Determination of L-Theanine and Caffeine Contents in Tea Infusions with Different Fermentation Degrees and Brewing Conditions Using the Chromatographic Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Selection and Procurement
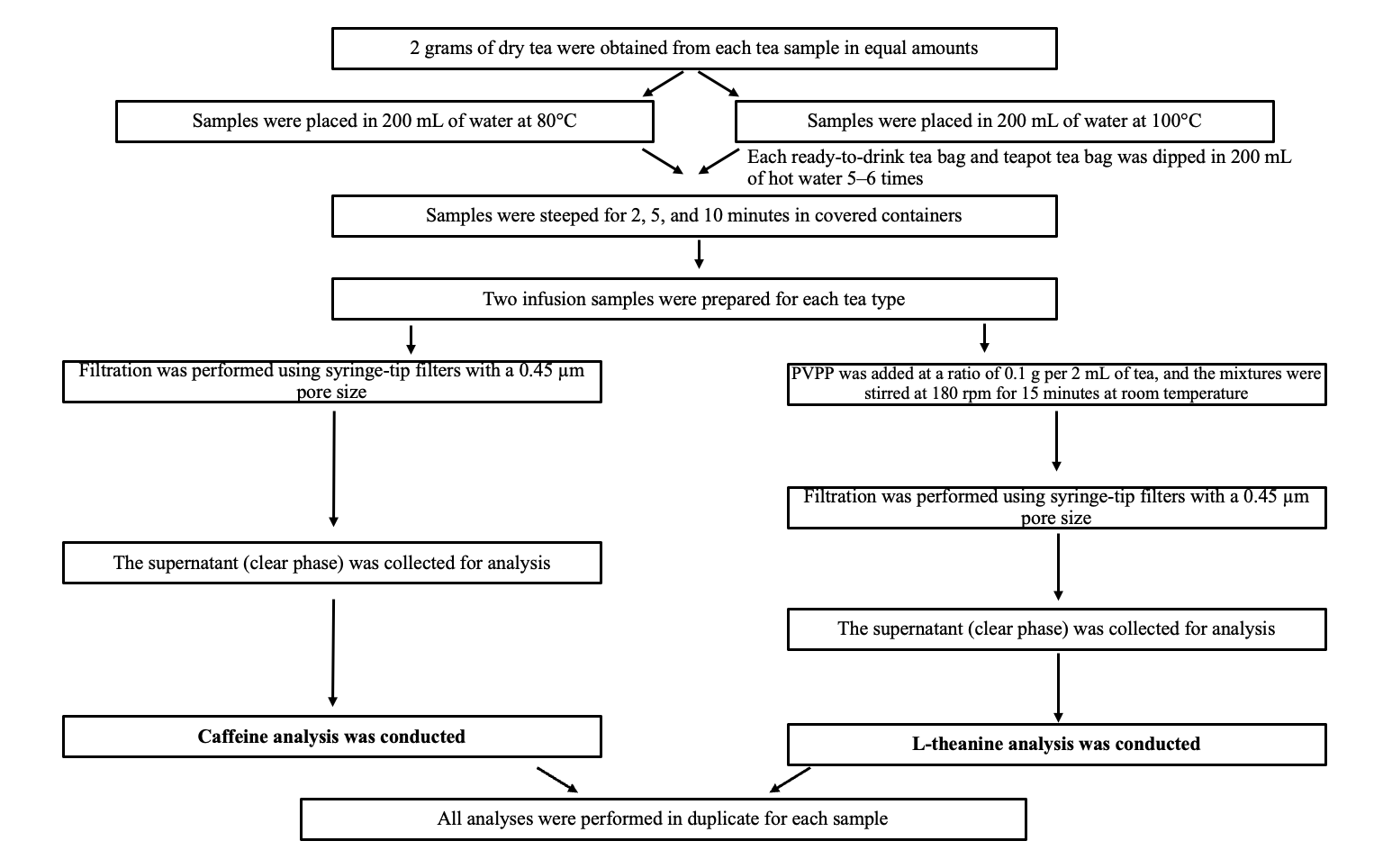
2.2. Preparation of Tea Infusions
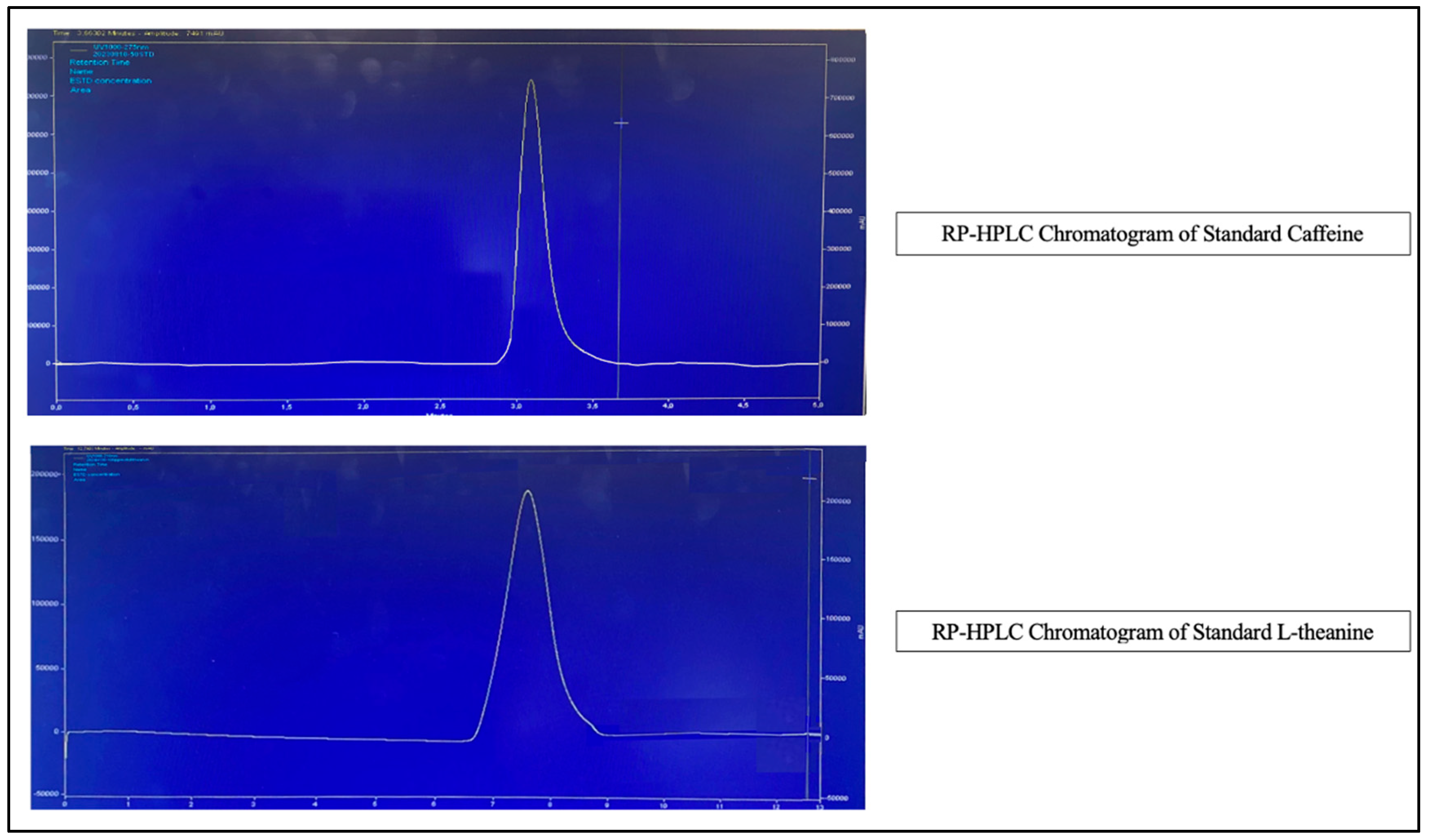
2.3. Chromatographic Analyses
2.3.1. Caffeine
2.3.2. L-Theanine
2.3.3. Method Validation
2.4. Statistical Analysis
3. Results
3.1. Caffeine
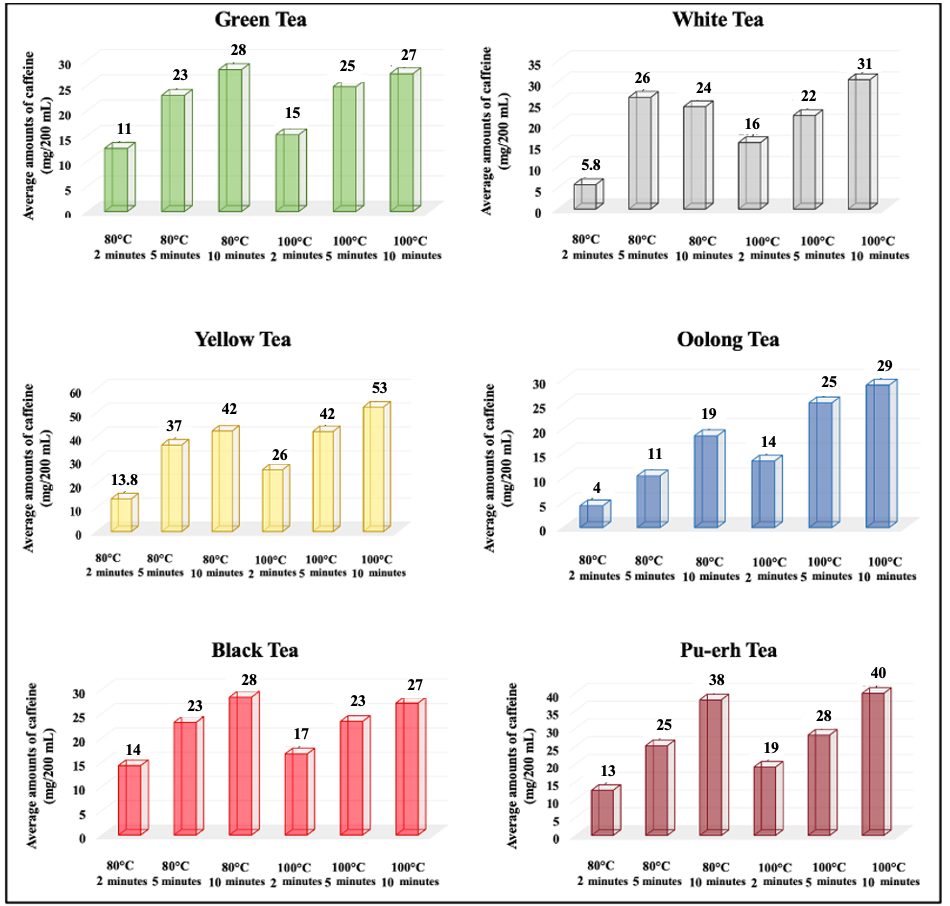
3.1.1. Caffeine Levels by Tea Type
3.1.2. Effects of Brewing Conditions on Caffeine Levels
3.1.3. Effect of the Serving Method on Caffeine Levels
3.2. L-Theanine
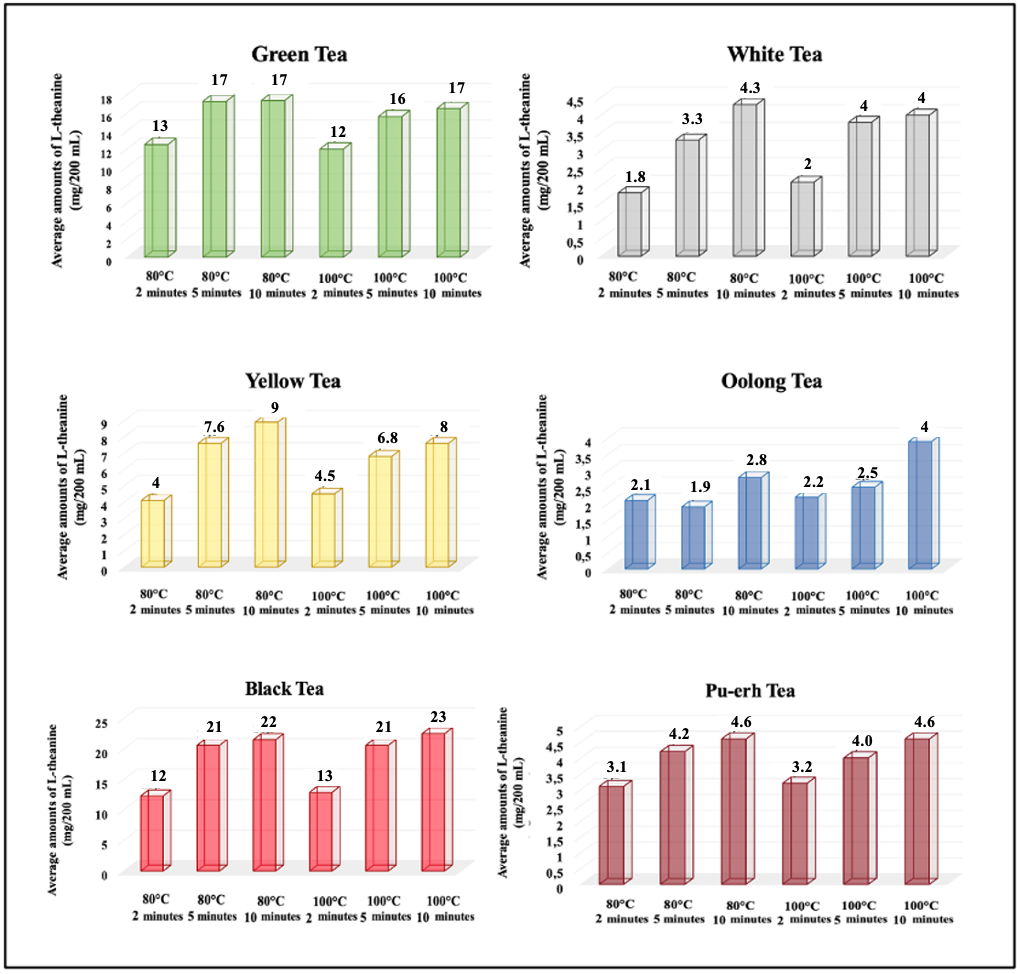
3.2.1. Caffeine Levels by Tea Type
3.2.2. Effects of Brewing Conditions on L-Theanine Levels
3.2.3. Effects of the Serving Method on L-Theanine Levels
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GABA | Gamma aminobutyric acid |
| GC | Gas chromatography |
| HPLC | High performance liquid chromatography |
| HPLC-DAD | HPLC-diode array detector |
| HPLC-MS | HPLC-mass spectrometer |
| HPLC-UV | HPLC-ultraviolet |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| POD | Peroxidase |
| PPO | Polyphenol peroxidase |
| PVPP | Polyvinylpolypyrrolidone |
| RP-HPLC | Revere phase-HPLC |
References
- Zeng, L.; Watanabe, N.; Yang, Z. Understanding the Biosyntheses and Stress Response Mechanisms of Aroma Compounds in Tea (Camellia sinensis) to Safely and Effectively Improve Tea Aroma. Crit. Rev. Food Sci. Nutr. 2019, 59, 2321–2334. [Google Scholar] [CrossRef]
- Türközü, D.; Şanlier, N. L-Theanine, Unique Amino Acid of Tea, and Its Metabolism, Health Effects, and Safety. Crit. Rev. Food Sci. Nutr. 2017, 57, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xia, G.; Luo, Z.; Liu, S. Uhplc Analysis of Major Functional Components in Six Types of Chinese Teas: Constituent Profile and Origin Consideration. LWT 2019, 102, 52–57. [Google Scholar] [CrossRef]
- Boros, K.; Jedlinszki, N.; Csupor, D. Theanine and Caffeine Content of Infusions Prepared from Commercial Tea Samples. Pharmacogn. Mag. 2016, 12, 75–79. [Google Scholar] [PubMed]
- Hayat, K.; Iqbal, H.; Malik, U.; Bilal, U.; Mushtaq, S. Tea and Its Consumption: Benefits and Risks. Crit. Rev. Food Sci. Nutr. 2015, 55, 939–954. [Google Scholar] [CrossRef] [PubMed]
- Keenan, E.K.; Finnie, M.D.A.; Jones, P.S.; Rogers, P.J.; Priestley, C.M. How Much Theanine in a Cup of Tea? Effects of Tea Type and Method of Preparation. Food Chem. 2011, 125, 588–594. [Google Scholar] [CrossRef]
- Dou, Q.P. Tea in Health and Disease. Nutrients 2019, 11, 929. [Google Scholar] [CrossRef]
- Camfield, D.A.; Stough, C.; Farrimond, J.; Scholey, A.B. Acute Effects of Tea Constituents L-Theanine, Caffeine, and Epigallocatechin Gallate on Cognitive Function and Mood: A Systematic Review and Meta-Analysis. Nutr. Rev. 2014, 72, 507–522. [Google Scholar] [CrossRef]
