Effect of Artichoke Outer Bract Powder Addition on the Nutritional Profile of Gluten-Free Rusks
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Chemicals
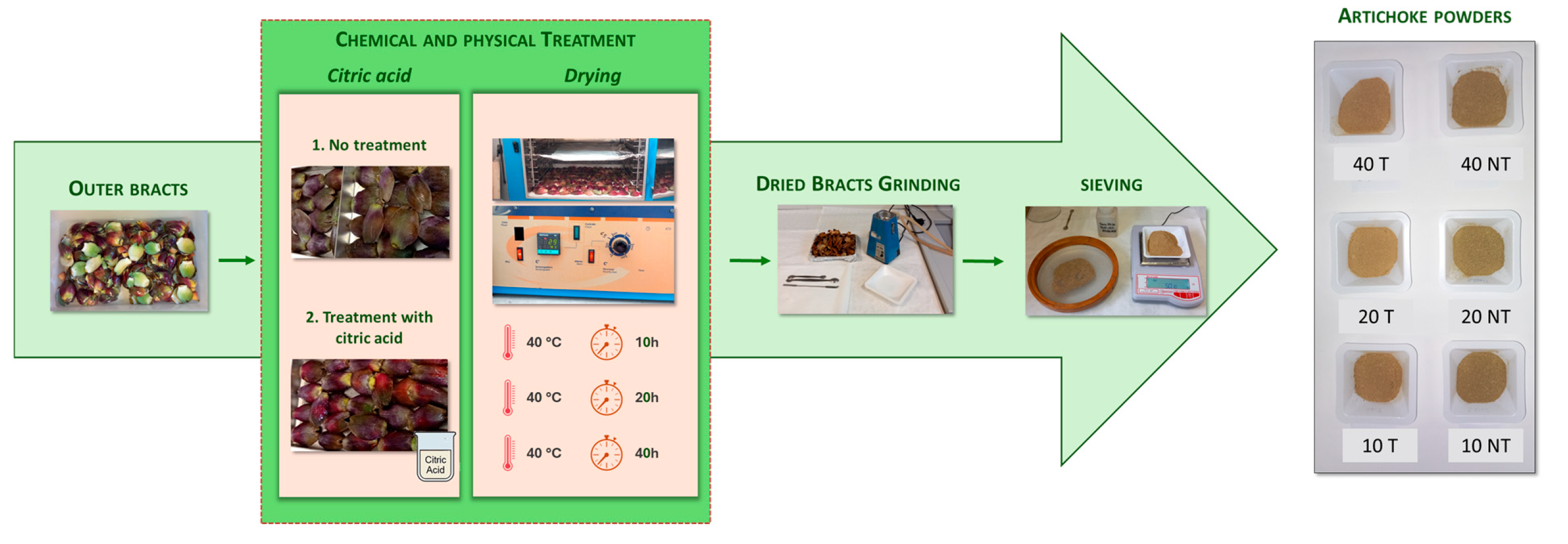
2.2. Upcycling of Artichoke Outer Bracts and Preparation of Powders
2.3. Preparation of Gluten-Free Rusks
2.4. Selection of Artichoke Outer Bract Powders for GF Rusk Enrichment
2.4.1. Measurement of Color by the CIEL*a*b* System
2.4.2. Determination of Inulin Content in Artichoke Powders
2.4.3. Determination of Free Phenolic Compound Content in Artichoke Powders
2.5. Nutritional Characterization of the Artichoke Bract Powder Selected for Enrichment and Gluten-Free Rusks
2.5.1. Proximate Analysis
2.5.2. Analysis of Non-Digestible Carbohydrates
Determination of Inulin Content
Determination of Total Dietary Fiber
2.5.3. Determination of Free Phenolic Compound Content
2.5.4. Determination of Total Flavonoid Compound Content
2.6. Statistical Analysis
3. Results and Discussion
3.1. Drying Curve
3.2. Effect of Pre-Drying Treatment and Drying Conditions on Artichoke Bract Powders
3.3. Proximate Composition and Bioactive Compounds in the Selected Artichoke Bract Powder, in Gluten-Free Flours, and in Enriched Rusks
3.3.1. Proximate Composition
3.3.2. Non-Digestible Carbohydrates
3.3.3. Phenolic Compounds
3.4. Limitations and Future Perspectives of the Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FCR | Folin–Ciocalteu Reagent |
| UAE | Ultrasound-Assisted Extraction |
| FPCs | Free Phenolic Compounds |
| TDF | Total Dietary Fiber |
| BPC | Bound Phenolic Compounds |
| GAEs | Gallic Acid Equivalents |
| TFCs | Total Flavonoid Compounds |
| NDCs | Non-Digestible Carbohydrates |
References
- Melini, V.; Melini, F.; Luziatelli, F.; Ruzzi, M. Functional ingredients from agri-food waste: Effect of inclusion thereof on phenolic compound content and bioaccessibility in bakery products. Antioxidants 2020, 9, 1216. [Google Scholar] [CrossRef]
- Gómez-García, R.; Vilas-Boas, A.A.; Campos, D.A.; Pintado, M.M.; Aguilar, C.N. Food Byproducts Management and Their Utilization; Gómez-García, R., Vilas-Boas, A.A., Campos, D.A., Pintado, M.M., Aguilar, C.N., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2024; ISBN 9781000852929. [Google Scholar]
