Fatty Acids Are Responsible for the Discrepancy of Key Aroma Compounds in Naturally Dried Red Goji Berries and Hot-Air-Dried Red Goji Berries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals and References
2.3. Analysis of Aroma Compounds
2.3.1. Isolation of the Volatiles
2.3.2. Gas Chromatography with Olfactory (GC/O)
2.3.3. Aroma Extract Dilution Analysis (AEDA)
2.3.4. Gas Chromatography with Mass Spectrometry (GC/MS) for Aroma Analysis
2.3.5. Identification of Aroma-Active Compounds
2.3.6. Determination of the Contents of Aroma-Active Compounds
2.3.7. Sensory Assessment
Aroma Profile Analysis (APA)
Aroma Recombination
2.4. Analysis of Fatty Acids
2.4.1. Transesterification of Fatty Acids
2.4.2. Gas Chromatography with Mass Spectrometry (GC/MS) for Fatty Acid Analysis
2.5. Statistical Analysis
3. Results and Discussion
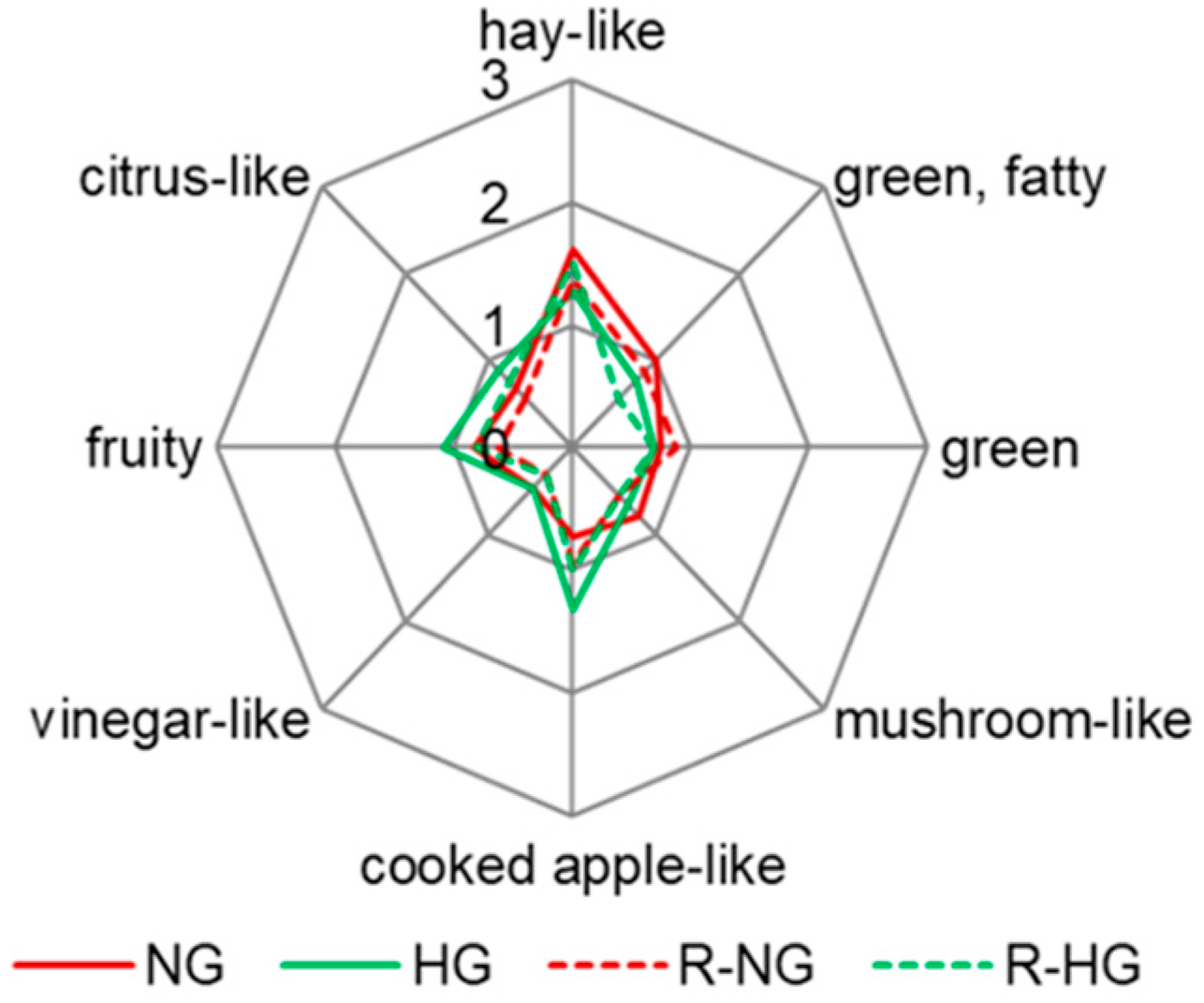
3.1. Sensory Analysis of Natural-Dried and Hot-Air-Dried Red Goji Berries
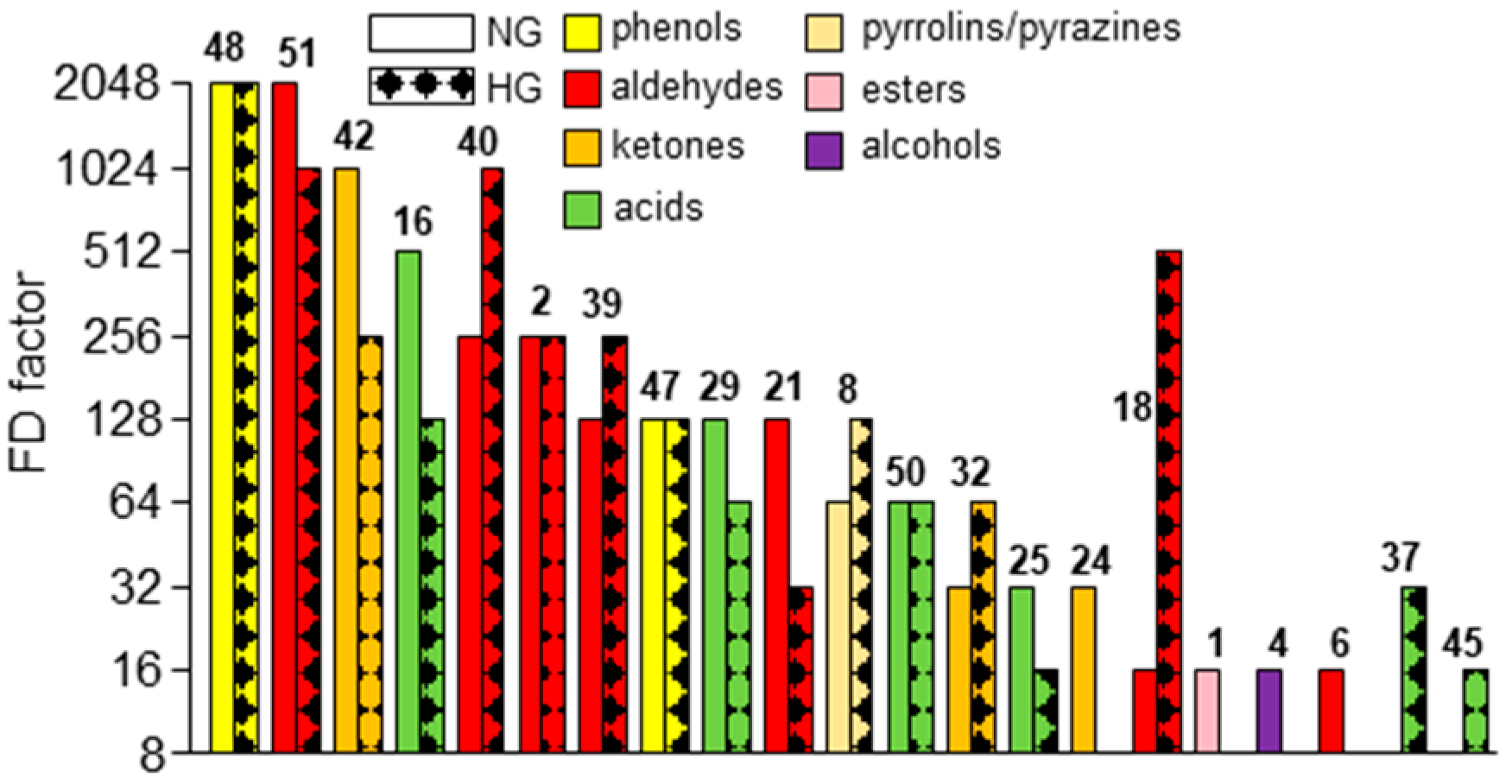
3.2. Identification of Aroma-Active Compounds in Both Dried Red Goji Berries
3.3. (Semi-)Quantitation of Aroma-Active Compounds and Calculation of OAVs
3.4. Aroma Recombination of Both Dried Red Goji Berries
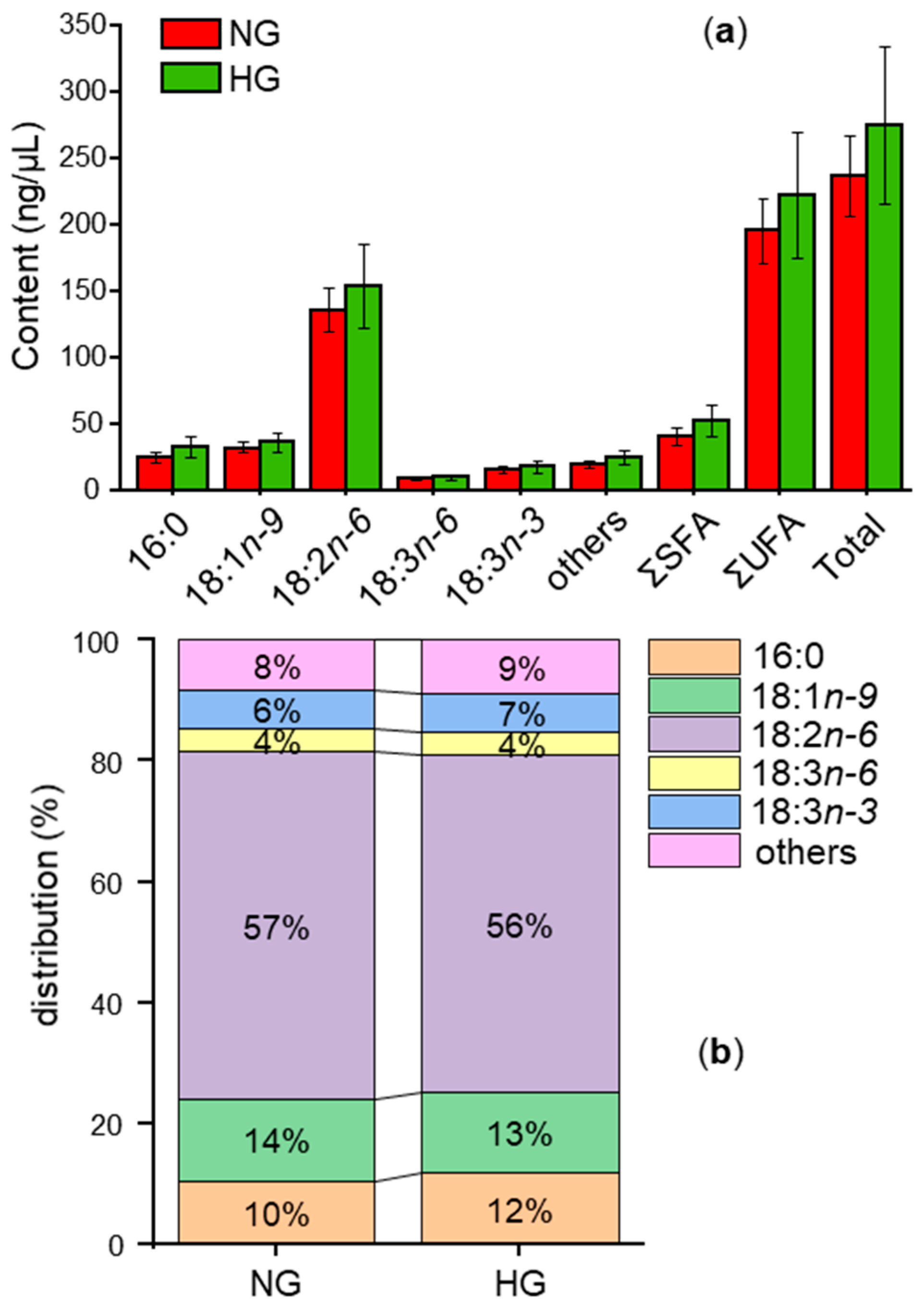
3.5. Comparison of Fatty Acids in NG and HG
