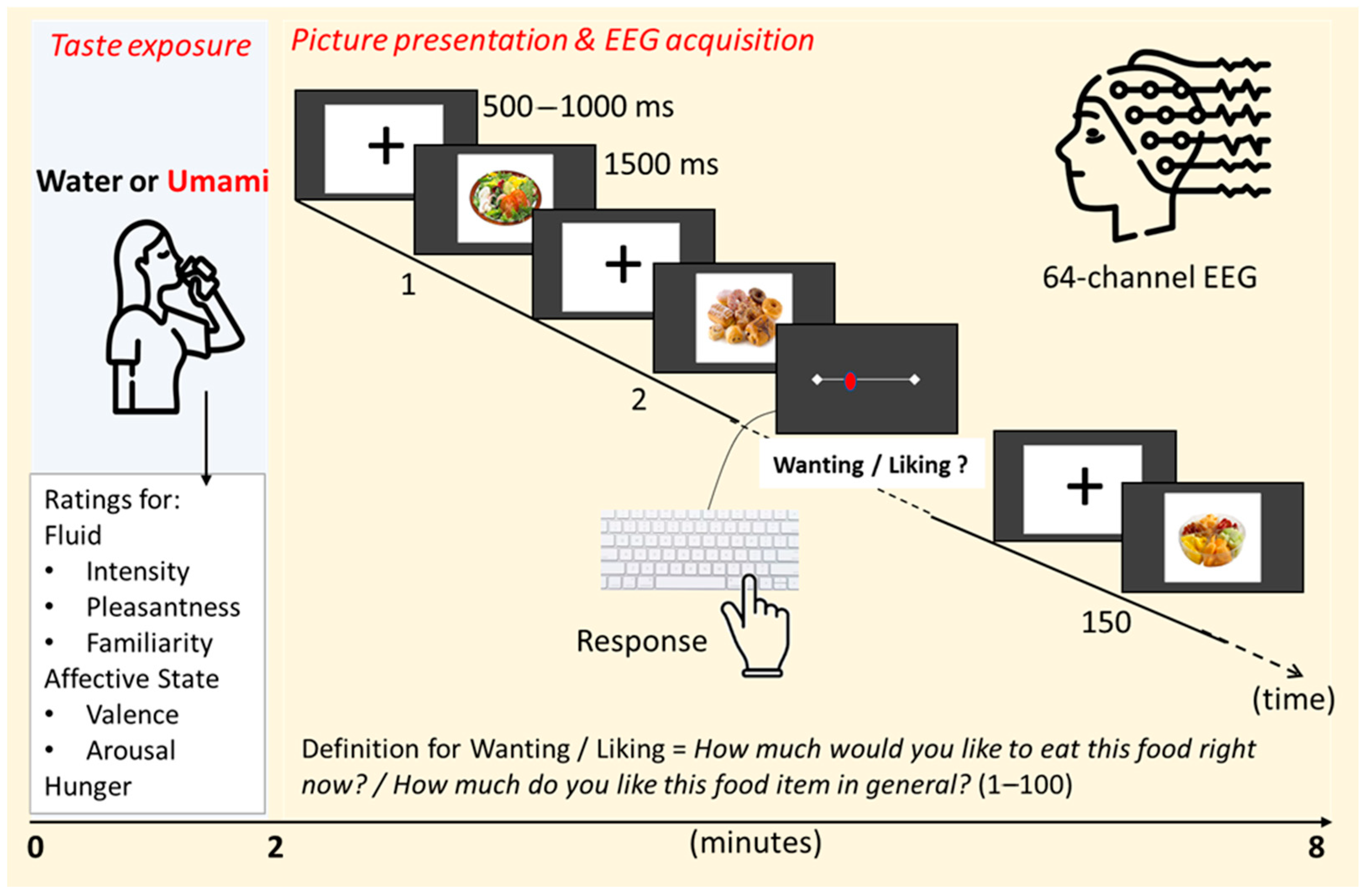
2.2. Stimuli
2.3. Experimental Procedure
2.4. EEG Recordings and Data Analysis
2.5. Source Localization
2.6. Statistical Analyses of the Rating Data
3. Results
3.1. Ratings
3.2. Event-Related Potentials
3.3. Source Localization and Estimated Current Source Density (eCSD)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loï, C.; Cynober, L. Glutamate: A safe nutrient, not just a simple additive. Ann. Nutr. Metab. 2022, 78, 133–146. [Google Scholar] [CrossRef]
- Wu, B.; Eldeghaidy, S.; Ayed, C.; Fisk, I.D.; Hewson, L.; Liu, Y. Mechanisms of umami taste perception: From molecular level to brain imaging. Crit. Rev. Food Sci. Nutr. 2022, 62, 7015–7024. [Google Scholar] [CrossRef]
- Lindemann, B. Receptors and transduction in taste. Nature 2001, 413, 219–225. [Google Scholar] [CrossRef]
- Kurihara, K. Umami the Fifth Basic Taste: History of Studies on Receptor Mechanisms and Role as a Food Flavor. BioMed Res. Int. 2015, 2015, 189402. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Ninomiya, K. Umami and food palatability. J. Nutr. 2000, 130, S921–S926. [Google Scholar] [CrossRef]
- Baryłko-Pikielna, N.; Kostyra, E. Sensory interaction of umami substances with model food matrices and its hedonic effect. Food Qual. Prefer. 2007, 18, 751–758. [Google Scholar] [CrossRef]
- Masic, U.; Yeomans, M.R. Umami flavor enhances appetite but also increases satiety. Am. J. Clin. Nutr. 2014, 100, 532–538. [Google Scholar] [CrossRef]
- McCrickerd, K.; Forde, C.G. Sensory influences on food intake control: Moving beyond palatability. Obes. Rev. 2016, 17, 18–29. [Google Scholar] [CrossRef]
- Wabnegger, A.; Schwab, D.; Schienle, A. Aversive aftertaste changes visual food cue reactivity: An fMRI study on cross-modal perception. Neurosci. Lett. 2018, 673, 56–60. [Google Scholar] [CrossRef]
- Schwab, D.; Giraldo, M.; Spiegl, B.; Schienle, A. Disgust evoked by strong wormwood bitterness influences the processing of visual food cues in women: An ERP study. Appetite 2017, 108, 51–56. [Google Scholar] [CrossRef]
- Hajcak, G.; MacNamara, A.; Olvet, D.M. Event-related potentials, emotion, and emotion regulation: An integrative review. Dev. Neuropsychol. 2010, 35, 129–155. [Google Scholar] [CrossRef]
- Sarlo, M.; Übel, S.; Leutgeb, V.; Schienle, A. Cognitive reappraisal fails when attempting to reduce the appetitive value of food: An ERP study. Biol. Psychol. 2013, 94, 507–512. [Google Scholar] [CrossRef]
- Asmaro, D.; Jaspers-Fayer, F.; Sramko, V.; Taake, I.; Carolan, P.; Liotti, M. Spatiotemporal dynamics of the hedonic processing of chocolate images in individuals with and without trait chocolate craving. Appetite 2012, 58, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Biehl, S.C.; Keil, J.; Naumann, E.; Svaldi, J. ERP and oscillatory differences in overweight/obese and normal-weight adolescents in response to food stimuli. J. Eat. Disord. 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Nijs, I.M.; Muris, P.; Euser, A.S.; Franken, I.H. Differences in attention to food and food intake between overweight/obese and normal-weight females under conditions of hunger and satiety. Appetite 2010, 54, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Keil, A.; Borovsky, A.; Bradley, M.M.; Lang, P.J. Affective modulation of steady-state visual evoked potentials: Source distribution and interindividual differences. Psychophysiology 2002, 39, S45. [Google Scholar]