- Ratu, R.N.; Ionut, D.V.; Stoica, F.; Usturoi, A.; Arsenoaia, V.N.; Crivei, I.C.; Postolache, A.N.; Lipsa, F.D.; Filipov, F.; Florea, A.M.; et al. Application of Agri-Food By-Products in the Food Industr. Agriculture 2023, 13, 1559. [Google Scholar] [CrossRef]
- Lau, K.Q.; Sabran, M.R.; Shafie, S.R. Utilization of Vegetable and Fruit By-products as Functional Ingredient and Food. Front. Nutr. 2021, 8, 661693. [Google Scholar] [CrossRef]
- Vilas-Franquesa, A.; Montemurro, M.; Casertano, M.; Fogliano, V. The food by-products bioprocess wheel: A guidance tool for the food industry. Trends Food Sci. Technol. 2024, 152, 104652. [Google Scholar] [CrossRef]
- Gómez-García, R.; Campos, D.A.; Aguilar, C.N.; Madureira, A.R.; Pintado, M. Valorisation of food agro-industrial by-products: From the past to the present and perspectives. J. Environ. Manag. 2021, 299, 113571. [Google Scholar] [CrossRef]
- Nations, U. The 17 Goals | Sustainable Development. Available online: https://sdgs.un.org/goals (accessed on 12 May 2022).
- Chaouch, M.A.; Benvenuti, S. The role of fruit by-products as bioactive compounds for intestinal health. Foods 2020, 9, 1716. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Matias, A. Food industry processing by-products in foods. In The Role of Alternative and Innovative Food Ingredients and Products in Consumer Wellness; Galanakis, C.M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 239–281. ISBN 9780128164532. [Google Scholar]
- Melini, V.; Melini, F.; Comendador, F.J. Response Surface Methodology as an Experimental Strategy for Ultrasound-Assisted Extraction of Phenolic Compounds from Artichoke Heads. Antioxidants 2023, 12, 1360. [Google Scholar] [CrossRef]
- Francavilla, M.; Marone, M.; Marasco, P.; Contillo, F.; Monteleone, M. Artichoke biorefinery: From food to advanced technological applications. Foods 2021, 10, 112. [Google Scholar] [CrossRef]
- Zayed, A.; Farag, M.A. Valorization, extraction optimization and technology advancements of artichoke biowastes: Food and non-food applications. LWT 2020, 132, 109883. [Google Scholar] [CrossRef]
- FAO. FAOSTAT—Food and Agriculture Data. Available online: https://www.fao.org/faostat/en/#home (accessed on 13 May 2022).