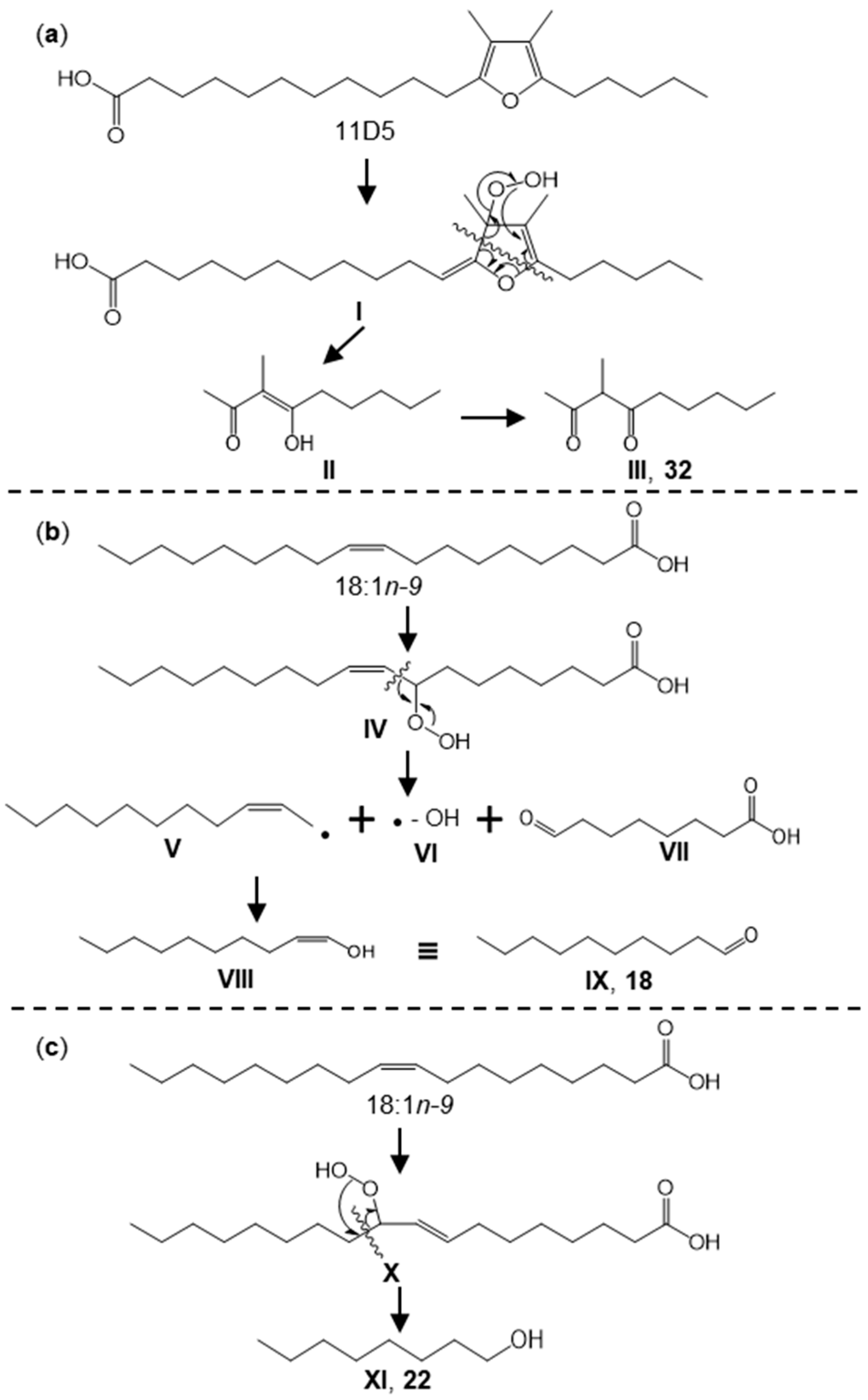
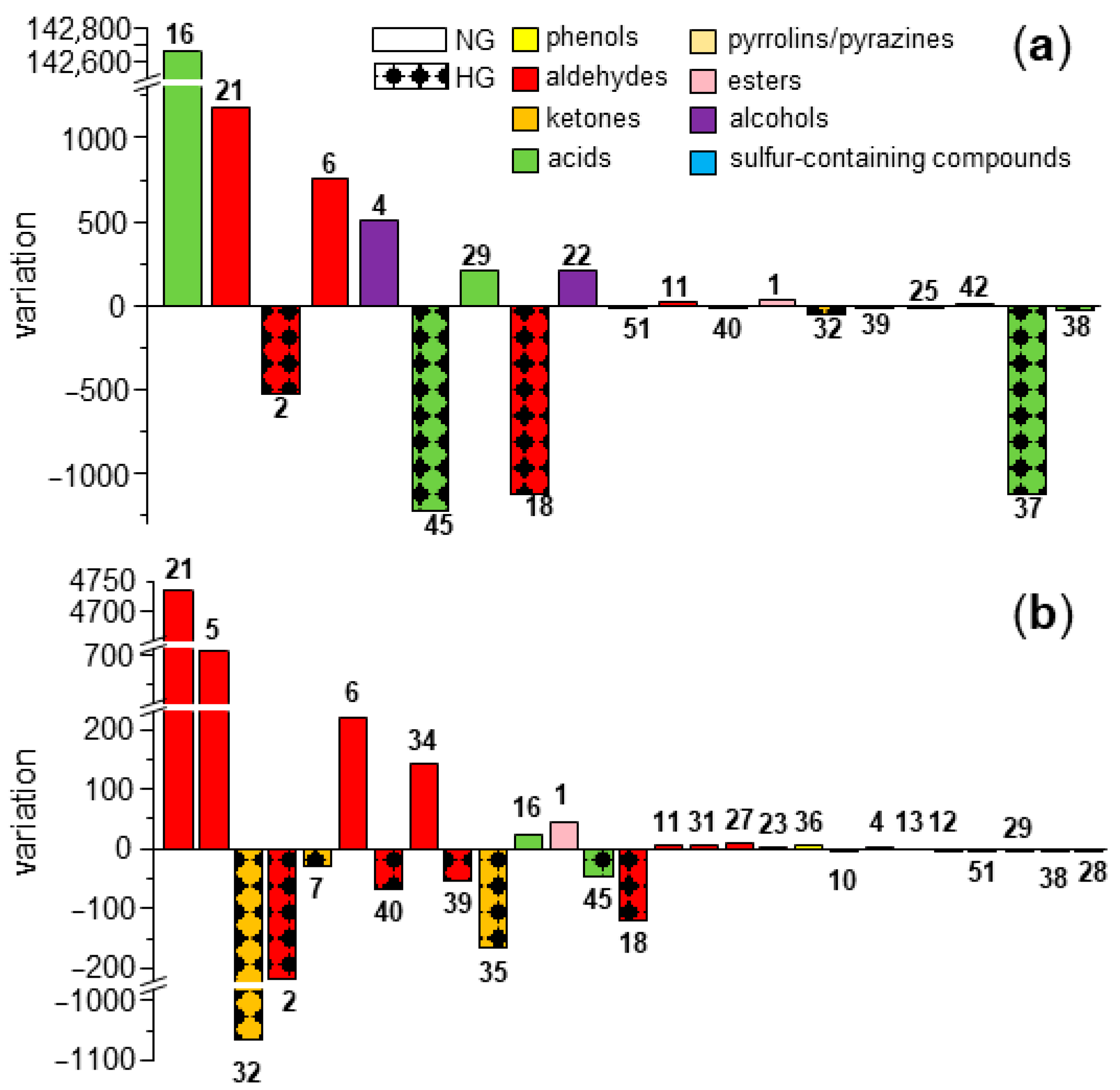
3.6. Comparison of Key Aroma-Active Compounds in NG and HG
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poggioni, L.; Romi, M.; Guarnieri, M.; Cai, G.; Cantini, C. Nutraceutical profile of goji (Lycium barbarum L.) berries in relation to environmental conditions and harvesting period. Food Biosci. 2022, 49, 101954. [Google Scholar] [CrossRef]
- Bertoldi, D.; Cossignani, L.; Blasi, F.; Perini, M.; Barbero, A.; Pianezze, S.; Montesano, D. Characterisation and geographical traceability of Italian goji berries. Food Chem. 2019, 275, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Rehman, F.; Ma, Y.; A, B.; Zeng, S.; Yang, T.; Huang, J.; Li, Z.; Wu, D.; Wang, Y. Germplasm resources and strategy for genetic breeding of Lycium species: A review. Front. Plant Sci. 2022, 13, 802936. [Google Scholar] [CrossRef] [PubMed]
- Potterat, O. Goji (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010, 76, 7–19. [Google Scholar] [CrossRef]
- Wu, L.; Chu, L.; Cao, H.; Tian, Q.; Gao, H.; Huo, J.; Gao, Q. Effect of Lycium ruthenicum and Lycium barbarum intake on Parkinson based on microbiology and metabonomics: A randomized pilot trial. Food Biosci. 2024, 57, 103548. