Selection of Fructophilic Yeast from Sun-Dried Pedro Ximénez Grape Must for the Development of New Vinegars Containing Gluconic Acid
Abstract
1. Introduction
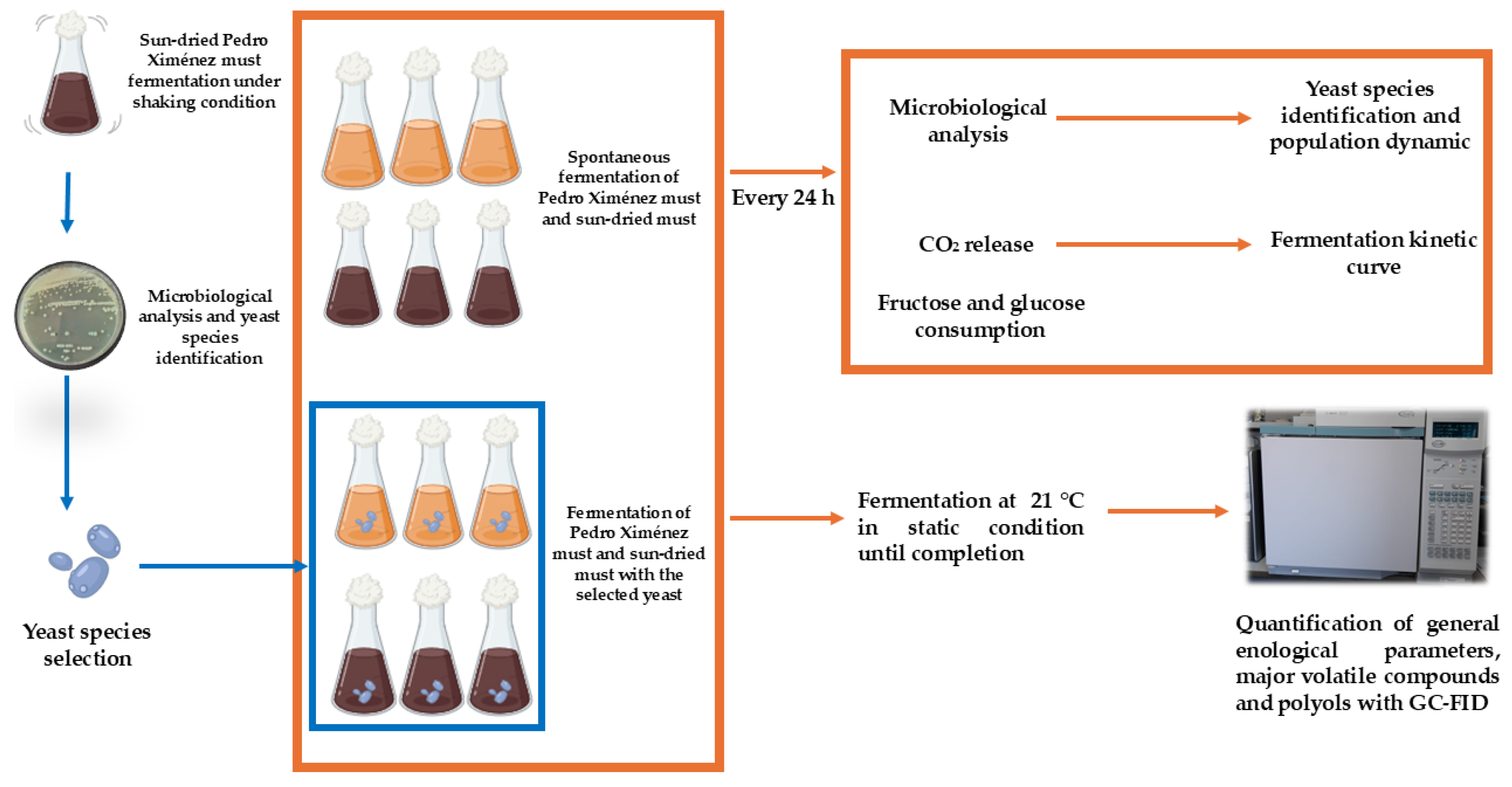
2. Materials and Methods
2.1. Isolation and Identification of Yeasts from Sun-Dried Pedro Ximénez Must Spontaneous Fermentation
2.2. Identification and Characterization of Yeast Isolates
2.3. Must Fermentation Conditions
2.4. Measurement of Enological Parameters
2.5. Quantification of Major Volatile Aroma Compounds and Polyols
2.6. Microbiological Analysis
2.7. Statistical Analyses
3. Results
3.1. Isolation, Identification, and Characterization of Yeast from Sun-Dried Grape Must
3.2. Inoculation of Selected Yeasts in Fresh and Sun-Dried Grape Must
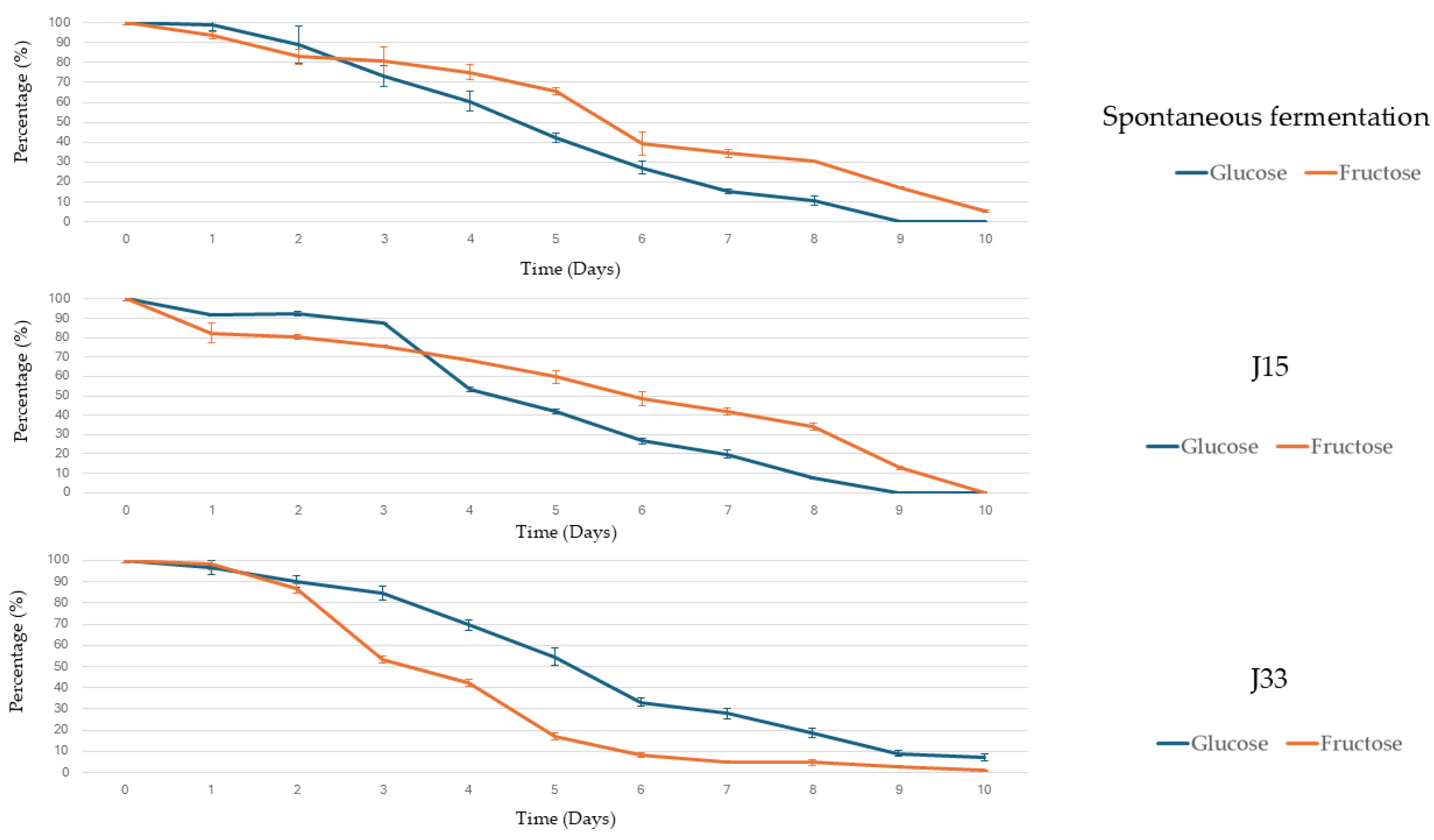
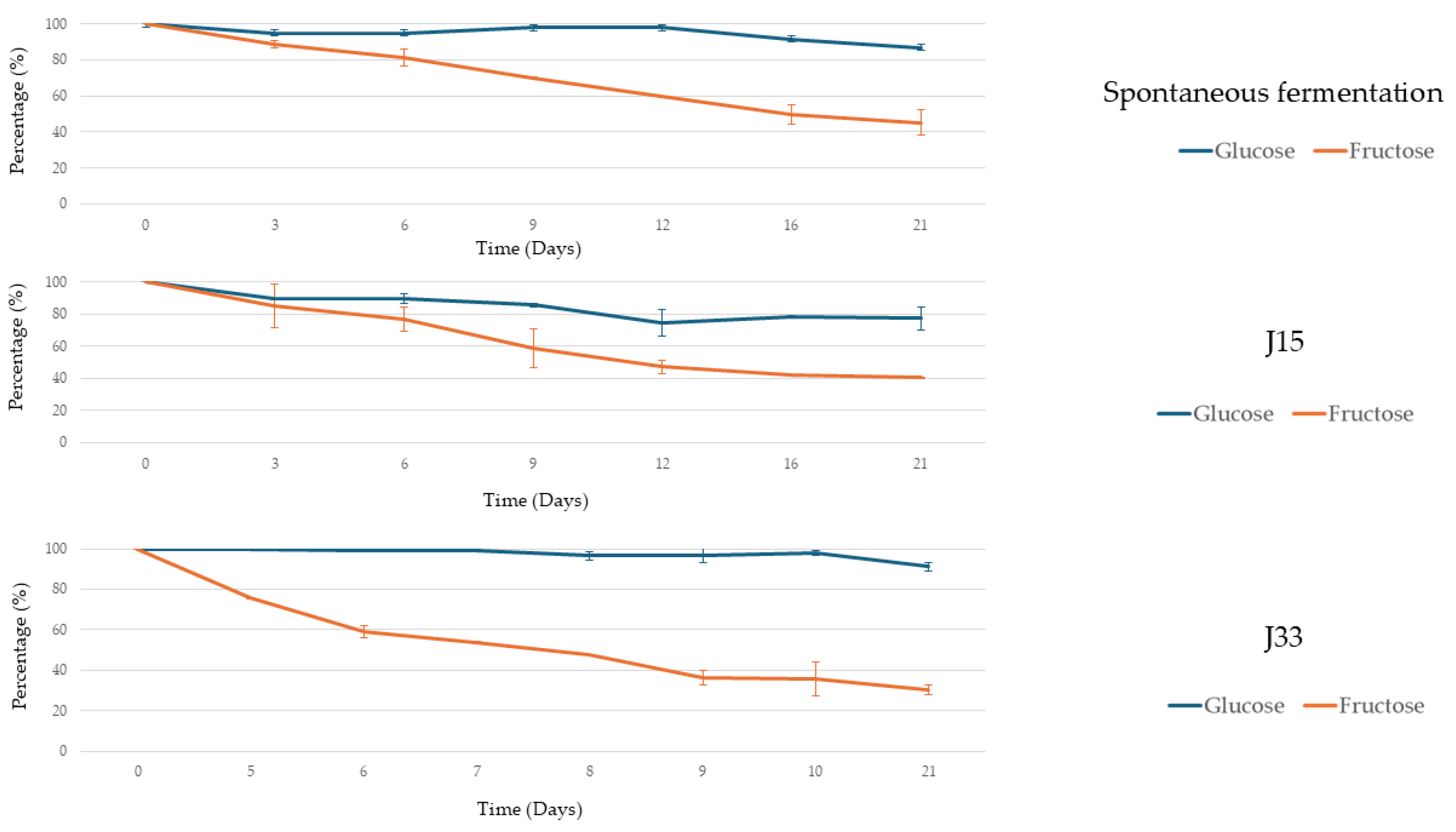
