Contributions to Estimating the Water-Holding Capacity in Fresh Pork Hams Using NMR Relaxometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Meat Sampling
2.2. Determination of Fat Content, pH, and Weight of Fresh Hams
2.3. Physicochemical Characterization of Different Muscles
2.4. Nuclear Magnetic Resonance Analysis
2.4.1. Magnetic Resonance Imaging (MRI)
High Magnetic Field MRI (4.7 T)
Medium Magnetic Field MRI (1 T)
Relaxation Time Calculation from MR Image Series
2.4.2. Time-Domain Nuclear Magnetic Resonance (TD-NMR)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Categorization and Characterization of Fresh Hams
3.2. Physicochemical Analysis of the Different Muscles of the Fresh Hams
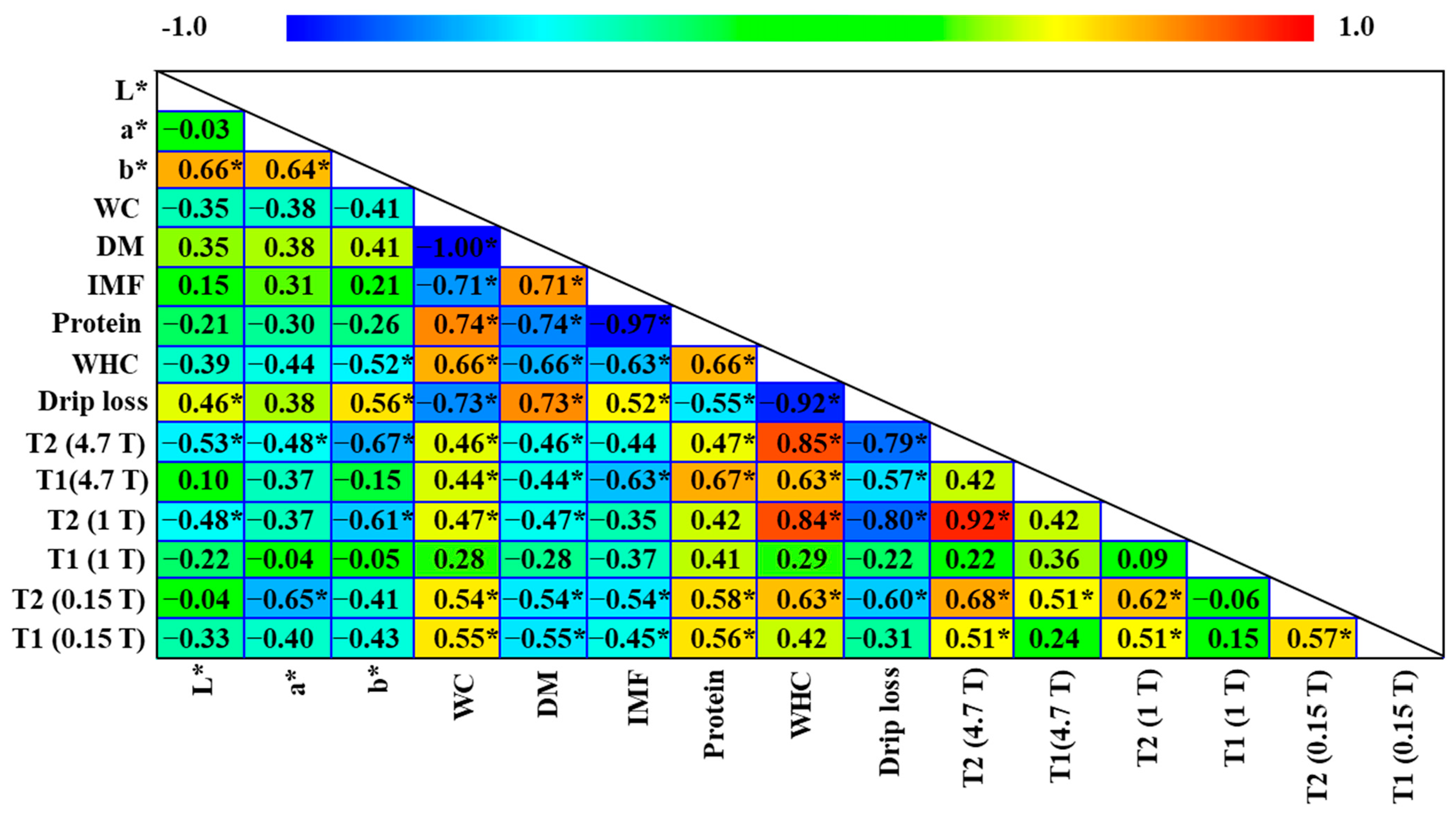
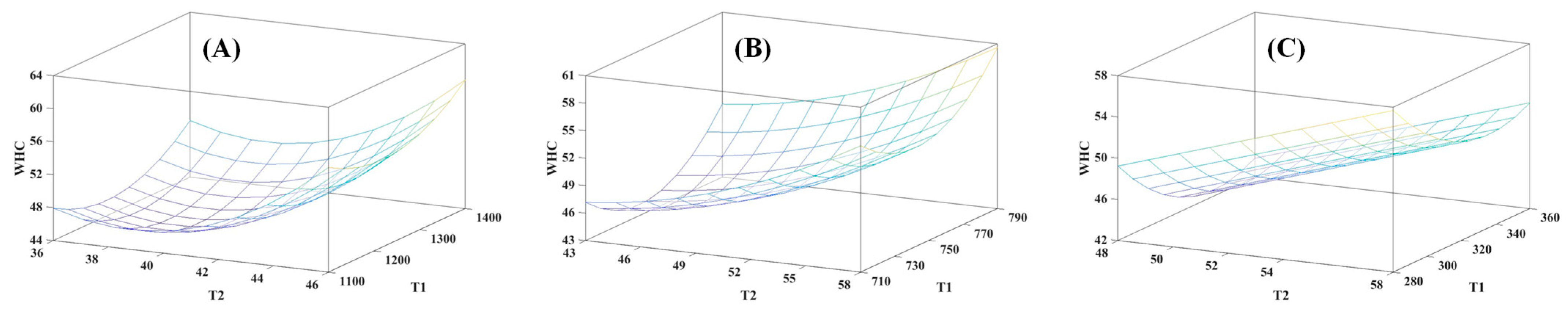
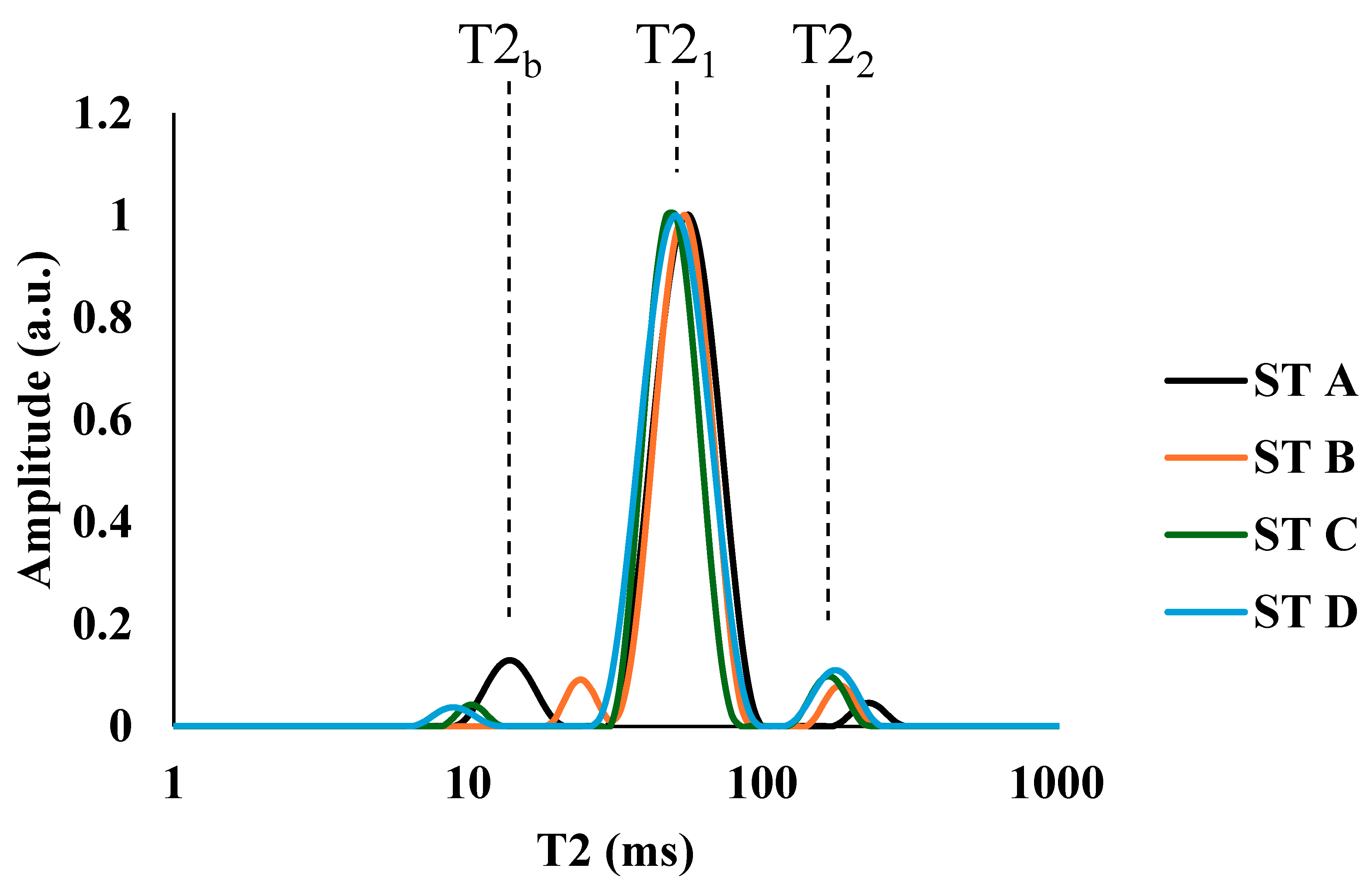
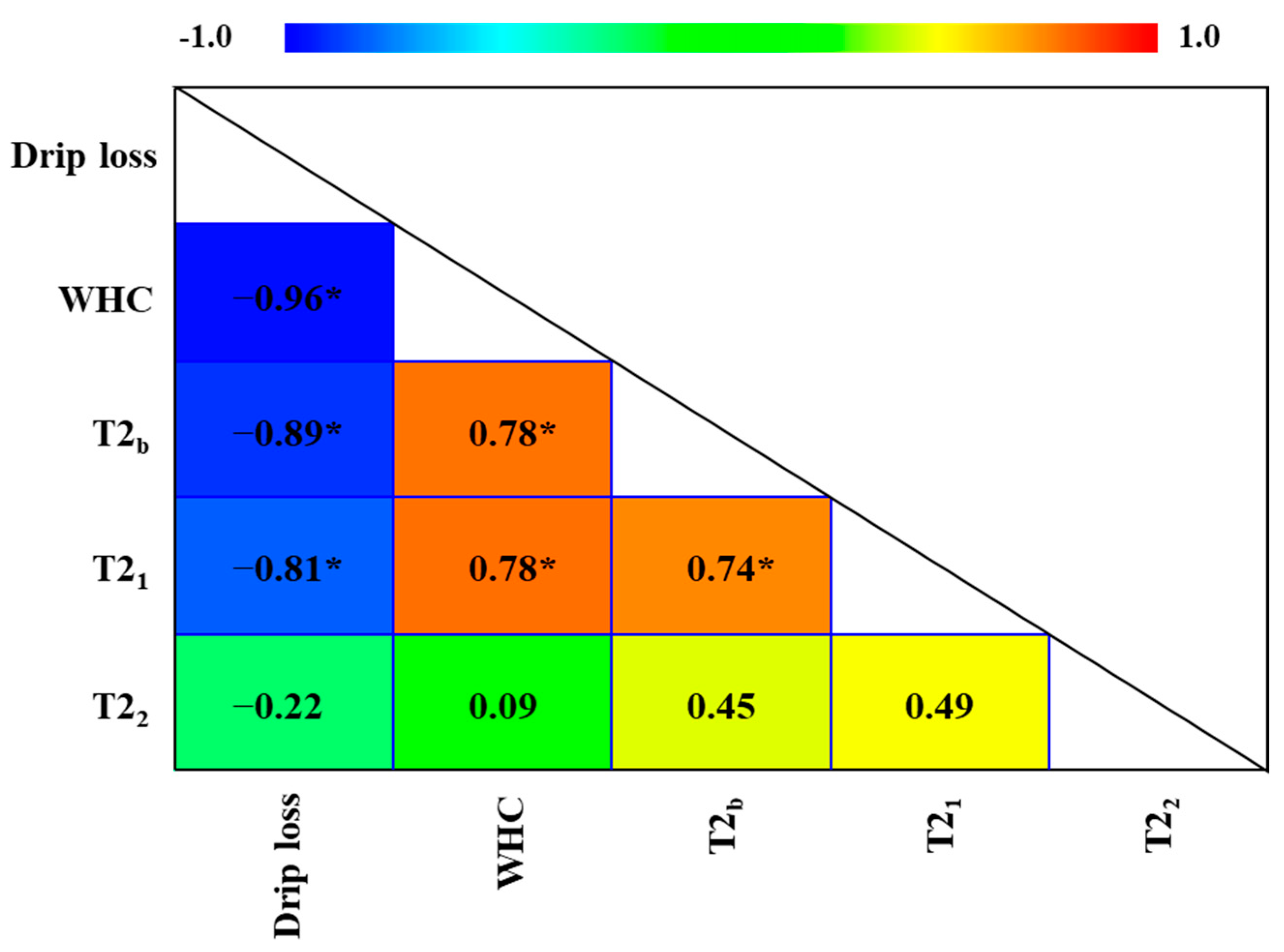
3.3. MRI and TD-NMR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tornberg, E.V.A. Effects of heat on meat proteins–Implications on structure and quality of meat products. Meat Sci. 2005, 70, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef]
- Warner, R.D. The eating quality of meat: IV—Water holding capacity and juiciness. In Lawrie’s Meat Science, 9th ed.; Woodhead Publishing: Sawston, UK, 2023; pp. 457–508. [Google Scholar]
- Barbut, S. Measuring water holding capacity in poultry meat. Poult. Sci. 2024, 103, 103577. [Google Scholar] [CrossRef] [PubMed]
- Oswell, N.J.; Gilstrap, O.P.; Pegg, R.B. Variation in the terminology and methodologies applied to the analysis of water holding capacity in meat research. Meat Sci. 2021, 178, 108510. [Google Scholar] [CrossRef]
- Günther, H. NMR Spectroscopy: Basic Principles, Concepts and Applications in Chemistry, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Keeler, J. Understanding NMR Spectroscopy, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Liang, Z.P.; Lauterbur, P.C. Principles of Magnetic Resonance Imaging: A Signal Processing Perspective; Wiley-IEEE Press: Hoboken, NJ, USA, 1999. [Google Scholar]
- García-García, A.B.; Fernández-Valle, M.E.; Castejón, D.; Escudero, R.; Cambero, M.I. Use of MRI as a predictive tool for physicochemical and rheological features during cured ham manufacturing. Meat Sci. 2019, 148, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Bertram, H.; Andersen, H. Proton NMR Relaxometry in Meat Science. In Modern Magnetic Resonance; Springer: Dordrecht, The Netherlands, 2006; pp. 1729–1733. [Google Scholar]
