Physicochemical Properties and Volatile Profile of Chito: A Traditional Dry-Cured Goat Meat Product
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Analysis of the Meat Product
2.2.1. Physicochemical Analysis
2.2.2. Color Measurement
2.2.3. Metmyoglobin (MMb) and Heme Iron Content
2.2.4. Instrumental Texture Measurement
2.2.5. Microbiological Analysis
2.2.6. Analysis of Volatile Compounds
2.2.7. Odor Activity Value (OAV) Analysis
2.3. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics
3.2. Instrumental Color and Metmyoglobin Content
3.3. Texture Evaluation
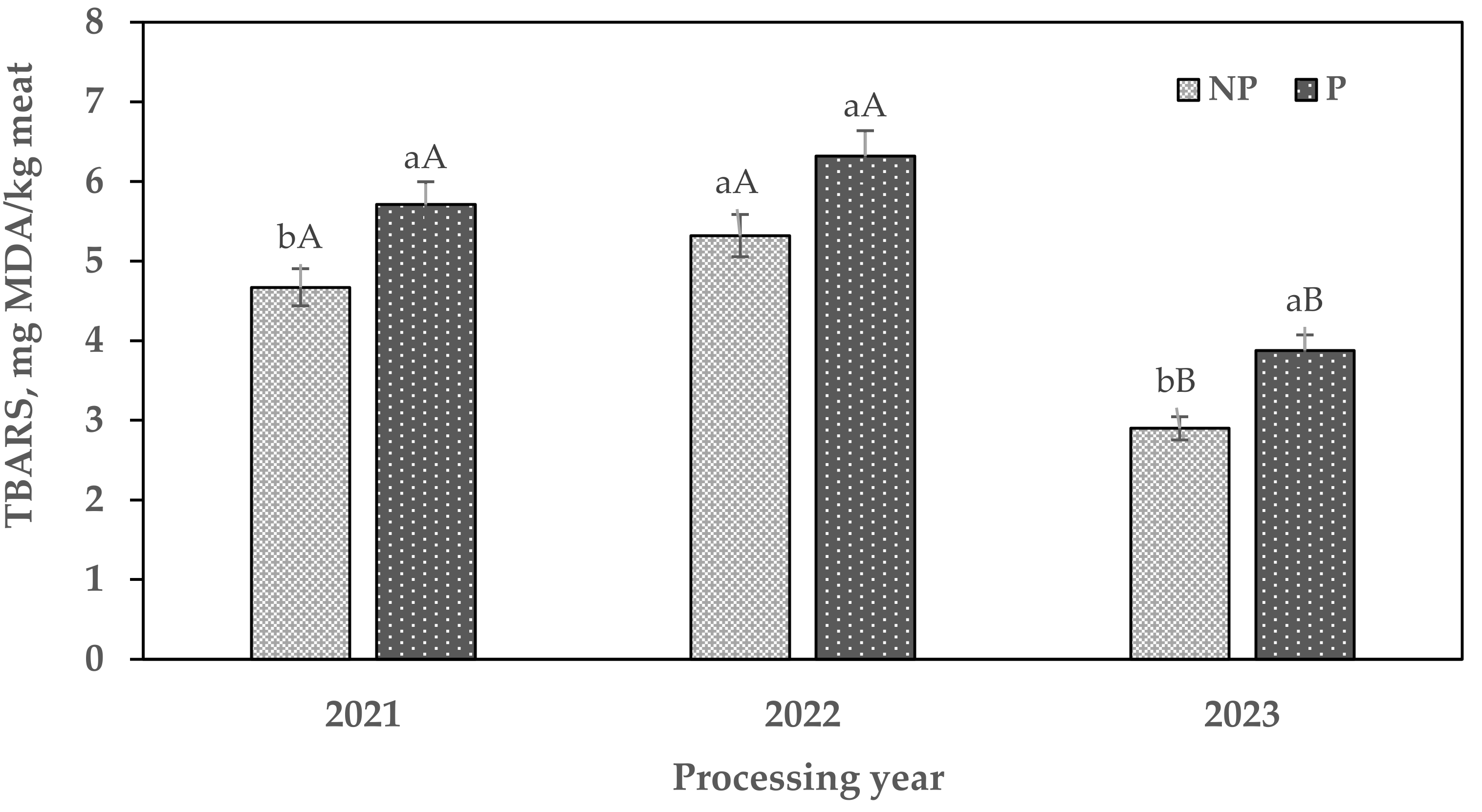
3.4. Lipid Oxidation
3.5. Microbial Counts
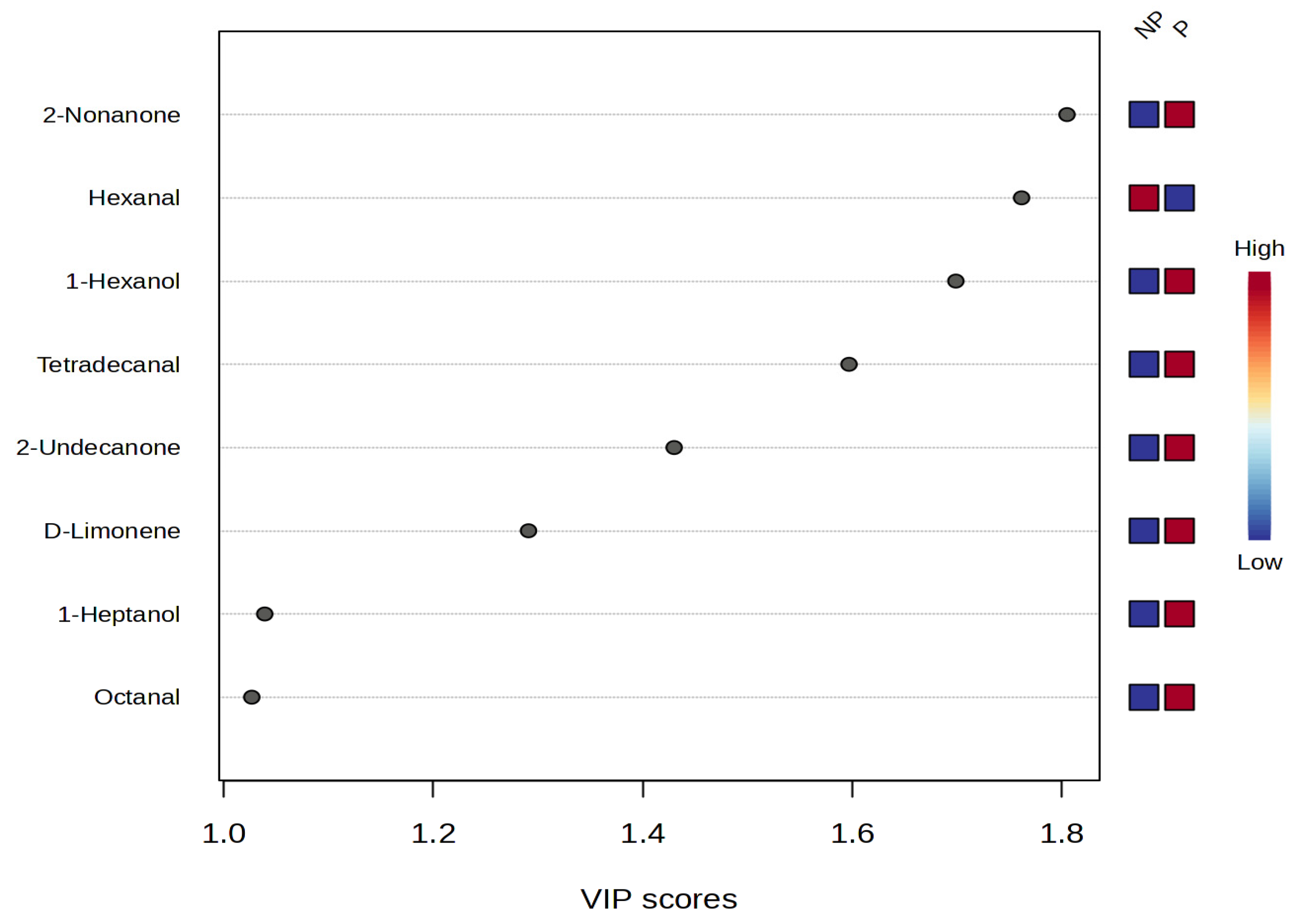
3.6. Volatile Compounds
3.7. Odor Activity Value (OAV)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petit, T.; Caro, Y.; Petit, A.S.; Santchurn, S.J.; Collignan, A. Physicochemical and Microbiological Characteristics of Biltong, a Traditional Salted Dried Meat of South Africa. Meat Sci. 2014, 96, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Molinero, C.; Martínez, B.; Rubio, B.; Rovira, J.; Jaime, I. The Effects of Extended Curing on the Microbiological, Physicochemical and Sensorial Characteristics of Cecina de León. Meat Sci. 2008, 80, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Hierro, E.; De La Hoz, L.; Ordóñez, J.A. Headspace Volatile Compounds from Salted and Occasionally Smoked Dried Meats (Cecinas) as Affected by Animal Species. Food Chem. 2004, 85, 649–657. [Google Scholar] [CrossRef]
- Mediani, A.; Hamezah, H.S.; Jam, F.A.; Mahadi, N.F.; Chan, S.X.Y.; Rohani, E.R.; Che Lah, N.H.; Azlan, U.K.; Khairul Annuar, N.A.; Azman, N.A.F.; et al. A Comprehensive Review of Drying Meat Products and the Associated Effects and Changes. Front. Nutr. 2022, 9, 1057366. [Google Scholar] [CrossRef]
- Reyes-Cano, R.; Dorantes-Alvarez, L.; Hernandez-Sanchez, H.; Gutierrez-Lopez, G.F. A Traditional Intermediate Moisture Meat: Beef Cecina. Meat Sci. 1994, 36, 365–370. [Google Scholar] [CrossRef]
- Paleari, M.A.; Moretti, V.M.; Beretta, G.; Caprino, F. Chemical Parameters, Fatty Acids and Volatile Compounds of Salted and Ripened Goat Thigh. Small Rumin. Res. 2008, 74, 140–148. [Google Scholar] [CrossRef]