3.2.1. Alcoholic Fermentation Rates and Sugar Consumption
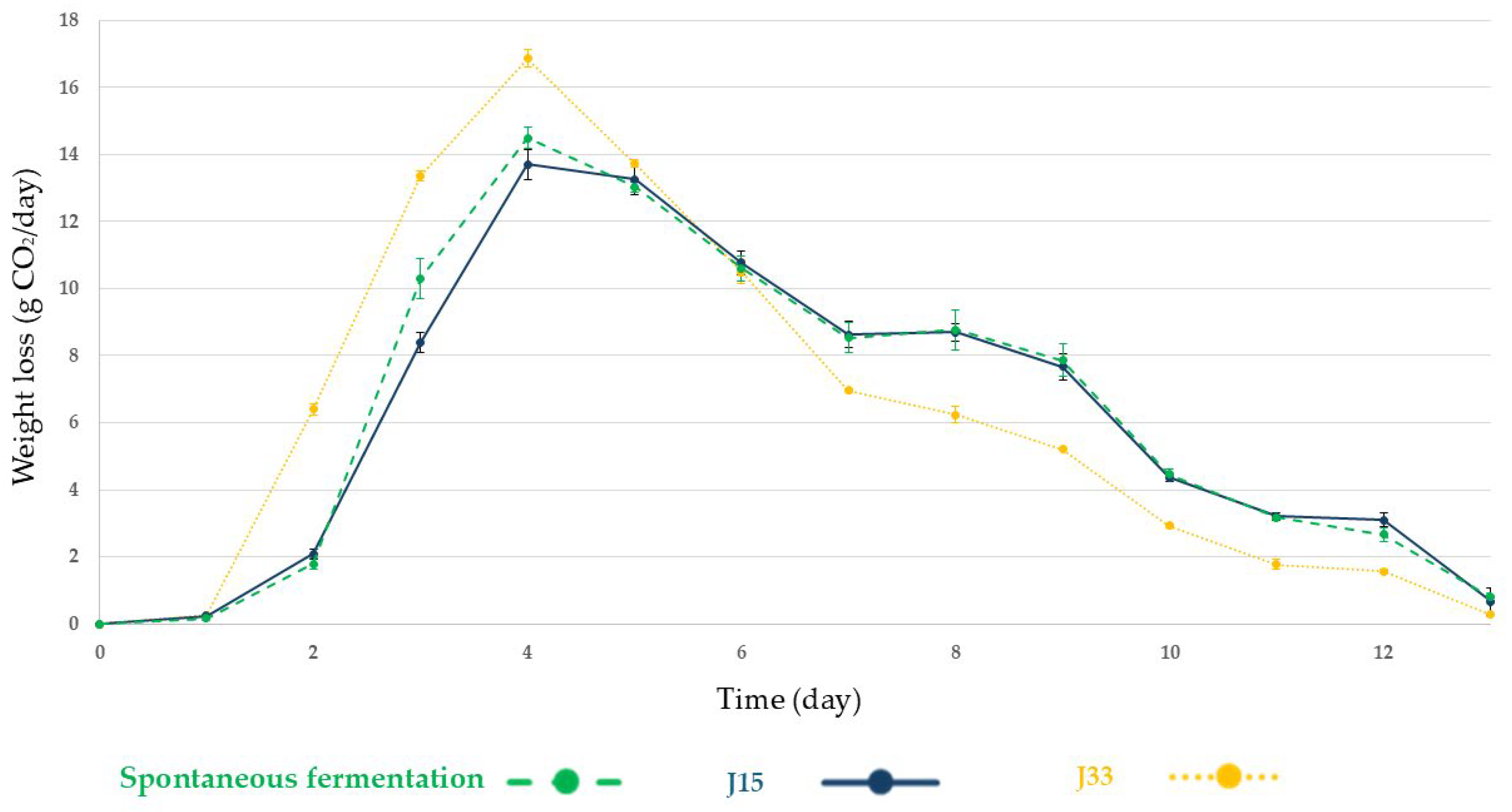
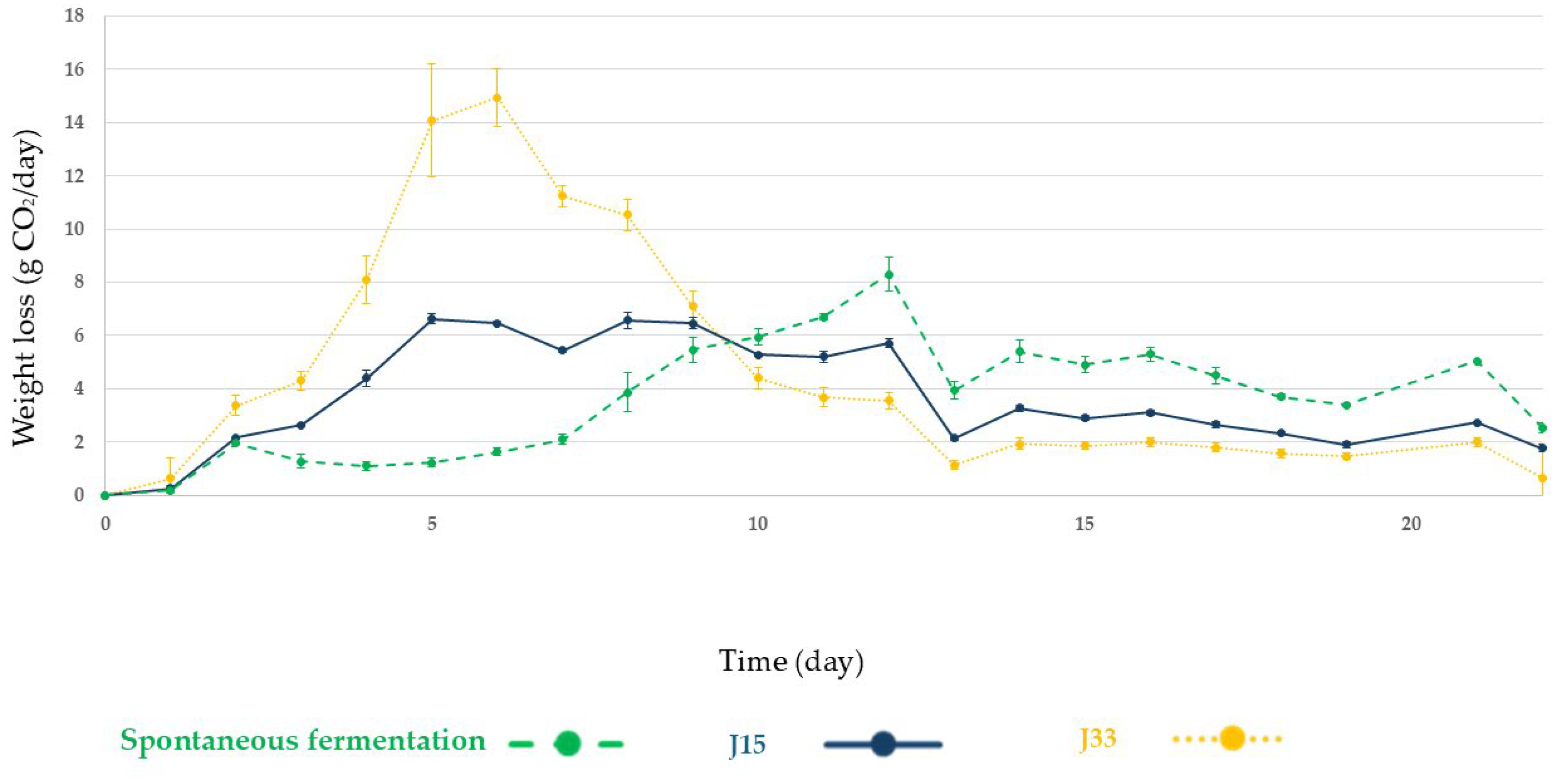
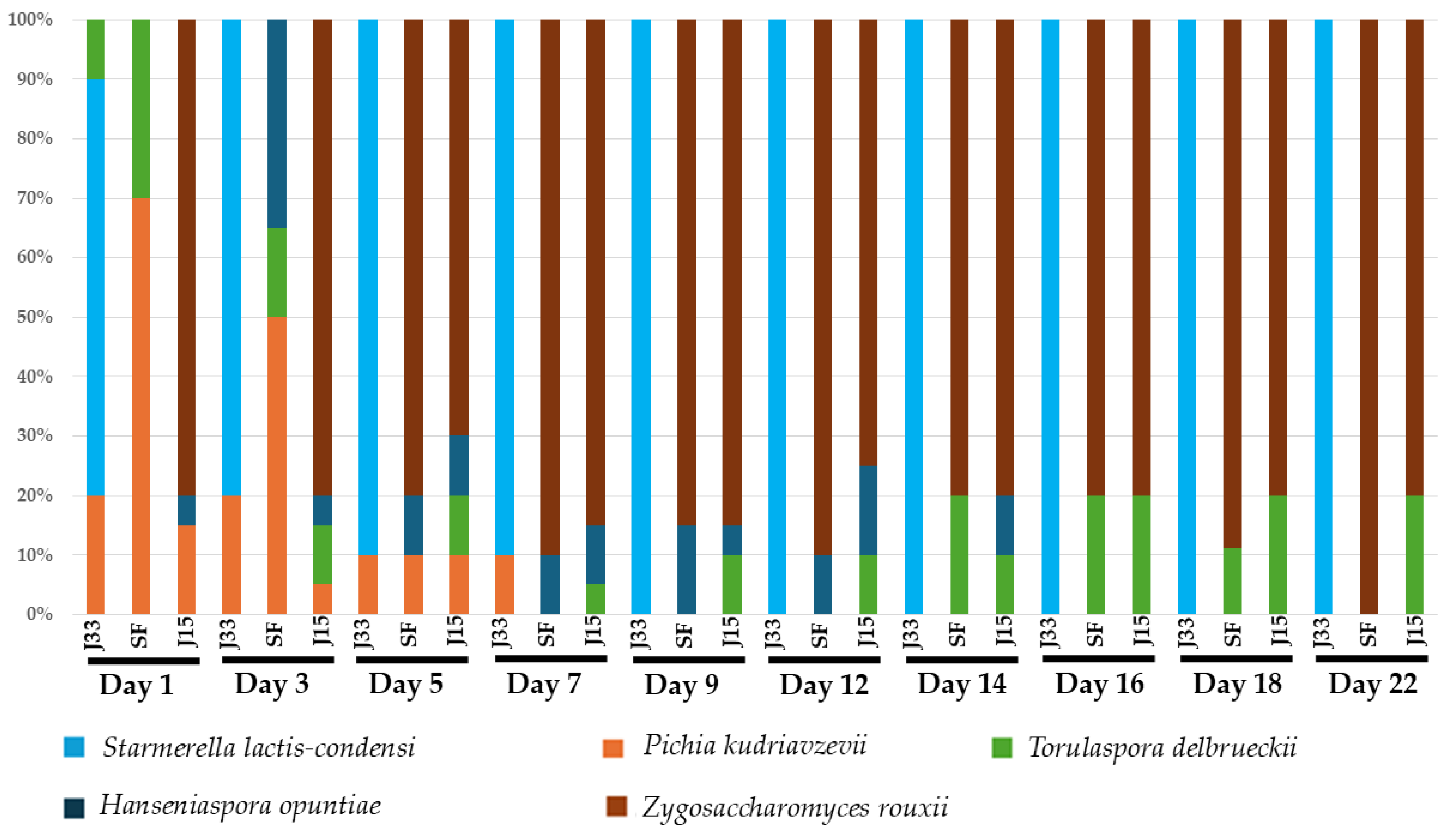
3.2.2. Microbiological Analysis
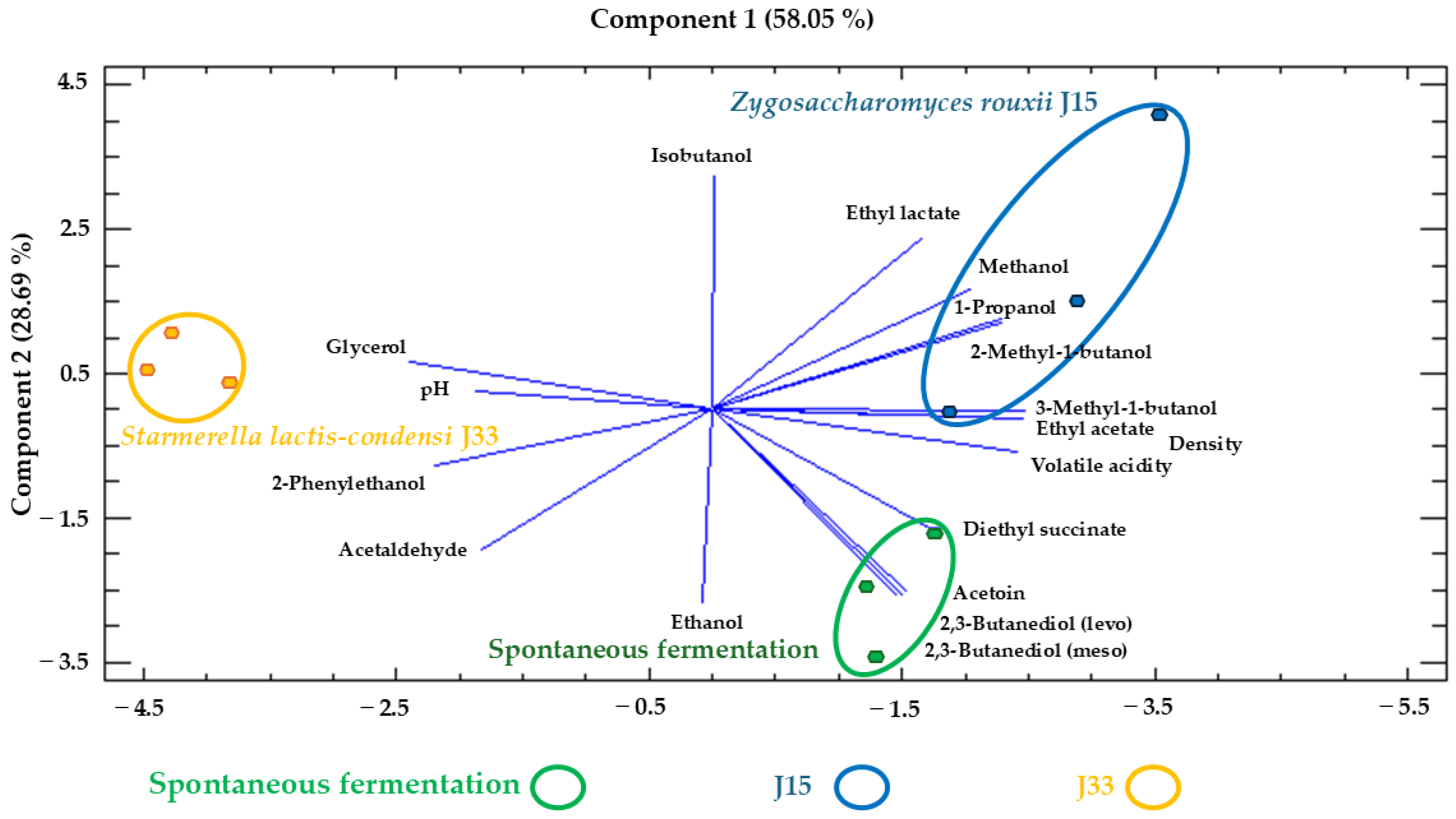
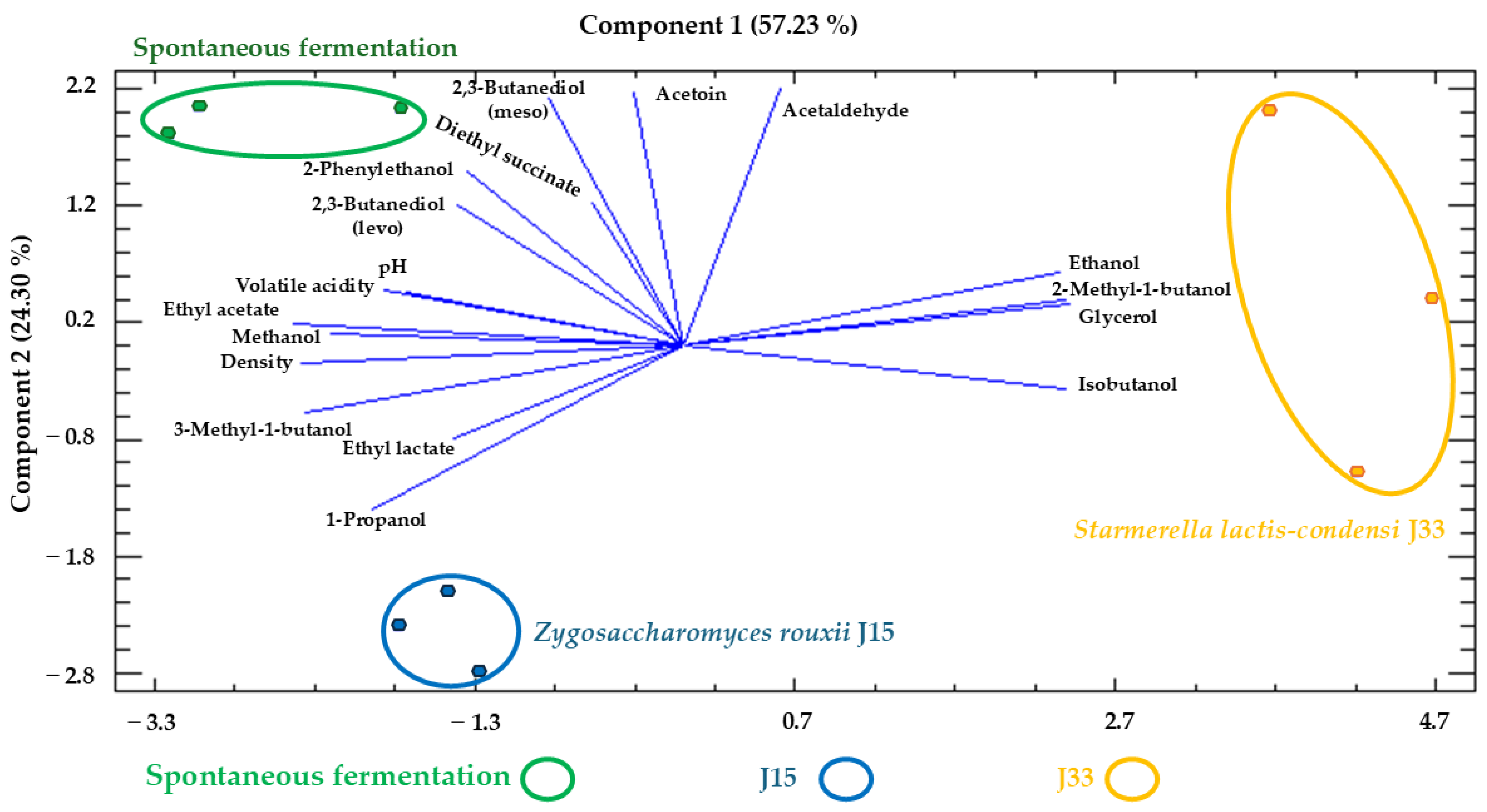
3.2.3. Enological Parameters and Major Aroma Compounds and Polyols
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Román-Camacho, J.J.; García-García, I.; Santos-Dueñas, I.M.; García-Martínez, T.; Mauricio, J.C. Latest Trends in Industrial Vinegar Production and the Role of Acetic Acid Bacteria: Classification, Metabolism, and Applications—A Comprehensive Review. Foods 2023, 12, 3705. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.W.; Lazim, A.M.; Fazry, S.; Zaki, U.K.H.H.; Lim, S.J. Varieties, Production, Composition and Health Benefits of Vinegars: A Review. Food