- García-García, A.B.; Cambero, M.I.; Castejón, D.; Escudero, R.; Fernández-Valle, M.E. Dry cured-ham microestructure: A T2 NMR relaxometry, SEM and uniaxial tensile test combined study. Food Struct. 2019, 19, 100104. [Google Scholar] [CrossRef]
- Khan, M.A.; Ahmad, B.; Kamboh, A.A.; Qadeer, Z. Use of NMR relaxometry for determination of meat properties: A brief review. Food Mater. Res. 2022, 2, 1–8. [Google Scholar] [CrossRef]
- Remiro, V.; Cambero, M.I.; Romero-de-Ávila, M.D.; Castejón, D.; Moreno-Molera, D.; Segura, J.; Fernández-Valle, M.E. Monitoring salting kinetics of pork loin using magnetic resonance imaging (MRI) and time-domain nuclear magnetic resonance (TD-NMR). LWT 2025, 222, 117635. [Google Scholar] [CrossRef]
- Cônsolo, N.R.; de Paula, A.P.; Rezende-de-Souza, J.H.; Herreira, V.L.; Gôngora, A.L.S.; Colnago, L.A.; Moraes, T.B.; Santos, P.M.; Nassu, R.T.; Pflanzer, S.B. Assessment of water relaxometry of meat under different ageing processes using time domain nuclear magnetic resonance relaxometry. Food Res. Int. 2024, 190, 114566. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, C.; Ma, G.; Yu, Q.; Zhang, L. LF-NMR determination of water distribution and its relationship with protein- related properties of yak and cattle during postmortem aging. Food Chem. X 2023, 20, 100891. [Google Scholar] [CrossRef]
- Bianchi, M.; Capozzi, F.; Cremonini, M.A.; Laghi, L.; Petracci, M.; Placucci, G.; Cavani, C. Influence of the season on the relationships between NMR transverse relaxation data and water-holding capacity of turkey breast meat. J. Sci. Food Agric. 2004, 84, 1535–1540. [Google Scholar] [CrossRef]
- Hu, H.H.; Nayak, K.S. Change in the proton T1 of fat and water in mixture. Magn. Reson. Med. 2010, 63, 494–501. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official method 991.36. Fat (crude) in meat and meat products. Soxhlet extraction method. In Official Methods of Analysis, 16th ed.; AOAC International: Washington, DC, USA, 1996. [Google Scholar]
- AOAC. Official method 950.46. Moisture in Meat. In Official Methods of Analysis, 18th ed.; AOAC International: Washington, DC, USA, 2006. [Google Scholar]