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Q.; Fang, J.; Wang, C.; Wang, D.; Li, M. The anti-aging activity of Lycium barbarum polysaccharide extracted by yeast fermentation: In vivo and in vitro studies. Int. J. Biol. Macromol. 2022, 209, 2032–2041. [Google Scholar] [CrossRef]
- Zheng, Y.; Lehnert, K.; Vetter, W. The high share of steryl esters is responsible for the unusual sterol pattern of black goji berries. J. Food Compos. Anal. 2025, 137, 106921. [Google Scholar] [CrossRef]
- Xie, L.; Mujumdar, A.S.; Zhang, Q.; Wang, J.; Liu, S.; Deng, L.; Wang, D.; Xiao, H.W.; Liu, Y.H.; Gao, Z.J. Pulsed vacuum drying of wolfberry: Effects of infrared radiation heating and electronic panel contact heating methods on drying kinetics, color profile, and volatile compounds. Dry. Technol. 2017, 35, 1312–1326. [Google Scholar] [CrossRef]
- Batu, H.S.; Kadakal, Ç. Drying characteristics and degradation kinetics in some parameters of goji berry (Lycium Barbarum L.) fruit during hot air drying. Ital. J. Food Sci. 2021, 33, 16–28. [Google Scholar] [CrossRef]
- Cui, C.; Zhao, D.; Huang, J.; Hao, J. Progress on research and development of goji berry drying: A review. Int. J. Food Prop. 2022, 25, 435–449. [Google Scholar] [CrossRef]
- GBT18672-2014; National Standard of Goji Berries. National Standards of People’s Republic of China: Beijing, China, 27 October 2014.