- ISO 1841-1:1996; Meat and Meat Products–Determination of Chloride Content–Part 1: Volhard Method. International Standards Organization: Geneva, Switzerland, 1996.
- AOAC. Official Methods of Analysis of the AOAC International, 16th ed.; Cunniff, P., Ed.; AOAC International: Gaithersburg, MD, USA, 1999; Volume II. [Google Scholar]
- Villalobos-Delgado, L.H.; Caro, I.; Blanco, C.; Morán, L.; Prieto, N.; Bodas, R.; Giráldez, F.J.; Mateo, J. Quality Characteristics of a Dry-Cured Lamb Leg as Affected by Tumbling after Dry-Salting and Processing Time. Meat Sci. 2014, 97, 115–122. [Google Scholar] [CrossRef]
- Villalobos-Delgado, L.H.; González-Mondragón, E.G.; Salazar Govea, A.Y.; Andrade, J.R.; Santiago-Castro, J.T. Potential Application of Epazote (Chenopodium Ambrosioides L.) as Natural Antioxidant in Raw Ground Pork. LWT 2017, 84, 306–313. [Google Scholar] [CrossRef]
- American Meat Science Association Meat Color Measurement Guidelines: AMSA; AMSA: Champaign, IL, USA, 2012; Available online: https://meatscience.org/docs/default-source/publications-resources/hot-topics/2012_12_meat_clr_guide.pdf (accessed on 9 October 2022).
- Shi, S.; Zhao, M.; Li, Y.; Kong, B.; Liu, Q.; Sun, F.; Yu, W.; Xia, X. Effect of Hot Air Gradient Drying on Quality and Appearance of Beef Jerky. LWT 2021, 150, 111974. [Google Scholar] [CrossRef]
- Cheng, J.H.; Ockerman, H.W. Effect of Phosphate with Tumbling on Lipid Oxidation of Precooked Roast Beef. Meat Sci. 2003, 65, 1353–1359. [Google Scholar] [CrossRef]
- Bourne, M.C. Texture Profile Analysis. Food Technol. 1978, 32, 62–66. [Google Scholar]
- Sánchez-Gamboa, C.; Hicks-Pérez, L.; Gutiérrez-Méndez, N.; Heredia, N.; García, S.; Nevárez-Moorillón, G.V. Seasonal Influence on the Microbial Profile of Chihuahua Cheese Manufactured from Raw Milk. Int. J. Dairy Technol. 2018, 71, 81–89. [Google Scholar] [CrossRef]
- NOM-210-SSA1-2014; Productos y Servicios. Métodos de Prueba Microbiológicos. Determinación de Microorganismos Indicadores. Determinación de Microorganismos Patógenos. Diario Oficial de la Federación: Mexico City, Mexico, 2014.