Chem. 2017, 221, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Bekatorou, A. Advances in Vinegar Production, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9781351208475. [Google Scholar]
- Luzón-Quintana, L.M.; Castro, R.; Durán-Guerrero, E. Biotechnological Processes in Fruit Vinegar Production. Foods 2021, 10, 945. [Google Scholar] [CrossRef]
- Deppenmeier, U.; Hoffmeister, M.; Prust, C. Biochemistry and Biotechnological Applications of Gluconobacter Strains. Appl. Microbiol. Biotechnol. 2002, 60, 233–242. [Google Scholar]
- Matsushita, K.; Toyama, H.; Adachi, O. Respiratory Chains and Bioenergetics of Acetic Acid Bacteria. In Advances in Microbial Physiology; Academic Press: Cambridge, MA, USA, 1994; Volume 36, pp. 247–301. [Google Scholar]
- Giudici, P.; De Vero, L.; Gullo, M.; Solieri, L.; Lemmetti, F. Fermentation Strategy to Produce High Gluconate Vinegar. Acetic Acid Bact. 2016, 5, 1–28. [Google Scholar] [CrossRef]
- Giudici, P.; Lemmetti, F.; Mazza, S. Balsamic Vinegars: Tradition, Technology, Trade, 2015th ed.; Springer International Publishing: Cham, Switzerland, 2015; ISBN 9783319137575. [Google Scholar]
- Csoma, H.; Kállai, Z.; Antunovics, Z.; Czentye, K.; Sipiczki, M. Vinification without: Interacting Osmotolerant and “Spoilage” Yeast Communities in Fermenting and Ageing Botrytised High-Sugar Wines (Tokaj Essence). Microorganisms 2020, 9, 19. [Google Scholar] [CrossRef]
- Pina, C.; Gonçalves, P.; Prista, C.; Loureiro-Dias, M.C. Ffz1, a New Transporter Specific for Fructose from Zygosaccharomyces bailii. Microbiology 2004, 150, 2429–2433. [Google Scholar] [CrossRef]