- Huerta, C.I.; Sarkar, P.R.; Duong, T.Q.; Laird, A.R.; Fox, P.T. Neural bases of food perception: Coordinate-based meta-analyses of neuroimaging studies in multiple modalities. Obesity 2014, 22, 1439–1446. [Google Scholar] [CrossRef]
- Cecchini, M.P.; Knaapila, A.; Hoffmann, E.; Boschi, F.; Hummel, T.; Iannilli, E. A cross-cultural survey of umami familiarity in European countries. Food Qual. Prefer. 2019, 74, 172–178. [Google Scholar] [CrossRef]
- Blechert, J.; Meule, A.; Busch, N.A.; Ohla, K. Food-pics: An image database for experimental research on eating and appetite. Front. Psychol. 2014, 5, 617. [Google Scholar] [CrossRef]
- Peirce, J.; Gray, J.R.; Simpson, S.; MacAskill, M.; Höchenberger, R.; Sogo, H.; Kastman, E.; Lindeløv, J.K. PsychoPy2: Ex-periments in behavior made easy. Behav. Res. Methods 2019, 51, 195–203. [Google Scholar] [CrossRef]
- Brunet, D.; Murray, M.M.; Michel, C.M. Spatiotemporal Analysis of Multichannel EEG: CARTOOL. Comput. Intell. Neurosci. 2011, 2011, 813870. [Google Scholar] [CrossRef]
- Michel, C.M.; Brunet, D. EEG Source Imaging: A Practical Review of the Analysis Steps. Front. Neurol. 2019, 10, 325. [Google Scholar] [CrossRef]
- Evans, A.C.; Janke, A.L.; Collins, D.L.; Baillet, S. Brain templates and atlases. NeuroImage 2012, 62, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Mai, J.K.; Majtanik, M.; Paxinos, G. Atlas of the Human Brain; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- de Peralta Menendez, R.G.; Murray, M.M.; Michel, C.M.; Martuzzi, R.; Andino, S.L.G. Electrical neuroimaging based on biophysical constraints. NeuroImage 2004, 21, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Scinska-Bienkowska, A.; Wrobel, E.; Turzynska, D.; Bidzinski, A.; Jezewska, E.; Sienkiewicz-Jarosz, H.; Golembiowska, K.; Kostowski, W.; Kukwa, A.; Plaznik, A.; et al. Glutamate concentration in whole saliva and taste responses to monosodium glutamate in humans. Nutr. Neurosci. 2006, 9, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Zhi, R.; Hu, X.; Wang, C.; Liu, S. Development of a direct mapping model between hedonic rating and facial responses by dynamic facial expression representation. Food Res. Int. 2020, 137, 109411. [Google Scholar] [CrossRef] [PubMed]
- Potthoff, J.; Jurinec, N.; Schienle, A. Placebo Effects on Visual Food Cue Reactivity: An Eye-Tracking Investigation. Front. Psychiatry 2019, 10, 525. [Google Scholar] [CrossRef]
- Beauchamp, G.K. Sensory and receptor responses to umami: An overview of pioneering work. Am. J. Clin. Nutr. 2009, 90, 723S–727S. [Google Scholar] [CrossRef]
- Ninomiya, K. Natural occurrence. Food Rev. Int. 1998, 14, 177–211. [Google Scholar] [CrossRef]
- Niemeyer, J.E.; Akers-Campbell, S.; Gregoire, A.; Paradiso, M.A. Perceptual enhancement and suppression correlate with V1 neural activity during active sensing. Curr. Biol. 2022, 32, 2654–2667. [Google Scholar] [CrossRef]