- Rodak, K.; Kokot, I.; Kratz, E.M. Caffeine as a Factor Influencing the Functioning of the Human Body—Friend or Foe? Nutrients 2021, 13, 3088. [Google Scholar] [CrossRef]
- Anas Sohail, A.; Ortiz, F.; Varghese, T.; Fabara, S.P.; Batth, A.S.; Sandesara, D.P.; Sabir, A.; Khurana, M.; Datta, S.; Patel, U.K. The Cognitive-Enhancing Outcomes of Caffeine and L-Theanine: A Systematic Review. Cureus 2021, 13, e20828. [Google Scholar] [CrossRef]
- Zhao, J.; Li, P.; Xia, T.; Wan, X. Exploring Plant Metabolic Genomics: Chemical Diversity, Metabolic Complexity in the Biosynthesis and Transport of Specialized Metabolites with the Tea Plant as a Model. Crit. Rev. Biotechnol. 2020, 40, 667–688. [Google Scholar] [CrossRef]
- Mejia, E.G.d.; Ramirez-Mares, M.V. Impact of Caffeine and Coffee on Our Health. Trends Endocrinol. Metab. 2014, 25, 489–492. [Google Scholar] [CrossRef]
- Mitchell, D.C.; Knight, C.A.; Hockenberry, J.; Teplansky, R.; Hartman, T. J Beverage Caffeine Intakes in the U.S. Food Chem. Toxicol. 2014, 63, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Chen, Z.; Chen, T.; Deng, W.; Wan, X.; Zhang, Z. Theanine Metabolism and Transport in Tea Plants (Camellia sinensis L.): Advances and Perspectives. Crit. Rev. Biotechnol. 2022, 43, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Vuong, Q.V.; Bowyer, M.C.; Roach, P.D. L-Theanine: Properties, Synthesis and Isolation from Tea. J. Sci. Food Agric. 2011, 91, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Horie, H.; Matsunaga, A.; Hirono, Y. Effect of Shading Intensity on Morphological and Color Traits and on Chemical Components of New Tea (Camellia sinensis L.) Shoots under Direct Covering Cultivation. J. Sci. Food Agric. 2018, 98, 5666–5676. [Google Scholar] [CrossRef]
- Komes, D.; Horžić, D.; Belščak, A.; Ganić, K.K.; Vulić, I. Green Tea Preparation and Its Influence on the Content of Bioactive Compounds. Food Res. Int. 2010, 43, 167–176. [Google Scholar] [CrossRef]
- Kaczyński, P.; Iwaniuk, P.; Jankowska, M.; Orywal, K.; Socha, K.; Perkowski, M.; Farhan, J.A.; Łozowicka, B. Pesticide Residues in Common and Herbal Teas Combined with Risk Assessment and Transfer to the Infusion. Chemosphere 2024, 367, 143550. [Google Scholar] [CrossRef]
- Lakenbrink, C.; Lapczynski, S.; Maiwald, B.; Engelhardt, U.H. Flavonoids and Other Polyphenols in Consumer Brews of Tea and Other Caffeinated Beverages. J. Agric. Food Chem. 2000, 48, 2848–2852. [Google Scholar] [CrossRef]
- Yang, X.R.; Ye, C.X.; Xu, J.K.; Jiang, Y.M. Simultaneous Analysis of Purine Alkaloids and Catechins in Camellia sinensis, Camellia ptilophylla and Camellia assamica Var. Kucha by Hplc. Food Chem. 2007, 100, 1132. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Giménez, R.; Rufián-Henares, J.A.; Pastoriza, S. Effect of Brewing Time and Temperature on Antioxidant Capacity and Phenols of White Tea: Relationship with Sensory Properties. Food Chem. 2018, 248, 111–118. [Google Scholar] [CrossRef]