- Devpal, R.; Verma, T.; Aggarwal, A.; Sharma, R. Formulation and quality attributes of nutrient-enriched black wheat rusks. J. Cereal Sci. 2025, 123, 104156. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F. Gluten-free diet: Gaps and needs for a healthier diet. Nutrients 2019, 11, 170. [Google Scholar] [CrossRef]
- Branca, F.; Arena, D.; Argento, S.; Frustaci, F.; Ciccarello, L.; Melilli, M.G. Recovery of healthy compounds in waste bracts of globe artichoke heads. In Proceedings of the III International Organic Fruit Symposium and I International Organic Vegetable Symposium, Catania, Italy, 14–17 December 2021; International Society for Horticultural Science: Leuven, Belgium, 2022; Volume 1354, pp. 361–366. [Google Scholar]
- Cannas, M.; Conte, P.; Urgeghe, P.P.; Piga, A.; Alañón, M.E.; Del Caro, A. Artichoke by-products: Promising ingredients for breadstick fortification. LWT 2024, 202, 116307. [Google Scholar] [CrossRef]
- Cavini, S.; Guzzetti, L.; Givoia, F.; Regonesi, M.E.; Di Gennaro, P.; Magoni, C.; Campone, L.; Labra, M.; Bruni, I. Artichoke (Cynara cardunculus var. scolymus L.) by-products as a source of inulin: How to valorise an agricultural supply chain extracting an added-value compound. Nat. Prod. Res. 2022, 36, 2140–2144. [Google Scholar] [CrossRef]
- Ciancolini, A.; Alignan, M.; Pagnotta, M.A.; Miquel, J.; Vilarem, G.; Crinò, P. Morphological characterization, biomass and pharmaceutical compounds in Italian globe artichoke genotypes. Ind. Crops Prod. 2013, 49, 326–333. [Google Scholar] [CrossRef]
- Ferioli, F.; D’Antuono, L.F. Phenolic compounds in local Italian types of cultivated cardoon (Cynara cardunculus L. var. altilis DC) stalks and artichoke (Cynara cardunculus L. var. scolymus L.) edible sprouts. J. Food Compos. Anal. 2022, 106, 104342. [Google Scholar] [CrossRef]
- López-Salas, L.; Borrás-Linares, I.; Quintin, D.; García-Gomez, P.; Giménez-Martínez, R.; Segura-Carretero, A.; Lozano-Sánchez, J. Artichoke by-products as natural source of phenolic food ingredient. Appl. Sci. 2021, 11, 3788. [Google Scholar] [CrossRef]
- Quintero Ruiz, N.A.; Paolucci, M.; Siano, F.; Mamone, G.; Picariello, G.; Puppo, M.C.; Cascone, G.; Volpe, M.G. Characterization of soluble and insoluble fibers in artichoke by-products by ATR-FTIR spectroscopy coupled with chemometrics. Int. J. Food Prop. 2021, 24, 1693–1704. [Google Scholar] [CrossRef]
- Mena-García, A.; Rodríguez-Sánchez, S.; Ruiz-Matute, A.I.; Sanz, M.L. Exploitation of artichoke byproducts to obtain bioactive extracts enriched in inositols and caffeoylquinic acids by Microwave Assisted Extraction. J. Chromatogr. A 2020, 1613, 460703. [Google Scholar] [CrossRef]
- Rejeb, I.B.; Dhen, N.; Gargouri, M.; Boulila, A. Chemical Composition, Antioxidant Potential and Enzymes Inhibitory Properties of Globe Artichoke By-Products. Chem. Biodivers. 2020, 17, e2000073. [Google Scholar] [CrossRef]
- Vacca, M.; Pinto, D.; Annunziato, A.; Ressa, A.; Calasso, M.; Pontonio, E.; Celano, G.; De Angelis, M. Gluten-Free Bread Enriched with Artichoke Leaf Extract In Vitro Exerted Antioxidant and Anti-Inflammatory Properties. Antioxidants 2023, 12, 845. [Google Scholar] [CrossRef]
- Boubaker, M.; Damergi, C.; Ben Marzouk, C.; Blecker, C.; Bouzouita, N. Effect of artichoke (Cynara scolymus L.) by-product on the quality and total phenol content of bread. Mediterr. J. Chem. 2016, 5, 548–553. [Google Scholar] [CrossRef]
- Mejri, F.; Baati, T.; Martins, A.; Selmi, S.; Luisa Serralheiro, M.; Falé, P.L.; Rauter, A.; Casabianca, H.; Hosni, K. Phytochemical analysis and in vitro and in vivo evaluation of biological activities of artichoke (Cynara scolymus L.) floral stems: Towards the valorization of food by-products. Food Chem. 2020, 333, 127506. [Google Scholar] [CrossRef]
- Suttirak, W.; Manurakchinakorn, S. Potential Application of Ascorbic Acid, Citric Acid and Oxalic Acid for Browning Inhibition in Fresh-Cut Fruits and Vegetables. Walailak J. Sci. Technol. 2010, 7, 5–14. [Google Scholar] [CrossRef]
- AOAC International Official Methods of Analysis of AOAC International. Available online: https://www.aoac.org/scientific-solutions/standards-and-official-methods/ (accessed on 17 June 2022).