- Pavlidis, D.E.; Mallouchos, A.; Ercolini, D.; Panagou, E.Z.; Nychas, G.J.E. A Volatilomics Approach for Off-Line Discrimination of Minced Beef and Pork Meat and Their Admixture Using HS-SPME GC/MS in Tandem with Multivariate Data Analysis. Meat Sci. 2019, 151, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xu, Y.; Wang, H.; Bai, T.; Wang, X.; Liu, D.; Zhang, Y.; Zhang, L.; Zhang, J. Investigation of Meat Quality, Volatilome, and Fatty Acid Composition of Meat Parts from Liangshan Semi-Fine Wool Sheep. Vet. Sci. 2025, 12, 591. [Google Scholar] [CrossRef]
- Şayin Sert, T.; Coşkun, F. The Effects of High-Pressure Processing on PH, Thiobarbituric Acid Value, Color and Texture Properties of Frozen and Unfrozen Beef Mince. Molecules 2022, 27, 3974. [Google Scholar] [CrossRef]
- Salvá, B.K.; Fernández-Diez, A.; Ramos, D.D.; Caro, I.; Mateo, J. Chemical Composition of Alpaca (Vicugna pacos) Charqui. Food Chem. 2012, 130, 329–334. [Google Scholar] [CrossRef]
- Teixeira, A.; Fernandes, A.; Pereira, E.; Manuel, A.; Rodrigues, S. Effect of Salting and Ripening on the Physicochemical and Sensory Quality of Goat and Sheep Cured Legs. Meat Sci. 2017, 134, 163–169. [Google Scholar] [CrossRef]
- Zdanowska-Sasiadek, Z.; Marchewka, J.; Horbanczuk, J.O.; Wierzbicka, A.; Lipinska, P.; Józwik, A.; Atanasov, A.G.; Huminiecki, Ł.; Sieron, A.; Sieron, K.; et al. Nutrients Composition in Fit Snacks Made from Ostrich, Beef and Chicken Dried Meat. Molecules 2018, 23, 1267. [Google Scholar] [CrossRef]
- Ortega, A.; Chito, D.; Teixeira, A. Comparative Evaluation of Physical Parameters of Salted Goat and Sheep Meat Blankets “Mantas” from Northeastern Portugal. J. Food Meas. Charact. 2016, 10, 670–675. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.; Lu, S.; Wang, J.; Fu, H.; Gu, B.; Lyu, B.; Wang, Q. Changes in Proteolysis, Protein Oxidation, Flavor, Color and Texture of Dry-Cured Mutton Ham during Storage. LWT 2021, 149, 111860. [Google Scholar] [CrossRef]
- Aykın-Dinçer, E.; Erbaş, M. Quality Characteristics of Cold-Dried Beef Slices. Meat Sci. 2019, 155, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Gómez, I.; Janardhanan, R.; Ibañez, F.C.; Beriain, M.J. The Effects of Processing and Preservation Technologies on Meat Quality: Sensory and Nutritional Aspects. Foods 2020, 9, 1416. [Google Scholar] [CrossRef] [PubMed]
- Aktaş, N.; Aksu, M.I.; Kaya, M. Changes in Myofibrillar Proteins during Processing of Pastirma (Turkish Dry Meat Product) Produced with Commercial Starter Cultures. Food Chem. 2005, 90, 649–654. [Google Scholar] [CrossRef]
- Bermúdez, R.; Franco, D.; Carballo, J.; Lorenzo, J.M. Physicochemical Changes during Manufacture and Final Sensory Characteristics of Dry-Cured Celta Ham. Effect of Muscle Type. Food Control. 2014, 43, 263–269. [Google Scholar] [CrossRef]
- Kim, J.; Kim, T.K.; Cha, J.Y.; Ku, S.K.; Jung, S.; Choi, Y.S. Effect of Drying Methods on Physicochemical Characteristics and Functional Properties of Duck Blood Gel. Food Sci. Anim. Resour. 2022, 42, 861–873. [Google Scholar] [CrossRef]
- Oliveira, A.F.; Rodrigues, S.; Leite, A.; Paulos, K.; Pereira, E.; Teixeira, A. Short Communication: Quality of Ewe and Goat Meat Cured Product Mantas. An Approach to Provide Value Added to Culled Animals. Can. J. Anim. Sci. 2014, 94, 459–462. [Google Scholar] [CrossRef]
- Campo, M.M.; Nute, G.R.; Hughes, S.I.; Enser, M.; Wood, J.D.; Richardson, R.I. Flavour Perception of Oxidation in Beef. Meat Sci. 2006, 72, 303–311. [Google Scholar] [CrossRef]
- Feiner, G. The Protein and Fat Content of Meat. In Meat Products Handbook; Elsevier: Cambridge, UK, 2006; pp. 3–32. [Google Scholar] [CrossRef]
- Sampels, S.; Pickova, J.; Wiklund, E. Fatty Acids, Antioxidants and Oxidation Stability of Processed Reindeer Meat. Meat Sci. 2004, 67, 523–532. [Google Scholar] [CrossRef]
- Vilar, I.; Garcia Fontan, M.C.; Prieto, B.; Tornadijo, M.E.; Carballo, J. A Survey on the Microbiological Changes during the Manufacture of Dry-Cured Lacon, a Spanish Traditional Meat Product. J. Appl. Microbiol. 2000, 89, 1018–1026. [Google Scholar] [CrossRef]
- Heo, S.; Lee, J.H.; Jeong, D.W. Food-Derived Coagulase-Negative Staphylococcus as Starter Cultures for Fermented Foods. Food Sci. Biotechnol. 2020, 29, 1023–1035. [Google Scholar] [CrossRef]
- NOM-034-SSA1-1993; Productos de La Carne. Carne Molida y Carne Molida Moldeada. Envasadas. Diario Oficial de la Federación: Mexico City, Mexico, 1993.
