Comprehensive Analysis of Bacterial Communities and Microbiological Quality of Frozen Edible Insects
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Extraction
2.3. 16S rRNA Gene Amplicon Sequencing
2.4. Microbiome Sequencing and Analysis
2.5. Microbiological Analysis of Frozen Edible Insects
2.5.1. Sample Preparation
2.5.2. Enumeration of Microbial Flora
2.5.3. Analysis of Indicator Microorganisms
2.5.4. Analysis of Bacterial Pathogens
3. Results and Discussion
3.1. Analysis of Bacterial Diversity Using 16S rRNA Gene High-Throughput Sequencing
3.2. Bacterial Community Diversity and Structure
3.3. Relative Abundance of Selected Microorganisms at Family Levels
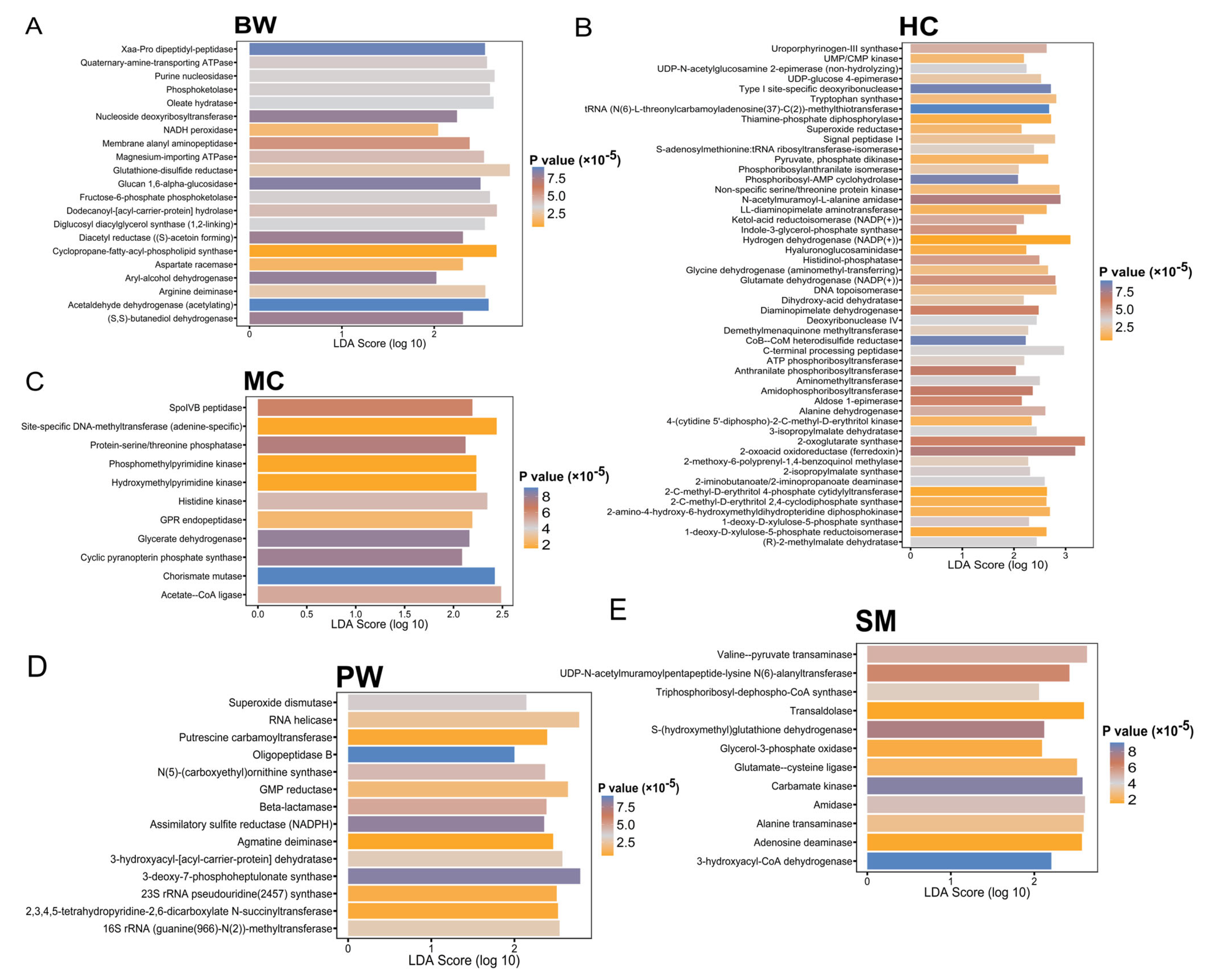
3.4. Functional Prediction and Analysis of 16S rRNA Gene
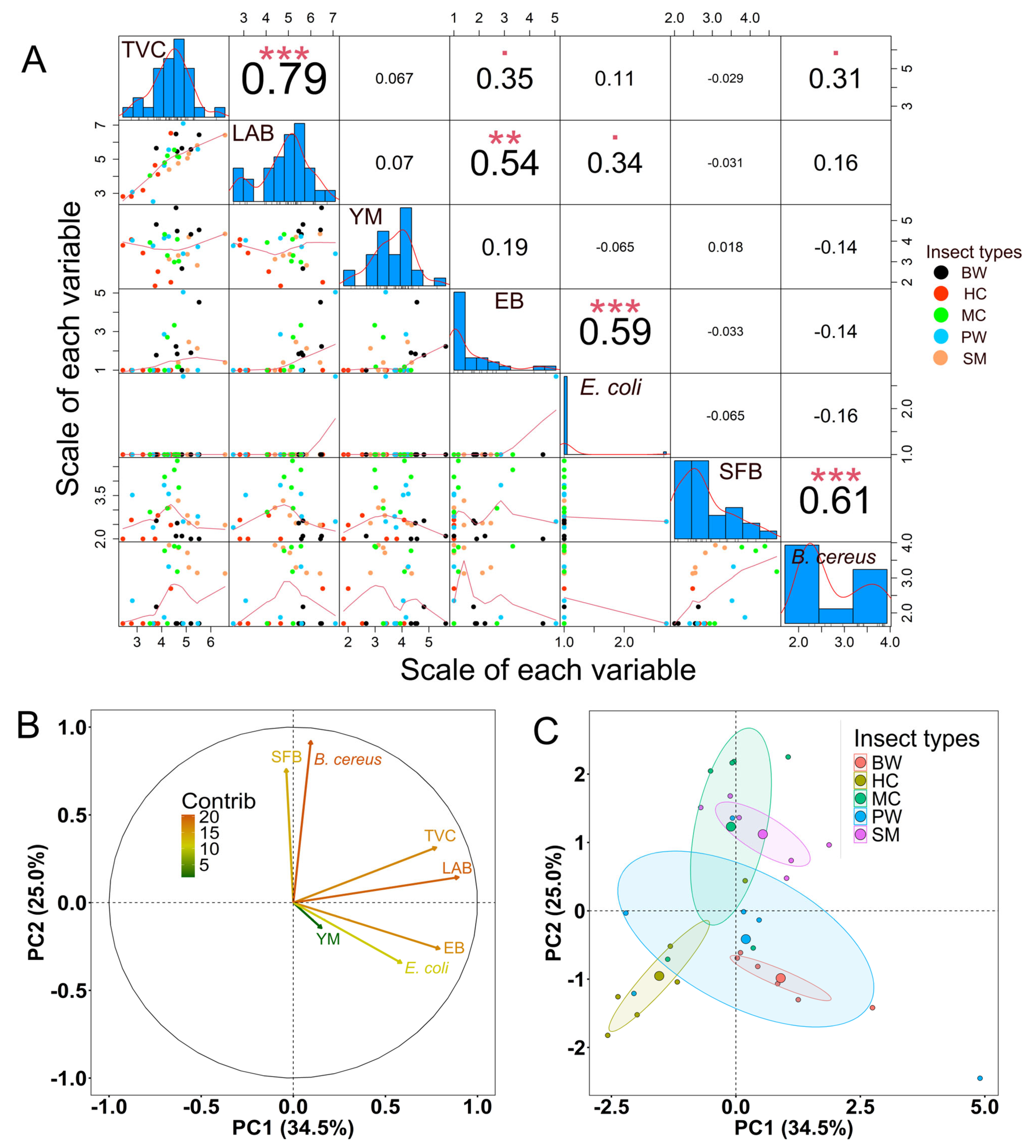
3.5. Microbiological Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olivadese, M.; Dindo, M.L. Edible insects: A historical and cultural perspective on entomophagy with a focus on western societies. Insects 2023, 14, 690. [Google Scholar] [CrossRef] [PubMed]
- Imathiu, S. Benefits and food safety concerns associated with consumption of edible insects. NFS J. 2020, 18, 1–11. [Google Scholar] [CrossRef]
- Kouřimská, L.; Adámková, A. Nutritional and sensory quality of edible insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef]
- Liang, Z.; Zhu, Y.; Leonard, W.; Fang, Z. Recent advances in edible insect processing technologies. Food Res. Int. 2024, 182, 114137. [Google Scholar] [CrossRef]
- Aidoo, O.F.; Osei-Owusu, J.; Asante, K.; Dofuor, A.K.; Boateng, B.O.; Debrah, S.K.; Ninsin, K.D.; Siddiqui, S.A.; Chia, S.Y. Insects as food and medicine: A sustainable solution for global health and environmental challenges. Front. Nutr. 2023, 10, 1113219. [Google Scholar] [CrossRef]
- Fu, C.; Cheema, W.A.; Mobashar, M.; Shah, A.A.; Alqahtani, M.M. Insects as Sustainable Feed: Enhancing animal nutrition and reducing livestock environmental impression. J. Anim. Physiol. Anim. 2025, 109, 280–290. [Google Scholar] [CrossRef]
- Moruzzo, R.; Mancini, S.; Guidi, A. Edible insects and sustainable development goals. Insects 2021, 12, 557. [Google Scholar] [CrossRef]
- Garofalo, C.; Milanović, V.; Cardinali, F.; Aquilanti, L.; Clementi, F.; Osimani, A. Current knowledge on the microbiota of edible insects intended for human consumption: A state-of-the-art review. Food Res. Int. 2019, 125, 108527. [Google Scholar] [CrossRef]
- Grabowski, N.T.; Klein, G. Microbiology of processed edible insect products—Results of a preliminary survey. Int. J. Food Microbiol. 2017, 243, 103–107. [Google Scholar] [CrossRef]
- Goumperis, T. Insects as food: Risk assessment and their future perspective in Europe. In Edible Insects in the Food Sector: Methods, Current Applications and Perspectives; Sogari, G., Mora, C., Menozzi, D., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–9. [Google Scholar]
- Spatola, G.; Giusti, A.; Mancini, S.; Tinacci, L.; Nuvoloni, R.; Fratini, F.; Di Iacovo, F.; Armani, A. Assessment of the information to consumers on insects-based products (Novel Food) sold by e-commerce in the light of the EU legislation: When labelling compliance becomes a matter of accuracy. Food Control 2024, 162, 110440. [Google Scholar] [CrossRef]