- Melini, V.; Melini, F. Modelling and Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds from Black Quinoa by Response Surface Methodology. Molecules 2021, 26, 3616. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F.; Luziatelli, F.; Ruzzi, M. Development of an Ultrasound-Assisted Extraction Procedure for the Simultaneous Determination of Anthocyanins and Phenolic Acids in Black Beans. Foods 2023, 12, 3566. [Google Scholar] [CrossRef]
- Sompong, R.; Siebenhandl-Ehn, S.; Linsberger-Martin, G.; Berghofer, E. Physicochemical and antioxidative properties of red and black rice varieties from Thailand, China and Sri Lanka. Food Chem. 2011, 124, 132–140. [Google Scholar] [CrossRef]
- International Association for Cereal Science and Technology ICC Standards. Available online: https://icc.or.at/icc-standards/standards-overview (accessed on 30 June 2025).
- Alshikh, N.; de Camargo, A.C.; Shahidi, F. Phenolics of selected lentil cultivars: Antioxidant activities and inhibition of low-density lipoprotein and DNA damage. J. Funct. Foods 2015, 18, 1022–1038. [Google Scholar] [CrossRef]
- Bi, Y.X.; Zielinska, S.; Ni, J.B.; Li, X.X.; Xue, X.F.; Tian, W.L.; Peng, W.J.; Fang, X.M. Effects of hot-air drying temperature on drying characteristics and color deterioration of rape bee pollen. Food Chem. X 2022, 16, 100464. [Google Scholar] [CrossRef]
- Canale, M.; Spina, A.; Summo, C.; Strano, M.C.; Bizzini, M.; Allegra, M.; Sanfilippo, R.; Amenta, M.; Pasqualone, A. Waste from Artichoke Processing Industry: Reuse in Bread-Making and Evaluation of the Physico-Chemical Characteristics of the Final Product. Plants 2022, 11, 3409. [Google Scholar] [CrossRef]
- Boubaker, M.; Omri, A.E.L.; Blecker, C.; Bouzouita, N. Fibre concentrate from artichoke (Cynara scolymus L.) stem by-products: Characterization and application as a bakery product ingredient. Food Sci. Technol. Int. 2016, 22, 759–768. [Google Scholar] [CrossRef]
- Ruiz-Cano, D.; Pérez-Llamas, F.; Frutos, M.J.; Arnao, M.B.; Espinosa, C.; López-Jiménez, J.Á.; Castillo, J.; Zamora, S. Chemical and functional properties of the different by-products of artichoke (Cynara scolymus L.) from industrial canning processing. Food Chem. 2014, 160, 134–140. [Google Scholar] [CrossRef]
- Bas-Bellver, C.; Barrera, C.; Betoret, N.; Seguí, L. Impact of Disruption and Drying Conditions on Physicochemical, Functional and Antioxidant Properties of Powdered Ingredients Obtained from Brassica Vegetable By-Products. Foods 2022, 11, 3663. [Google Scholar] [CrossRef]
- Kheto, A.; Bist, Y.; Awana, A.; Kaur, S.; Kumar, Y.; Sehrawat, R. Utilization of inulin as a functional ingredient in food: Processing, physicochemical characteristics, food applications, and future research directions. Food Chem. Adv. 2023, 3, 100443. [Google Scholar] [CrossRef]
- Sharif, M.K.; Butt, M.S.; Sharif, H.R.; Nasir, M. Sensory Evaluation and Consumer Research. Food Sci. Technol. 2017, 10, 362–386. [Google Scholar]