- Ivanovic, S.; Nesic, K.; Pisinov, B.; Pavlovic, I. The Impact of Diet on the Quality of Fresh Meat and Smoked Ham in Goat. Small Rumin. Res. 2016, 138, 53–59. [Google Scholar] [CrossRef]
- Sohail, A.; Al-Dalali, S.; Wang, J.; Xie, J.; Shakoor, A.; Asimi, S.; Shah, H.; Patil, P. Aroma Compounds Identified in Cooked Meat: A Review. Food Res. Int. 2022, 157, 111385. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, M.; Feng, T.; Liu, H.; Lin, Y.; Bai, B. Comparative Characterization of Flavor Precursors and Volatiles in Chongming White Goat of Different Ages by UPLC-MS/MS and GC–MS. Food Chem. X 2024, 24, 101929. [Google Scholar] [CrossRef] [PubMed]
- Bleicher, J.; Ebner, E.E.; Bak, K.H. Formation and Analysis of Volatile and Odor Compounds in Meat—A Review. Molecules. 2022, 27, 6703. [Google Scholar] [CrossRef] [PubMed]
- Indriani, S.; Srisakultiew, N.; Sangsawad, P.; Paengkoum, P.; Pongsetkul, J. Characterization of the Non-Volatiles and Volatiles in Correlation with Flavor Development of Cooked Goat Meat as Affected by Different Cooking Methods. Food Sci. Anim. Resour. 2024, 44, 662–683. [Google Scholar] [CrossRef]
- Park, M.K.; Kim, B.G.; Kang, M.C.; Kim, T.K.; Choi, Y.S. Distinctive Volatile Compound Profile of Different Raw Meats, Including Beef, Pork, Chicken, and Duck, Based on Flavor Map. Appl. Food Res. 2025, 5, 100655. [Google Scholar] [CrossRef]
- Van Gemert, L.J. Odour Thresholds:Compilations of Odour Threshold Values in Air, Water and Other Media, 2nd ed.; Oliemans Punter: Zeist, The Netherlands, 2011. [Google Scholar]
- Pérez-Santaescolástica, C.; Carballo, J.; Fulladosa, E.; Garcia-Perez, J.V.; Benedito, J.; Lorenzo, J.M. Effect of Proteolysis Index Level on Instrumental Adhesiveness, Free Amino Acids Content and Volatile Compounds Profile of Dry-Cured Ham. Food Res. Int. 2018, 107, 559–566. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Yang, X.; Pei, Z.; Du, W.; Xie, J. Characterization of Volatile Flavor Compounds in Dry-Rendered Beef Fat by Different Solvent-Assisted Flavor Evaporation (SAFE) Combined with GC–MS, GC–O, and OAV. Foods 2023, 12, 3162. [Google Scholar] [CrossRef]
- Al-Dalali, S.; Li, C.; Xu, B. Effect of Frozen Storage on the Lipid Oxidation, Protein Oxidation, and Flavor Profile of Marinated Raw Beef Meat. Food Chem. 2022, 376, 131881. [Google Scholar] [CrossRef]
- Pisinov, B.; Ivanović, S.; Živković, D.; Vranić, D.; Stajić, S. Profile of Volatile Compounds in Frankfurters from Culled Goat Meat during Cold Storage. J. Food Process Preserv. 2021, 45, e15410. [Google Scholar] [CrossRef]
- Sivadier, G.; Ratel, J.; Engel, E. Persistence of Pasture Feeding Volatile Biomarkers in Lamb Fats. Food Chem. 2010, 118, 418–425. [Google Scholar] [CrossRef]
- Oshiro, S.; Silvério, F.; Pinho, G. Determination of p -Cresol Levels in Smoked Meat Products Using QuEChERS Method and Gas Chromatography-Mass Spectrometry. J. Environ. Sci. Health B 2022, 57, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.K.; Lindsay, R.C. Volatile Alkylphenols and Thiophenol in Species-Related Characterizing Flavors of Red Meats. J. Food Sci. 1991, 56, 1197–1202. [Google Scholar] [CrossRef]
- Miao, Y.; Wang, L.; Bai, F.; Zheng, S.; Yan, J.; Wei, H.; Meng, Q.; Tong, H. Evaluation of Chongqing Tuo Tea at Different Grades: An Integrated Approach by Artificial and Intelligent Sensory, Non-Volatile, and Volatile Compounds Analysis. Foods 2024, 13, 865. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, C.; Ren, D.; Bai, R.; Li, W.; Wang, J.; Shan, Z.; Dong, W.; Yi, L. Headspace Solid-Phase Microextraction Coupled with Gas Chromatography-Mass Spectrometry (HS-SPME-GC-MS) and Odor Activity Value (OAV) to Reveal the Flavor Characteristics of Ripened Pu-Erh Tea by Co-Fermentation. Front. Nutr. 2023, 10, 1138783. [Google Scholar] [CrossRef]
| Processing Year | Treatments | SEM | P-Level | ||
|---|---|---|---|---|---|
| NP | P | ||||
| pH | 2021 | 5.08 bB | 5.75 aA | 0.05 | * |
| 2022 | 5.28 bA | 5.65 aB | 0.03 | *** | |
| 2023 | 5.27 bA | 5.74 aA | 0.03 | *** | |
| SEM | 0.06 | 0.06 | |||
| P-level | ** | ** | |||
| aw | 2021 | 0.72 aA | 0.71 aA | 0.01 | NS |
| 2022 | 0.68 aB | 0.68 aB | 0.03 | NS | |
| 2023 | 0.70 aAB | 0.69 aAB | 0.04 | NS | |