- Gonçalves, C.; Wisecaver, J.H.; Kominek, J.; Oom, M.S.; Leandro, M.J.; Shen, X.-X.; Opulente, D.A.; Zhou, X.; Peris, D.; Kurtzman, C.P.; et al. Evidence for Loss and Reacquisition of Alcoholic Fermentation in a Fructophilic Yeast Lineage. Elife 2018, 7, e33034. [Google Scholar] [CrossRef]
- Franco, M.; Peinado, R.A.; Medina, M.; Moreno, J. Off-Vine Grape Drying Effect on Volatile Compounds and Aromatic Series in Must from Pedro Ximénez Grape Variety. J. Agric. Food Chem. 2004, 52, 3905–3910. [Google Scholar] [CrossRef]
- García-Martínez, T.; de Lerma, N.L.; Moreno, J.; Peinado, R.A.; Millán, M.C.; Mauricio, J.C. Sweet Wine Production by Two Osmotolerant Saccharomyces cerevisiae Strains. J. Food Sci. 2013, 78, M874–M879. [Google Scholar] [CrossRef]
- Montilla-Moriles PDO Vinegar Legislation. Available online: https://www.montillamoriles.es/wp-content/uploads/2023/05/2.-PC-DOP-VINAGRE-DE-MONTILLA-MORILES-2023.pdf (accessed on 18 May 2025).