- Polat, A.; Kalcıoğlu, Z.; Müezzinoğlu, N. Effect of Infusion Time on Black Tea Quality, Mineral Content and Sensory Properties Prepared Using Traditional Turkish Infusion Method. Int. J. Gastron. Food Sci. 2022, 29, 100559. [Google Scholar] [CrossRef]
- Araslanova, K.; Nastos, J.M.; Sommerfeld, J.; Megill, W.; Struck, A.; Shirtcliffe, N.J. On the Relative Extraction Rates of Colour Compounds and Caffeine During Brewing, an Investigation of Tea over Time and Temperature. Open Chem. 2022, 20, 708–715. [Google Scholar] [CrossRef]
- Alcázar, A.; Ballesteros, O.; Jurado, J.M.; Pablos, F.; Martín, M.J.; Vilches, J.L.; Navalón, A. Differentiation of Green, White, Black, Oolong, and Pu-Erh Teas According to Their Free Amino Acids Content. J. Agric. Food Chem. 2007, 55, 5960–5965. [Google Scholar] [CrossRef]
- Syu, K.-Y.; Lin, C.-L.; Huang, H.-C.; Lin, J.-K. Determination of Theanine, Gaba, and Other Amino Acids in Green, Oolong, Black, and Pu-Erh Teas with Dabsylation and High-Performance Liquid Chromatography. J. Agric. Food Chem. 2008, 56, 7637–7643. [Google Scholar] [CrossRef] [PubMed]
- Thippeswamy, R.; Gouda, K.G.M.; Rao, D.H.; Martin, A.; Gowda, L.R. Determination of Theanine in Commercial Tea by Liquid Chromatography with Fluorescence and Diode Array Ultraviolet Detection. J. Agric. Food Chem. 2006, 54, 7014–7019. [Google Scholar] [CrossRef]
- Ying, Y.; Ho, J.W.; Chen, Z.Y.; Wang, J. Analysis of Theanine in Tea Leaves by Hplc with Fluorescence Detection. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 727–737. [Google Scholar] [CrossRef]
- Kowalska, J.; Marzec, A.; Domian, E.; Galus, S.; Ciurzyńska, A.; Brzezińska, R.; Kowalska, H. Influence of Tea Brewing Parameters on the Antioxidant Potential of Infusions and Extracts Depending on the Degree of Processing of the Leaves of Camellia sinensis. Molecules 2021, 26, 4773. [Google Scholar] [CrossRef]
- Stapley, A.G.F. Modelling the Kinetics of Tea and Coffee Infusion. J. Sci. Food Agric. 2002, 82, 1661–1671. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Golding, J.B.; Nguyen, M.H.; Roach, P.D. Preparation of Decaffeinated and High Caffeine Powders from Green Tea. Powder Technol. 2013, 233, 169–175. [Google Scholar] [CrossRef]
- Pokhrel, P.; Shrestha, S.; Rijal, S.; Rai, K. A Simple Hplc Method for the Determination of Caffeine Content in Tea and Coffee. J. Food Sci. Technol. Nepal 2016, 9, 74. [Google Scholar] [CrossRef]
- Henríquez-Aedo, K.; Vega, M.; Aranda, M. Evaluation of Tea Functionality: Determination of L-Theanine Content in Green and Black Teas by Liquid Chromatography. J. Chil. Chem. Soc. 2013, 58, 1651–1654. [Google Scholar] [CrossRef]
- Hess, A.S.; Hess, J.R. Understanding Standard Deviations and Standard Errors. Transfusion 2016, 56, 1259–1261. [Google Scholar] [CrossRef]