| SEM | 0.04 | 0.05 | |||
| P-level | ** | * | |||
| Proximate composition | |||||
| Moisture | 2021 | 33.16 aA | 30.17 aA | 1.87 | NS |
| 2022 | 33.42 aA | 22.74 bB | 2.22 | ** | |
| 2023 | 31.31 aA | 23.46 bB | 1.51 | *** | |
| SEM | 0.71 | 1.53 | |||
| P-level | NS | * | |||
| Protein | 2021 | 39.60 aA | 40.19 aA | 2.47 | NS |
| 2022 | 38.27 aA | 41.98 aA | 1.74 | NS | |
| 2023 | 38.77 aA | 39.55 aA | 1.80 | NS | |
| SEM | 1.41 | 1.45 | |||
| P-level | NS | NS | |||
| Fat | 2021 | 6.23 aB | 6.44 aB | 0.78 | NS |
| 2022 | 11.59 bA | 15.03 aA | 1.90 | ** | |
| 2023 | 4.82 bB | 9.34 aB | 2.14 | * | |
| SEM | 1.55 | 1.56 | |||
| P-level | ** | ** | |||
| Ash | 2021 | 18.01 aA | 18.45 aA | 0.60 | NS |
| 2022 | 15.86 aA | 17.37 aA | 0.44 | NS | |
| 2023 | 16.76 aA | 19.02 aA | 1.39 | NS | |
| SEM | 0.44 | 0.94 | |||
| P-level | NS | NS | |||
| Heme iron (µg/g) | 2021 | 32.21 aC | 30.82 aB | 0.59 | NS |
| 2022 | 81.82 aA | 49.30 bA | 2.14 | *** | |
| 2023 | 57.56 aB | 20.82 bC | 2.23 | *** | |
| *** | *** | ||||
| 2.01 | 2.23 | ||||
| NaCl | 2021 | 10.39 bA | 10.93 aB | 0.10 | * |
| 2022 | 10.55 bA | 11.21 aA | 0.28 | ** | |
| 2023 | 10.74 bA | 11.07 aAB | 0.15 | * | |
| SEM | 0.18 | 0.05 | |||
| P-level | NS | * | |||
| WSN | 2021 | 21.52 aA | 18.28 aA | 1.53 | NS |
| 2022 | 10.31 aB | 8.37 aB | 2.04 | NS | |
| 2023 | 9.26 aB | 8.43 aB | 0.26 | NS | |
| SEM | 2.07 | 1.79 | |||
| P-level | ** | * | |||
| Processing Year | Treatments | SEM | P-Level | ||
|---|---|---|---|---|---|
| NP | P | ||||
| Color | |||||
| L* | 2021 | 25.15 aA | 26.79 aA | 1.70 | NS |
| 2022 | 25.92 aA | 20.11 aB | 0.73 | NS | |
| 2023 | 20.31 aB | 20.56 aB | 0.83 | NS | |
| SEM | 0.99 | 1.10 | |||
| P-level | ** | * | |||
| a* | 2021 | 7.48 aA | 6.16 bA | 0.75 | * |
| 2022 | 5.92 aB | 5.76 aB | 0.73 | NS | |
| 2023 | 7.86 aA | 6.18 bA | 0.74 | * | |
| SEM | 0.56 | 0.54 | |||
| P-level | ** | * | |||
| b* | 2021 | 24.74 aA | 29.97 aA | 1.64 | NS |
| 2022 | 29.41 aA | 30.09 aA | 0.49 | NS | |
| 2023 | 28.96 aA | 28.52 aA | 0.82 | NS | |
| SEM | 0.78 | 0.98 | |||
| P-level | NS | NS | |||
| Chroma | 2021 | 30.91 aA | 26.23 aA | 1.71 | NS |
| 2022 | 30.69 aA | 30.01 aA | 0.54 | NS | |
| 2023 | 30.81 aA | 29.26 aA | 0.76 | NS | |
| SEM | 0.77 | 1.10 | |||
| P-level | NS | NS | |||
| Hue | 2021 | 72.00 bB | 77.04 aA | 1.28 | * |
| 2022 | 78.90 aA | 79.14 aA | 0.56 | NS | |
| 2023 | 70.68 bB | 78.01 aA | 1.57 | * | |
| SEM | 1.12 | 0.99 | |||
| P-level | ** | NS | |||
| Metmyoglobin (%) | 2021 | 33.61 aB | 36.45 aB | 1.33 | NS |
| 2022 | 36.32 bA | 53.21 aA | 1.24 | *** | |
| 2023 | 30.66 aB | 32.02 aB | 0.80 | NS | |
| SEM | 0.67 | 1.04 | |||
| P-level | *** | *** | |||
| Processing Year | Treatments | SEM | P-Level | ||
|---|---|---|---|---|---|
| NP | P | ||||
| Texture profile analysis | |||||
| Hardness (N) | 2021 | 55.39 bB | 223.67 aB | 55.19 | * |
| 2022 | 154.4 bA | 574.01 aA | 84.31 | *** | |
| 2023 | 75.75 bB | 328.01 aAB | 77.86 | * | |
| SEM | 29.55 | 53.55 | |||
| P-level | ** | *** | |||
| Springiness | 2021 | 0.54 aA | 0.66 aA | 2.91 | NS |
| 2022 | 0.59 aA | 0.69 aA | 2.10 | NS | |
| 2023 | 0.69 aA | 0.56 aA | 3.28 | NS | |
| SEM | 3.21 | 3.09 | |||
| P-level | NS | NS | |||
| Cohesiveness | 2021 | 0.56 bA | 0.63 aA | 3.52 | NS |
| 2022 | 0.59 aA | 0.69 aA | 4.27 | NS | |
| 2023 | 0.72 aA | 0.57 aA | 5.08 | NS | |
| SEM | 3.35 | 3.38 | |||
| P-level | NS | NS | |||
| Resilience | 2021 | 0.19 aA | 0.20 aA | 1.24 | NS |
| 2022 | 0.09 aB | 0.06 aB | 2.19 | NS | |
| 2023 | 0.09 aB | 0.05 aB | 2.64 | NS | |
| SEM | 1.74 | 2.07 | |||
| P-level | * | ** | |||
| Processing Year | Treatments | SEM | P-Level | ||
|---|---|---|---|---|---|
| NP | P | ||||
| TMBC | 2021 | 5.92 aB | 5.95 aB | 0.13 | NS |
| 2022 | 6.68 aA | 7.00 aA | 0.20 | NS | |
| 2023 | 6.62 aA | 6.10 aB | 0.16 | NS | |
| SEM | 0.11 | 0.19 | |||
| P-level | ** | * | |||
| LAB | 2021 | 5.50 aA | 5.68 aA | 0.29 | NS |
| 2022 | 4.56 aA | 4.91 aA | 0.63 | NS | |
| 2023 | 5.57 aA | 6.54 aA | 0.65 | NS | |
| SEM | 0.50 | 0.39 | |||
| P-level | NS | NS | |||
| Concentration (µg/kg) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | RT (min) | Chemical Formula | Odor Description a | CAS# | NP 2021 | P 2021 | NP 2022 | P 2022 | NP 2023 | P 2023 |