- Kauffman, R.G.; Eikelenboom, G.; Van der Wal, P.G.; Engel, B.; Zaar, M. A comparison of methods to estimate water-holding capacity in post-rigor porcine muscle. Meat Sci. 1986, 18, 307–322. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official method 928.08. Protein in meat. Kjeldahl method. In Official Methods of Analysis, 19th ed.; AOAC International: Washington, DC, USA, 2012. [Google Scholar]
- Bjarnason, T.A.; Mitchell, J.R. AnalyzeNNLS: Magnetic resonance multiexponential decay image analysis. J. Magn. Reson. 2010, 206, 200–204. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hadi, A.S. Variable selection procedures. In Regression Analysis by Example, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 299–334. [Google Scholar]
- Ventanas, J. Jamón Ibérico y Serrano. Fundamentos de la Elaboración y de la Calidad, 1st ed.; Ediciones Mundi-Prensa: Madrid, Spain, 2012. [Google Scholar]
- Lebret, B.; Čandek-Potokar, M. Pork quality attributes from farm to fork. Part II. Processed pork products. Animal 2022, 16, 100383. [Google Scholar] [CrossRef]
- Cernadas, E.; Fernández-Delgado, M.; Fulladosa, E.; Muñoz, I. Automatic marbling prediction of sliced dry-cured ham using image segmentation, texture analysis and regression. Expert. Syst. Appl. 2022, 206, 117765. [Google Scholar] [CrossRef]
- Romero de Ávila, M.D.; Escudero, R.; Ordóñez, J.A.; Cambero, M.I. Weibull analysis characterizes the breaking properties of dry-cured ham slices. Meat Sci. 2014, 97, 451–458. [Google Scholar] [CrossRef]
- Kim, J.H.; Seong, P.N.; Cho, S.H.; Park, B.Y.; Hah, K.H.; Yu, L.H.; Lim, D.G.; Hwang, I.H.; Kim, D.H.; Lee, J.M.; et al. Characterization of nutritional value for twenty-one pork muscles. Asian-Australas. J. Anim. Sci. 2008, 21, 138–143. [Google Scholar] [CrossRef]
- Font-i-Furnols, M.; Brun, A.; Gispert, M. Intramuscular fat content in different muscles, locations, weights and genotype-sexes and its prediction in live pigs with computed tomography. Animal 2019, 13, 666–674. [Google Scholar] [CrossRef]
- Ruusunen, M.; Puolanne, E. Histochemical properties of fibre types in muscles of wild and domestic pigs and the effect of growth rate on muscle fibre properties. Meat Sci. 2004, 67, 533–539. [Google Scholar] [CrossRef]