- Vale-Hagan, W.; Singhal, S.; Grigoletto, I.; Totaro-Fila, C.; Theodoridou, K.; Koidis, A. Edible insects in mixed-sourced protein meals for animal feed and food: An EU focus. Food Humanit. 2023, 1, 1180–1187. [Google Scholar] [CrossRef]
- Krongdang, S.; Phokasem, P.; Venkatachalam, K.; Charoenphun, N. Edible insects in Thailand: An overview of status, properties, processing, and utilization in the food industry. Foods 2023, 12, 2162. [Google Scholar] [CrossRef] [PubMed]
- Hanboonsong, A.; Durst, P. Guidance on Sustainable Cricket Farming—A Practical Manual for Farmers and Inspectors; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Rampanti, G.; Cardinali, F.; Ferrocino, I.; Milanović, V.; Garofalo, C.; Osimani, A.; Aquilanti, L. Deciphering the microbiota of edible insects sold by street vendors in Thailand using metataxonomic analysis. Insects 2025, 16, 122. [Google Scholar] [CrossRef] [PubMed]
- Vandeweyer, D.; De Smet, J.; Van Looveren, N.; Van Campenhout, L. Biological contaminants in insects as food and feed. J. Insects Food Feed. 2021, 7, 807–822. [Google Scholar] [CrossRef]
- Nyangena, D.N.; Mutungi, C.; Imathiu, S.; Kinyuru, J.; Affognon, H.; Ekesi, S.; Nakimbugwe, D.; Fiaboe, K.K.M. Effects of traditional processing techniques on the nutritional and microbiological quality of four edible insect species used for food and feed in East Africa. Foods 2020, 9, 574. [Google Scholar] [CrossRef]
- Doyle, J.J. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Wasimuddin; Schlaeppi, K.; Ronchi, F.; Leib, S.L.; Erb, M.; Ramette, A. Evaluation of primer pairs for microbiome profiling from soils to humans within the One Health framework. Mol. Ecol. Resour. 2020, 20, 1558–1571. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Maturin, L.; Peeler, J.T. Bacteriological Analytical Manual Chapter 3: Aerobic Plate Count; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2001; pp. 1–12.
- Ngamsomchat, A.; Kaewkod, T.; Konkit, M.; Tragoolpua, Y.; Bovonsombut, S.; Chitov, T. Characterisation of Lactobacillus plantarum of dairy-product origin for probiotic Chèvre cheese production. Foods 2022, 11, 934. [Google Scholar] [CrossRef] [PubMed]
- Tournas, V.; Stack, M.E.; Mislivec, P.B.; Koch, H.A.; Bandler, R. Bacteriological Analytical Manual Chapter 18: Yeasts, Molds and Mycotoxins; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2001; pp. 1–14.
- Vieira, F.C.S.; Nahas, E. Comparison of microbial numbers in soils by using various culture media and temperatures. Microbiol. Res. 2005, 160, 197–202. [Google Scholar] [CrossRef] [PubMed]
- ISO 21528-2:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part 2: Colony-Count Technique; Corrected Version 2018-06-01. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 16649-2; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of-Glucuronidase-Positive Escherichia Coli—Part 2: Colony-Count Technique at 44 °C Using 5-Bromo-4-chloro-3-indolyl-D-glucuronid. International Organization for Standardization: Geneva, Switzerland, 2001.
- Tallent, S.; Hait, J.; Bennett, R.W.; Lancette, G.A. Bacteriological Analytical Manual Chapter 12: Staphylococcus aureus; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2016; pp. 1–7.