| Aldehydes | ||||||||||
| Hexanal | 3.72 | C6H12O | Green leaves, fresh grass, fatty, rancid, unpleasant, tallowy, muttony | 66-25-1 | 2.21 | 1.32 | 2645.61 | 975.37 | 829.6 | 434.85 |
| Benzaldehyde | 4.62 | C7H6O | Bitter almond, almond, burnt sugar, roasted pepper, nutty | 100-52-7 | 32.84 | 52.6 | nd | nd | nd | nd |
| Heptanal | 4.9 | C7H14O | Potato, pleasant meaty notes, nutty, fruity green, aldehyde, fatty | 111-71-7 | nd | nd | 788.22 | 839.55 | 520.57 | 533.24 |
| Octanal | 6.02 | C8H16O | Green, citrus, lemon, meaty, fresh, fruity, grass, fatty | 124-13-0 | 3.38 | 9.61 | 455.61 | 784.73 | 556.67 | 621.17 |
| 2-Octenal | 6.7 | C8H14O | Grilled meat, peanut cake, fat green leaf, floral | 2548-87-0 | nd | nd | 128.69 | 146.43 | 141.01 | 112.37 |
| Benzaldehyde, 4-methoxy- | 6.98 | C8H8O2 | Similar hawkthorn | 123-11-5 | 2.48 | 2.59 | nd | nd | nd | nd |
| Nonanal | 7.1 | C9H18O | Fruity, sweet, pleasant meaty notes, citrus, green, citronella, grass, fat, waxy | 124-19-6 | 26.54 | 20.86 | 1361.95 | 2067.41 | 1517.25 | 1808.72 |
| 2-Nonenal | 7.71 | C9H16O | Fruity–floral, vegetable, herbaceous, and/or chemical, earthy, fermented, burnt | 18829-56-6 | nd | nd | 401.36 | 551.45 | 557.66 | 475.66 |
| Decanal | 8.13 | C10H20O | Green, fishy, fatty, rancid, meaty, burnt, soap, orange peel, tallow | 112-31-2 | 3.22 | nd | 165.74 | 217.16 | 153.83 | 123.99 |
| 2-Decenal | 8.7 | C10H18O | Fruity–floral, vegetable, herbaceous, citrus fruit, lemon, mint | 3913-81-3 | nd | nd | 1262.19 | 1554.77 | 1031.31 | 1285.06 |
| 2-Undecenal | 9.62 | C11H20O | Sweet | 2463-77-6 | nd | nd | 1763 | 1955.27 | 870.76 | 1574.87 |
| Undecanal | 9.09 | C11H22O | Floral, green, mild | 112-44-7 | nd | nd | 110.06 | 125.32 | 61.95 | 116.77 |
| Benzaldehyde, 4-pentyl- | 10.11 | C12H16O | Sweet, woody | 77961-30-1 | 3.26 | 2.56 | nd | nd | nd | nd |
| Tridecanal | 10.5 | C13H26O | Fatty, sweet | 629-62-9 | nd | nd | 216.23 | 236.15 | 95.49 | 188.64 |
| Tetradecanal | 11.66 | C14H28O | Roasted, fried | 629-59-4 | nd | nd | 279.07 | 214.57 | 116.05 | 579.44 |
| Pentadecanal | 12.21 | C15H30O | Waxy | 629-62-9 | 3.96 | 7.14 | 122.57 | 58.81 | 54.22 | 222.25 |
| Hexadecanal | 13.14 | C16H32O | Fatty | 629-80-1 | 3.76 | 51.09 | 4470.65 | 875.28 | 1058.8 | 11,980.47 |
| 9-Octadecenal | 14.29 | C18H34O | Fatty, green | 5090-41-5 | nd | nd | 117.12 | 13.76 | 26.27 | 385.9 |
| Octadecanal | 14.58 | C18H36O | Fatty, candle | 638-66-4 | nd | nd | 65.08 | 137.77 | 156.43 | 4439.1 |
| Ketones | ||||||||||
| 2-Heptanone | 4.77 | C7H14O | Fruity, citrus, grapefruit, limonene, floral, spicy cinnamon, spicy, blue cheese, acorn, soapy | 110-43-0 | nd | nd | 256.95 | 136.35 | 56.64 | 90.71 |
| 2-Nonanone | 6.98 | C9H18O | Blue cheese, fruity, floral, flower petal | 821-55-6 | nd | nd | 176.85 | 219.15 | 88.57 | 282.08 |
| 2-Decanone | 8 | C10H20O | Ethereal, butter, spicy, blue cheese, heavy, sweet | 693-54-9 | nd | nd | 234.65 | 191.87 | 118.86 | 228.37 |
| 2-Undecanone | 8.97 | C11H22O | Fruity, fatty | 112-12-9 | nd | nd | 246.98 | 389.7 | 129.85 | 228.83 |
| Geranylacetone | 10.39 | C13H22O | Floral, rose, sweet | 3796-70-1 | nd | nd | 145.64 | 111.04 | 58.82 | 117.94 |
| 2-Pentadecanone | 12.29 | C15H30O | Fatty, sweet | 2345-28-0 | nd | 28.52 | 91.85 | 68.31 | 48.55 | 338.67 |
| 2-Heptadecanone | 13.72 | C17H34O | Waxy, floral | 13922-62-8 | nd | nd | 101.41 | 71.61 | 56.71 | 466.24 |
| Alcohols | ||||||||||
| 1-Hexanol | 4.58 | C6H14O | Green, sweet, spicy notes | 111-27-3 | nd | nd | 43.19 | 95.17 | 53.16 | 70.52 |
| Benzyl alcohol | 5.17 | C7H8O | Sweet, Grass | 100-51-6 | 3.84 | 9.42 | nd | nd | nd | nd |
| 1-Heptanol | 5.72 | C7H16O | Woody, oily, floral | 111-70-6 | nd | 2.57 | 158.51 | 225.89 | 170.86 | 231.16 |
| 1-Octen-3-ol | 5.79 | C8H16O | Mushroom-like, earthy, grass | 3391-86-4 | 1.68 | 2 | 334 | 379.18 | 262.71 | 314.99 |
| 1-Octanol | 6.79 | C8H18O | Sharp, fatty, waxy, orange-rose, sweet | 111-87-5 | nd | nd | 267.07 | 355.33 | 265.15 | 281.82 |
| 1-Dodecanol | 7.39 | C12H26O | Wax, sweet | 112-53-8 | nd | nd | 54.22 | 88.2 | 136.49 | 54.77 |
| 2-Undecen-1-ol | 8.82 | C11H22O | Waxy, citrus-like | 112-42-5 | nd | nd | 72.51 | 127.09 | 49.83 | 93.7 |