- Realini, C.E.; Vénien, A.; Gou, P.; Gatellier, P.; Pérez-Juan, M.; Danon, J.; Astruc, T. Characterization of Longissimus thoracis, Semitendinosus and Masseter muscles and relationships with technological quality in pigs. 1. Microscopic analysis of muscles. Meat Sci. 2013, 94, 408–416. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Kim, G.D.; Jeong, J.Y.; Hur, S.J.; Joo, S.T. The relationship between muscle fiber characteristics and meat quality traits of highly marbled Hanwoo (Korean native cattle) steers. Meat Sci. 2010, 86, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Lebedová, N.; Stupka, R.; Čítek, J.; Zadinová, K.; Kudrnáčová, E.; Okrouhlá, M.; Dundáčková, P. Muscle fibre types and their relation to meat quality traits in pigs. Sci. Agric. Bohem. 2019, 50, 164–170. [Google Scholar] [CrossRef]
- Watanabe, G.; Motoyama, M.; Nakajima, I.; Sasaki, K. Relationship between water-holding capacity and intramuscular fat content in Japanese commercial pork loin. Asian-Australas. J. Anim. Sci. 2017, 31, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Daszkiewicz, T.; Bąk, T.; Denaburski, J. Quality of pork with a different intramuscular fat (IMF) content. Pol. J. Food Nutr. Sci. 2005, 14/55, 31–36. [Google Scholar]
- Graziotti, G.H.; Rodríguez Menéndez, J.; Ríos, M.C.; Salinas, M.; Paltenghi Ceschel, A.; Affricano, O.; Bosco, A.; Victorica, C.; Basso, L. Perfil metabólico del músculo semitendinoso del cerdo. InVet 2007, 9, 19–26. [Google Scholar]
- Semenova, A.A.; Pchelkina, V.A.; Nasonova, V.V.; Loskutov, S.I.; Bogolyubova, N.V.; Nekrasov, R.V.; Motovilina, A.A.; Bogdanova, Y.I. Histological characteristics and functional properties of red and white parts of m. semitendinosus of slaughter pigs. Theory Pract. Meat Process. 2023, 8, 191–202. [Google Scholar] [CrossRef]
- Stanisz, G.J.; Odrobina, E.E.; Pun, J.; Escaravage, M.; Graham, S.J.; Bronskill, M.J.; Henkelman, R.M. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn. Reson. Med. 2005, 54, 507–512. [Google Scholar] [CrossRef]
- Marty, B.; Carlier, P.G. Physiological and pathological skeletal muscle T1 changes quantified using a fast inversion-recovery radial NMR imaging sequence. Sci. Rep. 2019, 9, 6852. [Google Scholar] [CrossRef]
- Jo, K.; Lee, S.; Jeong, H.G.; Lee, D.H.; Yoon, S.; Chung, Y.; Jung, S. Utilization of electrical conductivity to improve prediction accuracy of cooking loss of pork loin. Food Sci. Anim. Resour. 2023, 43, 113–123. [Google Scholar] [CrossRef]