- Tallent, S.M.; Knolhoff, A.; Rhodehamel, J.E.; Harmon, S.M.; Bennett, R.W. Bacteriological Analytical Manual Chapter 14: Bacillus cereus; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2020; pp. 1–12.
- Rhodehamel, E.J.; Harmon, S.M. Bacteriological Analytical Manual, Chapter 16: Clostridium perfringens; January 2001 Edition; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2001; pp. 1–8.
- Vandeweyer, D.; Crauwels, S.; Lievens, B.; Van Campenhout, L. Metagenetic analysis of the bacterial communities of edible insects from diverse production cycles at industrial rearing companies. Int. J. Food Microbiol. 2017, 261, 11–18. [Google Scholar] [CrossRef]
- Garofalo, C.; Osimani, A.; Milanović, V.; Taccari, M.; Cardinali, F.; Aquilanti, L.; Riolo, P.; Ruschioni, S.; Isidoro, N.; Clementi, F. The microbiota of marketed processed edible insects as revealed by high-throughput sequencing. Food Microbiol. 2017, 62, 15–22. [Google Scholar] [CrossRef]
- Chen, B.; Yu, T.; Xie, S.; Du, K.; Liang, X.; Lan, Y.; Sun, C.; Lu, X.; Shao, Y. Comparative shotgun metagenomic data of the silkworm Bombyx mori gut microbiome. Sci. Data 2018, 5, 180285. [Google Scholar] [CrossRef]
- Aleknavičius, D.; Lukša, J.; Strazdaitė-Žielienė, Ž.; Servienė, E. The bacterial microbiota of edible insects Acheta domesticus and Gryllus assimilis revealed by high content analysis. Foods 2022, 11, 1073. [Google Scholar] [CrossRef]
- Osimani, A.; Milanović, V.; Cardinali, F.; Garofalo, C.; Clementi, F.; Pasquini, M.; Riolo, P.; Ruschioni, S.; Isidoro, N.; Loreto, N.; et al. The bacterial biota of laboratory-reared edible mealworms (Tenebrio molitor L.): From feed to frass. Int. J. Food Microbiol. 2018, 272, 49–60. [Google Scholar] [CrossRef]
- Pal, A.; Mann, A.; den Bakker, H.C. Analysis of microbial composition of edible insect products available for human consumption within the United States using traditional microbiological methods and whole genome sequencing. J. Food Prot. 2024, 87, 100277. [Google Scholar] [CrossRef]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.; Dominguez, R.; Pateiro, M.; Saraiva, J.A.; Franco, D. Chapter 3—Main groups of microorganisms of relevance for food safety and stability: General aspects and overall description. In Innovative Technologies for Food Preservation; Barba, F.J., Sant’Ana, A.S., Orlien, V., Koubaa, M., Eds.; Academic Press: London, UK, 2018; pp. 53–107. [Google Scholar]
- Jones Joshua, A.; Newton Irene, G.; Moczek Armin, P. Microbiome composition and turnover in the face of complex lifecycles and bottlenecks: Insights through the study of dung beetles. Appl. Environ. Microbiol. 2024, 91, e0127801224. [Google Scholar] [CrossRef]
- Łukasik, P.; Kolasa, M.R. With a little help from my friends: The roles of microbial symbionts in insect populations and communities. Philos. Trans. R. Soc. 2024, 379, 20230122. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Han, G.; Sun, J.; Huang, L.; Lu, Y.; Xia, Y.; Liu, Q.; Xu, J. The gut microbiota composition of Cnaphalocrocis medinalis and their predicted contribution to larval nutrition. Front. Microbiol. 2022, 13, 909863. [Google Scholar] [CrossRef] [PubMed]
- Kipkoech, C. Beyond proteins—Edible insects as a source of dietary fiber. Polysaccharides 2023, 4, 116–128. [Google Scholar] [CrossRef]
- Syahrulawal, L.; Torske, M.O.; Sapkota, R.; Næss, G.; Khanal, P. Improving the nutritional values of yellow mealworm Tenebrio molitor (Coleoptera: Tenebrionidae) larvae as an animal feed ingredient: A review. J. Anim. Sci. Biotechnol. 2023, 14, 146. [Google Scholar] [CrossRef]
- Stull, V.J.; Weir, T.L. Chitin and omega-3 fatty acids in edible insects have underexplored benefits for the gut microbiome and human health. Nat. Food 2023, 4, 283–287. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Xu, L. The pivotal roles of gut microbiota in insect plant interactions for sustainable pest management. NPJ Biofilms Microbiomes 2023, 9, 66. [Google Scholar] [CrossRef]
- Li, G.; Sun, J.; Meng, Y.; Yang, C.; Chen, Z.; Wu, Y.; Tian, L.; Song, F.; Cai, W.; Zhang, X.; et al. The impact of environmental habitats and diets on the gut microbiota diversity of true bugs (Hemiptera: Heteroptera). Biology 2022, 11, 1039. [Google Scholar] [CrossRef]
- The European Commission. Commission Implementing Regulation (EU) 2022/188 of 10 February 2022—Authorising the placing on the market of frozen, dried and powder forms of Acheta domesticus as a novel food under Regulation (EU) 2015/2283 of the European Parliament and of the Council and amending Commission Implementing Regulation (EU) 2017/2470. OJEU 2022, L30, 108–114. [Google Scholar]
- The European Commission. Commission Implementing Regulation (EU) 2022/169 of 8 February 2022—Authorising the placing on the market of frozen, dried and powder forms of yellow mealworm (Tenebrio molitor larva) as a novel food under Regulation (EU) 2015/2283 of the European Parliament and of the Council, and amending Commission Implementing Regulation (EU) 2017/2470. OJEU 2022, L28, 10–16. [Google Scholar]
- Vandeweyer, D.; Wynants, E.; Crauwels, S.; Verreth, C.; Viaene, N.; Claes, J.; Lievens, B.; Van Campenhout, L. Microbial dynamics during industrial rearing, processing, and storage of tropical house crickets (Gryllodes sigillatus) for human consumption. Appl. Environ. Microbiol. 2018, 84, e00255-18. [Google Scholar] [CrossRef]
- Messina, C.M.; Gaglio, R.; Morghese, M.; Tolone, M.; Arena, R.; Moschetti, G.; Santulli, A.; Francesca, N.; Settanni, L. Microbiological profile and bioactive properties of insect powders used in food and feed formulations. Foods 2019, 8, 400. [Google Scholar] [CrossRef] [PubMed]
- Kurdi, P.; Chaowiwat, P.; Weston, J.; Hansawasdi, C. Studies on microbial quality, proteiny yield, and antioxidant properties of some frozen edible insects. Int. J. Food Sci. 2021, 2021, 5580976. [Google Scholar] [CrossRef] [PubMed]
- Kewuyemi, Y.O.; Kesa, H.; Chinma, C.E.; Adebo, O.A. Fermented Edible Insects for Promoting Food Security in Africa. Insects 2020, 11, 283. [Google Scholar] [CrossRef] [PubMed]
- Dahal, S.; Jensen, A.B.; Lecocq, A. Effect of Probiotics on Tenebrio molitor Larval Development and Resistance against the Fungal Pathogen Metarhizium brunneum. Insects 2022, 13, 1114. [Google Scholar] [CrossRef]
- Savio, C.; Mugo-Kamiri, L.; Upfold, J.K. Bugs in Bugs: The Role of Probiotics and Prebiotics in Maintenance of Health in Mass-Reared Insects. Insects 2022, 13, 376. [Google Scholar] [CrossRef]
- Fasolato, L.; Cardazzo, B.; Carraro, L.; Fontana, F.; Novelli, E.; Balzan, S. Edible processed insects from e-commerce: Food safety with a focus on the Bacillus cereus group. Food Microbiol. 2018, 76, 296–303. [Google Scholar] [CrossRef]
- Pahalagedara, A.S.N.W.; Gkogka, E.; Hammershøj, M. A review on spore-forming bacteria and moulds implicated in the quality and safety of thermally processed acid foods: Focusing on their heat resistance. Food Control 2024, 166, 110716. [Google Scholar] [CrossRef]
- Lindbäck, T.; Granum, P.E. Chapter 20: Bacillus cereus. In Food Microbiology: Fundamentals And Frontiers, 5th ed.; Doyle, M.P., Diez-Gonzalez, F., Hill, C., Eds.; ASM Press: Washington, DC, USA, 2019; pp. 541–554. [Google Scholar]
- Ehling-Schulz, M.; Lereclus, D.; Koehler, T.M. The Bacillus cereus group: Bacillus species with pathogenic potential. Microbiol. Spectr. 2019, 7, 1–60. [Google Scholar] [CrossRef]
- Carroll, L.M.; Cheng, R.A.; Wiedmann, M.; Kovac, J. Keeping up with the Bacillus cereus group: Taxonomy through the genomics era and beyond. Crit. Rev. Food Sci. Nutr. 2022, 62, 7677–7702. [Google Scholar] [CrossRef]
- ISO 7932:2004/Amd 1:2020; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Presumptive Bacillus cereus—Colony-Count Technique at 30 Degrees C. International Organization for Standardization: Geneva, Switzerland, 2020.