| 6-Pentadecen-1-ol | 12.06 | C15H30O | Waxy, green, oily | 14652-30-5 | nd | nd | 53.14 | 41.22 | 108.63 | 148.39 |
| Hexadecanol | C16H34O | Waxy, floral, fatty | 36653-82-4 | nd | nd | 451 | 208.36 | 204.77 | 1166.33 | |
| Acids | ||||||||||
| Acetic acid | 2.52 | C2H4O2 | Pungent, acidic, cheesy, vinegar | 64-19-7 | 7.59 | nd | nd | nd | nd | nd |
| Hexanoic acid | 5.01 | C6H12O2 | Goaty, pungency, rancid, cheese, fatty, sweaty, sour | 142-62-1 | 5.25 | 8.37 | nd | nd | nd | nd |
| Octanoic acid | 7.56 | C8H16O2 | Rancid, fatty, coconut-like, vomit, cheese, rotten meat, waxy, sweaty | 124-07-2 | 7.2 | 6.57 | nd | nd | nd | nd |
| Benzoic acid | 8.4 | C7H6O2 | Sharp, sweet, balsamic | 65-85-0 | 6.64 | nd | nd | nd | nd | nd |
| n-Decanoic acid | 9.82 | C10H20O2 | Rancid, oily | 334-48-5 | 2.89 | 14.67 | nd | nd | nd | nd |
| Undecanoic acid | 10.57 | C11H22O2 | Waxy, citrus-like | 112-37-8 | 1.31 | 2.2 | nd | nd | nd | nd |
| Lauric acid | 11.32 | C12H24O2 | Soapy, fatty, sweet | 143-07-7 | 7.06 | 3.24 | nd | nd | nd | nd |
| Tridecanoic acid | 12.07 | C13H26O2 | Waxy, oily | 638-53-9 | 9.38 | nd | nd | nd | nd | nd |
| Myristic acid | 12.82 | C14H28O2 | Fatty, waxy | 544-63-8 | 14.52 | 10.81 | 260.65 | 522.11 | 83.55 | 579.01 |
| Pentadecanoic acid | 13.53 | C15H30O2 | Oily, waxy | 1002-84-2 | 4.41 | nd | nd | nd | nd | nd |
| Palmitoleic acid | 14.06 | C16H30O2 | Waxy, oily | 373-49-9 | nd | nd | 104.91 | 163.07 | 19.78 | 277.53 |
| Palmitic acid | 14.24 | C16H32O2 | Fatty, waxy, mild | 57-10-3 | nd | nd | 1527.09 | 1731.92 | 153.63 | 4556.27 |
| Oleic acid | 15.34 | C18H34O2 | Fatty, oily | 112-80-1 | nd | nd | 362.36 | 452.32 | 234.89 | 1425.92 |
| Stearic acid | 15.45 | C18H36O2 | Waxy, fatty | 57-11-4 | nd | nd | 135.68 | 257.99 | 34.22 | 838.1 |
| Terpenes | ||||||||||
| p-Cymene | 6.29 | C10H14 | Sweet, citrus, weak, spicy herbaceous, fresh | 99-87-6 | nd | nd | 43.05 | 51.22 | 21.58 | 24.81 |
| D-Limonene | 6.34 | C10H16 | Sweet, fruity, citrus, lemon | 5989-27-5 | nd | nd | 45.68 | 125.33 | 43.11 | 30.99 |
| Menthene | 7.38 | C10H18 | Minty, herbal | 5502-88-5 | nd | nd | 43.96 | 63.69 | 51.76 | 80.93 |
| Cubenol | 9.53 | C15H26O | Woody, sweet | 502-99-8 | nd | nd | 112.73 | 121.74 | 80 | 62.23 |
| Copaene | 9.81 | C15H24 | Woody, spicy | 3856-25-5 | nd | nd | 118.12 | 134.73 | 116.61 | 126.93 |
| β-Bourbonene | 9.87 | C15H24 | Woody spicy, sickly sweet, wallflowers, balsamic | 5208-59-3 | nd | nd | 167.79 | 65.83 | 133.79 | 171.96 |
| Isocaryophillene | 10.1 | C15H24 | Spicy, herbal | 6753-98-6 | nd | nd | 83.64 | 85.11 | 73.95 | 40.78 |
| α-Cedrene | 10.19 | C15H24 | Woody | 469-61-4 | nd | nd | 60.24 | 49.95 | 30.74 | 43.95 |
| Caryophyllene | 10.22 | C15H24 | Spicy, peppery | 87-44-5 | 9.87 | 3.25 | 216.68 | 341.57 | 310.82 | 123.06 |
| Caryophylladienol | 10.28 | C15H26O | Woody, sweet | nd | nd | 38.74 | 42.13 | 40.16 | 21.62 | |
| α-Humulene | 10.51 | C15H24 | Woody, hop-like, musty earthy | 6753-98-6 | nd | nd | 122.23 | 106.25 | 41.03 | 97.15 |
| Eudesmene | 10.79 | C15H24 | Woody, balsamic | 5153-27-3 | nd | nd | 48.41 | 44.4 | 26 | 16.59 |
| Isopatchoulane | 10.84 | C15H26 | Earthy, woody | 10094-06-9 | nd | nd | 157.86 | 154.38 | 125.78 | 167.37 |
| γ-Muurolene | 10.99 | C15H24 | Woody, earthy, herbal | 473-15-4 | nd | nd | 27.45 | nd | 16.33 | nd |
| α-Calacorene | 11.23 | C15H24 | Woody, spicy, herbal | 1131-62-0 | nd | nd | 89.45 | 52.85 | 126.86 | 136.11 |
| Caryophyllene oxide | 11.61 | C15H24O | Woody, spicy, green | 1139-30-6 | nd | nd | 32.34 | 36.43 | 18.8 | 30.78 |
| Squalene | 13.48 | C30H50 | Odorless to slightly oily | 111-02-4 | nd | nd | 55.24 | 284.96 | 22.29 | 171.54 |
| Hydrocarbons | ||||||||||
| Heptane | 2.85 | C7H16 | Hydrocarbon (weak), sweet, gasoline-like, light petroleum | 142-82-5 | nd | nd | 25.78 | 100.45 | 23.43 | 35.06 |
| Octane | 3.78 | C8H18 | Hydrocarbon (weak), fatty, solvent | 111-65-9 | nd | nd | 324.67 | 194.8 | 212.97 | 84.55 |
| Nonane | 4.86 | C9H20 | Sweet, mild gasoline odor | 111-84-2 | nd | nd | 13.74 | 34.64 | 9.66 | 7.98 |
| Pentadecane | 10.72 | C15H32 | Waxy, oily, mild | 629-62-9 | nd | nd | 245.72 | 221.3 | 98.93 | 236.65 |