- U.S. Department of Agriculture. Fruits_Agricultural Marketing Service. Available online: https://www.ams.usda.gov/grades-standards/fruits (accessed on 2 December 2024).
- Schieberle, P.; Hofmann, T. Mapping the combinatorial code of food flavors by means of molecular sensory science approach. In Food Flavors: Chmical, Sensory and Technological Propoerties; Jeleń, H., Ed.; CRC Press: Boca Raton, FL, USA, 2011; Chapter 18; pp. 439–456. [Google Scholar]
- Lu, J.; Li, H.; Quan, J.; An, W.; Zhao, J.; Xi, W. Identification of characteristic aroma volatiles of Ningxia goji berries (Lycium barbarum L.) and their developmental changes. Int. J. Food Prop. 2017, 20, S214–S227. [Google Scholar] [CrossRef]
- Zheng, Y.; Oellig, C.; Zhu, L.; Bauer, V.; Vetter, W.; Zhang, Y. Revealing the key aroma codes and (furan) fatty acids in fresh red goji berries and the impacts of the hot-air drying process. Food Chem. 2025, 484, 144336. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Huang, H.; Wang, J.; Liu, N.; Chen, X.; Jiang, T.; Xu, H.; Lei, H. Insights into the improvement of bioactive phytochemicals, antioxidant activities and flavor profiles in Chinese wolfberry juice by select lactic acid bacteria. Food Biosci. 2021, 43, 101264. [Google Scholar] [CrossRef]
- Zheng, Y.; Oellig, C.; Zhang, Y.; Liu, Y.; Chen, Y.; Zhang, Y. Characterization of the key odorants in goji wines in three levels of sweetness by applications of sensomics approach. Food Chem. 2024, 140803. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, D.; Duan, H.; Zhou, S.; Guo, J.; Yan, W. Detection and analysis of volatile flavor compounds in different varieties and origins of goji berries using HS-GC-IMS. LWT 2023, 187, 115322. [Google Scholar] [CrossRef]
- Zheng, Y.; Oellig, C.; Zhu, L.; Granvogl, M.; Liu, Y.; Chen, Y.; Feng, X.; Wang, W.; Zhang, Y. In-depth profiling of the key odor-active compounds in commercially dried red and black goji berries. Eur. Food Res. Technol. 2025. [Google Scholar] [CrossRef]
- Lu, W.; Chen, J.; Li, X.; Qi, Y.; Jiang, R. Flavor components detection and discrimination of isomers in Huaguo tea using headspace-gas chromatography-ion mobility spectrometry and multivariate statistical analysis. Anal. Chim. Acta 2023, 1243, 340842. [Google Scholar] [CrossRef]
- Vetter, W.; Wendlinger, C. Furan fatty acids—Valuable minor fatty acids in food. Lipid Technol. 2013, 25, 7–10. [Google Scholar] [CrossRef]
- Wagner, J.; Granvogl, M.; Schieberle, P. Characterization of the key aroma compounds in raw licorice (Glycyrrhiza glabra L.) by means of molecular sensory science. J. Agric. Food Chem. 2016, 64, 8388–8396. [Google Scholar] [CrossRef]
- Zhai, X.; Granvogl, M. Key odor-active compounds in raw green and red Toona sinensis (A. Juss.) Roem. and their changes during blanching. J. Agric. Food Chem. 2020, 68, 7169–7183. [Google Scholar] [CrossRef]
- Thurnhofer, S.; Lehnert, K.; Vetter, W. Exclusive quantification of methyl-branched fatty acids and minor 18:1-isomers in foodstuff by GC/MS in the SIM mode using 10,11-dichloroundecanoic acid and fatty acid ethyl esters as internal standards. Eur. Food Res. Technol. 2008, 226, 975–983. [Google Scholar] [CrossRef]
- Engel, W.; Bahr, W.; Schieberle, P. Solvent assisted flavour evaporation—A new and versatile technique for the careful and direct isolation of aroma compounds from complex food matrices. Eur. Food Res. Technol. 1999, 237–241. [Google Scholar] [CrossRef]
- Grosch, W. Detection of potent odorants in foods by aroma extract dilution analysis. Trends Food Sci. Technol 1993, 4, 68–73. [Google Scholar] [CrossRef]
- Frankel, E.N. Chemistry of free radical and singlet oxidation of lipids. Prog. Lipid Res. 1985, 23, 197–221. [Google Scholar] [CrossRef]
- Lin, J.; Blank, I. Odorants generated by thermally induced degradation of phospholipids. J. Agric. Food Chem. 2003, 51, 4364–4369. [Google Scholar] [CrossRef]
- Blank, I.; Fay, L.B.; Lakner, F.J.; Schlosser, M. Determination of 4-hydroxy-2,5-dimethyl-3(2H)-furanone and 2(or 5)-ethyl-4-hydroxy-5(or 2)-methyl-3(2H)-furanone in pentose sugar-based Maillard model systems by isotope dilution assays. J. Agric. Food Chem. 1997, 2642–2648. [Google Scholar] [CrossRef]
- Zabetakis, I.; Gramshaw, J.W.; Robinson, D.S. 2,5-Dimethyl-4-hydroxy-2H-furan-3-one and its derivatives: Analysis, synthesis and biosynthesis—A review. Food Chem. 1999, 139–151. [Google Scholar] [CrossRef]
- Sauvaire, Y.; Girardon, P.; Baccou, J.C.; Risterucci, A.M. Changes in growth, proteins and free amino acids of developing seed and pod of fenugreek. Phytochemistry 1984, 23, 479–486. [Google Scholar] [CrossRef]