- Qi, D.; Miao, A.; Cao, J.; Wang, W.; Chen, W.; Pang, S.; He, X.; Ma, C. Study on the Effects of Rapid Aging Technology on the Aroma Quality of White Tea Using Gc–Ms Combined with Chemometrics: In Comparison with Natural Aged and Fresh White Tea. Food Chem. 2018, 265, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S. Potential Bioactive Components and Health Promotional Benefits of Tea (Camellia sinensis). J. Am. Nutr. Assoc. 2022, 41, 65–93. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, L.; Zhou, S.; Hong, Y.; Zhang, X.; Chen, H. Analysis of Differences in the Accumulation of Tea Compounds under Various Processing Techniques, Geographical Origins, and Harvesting Seasons. Food Chem. 2024, 430, 137000. [Google Scholar] [CrossRef] [PubMed]
- Sari, F.; Velioglu, Y.S. Changes in Theanine and Caffeine Contents of Black Tea with Different Rolling Methods and Processing Stages. Eur. Food Res. Technol. 2013, 237, 229–236. [Google Scholar] [CrossRef]
- Zhang, L.; Ho, C.-T.; Zhou, J.; Santos, J.S.; Armstrong, L.; Granato, D. Chemistry and Biological Activities of Processed Camellia sinensis Teas: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1474–1495. [Google Scholar] [CrossRef]
- Venditti, E.; Bacchetti, T.; Tiano, L.; Carloni, P.; Greci, L.; Damiani, E. Hot Vs. Cold Water Steeping of Different Teas: Do They Affect Antioxidant Activity? Food Chem. 2010, 119, 1597–1604. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, F.; Qin, L.; Zhang, N.; Cao, Q.; Schwab, W.; Li, D.; Song, C. Dynamic Change in Amino Acids, Catechins, Alkaloids, and Gallic Acid in Six Types of Tea Processed from the Same Batch of Fresh Tea (Camellia sinensis L.) Leaves. J. Food Compos. Anal. 2019, 77, 28–38. [Google Scholar] [CrossRef]
- Wang, Y.; Kan, Z.; Thompson, H.J.; Ling, T.; Ho, C.-T.; Li, D.; Wan, X. Impact of Six Typical Processing Methods on the Chemical Composition of Tea Leaves Using a Single Camellia sinensis Cultivar, Longjing 43. J. Agric. Food Chem. 2018, 67, 5423–5436. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Chen, H.; Deng, Y. Simultaneous Determination of Catechins, Caffeine and Gallic Acids in Green, Oolong, Black and Pu-Erh Teas Using Hplc with a Photodiode Array Detector. Talanta 2002, 57, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Paiva, L.; Rego, C.; Lima, E.; Marcone, M.; Baptista, J. Comparative Analysis of the Polyphenols, Caffeine, and Antioxidant Activities of Green Tea, White Tea, and Flowers from Azorean Camellia sinensis Varieties Affected by Different Harvested and Processing Conditions. Antioxidants 2021, 10, 183. [Google Scholar] [CrossRef]
- Khokhar, S.; Magnusdottir, S. Total Phenol, Catechin, and Caffeine Contents of Teas Commonly Consumed in the United Kingdom. J. Agric. Food Chem. 2002, 50, 565–570. [Google Scholar] [CrossRef]
- Fernández, P.L.; Martín, M.J.; González, A.G.; Pablos, F. Hplc Determination of Catechins and Caffeine in Tea. Differentiation of Green, Black and Instant Teas. Analyst 2000, 125, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Erol, N.T.; Sarı, F.; Velioglu, Y.S. Polyphenols, Alkaloids and Antioxidant Activity of Different Grades Turkish Black Tea. GIDA 2010, 35, 161–168. [Google Scholar]
- Horanni, R.; Engelhardt, U.H. Determination of Amino Acids in White, Green, Black, Oolong, Pu-Erh Teas and Tea Products. J. Food Compos. Anal. 2013, 31, 94–100. [Google Scholar] [CrossRef]