| Hexadecane | 11.5 | C16H34 | Odorless or very faint hydrocarbon smell | 544-76-3 | nd | nd | 161.51 | 187.58 | 35.16 | 187.33 |
| Heptadecane | 12.26 | C17H36 | Odorless | 629-78-7 | nd | nd | 138.78 | 96.3 | 33.09 | 187.5 |
| Octadecane | 12.98 | C18H38 | Odorless | 593-45-3 | nd | nd | 90.29 | 102.84 | 32.98 | 205.2 |
| Nonadecane | 13.66 | C19H40 | Odorless | 629-92-5 | nd | nd | 280.88 | 62.41 | 51.36 | 910.16 |
| Phytane | 13.06 | C20H42 | Odorless | 638-36-8 | nd | nd | 127.54 | 93.26 | 48.42 | 366.28 |
| Phenols | ||||||||||
| Phenol | 11.31 | C6H6O | Phenolic medicinal | 108-95-2 | 1.69 | 2.93 | nd | nd | nd | nd |
| p-Cresol | 13.06 | C7H8O | Phenolic, horse-stable-like | 106-44-5 | 7.51 | 4.26 | nd | nd | nd | nd |
| m-Cresol | 13.07 | C7H8O | Tar, medicinal, phenolic | 108-39-4 | 23.38 | 6.13 | nd | nd | nd | nd |
| OAV | ||||||||
|---|---|---|---|---|---|---|---|---|
| Volatile Compound | Odor Threshold (µg/kg) | Ref. | NP 2021 | P 2021 | NP 2022 | P 2022 | NP 2023 | P 2023 |
| Aldehydes | ||||||||
| Hexanal | 5 | [38,39] | 0.44 | 0.26 | 529.12 | 195.07 | 165.92 | 86.97 |
| Heptanal | 2.8 | [38,39] | nd | nd | 281.51 | 299.84 | 185.92 | 190.44 |
| Octanal | 0.59 | [38,52] | 5.73 | 16.29 | 772.22 | 1330.0 | 943.51 | 1052.8 |
| 2-Octenal | 3 | [53] | nd | nd | 42.90 | 48.81 | 47.00 | 37.46 |
| Nonanal | 1.1 | [38,53] | 24.13 | 18.96 | 1238.14 | 1879.46 | 1379.32 | 1644.29 |
| 2-Nonenal | 0.19 | [38,53] | nd | nd | 2112.42 | 2902.37 | 2935.05 | 2503.47 |
| Decanal | 3 | [38,39] | 1.07 | nd | 55.25 | 72.39 | 51.28 | 41.33 |
| 2-Decenal | 1 | [38] | nd | nd | 4207.30 | 5182.57 | 3437.70 | 4283.53 |
| 2-Undecenal | 0.78 | [38] | nd | nd | 1259.29 | 1396.62 | 621.97 | 1124.91 |
| Tridecanal | 10 | [38] | nd | nd | 21.62 | 23.62 | 9.55 | 18.86 |
| Tetradecanal | 110 | [38] | nd | nd | 2.54 | 1.95 | 1.06 | 5.27 |
| Ketones | ||||||||
| 2-Heptanone | 140 | [38,52] | nd | nd | 1.84 | 0.97 | 0.40 | 0.65 |
| 2-Nonanone | 41~82 | [38] | nd | nd | 4.31 | 5.35 | 2.16 | 6.88 |
| 2-Decanone | 8~41 | [38] | nd | nd | 28.27 | 23.12 | 14.32 | 27.51 |
| 2-Undecanone | 5.5 | [38] | nd | nd | 44.91 | 70.85 | 23.61 | 41.61 |
| Geranylacetone | 0.06 | [43] | nd | nd | 2.43 | 1.85 | 0.98 | 1.97 |
| Alcohols | ||||||||
| 1-Hexanol | 5.6 | [39] | nd | nd | 7.71 | 16.99 | 9.49 | 12.59 |
| 1-Heptanol | 5.4 | [39] | nd | 0.48 | 29.35 | 41.83 | 31.64 | 42.81 |
| 1-Octen-3-ol | 1.5 | [38,39] | 1.12 | 1.33 | 222.67 | 252.79 | 175.14 | 209.99 |
| 1-Octanol | 120 | [38] | nd | nd | 2.12 | 2.82 | 2.11 | 2.24 |
| 1-Dodecanol | 16 | [38] | nd | nd | 3.39 | 5.51 | 8.53 | 3.42 |
| Terpenes | ||||||||
| p-Cymene | 5.01 | [38] | nd | nd | 8.59 | 10.22 | 4.31 | 4.95 |
| D-Limonene | 10 | [53] | 0.00 | 0.00 | 1.34 | 3.69 | 1.27 | 0.91 |
| Caryophyllene | 0.064 | [43] | 0.15 | 0.05 | 3.39 | 5.34 | 4.86 | 1.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villalobos-Delgado, L.H.; Martínez-Martínez, Y.Y.; Nevárez-Moorillón, G.V.; Santiago-Castro, J.T.; Soto-Simental, S.; Juárez-Palomo, C.I.; Guadarrama-Mendoza, P.C. Physicochemical Properties and Volatile Profile of Chito: A Traditional Dry-Cured Goat Meat Product. Foods 2025, 14, 2341. https://doi.org/10.3390/foods14132341
Villalobos-Delgado LH, Martínez-Martínez YY, Nevárez-Moorillón GV, Santiago-Castro JT, Soto-Simental S, Juárez-Palomo CI, Guadarrama-Mendoza PC. Physicochemical Properties and Volatile Profile of Chito: A Traditional Dry-Cured Goat Meat Product. Foods. 2025; 14(13):2341. https://doi.org/10.3390/foods14132341
Chicago/Turabian StyleVillalobos-Delgado, Luz Hermila, Yaneisy Y. Martínez-Martínez, Guadalupe Virginia Nevárez-Moorillón, Joaquín T. Santiago-Castro, Sergio Soto-Simental, Carlos Ignacio Juárez-Palomo, and Paula Cecilia Guadarrama-Mendoza. 2025. "Physicochemical Properties and Volatile Profile of Chito: A Traditional Dry-Cured Goat Meat Product" Foods 14, no. 13: 2341. https://doi.org/10.3390/foods14132341
APA StyleVillalobos-Delgado, L. H., Martínez-Martínez, Y. Y., Nevárez-Moorillón, G. V., Santiago-Castro, J. T., Soto-Simental, S., Juárez-Palomo, C. I., & Guadarrama-Mendoza, P. C. (2025). Physicochemical Properties and Volatile Profile of Chito: A Traditional Dry-Cured Goat Meat Product. Foods, 14(13), 2341. https://doi.org/10.3390/foods14132341