- Cedola, A.; Cardinali, A.; D’Antuono, I.; Conte, A.; Del Nobile, M.A. Cereal foods fortified with by-products from the olive oil industry. Food Biosci. 2020, 33, 100490. [Google Scholar] [CrossRef]
- Plazzotta, S.; Sillani, S.; Manzocco, L. Exploitation of lettuce waste flour to increase bread functionality: Effect on physical, nutritional, sensory properties and on consumer response. Int. J. Food Sci. Technol. 2018, 53, 2290–2297. [Google Scholar] [CrossRef]
- Lafarga, T.; Gallagher, E.; Bademunt, A.; Vinas, I.; Bobo, G.; Villarò, S.; Aguiló-Aguayo, I. Bioaccessibility, physicochemical, sensorial, and nutritional characteristics of bread containing broccoli co-products. J. Food Process. Preserv. 2019. [Google Scholar] [CrossRef]
- Umaña, M.; Wawrzyniak, P.; Rosselló, C.; Llavata, B.; Simal, S. Evaluation of the addition of artichoke by-products to O/W emulsions for oil microencapsulation by spray drying. LWT 2021, 151. [Google Scholar] [CrossRef]
- Alfano, V.; Stefanoni, W.; Latterini, F.; Liuzzi, F.; De Bari, I.; Viola, E.; Ciancolini, A.; Pari, L. Inulin Content in Chipped Roots of Cardoon Stored at Different Initial Moisture Contents After Six-Month Storage. Front. Energy Res. 2022, 10, 1–9. [Google Scholar] [CrossRef]
- Moscetti, R.; Benelli, A.; Ibba, P.; Mannozzi, C.; Melini, F.; Massantini, R. Instrumentation Techniques and Analyses of Bioactive Compounds in Different Dried Fruit Products. In Dried Fruit Products; Richter Reis, F., Shirkole, S.S., Eds.; CRC Press: Boca Raton, FL, USA, 2024; pp. 129–166. ISBN 9781032495750. [Google Scholar]
- Cauvain, S.P.; Young, L.S. Baked Products: Science, Technology and Practice. Baked Prod. Sci. Technol. Pract. 2007, 1–228. [Google Scholar] [CrossRef]
- Suhag, R.; Kellil, A.; Razem, M. Factors Influencing Food Powder Flowability. Powders 2024, 3, 65–76. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G. The influence of pre-harvest factors on the quality of globe artichoke. Sci. Hortic. 2018, 233, 479–490. [Google Scholar] [CrossRef]
- Melilli, M.G.; Tringali, S.; Bognanni, R.; Argento, S.; Calderaro, P.; Raccuia, S.A. Nutritional quality of globe artichoke [Cynara cardunculus L. subsp. scolymus (L.) Hegi] head as affected by genotype and environment of cultivation. Acta Hortic. 2014, 1040, 187–192. [Google Scholar] [CrossRef]
- Prieto-Vázquez del Mercado, P.; Mojica, L.; Morales-Hernández, N. Protein Ingredients in Bread: Technological, Textural and Health Implications. Foods 2022, 11, 2399. [Google Scholar] [CrossRef]
- Zhai, J.; Li, X.; Svensson, B.; Jin, Z.; Bai, Y. Increasing Protein Content of Rice Flour with Maintained Processability by Using Granular Starch Hydrolyzing Enzyme. Molecules 2023, 28, 3522. [Google Scholar] [CrossRef]