- Blank, I.; Lin, J.; Fumeaux, R.; Welti, D.H.; Fay, L.B. Formation of 3-hydroxy-4,5-dimethyl-2(5H)-furanone (sotolone) from 4-hydroxy-L-isoleucine and 3-amino-4,5-dimethyl-3,4-dihydro-2(5H)-furanone. J. Agric. Food Chem. 1996, 1851–1856. [Google Scholar] [CrossRef]
- König, T.; Gutsche, B.; Hartl, M.; Hübscher, R.; Schreier, P.; Schwab, W. 3-Hydroxy-4,5-dimethyl-2(5H)-furanone (Sotolon) causing an off-flavor: Elucidation of its formation pathways during storage of citrus soft drinks. J. Agric. Food Chem. 1999, 47, 3288–3291. [Google Scholar] [CrossRef]
- Sefton, M.A.; Skouroumounis, G.K.; Elsey, G.M.; Taylor, D.K. Occurrence, sensory impact, formation, and fate of damascenone in grapes, wines, and other foods and beverages. J. Agric. Food Chem. 2011, 59, 9717–9746. [Google Scholar] [CrossRef] [PubMed]
- Masanetz, C.; Guth, H.; Grosch, W. Fishy and hay-like off-flavours of dry spinach. Zeitschrift für Lebensmitteluntersuchung und-Forschung A 1998, 206, 108–113. [Google Scholar] [CrossRef]
- Frankel, E.N. Volatile lipid oxidation products. Prog. Lipid Res. 1982, 22, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Chung, H.Y. GC-MS analysis of the volatile components in dried boxthorn (Lycium chinensis) fruit. J. Appl. Biol. Chem. 2009, 52, 516–524. [Google Scholar] [CrossRef]
- Guth, H.; Grosch, W. Detection of furanoid fatty acids in soya-bean oil—Cause for the light-induced off-flavour. Fett/Lipid 1991, 93, 249–255. [Google Scholar] [CrossRef]
- Vetter, W.; Ulms, K.; Wendlinger, C.; van Rijn, J. Novel non-methylated furan fatty acids in fish from a zero discharge aquaculture system. NFS J. 2016, 2, 8–14. [Google Scholar] [CrossRef]
- Müller, F.; Hogg, M.; Vetter, W. Valuable furan fatty acids in soybeans and soy products. Eur. Food Res. Technol. 2020, 246, 1383–1392. [Google Scholar] [CrossRef]
- Chen, F.; Su, Y.; Zhang, F.; Guo, Y. Low-temperature headspace-trap gas chromatography with mass spectrometry for the determination of trace volatile compounds from the fruit of Lycium barbarum L. J. Sep. Sci. 2015, 38, 670–676. [Google Scholar] [CrossRef]
- Wang, C.; Chin, C.-K.; Ho, C.-T.; Hwang, C.F.; Polashock, J.J.; Martin, C.E. Changes of fatty acids and fatty acid-derived flavor compounds by expressing the yeast Δ-9 desaturase gene in tomato. J. Agric. Food Chem. 1996, 44, 3399–3402. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Kamal-Eldin, A. Lipid Oxidation Pathways; AOCS Press: Champaign, IL, USA, 2003; ISBN 9781003040316. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.; Chalkia, A.; Taoukis, P. Application of osmotic dehydration to improve the quality of dried goji berry. J. Food Eng. 2018, 232, 36–43. [Google Scholar] [CrossRef]
- Fratianni, A.; Niro, S.; Alam, M.; Cinquanta, L.; Di Matteo, M.; Adiletta, G.; Panfili, G. Effect of a physical pre-treatment and drying on carotenoids of goji berries (Lycium barbarum L.). LWT 2018, 92, 318–323. [Google Scholar] [CrossRef]
- Pino, J.A.; Mesa, J.; Muñoz, Y.; Martí, M.P.; Marbot, R. Volatile components from mango (Mangifera indica L.) cultivars. J. Agric. Food Chem. 2005, 53, 2213–2223. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Ding, C.; Zhang, Y.; Song, Z. Impact of different pretreatment methods on drying characteristics and microstructure of goji berry under electrohydrodynamic (EHD) drying process. Innov. Food Sci. Emerg. Technol. 2020, 61, 102318. [Google Scholar] [CrossRef]
| No. a | Compound | RI b | Odor Quality | FD Factor c | ||
|---|---|---|---|---|---|---|
| DB-5 | DB-FFAP | NG | HG | |||
| 1 | ethyl butanoate | 801 | 1032 | fruity | 16 | |
| 2 | hexanal | 801 | 1078 | green, grassy | 256 | 256 |
| 3 | methyl hexanoate | 923 | 1181 | fruity, musty | 4 | 4 |
| 4 | 3-methyl-1-butanol | <800 | 1198 | malty | 16 | 2 |
| 5 | (Z)-4-heptenal | 900 | 1237 | fish-like, train oil-like | 2 | 1 |
| 6 | octanal | 1004 | 1283 | citrus-like, green | 16 | 4 |
| 7 | 1-octen-3-one | 978 | 1295 | mushroom-like | 8 | 8 |
| 8 | 2-acetyl-1-pyrrolin d | 909 | 1314 | popcorn-like, roasty | 64 | 128 |
| 9 | (E)-2-heptenal | 958 | 1319 | green apple-like, bitter almond-like | 1 | 1 |
| 10 | dimethyl trisulfide | 973 | 1375 | sulfuric, cabbage-like | 2 | 1 |
| 11 | nonanal | 1106 | 1386 | citrus-like, soapy | 1 | 2 |
| 12 | (E)-2-octenal | 1060 | 1425 | fatty, nutty | 1 | 1 |
| 13 | 2-ethyl-3,5-dimethylpyrazine | 1077 | 1436 | earthy | 8 | 8 |
| 14 | 1-octen-3-ol | 981 | 1439 | mushroom-like | 1 | 1 |
| 15 | ethyl cyclohexanoate d | 1136 | 1414 | fruity, sweet | 8 | 1 |
| 16 | acetic acid | <700 | 1444 | vinegar-like | 512 | 128 |