- Majed, R.; Faille, C.; Kallassy, M.; Gohar, M. Bacillus cereus biofilms—Same, only different. Front. Microbiol. 2016, 7, 1054. [Google Scholar] [CrossRef]
- Osimani, A.; Milanović, V.; Garofalo, C.; Cardinali, F.; Roncolini, A.; Sabbatini, R.; Filippis, F.D.; Ercolini, D.; Gabucci, C.; Petruzzelli, A.; et al. Revealing the microbiota of marketed edible insects through PCR-DGGE, metagenomic sequencing and real-time PCR. Int. J. Food Microbiol. 2018, 276, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Stoops, J.; Crauwels, S.; Waud, M.; Claes, J.; Lievens, B.; Van Campenhout, L. Microbial community assessment of mealworm larvae (Tenebrio molitor) and grasshoppers (Locusta migratoria migratorioides) sold for human consumption. Food Microbiol. 2016, 53, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Trakulchang, S.P.; Kraft, A.A. Survival of Clostridium perfringens in refrigerated and frozen meat and poultry items. J. Food Sci. 1977, 42, 518–521. [Google Scholar] [CrossRef]
- Mujuru, F.M.; Kwiri, R.; Nyambi, C.; Winini, C.; Moyo, D.N. Microbiological quality of Gonimbrasia belina processed under different traditional practices in Gwanda, Zimbabwe. Food Control 2014, 43, 181–186. [Google Scholar]
- Mugo-Kamiri, L.; Imungi, J.K.; Njue, L.; Diiro, G.; Ombura, F.L.O.; Akutse, K.S.; Chrysantus, T.M.; Khamis, F.M.; Subramanian, S. Vendors’ handling practices of edible long-horned grasshoppers (Ruspolia differens) products and implications on microbial safety. Front. Microbiol. 2024, 15, 138543. [Google Scholar] [CrossRef]
- Mohd Zaini, N.S.; Lim, E.J.; Ahmad, N.H.; Gengatharan, A.; Wan-Mohtar, W.A.A.Q.I.; Abd Rahim, M.H. The review of cooking, drying, and green extraction methods on general nutritional properties of mealworms and locusts. Food Bioprocess Technol. 2023, 16, 1904–1918. [Google Scholar] [CrossRef]
- Mancini, S.; Fratini, F.; Tuccinardi, T.; Degl’Innocenti, C.; Paci, G. Tenebrio molitor reared on different substrates: Is it gluten free? Food Control 2020, 110, 107014. [Google Scholar] [CrossRef]
- Żuk-Gołaszewska, K.; Gałęcki, R.; Obremski, K.; Smetana, S.; Figiel, S.; Gołaszewski, J. Edible insect farming in the context of the EU regulations and marketing—An overview. Insects 2022, 13, 446. [Google Scholar] [CrossRef]
- Bharti, R.; Grimm, D.G. Current challenges and best-practice protocols for microbiome analysis. Brief. Bioinform. 2021, 22, 178–193. [Google Scholar] [CrossRef]

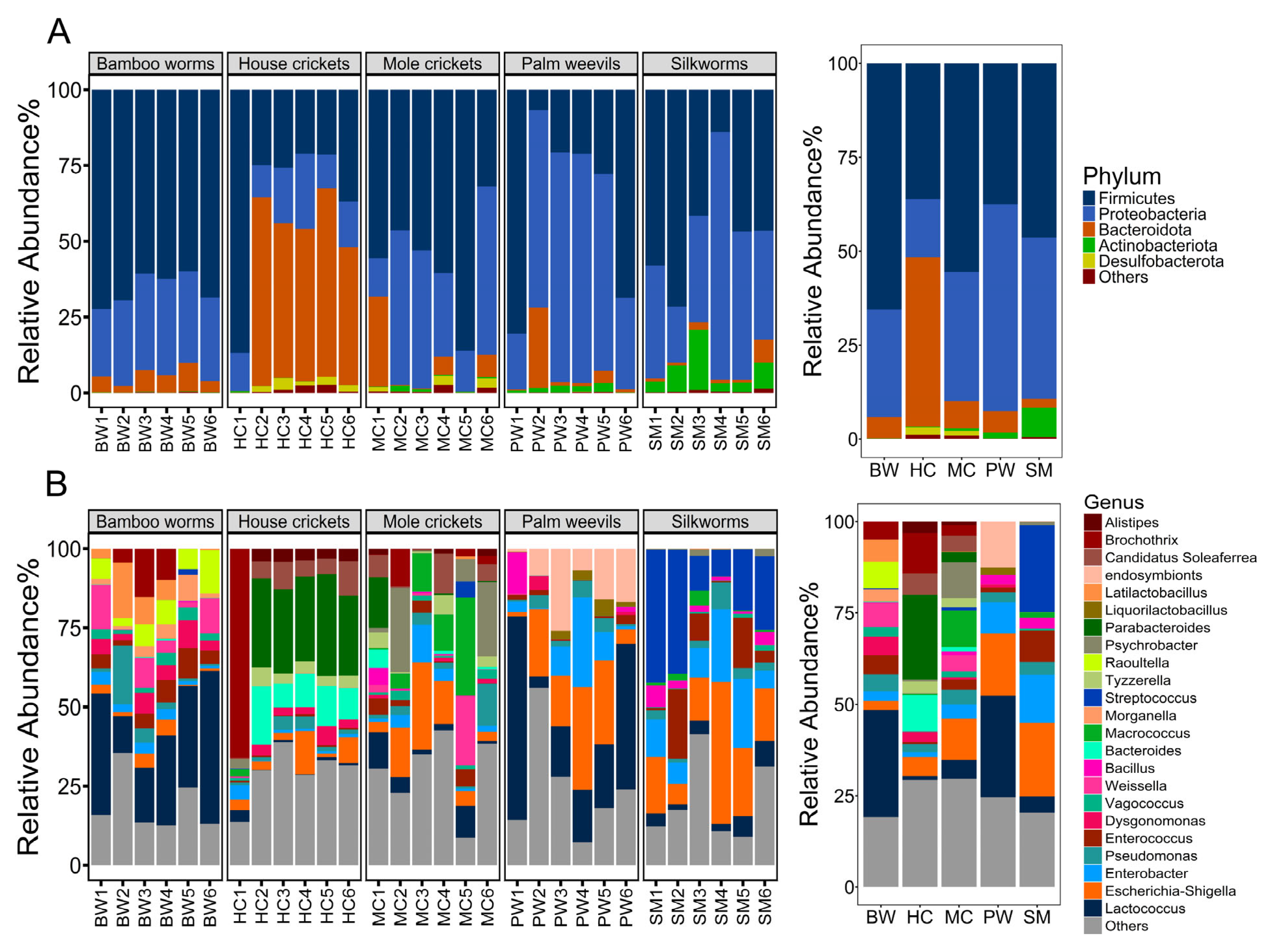
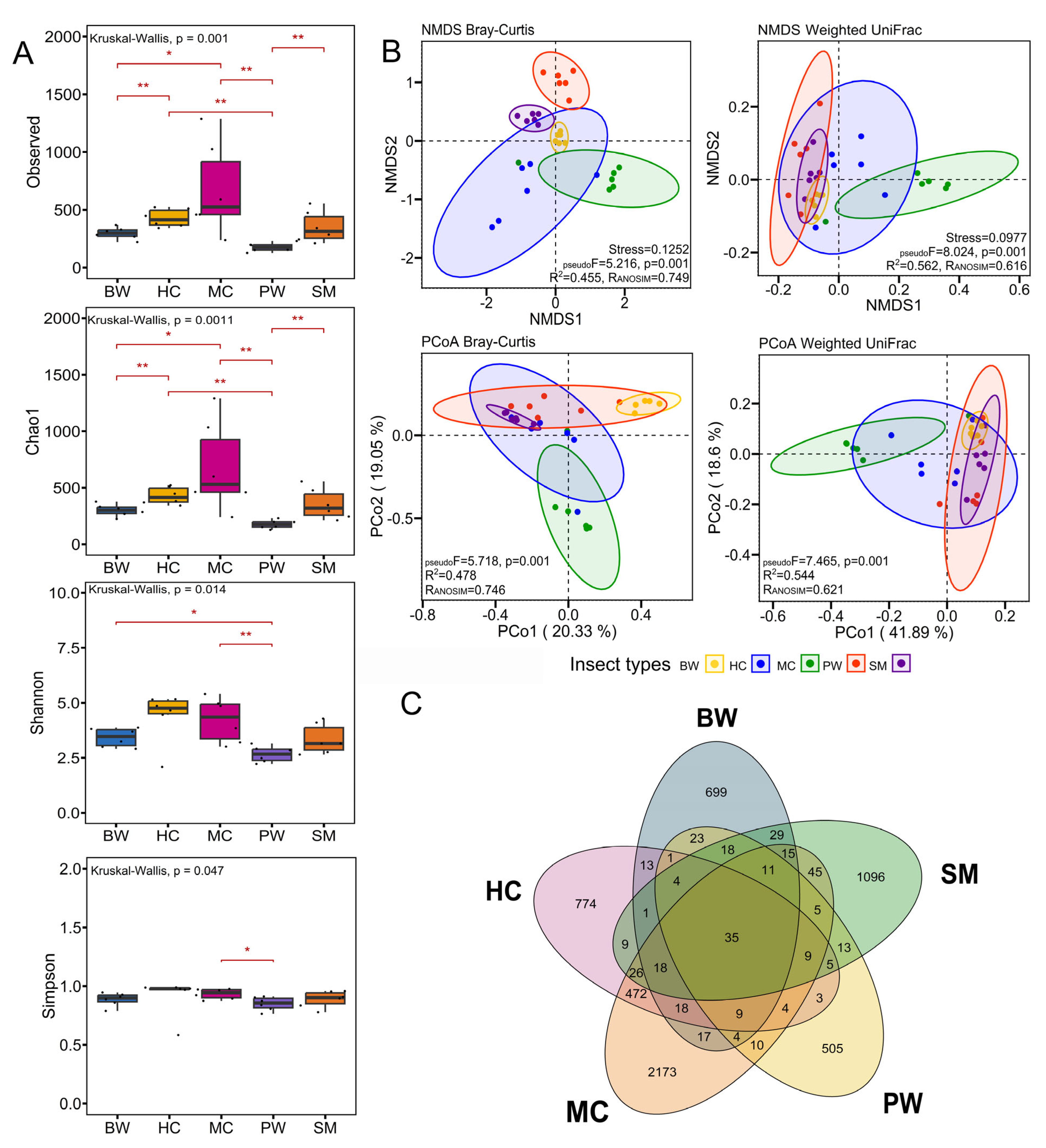
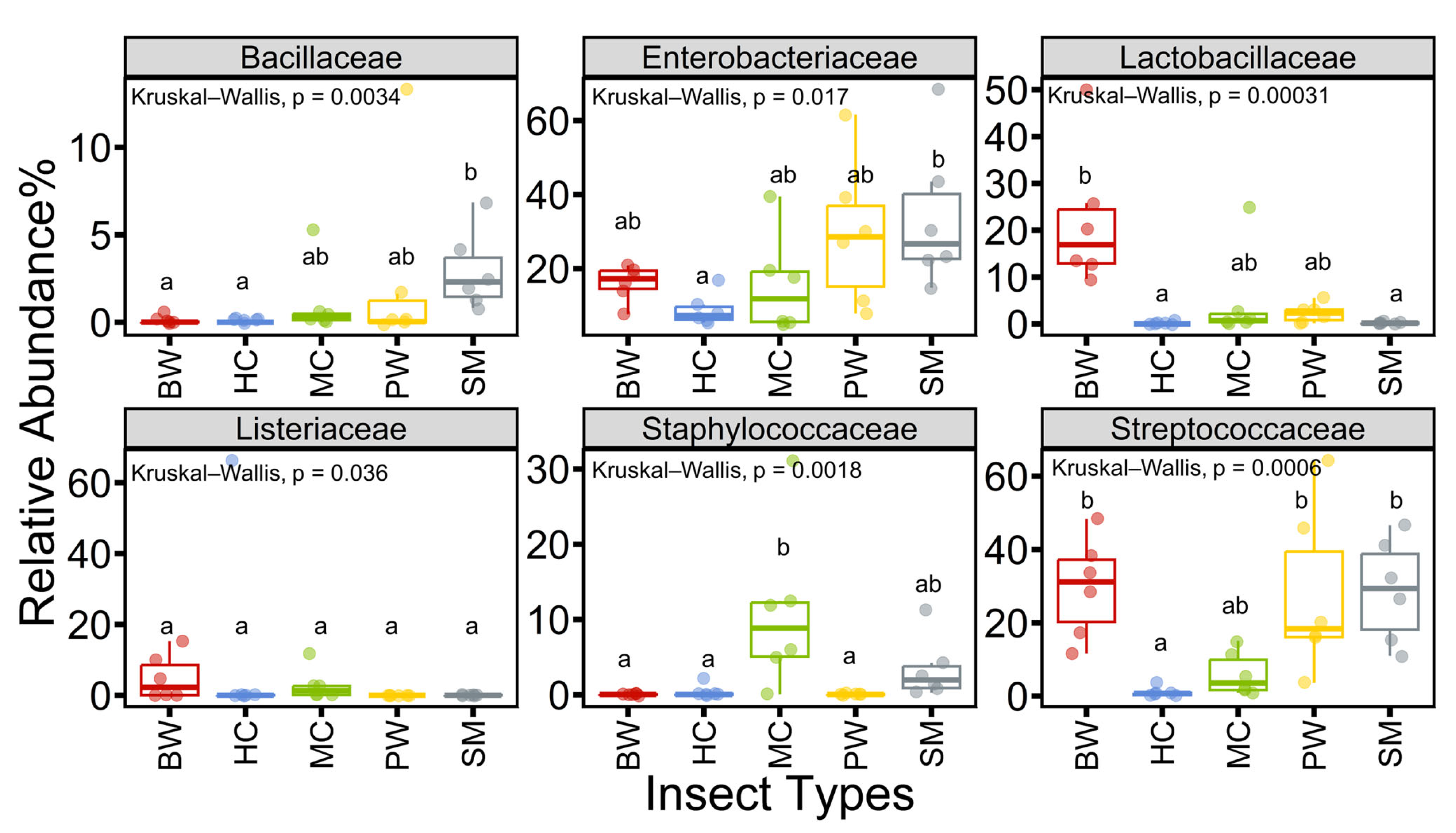