- Szmańko, T.; Lesiów, T.; Górecka, J. The water-holding capacity of meat: A reference analytical method. Food Chem. 2021, 357, 129727. [Google Scholar] [CrossRef] [PubMed]
- Bertram, H.C.; Andersen, H.J. NMR and the water-holding issue of pork. J. Anim. Breed. Genet. 2007, 124, 35–42. [Google Scholar] [CrossRef]
- Mariette, F. Investigations of food colloids by NMR and MRI. Curr. Opin. Colloid Interface Sci. 2009, 14, 203–211. [Google Scholar] [CrossRef]
- Bertram, H.C.; Andersen, H.J.; Karlsson, A.H. Comparative study of low-field NMR relaxation measurements and two traditional methods in the determination of water holding capacity of pork. Meat Sci. 2001, 57, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Brøndum, J.; Munck, L.; Henckel, P.; Karlsson, A.; Tornberg, E.; Engelsen, S.B. Prediction of water-holding capacity and composition of porcine meat by comparative spectroscopy. Meat Sci. 2000, 55, 177–185. [Google Scholar] [CrossRef]
- Zhu, H.; O’Farrell, M.; Bouquet, G.; Lunde, K.; Egelandsdal, B.; Alvseike, O.; Berg, P.; Gjerlaug-Enger, E.; Hansen, E.W. Evaluating nuclear magnetic resonance (NMR) as a robust reference method for online spectroscopic measurement of water holding capacity (WHC). J. Food Eng. 2016, 175, 51–57. [Google Scholar] [CrossRef]
- Straadt, I.K.; Rasmussen, M.; Andersen, H.J.; Bertram, H.C. Aging-induced changes in microstructure and water distribution in fresh and cooked pork in relation to water-holding capacity and cooking loss–A combined confocal laser scanning microscopy (CLSM) and low-field nuclear magnetic resonance relaxation study. Meat Sci. 2007, 75, 687–695. [Google Scholar] [CrossRef]
| (A) | |||||||||
| Muscle | Fat Category | DM (%) | Ash (DM) (%) | IMF (DM) (%) | Protein (DM) (%) | ||||
| BF | A | 25.88 ± 0.73 | b, γ | 4.18 ± 0.14 | a, α | 14.05 ± 0.81 | b, γ | 81.28 ± 1.02 | b, α |
| SM | A | 26.01 ± 0.34 | b, βγ | 4.49 ± 0.09 | a, β | 11.13 ± 0.54 | c, γ | 83.92 ± 0.42 | a, α |
| ST | A | 27.23 ± 0.67 | a, β | 4.11 ± 0.52 | a, αβ | 21.69 ± 0.89 | a, β | 73.62 ± 0.78 | c, α |
| BF | B | 26.75 ± 0.67 | a, βγ | 3.73 ± 0.34 | a, β | 16.11 ± 1.76 | b, β | 79.75 ± 1.70 | b, β |
| SM | B | 25.52 ± 0.30 | b, γ | 4.12 ± 0.30 | a, γ | 13.32 ± 0.68 | c, β | 82.10 ± 0.48 | a, β |
| ST | B | 26.35 ± 0.25 | a, γ | 3.73 ± 0.38 | a, β | 20.87 ± 0.82 | a, β | 74.90 ± 0.78 | c, α |