- Yi, T.; Zhu, L.; Peng, W.-L.; He, X.-C.; Chen, H.-L.; Li, J.; Yu, T.; Liang, Z.-T.; Zhao, Z.-Z.; Chen, H.-B. Comparison of Ten Major Constituents in Seven Types of Processed Tea Using Hplc-Dad-Ms Followed by Principal Component and Hierarchical Cluster Analysis. LWT-Food Sci. Technol. 2015, 62, 194–201. [Google Scholar] [CrossRef]
- Hilal, Y.; Engelhardt, U. Characterisation of White Tea—Comparison to Green and Black Tea. J. Verbraucherschutz Leb. 2007, 2, 414–421. [Google Scholar] [CrossRef]
- Zhou, P.; Zhao, F.; Chen, M.; Ye, N.; Lin, Q.; Ouyang, L.; Cai, X.; Meng, P.; Gong, X.; Wang, Y. Determination of 21 Free Amino Acids in 5 Types of Tea by Ultra-High Performance Liquid Chromatography Coupled with Tandem Mass Spectrometry (Uhplc–Ms/Ms) Using a Modified 6-Aminoquinolyl-N-Hydroxysuccinimidyl Carbamate (Aqc) Method. J. Food Compos. Anal. 2019, 81, 46–54. [Google Scholar] [CrossRef]
- Zhao, C.-N.; Tang, G.-Y.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Liu, Q.; Mao, Q.-Q.; Shang, A.; Li, H.-B. Phenolic Profiles and Antioxidant Activities of 30 Tea Infusions from Green, Black, Oolong, White, Yellow and Dark Teas. Antioxidants 2019, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-X.; Yang, L.; Lei, Y.-S.; Ju, R.-N.; Miao, S.-G.; Jin, S.-H. Integrated Transcriptome and Amino Acid Profile Analyses Reveal Novel Insights into Differential Accumulation of Theanine in Green and Yellow Tea Cultivars. Tree Physiol. 2022, 42, 1501–1516. [Google Scholar] [CrossRef]
- Fan, F.-Y.; Zhou, S.-J.; Qian, H.; Zong, B.-Z.; Huang, C.-S.; Zhu, R.-L.; Guo, H.-W.; Gong, S.-Y. Effect of Yellowing Duration on the Chemical Profile of Yellow Tea and the Associations with Sensory Traits. Molecules 2022, 27, 940. [Google Scholar] [CrossRef]
- Ekborg-Ott, K.H.; Taylor, A.; Armstrong, D.W. Varietal Differences in the Total and Enantiomeric Composition of Theanine in Tea. J. Agric. Food Chem. 1997, 45, 353–363. [Google Scholar] [CrossRef]
- Ho, K.K.; Haufe, T.C.; Ferruzzi, M.G.; Neilson, A.P. Production and Polyphenolic Composition of Tea. Nutr. Today 2018, 53, 268–278. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Li, K.; Li, C.; Li, L.; Li, J.; Huang, H.; He, Y.; Ye, C.; Song, X. Simultaneous Determination of Theanine, Gallic Acid, Purine Alkaloids, Catechins, and Theaflavins in Black Tea Using Hplc. Int. J. Food Sci. Technol. 2010, 45, 1263–1269. [Google Scholar] [CrossRef]
- Suteerapataranon, S.; Butsoongnern, J.; Punturat, P.; Jorpalit, W.; Thanomsilp, C. Caffeine in Chiang Rai Tea Infusions: Effects of Tea Variety, Type, Leaf Form, and Infusion Conditions. Food Chem. 2009, 114, 1335–1338. [Google Scholar] [CrossRef]
- Lin, Y.L.; Juan, I.M.; Chen, Y.L.; Liang, Y.C.; Lin, J.K. Composition of Polyphenols in Fresh Tea Leaves and Associations of Their Oxygen-Radical-Absorbing Capacity with Antiproliferative Actions in Fibroblast Cells. J. Agric. Food Chem. 1996, 44, 1387. [Google Scholar] [CrossRef]