- van der Laan, L.; de Ridder, D.; Viergever, M.; Smeets, P. The first taste is always with the eyes: A meta-analysis on the neural correlates of processing visual food cues. NeuroImage 2011, 55, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Wang, A.; Henderson, M.M.; Lin, R.; Prince, J.S.; Tarr, M.J.; Wehbe, L. Selectivity for food in human ventral visual cortex. Commun. Biol. 2023, 6, 175. [Google Scholar] [CrossRef] [PubMed]
- Adamson, K.; Troiani, V. Distinct and overlapping fusiform activation to faces and food. NeuroImage 2018, 174, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Dupuis-Roy, N.; Yanga XLQiua, J.F.; Zhang, Q.L. The taste-visual cross-modal Stroop effect: An event-related brain potential study. Neuroscience 2014, 263, 250–256. [Google Scholar] [CrossRef] [PubMed]


| Monosodium Glutamate M (SD) | Water M (SD) | |
|---|---|---|
| Age (years) | 24.93 (8.42) | 25.98 (7.92) |
| Body mass index | 22.36 (2.89) | 23.85 (4.06) |
| Hunger level (pre) | 51.20 (27.44) | 46.34 (25.36) |
| Hunger level (post) | 61.47 (28.35) | 55.06 (33.32) |
| Valence (pre) | 77.50 (14.65) | 76.39 (20.26) |
| Valence (post) | 71.32 (19.31) | 71.17 (20.07) |
| Arousal (pre) | 27.33 (22.25) | 30.02 (24.50) |
| Arousal (post) | 18.55 (18.34) | 22.58 (21.83) |
| Fluid ratings | ||
| Pleasantness | 29.44 (16.95) | 72.43 (20.62) |
| Intensity | 55.18 (23.08) | 13.02 (16.40) |
| Familiarity | 32.03 (22.27) | 86.03 (21.25) |
| Image ratings (wanting) | ||
| Sweets | 41.85 (19.98) | 41.12 (23.70) |
| Meat | 31.37 (25.39) | 31.36 (24.43) |
| Fruits | 63.03 (15.57) | 65.94 (18.93) |
| Vegetables | 50.79 (20.93) | 45.35 (19.56) |
| Image ratings (liking) | ||
| Sweets | 64.39 (16.54) | 63.82 (20.12) |
| Meat | 47.34 (27.14) | 51.00 (23.30) |
| Fruits | 79.07 (11.85) | 81.33 (11.96) |
| Vegetables | 73.31 (16.38) | 69.87 (14.67) |
| TF (ms) | Solution Point | Coordinates (Talairach, mm) | Label |
|---|---|---|---|
| P300 | RAI202 | (25, 3, −34) | Right fusiform gyrus |
| LPP | LAI236 RAS493 LPI425 | (−34, 1, −34) (3, 58, 5) (−21, −100, 2) | Left fusiform gyrus Right medial transversal frontopolar gyrus Left occipital gyrus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schienle, A.; Osmani, F.; Iannilli, E. Effects of Umami Taste on Visual Food Cue Processing: An ERP Study with Source Localization. Foods 2025, 14, 2409. https://doi.org/10.3390/foods14142409
Schienle A, Osmani F, Iannilli E. Effects of Umami Taste on Visual Food Cue Processing: An ERP Study with Source Localization. Foods. 2025; 14(14):2409. https://doi.org/10.3390/foods14142409
Chicago/Turabian StyleSchienle, Anne, Florian Osmani, and Emilia Iannilli. 2025. "Effects of Umami Taste on Visual Food Cue Processing: An ERP Study with Source Localization" Foods 14, no. 14: 2409. https://doi.org/10.3390/foods14142409
APA StyleSchienle, A., Osmani, F., & Iannilli, E. (2025). Effects of Umami Taste on Visual Food Cue Processing: An ERP Study with Source Localization. Foods, 14(14), 2409. https://doi.org/10.3390/foods14142409