- Carbonero-Pacheco, J.; García-Jiménez, Á.; García-García, J.C.; Santos-Dueñas, I.M.; García-Martínez, T.; Moreno, J.; Moreno-García, J.; Mauricio, J.C. Use of Wickerhamomyces anomalus Strains from Biologically Aged Wines to Improve the Sensorial Profile of Young White Wines. Appl. Sci. 2025, 15, 1546. [Google Scholar] [CrossRef]
- Lepe, J.A.S.; Leal, B.Í. Microbiología Enológica: Fundamentos de Vinificación; Mundi-Prensa Libros: Madrid, Spain, 2004; ISBN 9788484761846. [Google Scholar]
- OIV 2025. Available online: https://www.oiv.int/ (accessed on 18 May 2025).
- Peinado, R.A.; Moreno, J.A.; Muñoz, D.; Medina, M.; Moreno, J. Gas Chromatographic Quantification of Major Volatile Compounds and Polyols in Wine by Direct Injection. J. Agric. Food Chem. 2004, 52, 6389–6393. [Google Scholar] [CrossRef] [PubMed]
- Carbonero-Pacheco, J.; Constanta-Mustafa, F.; Muñoz-Castells, R.; Mauricio, J.C.; Moreno, J.; García-Martínez, T.; Moreno-García, J. Microbial Biocapsules as Generally Recognized-As-Safe Fungal-Based Immobilized Cell Technology for Precision Sequential Fermentations of Grape Must. Fermentation 2024, 10, 498. [Google Scholar] [CrossRef]
- Sola, I.M.M.S.; Evers, L.D.; Wojeicchowski, J.P.; de Assis, T.M.; Marinho, M.T.; Demiate, I.M.; Alberti, A.; Nogueira, A. Impact of Pure, Co-, and Sequential Fermentations with Hanseniaspora Sp. and Saccharomyces cerevisiae on the Volatile Compounds of Ciders. Fermentation 2024, 10, 177. [Google Scholar] [CrossRef]
- Seixas, I.; Barbosa, C.; Mendes-Faia, A.; Güldener, U.; Tenreiro, R.; Mendes-Ferreira, A.; Mira, N.P. Genome Sequence of the Non-Conventional Wine Yeast Hanseniaspora guilliermondii UTAD222 Unveils Relevant Traits of This Species and of the Hanseniaspora Genus in the Context of Wine Fermentation. DNA Res. 2019, 26, 67–83. [Google Scholar] [CrossRef]
- Alcalá-Jiménez, M.T.; García-Martínez, T.; Mauricio, J.C.; Moreno, J.; Peinado, R.A. Influence of Terroir on Microbial Diversity and Wine Volatilome. Appl. Sci. 2025, 15, 3237. [Google Scholar] [CrossRef]
- Csoma, H.; Kállai, Z.; Czentye, K.; Sipiczki, M. Starmerella lactis-condensi, a Yeast That Has Adapted to the Conditions in the Oenological Environment. Int. J. Food Microbiol. 2023, 401, 110282. [Google Scholar] [CrossRef]
- Francesca, N.; Naselli, V.; Prestianni, R.; Pirrone, A.; Viola, E.; Guzzon, R.; Settanni, L.; Maggio, A.; Vaglica, A.; Bruno, M.; et al. Impact of Two New Non-Conventional Yeasts, Candida oleophila and Starmerella lactis-condensi, Isolated from Sugar-Rich Substrates, on Frappato Wine Aroma. Food Biosci. 2024, 57, 103500. [Google Scholar] [CrossRef]
- Craparo, V.; Viola, E.; Vella, A.; Prestianni, R.; Pirrone, A.; Naselli, V.; Amato, F.; Oliva, D.; Notarbartolo, G.; Guzzon, R.; et al. Oenological Capabilities of Yeasts Isolated from High-Sugar Matrices (Manna and Honey) as Potential Starters and Co-Starters for Winemaking. Beverages 2024, 10, 48. [Google Scholar] [CrossRef]
- Leandro, M.J.; Cabral, S.; Prista, C.; Loureiro-Dias, M.C.; Sychrová, H. The High-Capacity Specific Fructose Facilitator ZrFfz1 Is Essential for the Fructophilic Behavior of Zygosaccharomyces rouxii CBS 732T. Eukaryot Cell 2014, 13, 1371–1379. [Google Scholar] [CrossRef]
- Rojo, M.C.; Torres Palazzolo, C.; Cuello, R.; González, M.; Guevara, F.; Ponsone, M.L.; Mercado, L.A.; Martínez, C.; Combina, M. Incidence of Osmophilic Yeasts and Zygosaccharomyces rouxii during the Production of Concentrate Grape Juices. Food Microbiol. 2017, 64, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Rigou, P.; Mekoue, J.; Sieczkowski, N.; Doco, T.; Vernhet, A. Impact of Industrial Yeast Derivative Products on the Modification of Wine Aroma Compounds and Sensorial Profile. A Review. Food Chem. 2021, 358, 129760. [Google Scholar] [CrossRef] [PubMed]
- Pirrone, A.; Naselli, V.; Gugino, I.M.; Porrello, A.; Viola, E.; Craparo, V.; Vella, A.; Alongi, D.; Seminerio, V.; Carusi, M.; et al. Use of Non-Conventional Yeasts for Enhancing the Sensory Quality of Craft Beer. Food Res. Int. 2025, 208, 116164. [Google Scholar] [CrossRef] [PubMed]
- Milanovic, V.; Ciani, M.; Oro, L.; Comitini, F. Starmerella bombicola Influences the Metabolism of Saccharomyces cerevisiae at Pyruvate Decarboxylase and Alcohol Dehydrogenase Level during Mixed Wine Fermentation. Microb. Cell Factories 2012, 11, 18. [Google Scholar] [CrossRef]
- Tedesco, F.; Siesto, G.; Pietrafesa, R.; Romano, P.; Salvia, R.; Scieuzo, C.; Falabella, P.; Capece, A. Chemical Methods for Microbiological Control of Winemaking: An Overview of Current and Future Applications. Beverages 2022, 8, 58. [Google Scholar] [CrossRef]