- Alasalvar, C.; Topal, B.; Serpen, A.; Bahar, B.; Pelvan, E.; Gökmen, V. Flavor Characteristics of Seven Grades of Black Tea Produced in Turkey. J. Agric. Food Chem. 2012, 60, 6323–6332. [Google Scholar] [CrossRef]
- Deng, S.; Zhou, X.; Dong, H.; Xu, Y.; Gao, Y.; Wang, B.; Liu, X. . Mellow and Thick Taste of Pu−Erh Ripe Tea Based on Chemical Properties by Sensory−Directed Flavor Analysis. Foods 2022, 11, 2285. [Google Scholar] [CrossRef]
- Kim, Y.; Goodner, K.L.; Park, J.-D.; Choi, J.; Talcott, S.T. Changes in Antioxidant Phytochemicals and Volatile Composition of Camellia sinensis by Oxidation During Tea Fermentation. Food Chem. 2011, 129, 1331–1342. [Google Scholar] [CrossRef]
- Pan, J.; Jiang, Y.; Lv, Y.; Li, M.; Zhang, S.; Liu, J.; Zhu, Y.; Zhang, H. Comparison of the Main Compounds in Fuding White Tea Infusions from Various Tea Types. Food Sci. Biotechnol. 2018, 27, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Perva-Uzunalić, A.; Škerget, M.; Knez, Ž.; Weinreich, B.; Otto, F.; Grüner, S. . Extraction of Active Ingredients from Green Tea (Camellia sinensis): Extraction Efficiency of Major Catechins and Caffeine. Food Chem. 2006, 96, 597–605. [Google Scholar] [CrossRef]
- Astill, C.; Birch, M.R.; Dacombe, C.; Humphrey, P.G.; Martin, P.T. Factors Affecting the Caffeine and Polyphenol Contents of Black and Green Tea Infusions. J. Agric. Food Chem. 2001, 49, 5340–5347. [Google Scholar] [CrossRef]
- Saklar, S.; Ertas, E.; Ozdemir, I.S.; Karadeniz, B. Effects of Different Brewing Conditions on Catechin Content and Sensory Acceptance in Turkish Green Tea Infusions. J. Food Sci. Technol. 2015, 52, 6639–6646. [Google Scholar] [CrossRef]
| Caffeine | L-theanine | ||
|---|---|---|---|
| Tea Types | Brand | Tea Types | Brand |
| Green Tea (n:12) | Green Tea (n:4) | ||
| Loose-leaf (n:6) | A (n:1), C (n:2), D (n:1), E (n:1), K (n:1) | Loose-leaf (n:2) | D (n:1), E (n:1) |
| Cup of Tea Bags (n:6) | A (n:2), B (n:1), C (n:1), D (n:1), E (n:1) | Cup of Tea Bags (n:2) | D (n:1), E (n:1) |
| White Tea (n:5) | White Tea (n:3) | ||
| Loose-leaf (n:3) | E (n:1), F (n:1), G (n:1) | Loose-leaf (n:2) | E (n:1), F (n:1) |
| Cup of Tea Bags (n:2) | A (n:1), L (n:1) | Cup of Tea Bags (n:1) | A (n:1) |
| Yellow Tea (n:2) | Yellow Tea (n:2) | ||
| Loose-leaf (n:2) | F (n:1), L (n:1) | Loose-leaf (n:2) | F (n:1), L (n:1) |
| Oolong Tea (n:4) | Oolong Tea (n:3) | ||
| Loose-leaf (n:3) | F (n:1), G (n:1), L (n:1) | Loose-leaf (n:2) | F (n:1), G (n:1) |
| Cup of Tea Bags (n:1) | F (n:1) | Cup of Tea Bags (n:1) | F (n:1) |
| Black Tea (n:41) | Black Tea (n:6) | ||
| Loose-leaf (n:19) | A (n:1), B (n:4), C (n:4), D (n:2), E (n:3), F (n:1), G (n:2), H (n:1), I (n:1) | Loose-leaf (n:3) | C (n:1), D (n:1), E (n:1) |
| Teapot Bag (n:10) | A (n:2), B (n:3), C (n:1), D (n:2), J (n:2) | Cup of Tea Bags (n:3) | C (n:1), D (n:1), E (n:1) |
| Cup of Tea Bags (n:12) | A (n:2), B (n:2), C (n:2), D (n:1), E (n:2), F (n:1), J (n:2) | ||