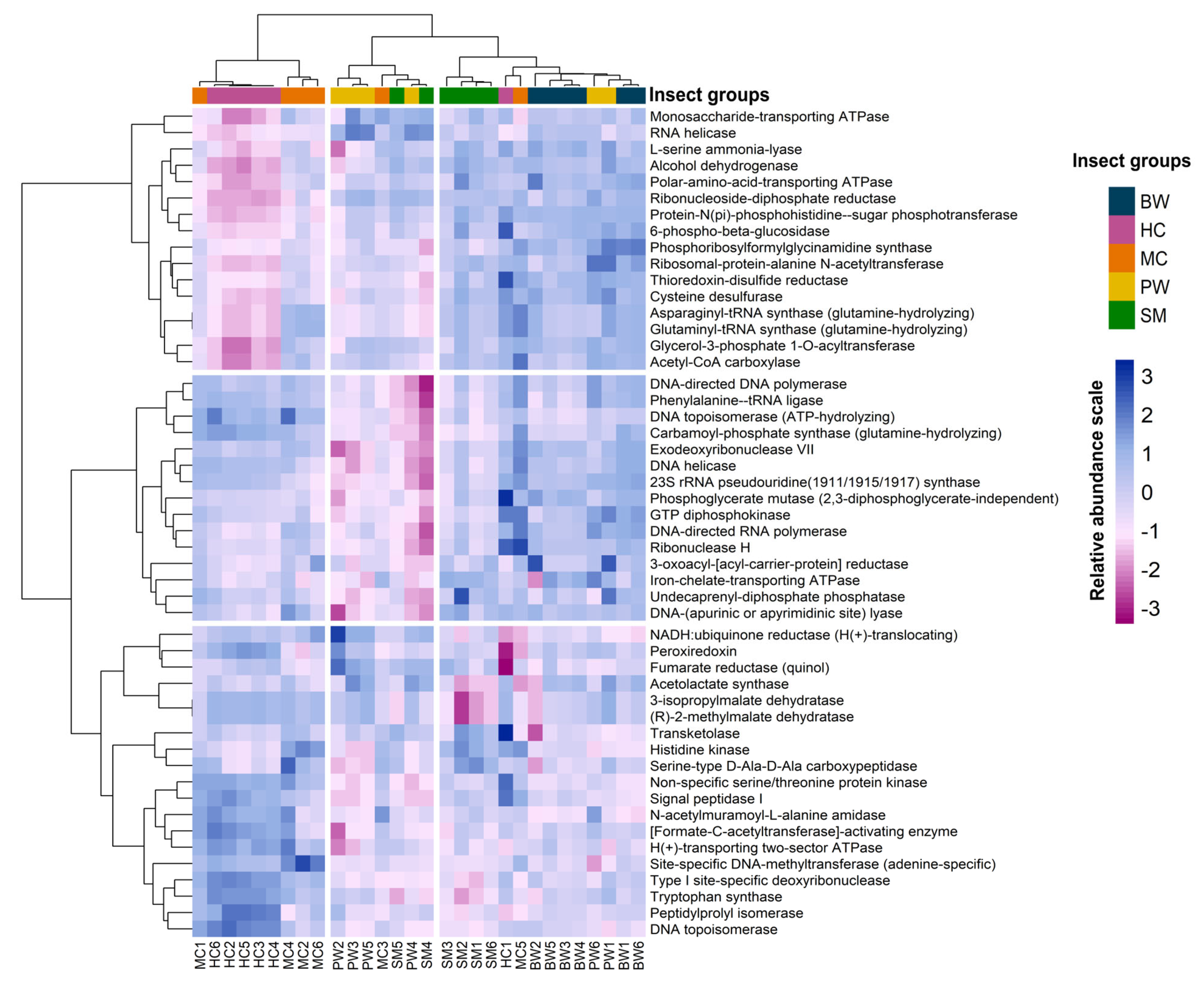
| Insect Group | Relative Abundance (%) | |||||
|---|---|---|---|---|---|---|
| Firmicutes | Proteobacteria | Bacteroidota | Actinobacteriota | Desulfobacterota | Others | |
| BW | 65.60 ± 5.20 | 28.60 ± 3.59 ab | 5.58 ± 2.56 ab | 0.09 ± 0.05 a | 0.04 ± 0.02 ab | 0.10 ± 0.10 a |
| HC | 36.10 ± 25.50 | 15.40 ± 5.45 a | 45.2 ± 23.1 b | 0.11 ± 0.17 a | 2.01 ± 1.30 a | 0.18 ± 1.14 b |
| MC | 55.60 ± 19.90 | 34.30 ± 18.90 b | 7.26 ± 11.4 a | 0.65 ± 0.66 ab | 1.29 ± 1.48 a | 0.90 ± 1.02 b |
| PW | 37.60 ± 29.70 | 55.00 ± 24.50 b | 5.71 ± 10.3 a | 1.58 ± 1.03 bc | 0.03 ± 0.04b c | 0.09 ± 0.11 a |
| SM | 46.40 ± 19.20 | 42.90 ± 21.40 b | 2.35 ± 2.62 a | 7.79 ± 6.51 c | 0.00 ± 0.00 c | 0.56 ± 0.50 b |
| BW | HC | MC | PW | SM | |||||
|---|---|---|---|---|---|---|---|---|---|
| Genus | % Relative Abundance | Genus | % Relative Abundance | Genus | % Relative Abundance | Genus | % Relative Abundance | Genus | % Relative Abundance |
| Lactococcus | 29.37 ± 13.43 | Others | 29.31 ± 8.43 | Others | 29.66 ± 12.34 | Lactococcus | 27.73 ± 22.68 | Streptococcus | 23.92 ± 14.11 |
| Others | 19.14 ± 9.18 | Parabacter-oides | 23.21 ± 11.62 | Escherichia-Shigella | 11.28 ± 9.65 | Others | 24.60 ± 17.01 | Others | 20.33 ± 13.12 |
| Raoultella | 7.28 ± 3.66 | Bacteroides | 9.80 ± 6.03 | Macrococcus | 9.96 ± 11.55 | Escherichia-Shigella | 17.01 ± 12.22 | Escherichia-Shigella | 20.15 ± 13.09 |
| Weissella | 6.72 ± 5.60 | Candidatus Soleaferrea | 5.89 ± 3.65 | Psychrob-acter | 9.82 ± 12.12 | Endosymb-ionts | 12.54 ± 8.90 | Enterobacter | 13.06 ± 7.57 |
| Latilactob-acillus | 6.05 ± 6.56 | Alistipes | 3.14 ± 1.57 | Lactococcus | 5.17 ± 4.60 | Enterobacter | 8.55 ± 10.47 | Enterococcus | 8.53 ± 8.77 |
| Enterococcus | 5.24 ± 2.66 | Tyzzerella | 3.28 ± 1.90 | Candidatus Soleaferrea | 4.36 ± 5.02 | Pseudomonas | 2.78 ± 2.05 | Lactococcus | 4.5 ± 2.46 |
| Dysgonom-onas | 5.02 ± 2.40 | Escherichia-Shigella | 5.23 ± 4.83 | Weissella | 4.32 ± 8.64 | Bacillus | 2.47 ± 5.26 | Pseudom-onas | 3.47 ± 2.55 |
| Brochothrix | 4.94 ± 6.38 | Dysgonom-onas | 2.77 ± 1.95 | Pseudomonas | 3.96 ± 4.71 | Liquorilactobacillus | 2.11 ± 1.97 | Bacillus | 2.9 ± 2.23 |
| Pseudomonas | 4.59 ± 6.93 | Pseudomonas | 2.15 ± 1.55 | Enterobacter | 3.90 ± 4.12 | Enterococcus | 1.21 ± 1.03 | Macrococcus | 1.44 ± 1.75 |
| Morganella | 3.36 ± 2.73 | Enterobacter | 1.40 ± 1.58 | Brochothrix | 2.86 ± 4.57 | Dysgonom-onas | 0.91 ± 1.79 | Psychrob-acter | 0.88 ± 1.02 |