- Tambo Tene, S.; Klang, J.M.; Ndomou Houketchang, S.C.; Teboukeu Boungo, G.; Womeni, H.M. Characterization of corn, cassava, and commercial flours: Use of amylase-rich flours of germinated corn and sweet potato in the reduction of the consistency of the gruels made from these flours—Influence on the nutritional and energy value. Food Sci. Nutr. 2019, 7, 1190–1206. [Google Scholar] [CrossRef]
- Alonso-Miravalles, L.; O’Mahony, J.A. Composition, protein profile and rheological properties of pseudocereal-based protein-rich ingredients. Foods 2018, 7, 73. [Google Scholar] [CrossRef]
- Sofi, S.A.; Ahmed, N.; Farooq, A.; Rafiq, S.; Zargar, S.M.; Kamran, F.; Dar, T.A.; Mir, S.A.; Dar, B.N.; Mousavi Khaneghah, A. Nutritional and bioactive characteristics of buckwheat, and its potential for developing gluten-free products: An updated overview. Food Sci. Nutr. 2022, 2256–2276. [Google Scholar] [CrossRef]
- Melini, F.; Melini, V.; Luziatelli, F.; Ruzzi, M. Current and Forward-Looking Approaches to Technological and Nutritional Improvements of Gluten-Free Bread with Legume Flours: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2017, 16. [Google Scholar] [CrossRef]
- Schmidt, M.; Sciurba, E. Determination of FODMAP contents of common wheat and rye breads and the effects of processing on the final contents. Eur. Food Res. Technol. 2021, 247, 395–410. [Google Scholar] [CrossRef]
- Mysonhimer, A.R.; Holscher, H.D. Gastrointestinal Effects and Tolerance of Nondigestible Carbohydrate Consumption. Adv. Nutr. 2022, 13, 2237–2276. [Google Scholar] [CrossRef]
- Verbeke, C.; Debonne, E.; Versele, S.; Van Bockstaele, F.; Eeckhout, M. Technological Evaluation of Fiber Effects in Wheat-Based Dough and Bread. Foods 2024, 13, 2582. [Google Scholar] [CrossRef]
- Colantuono, A.; Ferracane, R.; Vitaglione, P. Potential bioaccessibility and functionality of polyphenols and cynaropicrin from breads enriched with artichoke stem. Food Chem. 2018, 245, 838–844. [Google Scholar] [CrossRef]
- Borsini, A.A.; Llavata, B.; Umaña, M.; Cárcel, J.A. Artichoke by products as a source of antioxidant and fiber: How it can be affected by drying temperature. Foods 2021, 10, 459. [Google Scholar] [CrossRef]
- Cannas, M.; Conte, P.; Piga, A.; Del Caro, A. Green recovery optimization of phenolic compounds from “Spinoso sardo” globe artichoke by-products using response surface methodology. Front. Sustain. Food Syst. 2023, 7, 1–14. [Google Scholar] [CrossRef]


| Sample ID | Pretreatment | Drying Time (h) | L* | a* | b* | ΔE | Inulin (%) | FPCs (mg GAE/100 g dm) |
|---|---|---|---|---|---|---|---|---|
| 10 T | Citric acid | 10 | 59.00 ± 0.21 b,E | 1.82 ± 0.10 b,E | 21.83 ± 0.20 a,E | 38.02 ± 0.18 a | 18.90 ± 0.51 c,B | 336.50 ± 18.30 c,B |
| 10 NT | - | 10 | 58.27 ± 0.42 c,d,F | 0.18 ± 0.16 e,F | 21.19 ± 0.14 b,F | 38.32 ± 0.36 a | 22.03 ± 0.16 a,A | 331.80 ± 14.40 c,B |
| 20 T | Citric acid | 20 | 61.81 ± 0.26 a,A | 2.93 ± 0.09 a,A | 21.74 ± 0.17 a,A | 35.58 ± 0.30 c | 18.69 ± 0.07 c,D | 783.50 ± 10.10 a,A |