| Pu-erh Tea (n:4) | Pu-erh Tea (n:2) | ||
| Loose-leaf (n:4) | F (n:2), G (n:1), L (n:1) | Loose-leaf (n:2) | F (n:1), L (n:1) |
| Tea Types | Brewing Temperature (°C) | Brewing Time (min) | |||||
|---|---|---|---|---|---|---|---|
| 2 | 5 | 10 | |||||
| ± Se | CV (%) | ± Se | CV (%) | ± Se | CV (%) | ||
| Green Tea (n:12) | 80 | 11 ± 2.1 | 63.8 | 23 ± 2.8 | 42.2 | 28 ± 4.6 | 56.7 |
| 100 | 15 ± 2.5 | 57.0 | 25 ± 3.1 | 43.5 | 27 ± 4.4 | 55.8 | |
| White Tea (n:5) | 80 | 5.8 ± 0.7 | 47.7 | 26 ± 10.0 | 78.2 | 24 ± 3.1 | 54.5 |
| 100 | 16 ± 2.2 | 31.3 | 22 ± 4.1 | 31.2 | 31 ± 2.5 | 27.5 | |
| Yellow Tea (n:2) | 80 | 13.8 ± 0.2 | 2.0 | 37 ± 3.3 | 12.8 | 42 ± 10.9 | 36.4 |
| 100 | 26 ± 1.4 | 7.6 | 42 ± 3.7 | 12.4 | 53 ± 16.9 | 45.5 | |
| Oolong Tea (n:4) | 80 | 4 ± 1.6 | 45.7 | 11 ± 2.1 | 40.0 | 19 ± 3.7 | 39.8 |
| 100 | 14 ± 3.9 | 57.8 | 25 ± 4.9 | 38.7 | 29 ± 2.4 | 16.6 | |
| Black Tea (n:41) | 80 | 14 ± 1.0 | 44.8 | 23 ± 1.4 | 38.6 | 28 ± 1.5 | 33.8 |
| 100 | 17 ± 1.0 | 38.3 | 23 ± 1.3 | 35.6 | 27 ± 1.5 | 35.3 | |
| Pu-erh Tea (n:2) | 80 | 13 ± 2.9 | 45.7 | 25 ± 5.1 | 40.5 | 38 ± 9.9 | 52.0 |
| 100 | 19± 9.5 | 57.0 | 28 ± 8.6 | 43.5 | 40 ± 9.9 | 55.8 | |
| Tea Type | Brewing Temperature (°C) | Brewing Time (min) | Cup Bag | Teapot Bag | Loose-Leaf | Test Statistics * | p | |||
|---|---|---|---|---|---|---|---|---|---|---|
| ± ss | CV (%) | ± -ss | CV (%) | ± ss | CV (%) | |||||
| Green Tea (nCB:6, nLT:6) | 80 | 2 | 13 ± 5.00 | 38.1 | - | 10 ± 5.70 | 54.8 | 0.869 | 0.405 | |
| 5 | 28 ± 5.60 | 20.5 | - | 17 ± 8.90 | 52.7 | 2.505 | 0.031 | |||
| 10 | 36 ± 7.90 | 22.0 | - | 19 ± 8.40 | 43.8 | 3.535 | 0.005 | |||
| 100 | 2 | 15 ± 5.70 | 37.9 | - | 15 ± 7.40 | 47.9 | −0.108 | 0.916 | ||
| 5 | 28 ± 6.90 | 24.9 | - | 20 ± 9.90 | 49.9 | 1.591 | 0.143 | |||
| 10 | 33 ± 10.90 | 33.5 | - | 22 ± 9.90 | 44.7 | 1.711 | 0.118 | |||
| White Tea (nCB:2, nLT:3) | 80 | 2 | 8 ± 2.00 | 25.2 | - | 4 ± 2.20 | 51.2 | 1.911 | 0.152 | |
| 5 | 18.2 ± 0.10 | 0.8 | - | 42 ± 30.70 | 73.8 | −1.022 | 0.382 | |||
| 10 | 22 ± 0.60 | 2.9 | - | 35 ± 20.40 | 58.6 | −0.865 | 0.451 | |||
| 100 | 2 | 15 ± 3.80 | 25.9 | - | 15 ± 6.10 | 39.8 | −0.109 | 0.920 | ||
| 5 | 18 ± 5.10 | 28.9 | - | 25 ± 7.10 | 28.1 | −1.244 | 0.302 | |||
| 10 | 28 ± 3.70 | 13.3 | - | 37 ± 10.40 | 28.4 | −1.134 | 0.339 | |||
| Black Tea (nCB:12, nTPB:10, nLT:19) | 80 | 2 | 20 ± 5.00 a | 24.7 | 8 ± 2.50 b | 30.7 | 15 ± 4.10 c | 27.7 | 24.366 | <0.001 |
| 5 | 30 ± 4.50 a | 14.9 | 18 ± 3.40 b | 18.8 | 23 ± 6.40 c | 28.0 | 15.374 | <0.001 | ||
| 10 | 38 ± 8.00 a | 21.2 | 25 ± 4.70 b | 18.7 | 26 ± 7.10 b | 27.5 | 12.930 | <0.001 | ||
| 100 | 2 | 23 ± 6.10 a | 26.6 | 11 ± 2.10 b | 19.9 | 18 ± 4.70 c | 26.7 | 19.060 | <0.001 | |
| 5 | 33 ± 7.10 a | 21.6 | 20 ± 2.10 b | 10.6 | 22 ± 5.80 b | 26.4 | 18.406 | <0.001 | ||
| 10 | 39 ± 9.90 a | 25.3 | 25 ± 3.50 b | 13.8 | 24 ± 7.20 b | 29.6 | 16.768 | <0.001 | ||
| Tea Types | Brewing Temperature (°C) | Brewing Time (min) | |||||
|---|---|---|---|---|---|---|---|
| 2 | 5 | 10 | |||||