| Type of Frozen Edible Insect | Source of Sample | General Quality Indicator | Hygiene Indicator | Bacterial Pathogen | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Viable Count (CFU/g) | Lactic Acid Bacteria (CFU/g) | Yeasts and Molds (CFU/g) | Spore-Forming Bacteria (CFU/g) | Enterob-acteriaceae (CFU/g) | E. coli (CFU/g) | Presumptive B. cereus (CFU/g) | C. perfringens (CFU/g) | S. aureus (CFU/g) | Salmonella (in 25 g) | ||
| Bamboo worms | Store A | 6.60 × 104 | 4.41 × 105 | 4.67 × 102 | 1.25 × 102 | <1.00 × 101 | <1.00 × 101 | <5.00 × 101 | <5.00 × 101 | <5.00 × 101 | ND |
| Store B | 3.80 × 104 | 3.03 × 106 | 4.20 × 105 | 1.25 × 102 | 1.70 × 102 | <1.00 × 101 | <5.00 × 101 | <5.00 × 101 | <5.00 × 101 | ND | |
| Store C | 4.26 × 104 | 2.86 × 105 | 3.13 × 104 | 3.50 × 102 | 7.00 × 101 | <1.00 × 101 | 5.00 × 101 | <5.00 × 101 | <5.00 × 101 | ND | |
| Store D | 1.53 × 105 | 3.78 × 105 | 2.16 × 104 | 1.00 × 102 | 8.00 × 101 | <1.00 × 101 | <5.00 × 101 | <5.00 × 101 | <5.00 × 101 | ND | |
| Store E | 3.30 × 105 | 2.73 × 106 | 3.56 × 104 | 1.00 × 102 | 3.35 × 104 | <1.00 × 101 | <5.00 × 101 | <5.00 × 101 | <5.00 × 101 | ND | |
| Store G | 6.00 × 103 | 4.46 × 105 | 6.50 × 104 | 4.25 × 102 | 6.00 × 101 | <1.00 × 101 | 1.50 × 102 | <5.00 × 101 | <5.00 × 101 | ND | |
| House cricket | Store A | 3.00 × 104 | 3.40 × 106 | 1.00 × 102 | 3.00 × 102 | <1.00 × 101 | <1.00 × 101 | 5.00 × 102 | <5.00 × 101 | <5.00 × 101 | ND |
| Store B | 6.96 × 103 | 1.30× 104 | 3.33 × 102 | 6.50 × 102 | <1.00 × 101 | <1.00 × 101 | <5.00 × 101 | <5.00 × 101 | <5.00 × 101 | ND | |
| Store C | 5.30 × 103 | 4.43 × 104 | 6.70 × 101 | 1.00 × 102 | <1.00 × 101 | <1.00 × 101 | <5.00 × 101 | <5.00 × 101 | <5.00 × 101 | ND | |
| Store D | 5.80 × 102 | 6.67 × 102 | 1.20 × 104 | 4.50 × 102 | <1.00 × 101 | <1.00 × 101 | <5.00 × 101 | <5.00 × 101 | <5.00 × 101 | ND | |
| Store E | 1.70 × 103 | 1.53 × 103 | 2.66 × 103 | 1.00 × 102 | <1.00 × 101 | <1.00 × 101 | <5.00 × 101 | <5.00 × 101 | <5.00 × 101 | ND | |
| Store F | 2.60 × 102 | 6.67 × 102 | 6.33 × 103 | 1.00 × 102 | <1.00 × 101 | <1.00 × 101 | <5.00 × 101 | <5.00 × 101 | <5.00 × 101 | ND | |
| Mole crickets | Store B | 1.31 × 104 | 6.35 × 104 | 2.16 × 104 | 1.40 × 104 | <1.00 × 101 | <1.00 × 101 | 5.93 × 103 | <5.00 × 101 | <5.00 × 101 | ND |
| Store C | 4.27 × 104 | 1.51 × 105 | 1.23 × 104 | 5.05 × 104 | 1.50 × 101 | <1.00 × 101 | 1.50 × 103 | <5.00 × 101 | <5.00 × 101 | ND | |
| Store D | 3.30 × 103 | 1.10 × 104 | 8.66 × 103 | 8.50 × 102 | <1.00 × 101 | <1.00 × 101 | <5.00 × 101 | <5.00 × 101 | <5.00 × 101 | ND | |