| 17 | (E,E)-2,4-heptadienal | 1013 | 1490 | fatty, floral | 4 | 1 |
| 18 | decanal | 1208 | 1491 | soapy, citrus-like | 16 | 512 |
| 19 | pentyl hexanoate | 1278 | 1506 | fruity, ethereal | 8 | |
| 20 | 3-isobutyl-2-methoxypyrazine d | 1177 | 1516 | earthy, green bell pepper-like | 4 | 2 |
| 21 | (E)-2-nonenal | 1161 | 1529 | fatty, green | 128 | 32 |
| 22 | 1-octanol | 1070 | 1550 | soapy, citrus-like, green | 8 | 1 |
| 23 | (E,Z)-2,6-nonadienal | 1155 | 1581 | cucumber-like | 8 | 4 |
| 24 | 2-undecanone | 1293 | 1590 | soapy, green | 32 | |
| 25 | butanoic acid | <800 | 1621 | sweaty | 32 | 16 |
| 26 | 2-acetylpyrazine d | 1024 | 1625 | popcorn-like, roasty | 8 | 8 |
| 27 | (E)-2-decenal | 1264 | 1638 | fatty, green | 2 | |
| 28 | phenylacetaldehyde | 1047 | 1642 | floral, honey-like | 8 | 8 |
| 29 | 2-, and 3-methylbutanoic acid | 857 | 1662 | sweaty | 128 | 64 |
| 30 | α-terpineol | 1199 | 1690 | floral, citrus-like | 1 | 1 |
| 31 | (E,E)-2,4-nonadienal | 1218 | 1697 | fatty, green | 4 | 4 |
| 32 | 3-methyl-2,4-nonanedione | 1262 | 1715 | hay-like, aniseed-like, fish-like | 32 | 64 |
| 33 | (E)-2-undecenal | 1366 | 1747 | soapy, metallic | 2 | 2 |
| 34 | (E,E)-2,4-decadienal | 1322 | 1807 | fatty, deep-fried | 1 | |
| 35 | (E)-β-damascenone | 1382 | 1817 | cooked apple-like | 4 | 4 |
| 36 | 2-methoxyphenol | 1087 | 1858 | smoky, gammon-like | 2 | 1 |
| 37 | heptanoic acid | 1075 | 1945 | rancid, sweaty | 1 | 32 |
| 38 | (E)-3-hexenoic acid d | 986 | 1947 | floral, cheesy | 1 | 2 |
| 39 | cis-4,5-epoxy-(E)-2-decenal | 1369 | 1986 | metallic | 128 | 256 |
| 40 | trans-4,5-epoxy-(E)-2-decenal | 1384 | 1993 | metallic | 256 | 1024 |
| 41 | phenol | 975 | 2005 | ink-like, phenolic | 1 | |
| 42 | 4-hydroxy-2,5-dimethyl-3(2H)-furanone | 1158 | 2033 | caramel-like | 1024 | 256 |
| 43 | p-cresol | 1072 | 2081 | fecal, phenolic, horse stable-like | 1 | 2 |
| 44 | 4-ethyl-2-methoxyphenol d | 1275 | 2032 | smoky, gammon-like | 8 | |
| 45 | nonanoic acid | 1282 | 2158 | moldy, pungent | 8 | 16 |
| 46 | eugenol | 1357 | 2166 | clove-like | 2 | 2 |
| 47 | 4-ethylphenol | 1164 | 2174 | phenolic | 128 | 128 |
| 48 | 2-methoxy-4-vinylphenol | 1311 | 2197 | smoky, clove-like | 2048 | 2048 |
| 49 | 3-hydroxy-4,5-dimethyl-2(5H)-furanone | 1100 | 2198 | fenugreek-like, lovage-like | 8 | 8 |
| 50 | phenylacetic acid | 1241 | 2561 | honey-like, beeswax-like | 64 | 64 |
| 51 | vanillin | 1401 | 2572 | vanilla-like, sweet | 2048 | 1024 |
| 52 | 3-phenylpropanoic acid | 1346 | 2629 | floral, cinnamon-like | 4 | 4 |
| No. | Compound | Quantifier Ion (m/z) a | Calibration Curve b | R2 | NG c | HG d | ||
|---|---|---|---|---|---|---|---|---|
| Con. | RSD | Con. | RSD | |||||
| 16 | acetic acid | 60 | y = 0.1199x − 0.0278 | 0.9996 | 320,000 | 14 | 170,000 | 11 |
| 21 | (E)-2-nonenal | 55 | y = 0.9263x + 0.0776 | 0.9983 | 1400 | 13 | 250 ** | 13 |
| 2 | hexanal | 56 | y = 0.2749x + 0.0211 | 0.9984 | 1200 | 14 | 1700 * | 2.8 |
| 6 | octanal e | 56 | y = 0.8613x − 0.0218 | 1 | 1100 | 14 | 390 *** | 12 |
| 4 | 3-methyl-1-butanol | 55 | y = 0.4732x − 0.076 | 0.9939 | 770 | 14 | 260 ** | 7.2 |
| 45 | nonanoic acid | 60 | y = 0.1318x − 0.0313 | 0.9984 | 620 | 5.4 | 1800 ** | 7.6 |
| 29 | 2, and 3-methylbutanoic acid f | 60 | y = 0.6866x − 0.1458 | 0.9998 | 590 | 10 | 390 ** | 6.0 |
| 18 | decanal | 57 | y = 0.0528x − 0.0104 | 0.9975 | 220 | 3.7 | 1300 *** | 6.1 |
| 22 | 1-octanol | 56 | y = 0.6225x − 0.0748 | 0.9993 | 210 | 6.6 | 4.8 ** | 3.2 |
| 51 | vanillin g | 151 | y = 0.9997x − 0.1974 | 0.9958 | 75 | 6.8 | 95 * | 9.7 |
| 11 | nonanal | 57 | y = 0.2182x − 0.0336 | 0.9999 | 39 | 7.7 | 18 ** | 2.7 |
| 40 | trans-4,5-epoxy-(E)-2-decenal | 68 | y = 0.0884x − 0.0023 | 0.9978 | 38 | 3.0 | 53 * | 9.0 |
| 1 | ethyl butanoate | 71 | y = 0.2292x − 0.0161 | 0.9916 | 33 | 15 | 0.64 ** | 3.5 |
| 32 | 3-methyl-2,4-nonanedione | 99 | y = 0.1156x + 0.0086 | 0.9973 | 32 | 13 | 81 ** | 2.9 |
| 39 | cis-4,5-epoxy-(E)-2-decenal | 68 | y = 0.0422x − 0.0013 | 0.9996 | 29 | 9.1 | 41 ** | 2.6 |
| 25 | butanoic acid h | 73 | y = 0.8392x + 0.1731 | 0.9952 | 25 | 3.4 | 22 ** | 1.6 |
| 42 | 4-hydroxy-2,5-dimethyl-3(2H)-furanone | 128 | y = 0.2055x + 0.0075 | 0.9925 | 24 | 4.9 | 6.5 ** | 2.1 |
| 37 | heptanoic acid | 60 | y = 0.354x − 0.0846 | 0.9929 | 21 | 4.1 | 1100 ** | 9.7 |
| 50 | phenylacetic acid i | 136 | y = 0.964x + 1.6184 | 0.999 | 14 | 4.7 | 14 | 10 |
| 5 | (Z)-4-heptenal | 41 | y = 0.1385x − 0.0331 | 0.9993 | 11 | 8.0 | 5.3 ** | 3.0 |
| 14 | 1-octen-3-ol | 57 | y = 0.9689x − 0.0926 | 0.9969 | 11 | 8.4 | 9.4 | 5.8 |