| ± Se | CV (%) | ± Se | CV (%) | ± Se | CV (%) | ||
| Green Tea (n:4) | 80 | 13 ± 2.5 | 24.3 | 17 ± 3.6 | 25.1 | 17 ± 4.1 | 28.1 |
| 100 | 12 ± 3.1 | 31.0 | 16 ± 3.8 | 28.8 | 17 ± 4.5 | 32.4 | |
| White Tea (n:3) | 80 | 1.8 ± 0.4 | 22.5 | 3.3 ± 0.6 | 19.4 | 4.3 ± 0.7 | 17.8 |
| 100 | 2 ± 1.0 | 51.1 | 4 ± 2.0 | 54.8 | 4.0 ± 0.7 | 18.5 | |
| Yellow Tea (n:2) | 80 | 4 ± 1.1 | 21.8 | 7.6 ± 0.9 | 10.2 | 9 ± 5.4 | 51.9 |
| 100 | 4.5 ± 0.4 | 7.5 | 6.8 ± 0.8 | 9.6 | 8 ± 6.3 | 70.5 | |
| Oolong Tea (n:3) | 80 | 2.1 ± 0.5 | 23.1 | 1.9 ± 0.5 | 28.9 | 2.8 ± 0.4 | 16.7 |
| 100 | 2.2 ± 0.3 | 14.1 | 2.5 ± 0.7 | 27.9 | 4 ± 1.6 | 41.6 | |
| Black Tea (n:6) | 80 | 12 ± 2.8 | 48.0 | 21 ± 3.4 | 27.6 | 22 ± 2.5 | 36.5 |
| 100 | 13 ± 2.5 | 56.3 | 21 ± 2.1 | 27.2 | 23 ± 4.2 | 39.8 | |
| Pu-erh Tea (n:2) | 80 | 3.1 ± 0.4 | 10.0 | 4.2 ± 0.1 | 1.8 | 4.6 ± 0.1 | 2.0 |
| 100 | 3.2 ± 0.8 | 22.2 | 4.0 ± 0.4 | 9.3 | 4.6 ± 0.3 | 4.9 | |
| Tea Type | Brewing Temperature (°C) | Brewing Time (min) | Tea Bag | Loose-Leaf | Test Statistics * | p | ||
|---|---|---|---|---|---|---|---|---|
| ± ss | CV (%) | CV (%) | ||||||
| Green Tea (nTB:2, nLT:2) | 80 | 2 | 13 ± 4.3 | 38.1 | 12 ± 2.7 | 54.8 | 0.424 | 0.713 |
| 5 | 19 ± 1.1 | 20.5 | 15 ± 6.2 | 52.7 | 0.946 | 0.444 | ||
| 10 | 20 ± 2.0 | 22.0 | 15 ± 5.9 | 43.8 | 1.278 | 0.330 | ||
| 100 | 2 | 12 ± 3.3 | 37.9 | 12 ± 5.5 | 47.9 | 0.164 | 0.885 | |
| 5 | 18.1 ± 0.6 | 24.9 | 13 ± 5.9 | 49.9 | 1.195 | 0.355 | ||
| 10 | 20.5 ± 0.1 | 33.5 | 13 ± 4.8 | 44.7 | 2.34 | 0.144 | ||
| Black Tea (nTB:3, nLT:3) | 80 | 2 | 11 ± 1.4 | 24.7 | 13 ± 8.5 | 27.7 | −0.475 | 0.679 |
| 5 | 20 ± 6.6 | 14.9 | 21 ± 5.9 | 28.0 | −0.216 | 0.840 | ||
| 10 | 22 ± 12.0 | 21.2 | 22 ± 4.0 | 27.5 | −0.068 | 0.949 | ||
| 100 | 2 | 13 ± 4.0 | 26.6 | 15 ± 11.5 | 26.7 | −0.327 | 0.760 | |
| 5 | 20 ± 4.3 | 21.6 | 23 ± 7.6 | 26.4 | −0.691 | 0.528 | ||
| 10 | 25 ± 13.0 | 25.3 | 22 ± 7.0 | 29.6 | 0.325 | 0.761 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayakdaş, G.; Ağagündüz, D. Determination of L-Theanine and Caffeine Contents in Tea Infusions with Different Fermentation Degrees and Brewing Conditions Using the Chromatographic Method. Foods 2025, 14, 2313. https://doi.org/10.3390/foods14132313
Ayakdaş G, Ağagündüz D. Determination of L-Theanine and Caffeine Contents in Tea Infusions with Different Fermentation Degrees and Brewing Conditions Using the Chromatographic Method. Foods. 2025; 14(13):2313. https://doi.org/10.3390/foods14132313
Chicago/Turabian StyleAyakdaş, Gamze, and Duygu Ağagündüz. 2025. "Determination of L-Theanine and Caffeine Contents in Tea Infusions with Different Fermentation Degrees and Brewing Conditions Using the Chromatographic Method" Foods 14, no. 13: 2313. https://doi.org/10.3390/foods14132313
APA StyleAyakdaş, G., & Ağagündüz, D. (2025). Determination of L-Theanine and Caffeine Contents in Tea Infusions with Different Fermentation Degrees and Brewing Conditions Using the Chromatographic Method. Foods, 14(13), 2313. https://doi.org/10.3390/foods14132313