| Store E | 3.23 × 104 | 3.59 × 105 | 9.67 × 102 | 2.45 × 104 | 2.15 × 103 | <1.00 × 101 | 7.95 × 103 | <5.00 × 101 | <5.00 × 101 | ND | |
| Store F | 1.70 × 104 | 1.60 × 105 | 2.00 × 103 | 1.15 × 103 | 5.10 × 102 | <1.00 × 101 | <5.00 × 101 | <5.00 × 101 | <5.00 × 101 | ND | |
| Store G | 4.91 × 104 | 1.36 × 105 | 1.06 × 103 | 6.00 × 103 | 1.50 × 101 | <1.00 × 101 | 7.50 × 103 | <5.00 × 101 | <5.00 × 101 | ND | |
| Palm weevil larvae | Store A | 7.43 × 104 | 1.26 × 107 | 2.46 × 103 | 4.00 × 102 | 1.11 × 105 | 5.05 × 102 | <5.00 × 101 | <5.00 × 101 | <5.00 × 101 | ND |
| Store D | 4.42 × 103 | 3.33 × 102 | 6.00 × 103 | 2.50 × 102 | <1.00 × 101 | <1.00 × 101 | 2.50 × 101 | <5.00 × 101 | <5.00 × 101 | ND | |
| Store E | 1.91 × 104 | 3.63 × 105 | 1.70 × 104 | 3.60 × 103 | <1.00 × 101 | <1.00 × 101 | 2.06 × 103 | <5.00 × 101 | <5.00 × 101 | ND | |
| Store F | 1.25 × 104 | 4.34 × 104 | 1.40 × 104 | 7.25 × 103 | 7.25 × 102 | <1.00 × 101 | 7.50 × 101 | <5.00 × 101 | <5.00 × 101 | ND | |
| Store H | 6.33 × 102 | 1.20 × 103 | 1.10 × 104 | 3.30 × 103 | <1.00 × 101 | <1.00 × 101 | 2.25 × 102 | <5.00 × 101 | <5.00 × 101 | ND | |
| Store I | 2.93 × 105 | 3.87 × 105 | 5.33 × 103 | 6.00 × 102 | <1.00 × 101 | <1.00 × 101 | 2.50 × 101 | <5.00 × 101 | <5.00 × 101 | ND | |
| Silkworms | Store A | 1.98 × 104 | 2.48 × 104 | 1.33 × 103 | 1.20 × 103 | <1.00 × 101 | <1.00 × 101 | 8.56 × 103 | <5.00 × 101 | <5.00 × 101 | ND |
| Store B | 2.79 × 105 | 6.46 × 105 | 6.67 × 102 | 3.50 × 102 | 1.35 × 102 | <1.00 × 101 | 1.36 × 103 | <5.00 × 101 | <5.00 × 101 | ND | |
| Store C | 4.70 × 104 | 5.76 × 104 | 2.00 × 103 | 2.10 × 103 | 2.50 × 101 | <1.00 × 101 | 7.25 × 103 | <5.00 × 101 | <5.00 × 101 | ND | |
| Store D | 3.77 × 106 | 2.59 × 107 | 2.26 × 104 | 3.00 × 102 | 2.50 × 101 | <1.00 × 101 | 1.33 × 103 | <5.00 × 101 | <5.00 × 101 | ND | |
| Store E | 1.26 × 105 | 2.15 × 105 | 1.60 × 104 | 3.50 × 102 | 2.90 × 102 | <1.00 × 101 | 1.97 × 103 | <5.00 × 101 | <5.00 × 101 | ND | |
| Store G | 1.11 × 105 | 1.15 × 105 | 2.30 × 103 | 6.50 × 102 | <1.00 × 101 | <1.00 × 101 | 5.18 × 103 | <5.00 × 101 | <5.00 × 101 | ND | |
| Microbiological Analysis | Results of Quantitative Analysis (Mean of log CFU/g (Range)) * or Detection | p-Value ** | Microbiological Limit (log CFU/g) *** | ||||
|---|---|---|---|---|---|---|---|
| BW | HC | MC | PW | SM | |||
| General quality indicator | |||||||
| Total viable count | 4.75 ± 0.59 ab | 3.41 ± 0.76 b | 4.28 ± 0.44 ab | 4.20 ± 0.93 ab | 5.19 ± 0.79 a | 0.0103 | ≤5.00 |