| BF | C | 28.31 ± 0.69 | a, α | 4.04 ± 0.44 | b, αβ | 21.34 ± 1.16 | b, α | 74.32 ± 0.60 | b, δ |
| SM | C | 26.19 ± 0.33 | b, β | 4.79 ± 0.21 | a, α | 11.65 ± 1.70 | c, βγ | 83.16 ± 1.41 | a, αβ |
| ST | C | 28.10 ± 0.16 | a, α | 4.40 ± 0.34 | ab, α | 23.53 ± 1.05 | a, α | 71.62 ± 1.15 | c, β |
| BF | D | 27.09 ± 0.51 | b, β | 3.96 ± 0.32 | b, αβ | 20.05 ± 0.49 | b, α | 75.81 ± 0.52 | a, γ |
| SM | D | 27.29 ± 0.48 | b, α | 4.40 ± 0.08 | a, β | 18.62 ± 2.13 | b, α | 76.51 ± 2.22 | a, γ |
| ST | D | 28.46 ± 0.36 | a, α | 4.15 ± 0.06 | ab, αβ | 23.89 ± 1.23 | a, α | 71.50 ± 1.06 | b, β |
| (B) | |||||||||
| Muscle | Fat Category | pH | WHC (%) | Drip Loss (%) | |||||
| BF | A | 5.77 ± 0.05 | ab, β | 48.44 ± 1.78 | a, αβ | 4.10 ± 1.03 | ab, αβ | ||
| SM | A | 5.72 ± 0.09 | b, α | 48.18 ± 2.31 | a, αβ | 5.07 ± 0.84 | a, βγ | ||
| ST | A | 5.88 ± 0.12 | a, α | 50.11 ± 0.83 | a, αβ | 3.44 ± 0.72 | b, βγ | ||
| BF | B | 5.94 ± 0.34 | a, αβ | 50.38 ± 1.42 | ab, α | 3.46 ± 0.49 | b, β | ||
| SM | B | 5.76 ± 0.22 | a, α | 48.71 ± 0.77 | b, α | 5.04 ± 0.54 | a, γ | ||
| ST | B | 5.88 ± 0.46 | a, α | 51.14 ± 1.38 | a, α | 3.19 ± 0.61 | b, γ | ||
| BF | C | 6.13 ± 0.25 | a, α | 46.91 ± 2.48 | a, β | 5.04 ± 1.30 | a, α | ||
| SM | C | 5.87 ± 0.17 | a, α | 46.35 ± 1.49 | a, β | 6.19 ± 0.78 | a, α | ||
| ST | C | 6.02 ± 0.27 | a, α | 46.84 ± 2.95 | a, γ | 4.73 ± 1.30 | a, α | ||
| BF | D | 6.10 ± 0.09 | a, α | 48.10 ± 2.48 | a, αβ | 4.74 ± 1.08 | b, αβ | ||
| SM | D | 5.92 ± 0.07 | b, α | 46.91 ± 1.18 | a, αβ | 5.97 ± 0.48 | a, αβ | ||
| ST | D | 6.11 ± 0.14 | a, α | 47.90 ± 1.42 | a, βγ | 4.56 ± 0.54 | b, αβ | ||
| (C) | |||||||||
| Muscle | Fat Category | L* | a* | b* | |||||
| BF | A | 51.13 ± 1.67 | a, α | 9.65 ± 0.79 | b, β | 4.96 ± 0.65 | b, α | ||
| SM | A | 53.66 ± 2.40 | a, α | 8.15 ± 0.93 | c, β | 5.76 ± 0.81 | b, α | ||
| ST | A | 51.67 ± 1.64 | a, α | 11.66 ± 1.30 | a, γ | 6.74 ± 0.38 | a, αβ | ||
| BF | B | 48.32 ± 0.48 | a, β | 11.85 ± 0.71 | a, α | 5.50 ± 0.40 | ab, α | ||
| SM | B | 50.61 ± 1.34 | a, β | 8.96 ± 0.53 | b, β | 4.72 ± 0.07 | b, β | ||
| ST | B | 48.73 ± 2.54 | a, β | 12.76 ± 1.61 | a, βγ | 6.45 ± 1.22 | a, β | ||
| BF | C | 46.95 ± 1.91 | b, β | 12.26 ± 0.63 | b, α | 5.07 ± 0.54 | b, α | ||
| SM | C | 46.78 ± 1.19 | b, γ | 10.57 ± 1.17 | c, α | 5.06 ± 0.26 | b, αβ | ||