| 20 NT | - | 20 | 57.74 ± 0.44 d,B | 0.73 ± 0.22 d,B | 20.89 ± 0.29 b,B | 38.73 ± 0.51 a | 21.73 ± 0.16 a,C | 777.50 ± 1.10 a,A |
| 40 T | Citric acid | 40 | 61.73 ± 0.27 a,C | 2.83 ± 0.11 a,C | 21.23 ± 0.18 b,C | 35.43 ± 0.27 c | 17.35 ± 0.01 d,F | 481.30 ± 63.00 b,C |
| 40 NT | - | 40 | 58.98 ± 0.37 b,c,D | 1.18 ± 0.05 c,D | 19.99 ± 0.21 c,D | 37.27 ± 0.30 b | 20.49 ± 0.01 b,E | 481.50 ± 83.80 b,C |
| Analyte | Artichoke Powder | GF Flours | Experimental Rusks | ||||
|---|---|---|---|---|---|---|---|
| F-CTRL | F-RSK5 | F-RSK10 | CTRL | RSK5 | RSK10 | ||
| Moisture (%) | 11.62 ± 0.04 | 10.94 ± 0.06 A | 11.03 ± 0.16 A | 10.56 ± 0.02 B | 8.04 ± 0.13 b | 7.95 ± 0.08 b | 8.49 ± 0.06 a |
| Protein (g/100 g dm) | 15.43 ± 0.02 | 3.32 ± 0.05 C | 3.88 ± 0.06 B | 4.34 ± 0.03 A | 3.98 ± 0.02 c | 4.72 ± 0.04 b | 5.30 ± 0.05 a |
| Fat (g/100 g dm) | 0.77 ± 0.08 | 0.61 ± 0.08 A | 0.58 ± 0.01 A | 0.23 ± 0.04 B | 3.61 ± 0.04 a | 3.44 ± 0.10 a | 3.36 ± 0.06 a |
| Carbohydrates (g/100 g dm) | 76.68 ± 0.15 | 94.91 ± 0.29 A | 94.12 ± 0.06 A | 93.77 ± 0.34 A | 90.24 ± 0.05 a | 89.40 ± 0.11 a | 88.69 ± 0.08 a |
| Ash (g/100 g dm) | 7.12 ± 0.12 | 1.16 ± 0.02 C | 1.42 ± 0.02 B | 1.66 ± 0.00 A | 2.17 ± 0.20 c | 2.44 ± 0.00 b | 2.65 ± 0.03 a |
| Total Dietary Fiber (g/100 g dm) | 39.69 ± 0.05 | 1.17 ± 0.02 C | 3.65 ± 0.10 B | 4.82 ± 0.09 A | 3.81 ± 0.25 c | 4.51 ± 0.59 b | 7.67 ± 0.72 a |
| Inulin (g/100 g dm) | 6.41 ± 0.11 | 0.57 ± 0.01 B | 0.54 ± 0.04 B | 0.79 ± 0.09 A | 0.21 ± 0.02 c | 0.33 ± 0.05 b | 0.83 ± 0.07 a |
| Phenolic compounds | |||||||
| FPCs (mg GAE 100 g−1 dm) | 708.65 ± 33.47 | 39.05 ± 3.39 C | 64.88 ± 2.82 B | 95.02 ± 3.73 A | 56.35 ± 1.25 c | 94.87 ± 7.06 b | 138.99 ± 11.45 a |
| TFCs (mg CE 100 g−1 dm) | 5903.06 ± 42.38 | 153.62 ± 31.6 C | 547.43 ± 121.47 B | 772.65 ± 48.48 A | 221.69 ± 9.48 c | 802.46 ± 15.65 b | 1123.43 ± 32.78 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melini, V.; Melini, F.; Salvati, A.; Luziatelli, F.; Ruzzi, M. Effect of Artichoke Outer Bract Powder Addition on the Nutritional Profile of Gluten-Free Rusks. Foods 2025, 14, 2395. https://doi.org/10.3390/foods14132395
Melini V, Melini F, Salvati A, Luziatelli F, Ruzzi M. Effect of Artichoke Outer Bract Powder Addition on the Nutritional Profile of Gluten-Free Rusks. Foods. 2025; 14(13):2395. https://doi.org/10.3390/foods14132395
Chicago/Turabian StyleMelini, Valentina, Francesca Melini, Alessandro Salvati, Francesca Luziatelli, and Maurizio Ruzzi. 2025. "Effect of Artichoke Outer Bract Powder Addition on the Nutritional Profile of Gluten-Free Rusks" Foods 14, no. 13: 2395. https://doi.org/10.3390/foods14132395
APA StyleMelini, V., Melini, F., Salvati, A., Luziatelli, F., & Ruzzi, M. (2025). Effect of Artichoke Outer Bract Powder Addition on the Nutritional Profile of Gluten-Free Rusks. Foods, 14(13), 2395. https://doi.org/10.3390/foods14132395