- Perpetuini, G.; Rossetti, A.P.; Rapagnetta, A.; Arfelli, G.; Prete, R.; Tofalo, R. Wine Barrel Biofilm as a Source of Yeasts with Non-Conventional Properties. Microorganisms 2024, 12, 880. [Google Scholar] [CrossRef]
- Sipiczki, M. Overwintering of vineyard yeasts: Survival of interacting yeast communities in grapes mummified on vines. Front. Microbiol. 2016, 7, 212. [Google Scholar] [CrossRef]
- Ding, Y.; Wei, R.; Wang, L.; Yang, C.; Li, H.; Wang, H. Diversity and Dynamics of Microbial Ecosystem on Berry Surface during the Ripening of Ecolly (Vitis vinifera L.) Grape in Wuhai, China. World J. Microbiol. Biotechnol. 2021, 37, 214. [Google Scholar] [CrossRef]
- Costantini, A.; Vaudano, E.; Pulcini, L.; Boatti, L.; Gamalero, E.; Garcia-Moruno, E. Yeast Biodiversity in Vineyard during Grape Ripening: Comparison between Culture Dependent and NGS Analysis. Processes 2022, 10, 901. [Google Scholar] [CrossRef]
- Aslankoohi, E.; Rezaei, M.N.; Vervoort, Y.; Courtin, C.M.; Verstrepen, K.J. Glycerol Production by Fermenting Yeast Cells Is Essential for Optimal Bread Dough Fermentation. PLoS ONE 2015, 10, e0119364. [Google Scholar] [CrossRef]
- Nadai, C.; da Silva Duarte, V.; Sica, J.; Vincenzi, S.; Carlot, M.; Giacomini, A.; Corich, V. Released in Vineyards at Different Concentrations Influences Wine Glycerol Content Depending on the Vinification Protocols. Foods 2022, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.L.; Fierro-Risco, J.; Ríos-Reina, R.; Ubeda, C.; Paneque, P. Influence of Saccharomyces cerevisiae and Lachancea thermotolerans Co-Inoculation on Volatile Profile in Fermentations of a Must with a High Sugar Content. Food Chem. 2019, 276, 427–435. [Google Scholar] [CrossRef] [PubMed]

| Species | T0 | T2 | T7 | T14 |
|---|---|---|---|---|
| Hanseniaspora opuntiae | 64.23 | 0.00 | 0.00 | 0.00 |
| Lachancea thermotolerans | 21.02 | 9.20 | 2.30 | 0.00 |
| Pichia kudriavzevii | 2.90 | 0.00 | 0.00 | 0.00 |
| Starmerella lactis-condensi | 0.00 | 0.00 | 1.40 | 74.76 |
| Torulaspora delbrueckii | 7.43 | 3.16 | 0.00 | 0.00 |
| Zygosaccharomyces rouxii | 4.43 | 87.65 | 96.30 | 25.25 |
| Species | Glucose | Fructose |
|---|---|---|
| Hanseniaspora opuntiae | 13.92 ± 2.64 | 20.96 ± 6.45 |
| Lachancea thermotolerans | 23.00 ± 4.19 | 18.59 ± 1.56 |
| Pichia kudriavzevii | 9.15 ± 0.91 | 8.25 ± 0.35 |
| Starmerella lactis-condensi | 26.32 ± 3.95 | 58.95 ± 6.92 |
| Torulaspora delbrueckii | 36.42 ± 1.21 | 29.76 ± 0.08 |
| Zygosaccharomyces rouxii | 28.76 ± 7.47 | 56.27 ± 4.09 |
| Compound | Method of Detection | CAS | OT (mg/L) | Odor/Flavor Description | J33 | J15 | SF |
|---|---|---|---|---|---|---|---|
| Parameters measured according OIV | |||||||
| Ethanol (% v/v) | 64-17-5 | 10 | Alcoholic | 11.88 ± 0.05 a | 11.74 ± 0.17 a | 12.12 ± 0.05 b | |
| Glycerol (mg/L) | 56-81-5 | - | Confers body and smoothness and a sweet taste | 8070 ± 34.00 b | 4890 ± 25.00 a | 5030 ± 19.00 b | |
| pH | - | - | - | 3.17 ± 0.01 b | 3.09 ± 0.03 a | 3.11 ± 0.06 ab | |
| Density | - | - | - | 0.9897 ± 0.0002 a | 0.9936 ± 0.0002 b | 0.9936 ± 0.0003 b | |
| Volatile acidity (g/L) | 64-19-7 | 200 | Vinegar | 0.11 ± 0.02 a | 0.74 ± 0.06 b | 0.74 ± 0.05 b | |
| Titratable acidity (g/L) | - | - | - | 7.08 ± 0.13 a | 9.08 ± 0.44 a | 8.69 ± 0.37 b | |
| Free SO2 (mg/L) | - | - | - | 8.30 ± 1.15 a | 7.30 ± 0.47 a | 8.30 ± 0.47 a | |
| Total SO2 (mg/L) | - | - | - | 31.33 ± 1.15 a | 38.30 ± 1.52 b | 39.66 ± 0.57 b | |
| Total extract (g/L) | - | - | - | 21.86 ± 0.25 b | 20.62 ± 0.19 a | 21.42 ± 0.07 b | |
| GC-FID | |||||||
| Acetaldehyde (mg/L) | 75-07-0 | 10 | Over-ripe apple | 56.98 ± 0.62 b | 50.75 ± 3.88 a | 55.20 ± 0.70 ab | |
| Ethyl acetate (mg/L) | 141-78-6 | 7.5 | Pineapple, varnish, balsamic | 28.27 ± 0.02 a | 331.19 ± 3.28 c | 262.87 ± 1.72 b | |
| 1,1-Diethoxyethane (mg/L) | 105-57-7 | 1 | Refreshing, pleasant, fruity-green | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Methanol (mg/L) | 67-56-1 | 668 | Chemical, medicinal | 63.73 ± 2.60 a | 78.43 ± 6.47 b | 69.66 ± 4.60 ab | |
| 1-Propanol (mg/L) | 71-23-8 | 830 | Ripe fruit, alcohol | 28.20 ± 0.28 a | 35.18 ± 2.31 c | 31.85 ± 0.95 b | |
| Isobutanol (mg/L) | 78-83-1 | 40 | Alcohol, wine like, nail polish | 57.45 ± 0.82 b | 59.79 ± 2.08 b | 52.16 ± 0,83 a | |
| 2-Methyl-1-butanol (mg/L) | 137-32-6 | 30 | N.f. | 30.67 ± 0.07 a | 33.62 ± 1.10 c | 32.26 ± 0.21 b | |
| 3-Methyl-1-butanol (mg/L) | 123-51-3 | 30 | Alcohol, nail polish | 124.45 ± 1.51 a | 194.54 ± 6.46 c | 180.98 ± 2.35 b | |
| Acetoin (mg/L) | 513-86-0 | 30 | Buttery, creamy | 37.10 ± 6.79 ab | 52.79 ± 4.68 b | 76.37 ± 8.89 c | |
| Ethyl lactate (mg/L) | 97-64-3 | 7.5 | Strawberry, raspberry, buttery | 15.16 ± 0.05 a | 15.61 ± 0.28 b | 15.21 ± 0.03 a | |
| 2,3-butanediol (levo) (mg/L) | 24347-58-8 | 668 | Buttery, creamy | 293.24 ± 16.73 a | 390.33 ± 46.84 b | 524.71 ± 67.42 c | |