| ST | C | 51.25 ± 1.94 | a, αβ | 14.75 ± 0.87 | a, α | 8.04 ± 0.97 | a, α | ||
| BF | D | 46.82 ± 0.85 | b, β | 12.25 ± 0.83 | a, α | 5.21 ± 0.22 | b, α | ||
| SM | D | 48.96 ± 1.84 | a, βγ | 10.70 ± 0.55 | b, α | 5.45 ± 0.69 | b, αβ | ||
| ST | D | 54.14 ± 5.85 | a, αβ | 12.18 ± 1.55 | a, αβ | 7.13 ± 1.28 | a, αβ | ||
| Parameters | Factors | |||||
|---|---|---|---|---|---|---|
| Muscle (M) | Fat Category (F) | MxF | ||||
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | |
| Chemical composition (%) | ||||||
| Dry matter (DM) | 34.01 | <0.0001 | 34.07 | <0.0001 | 8.20 | <0.0001 |
| Ash content (DM) | 12.81 | <0.0001 | 8.66 | 0.0001 | 0.70 | 0.6509 |
| Protein content (DM) | 281.56 | <0.0001 | 61.85 | <0.0001 | 16.62 | <0.0001 |
| Intramuscular fat content (DM) | 265.01 | <0.0001 | 54.55 | <0.0001 | 15.86 | <0.0001 |
| Physicochemical characteristics | ||||||
| pH | 3.46 | 0.0395 | 4.27 | 0.0094 | 0.28 | 0.9431 |
| Water parameters | ||||||
| Water holding capacity (WHC) (%) | 3.24 | 0.0479 | 9.73 | <0.0001 | 0.48 | 0.8216 |
| Drip loss (%) | 18.72 | <0.0001 | 9.52 | <0.0001 | 0.18 | 0.9823 |
| Color parameters | ||||||
| L* | 9.19 | 0.0004 | 15.35 | <0.0001 | 4.33 | 0.0014 |
| a* | 63.18 | <0.0001 | 21.53 | <0.0001 | 1.01 | 0.4327 |
| b* | 44.4 | <0.0001 | 1.27 | 0.2961 | 2.85 | 0.0185 |
| Fat | T2 (4.7 T) | T1 (4.7 T) | T2 (1 T) | T1 (1 T) | T2 (0.15 T) | T1 (0.15 T) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 43.50 ± 0.23 | a | 1312.20 ± 15.75 | a | 51.85 ± 1.06 | a | 739.47 ± 29.06 | b | 56.91 ± 0.86 | a | 337.55 ± 1.44 | b |
| B | 43.84 ± 1.14 | a | 1308.40 ± 44.83 | a | 52.70 ± 2.47 | a | 767.59 ± 5.91 | a | 54.67 ± 0.10 | b | 370.86 ± 0.64 | a |
| C | 40.65 ± 2.61 | b | 1265.40 ± 51.26 | a | 47.28 ± 3.57 | b | 752.78 ± 18.26 | ab | 50.02 ± 1.50 | d | 306.86 ± 18.08 | c |
| D | 42.96 ± 0.44 | a | 1266.00 ± 24.85 | a | 51.54 ± 1.39 | a | 741.97 ± 8.97 | b | 52.82 ± 1.08 | c | 346.04 ± 0.67 | b |
| Dependent Variable | Independent Variable | Regression Coefficient | SE | F-Value | p-Value | R2 Model |
|---|---|---|---|---|---|---|
| WHC | constant | 567.415 | 129.249 | 0.94 | ||
| T2 (4.7 T) (ms) | −15.4179 | 3.17987 | 23.51 | 0.0002 | Adj. R2 model | |
| T1 (4.7 T) (ms) | −0.367942 | 0.172053 | 4.57 | 0.0493 | 0.92 | |
| T22 (4.7 T) (ms) | 0.198722 | 0.0383775 | 26.81 | 0.0001 | SE model | |