| 24 | 2-undecanone | 58 | y = 1.4768x − 0.1106 | 0.9925 | 10 | 12 | 1.7 ** | 14 |
| 9 | (E)-2-heptenal | 83 | y = 0.33x − 0.0402 | 0.9908 | 9.1 | 14 | 6.4 | 12 |
| 12 | (E)-2-octenal | 55 | y = 0.395x − 0.0353 | 0.9981 | 6.8 | 5.4 | 5.8 * | 6.6 |
| 27 | (E)-2-decenal | 70 | y = 0.316x − 0.0353 | 0.9928 | 6.0 | 6.1 | 0.96 ** | 14 |
| 7 | 1-octen-3-one | 55 | y = 0.9102x − 0.2433 | 0.9938 | 5.6 | 4.2 | 6.1 | 7 |
| 36 | 2-methoxyphenol | 109 | y = 0.7453x − 0.11 | 0.9924 | 5.5 | 11 | 1.2 *** | 4.5 |
| 48 | 2-methoxy-4-vinylphenol | 135 | y = 0.7976x − 0.1984 | 0.9916 | 5.5 | 8.0 | 1.2 ** | 3.0 |
| 17 | (E,E)-2,4-heptadienal | 81 | y = 1.0704x − 0.2174 | 0.9965 | 5.4 | 6.1 | 1.3 ** | 12 |
| 47 | 4-ethylphenol | 107 | y = 1.5506x − 0.0697 | 0.9996 | 4.6 | 13 | 1.9 ** | 10 |
| 28 | phenylacetaldehyde | 91 | y = 1.8407x − 0.1792 | 0.9958 | 4.5 | 8.5 | 5.7 | 11 |
| 3 | methyl hexanoate | 74 | y = 0.9783x − 0.2711 | 0.9912 | 4.4 | 12 | 5.4 * | 2.6 |
| 34 | (E,E)-2,4-decadienal | 81 | y = 1.5075x − 0.3252 | 0.9912 | 4.4 | 13 | 0.56 ** | 20 |
| 38 | (E)-3-hexenoic acid | 55 | y = 0.1498x + 0.0073 | 0.9965 | 3.9 | 1.5 | 27 ** | 6.9 |
| 41 | phenol | 94 | y = 1.3524x − 0.124 | 0.9982 | 2.8 | 12 | ||
| 30 | α-terpineol | 59 | y = 0.7084x − 0.0662 | 0.9981 | 1.7 | 0.83 | 0.09 *** | 2.5 |
| 31 | (E,E)-2,4-nonadienal | 81 | y = 1.8019x + 0.1319 | 0.9991 | 0.82 | 11 | 0.45 * | 11 |
| 35 | (E)-β-damascenone | 69 | y = 0.0259x − 0.0029 | 0.9983 | 0.75 | 15 | 1.8 * | 12 |
| 52 | 3-phenylpropanoic acid | 91 | y = 0.1619x − 0.0517 | 0.9974 | 0.67 | 11 | 1.1 * | 14 |
| 13 | 2-ethyl-3,5-dimethylpyrazine | 135 | y = 0.2879x + 0.0422 | 1 | 0.56 | 13 | 0.55 | 6.4 |
| 49 | 3-hydroxy-4,5-dimethyl-2(5H)-furanone | 83 | y = 0.0871x + 0.0034 | 0.9964 | 0.35 | 13 | 0.71 * | 14 |
| 23 | (E,Z)-2,6-nonadienal | 70 | y = 0.71x − 0.073 | 0.9943 | 0.31 | 2.0 | 0.21 ** | 14 |
| 33 | (E)-2-undecenal | 70 | y = 0.439x − 0.094 | 0.9918 | 0.18 | 15 | 0.43 ** | 0.4 |
| 46 | eugenol | 164 | y = 0.4707x − 0.0426 | 0.9977 | 0.06 | 16 | 0.06 | 8.2 |
| 1 | dimethyl trisulfide | 126 | y = 0.9653x − 0.0892 | 0.9974 | 0.05 | 5.7 | 0.06 | 14 |
| 43 | p-cresol | 107 | y = 1.2465x − 0.0692 | 0.9997 | 0.05 | 22 | 0.14 ** | 7.2 |
| 19 | pentyl hexanoate | 70 | y = 0.6198x + 0.0138 | 0.9902 | 0.44 | 11 | ||
| No. | Compound | OT a | OAV b | |
|---|---|---|---|---|
| NG | HG | |||
| 21 | (E)-2-nonenal | 0.25 | 5800 | 1000 |
| 5 | (Z)-4-heptenal | 0.0087 | 1300 | 610 |
| 32 | 3-methyl-2,4-nonanedione | 0.046 | 700 | 1800 |
| 2 | hexanal | 2.4 | 510 | 730 |
| 7 | 1-octen-3-one | 0.016 | 350 | 380 |
| 6 | octanal | 3.4 | 340 | 110 |
| 40 | trans-4,5-epoxy-(E)-2-decenal | 0.22 | 170 | 240 |
| 34 | (E,E)-2,4-decadienal | 0.027 | 160 | 21 |
| 39 | cis-4,5-epoxy-(E)-2-decenal | 0.22 | 130 | 190 |
| 35 | (E)-β-damascenone | 0.006 | 130 | 290 |
| 16 | acetic acid | 5600 | 56 | 31 |
| 1 | ethyl butanoate | 0.75 | 45 | <1 |
| 45 | nonanoic acid | 26 c | 24 | 71 |
| 18 | decanal | 9.3 | 23 | 140 |
| 11 | nonanal | 2.8 | 14 | 7 |
| 31 | (E,E)-2,4-nonadienal | 0.062 | 13 | 7 |
| 27 | (E)-2-decenal | 0.49 c | 12 | 2 |
| 23 | (E,Z)-2,6-nonadienal | 0.03 | 10 | 7 |
| 36 | 2-methoxyphenol | 0.84 | 7 | 1 |
| 10 | dimethyl trisulfide | 0.0099 | 5 | 6 |
| 4 | 3-methyl-1-butanol | 220 | 3 | 1 |
| 13 | 2-ethyl-3,5-dimethylpyrazine | 0.28 | 2 | 2 |
| 12 | (E)-2-octenal | 4 | 2 | 2 |
| 51 | vanillin | 53 | 1 | 2 |
| 29 | 2-, and 3-methylbutanoic acid | 490 | 1 | <1 |
| 38 | (E)-3-hexenoic acid | 3.9 | <1 | 7 |
| 28 | phenylacetaldehyde | 5.2 | <1 | 1 |
| 9 | (E)-2-heptenal | 18 c | <1 | <1 |
| 24 | 2-undecanone | 24 c | <1 | <1 |
| 47 | 4-ethylphenol | 13 | <1 | <1 |
| 42 | 4-hydroxy-2,5-dimethyl-3(2H)-furanone | 87 | <1 | <1 |
| 48 | 2-methoxy-4-vinylphenol | 21 c | <1 | <1 |
| 14 | 1-octen-3-ol | 45 | <1 | <1 |
| 33 | (E)-2-undecenal | 0.78 | <1 | <1 |
| 49 | 3-hydroxy-4,5-dimethyl-2(5H)-furanone | 1.7 | <1 | <1 |
| 50 | phenylacetic acid | 68 | <1 | <1 |
| 3 | methyl hexanoate | 90 | <1 | <1 |
| 46 | eugenol | 1.8 | <1 | <1 |
| 43 | p-cresol | 3.9 | <1 | <1 |
| 25 | butanoic acid | 2400 | <1 | <1 |
| 52 | 3-phenylpropanoic acid | 120 | <1 | <1 |
| 30 | α-terpineol | 1200 | <1 | <1 |
| 41 | phenol | 3400 c | <1 | <1 |
| 44 | 4-ethyl-2-methoxyphenol | 50 | <1 | <1 |
| Fatty Acid | Structure | Content (µg/kg) | µg Fatty Acid/mg of Fat | Contribution (%) | |||
|---|---|---|---|---|---|---|---|
| NG a | HG b | NG | HG | NG | HG | ||
| caprylic | 8:0 | 0.47 ± 0.00 | 0.27 ± 0.01 *** | 0.20 ± 0.02 | 0.12 ± 0.00 * | 0.20 ± 0.02 | 0.10 ± 0.02 * |
| lauric | 12:0 | 0.06 ± 0.01 | 0.11 ± 0.03 | 0.03 ± 0.01 | 0.05 ± 0.01 | 0.03 ± 0.00 | 0.04 ± 0.00 * |