| 2,3-butanediol (meso) (mg/L) | 5341-95-7 | 668 | Buttery, creamy | 66.70 ± 7.61 a | 102.15 ± 21.33 ab | 146.35 ± 27.32 b | |
| Diethyl succinate (mg/L) | 123-25-1 | 100 | Over-ripe, lavender | 17.64 ± 0.27 b | 10.45 ± 1.90 a | 11.00 ± 0.28 a | |
| 2-Phenylethanol (mg/L) | 60-12-8 | 10 | Floral | 4.85 ± 0.27 a | 3.26 ± 0.20 b | 3.83 ± 0.66 a |
| Compound | Method of Detection | CAS | OT (mg/L) | Odor/Flavor Description | J33 | J15 | SF |
|---|---|---|---|---|---|---|---|
| Parameters measured according OIV | |||||||
| Ethanol (% v/v) | 64-17-5 | 10 | Alcoholic | 11.10 ± 0.15 c | 8.06 ± 0.07 a | 8.48 ± 0.19 b | |
| Glycerol (mg/L) | 56-81-5 | - | Confers body and smoothness and a sweet taste | 9950 ± 93.00 b | 4730 ± 90.00 a | 4950 ± 30.00 a | |
| pH | - | - | - | 3.47 ± 0.01 b | 3.51 ± 0.03 a | 3.53 ± 0.06 a | |
| Density | - | - | - | 1.130 ± 0.004 a | 1.145 ± 0.005 b | 1.147 ± 0.005 b | |
| Volatile acidity (g/L) | 64-19-7 | 200 | Vinegar | 0.24 ± 0.04 a | 0.31 ± 0.03 ab | 0.36 ± 0.06 b | |
| Titratable acidity (g/L) | - | - | - | 8.21 ± 0.31 a | 8.61 ± 0.15 a | 8.69 ± 0.34 a | |
| Free SO2 (mg/L) | - | - | - | 9.33 ± 1.16 a | 10.67 ± 1.16 a | 10.67 ± 1.16 a | |
| Total SO2 (mg/L) | - | - | - | 51.00 ± 3.46 a | 52.00 ± 1.73 a | 53.33 ± 0.58 a | |
| Total extract (g/L) | - | - | - | 441.40 ± 0.57 c | 396.38 ± 0.60 b | 392.45 ± 0.56 a | |
| GC-FID | |||||||
| Acetaldehyde (mg/L) | 75-07-0 | 10 | Over-ripe apple | 145.81 ± 15.38 b | 61.54 ± 6.61 a | 157.11 ± 7.92 b | |
| Ethyl acetate (mg/L) | 141-78-6 | 7.5 | Pineapple, varnish, balsamic | 23.23 ± 0.37 a | 34.42 ± 0.16 b | 38.37 ± 0.44 c | |
| 1,1-Diethoxyethane (mg/L) | 105-57-7 | 1 | Refreshing, pleasant, fruity-green | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Methanol (mg/L) | 67-56-1 | 668 | Chemical, medicinal | 80.22 ± 16.85 a | 114.54 ± 2.26 b | 134.21 ± 1.83 b | |
| 1-Propanol (mg/L) | 71-23-8 | 830 | Ripe fruit, alcohol | 21.05 ± 1.07 a | 29.33 ± 0.33 c | 25.60 ± 0.18 b | |
| Isobutanol (mg/L) | 78-83-1 | 40 | Alcohol, wine like, nail polish | 134.98 ± 0.76 c | 108.75 ± 0.05 b | 95.24 ± 0,67 a | |
| 2-Methyl-1-butanol (mg/L) | 137-32-6 | 30 | N.f. | 35.24 ± 0.41 b | 16.96 ± 0.20 a | 16.50 ± 0.85 a | |
| 3-Methyl-1-butanol (mg/L) | 123-51-3 | 30 | Alcohol, nail polish | 71.38 ± 1.60 a | 187.33 ± 0.42 c | 176.57 ± 0.93 b | |
| Acetoin (mg/L) | 513-86-0 | 30 | Buttery, creamy | 103.76 ± 15.49 ab | 83.78 ± 1.77 a | 124.62 ± 13.19 b | |
| Ethyl lactate (mg/L) | 97-64-3 | 7.5 | Strawberry, raspberry, buttery | 15.01 ± 0.12 a | 16.06 ± 0.44 a | 15.62 ± 1.10 a | |
| 2,3-butanediol (levo) (mg/L) | 24347-58-8 | 668 | Buttery, creamy | 159.60 ± 28.49 a | 170.04 ± 20.68 a | 215.31 ± 48.79 a | |
| 2,3-butanediol (meso) (mg/L) | 5341-95-7 | 668 | Buttery, creamy | 589.11 ± 58.68 a | 546.97 ± 37.06 a | 680.70 ± 13.96 b | |
| Diethyl succinate (mg/L) | 123-25-1 | 100 | Over-ripe, lavender | 12.30 ± 1.50 a | 12.26 ± 0.94 a | 13.05 ± 0.48 a | |
| 2-Phenylethanol (mg/L) | 60-12-8 | 10 | Floral | 9.21 ± 0.75 a | 8.81 ± 0.25 a | 14.55 ± 3.51 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbonero-Pacheco, J.; García-Jiménez, Á.; Mauricio, J.C.; García-García, J.C.; Román-Camacho, J.J.; García-Muñoz, E.; Santos-Dueñas, I.M.; García-Martínez, T.; García-García, I. Selection of Fructophilic Yeast from Sun-Dried Pedro Ximénez Grape Must for the Development of New Vinegars Containing Gluconic Acid. Foods 2025, 14, 2410. https://doi.org/10.3390/foods14142410
Carbonero-Pacheco J, García-Jiménez Á, Mauricio JC, García-García JC, Román-Camacho JJ, García-Muñoz E, Santos-Dueñas IM, García-Martínez T, García-García I. Selection of Fructophilic Yeast from Sun-Dried Pedro Ximénez Grape Must for the Development of New Vinegars Containing Gluconic Acid. Foods. 2025; 14(14):2410. https://doi.org/10.3390/foods14142410
Chicago/Turabian StyleCarbonero-Pacheco, Juan, Álvaro García-Jiménez, Juan C. Mauricio, Juan C. García-García, Juan J. Román-Camacho, Elena García-Muñoz, Inés M. Santos-Dueñas, Teresa García-Martínez, and Isidoro García-García. 2025. "Selection of Fructophilic Yeast from Sun-Dried Pedro Ximénez Grape Must for the Development of New Vinegars Containing Gluconic Acid" Foods 14, no. 14: 2410. https://doi.org/10.3390/foods14142410
APA StyleCarbonero-Pacheco, J., García-Jiménez, Á., Mauricio, J. C., García-García, J. C., Román-Camacho, J. J., García-Muñoz, E., Santos-Dueñas, I. M., García-Martínez, T., & García-García, I. (2025). Selection of Fructophilic Yeast from Sun-Dried Pedro Ximénez Grape Must for the Development of New Vinegars Containing Gluconic Acid. Foods, 14(14), 2410. https://doi.org/10.3390/foods14142410