| (3.78–5.52) | (2.41–4.48) | (3.52–4.69) | (2.80–5.47) | (4.30–6.58) | |||
| Lactic acid bacteria | 5.87 ± 0.46 | 4.02 ± 1.43 | 4.99 ± 0.52 | 4.75 ± 1.71 | 5.46 ± 1.07 | 0.0789 | NA |
| (5.46–6.48) | (2.82–6.54) | (4.04–5.56) | (2.52–7.10) | (4.40–7.41) | |||
| Yeasts and molds | 4.41 ± 0.97 | 2.94 ± 0.96 | 3.61 ± 0.58 | 3.89 ± 0.31 | 3.53 ± 0.61 | 0.0559 | ≤2.00 |
| (2.67–5.62) | (1.83–4.08) | (2.99–4.33) | (3.39–4.23) | (2.82–4.36) | |||
| Spore forming bacteria | 2.23 ± 0.28 b | 2.32 ± 0.37 b | 3.84 ± 0.72 a | 3.12 ± 0.6 ab | 2.80 ± 0.34 ab | 0.0016 | NA |
| (2.00–2.63) | (2.00–2.81) | (2.93–4.70) | (2.40–3.86) | (2.48–3.32) | |||
| Hygiene indicator | |||||||
| Enterobacteriaceae | <1.00–4.53 | <1.00 | <1.00–3.33 | <1.00–5.05 | <1.00–2.46 | 0.1000 | ≤2.00 (or <2.00) |
| Escherichia coli | <1.00 | <1.00 | <1.00 | <1.00–2.70 | <1.00 | 0.4060 | ≤1.70 |
| Bacterial pathogen | |||||||
| Presumptive Bacillus cereus | <1.70–2.18 c | <1.70–2.70 bc | <1.70–3.90 ab | <1.70–3.32 bc | 3.12–3.93 a | 0.0063 | ≤2.00 |
| Clostridium perfringens | <1.70 | <1.70 | <1.70 | <1.70 | <1.70 | NA | NA |
| Staphylococcus aureus | <1.70 | <1.70 | <1.70 | <1.70 | <1.70 | NA | NA |
| Salmonella (in 25 g) | ND | ND | ND | ND | ND | NA | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krongdang, S.; Sawongta, N.; Pheepakpraw, J.; Ngamsomchat, A.; Wangtueai, S.; Wongsa, J.; Parametthanuwat, T.; Charoenphun, N.; Chitov, T. Comprehensive Analysis of Bacterial Communities and Microbiological Quality of Frozen Edible Insects. Foods 2025, 14, 2347. https://doi.org/10.3390/foods14132347
Krongdang S, Sawongta N, Pheepakpraw J, Ngamsomchat A, Wangtueai S, Wongsa J, Parametthanuwat T, Charoenphun N, Chitov T. Comprehensive Analysis of Bacterial Communities and Microbiological Quality of Frozen Edible Insects. Foods. 2025; 14(13):2347. https://doi.org/10.3390/foods14132347
Chicago/Turabian StyleKrongdang, Sasiprapa, Nipitpong Sawongta, Jintana Pheepakpraw, Achirawit Ngamsomchat, Sutee Wangtueai, Jittimon Wongsa, Thanya Parametthanuwat, Narin Charoenphun, and Thararat Chitov. 2025. "Comprehensive Analysis of Bacterial Communities and Microbiological Quality of Frozen Edible Insects" Foods 14, no. 13: 2347. https://doi.org/10.3390/foods14132347
APA StyleKrongdang, S., Sawongta, N., Pheepakpraw, J., Ngamsomchat, A., Wangtueai, S., Wongsa, J., Parametthanuwat, T., Charoenphun, N., & Chitov, T. (2025). Comprehensive Analysis of Bacterial Communities and Microbiological Quality of Frozen Edible Insects. Foods, 14(13), 2347. https://doi.org/10.3390/foods14132347