| myristic | 14:0 | 0.53 ± 0.11 | 0.75 ± 0.21 | 0.22 ± 0.06 | 0.33 ± 0.08 | 0.22 ± 0.02 | 0.27 ± 0.02 |
| pentadecanoic | 15:0 | 0.06 ± 0.01 | 0.09 ± 0.02 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.00 | 0.03 ± 0.00 * |
| palmitic | 16:0 | 25 ± 4.0 | 33 ± 8.1 | 11 ± 2.4 | 15 ± 3.0 | 11 ± 0.32 | 12 ± 0.37 |
| heptadecanoic | 17:0 | 0.52 ± 0.07 | 0.53 ± 0.13 | 0.22 ± 0.05 | 0.24 ± 0.05 | 0.22 ± 0.00 | 0.19 ± 0.01 |
| isomer of stearic | isomer of 18:0 | 0.23 ± 0.03 | 0.32 ± 0.08 | 0.10 ± 0.02 | 0.15 ± 0.03 | 0.10 ± 0.00 | 0.12 ± 0.00 * |
| stearic | 18:0 | 5.7 ± 0.75 | 7.2 ± 1.45 | 2.4 ± 0.49 | 3.2 ± 0.51 | 2.4 ± 0.01 | 2.6 ± 0.04 ** |
| arachidic | 20:0 | 1.5 ± 0.25 | 1.6 ± 0.40 | 0.65 ± 0.15 | 0.72 ± 0.15 | 0.65 ± 0.02 | 0.58 ± 0.02 * |
| henicosanoic | 21:0 | 0.16 ± 0.03 | 0.16 ± 0.05 | 0.07 ± 0.02 | 0.07 ± 0.02 | 0.07 ± 0.01 | 0.06 ± 0.01 |
| behenic | 22:0 | 2.9 ± 0.52 | 3.0 ± 0.79 | 1.2 ± 0.31 | 1.3 ± 0.30 | 1.2 ± 0.06 | 1.1 ± 0.06 |
| tricosanoic | 23:0 | 0.17 ± 0.02 | 0.08 ± 0.00 * | 0.07 ± 0.01 | 0.04 ± 0.00 * | 0.07 ± 0.00 | 0.03 ± 0.01 ** |
| lignoceric | 24:0 | 2.24 ± 0.39 | 3.3 ± 0.71 | 0.95 ± 0.23 | 1.5 ± 0.26 | 0.95 ± 0.04 | 1.2 ± 0.00 ** |
| ΣSFA | 40 ± 6.3 | 52 ± 12 | 17 ± 3.9 | 23 ± 4.5 | 17 ± 0.47 | 19 ± 0.32 | |
| cis-10-pentadecenoic | 15:1n-5 | 0.26 ± 0.06 | 0.44 ± 0.11 | 0.11 ± 0.03 | 0.20 ± 0.04 | 0.11 ± 0.01 | 0.16 ± 0.01 * |
| palmitoleic | 16:1n-7 | 1.06 ± 0.20 | 1.6 ± 0.43 | 0.45 ± 0.12 | 0.72 ± 0.16 | 0.44 ± 0.03 | 0.59 ± 0.03 * |
| oleic | 18:1n-9c | 32 ± 4.0 | 37 ± 7.3 | 14 ± 2.7 | 16 ± 2.6 | 14 ± 0.07 | 13 ± 0.22 |
| elaidic | 18:1n-9t | 2.1 ± 0.29 | 2.7 ± 0.57 | 0.87 ± 0.19 | 1.2 ± 0.20 | 0.87 ± 0.01 | 0.99 ± 0.01 ** |
| cis-11-eicosenoic | 20:1n-9 | 0.20 ± 0.05 | 0.28 ± 0.04 * | 0.08 ± 0.03 | 0.13 ± 0.01 ** | 0.08 ± 0.01 | 0.10 ± 0.01 ** |
| linoleic | 18:2n-6 | 140 ± 16 | 150 ± 32 | 58 ± 11 | 69 ± 11 | 58 ± 0.69 | 56 ± 0.54 ** |
| γ-linolenic | 18:3n-6 | 9.2 ± 1.1 | 9.7 ± 2.6 | 3.9 ± 0.76 | 4.3 ± 0.96 | 3.9 ± 0.03 | 3.5 ± 0.17 * |
| α-linolenic | 18:3n-3 | 15 ± 2.6 | 18 ± 4.6 | 6.4 ± 1.5 | 8.0 ± 1.7 | 6.3 ± 0.27 | 6.5 ± 0.25 |
| ΣUFA | 200 ± 24 | 220 ± 47 | 83 ± 16 | 100 ± 17 | 83 ± 0.46 | 81 ± 0.32 | |
| sum | 240 ± 31 | 280 ± 60 | 100 ± 20 | 120 ± 21 | 100 | 100 | |
| No. | Aldehyde | Content Ratio of NG and HG | Potential Precursor a | Content Ratio of NG and HG | Hydroperoxide | Proposed Mechanism b |
|---|---|---|---|---|---|---|
| 21 | (E)-2-nonenal | 5.7 | LA (18:2n-6) | 0.89 | LA-10-OOH | AO, PO, EO |
| GLA (18:3n-6) | 0.95 | GLA-9-OOH | ||||
| 5 | (Z)-4-heptenal | 2.2 | ALA (18:3n-3) | 0.83 | ALA-10-OOH | PO, EO |
| 2 | hexanal | 0.70 | LA (18:2n-6) | 0.89 | LA-12-OOH | AO, PO, EO |
| GLA (18:3n-6) | 0.95 | GLA-11-OOH | ||||
| 34 | (E,E)-2,4-decadienal | 7.9 | LA (18:2n-6) | 0.89 | LA-9-OOH | AO, PO, EO |
| GLA (18:3n-6) | 0.95 | GLA-8-OOH | ||||
| 6 | octanal | 2.8 | OA (18:1n-9) | 0.75 | OA-11-OOH | AO |
| 40, 39 | trans- and cis- 4,5-epoxy-(E)-2-decenal | 0.72 | LA (18:2n-6) | 0.89 | LA-13-OOH | AO, PO, EO |
| 18 | decanal | 0.17 | OA (18:1n-9) | 0.75 | OA-8-OOH | AO |
| 11 | nonanal | 2.1 | OA (18:1n-9) | 0.75 | OA-10-OOH | AO, PO |
| 31 | (E,E)-2,4-nonadienal | 1.8 | ALA (18:3n-3) | 0.83 | ALA-10-OOH | PO, EO |
| 27 | (E)-2-decenal | 6.2 | OA (18:1n-9) | 0.75 | OA-9-OOH | AO, PO |
| 12 | (E)-2-octenal | 1.2 | PA (16:1n-7) | 0.65 | PA-9-OOH | AO, PO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Oellig, C.; Vetter, W.; Bauer, V.; Liu, Y.; Chen, Y.; Zhang, Y. Fatty Acids Are Responsible for the Discrepancy of Key Aroma Compounds in Naturally Dried Red Goji Berries and Hot-Air-Dried Red Goji Berries. Foods 2025, 14, 2388. https://doi.org/10.3390/foods14132388
Zheng Y, Oellig C, Vetter W, Bauer V, Liu Y, Chen Y, Zhang Y. Fatty Acids Are Responsible for the Discrepancy of Key Aroma Compounds in Naturally Dried Red Goji Berries and Hot-Air-Dried Red Goji Berries. Foods. 2025; 14(13):2388. https://doi.org/10.3390/foods14132388
Chicago/Turabian StyleZheng, Yan, Claudia Oellig, Walter Vetter, Vanessa Bauer, Yuan Liu, Yanping Chen, and Yanyan Zhang. 2025. "Fatty Acids Are Responsible for the Discrepancy of Key Aroma Compounds in Naturally Dried Red Goji Berries and Hot-Air-Dried Red Goji Berries" Foods 14, no. 13: 2388. https://doi.org/10.3390/foods14132388
APA StyleZheng, Y., Oellig, C., Vetter, W., Bauer, V., Liu, Y., Chen, Y., & Zhang, Y. (2025). Fatty Acids Are Responsible for the Discrepancy of Key Aroma Compounds in Naturally Dried Red Goji Berries and Hot-Air-Dried Red Goji Berries. Foods, 14(13), 2388. https://doi.org/10.3390/foods14132388