| T12 (4.7 T) (ms) | 0.00015104 | 0.0000663681 | 5.18 | 0.0380 | 0.69 | |
| p-value model | ||||||
| <0.0001 | ||||||
| F-value model | ||||||
| 55.10 | ||||||
| Dependent Variable | Independent Variable | Regression Coefficient | SE | F-Value | p-Value | R2 Model |
| WHC | constant | 1719.87 | 223.446 | 0.95 | ||
| T2 (1 T) (ms) | −2.63615 | 1.13718 | 5.37 | 0.0350 | Adj. R2 model | |
| T1 (1 T) (ms) | −4.3731 | 0.571921 | 58.47 | <0.0001 | 0.94 | |
| T22 (1 T) (ms) | 0.0324571 | 0.0113611 | 8.16 | 0.0120 | SE model | |
| T12 (1 T) (ms) | 0.00294693 | 0.000383083 | 59.18 | <0.0001 | 0.60 | |
| p-value model | ||||||
| <0.0001 | ||||||
| F-value model | ||||||
| 73.28 | ||||||
| Dependent Variable | Independent Variable | Regression Coefficient | SE | F-Value | p-Value | R2 Model |
| WHC | constant | 242.059 | 27.6622 | 0.91 | ||
| T2 (0.15 T) (ms) | 0.846445 | 0.0895625 | 89.32 | <0.0001 | Adj. R2 model | |
| T1 (0.15 T) (ms) | −1.42949 | 0.175391 | 66.43 | <0.0001 | 0.89 | |
| T12 (0.15 T) (ms) | 0.00212762 | 0.000261601 | 66.15 | <0.0001 | SE model | |
| 0.79 | ||||||
| p-value model | ||||||
| <0.0001 | ||||||
| F-value model | ||||||
| 42.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Remiro, V.; Cambero, M.I.; Romero-de-Ávila, M.D.; Castejón, D.; Segura, J.; Fernández-Valle, M.E. Contributions to Estimating the Water-Holding Capacity in Fresh Pork Hams Using NMR Relaxometry. Foods 2025, 14, 2329. https://doi.org/10.3390/foods14132329
Remiro V, Cambero MI, Romero-de-Ávila MD, Castejón D, Segura J, Fernández-Valle ME. Contributions to Estimating the Water-Holding Capacity in Fresh Pork Hams Using NMR Relaxometry. Foods. 2025; 14(13):2329. https://doi.org/10.3390/foods14132329
Chicago/Turabian StyleRemiro, Víctor, María Isabel Cambero, María Dolores Romero-de-Ávila, David Castejón, José Segura, and María Encarnación Fernández-Valle. 2025. "Contributions to Estimating the Water-Holding Capacity in Fresh Pork Hams Using NMR Relaxometry" Foods 14, no. 13: 2329. https://doi.org/10.3390/foods14132329
APA StyleRemiro, V., Cambero, M. I., Romero-de-Ávila, M. D., Castejón, D., Segura, J., & Fernández-Valle, M. E. (2025). Contributions to Estimating the Water-Holding Capacity in Fresh Pork Hams Using NMR Relaxometry. Foods, 14(13), 2329. https://doi.org/10.3390/foods14132329







